Abstract
Carotid plaque instability contributes to large vessel ischemic stroke. Although vascular smooth muscle cells (VSMCs) affect atherosclerotic growth and instability, no treatments aimed at improving VSMC function are available. Large genetic studies investigating atherosclerosis and carotid disease in relation to the risk of stroke have implicated polymorphisms at the HDAC9 locus. The HDAC9 protein has been shown to affect the VSMC phenotype; however, how this might affect carotid disease is unknown. We conducted a pilot investigation using single nuclei RNA sequencing of human carotid tissue to identify cells expressing HDAC9 and specifically investigate the role of the HDAC9 in carotid atherosclerosis. We found that carotid VSMCs express HDAC9 and genes typically associated with immune characteristics. Using cellular assays, we have demonstrated that recruitment of macrophages can be modulated by HDAC9 expression. HDAC9 expression might affect carotid plaque stability and progression through its effects on the VSMC phenotype and recruitment of immune cells.
Keywords: Atherosclerosis, Carotid, Single cell sequencing, Vascular smooth muscle cells
Carotid plaque instability contributes to large vessel ischemic stroke. Although vascular smooth muscle cells (VSMCs) affect atherosclerotic growth and instability, at present, no therapies aimed at improving VSMC function are available.1 Definitive invasive treatment of atherosclerotic lesions includes physical removal of plaque through surgical carotid endarterectomy or exclusion of the lesion through stenting. The bulk of these plaques are composed of VSMCs.2,3 However, most studies to date have focused on the immune-mediated progression of atherosclerosis.4,5 Frequently, single markers such as CD68 and LGALS3 have been used to identify cells of immune origin, despite the ability for VSMCs to express these same markers, suggesting that VSMC contribution to atherosclerosis might have been previously attributed to cells of immune origin.1, 2, 3 Genome-wide association studies investigating both atherosclerosis and stroke have implicated the HDAC9 locus.5, 6, 7, 8 However, controversy exist regarding whether HDAC9 is the gene mediating this effect. In experimental disease models, HDAC9 has been shown to play a role in VSMC phenotype modulation and to affect cardiovascular diseases such as aortic aneurysm and neointimal stenosis.9,10 We have previously shown that the HDAC9 protein joins a novel epigenetic complex involved in the remodeling of VSMC chromatin.9 It consists of HDAC9, the chromatin remodeling enzyme BRG1, and the long non-coding RNA MALAT-1 (metastasis-associated lung adenocarcinoma transcript 1). The HDAC9 complex assembles in the nucleus of diseased VSMCs and is associated with the promoters of genes that maintain cellular phenotypic stability.9 Targeting the HDAC9 complex through genetic deletion of Hdac9 or Malat1 and pharmacologic inhibition has palliated thoracic aortic aneurysms in models of experimental aortic aneurysms and reduced surgically induced neointimal hyperplasia in mice.9, 10, 11 These data have suggested an emerging role for HDAC9-mediated transcriptional silencing in VSMCs for a broad spectrum of vascular diseases. We, therefore, investigated whether HDAC9 plays a role in VSMC phenotype switching in carotid disease. We conducted single nuclear RNA sequencing (snRNA-seq) of human carotid tissue to identify the cells expressing HDAC9 and specifically investigated carotid VSMC biology.
Methods
Details of the methods used are available in the Supplementary Methods. All the patients provided written informed consent. Carotid plaque was isolated from patients who had undergone carotid endarterectomy for symptomatic and asymptomatic carotid disease (institutional review board approval no. 2000P001531). Control carotid tissue at the carotid bifurcation was obtained from patients without carotid atherosclerotic disease who had participated in a rapid autopsy protocol (institutional review board DCFI approval no. 13-1416). Carotid endarterectomy tissue and full-thickness control carotid tissue from the study participants provided medial VSMCs for comparison.12,13 Single cell data are available from the Single Cell Portal (available at: https://singlecell.broadinstitute.org).
Results
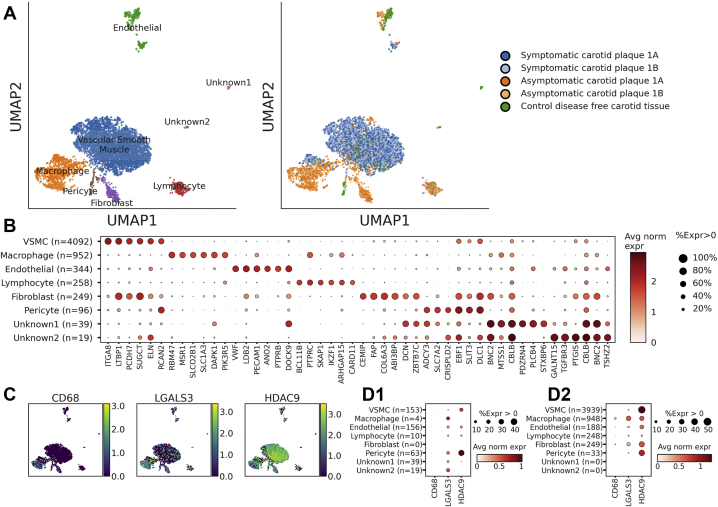
snRNA-seq of human carotid tissue showed that VSMCs express genes associated with immune cells
Internal carotid tissue was obtained from participants without carotid disease and those with symptomatic carotid plaque and asymptomatic carotid plaque. The samples underwent preservation, nuclear isolation, and processing for snRNA-seq, similar to previously described methods.14 The baseline characteristics of the patients and the methods of tissue processing and analysis are described in the Supplementary Methods. snRNA-seq was performed on carotid tissue from seven patients. Three samples met the quality control criteria for analysis of one individual or sample each of control tissue, asymptomatic plaque, and symptomatic plaque for exploratory analysis. Six distinct cell types were identified among the 6049 nuclei that had passed quality control. The cell types were identified across the patient samples using canonical marker expression.14 A depiction of our single nuclei data grouped by expression profiles from recent single cell data of carotid endarterectomy specimens is shown in Supplementary Fig 1, A.15 Immune cells demonstrated greater expression of CD68 and LGALS3. These markers were also expressed in carotid tissue VSMCs from patients with carotid atherosclerosis; however, their expression was not as prevalent in those without atherosclerotic disease (Fig 1). Furthermore, HDAC9 was highly expressed in the VSMCs from patients with carotid disease. An additional subclustering analysis of the VSMC population identified two subpopulations that might represent two distinct VSMC phenotypes, distinguished predominantly by PDE4D, ANK3, and LAMA2 expression. However, the interpretability was limited by the sample number (Supplementary Fig 1, B). Given the interest in HDAC9 biology in the setting of stroke,5,7,8,16 we also investigated the role of HDAC9 in VSMC phenotype modulation in the setting of healthy and diseased states.
Fig 1.
Observed cell types in carotid plaque and tissue. A, Combined uniform manifold approximation and projection (UMAP) plot of 6049 cells from three individuals shown by assigned cell types (Left) and specimen source (Right). B, Dot plot detailing the proportion of cells where each gene is detected (dot size) and the mean log-normalized expression (red) for representative markers of each cell type. C, UMAP plot of all nuclei, with color corresponding to the level of the log-normalized expression of indicated genes. D, Dot plot detailing the proportion of cells where each gene is detected (dot size) and mean log expression (red) for representative in (1) control (65-year-old woman without atherosclerotic disease) and (2) diseased tissue (70-year-old woman with symptomatic carotid disease and 71-year-old woman with asymptomatic carotid disease). VSMCs, Vascular smooth muscle cells.
VSMC phenotype modulation and recruitment of macrophages can be modulated by HDAC9 in cell models of atherosclerosis
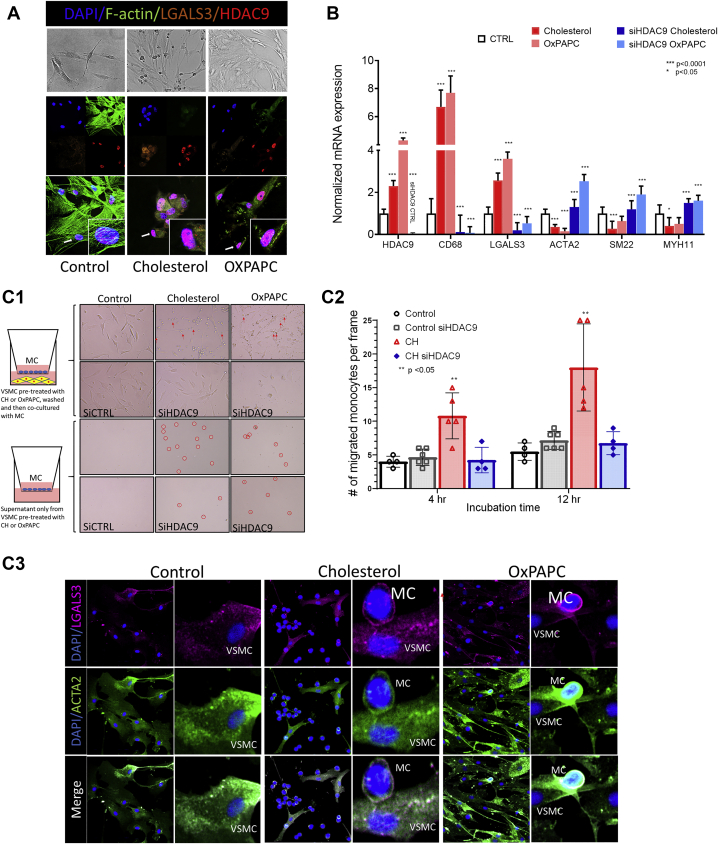
In the disease states, VSMCs have been shown to modulate phenotypes, express immune cell markers such as LGALS3 and CD68, and lose expression of contractile genes such ACTA2, SMTN1, and MYH11.1,3,17 To model atherosclerosis in cell culture, we treated human primary VSMCs with either cholesterol or oxidized phospholipids in a model of induced phenotypic modulation. We observed cell morphology changes, lipid uptake, and increased expression of LGALS3 and CD68 and decreased expression of contractile genes (Fig 2, A and B, and Supplementary Figs 2 and 3). The treated cells had viability and ability for migration similar to those of the control cells. However, VSMCs inhibited for HDAC9 had a decreased ability to migrate (Supplementary Figs 4 and 5). Decreased expression of classic immune markers and restoration of VSMC contractile gene expression was observed with small interfering RNA inhibition of HDAC9, despite cell treatment with lipids (Fig 2, B).
Fig 2.
A, Human primary vascular smooth muscle cells (VSMCs) treated with cholesterol or oxidized phospholipids (OxPAPC) demonstrated structural changes compared with control (CTRL) cells grown in normal media using bright field microscopy. Immunofluorescence staining showed migration of HDAC9 to the nucleus and increased HDAC9 expression in treated VSMCs. B, Treated VSMCs had significantly increased expression of HDAC9, CD68, and LGALS3 and decreased expression of ACTA2, SM22, and MYH11 compared with the control cells. Inhibition of HDAC9 in the control cells demonstrated efficacy of HDAC9 silencing, also confirmed by Western blot (Supplementary Fig 2). In the treated cells, HDAC9 silencing reduced expression of HDAC9, CD68, and LGALS3 and restored expression of ACTA2, SM22, and MYH11. P values reported for comparisons between control and cholesterol and control and OXPAPC groups and cholesterol and OXPAPC with small interfering HDAC9 (siHDAC9) treatment. C1, Transwell experiments using supernatant from treated VSMCs and VSMCs grown in coculture with THP-1 (MC) cells incubated for 4 and 12 hours. Treated VSMCs and their supernatant increased migration of macrophages (circled in red) across the 3-μm-pore Transwell plates. C2, The number of MCs that migrated to the bottom well were counted after 4 and 12 hours; each group was repeated in quadruplicate. P values reflect comparisons between the counts of the migrated MCs between the control group and each of the three treatment groups at 4 and 12 hours. The VSMCs that underwent silencing for HDAC9 showed no difference in the migration of monocytes compared with untreated, or control, group VSMCs, despite treatment with cholesterol. C3, Immunofluorescence staining of the Transwell plates depicts the interaction of macrophages and VSMCs showing increased staining of LGALS3 and ACTA2 in VSMCs that had undergone treatment, especially at the macrophage–VSMC interface.
When VSMCs and activated macrophages were grown in coculture Transwell plates, lipid-exposed VSMCs increased the migration of macrophages (Fig 2, C). Furthermore, isolated supernatant of VSMCs that had undergone lipid treatment was sufficient in recruiting macrophages, suggesting that VSMCs that have switched phenotype will secrete factors that increase macrophage migration. Proteomic analysis of the supernatant revealed potential targets for further analysis, such as PN-1. Pathway analysis of the proteins highly expressed in the supernatant of VSMCs treated with cholesterol suggested involvement in functions related to cell morphology, movement, growth, and proliferation (Supplementary Fig 6). Inhibition of HDAC9 in this model system significantly decreased the migration of macrophages in the experiments of coculture or supernatant only (Fig 2, C).
Effects of HDAC9 activity on VSMC phenotype observed in human carotid tissue
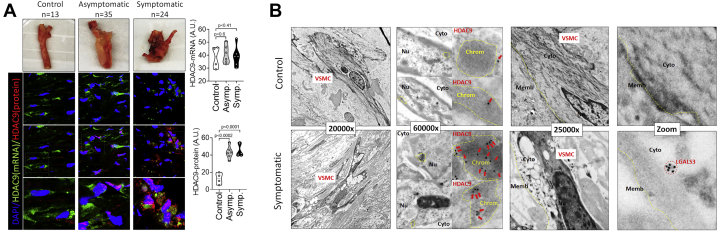
The observations were replicated in the human carotid tissues among the 13 patients free of disease, 35 patients with asymptomatic carotid disease, and 24 patients with symptomatic carotid disease. Transmission electron microscopy of VSMCs from the control, asymptomatic, and symptomatic tissue samples showed striking differences in cell morphology and staining of HDAC9 and LGALS3 between the disease states (Fig 3, A and B, and Supplementary Fig 9). VSMCs from carotid tissue free of disease demonstrated consistent organization of actin. In contrast, VSMCs from diseased tissue had large vacuoles and disorganized overall morphology and collagen fibrils (Supplementary Fig 9). Immunohistochemical staining of HDAC9 in the tissue showed localization in the electron dense areas of the nucleus and clustering of LGALS3 in the cell cytoplasm of symptomatic patients, which was absent in the control tissue (Fig 3, B).
Fig 3.
A, Carotid tissue isolated from control carotid artery and asymptomatic (Asympt.) and symptomatic (Sympt.) carotid artery plaque. Fluorescence in situ hybridization of nine specimens (three control, three asymptomatic, three symptomatic) showing similar expression of HDAC9 mRNA in human carotid tissue with and without disease but significantly increased protein levels of HDAC9 in diseased carotid tissue. B, Transmission electron microscopy showing striking phenotypic differences in vascular smooth muscle cells (VSMCs) from patients without and with carotid atherosclerosis. Consistent organization was found for actin and cell morphology in the control tissue. Diseased tissue displayed cells with large vacuoles and disorganized morphology. Localization of HDAC9 was found in the electron dense areas of the nucleus and clustering of LGALS3 in the cell cytoplasm of the diseased carotid tissue, which was absent in the control tissue. Samples from three control patients and three patients with symptomatic plaques were analyzed, with representative images shown. Chrom, Chromatin; Cyto, cytoplasm; Memb, membrane; Nu, nucleus.
Immunofluorescent analysis of the human carotid atherosclerotic tissue compared with the control tissue showed increased expression of HDAC9 and PN-1, the most highly expressed secretory protein found on proteomic analysis of the supernatant from lipid-treated VSMCs in culture (Fig 3, A). Silencing HDAC9 in VSMCs treated with cholesterol decreased the expression of PN-1 (Supplementary Fig 7). We also found increased protein and mRNA expression of HDAC9 in the nuclei of tissues from the patients with asymptomatic and symptomatic carotid disease (Fig 3, A). Furthermore, human carotid tissue with atherosclerosis had increased histone methylation, consistent with decreased contractile gene expression, compared with the control tissues (Supplementary Fig 8).
Discussion
We have provided the first example of single nuclei mapping of whole human carotid tissue in humans with and without carotid atherosclerosis. The findings from analysis of carotid tissue snRNA-seq offer additional support that HDAC9 expression and immune cell marker expression is not limited to cells of immune origin. The VSMCs in experimentally induced atherosclerotic conditions and human carotid atherosclerotic lesions expressed classic immune markers and suggested the occurrence of VSMC transdifferentiation or phenotype switching during atherogenesis. We have previously shown that inhibition of the HDAC9 complex allows for expression of contractile gene expression in VSMCs and reduced neointimal hyperplasia in mouse carotid ligation models.9,10 Our data suggest that VSMCs and the yet to be fully elucidated role of HDAC9 might have a larger influence on the pathogenesis and inflammatory effects of atherosclerosis than previously thought.
A comparison of our data with the limited single cell data available for carotid atherosclerotic disease revealed several important areas of interest for further exploration. Single cell analyses of carotid atherosclerotic disease thus far have focused on murine models, the immune cell population of plaque, or have not included control or healthy carotid tissue.4,15,18,19 All single cell or nuclei studies to date have also used a flow cytometry step to sort specifically for leukocyte populations or to select for cell viability, which could have resulted in a misrepresentation of cellular diversity owing to the bias inherent in sample preparation to target certain cell lineages instead of others.20 The protocols used thus far have shown that human carotid plaques and microdissected mouse plaques are mostly populated by macrophages, T cells, and monocytes.
However and most distinctively, our data, in contrast to the findings from most existing studies, have shown that the predominant cell population in human plaques is VSMCs. Similar to recent single cell data reported by Depuydt et al,15 our data have implicated the role of VSMCs in endarterectomy tissue and defined a similar range of cellular diversity. The findings from both studies have highlighted the importance of high-quality tissue processing, a sufficient single cell or nuclei quantity, the use of quality control in data analysis, and nuanced interpretation of the data for characterization of distinct cell “populations.” Thus, the characterization of synthetic VSMCs reported by Depuydt et al15 closely resembled the fibroblast population observed in our dataset. A defined fibroblast cell population, however, was not reported by Depuydt et al.15 This discrepancy has revealed the fluid nature of cell population assignments, especially for VSMCs, which have a range of phenotypes. The significant overlap in the expression profile between fibroblasts, pericytes, and VSMCs has made identifying isolated cell groups, as they have been defined at present, difficult or inappropriate. Other differences between studies could have resulted from the variability in gene capture between single cell sequencing vs snRNA-seq. Despite these limitations of single cell and single nuclei analyses, however, additional data and refinement in the techniques and data analysis could greatly change and challenge our current understanding and definitions of cell populations and phenotypes.
Bulk RNAseq-based transcriptomic studies of VSMCs from atherosclerotic plaque and single cell studies of VSMCs from coronary arteries from humans have suggested that VSMCs are regulators of plaque stability.21,22 Current human studies, however, have mostly explored the cardiac or coronary tissue, rather than the peripheral vascular tissue. Furthermore, studies have emphasized the exclusion of the medial layers of VSMCs in studying atherosclerotic disease,19 despite our data, and data from others, supporting that VSMCs with phenotypic changes in plaque might be from the media. The reported data have suggested that medial VSMCs are not terminally differentiated and are capable of phenotypic transition in culture, atherosclerosis, and after vascular injury—changes that might be modulated by HDAC9.1,10,23 Thus, although atherosclerotic tissue, itself, provides significant obstacles for analysis owing to its extremely heterogeneous composition of plaque, phenotypically diverse environment, cell–cell and cell–particle interactions,24, 25, 26 the findings from our single nuclei and tissue analysis of human carotid tissue support the evidence of modifiable, HDAC9-dependent phenotypic VSMC changes in atherosclerotic disease that could affect disease progression.
Study limitations
The limitations of our study included the small number of human participants in our single nuclei analysis. We consider our study a proof-of-concept exploratory effort in sequencing single nuclei from frozen, whole carotid tissue and the beginning of a larger effort to capture the cellular diversity of human carotid tissue. The underlying mechanisms and consequences of targeting VSMC phenotype switching via HDAC9 in the context of atherosclerotic disease progression remain to be fully defined. However, we found that VSMCs are an important cellular component of plaque, with contributions to the established inflammatory activity associated with atherosclerotic disease previously attributed mainly to cells of immune origin. Although symptomatic and asymptomatic carotid plaque samples were investigated in the present study, we were unable to discern any differences in the VSMC phenotype according to clinical status. This might have resulted from the overall low number of human specimens and an inherent overlap in the clinical characteristics of asymptomatic plaque, because patients had undergone carotid endarterectomy before becoming symptomatic.
Conclusions
The emerging findings in cell, animal, and human studies have suggested that HDAC9 influences carotid atherosclerosis in relation to the stroke risk in cells of the immune system. We found that HDAC9 modifies the VSMC phenotype and immune cell recruitment in carotid disease in vitro and in human tissue through histologic analysis and snRNA-seq of human carotid tissue. Further studies are warranted to investigate the underlying mechanisms and consequences of VSMC phenotype switching in atherosclerosis that might be modulated by HDAC9 targeting in carotid atherosclerosis.
Author Contributions
Conception and design: EC, CLC, PE, ML
Analysis and interpretation: EC, CLC, MC, AA, ML
Data collection: EC, DJ, JS, GL, ME, MC, EI, PE
Writing the article: EC
Critical revision of the article: EC, CLC, MC, AA, DJ, JS, GL, ME, MC, EI, PE, ML
Final approval of the article: EC, CLC, MC, AA, DJ, JS, GL, ME, MC, EI, PE, ML
Statistical analysis: EC, MC
Obtained funding: EC, PE, ML
Overall responsibility: EC
Acknowledgments
Data supporting the findings from the present study are available from the corresponding author on request.
Footnotes
E.L.C. is supported by the National Institutes of Health (grant T32HL007208) and a Vascular and Endovascular Surgery Society Resident Research Award. The rapid autopsy program has been generously supported by the Susan Eid Tumor Heterogeneity Initiative. P.T.E. is supported by the Fondation Leducq (grant 14CVD01), National Institutes of Health (grants 1RO1HL092577 and K24HL105780), and American Heart Association (grant 18SFRN34110082). M.E.L. and C.L.L. are supported by the National Institutes of Health (grant 1RO1HL130113). M.E.L. is supported by the Toomey Fund for Aortic Research.
Author conflict of interest: The Precision Cardiology Laboratory is a joint effort between the Broad Eli and Edythe L. Institute and Bayer AG. A.D.A. is an employee of Bayer US LLC (a subsidiary of Bayer AG). P.T.E. receives sponsored research support from Bayer AG and IBM Health and has served on advisory boards or consulted for Bayer AG, MyoKardia, Quest Diagnostics, and Novartis. E.L.C., C.L.L.C., M.C., D.J., J.R.S., G.M.L., M.J.E., M.F.C., and E.M.I. have no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix (online only)
References
- 1.Bennett M.R., Sinha S., Owens G.K. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Dubland J.A., Allahverdian S., Asonye E., Sahin B., Jaw J.E., et al. Smooth muscle cells contribute the majority of foam cells in Apoe (apolipoprotein e)-deficient mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:876–887. doi: 10.1161/ATVBAHA.119.312434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feil S., Fehrenbacher B., Lukowski R., Essmann F., Schulze-Osthoff K., Schaller M., et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez D.M., Rahman A.H., Fernandez N.F., Chudnovskiy A., Amir E.D., Amadori L., et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azghandi S., Prell C., van der Laan S.W., Schneider M., Malik R., Berer K., et al. Deficiency of the stroke relevant HDAC9 gene attenuates atherosclerosis in accord with allele-specific effects at 7p21.1. Stroke. 2015;46:197–202. doi: 10.1161/STROKEAHA.114.007213. [DOI] [PubMed] [Google Scholar]
- 6.Jaitin D.A., Kenigsberg E., Keren-Shaul H., Elefant N., Paul F., Zaretsky I., et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779. doi: 10.1126/science.1247651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shroff N., Ander B.P., Zhan X., Stamova B., Liu D., Hull H., et al. HDAC9 polymorphism alters blood gene expression in patients with large vessel atherosclerotic stroke. Transl Stroke Res. 2019;10:19–25. doi: 10.1007/s12975-018-0619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markus H.S., Makela K.M., Bevan S., Raitoharju E., Oksala N., Bis J.C., et al. Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke. 2013;44:1220–1225. doi: 10.1161/STROKEAHA.111.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lino Cardenas C.L., Kessinger C.W., Cheng Y., MacDonald C., MacGillivray T., Ghoshhajra B., et al. An HDAC9-MALAT1-BRG1 complex mediates smooth muscle dysfunction in thoracic aortic aneurysm. Nat Commun. 2018;9:1009. doi: 10.1038/s41467-018-03394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lino Cardenas C.L., Kessinger C.W., Chou E.L., Ghoshhajra B., Yeri A.S., Das S., et al. HDAC9 complex inhibition improves smooth muscle dependent stenotic vascular disease. JCI Insight. 2019;4:e124706. doi: 10.1172/jci.insight.124706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lino Cardenas C.L., Kessinger C.W., MacDonald C., Jassar A.S., Isselbacher E.M., Jaffer F.A., et al. Inhibition of the methyltranferase EZH2 improves aortic performance in experimental thoracic aortic aneurysm. JCI Insight. 2018;3:e97493. doi: 10.1172/jci.insight.97493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niizuma K., Shimizu H., Inoue T., Watanabe M., Tominaga T. Maximum preservation of the media in carotid endarterectomy. Neurol Med Chir (Tokyo) 2014;54:812–818. doi: 10.2176/nmc.tn.2014-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts W.C., Laborde N.J., Pearl G.J. Frequency and extent of media in the internal carotid artery in "endarterectomy" specimens. Am J Cardiol. 2007;99:990–992. doi: 10.1016/j.amjcard.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Tucker N.R., Chaffin M., Fleming S.J., Hall A.W., Parsons V.A., Bedi K.C., Jr., et al. Transcriptional and cellular diversity of the human heart. Circulation. 2020;142:466–482. doi: 10.1161/CIRCULATIONAHA.119.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Depuydt M.A.C., Prange K.H.M., Slenders L., Ord T., Elbersen D., Boltjes A., et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ Res. 2020;127:1437–1455. doi: 10.1161/CIRCRESAHA.120.316770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prestel M., Prell-Schicker C., Webb T., Malik R., Lindner B., Ziesch N., et al. The atherosclerosis risk variant rs2107595 mediates allele-specific transcriptional regulation of HDAC9 via E2F3 and Rb1. Stroke. 2019;50:2651–2660. doi: 10.1161/STROKEAHA.119.026112. [DOI] [PubMed] [Google Scholar]
- 17.Cherepanova O.A., Pidkovka N.A., Sarmento O.F., Yoshida T., Gan Q., Adiguzel E., et al. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circ Res. 2009;104:609–618. doi: 10.1161/CIRCRESAHA.108.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkels H., Ehinger E., Vassallo M., Buscher K., Dinh H.Q., Kobiyama K., et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122:1675–1688. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochain C., Vafadarnejad E., Arampatzi P., Pelisek J., Winkels H., Ley K., et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 20.Williams J.W., Winkels H., Durant C.P., Zaitsev K., Ghosheh Y., Ley K. Single cell RNA sequencing in atherosclerosis research. Circ Res. 2020;126:1112–1126. doi: 10.1161/CIRCRESAHA.119.315940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alloza I., Goikuria H., Idro J.L., Trivino J.C., Fernandez Velasco J.M., Elizagaray E., et al. RNAseq based transcriptomics study of SMCs from carotid atherosclerotic plaque: BMP2 and IDs proteins are crucial regulators of plaque stability. Sci Rep. 2017;7:3470. doi: 10.1038/s41598-017-03687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirka R.C., Wagh D., Paik D.T., Pjanic M., Nguyen T., Miller C.L., et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25:1280–1289. doi: 10.1038/s41591-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doran A.C., Meller N., McNamara C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhry F., Isherwood J., Bawa T., Patel D., Gurdziel K., Lanfear D.E., et al. Single-cell RNA sequencing of the cardiovascular system: new looks for old diseases. Front Cardiovasc Med. 2019;6:173. doi: 10.3389/fcvm.2019.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Kuijk K., Kuppe C., Betsholtz C., Vanlandewijck M., Kramann R., Sluimer J.C. Heterogeneity and plasticity in healthy and atherosclerotic vasculature explored by single-cell sequencing. Cardiovasc Res. 2019;115:1705–1715. doi: 10.1093/cvr/cvz185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M., Gomez D. Smooth muscle cell phenotypic diversity. Arterioscler Thromb Vasc Biol. 2019;39:1715–1723. doi: 10.1161/ATVBAHA.119.312131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.