Abstract
Purpose
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a necrotizing vasculitis syndrome characterized by the destruction of small vessels, leading to various organ disorders. Here, we report a case of posterior scleritis with AAV successfully treated with prednisolone and rituximab (RTX) combination therapy.
Observations
A 69-year-old female suffered from ocular pain and redness in her left eye for 2.5 years. She had been diagnosed with idiopathic otitis media before a year. At her initial visit, scleral injection with nodular elevated scleral lesions, vitreous haze, and serous retinal detachment (SRD) in the inferior periphery were observed in the left eye. Enhanced computed tomography revealed the enhancement and thickening of the left sclera. The results of laboratory analysis were positive for myeloperoxidase ANCA. Accordingly, she was diagnosed with AAV. Owing to the exacerbation of vitreous haze and SRD, topical treatment and steroid pulse therapy were initiated. Following therapy, anterior and posterior scleritis improved, and additional RTX was administered to maintain the remission. Following treatment, the patient has maintained remission with 10 mg/day prednisolone to date.
Conclusions and importance
We encountered a case of posterior scleritis with AAV in which inflammatory manifestations subsided with RTX and glucocorticoid combination therapy. RTX administration likely contributed to the maintenance of remission.
Keywords: Posterior scleritis, ANCA-Associated vasculitis, Rituximab
Abbreviations
- ANCA
anti-neutrophil cytoplasmic antibody
- AAV
ANCA-associated vasculitis
- PSL
prednisolone
- RTX
rituximab
- SRD
serous retinal detachment
- GPA
granulomatosis with polyangiitis
- BCVA
best-corrected visual acuity
- CME
cystoid macular edema
- AZA
azathioprine
- UBM
ultrasound biomicroscopy
- STTA
sub-tenon injection of triamcinolone acetonide
- RA
rheumatoid arthritis
- PR3-ANCA
protein-3 ANCA
- MPO-ANCA
myeloperoxidase ANCA
- GC
glucocorticoid
- CYC
cyclophosphamide
- CHCC
Chapel Hill Consensus Conference
1. Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a necrotizing vasculitis syndrome characterized by the destruction of small vessels, leading to the dysfunction of various organs, such as the ear, nose, throat, eye, respiratory tract, kidney, skin, and nervous system.1 Typical ocular complications include scleritis, episcleritis, orbital inflammation, lacrimal duct stenosis, and uveitis.2
Rituximab (RTX) is a chimeric monoclonal antibody against B-cell-specific CD20. RTX has recently been recommended for the treatment of granulomatosis with polyangiitis (GPA) in patients who are resistant to or intolerant of cyclophosphamide (CYC).3,4
Posterior scleritis is an uncommon form of scleral inflammation and often extends to optic disc edema, exudative retinal detachment, and choroidal detachment. Here, we report a case of posterior scleritis with AAV successfully treated with prednisolone (PSL) and RTX combination therapy.
2. Case report
A 69-year-old female patient suffered from ocular pain and redness in her left eye (OS) for 2.5 years, and she noted decreased visual acuity OS 1.5 years previously. She had been treated with topical betamethasone several times only when she had symptoms. At her visit to an eye clinic, scleral injection was detected and she was treated with topical betamethasone for two months. There was no improvement, and she was thus referred to our hospital. She had been diagnosed with otitis media of unknown etiology before a year, and her family history was unremarkable.
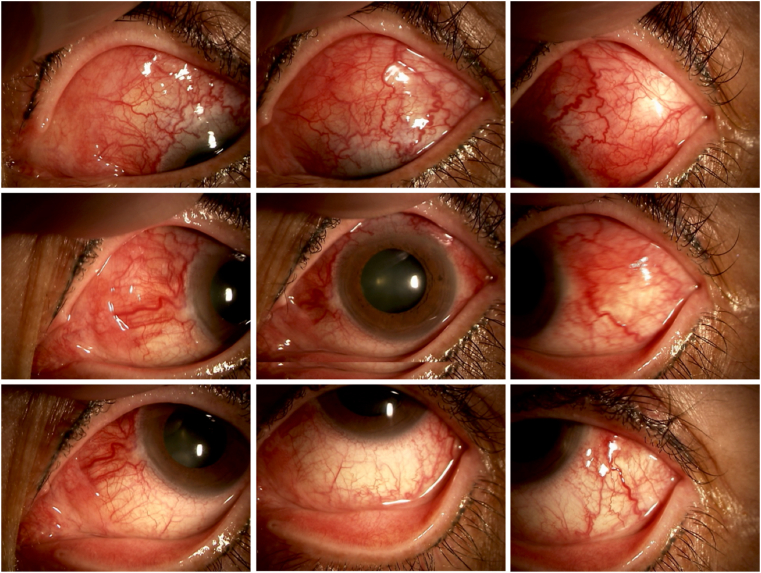
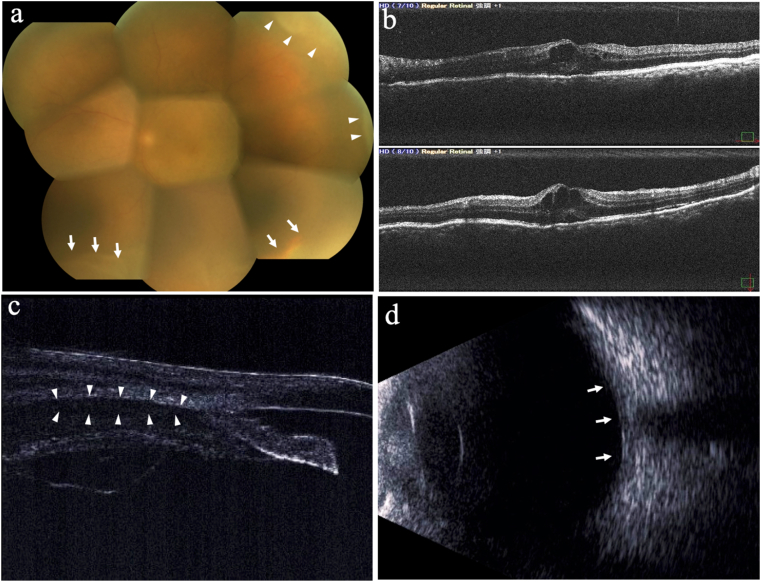
At her initial visit to our hospital, the best-corrected visual acuity (BCVA) was 0.9 in the right eye (OD) and 0.7 OS. Intraocular pressure was within the normal range in both eyes. Slit-lamp examination showed the injection of the sclera and conjunctiva, 1+ flare, 1+ cells of anterior chamber inflammation with fine keratic precipitates, and nodular-elevated lesion in the upper sclera OS (Fig. 1). Fundus examination revealed 1+ vitreous haze, a yellowish elevated lesion at the superotemporal periphery, and serous retinal detachment (SRD) at the inferotemporal periphery OS (Fig. 2a). Optical coherence tomography (OCT) revealed cystoid macular edema and retinal pigment epithelium (RPE) undulations OS (Fig. 2b). There were no abnormal findings, except mild superficial punctate keratopathy OD. Ultrasound biomicroscopy revealed enlargement of the supraciliary space OS (Fig. 2c), and B-mode ultrasonography revealed the thickening and flattening of the posterior coats OS (Fig. 2d).
Fig. 1.
Slit-lamp photographs of the left eye at the initial visitScleral
injection and nodular elevated lesion in the upper sclera were observed.
Fig. 2.
Ocular findings of the left eye at the initial visit. a. Fundus examination: 1+ vitreous haze, yellowish elevated lesion (white arrowheads), and serous retinal detachment (white arrows) were observed. b. Optical coherence tomography: Cystoid macular edema and retinal pigment epithelium undulations were observed. c. Ultrasound biomicroscopy: Enlargement of the supraciliary space (white arrowheads) was observed. d. B-mode ultrasonography: Thickening and flattening of the posterior coats (white arrows) were observed.
Laboratory analysis revealed high titers of antinuclear antibody (ANA) (1:160) and the CRP level was as high as 0.4 mg/dl. The results were positive for myeloperoxidase ANCA (MPO-ANCA) and negative for proteinase 3 ANCA (PR3-ANCA) (Table 1). Others results were not significant.
Table 1.
Blood test results at the initial visit.
| Items | Result | Reference Range | Unit |
|---|---|---|---|
| WBC | 7.2 | 3.3–8.6 | × 103/μL |
| PR3-ANCA | <20 | <20 | RU/ml |
| MPO-ANCA | 124.2 | <20 | RU/ml |
| RF | 29.6 | <15 | IU/ml |
| Antinuclear antibody | × 160 | Negative | |
| CRP | 0.40 | 0.00–0.14 | mg/dl |
| ESR 1 Hour | 7 | mm | |
| ESR 2 Hour | 15 | mm |
WBC, white blood cell; PR3-ANCA, proteinase 3 anti-neutrophil cytoplasmic antibodies; MPO-ANCA, myeloperoxidase anti-neutrophil cytoplasmic antibodies; RF, rheumatoid factor; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
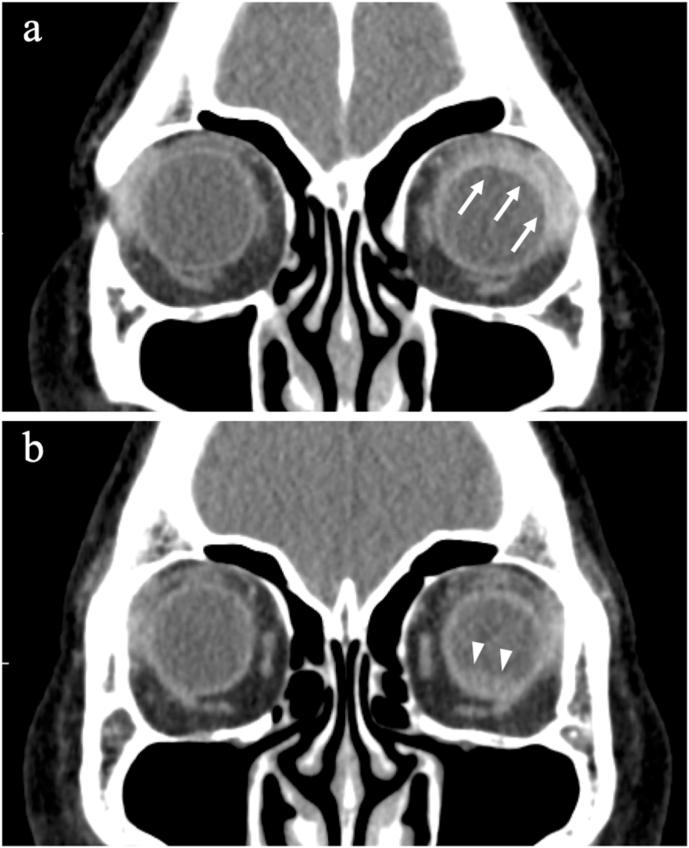
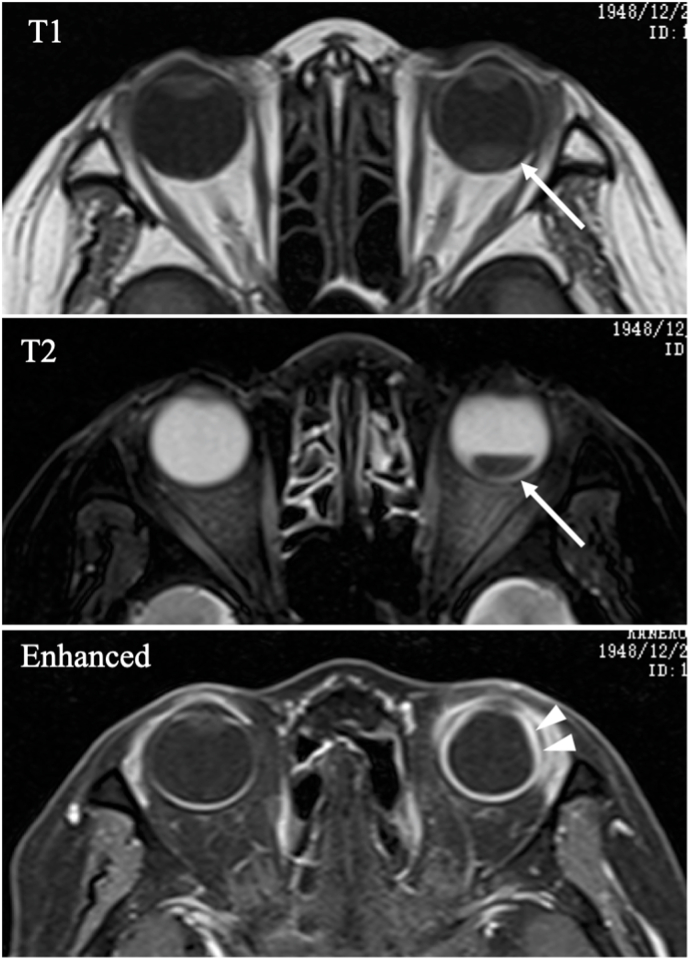
Computed tomography (CT) revealed high brightness of the soft tissue enhanced by the contrast agent in the left orbit and inferior retinal detachment OS (Fig. 3a–b). Magnetic resonance imaging (MRI) revealed enlargement of the supraciliary space and retinal detachment OS (Fig. 4).
Fig. 3.
Enhanced computed tomographic scans at the initial visit. a. High brightness of the soft tissue enhanced by the contrast agent in the left orbit (white arrows) was observed. b. Retinal detachment in the left eye (white arrowheads) was observed.
Fig. 4.
Magnetic resonance image at the initial visit Retinal
detachment in T1 and T2 (white arrows) can be observed. Enhanced magnetic resonance image showed enlargement of the supraciliary space (white arrow heads).
This patient was diagnosed with AAV in consultation with a rheumatologist and categorized as GPA on the basis of the Watts classification.5
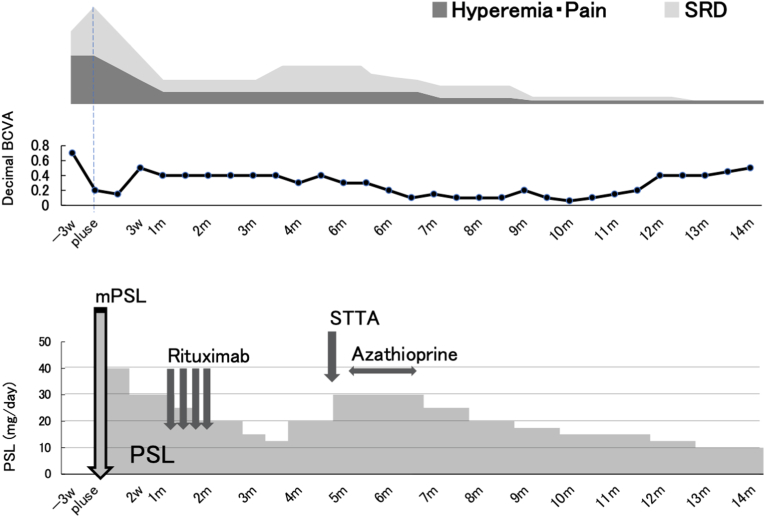
She was treated with topical betamethasone for three weeks after her initial visit. However, BCVA decreased to 0.15 and anterior chamber inflammation and vitreous haze OS exacerbated. OCT revealed the aggravation of SRD and RPE undulations OS. Therefore, she was treated with steroid pulse therapy, and her ocular findings improved (Fig. 5). Three weeks after the treatment, RTX was additionally administered at a dose of 500 mg/week for 4 weeks to maintain the remission. As the injection of the sclera and conjunctiva, vitreous haze, and SRD were resolved, PSL dose was gradually tapered. However, 3 months after the initial treatment when the PSL dose was tapered to 12.5 mg/day, SRD was observed in the macula OS (Fig. 6). Therefore, PSL dose was increased to 30 mg/day along with the administration of azathioprine and subtenon injection of 40 mg of triamcinolone acetonide OS. In response to these additional treatments, SRD disappeared OS. Fourteen months after the initial treatment, BCVA increased to 0.5 OS. Although focal thinning and vasodilatation of the sclera persisted (Fig. 7a) and epiretinal membrane developed (Fig. 7b and c), the scleral injection, peripheral yellowish lesions, and SRD disappeared OS. No recurrence has been noted to date, and the patient is currently receiving 10 mg/day of PSL.
Fig. 5.
Clinical course Three
weeks after the initial visit, methylprednisolone (mPSL) pulse therapy (1g/day × 3) was initiated, followed by rituximab (0.5g/week × 4) administration. Three months after the treatment, when serous retinal detachment (SRD) appeared in the macular area, prednisolone (PSL) dose was increased along with the administration of oral azathioprine and subtenon triamcinolone acetonide (STTA) (40mg). In response to these treatments, SRD disappeared, and the remission has been maintained to date.
Fig. 6.
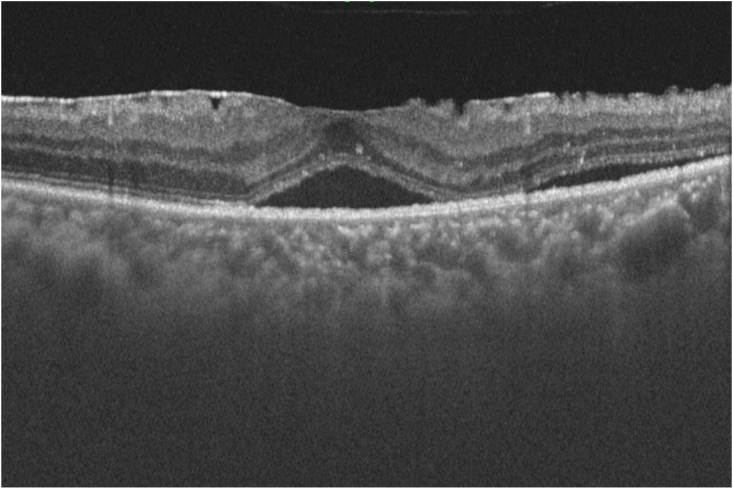
Optical coherence tomographic scan of the left eye three months after the initial treatment
Serous retinal detachment in the macula of the left eye was observed.
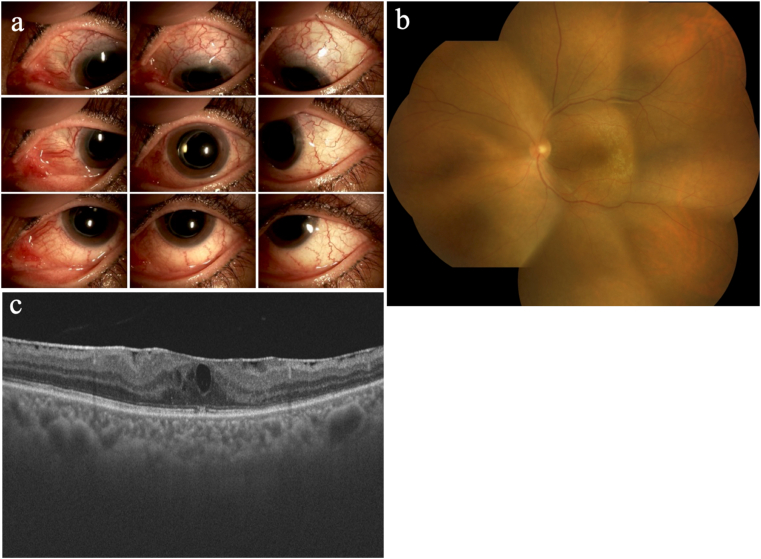
Fig. 7.
Ocular findings of the left eye 14 months after the initial treatment. a. Slit-lamp photographs: Focal thinning of the sclera and remaining scleral vasodilatation were observed. b. Fundus examination: Elevated lesions and serous retinal detachment (SRD) disappeared. c. Optical coherence tomography: SRD in the macula disappeared, and the epiretinal membrane developed.
3. Discussion
Posterior scleritis accounts for 2–12% of all scleritis cases,6 and it often requires aggressive therapy7 because of vision-threatening complications including vitritis, macular edema, and exudative retinal detachment.8 Scleritis associated with systemic diseases, such as rheumatoid arthritis, recurrent polychondritis, and AAV, is often refractory to treatment. The incidence of scleritis associated with such autoimmune diseases has been reported to be approximately 20–40%.7,9, 10, 11 In a previous study, 66% cases of scleritis associated with systemic diseases were refractory and only 6% were mild.12 AAV can be categorized into three distinct forms, namely GPA (previously known as Wegener's granulomatosis), microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis (previously known as Churg-Strauss syndrome).3
In the present case, although MPO-ANCA was positive, the patient presented no history of allergic diseases, pulmonary and renal abnormalities, or eosinophilia. Moreover, she presented with symptoms such as eye pain, vision loss, otitis media, and episcleritis.
Watts and colleagues proposed an AAV classification algorithm.5 This stepwise algorithm incorporated both the American College of Rheumatology (ACR) criteria for EGPA and GPA and the Chapel Hill Consensus Conference (CHCC)3 definition of EGPA, GPA, and MPA. In this case, she was diagnosed with AAV in consultation with a rheumatologist. According to the Watts classification,5 her AAV was categorized into GPA based on the findings of otitis media, posterior scleritis, and MPO-ANCA positivity.
Glucocorticoids (GCs) and immunosuppressants were the gold standard for AAV treatment. Although a combination therapy of a GC and an immunosuppressant is effective in the majority of GPA patients, the 2-year relapse rate of GPA is approximately 18–40%.13 In Japan, the combination therapy of CYC and GCs has been a gold standard to achieve remission in AAV.14 However, the frequency of treatment-associated side effects, such as infectious complications, cardiovascular diseases, myelosuppression, malignancy, and infertility, remains high.15, 16, 17
RTX is a cytotoxic monoclonal antibody, which depletes B cells upon CD20 binding. The efficacy of RTX against non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis18,19 has been proven, and this drug is now anticipated to be approved for the treatment of other chronic inflammatory conditions15,16. Moreover, RTX has been used to maintain remission in AAV since 2001.14,20 In 2011, the European League Against Rheumatism recommended RTX and GC combination therapy as the initial management of GPA. In Japan, RTX was approved for GPA treatment in 2013.14
In the present case of AAV associated with posterior scleritis, considering the risk of rapid vision loss, RTX and GC combination therapy was administered after consulting a collagen vascular disease specialist. The RTX treatment was considered effective, and the patient successfully maintained remission with a low dose of PSL, although she exhibited mild recurrence once.
The risk of impairment of visual function is much higher in posterior scleritis than anterior scleritis. Therefore, inducing and maintaining the remission is required more in posterior scleritis. Recillas-Gispert et al.21 and You et al.22 exhibited the case series of GPA patients with scleritis and revealed the effectiveness of RTX therapy, in which posterior scleritis was involved only one of 8 and one of 9 cases, respectively.
The present case is a rare case report of posterior scleritis associated with AAV providing the detail of clinical course and indicating the benefit of RTX in the management of posterior scleritis associated with AAV.
Based on the present case, RTX may contribute to the maintenance of remission. In addition, taking the various adverse effects of GCs into account, the trend of immune regulation is shifting toward minimizing their use and preferring RTX application.23 Nonetheless, the detailed mechanism of action of RTX in AAV remains unknown and warrants further research.
4. Conclusion
We encountered a case of posterior scleritis associated with MPO-ANCA-positive AAV, in which inflammatory manifestations subsided following RTX and PSL combination therapy. RTX administration likely contributed to the maintenance of remission in this case.
Patient consent
Institutional review board approval was not required for this case report of a single patient. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. This report does not contain any personal information that could reveal the patient's identify.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Authors’ contributions
Xinyu Weng: Data curation, Writing- Original draft preparation, Daiju Iwata: Conceptualization, Writing- Reviewing and Editing, Kenichi Namba: Conceptualization, Supervision.: Writing- Reviewing and Editing, Kayo Suzuki: Data curation, KazuomiMizuuchi: Data curation, Hiroyuki Nakamura: Supervision. Tatsuya Atsumi: Supervision. Susumu Ishida: Supervision.
Declaration of competing interest
The following authors have no financial disclosures: (XW, DI, KN, KS, KM, HN, TA, SI).
Acknowledgements
None.
Contributor Information
Xinyu Weng, Email: wengwengxinyu@gmail.com.
Daiju Iwata, Email: d.iwata@med.hokudai.ac.jp.
Kenichi Namba, Email: knamba@med.hokudai.ac.jp.
Kayo Suzuki, Email: snow-rabbit.kayo@h9.dion.ne.jp.
Kazuomi Mizuuchi, Email: mizuuchi@peach.plala.or.jp.
Hiroyuki Nakamura, Email: hirohironakamu@gmail.com.
Tatsuya Atsumi, Email: at3tat@med.hokudai.ac.jp.
Susumu Ishida, Email: ishidasu@med.hokudai.ac.jp.
References
- 1.Isobe M., Amano K., Arimura Y., et al. 2017. Guidelines for Management of Vasculitis Syndrome; pp. 1–109. [Google Scholar]
- 2.Patompong U., Cynthia S.C., Rodrigo C.C., et al. Clinical characteristics of inflammatory ocular disease in anti-neutrophil cytoplasmic antibody associated vasculitis: a retrospective cohort study. Rheumatology. 2017 Oct;56(10):1763–1770. doi: 10.1093/rheumatology/kex261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates M., Watts R. ANCA-associated vasculitis. Clin Med. 2017 Feb;17(1):60–64. doi: 10.7861/clinmedicine.17-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozaki S., Makino H., Matuo S., et al. 2011. Anca-related Vasculitis Medical Treatment Guideline; pp. 1–105. [Google Scholar]
- 5.Richard W., Suzanne L., Thomas H., et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007 Feb;66(2):222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson W.E. Posterior scleritis. Surv Ophthalmol. Mar-Apr 1988;32(5):297–316. doi: 10.1016/0039-6257(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 7.Keino H., Watanabe T., Taki W., et al. Clinical features and visual outcomes of Japanese patients with scleritis. Br J Ophthalmol. 2010 Nov;94(11):1459–1463. doi: 10.1136/bjo.2009.171744. [DOI] [PubMed] [Google Scholar]
- 8.Jabs D.A., Mudun A., Dunn J.P., et al. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000 Oct;130(4):469–476. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 9.Raiji V.R., Palestine A.G., Parver D.L. Scleritis and systemic disease association in a community-based referral practice. Am J Ophthalmol. 2009;148(6):946–950. doi: 10.1016/j.ajo.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Wieringa W.G., Wieringa J.E., Ten Dam-van Loon N.H., et al. Visual outcome, treatment results, and prognostic factors in patients with scleritis. Ophthalmology. 2013;120(2):379–386. doi: 10.1016/j.ophtha.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Akpek E.K., Thorne J.E., Qazi F.A., Do D.V., Jabs D.A. Evaluation of patients with scleritis for systemic disease. Ophthalmology. 2004 Mar;111(3):501–506. doi: 10.1016/j.ophtha.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Bernauer W., Pleisch B., Brunner M. Five-year outcome in immune-mediated scleritis. Graefe’s Arch Clin Exp Ophthalmol. 2014;252(9):1477–1481. doi: 10.1007/s00417-014-2696-1. [DOI] [PubMed] [Google Scholar]
- 13.Mukhtyar C., Guillevin L., Cid M.C., et al. European Vasculitis Study Group. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009 Mar;68(3):310–317. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 14.Arimura Y., Maruyama S., Homma S., et al. 2017. Clinical Practice Guideline for ANCA-Associated Vasculitis; pp. 1–46. [Google Scholar]
- 15.Specks U., Merkel P.A., Seo P., et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone J.H., Merkel P.A., Spiera R., et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letaief H., Lukas C., Barnetche T., Gaujoux-Viala C., Combe B., Morel J. Efficacy and safety of biological DMARDs modulating B cells in primary Sjögren’s syndrome: systematic review and meta-analysis. Joint Bone Spine. Joint Bone Spine. 2018 Jan;85(1):15–22. doi: 10.1016/j.jbspin.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Chatzidionysiou K., Lie E., Nasonov E., et al. Effectiveness of two different doses of rituximab for the treatment of rheumatoid arthritis in an international cohort: data from the CERERRA collaboration. Arthritis Res Ther. 2016;18:50. doi: 10.1186/s13075-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randall K.L. Rituximab in autoimmune diseases. Aust Prescr. 2016 Aug;39(4):131–134. doi: 10.18773/austprescr.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yates M., Watts R.A., Bajema I.M., et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016 Sep;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 21.Recillas-Gispert C., Serna-Ojeda J.C., Flores-Suarez L.F., et al. Rituximab in the treatment of refractory scleritis in patients with granulomatosis with polyangiitis (Wegener's) Graefes Arch Clin Exp Ophthalmol. 2015;253:2279–2284. doi: 10.1007/s00417-015-3198-5. [DOI] [PubMed] [Google Scholar]
- 22.You C., Ma L., Lasave A.F., et al. Rituximab induction and maintenance treatment in patients with scleritis and granulomatosis with polyangiitis (Wegener's) Ocul Immunol Inflamm. 2018;26(8):1166–1173. doi: 10.1080/09273948.2017.1327602. [DOI] [PubMed] [Google Scholar]
- 23.Smith R.M. Update on the management of ANCA-associated vasculitis. Presse Med. 2015 Jun;44(6 Pt 2):e241–249. doi: 10.1016/j.lpm.2015.04.008. [DOI] [PubMed] [Google Scholar]