Abstract
Physical activity relates to reduced dementia risk, but the cellular and molecular mechanisms are unknown. We translated animal and in vitro studies demonstrating a causal link between physical activity and microglial homeostasis into humans. Decedents from Rush Memory and Aging Project completed actigraphy monitoring (average daily activity) and cognitive evaluation in life, and neuropathological examination at autopsy. Brain tissue was analyzed for microglial activation via immunohistochemistry (anti-human HLA-DP-DQ-DR) and morphology (% Stage I, II, or III), and synaptic protein levels (SNAP-25, synaptophysin, complexin-I, VAMP, syntaxin, synaptotagmin-1). Proportion of morphologically activated microglia (PAM) was estimated in ventromedial caudate, posterior putamen, inferior temporal (IT), and middle frontal gyrus. The 167 decedents averaged 90 years at death, two-thirds were nondemented, and 60% evidenced pathologic Alzheimer's disease (AD). Adjusting for age, sex, education, and motor performances, greater physical activity associated with lower PAM in the ventromedial caudate and IT. Relationships between physical activity and PAM in the ventromedial caudate or IT were particularly prominent in adults evidencing microinfarcts or AD pathology, respectively. Mediational analyses indicated that PAM IT mediated ∼30% of the relationships between (1) physical activity and synaptic protein in IT, and (2) physical activity and global cognition, in separate models. However, the size of the mediation depended on AD pathology ranging from >40% in adults with high AD burden, but <10% in adults with low AD burden. Lower microglial activation may be a pathway linking physical activity to age-related brain health in humans. Physical activity may promote AD-related synaptic and cognitive resilience through reduction of pro-inflammatory microglial states.
SIGNIFICANCE STATEMENT Physical activity relates to better cognitive aging and reduced risk of neurodegenerative disease, yet the cellular and molecular pathways linking behavior-to-brain in humans are unknown. Animal studies indicate that increasing physical activity leads to decreased microglial activation and corresponding increases in synaptogenesis and neurogenesis. We objectively monitored physical activity (accelerometer-based actigraphy) and cognitive performances in life, and quantified microglial activation and synaptic markers in brain tissue at death in older adults. These are the first data supporting microglial activation as a physiological pathway by which physical activity relates to brain heath in humans. Although more interventional work is needed, we suggest that physical activity may be a modifiable behavior leveraged to reduce pro-inflammatory microglial states in humans.
Keywords: Alzheimer's disease, exercise, HLA, major histocompatibility complex II
Introduction
Physical activity is associated with reduced risk of dementia (Buchman et al., 2012; Hörder et al., 2018) and dose-dependent cognitive benefits in older adults (Sanders et al., 2019; Ludyga et al., 2020). At the system level, physical activity interventions show hippocampal growth (Erickson et al., 2011), and increased cerebral metabolism, perfusion (Boraxbekk et al., 2016; Chapman et al., 2016; Gaitán et al., 2019), and white matter integrity (Voss et al., 2013; Tarumi et al., 2020). Even in autosomal dominant forms of dementia, greater physical activity associates with slower symptom manifestation (Müller et al., 2018; Casaletto et al., 2020a). Yet, the cellular and molecular mechanisms by which physical activity, a systemic intervention, promotes CNS health remain largely unknown. Indeed, not all physical activity interventions show benefits (Barnes et al., 2013; Lamb et al., 2018). Characterization of the active biological pathways linking physical activity to the brain are needed to identify those who stand to benefit most and resilience-related pathways amenable for therapeutic development.
Microglia are the innate immune cells of the brain that shape synaptic connections, become dysregulated with age and neurodegeneration, and are a potential beneficial target of physical activity (Hammond et al., 2018; Li and Barres, 2018). Under homeostatic conditions, microglia promote phagocytic clearance of debris, support neural differentiation, and refine synaptic pruning, whereas pathogenic inflammatory signaling can polarize microglia to inhibit neurogenesis and participate in excessive synaptic engulfment (Hammond et al., 2018; Li and Barres, 2018). A growing number of animal and human GWAS studies implicate microglial dysregulation as a contributing factor in neurodegenerative disease (Sims et al., 2017; Podleśny-Drabiniok et al., 2020) and clinical trials (e.g., CSF1R inhibitor) are underway targeting microglial function. Identification of readily available behavioral strategies that promote microglial homeostasis may be highly complementary to these ongoing efforts.
Decades of seminal works demonstrate that enhancing physical activity modulates glial number and signaling (Diamond et al., 1964; Hare, 1989). Exercised animals show less microglia activation, lower expression of hippocampal inflammatory proteins (e.g., IL1B, TNFα), and greater microglial proliferation and expression of neuroprotective factors (e.g., IGF1) (Ehninger and Kempermann, 2003; Kohman et al., 2012, 2013; Littlefield et al., 2015; He et al., 2017; Mee-inta et al., 2019). Activity-induced microglial differences correspond with and appear to mediate the neurogenic and synaptogenic benefits of physical activity. In vitro experiments show that activity-related increases in neural progenitor cells are abolished when microglia are removed from cell culture and microglia isolated from exercised animals can activate neural progenitor cells in sedentary hippocampal cell cultures (Vukovic et al., 2012). In humans, physical activity is associated with peripheral immune system functioning, including acute cytokine changes and longer-term lowered inflammatory markers (Beavers et al., 2010; Woods et al., 2012; Nascimento et al., 2014; Stigger et al., 2019). Few studies directly link physical activity to markers of neuroinflammation or microglial functioning in humans. A recent 16 week randomized exercise trial in adults with mild-to-moderate Alzheimer's disease (AD) found minimal changes in most cytokines in CSF (Jensen et al., 2019); however, there were mild increases in CSF-soluble TREM2 (key microglial receptor) among the highest exercisers. Given the important role for microglia guiding brain aging and neurodegeneration and the casual relationship between physical activity and microglial homeostasis in animals, a more in-depth understanding of this relationship in humans is needed.
We monitored objective activity levels via accelerometer-based actigraphy in older adults during life who completed autopsy with brain tissue quantified for immunohistochemistry-based microglial activation markers (anti-human HLA-DP-DQ-DR, plus morphology staging) as part of the Rush Memory and Aging Project (MAP). Prior MAP studies demonstrate that actigraphy-based physical activity is associated with better cognitive trajectories, reduced incidence of Alzheimer's dementia (Buchman et al., 2012, 2018), and higher levels of synaptic proteins in brain tissue (Casaletto et al., 2021). We aimed to characterize the relationship between late life physical activity and markers of microglial activation in MAP participants, including regional and pathology-specific effects. We additionally tested the mediational role of activated microglia on the relationships between physical activity and (1) synaptic and (2) cognitive outcomes.
Materials and Methods
Participants
A total of 167 decedents from the MAP were included (Bennett et al., 2018). All participants completed annual actigraphy monitoring visits and underwent comprehensive neuropathological evaluations with brain tissue quantified for microglial markers. MAP data collection began in 1997, and actigraphy data collection started in 2005. Exclusion criteria were inability to sign an informed consent and the Anatomical Gift Act. All included participants also signed a repository consent to allow their data to be repurposed. MAP was approved by a Rush University Medical Center Institutional Review Board and is conducted in accordance with the latest Declaration of Helsinki, including written informed consent from all participants.
Clinical assessments
Assessment of daily physical activity
Physical activity was measured continuously for 24 h/day for up to 10 d per visit with an omnidirectional accelerometer worn on the nondominant wrist (Actical; Mini Mitter). Actical provides estimates of activity counts per 15 s epochs. Records were visually examined for periods of suspected device removal. Incomplete days of data were excluded from analyses. Incomplete data were determined based on inspection of the recordings via an automated program that flagged average daily counts at the extremes: ∼0/d or >500/d. Only participants with valid data for 1+ days were included in analyses. Daily raw activity counts were then averaged based on all 15 s epochs for each day with full actigraphy data. On average in the MAP cohort, there were >3 × 105 activity counts/day; raw counts were divided by 1 × 105 (∼1 SD) to facilitate presentation (Buchman et al., 2012, 2018). Daily actigraphy counts were averaged across available visits of actigraphy data (mean visits = 1.05 [SD = 1.2], range: 1-6; Table 1) to create an indicator of late life activity less heavily influenced by a single time point. We examined how associations differed using actigraphy averaged across visits versus actigraphy at first visit or last visit. The pattern of results was the same. To use a more representative indicator of late life physical activity levels, average actigraphy across visits was used in all subsequent analyses following a similar approach in a prior MAP study (Lim et al., 2020).
Table 1.
Participant clinical and pathologic characteristics at baseline actigraphy visit (N = 167)a
| Characteristic | Value |
|---|---|
| Age, yr | 85.9 (5.8) |
| Education, yr | 14.6 (2.6) |
| Sex (%, n; female) | 66.5% (111) |
| MMSE (median, IQR) | 27 (25, 29) |
| Average physical activity (daily counts) | 2.10 (1.15) |
| Actigraphy visits (%, n) | 1.05 (1.2) range: 0-6 |
| 1 | 100% (167) |
| 2 | 64.1% (107) |
| 3 | 34.7% (58) |
| 4 | 18.6% (31) |
| 5 | 10.8% (18) |
| 6 | 1.8% (3) |
| Neurocognitive diagnosis (%, n) | |
| Clinically normal | 39.5% (66) |
| Mild cognitive impairment | 27.5% (46) |
| Dementia | 32.9% (55) |
| Years to death | 3.2 (1.6) |
| Postmortem interval, h (median, IQR) | 6.3 (5.2, 8.3) |
| Pathologic AD (Reagan criteria, %, n) | 59.9% (100) |
| Proportion activated microglia | |
| Inferior temporal gyrus | 0.08 (0.06) |
| Middle frontal gyrus | 0.09 (0.06) |
| VM caudate | 0.06 (0.05) |
| Posterior putamen | 0.09 (0.05) |
a>Data are mean and SD. MMSE, Mini Mental Status Examination; IQR, interquartile range. Daily physical activity counts divided by 1 × 105 (∼1 SD) to facilitate presentation.
Assessment of motor function
Ten motor performances were assessed at baseline and at each annual follow-up visit, including grip strength, pegboard test, finger tapping, gait speed and steps, turn time and steps, pinch strength, leg stand, and toe stand (Buchman et al., 2012). These performances were summarized into a single global performance metric (motor10). Motor performance measures were averaged to align this metric with averaged physical activity. All models adjusted for the summary motor performance metric (i.e., physical activity levels accounting for level of motor function).
Assessment of cognitive function
Global cognition was estimated using a z score composite of 17 cognitive tests examining episodic memory, visuospatial processing, semantic memory, working memory, and processing speed. Cognitive testing is collected on all participants annually since the inception of MAP in 1997. In the subset of participants who also completed actigraphy monitoring and microglial markers, participants completed 6.4 annual testing visits on average (SD = 2.8, range = 1-14 visits). All available cognitive data were incorporated into linear mixed effects models to estimate the effect of time (years) on cognitive performance. Individual-specific random cognitive slopes were then extracted from linear mixed models for analyses, per prior publications (Yang et al., 2018).
Other clinical covariates
Demographics variables used in these analyses included age, which was based on birth date obtained at baseline examination, as well as sex and years of education.
Postmortem indices
Microglial activation quantification
Brain tissue was analyzed for the presence of major histocompatibility complex II-related microglia activation at three stages of severity (Stage I, II, III) in four brain regions: midfrontal gyrus, inferior temporal (IT) gyrus, posterior putamen, and ventromedial (VM) caudate. These four regions were originally selected as part of the larger MAP study to capture both cortical and subcortical areas affected by microglial activation across different pathologies and cognitive/motor outcomes.
Activated microglia were tagged via immunohistochemistry using an Automated Leica Bond immunostainer (Leica Microsystems) and anti-human HLA-DP-DQ-DR antibodies (clone CR3/43; DaktoCytomation; 1:100 dilution; catalog #MA1-25 914). Separately, cells were staged by a blinded investigator based on morphology: Stage I (thin ramified processes), Stage II (rounded cell body >14 μm with thickened processes), or Stage III (appearance of macrophages, cell body > 14 μm) using Stereo Investigator 8.0 software (4.0% region sample with 200 × 150 μm counting frame at 400× magnification). Two adjacent blocks of tissue (0.5-1 cm apart) were quantified, and activated microglia counts were upweighted by stereology software to estimate total number by stage in defined regions. For each stage, number of activated microglia were summed, divided by area, and multiplied by 106 to obtain a composite average density by region. In the brain, major histocompatibility complex II is primarily expressed on microglia and generally considered to be a marker of reactive cells (Hopperton et al., 2018; Swanson et al., 2020); therefore, the HLA-DP-DQ-DR isoforms quantified here generally reflect densities of “activated microglia” versus total microglial count.
A summary index of the proportion of morphologically activated microglia (PAM) was calculated following a recently validated approach (Felsky et al., 2019) as follows:
r represents each of the four regions and S1-S3 represent microglial densities across activation stages. PAM was developed to be a sensitive index of the most activated microglia states consistent with disease (Felsky et al., 2019). For example, iterative model fitting and sensitivity analyses demonstrated that PAM was a better discriminator of AD pathology compared with examination of microglia at individual stages. As such, we selected PAM as our primary outcome to reflect a global index of microglia activation, which also reduced issues regarding multiple comparisons.
Assessment of brain neuropathologies
Global AD pathology was summarized from counts of three indicators: neuritic plaques, diffuse plaques, and neurofibrillary tangles, as determined by microscopic examination of silver-stained slides from five regions (midfrontal cortex, midtemporal cortex, inferior parietal cortex, entorhinal cortex, and hippocampus). The regional measures for each pathology type were averaged to obtain summary measures, and the three summary measures were averaged to obtain measures of global AD pathology. Hippocampal sclerosis was quantified unilaterally in a coronal section of the midhippocampus at the level of the lateral geniculate body, and graded as present or absent based on severity of neuronal loss and gliosis in CA1 and/or subiculum. A semiquantitative summary of cerebral amyloid angiopathy (CAA) in four neocortical regions (midfrontal, midtemporal, parietal, and calcarine cortices) was used using a severity rating scale (0 = none; 1 = mild; 2 = moderate; 3 = severe). Lewy body disease was measured using four stages of α-synuclein distribution in the brain based on a standardized procedure and neuropathologist's evaluation (0 = not present; 1 = nigral-predominant; 2 = limbic-type; 3 = neocortical-type). Presence of TDP-43 cytoplasmic inclusions in neurons and glia were determined for each region (yes vs no) and four stages of TDP-43 distribution were identified (0 = none; 1 = amygdala; 2 = amygdala + limbic; 3 = amygdala + limbic + neocortical). Macroinfarcts and microinfarcts were determined as present or missing (0 = none; 1 = 1 or more). Arteriolosclerosis was defined based on a semiquantitative grading system (0 = none; 1 = mild; 2 = moderate; 3 = severe) of histologic changes commonly found in the small vessels of the brain during aging, including intimal deterioration, smooth muscle degeneration, and fibrohyalinotic thickening of arterioles with consequent narrowing of the vascular lumen in the vessels of the anterior basal ganglia. Atherosclerosis severity was determined by visual inspection and ranged from none or possible (0) to severe (3; in more than half of all visualized arteries and/or >75% occlusion of one or more vessels).
Synaptic protein
Synaptic integrity was quantified as average levels of six synaptic proteins previously shown to demonstrate significant relationships with physical activity (SNAP-25, complexin-I, synaptophysin, synpatotagmin-1, syntaxin, VAMP) (Casaletto et al., 2021).
Frozen gray matter samples were used to prepare homogenates at a consistent protein concentration, followed by serial dilution for ELISA (Honer et al., 2012). Average synaptic levels were estimated within the VM caudate, posterior putamen, and IT and middle frontal gyri, separately, to correspond with microglial marker regionality. Monoclonal antibodies quantified synaptophysin, synaptotagmin-1, SNAP-25, syntaxin, VAMP, and complexin-I (Matthew et al., 1981; Honer et al., 1989, 1993; Takahashi et al., 1995). Values were expressed in log10 units and standardized within regions for each participant. Individual protein levels provide information regarding integrity of the presynaptic compartment; higher values indicate more protein available and have been associated with better cognitive aging (Honer et al., 2012; Boyle et al., 2013).
Statistical analyses
Our overarching goal was to determine the relationship between physical activity and microglial markers in older adults, including examination of the moderating effect of neuropathologies. We additionally tested microglial activation as a mediating pathway between physical activity and (1) synaptic or (2) cognitive outcomes.
We evaluated bivariate correlations between physical activity (daily actigraphy counts) and PAM globally, as well as in the VM caudate, posterior putamen, IT gyrus, and middle frontal gyrus. We adjusted p values for false discovery rate (FDR) associated with four comparisons; FDR adjusted p values <0.05 were considered statistically significant.
Next, we examined how relationships between physical activity and microglial activation may be altered in the context of other pathologies. To reduce potential for overfitting with our relatively small sample size, we entered nine pathology predictors as covariates in a single multivariable model and applied a backward stepwise regression approach (i.e., dementia with Lewy bodies, CAA, macroinfarcts, microinfarcts, TDP43, hippocampal sclerosis, arteriolosclerosis, cerebral atherosclerosis, global AD). The model included fixed covariates adjusting for age, sex, education, and motor10 performances and optimized for minimum Bayesian information criterion using combined removal rules for pathology indicators. Next, we evaluated a series of individual models testing the moderating effect of each pathology (average physical activity × pathology) predicting microglial activation to determine whether observed relationships were more relevant in individuals with specific pathologies. In sensitivity analyses, we further tested whether relationships between physical activity and PAM were driven by individuals at specific clinical disease stages (no cognitive impairment vs mild cognitive impairment vs dementia) or by sex via regression interaction models.
Last, we examined relationships between PAM indicators with global cognition and synaptic integrity, separately. We only examined PAM regions that showed significant associations with physical activity. Finally, based on our findings, models were integrated and tested in two comprehensive moderated mediation models (see Fig. 3). Specifically, we tested whether IT PAM mediated the relationship between physical activity and synaptic or cognitive outcomes (separately), and whether this mediation was further moderated by AD pathology. We tested hypotheses of moderated mediation (Model 7) using SPSS PROCESS macro package (Hayes, 2018). The conditional indirect effect (i.e., mediating effect of IT PAM at differing levels of AD pathology) was estimated using a 5000-sample bootstrap procedure via bias-corrected 95% CIs to test significance. If CIs do not contain 0, the conditional indirect relationship is considered statistically significant. The magnitude of the mediation (i.e., proportion of the X-Y relationship explained by mediator) was estimated by calculating: (indirect effect)/(total effect) × 100%. We adjusted for age, education, sex, and motor10 composite scores in all final models. Moderated mediation models were conducted using packages in SPSS, and all other analyses were conducted in JMP16.
Figure 3.
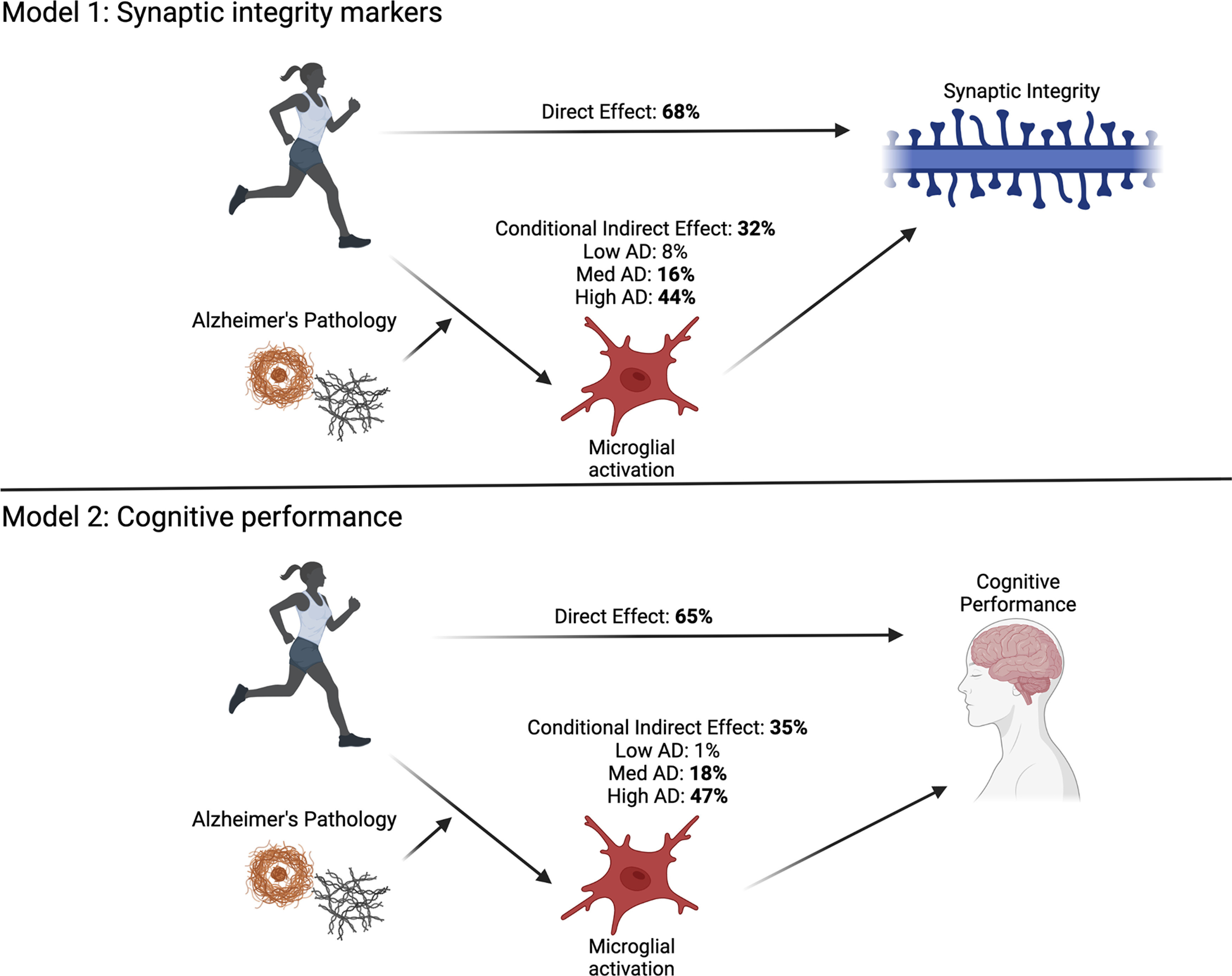
Conceptual diagram of moderated mediation models. Models examine the mediating effect of IT microglial activation (PAM IT) on the relationship between physical activity and global cognitive outcomes (Model 1, N = 156) or synaptic integrity markers in the IT gyrus (Model 2, N = 154). Both models estimated indirect effects of PAM IT (mediation) conditional on AD pathology burden (moderation). Values indicate unstandardized estimates. Created with Biorender.com.
Results
A total of 167 participants completed actigraphy monitoring and neuropathological evaluation for microglial activation markers in brain tissue. Participants averaged 86 years of age at first actigraphy evaluation and 3 years until autopsy. The cohort was roughly evenly distributed across clinical diagnoses (i.e., no cognitive impairment, mild cognitive impairment, dementia), and ∼60% met National Institute on Aging-Reagan criteria for neuropathological AD (Table 1).
Correlational analyses examining the four regions of microglia activation evidenced moderate, positive correlations between PAM striatal markers (PAM VM caudate and posterior putamen: r = 0.66, p < 0.001) and between PAM cortical markers (PAM IT and middle frontal gyrus: r = 0.67, p < 0.001), but small associations in PAM markers across striatal to cortical regions (r range = 0.20-0.27, p values < 0.02). Greater global PAM activation was not strongly associated with age at death (r = 0.13, p = 0.15), education (r = 0.01, p = 0.87), or sex (t = −0.74, p = 0.45). Greater average physical activity was associated with younger age (r = −0.25, p = 0.002) and better average motor performance scores (r = 0.37, p < 0.001), but did not significantly relate to sex (t = −0.49, p = 0.63) or education (r = −0.07, p = 0.37).
Bivariate associations demonstrated a modest, inverse relationship between average physical activity and global PAM that did not reach significance (r = −0.17, p = 0.053). Regionally, this relationship appeared to be driven by PAM in the VM caudate (r = −0.20, FDR-adjusted p = 0.04) and IT gyrus (r = −0.19, FDR-adjusted p = 0.04; Fig. 1). Relatively smaller associations were observed between physical activity and PAM in the posterior putamen (r = −0.15, FDR-adjusted p = 0.08) and middle frontal gyrus (r = −0.07, FDR-adjusted p = 0.36). Given the observed region-specific associations, all subsequent models examined PAM in the VM caudate and IT gyrus as primary outcomes of interest.
Figure 1.
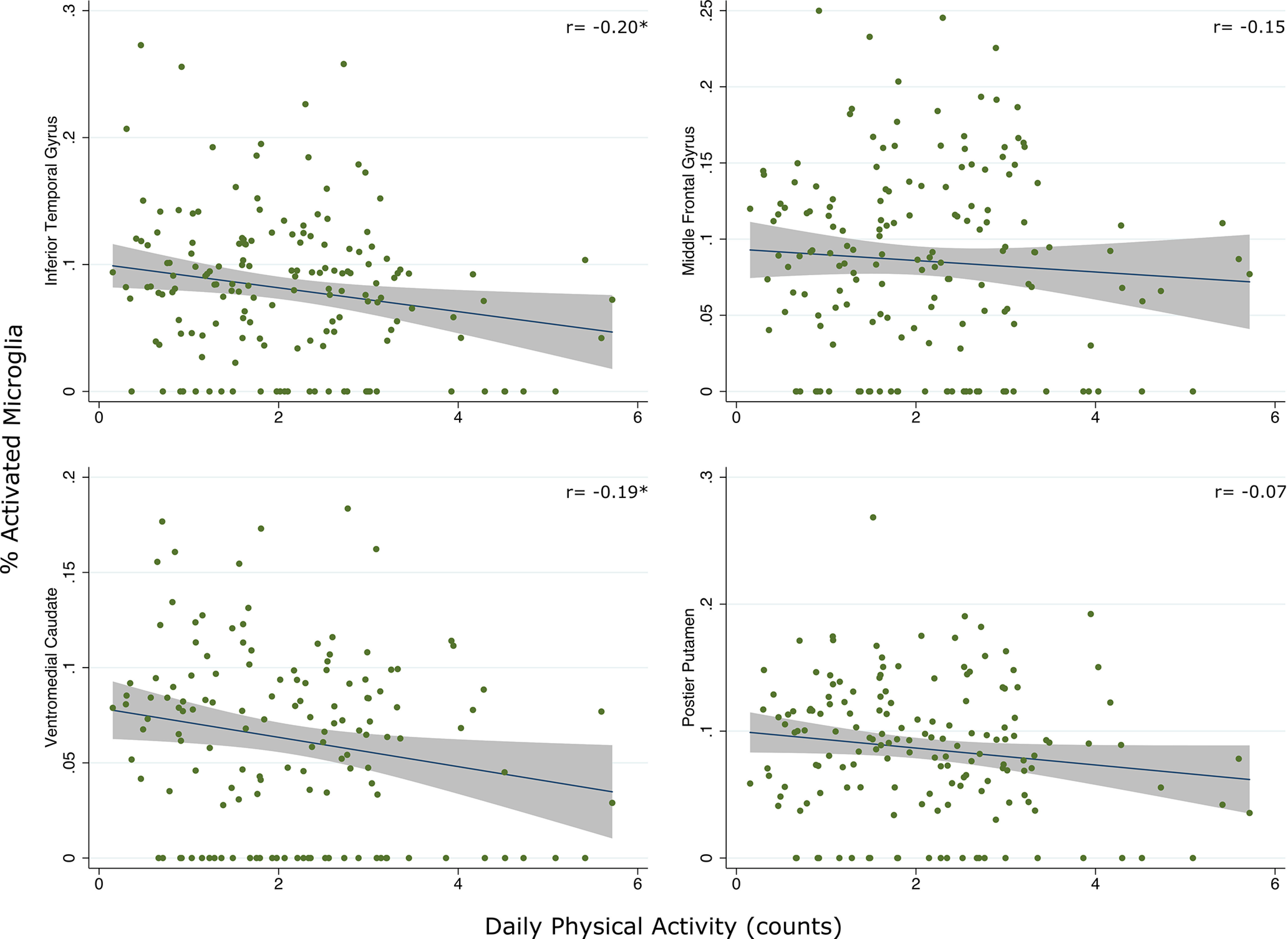
Scatterplots represent bivariate relationships between average physical activity and PAM across regions (raw data). Fitted regression line and 95% CIs are shown. Daily physical activity counts divided by 1 × 105 (∼1 SD) to facilitate presentation. *p < 0.05.
Physical activity and microglia activation: pathology-specific effects
Adjusting for age, sex, and education, backward stepwise regression tested simultaneous entry of nine common pathology indicators on the relationship between physical activity and PAM in the VM caudate or IT. Models indicated that adjustment for pathology burden did not explain further variance or alter the size of the relationship between physical activity and PAM in the VM caudate (Table 2). However, adjustment for global AD pathology increased the effect size of the relationship between physical activity and PAM in the IT gyrus (Table 2). We further covaried for average motor performance scores in both models to adjust for effect of motor symptoms contributing to physical activity engagement (i.e., reverse directionality). With adjustment, the relationship between physical activity and VM caudate PAM slightly increased (β = −0.19 to −0.22), whereas the relationship between physical activity and IT PAM slightly decreased (β = −0.20 to −0.17); in both final models, physical activity remained a statistically significant predictor, whereas motor performance was not (p values > 0.33; Table 2).
Table 2.
Final backward stepwise regression models evaluating the relationship between physical activity and regional PAM. Unstandardized b (SE); Standardized β depicted
| PAM VM caudate | PAM IT gyrus | |
|---|---|---|
| Age | 0.001 (0.001); 0.09 |
0.001 (0.001); 0.06 |
| Sex | −0.004 (0.005); −0.08 |
0.01 (0.005); 0.12 |
| Education | 0.0003 (0.002); 0.02 |
0.002 (0.002); 0.07 |
| motor10 | 0.03 (0.03); 0.10 |
−0.02 (0.03); −0.06 |
| AD pathology | — | 0.04 (0.007); 0.39** |
| Physical activity | −0.01 (0.004); −0.23* |
−0.009 (0.004); −0.17* |
Age, sex, education, motor10, and physical activity were locked effects in models, and nine pathology indicators were evaluated (AD pathology, arteriolosclerosis, CAA, TDP-43, Lewy body disease, microinfarcts, macroinfarcts, cerebral atherosclerosis, hippocampal sclerosis) and optimized for minimum Bayesian information criterion using combined entry rules.
**p < 0.01;
*p < 0.05.
Next, we tested the moderating effect of individual pathology indicators to determine whether the relationship between physical activity and reduced regional PAM was specific to adults with certain pathologies. There was a significant interaction between physical activity and microinfarcts on VM caudate PAM (interaction b = −0.02, SE = 0.01, β = −0.19, p = 0.037), as well as between physical activity and AD pathology on IT PAM (interaction b = −0.02, SE = 0.006, B = −0.18, p = 0.01; Fig. 2). Individuals with microinfarcts demonstrated a stronger relationship between physical activity and decreased PAM in the VM caudate. Similarly, individuals with greater AD pathology burden demonstrated a stronger relationship between physical activity and lower PAM IT. Framed another way, high physical activity adults with microinfarcts or high AD pathology burden demonstrated disproportionately lower microglial activation compared with their low-activity peers with the same pathology burdens. No other statistically significant interactions emerged (all p values > 0.10).
Figure 2.
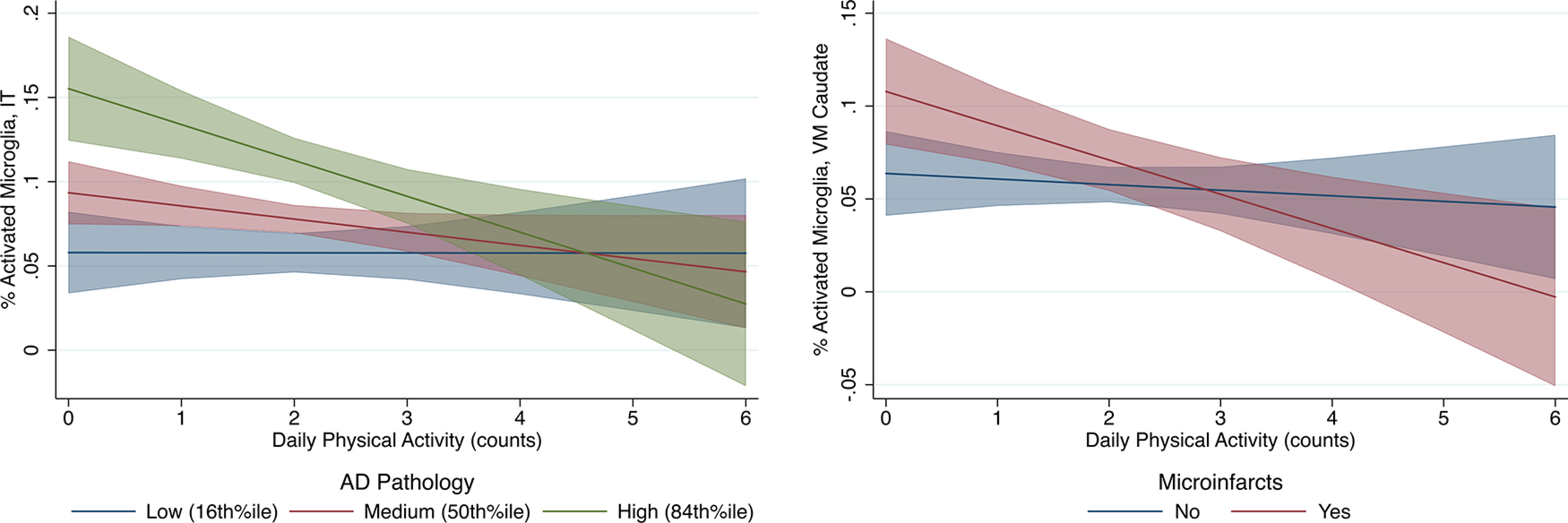
Plots of predicted relationship between physical activity and microglial activation as moderated by pathology. Fitted regression lines and 95% CIs are shown. Interaction regression models adjusted for age, sex, education, and motor10 composite. Daily physical activity counts divided by 1 × 105 (∼1 SD) to facilitate presentation.
Further adjusting for clinical diagnosis (no cognitive impairment vs mild cognitive impairment vs dementia), model estimates remained unchanged and no interactions were observed (all p values > 0.30). Additionally, there were no interactions between physical activity and sex (all p values > 0.46).
Physical activity, microglial activation, and synaptic or cognitive outcomes
To build on prior works showing an association of physical activity with synaptic and cognitive outcomes (Buchman et al., 2012, 2018; Casaletto et al., 2021) and to inform clinical relevance of our findings, we evaluated relationships between PAM in the VM caudate or IT gyrus with synaptic proteins and cognitive function.
Adjusting for age, sex, and education, higher PAM IT was associated with declining global cognition (b = −0.57, SE = 0.12, B = −0.36, p < 0.001) consistent with a recent report (Felsky et al., 2019), as well as lower synaptic integrity markers in the IT gyrus (b = −3.67, SE = 1.31, B = −0.22, p = 0.006). VM caudate PAM did not relate to cognition (b = −0.10, SE = 0.17, B = −0.05, p = 0.57) or synaptic markers in the VM caudate (b = −1.90, SE = 1.51, B = −0.11, p = 0.21).
Given observed relationships between PAM IT with (1) physical activity, moderated by AD pathology and (2) cognitive and synaptic outcomes, we integrated models to test two final (separate) moderated mediation models (Fig. 3). We hypothesized that the positive relationship between physical activity and cognitive or synaptic integrity outcomes would be mediated by lower PAM in the IT gyrus, and that this effect would be most prominent in individuals harboring AD pathology. The conditional indirect effects in both final moderated mediation models were significant, supporting the hypothesized relationships (Table 3; Fig. 3). Examining direct and indirect effects, ∼35% of the relationship between physical activity and synaptic proteins in the IT was estimated to be mediated through PAM IT (in individuals with average AD burden). The magnitude of the mediational effect was larger in those with greater AD pathology (i.e., 47% in those with high AD pathology [84th percentile]) and smaller in those with lower AD pathology (i.e., 1% in those with low AD pathology [16th percentile]). Similarly, ∼32% of the relationship between physical activity and cognition was estimated to be mediated through PAM IT. Again, the magnitude of the mediation was larger in individuals with greater AD pathology (i.e., 44% in those with high AD pathology burden [84th percentile]) and smaller in those with lower AD pathology burdens (i.e., 8% in those with low AD pathology [16th percentile SD]).
Table 3.
Moderated mediation models examining the mediating effect of IT microglial activation (PAM IT) on the relationship between physical activity and (Model 1, N = 156) global cognitive outcomes or (Model 2, N = 154) synaptic integrity markers in the IT gyrus
| Effect | SE | p | 95% CI | |
|---|---|---|---|---|
| Moderation effect (physical activity × AD pathology → PAM IT) | ||||
| Age | 0.0005 | 0.0008 | 0.49 | −0.001, 0.002 |
| Sex | −0.015 | 0.009 | 0.11 | −0.032, 0.033 |
| Education | 0.0017 | 0.0017 | 0.32 | −0.0017, 0.005 |
| motor10 | −0.026 | 0.030 | 0.39 | −0.085, 0.033 |
| AD pathology | 0.076 | 0.016 | <0.001 | 0.045, 0.011 |
| Physical activity | 0.001 | 0.006 | 0.89 | −0.011, 0.012 |
| Physical activity × AD | −0.016 | 0.007 | 0.014 | −0.029, −0.003 |
| Model 1: Direct effect (physical activity → cognition) | ||||
| Age | 0.005 | 0.0012 | <0.001 | 0.002, 0.007 |
| Sex | −0.024 | 0.015 | 0.11 | −0.052, 0.005 |
| Education | −0.002 | 0.003 | 0.54 | −0.007, 0.004 |
| motor10 | 0.089 | 0.048 | 0.065 | −0.006, 0.184 |
| PAM IT | −0.50 | 0.12 | <0.001 | −0.734, −0.267 |
| Physical activity | 0.017 | 0.007 | 0.011 | 0.004, 0.030 |
| Model 1: Conditional indirect effects (physical activity → PAM IT → cognition, at levels of AD pathology) | ||||
| Index of moderated mediation | Index: 0.008 | 0.005 | — | 0.002, 0.019 |
| AD pathology | — | |||
| Low (16th percentile) | 0.002 | 0.003 | −0.005, 0.006 | |
| Medium (50th percentile) | 0.004 | 0.003 | 0.002, 0.01 | |
| High (84th percentile) | 0.011 | 0.005 | 0.003, 0.023 | |
| Model 2: Direct effect (physical activity → synapse IT) | ||||
| Age | 0.026 | 0.014 | 0.055 | −0.005, 0.053 |
| Sex | −0.032 | 0.161 | 0.84 | −0.351, 0.287 |
| Education | 0.012 | 0.030 | 0.69 | −0.047, 0.072 |
| motor10 | −0.036 | 0.53 | 0.95 | −1.084, 1.012 |
| PAM IT | −3.573 | 1.34 | 0.008 | −6.22, −0.928 |
| Physical activity | 0.105 | 0.073 | 0.154 | −0.040, 0.249 |
| Model 2: Conditional indirect effects (physical activity → PAM IT → synapse IT, at levels of AD pathology) | ||||
| Index of moderated mediation | Index: 0.057 | 0.033 | — | 0.007, 0.135 |
| AD pathology | — | |||
| Low (16th percentile) | 0.002 | 0.020 | −0.038, 0.042 | |
| Medium (50th percentile) | 0.029 | 0.018 | 0.001, 0.070 | |
| High (84th percentile) | 0.076 | 0.037 | 0.016, 0.161 |
Both models estimated indirect effects of PAM IT (mediation) conditional on AD pathology burden (moderation) using 5000-sample bootstrapping. 16th percentile roughly corresponds to −1 SD, and 84th percentile roughly corresponds to 1 SD.
IT PAM cognitive models were particularly driven by performances in semantic and episodic memory (Table 4). To support specificity, we further show that moderated mediation models examining VM caudate PAM did not reach significance (Table 5), suggesting that the observed relationships may be most clinically relevant in the IT.
Table 4.
Moderated mediation models examining the mediating effect of IT microglial activation (PAM) on the relationship between physical activity and episodic or semantic memory slopes (N = 156)
| Effect | SE | p | 95% CI | |
|---|---|---|---|---|
| Episodic memory: direct effect (physical activity → episodic memory) | ||||
| Age | 0.004 | 0.0014 | 0.002 | 0.002, 0.007 |
| Sex | −0.026 | 0.016 | 0.11 | −0.058, 0.006 |
| Education | −0.002 | 0.003 | 0.49 | −0.008, 0.004 |
| motor10 | 0.067 | 0.053 | 0.21 | −0.038, 0.172 |
| PAM IT | −0.458 | 0.131 | <0.001 | −0.716, −0.199 |
| Physical activity | 0.014 | 0.007 | 0.070 | −0.001, 0.028 |
| Conditional indirect effects (physical activity → PAM IT → episodic memory, at levels of AD pathology) | ||||
| Index of moderated mediation | Index: 0.008 | 0.004 | — | 0.001, 0.016 |
| AD pathology | — | |||
| Low (16th percentile) | 0.002 | 0.003 | −0.005, 0.005 | |
| Medium (50th percentile) | 0.004 | 0.002 | 0.001, 0.009 | |
| High (84th percentile) | 0.010 | 0.004 | 0.003, 0.020 | |
| Semantic memory: direct effect (physical activity → semantic memory) | ||||
| Age | 0.003 | 0.014 | 0.014 | 0.007, 0.006 |
| Sex | −0.031 | 0.160 | 0.056 | −0.351, 0.287 |
| Education | 0.012 | 0.030 | 0.69 | −0.062, 0.008 |
| motor10 | 0.006 | 0.053 | 0.92 | −0.098, 0.110 |
| PAM IT | −0.538 | 0.130 | 0.0001 | −0.794, −0.282 |
| Physical activity | 0.021 | 0.007 | 0.005 | 0.007, 0.036 |
| Conditional indirect effects (physical activity → PAM IT → semantic memory, at levels of AD pathology) | ||||
| Index of moderated mediation | Index: 0.009 | 0.005 | — | 0.002, 0.020 |
| AD pathology | — | |||
| Low (16th percentile) | 0.002 | 0.003 | −0.006, 0.006 | |
| Medium (50th percentile) | 0.004 | 0.003 | 0.002, 0.010 | |
| High (84th percentile) | 0.011 | 0.005 | 0.003, 0.024 |
Models estimated indirect effects of PAM IT (mediation) conditional on AD burden (moderation) using 5000-sample bootstrapping.
Table 5.
Moderated mediation models examining the mediating effect of ventromedial caudate microglial activation (PAM) on the relationship between physical activity and (Model 3, N = 134) global cognitive outcomes or (Model 4, N = 131) synaptic integrity markers in the VM caudate
| Effect | SE | p | 95% CI | |
|---|---|---|---|---|
| Moderation effect (physical activity × microinfarct → PAM VM caudate) | ||||
| Age | 0.001 | 0.002 | 0.16 | −0.0004, 0.0026 |
| Sex | 0.004 | 0.009 | 0.63 | −0.0136, 0.022 |
| Education | 0.001 | 0.0017 | 0.32 | −0.002, 0.005 |
| motor10 | 0.033 | 0.031 | 0.30 | −0.030, 0.095 |
| Microinfarcts | 0.044 | 0.018 | 0.016 | 0.008, 0.079 |
| Physical activity | −0.003 | 0.005 | 0.51 | −0.013, 0.006 |
| Physical activity × microinfarcts | −0.015 | 0.007 | 0.042 | −0.030, −0.005 |
| Model 3: Direct effect (physical activity → cognition) | ||||
| Age | 0.005 | 0.002 | 0.001 | 0.002, 0.008 |
| Sex | −0.009 | 0.017 | 0.61 | −0.043, 0.026 |
| Education | −0.007 | 0.003 | 0.058 | −0.013, 0.0002 |
| motor10 | 0.10 | 0.057 | 0.078 | −0.012, 0.216 |
| PAM VM caudate | 0.005 | 0.17 | 0.98 | −0.330, 0.339 |
| Physical activity | 0.020 | 0.008 | 0.010 | 0.005, 0.035 |
| Model 3: Conditional indirect effects (physical activity → PAM VM caudate → cognition, with or without microinfarcts) | ||||
| Index of moderated mediation | Index: −0.001 | 0.0024 | — | −0.004, 0.005 |
| Microinfarcts | — | |||
| No | 0.000 | 0.009 | −0.002, 0.002 | |
| Yes | −0.001 | 0.003 | −0.005, 0.006 | |
| Model 4: Direct effect (physical activity → synapse VM caudate) | ||||
| Age | 0.018 | 0.014 | 0.19 | −0.009, 0.045 |
| Sex | 0.073 | 0.159 | 0.65 | −0.243, 0.388 |
| Education | 0.068 | 0.032 | 0.63 | −0.003, 0.005 |
| motor10 | 0.029 | 0.032 | 0.04 | 0.004, 0.132 |
| PAM VM Caudate | −1.685 | 1.557 | 0.28 | −4.77, 1.40 |
| Physical activity | 0.084 | 1.39 | 0.16 | −4.72, 0.771 |
| Model 4: Conditional indirect effects (physical activity → PAM VM caudate → synapse VM caudate, with or without microinfarcts) | ||||
| Index of moderated mediation | Index: 0.0234 | 0.0261 | — | −0.021, 0.082 |
| Microinfarcts | — | |||
| No | 0.005 | 0.012 | −0.016, 0.036 | |
| Yes | 0.029 | 0.030 | −0.026, 0.094 |
Models estimated indirect effects of PAM VM caudate (mediation) conditional on microinfarct burden (moderation) using 5000-sample bootstrapping.
Discussion
Physical activity induces glial changes and microglia mediate activity-related neurogenesis in animal models (Diamond et al., 1964; Ehninger and Kempermann, 2003; Choi et al., 2008; Vukovic et al., 2012). We describe the first human data showing that greater physical activity is associated with a lower proportion of microglial activation in brain tissue, and this effect mediates the relationship between activity and synaptic or cognitive outcomes. The observed relationships were independent of motor function and severity of cognitive impairment, both of which could impact physical activity engagement. The characteristics of the relationship demonstrated regional specificity to microglia in the VM caudate and IT gyrus and were particularly pronounced in individuals with microinfarcts and/or AD pathology. Of clinical relevance, only PAM in the IT gyrus mediated the relationship between physical activity and synaptic or cognitive outcomes, and this relationship was dependent on degree of AD burden. Namely, lower IT microglial activation explained >40% of the beneficial relationship between physical activity and synaptic or cognitive outcomes in adults with greater AD burden; only minimal variance (<10%) was mediated by IT microglial activation in adults with low AD pathology. Our data are limited by the observational design, preventing implications regarding causality and bidirectionality of effects are likely (i.e., microglial activation causing less physical activity engagement). Nonetheless, these data suggest that reduced microglial activation may be a meaningful pathway linking physical activity to age-related brain health in humans, and adults who stand to clinically benefit most from physical activity may be those with AD-related microglial activation.
Our data directly build on and translate a growing number of animal studies highlighting microglial functioning as a nexus between physical activity and brain health. Longstanding in vivo studies of exercised animals show proliferation of glia, increased expression of neurotrophic factors by microglia, as well as reductions in pro-inflammatory microglia activation states in brain tissue (Ehninger and Kempermann, 2003; Kohman et al., 2012, 2013; Littlefield et al., 2015; He et al., 2017; Mee-inta et al., 2019). It is important to note that microglial quantification in our data used a common antibody indicative of reactive microglial (anti-human HLA-D- DQ-DR) along with morphologic staging. Our microglial outcome therefore represents the proportion of highest activated microglia (Felsky et al., 2019) thought to be reflective of “M1” pro-inflammatory microglial phenotypes. Although we show that greater physical activity is related to lower proportion of microglial activation, we cannot yet evaluate potential relationships between physical activity with total microglial number or preponderance of protective states (e.g., “M2” states). However, recent data demonstrate that HLA-DR expression at least modestly correlates with expression of a panel of other proteins reflective of other microglial states and functions (e.g., TMEM119, CD163, CD32, L-Ferritin) in Iba-1+ cells from human brain tissue, supporting utility and some generalizability of this approach (Swanson et al., 2020). Interestingly, HLA-DR expression appears to be equally represented in gray and white matter tissue and may be present on both microglia and perivascular monocyte cells. Together, it is possible the observed relationship between physical activity and lower HLA-DP-DQ-DR expression may reflect both “true” microglial and/or perivascular monocyte activation states in gray and white matter. Microglia are highly heterogeneous and dynamic cells with a host of physiologic functions. It is possible that we did not fully capture other forms of microglial activation not reflected by HLA isoforms. Additionally, the precise molecular cascade through which physical activity promotes microglial homeostasis is not clear, although complement and coagulation signaling are highly implicated (De Miguel et al., 2019; Horowitz et al., 2020). Further work is needed to more systematically elucidate questions regarding upstream microglial signals, proliferation, and protective phenotypes induced by activity behaviors in humans.
Further, we found that the relationship between physical activity and lower PAM was regionally specific and dependent on burden of pathologies common within those regions. For instance, the physical activity-PAM relationship in the VM caudate was particularly driven by individuals with microinfarcts (common region for cerebrovascular disease), whereas in the IT gyrus, the relationship was driven by individuals with AD pathology (prominent region of AD accumulation). These data suggest that older adults with greater pathology burden but high-activity levels show disproportionately lower disease-related microglial activation compared with low-activity peers with the same pathology burden. Or framed another way, among adults with low-activity levels, pathology burden shows a stronger relationship with microglial activation, an effect that is attenuated among those with high physical activity levels. The apparent regionality of the activity-PAM relationship was not necessarily hypothesized. However, given that microinfarcts and AD pathologies preferentially affect caudate and IT regions, perhaps physical activity is most relevant for disease-related microglial activation and these regions were simply susceptible to disease. Together, it is possible that these “regional” relationships reflect disease effects. This may be particularly relevant in the AD models. HLA-DR was originally identified and developed to represent microglial activation because of its proclivity to surround amyloid plaques in AD; in other words, our use of HLA-DR may be particularly sensitive to AD-related microglial activation. Indeed, we found that AD-related microglial activation significantly mediated the beneficial relationship between physical activity and synaptic or cognitive functioning. These latter data converge closely with in vivo animal exercise studies showing decreased microglial activation concurrently with increased synaptic integrity markers and improved behavior in AD transgenic mice (Xiong et al., 2015; He et al., 2017), as well as AD in vitro experiments demonstrating microglia as a mediator the synaptogenic and neurogenic benefits of exercise (Choi et al., 2008; Vukovic et al., 2012). In the context of typical brain development, microglia are critical modulators of healthy synaptic pruning, a process hypothesized to go awry and become destructive in neurodegenerative states (Schafer et al., 2012, 2013; Li and Barres, 2018). Our data lend further human support toward these models, suggesting that activity-related reductions in neurotoxic microglial states may be intimately related to maintenance of synaptic integrity in the context of amyloid and tau pathology. Given that AD is the most common form of dementia and a majority (>65%) of older adults die with AD pathology (regardless of clinical status) (Boyle et al., 2013; Ossenkoppele et al., 2015), our findings help guide our understanding of how and in whom physical activity may confer “resilience” for a substantial portion of the aging population.
These data have several implications for therapeutic approaches to cognitive aging. First, these data help inform more precision-health approaches for physical activity recommendations; namely, older adults with greater AD pathology burden and particularly AD-related microglial activation may demonstrate greatest gains in synaptic and cognitive outcomes following exercise. Given the increased accessibility and reliability of AD biomarkers (e.g., plasma), risk stratification may be a useful approach to prescribe exercise in clinic and useful for prospective behavioral intervention studies aiming to capture the largest effects. Although reliable microglial markers are not yet clinically available, in research, CSF proteins (e.g., sTREM2, MCP-1, YKL-40) or molecular PET ligands (e.g., TSPO) (Werry et al., 2019) reflecting glial activation are underway and may have utility as risk stratification and/or exercise prognostication and monitoring tools. It is possible that not all older adults will benefit from exercise (Barnes et al., 2013; Lamb et al., 2018). We suggest that more precise targeting and monitoring tools (e.g., pathology risk or biomarker stratification) are needed to identify individuals who may benefit. Physical activity is also associated with development of age-related cognitive reserve and resilience (i.e., clinical outperformance of neuropathological burden). For instance, physical activity attenuates brain-to-cognition relationships, such that high-activity older adults demonstrate better cognitive performances than their low-activity peers, despite comparable levels of neurodegeneration or white matter injury (Casaletto et al., 2020a,b). Our data suggest that reduced microglial activation may be one pathway through which physical activity promotes cognitive resilience, particularly in the context of AD pathology. Identification of biological pathways, and ultimately molecules, underlying cognitive reserve and resilience are high import targets for cognitive aging. Characterization of resilience-related behaviors may be an effective strategy to uncover these pathways.
There are several important limitations. First, our observational design precludes conclusions regarding directionality or causality of effects. It is likely that the relationship between physical activity and microglial activation is bidirectional, and some effects are at least in part related to reverse causality (i.e., microglial activation leading to reductions in physical activity). However, these data build on experimental animal models showing at least some directional beneficial effects of activity on microglial homeostasis. We also statistically adjusted for factors that may contribute to reverse causality (i.e., motor symptoms, clinical severity). Regarding pathology-specific findings, our metric of global AD pathology was continuous, which may have allowed detection of effects with higher sensitivity compared with other categorical pathology indicators (e.g., ordinal grading of Lewy body disease, TDP-43, etc.). Additionally, microglial and other pathology markers may be influenced by agonal and other effects of death on tissue. We did not observe a significant relationship between postmortem interval and microglial markers (r range = 0.09-0.12, all p values >0.11) or an effect of postmortem interval in our models (data not shown). In most pathologic processes, the histologic evidence of microglia activation takes hours, if not days. Indeed, with hypoxia there appears to be minimal effect on microglia activation if death occurs within hours, such that agonal events are unlikely to change morphology. However, illnesses that are more subacute or chronic proximate to death could potentially influence microglia activation and may confound results. Regarding physical activity quantification, although actigraphy provides a more objective indicator of activity levels, these analyses cannot determine how engagement in structured or specific exercise activities may specifically impact brain outcomes. Last, the cohort of older adults from MAP who completed both actigraphy monitoring and quantification of microglial markers was relatively small and homogeneous. Comprehensive characterization of these relationships in cohorts representing diverse cultural, socioeconomic, and pathologic risk factors is needed to determine generalizability and inform precision behavioral intervention goals.
Despite the limitations, these are the first data supporting microglial activation as a physiological pathway by which physical activity supports brain heath in humans. These findings contribute to our fundamental understanding of brain development in late life and the biological pathways that may connect everyday behaviors to the brain. Although more interventional work is needed, we suggest that physical activity may be a modifiable behavior leveraged to reduce pro-inflammatory microglial states in humans.
Footnotes
This work was supported by National Institutes of Health-National Institute on Aging Grants R01AG17917 (PI: D.A.B.), R01AG072475 (PI: K.B.C.), and K23AG058752 (PI: K.B.C.); and Alzheimer's Association AARG-20-683875 (PI: K.B.C.). Rush Memory and Aging Project data can be requested at https://www.radc.rush.edu.
The authors declare no competing financial interests.
References
- Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, Yaffe K (2013) The Mental Activity and eXercise (MAX) Trial. JAMA Intern Med 173:797–804. 10.1001/jamainternmed.2013.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavers KM, Brinkley TE, Nicklas BJ (2010) Effect of exercise training on chronic inflammation. Clin Chim Acta 411:785–793. 10.1016/j.cca.2010.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious orders study and rush memory and aging project. J Alzheimers Dis 64:S161–S189. 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraxbekk CJ, Salami A, Wåhlin A, Nyberg L (2016) Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network: a multimodal approach. Neuroimage 131:133–141. 10.1016/j.neuroimage.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA (2013) Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 74:478–489. 10.1002/ana.23964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA (2012) Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78:1323–1329. 10.1212/WNL.0b013e3182535d35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Dawe RJ, Yu L, Lim A, Wilson RS, Schneider JA, Bennett DA (2018) Brain pathology is related to total daily physical activity in older adults. Neurology 90:E1911–E1919. 10.1212/WNL.0000000000005552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Ramos-Miguel A, VandeBunte A, Memel M, Buchman A, Bennett DA, Honer WG (2021) Late life physical activity relates to brain tissue synaptic integrity markers in older adults. Alzheimers Dement, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Staffaroni AM, Wolf A, Appleby B, Brushaber D, Coppola G, Dickerson B, Domoto-Reilly K, Elahi FM, Fields J, Fong JC, Forsberg L, Ghoshal N, Graff-Radford N, Grossman M, Heuer HW, Hsiung GY, Huey ED, Irwin D, Kantarci K, et al. (2020a) Active lifestyles moderate clinical outcomes in autosomal dominant frontotemporal degeneration. Alzheimers Dement 16:E1911–E1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Rentería MA, Pa J, Tom SE, Harrati A, Armstrong NM, Rajan KB, Mungas D, Walters S, Kramer J, Zahodne LB (2020b) Late-life physical and cognitive activities independently contribute to brain and cognitive resilience. J Alzheimers Dis 74:363–376. 10.3233/JAD-191114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Keebler MW, DeFina LF, Didehbani N, Perez AM, Lu H, D'Esposito M (2016) Distinct brain and behavioral benefits from cognitive vs. physical training: a randomized trial in aging adults. Front Hum Neurosci 10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS (2008) Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron 59:568–580. 10.1016/j.neuron.2008.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel Z, Betley M, Willoughby D, Lehallier B, Olsson N, Bonanno L, Fairchild K, Contrepois K, Elias J, Rando T, Wyss-Coray T (2019) Exercise conditioned plasma dampens inflammation via clusterin and boosts memory. bioRxiv. doi: 10.1101/775288. [DOI] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR (1964) The effects of an enriched environment on the histology of the rat cerebral cortex. J Comp Neurol 123:111–120. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G (2003) Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex 13:845–851. 10.1093/cercor/13.8.845 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108:3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsky D, Roostaei T, Nho K, Risacher SL, Bradshaw EM, Petyuk V, Schneider JA, Saykin A, Bennett DA, De Jager PL (2019) Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun 10:1–12. 10.1038/s41467-018-08279-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitán JM, Boots EA, Dougherty RJ, Oh JM, Ma Y, Edwards DF, Christian BT, Cook DB, Okonkwo OC (2019) Brain glucose metabolism, cognition, and cardiorespiratory fitness following exercise training in adults at risk for Alzheimer's disease. Brain Plast 5:83–95. 10.3233/BPL-190093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Robinton D, Stevens B (2018) Microglia and the brain: complementary partners in development and disease. Annu Rev Cell Dev Biol 34:523–544. [DOI] [PubMed] [Google Scholar]
- Hare E (1989) Enriching heredity: the impact of the environment on the anatomy of the brain. Br J Psychiatry 155:139–139. 10.1017/S0007125000177219 [DOI] [Google Scholar]
- Hayes AF (2018) Introduction to mediation, moderation, and conditional process analysis: A regression-based approach, Ed 2. New York: Guilford Press. [Google Scholar]
- He X, Liu D, Zhang Q, Liang F, Dai G, Zeng J, Pei Z, Xu G, Lan Y (2017) Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci 10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honer WG, Kaufmann CA, Kleinman JE, Casanova MF, Davies P (1989) Monoclonal antibodies to study the brain in schizophrenia. Brain Res 500:379–383. [DOI] [PubMed] [Google Scholar]
- Honer WG, Hu L, Davies P (1993) Human synaptic proteins with a heterogeneous distribution in cerebellum and visual cortex. Brain Res 609:9–20. 10.1016/0006-8993(93)90848-H [DOI] [PubMed] [Google Scholar]
- Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, Schneider JA, Bennett DA (2012) Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry 2:e114. 10.1038/tp.2012.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopperton KE, Mohammad D, Trépanier MO, Giuliano V, Bazinet RP (2018) Markers of microglia in post-mortem brain samples from patients with Alzheimer's disease: a systematic review. Mol Psychiatry 23:177–198. 10.1038/mp.2017.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörder H, Johansson L, Guo X, Grimby G, Kern S, Östling S, Skoog I (2018) Midlife cardiovascular fitness and dementia: a 44-year longitudinal population study in women. Neurology 91:763–763. 10.1212/WNL.0000000000006350 [DOI] [PubMed] [Google Scholar]
- Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, Gontier G, Casaletto KB, Kramer JH, Williams KE, Villeda SA (2020) Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369:167–173. 10.1126/science.aaw2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Bahl JM, Østergaard LB, Høgh P, Wermuth L, Heslegrave A, Zetterberg H, Heegaard NH, Hasselbalch SG, Simonsen AH (2019) Exercise as a potential modulator of inflammation in patients with Alzheimer's disease measured in cerebrospinal fluid and plasma. Exp Gerontol 121:91–98. 10.1016/j.exger.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS (2012) Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun 26:803–810. 10.1016/j.bbi.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS (2013) Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation 10:114–119. 10.1186/1742-2094-10-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, Dosanjh S, Slowther AM, Khan I, Petrou S, Lall R, DAPA Trial Investigators (2018) Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 361:k1675. 10.1136/bmj.k1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18:225–242. [DOI] [PubMed] [Google Scholar]
- Lim B, Sando SB, Grøntvedt GR, Bråthen G, Diamandis EP (2020) Cerebrospinal fluid neuronal pentraxin receptor as a biomarker of long-term progression of Alzheimer's disease: a 24-month follow-up study. Neurobiol Aging 93:97.e1–97.e7. 10.1016/j.neurobiolaging.2020.03.013 [DOI] [PubMed] [Google Scholar]
- Littlefield AM, Setti SE, Priester C, Kohman RA (2015) Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J Neuroinflammation 12:138. 10.1186/s12974-015-0362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludyga S, Gerber M, Pühse U, Looser VN, Kamijo K (2020) Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat Hum Behav 4:603–610. 10.1038/s41562-020-0851-8 [DOI] [PubMed] [Google Scholar]
- Matthew WD, Tsavaler L, Reichardt LF (1981) Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol 91:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee-inta O, Zhao ZW, Kuo YM (2019) Physical exercise inhibits inflammation and microglial activation. Cells 8:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Preische O, Sohrabi HR, Gräber S, Jucker M, Ringman JM, Martins RN, McDade E, Schofield PR, Ghetti B, Rossor M, Fox NN, Graff-Radford NR, Levin J, Danek A, Vöglein J, Salloway S, Xiong C, Benzinger T, Buckles V, et al. (2018) Relationship between physical activity, cognition, and Alzheimer pathology in autosomal dominant Alzheimer's disease. Alzheimers Dement 14:1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento C, Pereira J, Andrade L, Garuffi M, Talib L, Forlenza O, Cancela J, Cominetti M, Stella F (2014) Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res 11:799–805. 10.2174/156720501108140910122849 [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, Scheltens P, Visser PJ, Verfaillie SC, Zwan MD, Adriaanse SM, Lammertsma AA, Barkhof F, Jagust WJ, Miller BL, Rosen HJ, Landau SM, Villemagne VL, Rowe CC, Lee DY, et al. (2015) Prevalence of amyloid PET positivity in dementia syndromes. JAMA 313:1939–1949. 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podleśny-Drabiniok A, Marcora E, Goate AM (2020) Microglial phagocytosis: a disease-associated process emerging from Alzheimer's disease genetics. Trends Neurosci 43:965–979. 10.1016/j.tins.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LM, Hortobágyi T, la Bastide-van Gemert S, van der Zee EA, van Heuvelen MJ (2019) Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: a systematic review and meta-analysis. PLoS One 14:e0210036. 10.1371/journal.pone.0210036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B (2013) The 'quad-partite' synapse: microglia-synapse interactions in the developing and mature CNS. Glia 61:24–36. 10.1002/glia.22389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, Bis JC, Martin ER, Grenier-Boley B, Heilmann-Heimbach S, Chouraki V, Kuzma AB, Sleegers K, Vronskaya M, Ruiz A, Graham RR, Olaso R, et al. (2017) Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet 49:1373–1384. 10.1038/ng.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigger FS, Zago Marcolino MA, Portela KM, Della Méa Plentz R (2019) Effects of exercise on inflammatory, oxidative, and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 74:616–624. 10.1093/gerona/gly173 [DOI] [PubMed] [Google Scholar]
- Swanson ME, Scotter EL, Smyth LC, Murray HC, Ryan B, Turner C, Faull RL, Dragunow M, Curtis MA (2020) Identification of a dysfunctional microglial population in human Alzheimer's disease cortex using novel single-cell histology image analysis. Acta Neuropathol Commun 8:170. 10.1186/s40478-020-01047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Yamamoto H, Matsuda Z, Ogawa M, Yagyu K, Taniguchi T, Miyata T, Kaba H, Higuchi T, Okutani F, Fujimoto S (1995) Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Lett 368:455–460. [DOI] [PubMed] [Google Scholar]
- Tarumi T, Thomas BP, Tseng BY, Wang C, Womack KB, Hynan L, Lu H, Cullum CM, Zhang R (2020) Cerebral white matter integrity in amnestic mild cognitive impairment: a 1-year randomized controlled trial of aerobic exercise training. J Alzheimers Dis 73:489–501. 10.3233/JAD-190875 [DOI] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wójcicki TR, White SM, Gothe N, Mcauley E, Sutton BP, Kramer AF (2013) The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp 34:2972–2985. 10.1002/hbm.22119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF (2012) Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci 32:6435–6443. 10.1523/JNEUROSCI.5925-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry EL, Bright FM, Piguet O, Ittner LM, Halliday GM, Hodges JR, Kiernan MC, Loy CT, Kril JJ, Kassiou M (2019) Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. Int J Mol Sci 20:3161. 10.3390/ijms20133161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Wilund KR, Martin SA, Kistler BM (2012) Exercise, inflammation and aging. Aging Dis 3:130–140. [PMC free article] [PubMed] [Google Scholar]
- Xiong JY, Li SC, Sun YX, Zhang XS, Dong ZZ, Zhong P, Sun XR (2015) Long-term treadmill exercise improves spatial memory of male APPswe/PS1dE9 mice by regulation of BDNF expression and microglia activation. Biol Sport 32:295–300. 10.5604/20831862.1163692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Yu L, White CC, Chibnik LB, Chhatwal JP, Sperling RA, Bennett DA, Schneider JA, De Jager PL (2018) Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE ε4 haplotype status: a community-based cohort study. Lancet Neurol 17:773–781. 10.1016/S1474-4422(18)30251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


