Abstract
Introduction
Basal cell carcinoma (BCC) is the most common cancer in humans, but the reporting of patients with BCC is still not complete. There are a limited number of analyses in the literature on BCC epidemiology.
Aim
To study the epidemiological as well as clinical aspects of BCC by analysing a single centre’s experience in the Lower Silesia region of Poland.
Material and methods
We investigated 180 patients with BCC treated in the Unit of Dermatosurgery, Department of Dermatology, Venereology, and Allergology of Wroclaw Medical University between September 2017 and December 2019.
Results
The mean age of patients with BCC was 70.5 ±11.9 years. The most frequently diagnosed type of BCC was nodular type, at 72% of all patients. BCC occurred most commonly on the face and neck area, at 132 (73.3%), without a statistical difference between males and females. The vast majority of cancers were located on the nose. There was a personal history of skin cancer in 29% of our patients. In 127 (70.6%) subjects, the diameter of the BCC on the day of excision was less than 2 cm.
Conclusions
The clinical characteristics of our cohort of BCC patients has some similarities with that reported from central Poland and other European countries. However, we found an increase in the incidence of BCC among females.
Keywords: basal cell carcinoma, skin cancer, epidemiology
Introduction
Despite the well-known fact that basal cell carcinoma (BCC) is the most common cancer in humans worldwide [1], problems with adequate reporting of patients with BCC still occur. For this reason, estimating the prevalence and incidence poses a great challenge. There are a limited number of studies and analyses in the medical literature on BCC epidemiology in the European (including Polish) population. Moreover, pathogenesis, risk factors, and differences in clinical aspects of BCC remain areas in need further exploration and evaluation [2].
Aim
The aim of this study was to analyse the epidemiological and clinical aspects of BCC by investigating a single university centre’s experience in the Lower Silesia region of Poland.
Material and methods
We approached prospectively 240 consecutive patients with BCC confirmed by histological examination, treated in the Unit of Dermatosurgery, Department of Dermatology, Venereology, and Allergology of Wroclaw Medical University between September 2017 and December 2019. Of those 240 patients, 19 refused to participate in the study (response rate: 92.1%).
Furthermore, the following patients were excluded from the final analysis: subjects with Gorlin-Goltz syndrome, other syndromic and non-syndromic BCCs, and cases with more than one BCC at the current visit. This allowed us to limit the eventual bias in the results, having a more homogenous group concerning the background of the tumour. Therefore, the final study group consisted of 180 patients with BCC.
Detailed information on demographics and BCC characteristics (duration of the lesion, clinical type based on Polish Dermatological Society recommendations, and exact location) were recorded before the surgical treatment. Moreover, we evaluated environmental factors such as sun exposure, sunburns, the use of sun protector creams, smoking habits, and alcohol consumption.
Statistical analysis
All data were statistically analysed. The variables were assessed for normal or non-normal distribution, in order to apply corresponding statistical tests. Differences between groups were determined using the Mann-Whitney U test with reference to the non-normal distribution of evaluated continuous variables. Moreover, the comparisons between groups were conducted using Pearson’s χ2 test for categorical sets of variables. The level of significance was set at a = 0.05. The resulting p-values were considered significant if p < 0.05. Statistical analyses were performed using Statistica 13 software (StatSoft Inc., Tulsa, USA).
Results
General demographics and previous treatment
We recorded 180 BCCs, among which 83 were diagnosed in females (46.1%) and 97 in males (53.9%). All of the evaluated individuals were Caucasians, with skin phototype I (25%), skin phototype II (65%), and phototype III (10%), according to the Fitzpatrick scoring system.
The age of patients ranged from 17 to 95 years (mean: 70.5 ±11.9 years) (Tables 1, 2).
Table 1.
Basic demographic of the study subjects
| Parameter | Value |
|---|---|
| Gender (n): | 180 |
| Female | 83 (46.11%) |
| Male | 97 (53.88%) |
| Age [years] mean ± SD | 70.5 ±11.9 |
| Range | (17–95) |
| BMI [kg/m²] mean ± SD | 27.5 ±4.4 |
| Range | (17–95) |
| Clinical type of BCC: | |
| Nodular | 130 (72.2%) |
| Pigmented | 12 (6.7%) |
| Superficial | 16 (8.9%) |
| Ulcerative | 16 (8.9%) |
| Cystic | 4 (2.2%) |
| Morphoeic | 2 (1.1%) |
| BCC localization: | |
| Face and neck | 132 (73.3%) |
| Scalp | 20 (11.1%) |
| Trunk | 19 (10.6%) |
| Upper limbs | 5 (2.7%) |
| Lower limb | 4 (2.2%) |
| Level of education: | |
| Primary school | 56 (31.1%) |
| Secondary education | 60 (33.3%) |
| Higher education | 38 (21.1%) |
| No data | 26 (14.4%) |
| Place of life of patients: | |
| Small village < 1000 | 28 (15.6%) |
| Big village 1000–10,000 | 23 (12.8%) |
| Small town 10,00–100,000 | 20 (11.1%) |
| Big town 100,000–500,000 | 6 (3.3%) |
| Big city > 500,000 inhabitants | 100 (55.6%) |
| No data | 3 (1.7%) |
SD – standard deviation, BMI – body mass index.
Table 2.
Basic demographics and clinical aspects of BCC for the subsequent groups of subjects
| Parameter | Women | Men | P-value |
|---|---|---|---|
| Age [years] mean ± SD | 68.45 ±13.73 | 72.29 ±9.90 | 0.06 |
| Duration of tumour development [months] mean ± SD | 28.88 ±63.27 | 21 ±63.27 | NS |
| Clinical type of BCC: | NS | ||
| Nodular | 59 (71.1%) | 71 (73.2%) | |
| Pigmented | 7 (8.4%) | 5 (5.2%) | |
| Superficial | 7 (8.4 %) | 9 (9.3%) | |
| Ulcerative | 6 (7.2%) | 10 (10.3%) | |
| Cystic | 3 (3.6%) | 1 (1%) | |
| Morphoeic | 1 (1.2%) | 1 (1%) | |
| BCC localization: | Women | Men | NS |
| Face and neck | 60 (72.3%) | 72 (74.3%) | |
| Scalp | 7 (8.4%%) | 12 (12.4%) | |
| Trunk | 11 (13.3%) | 10 (10.3%) | |
| Upper limb | 3 (3.6%) | 2 (2.1%) | |
| Lower limb | 2 (2.4%) | 1 (1 %) |
Tables 1 and 2 present the age-specific prevalence for BCC in females and males. A clear trend was observed that BCC appeared in females in younger age compared to males (p = 0.06) (Table 1). Lesions occurred most commonly on the face and neck area 132 (73.3%), of which 123 (68.3%) specifically on the face, without a statistical difference between males and females (Table 2).
In 80.6% of all patients, BCC was the reason they visited a physician, whereas 19.4% of all analysed cases of BCC were detected during routine medical visits and physical examinations by family doctors (65.5%) or dermatologists (34.5%). The most frequent reasons the patients visited the doctor were: bleeding, changes in lesion area, and itching.
The vast majority of patients (71.1%) were untreated previously, while the remaining ones (28.9%) had had therapy (cryosurgery or laser treatment) applied in the past. There was not a single patient who had had more than 3 cryosurgeries or laser treatments.
There was no difference between females and males with BCC in terms of age (a trend towards older age of females was observed; p = 0.06), duration of BCC before the diagnosis (Table 2) and level of education (details not shown).
Risk factors/environmental factors
Out of the entire study group, 115 (63.9%) patients declared a positive history of excessive solar exposure (measured as the summed self-reported number of 3 weeks of holidays in sunny climates per year or an average number of 40 h spent outdoors per week). In addition, 102 (56.7%) declared regular sunburns during summers in the past (minimum 3 sunburns during a year), and 68 (37.8%) patients had never used sun protection creams. Nineteen (10.6%) subjects declared the use of sun protection factor (SPF) 50/50+ and 14 (7.8%) SPF 30, while 79 (43.9%) patients used a lower SPF (< 30).
There was personal history of skin cancer in 29% of our patients (melanoma (2%), non- melanoma skin cancer (98%)).
Among the studied group, 113 (62.8%) had never smoked cigarettes, 46 (25.5%) were regular smokers, and 21 (11.7%) patients had smoked in the past. Additionally, 104 (57.8%) patients abstained from alcohol, 65 (36.1%) subjects regularly drank alcohol, and 11 (6.1%) refused to answer the question.
Tumour characteristics
The most frequently diagnosed BCC was nodular type: 130 (72%) of all patients (Figure 1 A). The other types of BCC were less common: 16 (8.9%) – ulcerated/infiltrative subtype of BCC (Figure 1 B), 16 (8.9%) patients – superficial BCC (Figure 1 C), 12 (6.6%) – pigmented BCC (Figure 1 D), 4 (2.2%) – cystic BCC (Figure 1 E), 2 (1.1%) patients – morphoeic (sclerosing) BCC (Figure 1 F).
Figure 1.
Clinical types of BCC
The exact data about the location of BCC was collected from 179 (98.8%) patients (Table 1).
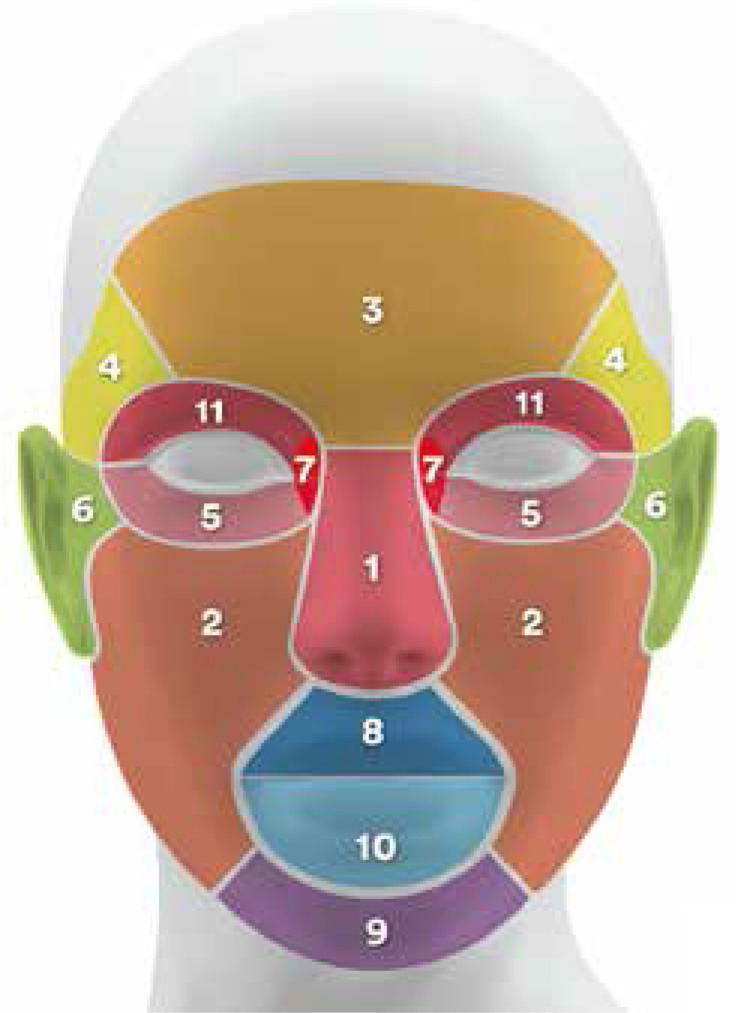
Of the group with BCC on the face, the vast majority were located on the nose – 48 (39%) patients. Other sites of BCCs on the face were as follows: cheeks 19 (15.6%), forehead 18 (14.6%), temples 11 (8.9%), lower eyelid 8 (6.5%), ears and preauricular area 8 (6.5%), angle of the eyes 4 (3.3%), upper lip 3 (2.4%), chin 2 (1.6%), lower lip 1 (0.8 %), and upper eyelid 1 (0.8%) (Figure 2).
Figure 2.
BCC sites
In 127 (70.6%) patients, the diameter of the BCC on the day of excision was less than 2 cm, in 53 (29.4%) patients it was larger than 2 cm.
Discussion
BCC is the most frequent malignant skin cancer. It was first described in 1824 by Jacob, and since then its incidence has been constantly rising [3].
Unfortunately, there are insufficient epidemiological data due to the lack of proper national registry systems in many European countries, including Poland. There are a limited number of studies in the various regions of Poland regarding BCC [1, 4].
In general, the incidence of BCC increases with age. In our study, the mean age was 70.5 ±11.9 years, which is quite similar to the other studies with Caucasians reported in the literature [4, 5].
BCC without a genetic condition or iatrogenic risk factors is relatively rare under the age of 40 years and very uncommon in the paediatric population. In our studied population, there were only 2 cases of BCC in patients under 40 years old, including 1 teenager without the above-mentioned risk factors.
However, Huang et al. [6] suggest that 26% of BCCs in the paediatric population did not exhibit these 2 risk factors (genetic and iatrogenic).
In contrast to adults, most children have not had sufficient sun exposure to develop BCC as a consequence of photodamage. However, it is important to be aware that it could happen as a result of behavioural changes (tanning beds) and maybe other not very well established environmental factors [6].
There are a number of studies that have reported that BCC is more common in males than in females (with a male-to-female ratio of about 2 : 0) [4, 7].
However, recently, there has been a documented increase in the incidence of BCCs in female patients [3, 8].
Furthermore, a study found that female patients were the dominant group [1]. In our study, there was a slight male predominance. Some data suggest an increasing number of younger females with BCC [8]. Our observations are in agreement with this. Although the age-specific incidence rate for BCC in females and males was not statistically significant, there was a tendency towards younger age of female patients.
In the literature, the most important potential risk factors are tanning beds and sun exposure, which may be the cause of the higher incidence in females [8]. Because older age is a risk factor for BCC, the next suggested reason for the increasing number of females with BCC could be their longer life expectancy [1].
What is interesting in this study is that 2 “peaks” of incidence of BCC were observed, which may be an explanation for those 2 aspects.
Of all BCCs 70–80% occur on the head and neck area. The trunk typically accounts for 25% of cases [2, 9]. Our analysis presents similar results – more than 85% of BCCs were located on sun-exposed areas. This is due to the fact that the sun exposure is one of the main risk factors for all BCCs [10].
The risk was especially high given that 56.7% of the studied patients declared regular sunburns during summers in the past, 63.9% of subjects had a positive history of excessive solar exposure, and 37.8% of BCC patients had never used sun protection creams.
In terms of face distribution, BCC was particularly concentrated on the centre of the face (nose, cheeks, and forehead). A similar distribution was found in other analyses [3, 11, 12].
Such knowledge is essential for dermatosurgery education, which should focus on surgical treatment of BCCs of the nose and forehead, because surgery in these areas is especially difficult and requires advanced surgical skills.
The nodular type of BCC (nBCC) is the most common clinical type of BCC, which is calculated for 50–79% of all BCCs. The second most common type of BCC is superficial BCC (sBCC), which accounts for 15% of all BCCs (from 10–30%). In our studied group, only 16 (8.9%) patients were diagnosed with sBCC, which is in contrast to other data. This discrepancy was caused by the limited number of superficial BCC cases referred for surgery to our Dermatosurgery Unit. Surgery, which is the standard care for all skin cancer, is not always carried out with sBCC. On the basis of the Polish Dermatological Society’s recommendation, cryosurgery should be considered as the first-choice method of treatment for sBCC with low recurrence risk and size up to 2 cm [13].
There are no special guidelines for first-line treatment of sBCCs larger than 2 cm [13], but in everyday dermatological practice cryosurgery is often the treatment for sBCCs larger than 2 cm, with excellent therapeutic as well as aesthetic results [14].
Superficial BCCs usually occur on the trunk (40%) and distal extremities (14%), and often grow to large diameter (sometimes more than 10 cm) [15].
Only 1 case of sBCC in our study was located on the neck; the rest of the sBCCs were diagnosed outside the head and neck area. Many studies, including our own, suggest that UV radiation may not play such a significant role in the cancerogenesis process in this type of BCC [16, 17].
In our study, the diameter of all sBCCs was greater than 2 cm (median 5 cm). Although they were not small tumours, we were able to perform successfully simple excision in all of our patients. We postulate the establishment of criteria for surgical treatment of sBCC. Our proposal is to consider lesions that could be treated with primary closure (even, or especially, those which are larger than T2-2 cm in diameter) in all patients who have no contraindication for surgical treatment. Before the treatment, a biopsy should be performed on all lesions suspected of superficial BCC.
Our study revealed that the duration of BCC before the diagnosis did not correlate with the level of education of patients or the size of the place the patients were living in. These findings are surprising because well-educated patients living in big cities have easier access to specialists. Therefore, it is essential to educate people both from villages and big cities via public service announcements. We were not able to find similar studies regarding these aspects.
Furthermore, a rather large group of patients, 19.4%, had their BCCs detected by doctors during routine visits. The importance of educating patients regarding the risk factors of BCC and self-examination should be emphasised because they are essential to improving prognoses.
On the whole, the main proven environmental risk factor for BCC development is UVR exposure [13, 17]. Additionally, the time of first UVR exposure is highly significant, and patients with high UVR exposure before the age of 20 years have a greater likelihood of developing BCC later in life [18]. In our studied population, 63.9% of patients revealed excessive sunlight exposure, 56.7% of patients had multiple sunburns in the past, and 37.8% of patients had never used sun protection creams.
Moreover, only 18.4% of patients used protection against the sun correctly; primary prophylaxis should be based on the use of SPF 30–50+ preparations [13].
Our study displayed observations similar to those of others in terms of sun protection in the general population [19] as well as among patients with a history of BCC excision [20]. Although most patients with a history of BCC (71.2% in the de Blacam study) were aware of the harmful and carcinogenic effect of UV on the skin, only 22.8% of patients used sunscreen on a daily basis [20].
Moreover, cigarette smoke contains several carcinogenic ingredients and has been shown to have an aetiological effect in at least 18 types of cancers (e.g. squamous cell carcinoma) [21, 22]. However, it is unclear if smoking modifies the risk of BCC. In some previous studies no association with smoking was found [22, 23].
In our study, BCC was more frequently present in the non-smoking population; namely, 62.8% of our BCC patients had never smoked cigarettes and 11.7% were currently non-smokers. The findings of previous studies suggest that alcohol intake is positively associated with risk of development of BCC [24–27]. However, in our analysis 104 (57.8%) BCC patients declared alcohol abstinence. Due to the fact that our questionnaires were not anonymous, it is important to note the observer-expectancy effect.
We are aware of the limitations of our study. This was a single-centre study, and only subjects referred for surgical procedures were included in the analysis. Therefore, the number of subjects is relatively small, and this study should be considered of an exploratory nature.
Conclusions
BCC is a very common cancer in southern Poland. The clinical characteristics of BCC have some similarities with central Poland and other European countries. We found an increase in incidence among females, which coincides with other recent studies. In a number of previous studies, males were at risk of developing BCC.
Overall, in our analysis, the age of females was lower than that of males, but the difference was not statistically significant. However, it may be a trend, and thus more studies would deepen knowledge on this aspect.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ciążyńska M, Narbutt J, Woźniacka A, Lesiak A. Trends in basal cell carcinoma incidence rates: a 16-year retrospective study of a population in central Poland. Adv Dermatol Allergol. 2018;35:47–52. doi: 10.5114/ada.2018.73164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015 [PMC free article] [PubMed] [Google Scholar]
- 3.Kasumagic-Halilovic E, Hasic M, Ovcina-Kurtovic NA. Clinical study of basal cell carcinoma. Med Arch. 2019;73:394–8. doi: 10.5455/medarh.2019.73.394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szewczyk M, Pazdrowski J, Golusiński P, et al. Basal cell carcinoma in farmers: an occupation group at high risk. Int Arch Occup Environm Health. 2016;89:497–501. doi: 10.1007/s00420-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesiak A, Slowik-Rylska M, Rogowski-Tylman M, et al. Risk factors in Central Poland for the development of superficial and nodular basal cell carcinomas. Arch Med Sci. 2010;6:270–5. doi: 10.5114/aoms.2010.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang JT, Coughlin CC, Hawryluk EB, et al. Risk factors and outcomes of nonmelanoma skin cancer in children and young adults. J Pediatr. 2019;211:152–8. doi: 10.1016/j.jpeds.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourlidou E, Vahtsevanos K, Kyrgidis A, et al. Risk factors for local recurrence of basal cell carcinoma and cutaneous squamous cell carcinoma of the middle third of the face: a 15-year retrospective analysis based on a single centre. Eur J Dermatol. 2019;29:490–9. doi: 10.1684/ejd.2019.3643. [DOI] [PubMed] [Google Scholar]
- 8.Chinem VP, Miot HA. Epidemiologia do carcinoma basocelular. An Brasil Dermatol. 2011;86:292–305. doi: 10.1590/s0365-05962011000200013. [DOI] [PubMed] [Google Scholar]
- 9.Chung S. Basal cell carcinoma. Arch Plast Surg. 2012;39:166–70. doi: 10.5999/aps.2012.39.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer – the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 11.Al-Qarqaz F, Marji M, Bodoor K, et al. Clinical and demographic features of basal cell carcinoma in North Jordan. J Skin Cancer. 2018;2018:2624054. doi: 10.1155/2018/2624054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Custódio G, Henrique Locks L, Fernanda Coan M, et al. Epidemiology of basal cell carcinomas in Tubarão, Santa Catarina (SC), Brazil between 1999 and 2008. An Bras Dermatol. 2010;85:819–26. doi: 10.1590/s0365-05962010000600007. [DOI] [PubMed] [Google Scholar]
- 13.Lesiak A, Czuwara J, Kamińska-Winciorek G, et al. Basal cell carcinoma. Diagnostic and therapeutic recommendations of Polish Dermatological Society. Dermatol Rev. 2019;106:107–26. [Google Scholar]
- 14.Buckley D, Marczuk C, Kennedy T. Cryosurgery for basal cell carcinoma treated in primary care. Ir J Med Sci. 2020;189:1183–7. doi: 10.1007/s11845-020-02188-5. [DOI] [PubMed] [Google Scholar]
- 15.Manstein CH, Manstein ME, Beidas OE. Giant basal cell carcinoma. Plast Reconstr Surg. 2011;128:1105–6. doi: 10.1097/PRS.0b013e31822b67e3. [DOI] [PubMed] [Google Scholar]
- 16.Bastiaens MT, Hoefnagel JJ, Vermeer BJ, et al. Differences in age, site distribution, and sex between nodular and superficial basal cell carcinomas indicate different types of tumors. J Investig Dermatol. 1998;110:880–4. doi: 10.1046/j.1523-1747.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 17.Gruber F, Zamolo G, Kastelan M, et al. Photocarcinogenesis: molecular mechanisms. Coll Antropol. 2007;31(Suppl 1):101–6. [PubMed] [Google Scholar]
- 18.Walther U, Kron M, Sander S, et al. Risk and protective factors for sporadic basal cell carcinoma: results of a two-centre case-control study in southern Germany. Clinical actinic elastosis may be a protective factor. Br J Dermatol. 2004;151:170–8. doi: 10.1111/j.1365-2133.2004.06030.x. [DOI] [PubMed] [Google Scholar]
- 19.Modenese A, Loney T, Pio Ruggieri F, et al. Sun protection habits and behaviors of a group of outdoor workers and students from the agricultural and construction sectors in north-Italy. Med Lav. 2020;111:116–25. doi: 10.23749/mdl.v111i2.8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Blacam C, Dermott CM, Sugrue C, et al. Patient awareness and sun protection behaviour following excision of basal cell carcinoma. Surgeon. 2017;15:12–7. doi: 10.1016/j.surge.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Secretan B, Straif K, Baan R, et al. A review of human carcinogens – Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–4. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 22.De Hertog SAE, Wensveen CAH, Bastiaens MT, et al. Relation between smoking and skin cancer. J Clin Oncol. 2001;19:231–8. doi: 10.1200/JCO.2001.19.1.231. [DOI] [PubMed] [Google Scholar]
- 23.Lukic D, Karabeg R, Jahic V, et al. Analysis of the skin basocellular carcinoma (BCC) among the smokers in Bosnia and Herzegovina. Mater Sociomed. 2018;30:251–4. doi: 10.5455/msm.2018.30.251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen H, Dhana A, Okhovat JP, et al. Alcohol intake and risk of nonmelanoma skin cancer: a systematic review and dose-response meta-analysis. Br J Dermatol. 2017;177:696–707. doi: 10.1111/bjd.15647. [DOI] [PubMed] [Google Scholar]
- 25.Freedman de M, Sigurdson A, Morin Doody M, et al. Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer Epidemiol Biomarkers Prev. 2003;12:1540–3. [PubMed] [Google Scholar]
- 26.Fung TT, Hunter JD, Spiegelman D, et al. Intake of alcohol and alcoholic beverages and the risk of basal cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2002;11:1119–22. [PubMed] [Google Scholar]
- 27.Wu S, Li WQ, Qureshi AA, Cho E. Alcohol consumption and risk of cutaneous basal cell carcinoma in women and men: 3 prospective cohort studies. Am J Clin Nutr. 2015;102:1158–66. doi: 10.3945/ajcn.115.115196. [DOI] [PMC free article] [PubMed] [Google Scholar]