Abstract
Purpose:
Epigenetic age acceleration (EAA) is robustly linked with mortality and morbidity. This study examined risk factors of EAA and its association with overall survival (OS), progression-free survival (PFS), and quality of life (QOL) in patients with head and neck cancer (HNC) receiving radiotherapy.
Methods and Materials:
Patients without distant metastasis were enrolled and followed before and end of radiotherapy, and 6-months and 12-months post-radiotherapy. EAA was calculated with DNAmPhenoAge at all four times. Risk factors included demographics, lifestyle, clinical characteristics, treatment-related symptoms, and blood biomarkers. Survival data were collected until August 2020; QOL was measured using Functional Assessment of Cancer Therapy–HNC.
Results:
Increased comorbidity, HPV-unrelated, and severer treatment-related symptoms were associated with higher EAA (p=0.03 to <0.001). A non-linear association (quadratic) between body mass index (BMI) and EAA was observed: decreased BMI (when BMI<35,p=0.04) or increased BMI (when BMI≥35,p=0.01), was linked to higher EAA. Increased EAA (per year) was associated with worse OS (hazard ratio (HR)=1.11,95% CI=[1.03,1.18],p=0.004; HR=1.10,95% CI=[1.01,1.19], p=0.02, for EAA at 6-months and 12-months post-treatment, respectively), PFS (HR=1.10, 95% CI=[1.02,1.19], p=0.02; HR=1.14, 95% CI=[1.06,1.23], p<0.001; HR=1.08,95% CI=[1.02,1.14], p=0.01, for EAA before, end, and 6-months post-radiotherapy, respectively), and QOL over time (β=−0.61,p=0.001). An average of 3.25–3.33 years of age acceleration across time, which was responsible for 33% to 44% higher HRs of OS and PFS, was observed in those who died or developed recurrences compared to those who did not (all p<0.001).
Conclusion:
Compared to demographic and lifestyle factors, clinical characteristics were more likely to contribute to faster biological aging in patients with HNC. Acceleration in epigenetic age resulted in more aggressive adverse events including OS and PFS. EAA could be considered as a marker for cancer outcomes, and decelerating aging could improve survival and QOL.
Introduction
Increasing age is among the most significant risk factors for cancer.1 Although the mechanisms linking age and cancer are not completely understood, epigenetic alterations (e.g., DNA methylation) appear to be a hallmark of age and cancer.2,3 Epigenetic clocks calculated using DNA methylation data have emerged as a novel and reliable age biomarker.4,5 Epigenetic aging acceleration (EAA), a discrepancy between epigenetic and chronological age, is considered as an indicator of a biologically accelerated or decelerated aging process.4 Since the methods for measuring epigenetic age incorporate gene loci in pathways related to cancer development and aging in general (e.g., DNA damage, cellular proliferation, and oxidative stress),4,6 it is highly possible that EAA can be a biomarker for cancer survival in addition to serving as an indicator of aging.
Although EAA has been associated with inflammation,7 cancer onset,8 and all-cause mortality,9 EAA and its association with risk factors, disease outcomes, and patient-reported outcomes in cancer have not yet been studied. Very limited studies in cancer have investigated EAA. One study of 72 breast cancer patients found increased EAA post-adjuvant therapy compared to before the treatment.10 Another study used tumor tissues from 33 patients with lung cancer. This study found that that squamous cell carcinomas tissue exhibited a significantly decreased epigenetic age compared to adenocarcinomas tissue.11 Both investigations had a relatively small sample size and did not examine risk factors of EAA; nor did they examine EAA’s associations with survival and quality of life. A large epidemiology study of 442 health participants found that EAA (years before cancer diagnosis) was predictive of cancer risk and cancer-related mortality.12 In this epidemiology study, 132 participants developed cancer during follow-up (38 prostate, 50 skin, 44 other), and 34 died from cancer (4 prostate, 2 skin, 28 other).
There are approximately 66,630 new head and neck cancer (HNC) cases in 2021 in the U.S.13 Due to the emergence of human papillomavirus (HPV)-related HNC, generally involving younger populations with fewer comorbidities,14 newly diagnosed HNC cases have been increasing in the past decades.15 There are still approximately two-thirds of HNC patients diagnosed with HPV-unrelated tumors; these patients are more vulnerable to environmental carcinogens such as tobacco and alcohol than those with HPV-unrelated tumors.16,17 Given the impact of these environmental carcinogens and aging-related factors on DNA methylation,18–20 EAA could be a well-suited biomarker for cancer outcomes in HNC patients.
Our aim was to address the gap in the growing literature on EAA by examining: 1) risk factors associated with EAA; 2) association between EAA and survival outcomes; and 3) association between EAA and patient-reported outcomes as well as QOL for HNC patients actively receiving cancer treatment.
Methods and Materials
Study design:
This was a prospective study of patients treated for HNC. Data were collected prior to intensity-modulated radiation therapy (IMRT, approximately 6–7 weeks in duration) with or without chemotherapy, at the end of treatment, and 6 months and 12 months post-treatment. If surgery was performed, it typically took place approximately 1 month prior to IMRT. The Emory University institutional review board approved the study, and enrolled patients provided informed consent.
Patients:
Patients were eligible if they were newly diagnosed with histological proof of squamous cell carcinoma of the head and neck region, without distant metastasis, and without uncontrolled major organ diseases. The major exclusion criteria included: simultaneous primaries, major psychiatric disorders, and chronic health conditions involving the immune system or regular use of immunosuppressive medications.
Risk factor assessments:
Demographic factors, like age, gender, race, marital status, education, and income, were collected through a standard patient-reported questionnaire. Lifestyle factors included a history of tobacco use (at least a 1-year history of smoking vs. no), years since quitting tobacco (<1 year, 1–9 years, or 10–19 years), a history of alcohol use (at least 1 drink/week in the past 1 year vs. no), and body mass index (BMI); all collected using questionnaires or medical record review. Clinical characteristics consisted of functional status (Eastern Cooperative Oncology Group [ECOG)), comorbidities (Charlson Comorbidity Index), cancer sites, stage (TNM),21 human papillomavirus (HPV) status, treatment types, radiation dose, and feeding tubes, obtained via medical record review. Tumors with p16 or HPV positive from pathology reports were counted as HPV-related; otherwise they were counted as HPV-unrelated. Treatment-related symptoms/side effects were attained from the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).22 Symptoms included an HNC cluster (fatigue, pain, dry mouth, difficulty swallowing, skin burn from radiation, mouth or throat sores, and taste change) and a gastrointestinal cluster (nausea and vomiting) supported by our publication.23 Blood biomarkers, including albumin, hemoglobin, white blood cell counts, neutrophils, and lymphocytes, were acquired by medical record review. BMI, symptoms, and blood biomarkers were collected at all 4 times (prior to IMRT, end of treatment, and 6 months and 12 months post-treatment); others were collected at baseline or follow-up as appropriate.
Survival assessments:
Overall survival (OS) was defined as the time from the first day of radiotherapy to death due to any cause, or to the last contact up to August 2020, whichever occurred first. Vital status was ascertained through medical record linkage to the Georgia Cancer Registry. Progression-free survival (PFS) was defined as the time from the first day of radiotherapy to recurrence (i.e., locoregional, distant recurrence) on imaging, to the patients’ death, or to the last contact up to August 2020, whichever occurred first. Patients were followed based on the standard procedure at the Winship Cancer Institute: a PET scan and a CT of the neck at 3 months post-radiotherapy, then a CT of the neck and chest every 6 months for the first 2 years, then yearly until year 5; all patients had an endoscopic evaluation at least twice over the first 2 years of follow-up post definitive therapy.
QOL assessments:
The Functional Assessment of Cancer Therapy – Head & Neck Cancer (FACT-HN), a well-validated, self-reported tool, was used to assess QOL.24–26 This questionnaire consists of 27 core items assessing well-being and is supplemented by 12 items to assess HNC-related symptoms. The total score ranges from 0–148; higher scores represent better QOL. The FACT-H&N was completed 4 times (prior to IMRT, end of treatment, and 6 months and 12 months post-treatment) at the same day of collecting other questionnaires and blood biomarkers.
Epigenetic age acceleration assessments:
Whole blood was collected into EDTA tubes at all 4 times for isolation of blood leukocytes, which were stored at −80°C until batched extraction. DNA was extracted using a standard protocol (QIAamp DNA mini kit, Qiagen). DNA with concentration>20μg/ml was used for methylation analysis (MethylationEPIC BeadChip, Illumina, San Diego, CA) at the Emory Integrated Genomics Core.
Methylation data were preprocessed and quantile normalized with R package minfi.27 We filtered probes using similar criteria as our previous publication.28 After quality control, 807,104 out of 866,238 probes remained. Samples would be further removed based on the criteria similar to our previous publication;28 no samples were removed.
Epigenetic age or DNA methylation (DNAm) age was calculated using Levine’s DNAmPhenoAge.29 DNAmPhenoAge is predicted based on phenotypic age, which uses both chronological age and clinical biomarkers reflecting physiological status. A total of 513 CpG sites associated with phenotypic age was considered for Levine’s clock. Levine’s clock, as the second-generation epigenetic age measure, has been shown to outperform previous clocks in predicting cancer, mortality, and other age-related health conditions.29 Additionally, it has been recommended to use multiple methods of calculating epigenetic age, as different methods use different CpG sites and may reflect different molecular processes in aging.4,6,30,31 Thus, we applied 3 additional well-developed and widely-used methods: Hannum,6 Horvath 2013,4 and Horvath 2018.30
EAA was defined as the residual resulting from regressing DNAmAge on chronological age.8 A positive EAA suggests age acceleration in years, as the predicted DNAmAge is older than the chronological age. A negative EAA indicates a slower aging process, because the predicted DNAmAge is younger than the chronological age. To adjust for chronological age increases over the study period, patients’ age was estimated as their chronological age at baseline plus 7 weeks, 8 months, and 14 months to represent the chronological age at the end of treatment and 6 and 12 months post-treatment.
Statistical methods:
To examine the risk factors associated with EAA longitudinally over time, generalized estimating equation (GEE) models were performed in 2 steps. In the first step, a simple GEE model was conducted to test each risk factor’s association with EAA (outcome variable) adjusting for time. During the first step, we noticed a non-linear association between BMI and EAA over time, and BMI*BMI was added into further models. Also, post hoc analyses were performed to determine the BMI cutoff and the non-linear association. The second step was the GEE models involving all risk factors with a p < 0.1 at the first step; a backward model selection was used to select risk factors. After identifying the risk factors (demographic and lifestyle factors and clinical characteristics) in the final minimal GEE model, the two symptom clusters were added into the minimal GEE model, and then blood biomarkers were added.
The Cox proportional hazards (PH) regression model was used for OS and PFS analyses. The proportional hazard assumption of each covariate was tested in the Cox PH model via the Kolmogorov-type supremum test; no violation of this assumption was found. Covariates with p<0.1 in the univariable model were carried forward into the multivariable survival analysis with a backward selection. The final models for OS and PFS were performed at each time after adjusting for covariates. Sensitivity analyses were performed to add more covariates that were dropped from the backward selection, but were commonly used risk factors for survival analysis. These commonly used risks factors were: age, race (white vs. non-white), history of tobacco use (yes vs. no), stage (IV vs. ≤ III), functional status (ECOG = 0 vs. ≥ 1), comorbidity (CCI = 0 vs. ≥ 1), BMI, HNC cluster, HPV status, feeding tubes, treatment (radiation ± surgery vs. chemoradiation vs. chemoradiation + surgery), and surgery (no vs. yes). To examine whether EAA was superior to commonly used risk factors predicting OS and PFS, likelihood ratio tests by comparing −2log(L) between the models with and without EEA were performed. Furthermore, Cox models were used to examine whether EAA was also related to cancer-related mortality, instead of all-cause mortality. Moreover, a t-test was performed to examine EAA differences between patients who died or developed recurrences and those who did not.
The association between EAA and QOL longitudinally over time was examined using GEE, a similar approach as that for testing risk factors of EAA. The multivariable GEE model of QOL (outcome variable) included significant covariates via backward selection, and then EAA was tested after adjusting for selected covariates.
Analyses were conducted using relevant R packages and SAS 9.4; statistical tests were 2-tailed with a significance level of 0.05.
Results
This study consisted of 146 patients diagnosed with HNC (Table 1), with an average chronological age at baseline of 59 ± 10. The majority were male (73%), white (84%), and married (68%). Sixty percent reported at least a one-year history of smoking, and 43% had at least one drink/week in the past one year. Approximately half were diagnosed with oropharyngeal cancer (53%); among them, 90% had HPV-related cancer. Most were diagnosed with advanced stage disease (IV: 76%) and received concurrent chemoradiation (79%); 96% of patients completed prescribed regimens. Prior to radiotherapy, 38% of patients had positive values of Levine’s EAA, indicating age acceleration, while at the end of treatment, 73% had positive values. This number was dropped to 37% and 36% at 6 months and 12 months post-treatment, respectively. Epigenetic age calculated using the four methods were highly associated with each other and chronological age (Supplemental Table 1).
Table 1.
Baseline characteristics of the participants (N=146)
| Variables | Mean ± SD or N (%) | |
|---|---|---|
|
| ||
| Age | 59.06 ± 10.31 | |
| Gender | Male | 107 (73) |
| Female | 39 (27) | |
| Race | White | 122 (84) |
| Non-white | 24 (16) | |
| Marital statusa | Married | 100 (68) |
| Not married | 46 (32) | |
| Education* | <college | 92 (63) |
| >=college | 53 (37) | |
| Income* | <40,000 | 44 (38) |
| >=40,000 | 72 (62) | |
| History of alcohol use* | No | 82 (57) |
| Yes | 62 (43) | |
| History of tobacco use | No | 59 (40) |
| Yes | 87 (60) | |
| Quit smoking | Never | 9 (11) |
| <1 year | 28 (33) | |
| 1–9 years | 17 (20) | |
| 10–19 years | 6 (7) | |
| BMI* | 27.84 ± 5.13 | |
| ECOG* | 0 | 52 (37) |
| 1 | 71 (50) | |
| 2 | 19 (13) | |
| Comorbidityb | 0 | 91 (62) |
| >=1 | 55 (38) | |
| Cancer site | Oral cavity | 24 (16) |
| Oropharynx | 77 (53) | |
| Larynx | 23 (16) | |
| Others | 22 (15) | |
| Stage | ≤III | 35 (24) |
| IV | 111 (76) | |
| HPV status | Unrelated | 69 (47) |
| Related | 77 (53) | |
| Treatment | IMRT | 6 (4) |
| IMRT + Surgery | 25 (17) | |
| IMRT + Chemo | 77 (53) | |
| IMRT + Chemo + Surgery | 38 (26) | |
| Surgery | No | 83 (57) |
| Yes | 63 (43) | |
| Chemotherapy | No | 31 (21) |
| Yes | 115 (79) | |
| Chemotherapy drug | Cisplatin | 80 (70) |
| Carboplatin/Paclitaxel | 22 (19) | |
| Others | 13 (11) | |
| Radiation dose | 66.80 ± 5.08 | |
| Feeding tubes* | No | 65 (46) |
| Yes | 76 (54) | |
Note. BMI = Body Mass Index, ECOG = Eastern Cooperative Oncology Group Performance Status, HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy, SD = Standard deviation.
Having missing data: Education (1); Income (30); History of alcohol use (2); BMI (1); ECOG (4); Feeding tubes (5).
Married includes patients married or living as married; Unmarried includes patients single, separated, divorced, or widowed.
Comorbidity was assessed using the Charlson Comorbidity Index excluding tumor.
Risk factors and EAA
In our simple GEE model controlling for time (Supplemental Table 2), we observed higher EAA in those with lower income, history of tobacco use, and fewer smoking cessation years (p = 0.04 to 0.01). We noted a nonlinear association between BMI and EAA. For patients with BMI<35, EAA was higher in those with lower BMI (p = 0.04), while for patients with BMI ≥ 35, EAA was higher in those with higher BMI (p = 0.01). Among clinical characteristics, lower functional performance (ECOG ≥ 1), more comorbidities (≥ 1), non-oropharyngeal cancer, HPV-unrelated tumors, receiving chemoradiation plus surgery, receiving chemotherapy, and severer HNC symptom clusters were associated with higher EAA (p= 0.02 to < 0.001). Decreased albumin, hemoglobin, and lymphocyte were associated with higher EAA, while increased white blood cell count and neutrophils were associated with higher EAA (all p < 0.001). Fewer risk factors were associated with EAA derived from the Hannum, Horvath 2013, and Horvath 2018 clocks than Levine’s EAA clock (Supplemental Table 3).
In our multivariable model (Table 2), BMI, comorbidity, and HPV status remained significant in the minimal model. After adding treatment-related symptoms, those risk factors were still significant, along with the HNC symptom cluster (p = 0.02). In our final model (adding blood markers), comorbidity and HPV status, as well as hemoglobin and lymphocyte percentage, were significantly associated with EAA. Overall, compared to patients without comorbidity, patients with comorbidity ≥ 1 exhibited a 2–3 year increase in EAA (β = 2.30 to 2.90, p ≤ 0.001). Compared to those with HPV-related tumors, patients with HPV-unrelated tumors had increased EAA by approximately 1.5–2.5 years (β = 1.62 to 2.36, p = 0.029 to < 0.001).
Table 2.
Multivariable longitudinal models for risk factors of epigenetic age acceleration over time
| Model 1 Minimal | Model 2 Symptoms | Model 3 Blood markers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | p | 95% CI | Estimate | p | 95% CI | Estimate | p | 95% CI | |
|
| |||||||||
| Time: (Baseline) | - | - | - | - | - | - | - | - | - |
| End of IMRT | 4.65 | <0.001 | [3.73, 5.57] | 3.58 | <0.001 | [2.24, 4.91] | 1.87 | 0.07 | [−0.13, 3.88] |
| 6 months post-IMRT | −0.45 | 0.34 | [−1.38, 0.47] | −0.57 | 0.23 | [−1.49, 0.36] | −1.49 | 0.03 | [−2.85, −0.12] |
| 12 months post-IMRT | 0.20 | 0.68 | [−0.77, 1.18] | 0.11 | 0.82 | [−0.87, 1.10] | −0.96 | 0.14 | [−2.23, 0.30] |
| Comorbidity: (>=1) | - | - | - | - | - | - | - | - | - |
| 0 | −2.90 | <0.001 | [−4.24, −1.56] | −2.77 | <0.001 | [−4.12, −1.43] | −2.30 | <0.001 | [−3.65, −0.95] |
| BMI* | −0.74 | 0.007 | [−1.27, −0.20] | −0.62 | 0.02 | [−116, −0.09] | 0.03 | 0.93 | [−0.60, 0.65 |
| BMI × BMI* | 0.01 | 0.008 | [0.003, 0.02] | 0.01 | 0.02 | [0.001, 0.02] | −0.001 | 0.83 | [−0.01, 0.01] |
| HPV status: (related) | - | - | - | - | - | - | - | - | - |
| unrelated | 2.28 | 0.001 | [0.90, 3.66] | 2.36 | <0.001 | [0.99, 3.73] | 1.62 | 0.03 | [0.17, 3.07] |
| Treatment: (IMRT ± surgery) | - | - | - | - | - | - | - | - | - |
| IMRT + chemo | 1.01 | 0.28 | [−0.80, 2.83] | 1.16 | 0.19 | [−0.57, 2.89] | −1.23 | 0.41 | [−4.13, 1.67] |
| IMRT + chemo + surgery | 1.29 | 0.23 | [−0.82, 3.39] | 1.42 | 0.17 | [−0.62, 3.45] | −1.75 | 0.27 | [−4.84, 1.34] |
| HNC symptom cluster | 0.13 | 0.02 | [0.02, 0.25] | 0.11 | 0.11 | [−0.02, 0.25] | |||
| Gastrointestinal cluster | −0.08 | 0.58 | [−0.35, 0.19] | −0.16 | 0.33 | [−0.48, 0.16] | |||
| Albumin | −0.90 | 0.15 | [−2.12, 0.32] | ||||||
| Hemoglobin, g/dL | −0.53 | 0.002 | [−0.86, −0.19] | ||||||
| White blood cell counts | 0.0001 | 0.41 | [−0.0001, 0.0003 | ||||||
| Neutrophil, % | 0.004 | 0.94 | [−0.10, 0.10] | ||||||
| Lymphocyte, % | −0.16 | 0.009 | [−0.28, −0.04] | ||||||
Note. Reference or comparator groups were indicated in parentheses. BMI = Body Mass Index, CI = confidence interval, HNC = Head and neck cancer, HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy. Bold text indicates a statistically significant association with a p-value less than 0.05.
BMI × BMI indicating a quadratic association with epigenetic age acceleration.
EAA and survival
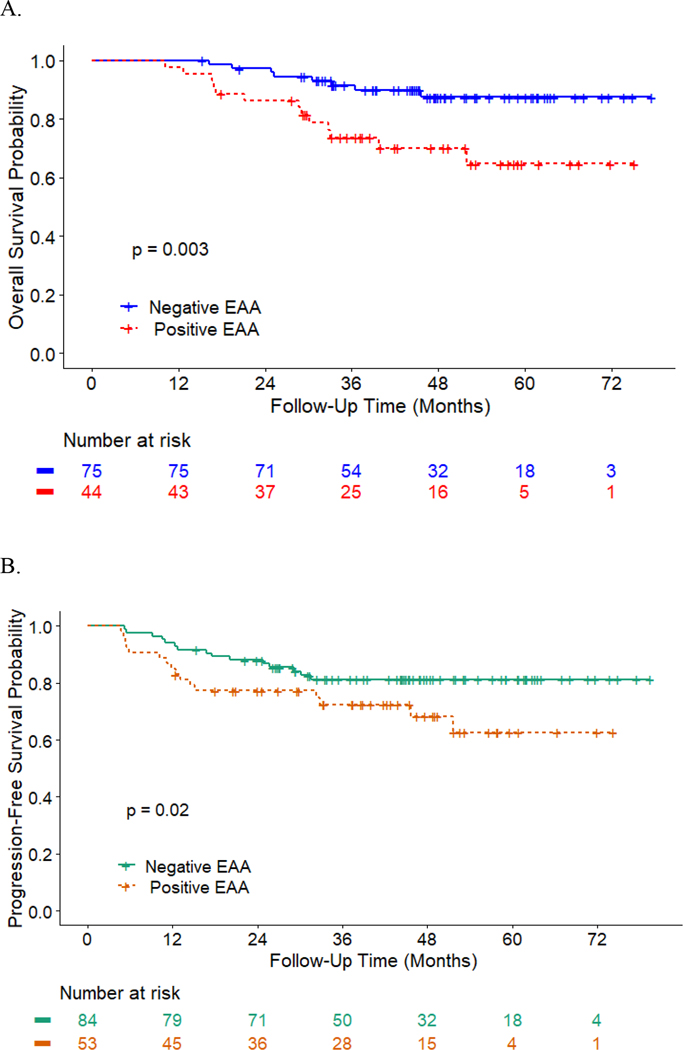
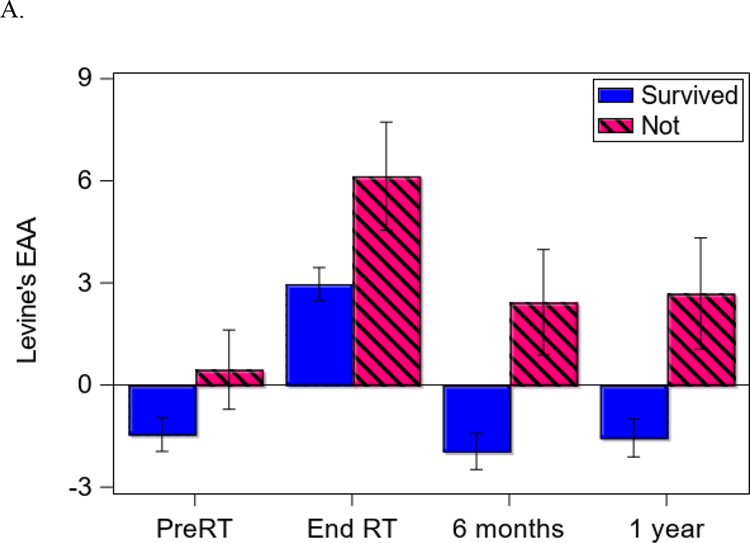
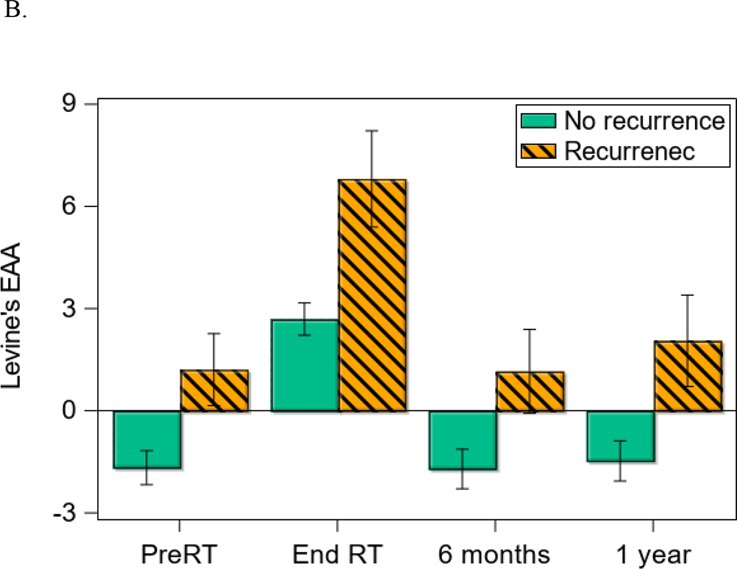
At the time of analysis (August 2020), 124 patients were alive, with a median follow-up time of 43 months (N = 16 for cancer-related death; N = 6 for non-cancer death). Eighteen patients had distant recurrence; 7 patients had local-regional recurrence; and the median follow-up time of recurrence was 40 months. Patients with higher EAA at 6 months (HR = 1.11, 95% CI = [1.03,1.18], p = 0.004; Figure 1A) and 12 months post-treatment (HR = 1.10, 95% CI = [1.01,1.19], p = 0.02) had worse OS controlling for covariates such as HPV and treatment (Table 3). Similarly, patients with higher EAA at baseline (HR = 1.10, 95% CI = [1.01,1.20], p = 0.03; Figure 1B) and end of treatment (HR = 1.13, 95% CI = [1.04,1.23], p = 0.004) had low PFS controlling for covariates. Supplemental Figure 1 further shows different cumulative hazard curves for any recurrence, locoregional recurrence, and distant recurrence, respectively, between positive EAA and negative EAA at baseline. Overall, an average of 3.25 and 3.33 years of age acceleration across time was noted for patients who died or developed recurrences compared to those who did not (EAA for OS: 2.78 ± 6.70 vs. −0.47 ± 5.57, p < 0.001; Figure 2A; EAA for PFS: 2.79 ± 6.22 vs. −0.54 ± 5.64, p < 0.001; Figures 2B). According to the estimated HRs of a 10% to 13% increase per year in EAA for OS and PFS, a 3-year increase in EAA would be responsible for 33% to 44% higher HRs of OS and PFS.
Figure 1.
The association between survival and epigenetic age acceleration (EAA). A: Overall survival curves (Kaplan Meier Estimates) for negative EAA vs. positive EAA at six months post treatment. B: Progression-free survival curves (Kaplan Meier Estimates) for negative EAA vs. positive EAA at prior to radiotherapy.
Note. P-value is obtained from the Cox proportional hazards regression model adjusting covariates as presented in Table 3 main model.
Table 3.
Association between epigenetic age acceleration and overall survival as well as progression-free survival cross-sectionally at different time points
| Overall survival model | Main model | Sensitivity Analysis* | ||||||
|---|---|---|---|---|---|---|---|---|
| N; events | Adjusted hazard ratio | p | 95% confidence interval | N; events | Adjusted hazard ratio | p | 95% confidence interval | |
| Baseline | 132; 21 | 1.06 | 0.23 | [0.96, 1.17] | 128; 21 | 1.02 | 0.78 | [0.90, 1.14] |
| End of IMRT | 117; 17 | 1.09 | 0.06 | [1.00, 1.20] | 108; 17 | 1.33 | <0.001 | [1.15, 1.62] |
| 6-month post-IMRT | 117; 20 | 1.11 | 0.004 | [1.03, 1.18] | 112; 20 | 1.07 | 0.13 | [0.98, 1.17] |
| 12-month post-IMRT | 105; 16 | 1.10 | 0.02 | [1.01, 1.19] | 100; 16 | 1.15 | 0.04 | [1.01, 1.33] |
| Progression free survival model | ||||||||
| Baseline | 132; 30 | 1.10 | 0.02 | [1.02, 1.19] | 128; 30 | 1.13 | 0.008 | [1.03, 1.24] |
| End of IMRT | 117; 26 | 1.14 | <0.001 | [1.06, 1.23] | 108; 26 | 1.32 | <0.001 | [1.16, 1.54] |
| 6-month post-IMRT | 117; 27 | 1.08 | 0.01 | [1.02, 1.14] | 112; 27 | 1.08 | 0.03 | [1.01, 1.17] |
| 12-month post-IMRT | 105; 22 | 1.07 | 0.05 | [1.00, 1.15] | 100; 22 | 1.04 | 0.46 | [0.95, 1.14] |
Note. All main models were adjusted for HPV status, feeding tubes, and treatment (radiation ± surgery vs. chemoradiation vs. chemoradiation + surgery). Sensitivity analysis further adjusted for age, race (white vs. non-white), history of tobacco use (yes vs. no), stage (IV vs. ≤III), functional status (ECOG=0 vs. ≥1), comorbidity (CCI =0 vs. ≥1), BMI×BMI (quadratic association), HNC cluster, and surgery (no vs. yes). HNC = head and neck symptom cluster, HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy. Bold text indicates a statistically significant association with a p-value less than 0.05.
The number of patients included in the sensitivity analysis was less than the main model due to a few missing from the added covariates.
Figure 2.
Difference in epigenetic age acceleration (EAA) between groups over time. A: Differences in EAA between survivors vs. not at different measurement times. B: Differences in EAA between those with recurrence vs. without at different measurement times.
Note: Error bar represents one standard error.
Functional status was excluded from the final Cox model during the backward selection. Sensitivity analysis (Table 3) of adding functional status, as well as other commonly used risk factors (e.g., age, race, BMI, history of tobacco use, comorbidity, cancer stage, surgery, and HNC symptom cluster) associated with survival, showed that EAA at 12 months post-treatment (HR = 1.15, 95% CI = [1.01, 1.33], p = 0.04) remained significant, plus end of treatment (HR = 1.33, 95% CI = [1.15, 1.62], p < 0.001), for predicting OS, whereas functional status was not significant. Comparable findings were noted for PFS with the addition of these covariates (Table 3). Further comparisons of the models with commonly used risk factors only to the models with additional EAA showed a significant model improvement after adding EAA for OS and PFS at above-described significant times (p = 0.03 to <0.001), with the exception of EAA at 12 months post-treatment. Analyses on cancer-related mortality also revealed that EAA at the end of treatment was a risk factor for cancer-related mortality (HR = 1.15, 95% CI = [1.18, 1.95], p = 0.003) adjusting for commonly used risk factors. EAA derived from Hannum’s clock was also predictive to OS and PFS, including prior to treatment, while less statistically significant findings were observed for Horvath’s 2013 and 2018 methods (Supplemental Table 4).
EAA and QOL
Lower Levine’s EAA was significantly associated with better QOL over time (β = −0.63, p = 0.001; Table 4; Supplemental Figure 2), controlling for covariates. For patients with negative EAA, their QOL total score was approximately 10.60 points higher than those with positive EAA over time (p<0.001), which met the minimal clinically important difference.25 Controlling for the same covariates, Hannum and Horvath’s 2018 EAA was not associated with QOL (p = 0.31; p = 0.06, respectively), while increased Horvath’s 2013 EAA was associated with better QOL (p = 0.04). QOL data were available for a subset of patients enrolled later (N = 102); demographic and clinical characteristics were well-balanced between patients with and without QOL data (Supplemental Table 5).
Table 4.
Association between epigenetic age acceleration and quality of life longitudinally over time (N=102)
| Estimate | p | 95% confidence interval | |
|---|---|---|---|
|
| |||
| Time: (baseline) | - | - | - |
| End of IMRT | −20.95 | <0.001 | [−26.43, −15.46] |
| 6-month post-IMRT | 3.82 | 0.07 | [−0.28, 7.93] |
| 12 months post-IMRT | 5.87 | 0.01 | [1.22, 10.51] |
| Marital status: (not married) | - | - | - |
| married | 12.62 | 0.01 | [2.52, 22.72] |
| ECOG: (>=1) | - | - | - |
| =0 | 8.06 | 0.09 | [−1.11, 17.23] |
| Comorbiditya: (>=1) | - | - | - |
| =0 | −2.83 | 0.52 | [−11.52, 5.86] |
| BMI* | 0.13 | 0.93 | [−2.87, 3.14] |
| BMI × BMI* | 0.01 | 0.79 | [−0.04, 0.05] |
| HPV status: (unrelated) | - | - | - |
| related | −2.61 | 0.54 | [−10.95, 5.73] |
| Treatment: (IMRT ± surgery) | - | - | - |
| IMRT + chemo | 9.46 | 0.05 | [−0.03, 18.96] |
| IMRT + chemo + surgery | 11.72 | 0.03 | [1.21, 22.23] |
| Feeding tubes (yes) | - | - | - |
| no | 11.08 | 0.002 | [4.08, 18.07] |
| Levine’s EAA | −0.63 | 0.001 | [−1.01, −0.26] |
Note. Reference or comparator groups were indicated in parentheses. BMI = Body Mass Index, EAA=Epigenetic age acceleration, ECOG = Eastern Cooperative Oncology Group performance status, HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy. Bold text indicates a statistically significant association with a p-value less than 0.05.
BMI × BMI indicating a quadratic association with epigenetic age acceleration.
Discussion
To our knowledge, this is the first study examining a wide range of risk factors associated with EAA, as well as EAA’s associations with survival and QOL among cancer patients. The results suggest that clinical characteristics, such as comorbidity, HPV status, and treatment-related symptoms/side effects, were more likely than demographic or lifestyle factors to be the dominant risk factors for higher EAA in HNC patients. Higher EAA was associated with worse survival outcomes and clinically meaningful declines in QOL. EAA outperformed functional status in predicting OS and PFS, suggesting that age acceleration derived from epigenetic clocks is a sensitive marker for cancer outcomes.
We observed that demographic and lifestyle characteristics, such as income, history of tobacco use, years since smoking cessation, and BMI, were associated with EAA in our simple GEE model. Most of these observed associations are consistent with available evidence.32,33 We found a dose-dependent effect for years since smoking cessation: longer duration of smoking cessation was associated with lower EAA, supporting the beneficial effect of smoking cessation on reversing epigenetic age.34 Interestingly but not surprisingly, we noted a non-linear association between EAA and BMI. For those with BMI < 35 (obesity class II)35, increased BMI was a protective factor associated with a slower aging process; however, if BMI ≥ 35, increased BMI was a risk factor linked to age acceleration. Although this U-shaped association is not fully consistent with published large studies where increased BMI is associated with increases in EAA,29,32 our finding could still be clinically meaningful. Evidence on non-EAA literature, including a meta-analysis of 414,587 participants, has shown this U-shaped association between BMI and QOL36 as well as mortality.37 Since a cutoff of BMI = 30 was defined as obesity class I,35 BMI = 30 was also tested for the U-shaped association; however, no significant effect was detected. Further research may verify this U-shaped association of BMI with EAA, and a larger sample size study may determine whether BMI = 30 could be a cutoff for the association.
In our multivariable model, BMI remained in the model, along with clinical characteristics, while other demographic and lifestyle factors were no longer significant. As expected, more comorbidities, HPV-unrelated tumors, and severer HNC symptom clusters were the risk factors for higher EAA, suggesting a “premature aging” phenomenon among patients with those risk factors. These findings are in agreement with published work showing that these risk factors are also associated with worse disease outcomes.14,38–40 Lower lymphocytes were significantly associated with higher EAA; this negative association is consistent with Levine’s model of calculating DNAmPhenoAge.29 Furthermore, one hallmark change in the immune system linked to aging or immunosenescence is the alteration of the number and composition of different types of lymphocytes, particularly decreased naïve T and B cells,41 which also occur during anti-tumor treatment.42,43
Having performed the earliest study addressing EAA, survival, and QOL in cancer patients, we cannot compare our results to other cancer populations, but our findings are in line with non-cancer publications.8,12,33,44 A meta-analysis of >13,000 participants demonstrated that EAA predicted all-cause mortality.9 More importantly, our analyses revealed that the association between EAA and OS as well as PFS is above and beyond traditional risk factors such as functional status and comorbidity, suggesting that EAA could be a superior prognostic factor.45 Other studies have also found that EAA’s predictive effect is independent of comorbidity or telomere length.9,12,46 Although it is unclear why acceleration in epigenetic age is linked to worse OS and PFS, our analysis of EAA’s risk factor suggests that more comorbidities, biologically more aggressive tumors (HPV-unrelated tumors), and severer treatment-related toxicities (HNC clusters), as the risk factors for higher EAA, could contribute to the unfavorable OS and PFS linked to higher EAA. Furthermore, our current study adds to the existing literature and suggests that EAA is linked to not only all-cause mortality, but also cancer-related mortality. Taken together, our findings suggest that EAA might reflect early methylation changes after cancer diagnosis and treatment and be a sensitive indicator for cancer-related fatal events. This blood-based biomarker is particularly well-suited for clinical screening and prediction (due to its minimally invasive collection method and easily repeated nature) compared to collecting tumor tissue.
Although the mechanisms of the link between EAA and cancer outcomes are unclear, recent evidence has shown that EAA captures key aspects of immunosenescence (e.g., decreased naïve T and B cells)9,29,47 T cells are the key effectors during antitumor immune responses.48 Senescent T cells with aging can result in reduced ability to eliminate tumor cells and subsequently associated with poor outcomes.49,50 Thus, EAA’s capability of capturing cellular senescence could explain in part its association with survival. This predictive effect could also be used for immunotherapy. Current immunotherapy functions mainly through T cell activation;51 immunosenescence is considered a parameter affecting the immunotherapy response.52,53 Being able to capture cellular senescence, EAA could represent a biomarker for immunotherapy efficacy.
In general, we observed that Levine’s EAA is more noticeable in all our analyses than EAA from other clocks. Using CPGs sites predicting both chronological age and clinical biomarkers may explain the outperformance of Levine’s clock, as other clocks are predictive of chronological age. Evidence also shows that Levine’s clock captures cell senescence induction, but other clocks do not,47 which may further explain that Levine’s EAA is a robust predictor of morbidity and mortality outcomes, compared to other clocks.9 In general, these clocks were calculated based on different CPG sites obtained from various methylation array platforms (Illumina 27K, 450K, or EPIC) and different tissue or blood sources.4,6,29 Thus, different clocks may reflect different biological processes and are connected with different phenotypes. Future studies could include these clocks or others to elucidate and differentiate their associations with phenotypes.
The findings of our study add to the existing literature and suggest that acceleration in biological age, such as epigenetic age, pre or post-radiotherapy (in most cases with chemotherapy), is associated with worse survival outcomes and poor quality of life. These findings indicate that interventions on decelerating aging might be meaningful to improve cancer patients’ outcomes. Results from our risk factor analyses further suggest that multiple risk factors of EAA could be considered for intervention to slow the aging process. For instance, controlling BMI, quitting smoking, and reducing side effects of cancer treatment could all contribute to decelerate aging. Decreasing the number of comorbidities could also promote healthy aging among cancer patients. Given the concurrent contributions of multiple risk factors on aging, a multicomponent intervention on a combination of different risk factors at the same time could be proposed and may act synergistically.54 These multicomponent interventions may also be personalized based on different risk factor profiles of each cancer patient to maximize the effects of promoting healthy aging on improving QOL and survival.
The major strength of the study is that it includes a relatively large sample size for this patient group. A prospective design with data collected at different times across and following cancer treatment is another strength that allows us to observe aging changes over cancer treatment. Being able to collect risk factors at multiple levels, including demographic factors, clinical characteristics, lifestyle, treatment-related toxicities, and blood markers, further added to the literature. There are limitations that are worth noting. The generalizability of our finding might be limited to patient groups with a similar disease profile as our data. As a single-institute study, the interpretation of our findings needs to be cautious. A limited number of deaths and recurrences may also produce biases in our conclusions. Missing data may bias the results; however, our QOL data comparison did not show significant differences between patients with and without QOL data.
CONCLUSIONS
BMI, comorbidities, HPV status, and HNC symptom clusters are the risk factors associated with aging faster biologically among patients with HNC receiving active cancer treatment. Our findings also provide novel evidence that EAA could be a marker for survival outcomes, above and beyond traditional risk factors. Acceleration in epigenetic age further contributes to worse QOL, and a clinically meaningful decline in QOL is observed for patients aging faster biologically. Large-scale studies in other cancer populations or patients receiving different treatments, such as immunotherapy, will further enrich the associations between EAA and cancer outcomes. Studies could also seek to develop interventions targeting the risk factors of EAA, such as controlling BMI, quitting smoking, and reducing side effects, to decelerate aging. Multicomponent interventions on the combination of various risk factors may be proposed for personalized aging management to improve survival and QOL among cancer patients.
Supplementary Material
Funding:
This work was supported by the National Institute of Nursing Research (K99/R00NR014587 and R01NR015783); and the National Cancer Institute (P30CA138292).
Footnotes
Disclosures:
Dr. Levine has a patent WO2019143845A1 with royalties paid. Dr. Saba reports consultant:: GSK, Merck, Bluprint, Biontech, Kura, and Pfizer. Dr. Higgins reports other from astra zeneca, grants from RefleXion Medical, other from precisCA, during the conduct of the study.
Data Sharing Statement:
The data underlying this article may be shared on reasonable request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Canhua Xiao, Emory University School of Nursing.
Andrew H. Miller, Emory University School of Medicine.
Gang Peng, Yale University School of Medicine.
Morgan E. Levine, Yale University School of Medicine.
Karen N Conneely, Emory University School of Medicine.
Hongyu Zhao, Yale University School of Medicine.
Ronald C. Eldridge, Emory University School of Nursing.
Evanthia C Wommack, Emory University School of Medicine.
Sangchoon Jeon, Yale University School of Nursing.
Kristin A. Higgins, Emory University School of Medicine.
Dong M. Shin, Emory University School of Medicine.
Nabil F. Saba, Emory University School of Medicine.
Alicia K. Smith, Emory University School of Medicine.
Barbara Burtness, Yale University School of Medicine.
Henry S. Park, Yale University School of Medicine.
Melinda L. Irwin, Yale University School of Public Health and Yale Cancer Center.
Leah M. Ferrucci, Yale University School of Public Health and Yale Cancer Center.
Bryan Ulrich, Emory University School of Medicine.
David C. Qian, Emory University School of Medicine.
Jonathan J. Beitler, Emory University School of Medicine.
Deborah W. Bruner, Emory University School of Nursing.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Esteller M Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. [DOI] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath S DNA methylation age of human tissues and cell types. Genome biology. 2013;14(10):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine AJ, Quach A, Moore DJ, et al. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. Journal of neurovirology. 2016;22(3):366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irvin MR, Aslibekyan S, Do A, et al. Metabolic and inflammatory biomarkers are associated with epigenetic aging acceleration estimates in the GOLDN study. Clinical epigenetics. 2018;10:56–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging. 2015;7(9):690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging. 2016;8(9):1844–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marwitz S, Heinbockel L, Scheufele S, et al. Fountain of youth for squamous cell carcinomas? On the epigenetic age of non-small cell lung cancer and corresponding tumor-free lung tissues. Int J Cancer. 2018;143(12):3061–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, Joyce BT, Colicino E, et al. Blood Epigenetic Age may Predict Cancer Incidence and Mortality. EBioMedicine. 2016;5:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 14.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 16.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. The Lancet Oncology. 2014;15(12):1319–1331. [DOI] [PubMed] [Google Scholar]
- 17.Dayyani F, Etzel CJ, Liu M, Ho C-H, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaz M, Hwang SY, Kagiampakis I, et al. Chronic Cigarette Smoke-Induced Epigenomic Changes Precede Sensitization of Bronchial Epithelial Cells to Single-Step Transformation by KRAS Mutations. Cancer Cell. 2017;32(3):360–376 e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varela-Rey M, Woodhoo A, Martinez-Chantar M-L, Mato JM, Lu SC. Alcohol, DNA methylation, and cancer. Alcohol Res. 2013;35(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath S, Zhang Y, Langfelder P, et al. Aging effects on DNA methylation modules in human brain and blood tissue. Genome biology. 2012;13(10):R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Office AE. AJCC 7th Ed Cancer Staging Manual. 2009 ed: Springer; 2009. [Google Scholar]
- 22.National Cancer Institute. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). http://outcomes.cancer.gov/tools/pro-ctcae.html. Published 2012. http://outcomes.cancer.gov/tools/pro-ctcae.html. Accessed.
- 23.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol 2013;49:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.List MA, D’Antonio LL, Cella DF, et al. The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale. A study of utility and validity. Cancer. 1996;77(11):2294–2301. [DOI] [PubMed] [Google Scholar]
- 25.Ringash J, Bezjak A, O’Sullivan B, Redelmeier DA. Interpreting differences in quality of life: the FACT-H&N in laryngeal cancer patients. Qual Life Res. 2004;13(4):725–733. [DOI] [PubMed] [Google Scholar]
- 26.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health and quality of life outcomes. 2003;1:79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortin JP, Triche TJ Jr., Hansen KD. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics (Oxford, England). 2017;33(4):558–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao C, Fedirko V, Beitler J, et al. The role of the gut microbiome in cancer-related fatigue: pilot study on epigenetic mechanisms. Support Care Cancer 2021;29:3173–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10(4):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath S, Oshima J, Martin GM, et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging. 2018;10(7):1758–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W, Ammous F, Ratliff S, et al. Education and Lifestyle Factors Are Associated with DNA Methylation Clocks in Older African Americans. Int J Environ Res Public Health. 2019;16(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quach A, Levine ME, Tanaka T, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9(2):419–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dugué PA, Bassett JK, Joo JE, et al. Association of DNA Methylation-Based Biological Age With Health Risk Factors and Overall and Cause-Specific Mortality. Am J Epidemiol. 2018;187(3):529–538. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Huang Q, Javed R, Zhong J, Gao H, Liang H. Effect of tobacco smoking on the epigenetic age of human respiratory organs. Clinical epigenetics. 2019;11(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 36.Laxy M, Teuner C, Holle R, Kurz C. The association between BMI and health-related quality of life in the US population: sex, age and ethnicity matters. International Journal of Obesity. 2018;42(3):318–326. [DOI] [PubMed] [Google Scholar]
- 37.Zaccardi F, Dhalwani NN, Papamargaritis D, et al. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: a systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia. 2017;60(2):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao C, Beitler JJ, Higgins KA, et al. Associations among human papillomavirus, inflammation, and fatigue in patients with head and neck cancer. Cancer 2018;124:3163–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacon CG, Giovannucci E, Testa M, Glass TA, Kawachi I. The association of treatment-related symptoms with quality-of-life outcomes for localized prostate carcinoma patients. Cancer. 2002;94(3):862–871. [DOI] [PubMed] [Google Scholar]
- 40.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(30):3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aiello A, Farzaneh F, Candore G, et al. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Frontiers in Immunology. 2019;10(2247). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waidhauser J, Schuh A, Trepel M, Schmälter A-K, Rank A. Chemotherapy markedly reduces B cells but not T cells and NK cells in patients with cancer. Cancer Immunology, Immunotherapy. 2020;69(1):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Research. 2016;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perna L, Zhang Y, Mons U, Holleczek B, Saum K-U, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clinical epigenetics. 2016;8(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–1587. [DOI] [PubMed] [Google Scholar]
- 46.Marioni RE, Harris SE, Shah S, et al. The epigenetic clock and telomere length are independently associated with chronological age and mortality. International journal of epidemiology. 2016;45(2):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Leung D, Thrush K, et al. Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell. 2020;in press:e13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. The Journal of experimental medicine. 1998;188(12):2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102(2–3):187–198. [DOI] [PubMed] [Google Scholar]
- 50.Chou JP, Effros RB. T cell replicative senescence in human aging. Current pharmaceutical design. 2013;19(9):1680–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews Immunology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. European journal of cancer (Oxford, England : 1990). 2017;82:155–166. [DOI] [PubMed] [Google Scholar]
- 53.Isidori A, Loscocco F, Ciciarello M, et al. Immunosenescence and Immunotherapy in Elderly Acute Myeloid Leukemia Patients: Time for a Biology-Driven Approach. Cancers. 2018;10(7):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guida JL, Agurs-Collins T, Ahles TA, et al. Strategies to Prevent or Remediate Cancer and Treatment-Related Aging. Journal of the National Cancer Institute. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.