Abstract
Over the past four decades, no class of drugs has had more impact on cardiovascular health than the 3-hydroxy-methylglutaryl coenzyme A reductase inhibitors or statins. Developed as potent lipid-lowering agents, statins were later shown to reduce morbidity and mortality of patients who are at risk for cardiovascular disease. However, retrospective analyses of some of these clinical trials have uncovered some aspects of their clinical benefits that may be additional to their lipid-lowering effects. Such ‘pleiotropic’ effects of statins garnered intense interest and debate over its contribution to cardiovascular risk reduction. This review will provide a brief background of statin pleiotropy, assess the available clinical evidence for and against their non-lipid-lowering benefits, and propose future research directions in this field.
Keywords: Cholesterol • HMG-CoA reductase inhibitors • Atherosclerosis • Inflammation
1. Introduction
Coronary heart disease (CHD) continues to be the leading cause of death in adults, accounting for one-third of all deaths in 2015.1 The 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors or statins play a crucial role in preventing and reducing cardiovascular disease. The emergence of statins followed decades of accumulating evidence that established the causal link between cholesterol and cardiovascular mortality, including epidemiological data from the Seven Countries Study, Framingham Study, and MRFIT trial2–4 that recognized low-density lipoprotein cholesterol (LDL-c) as the primary risk factor for CHD.
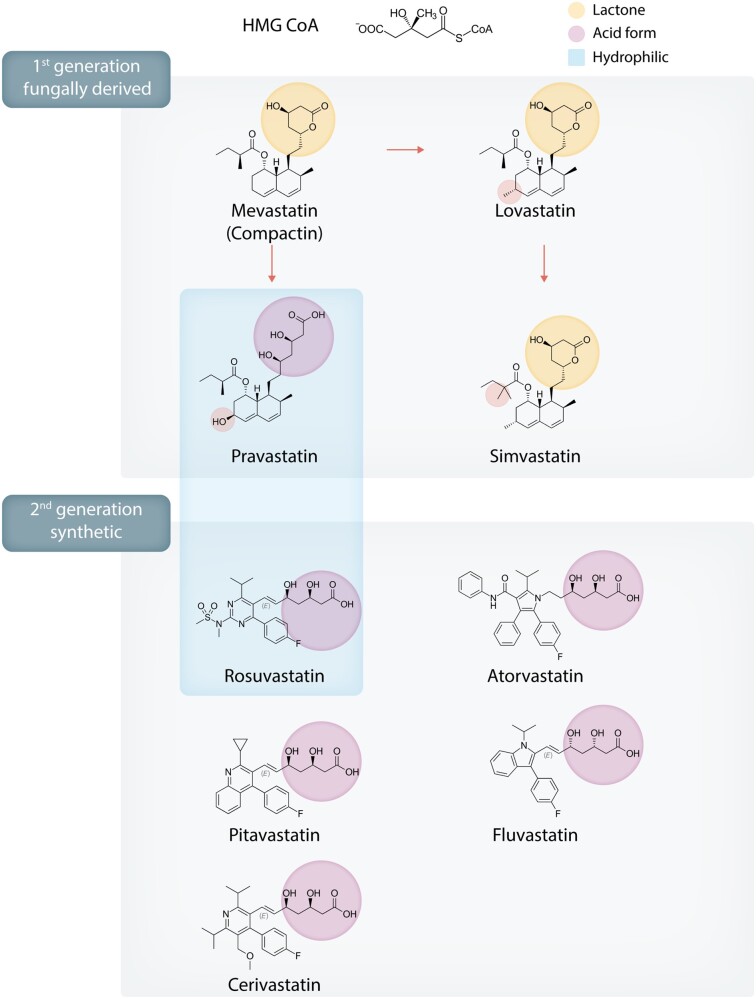
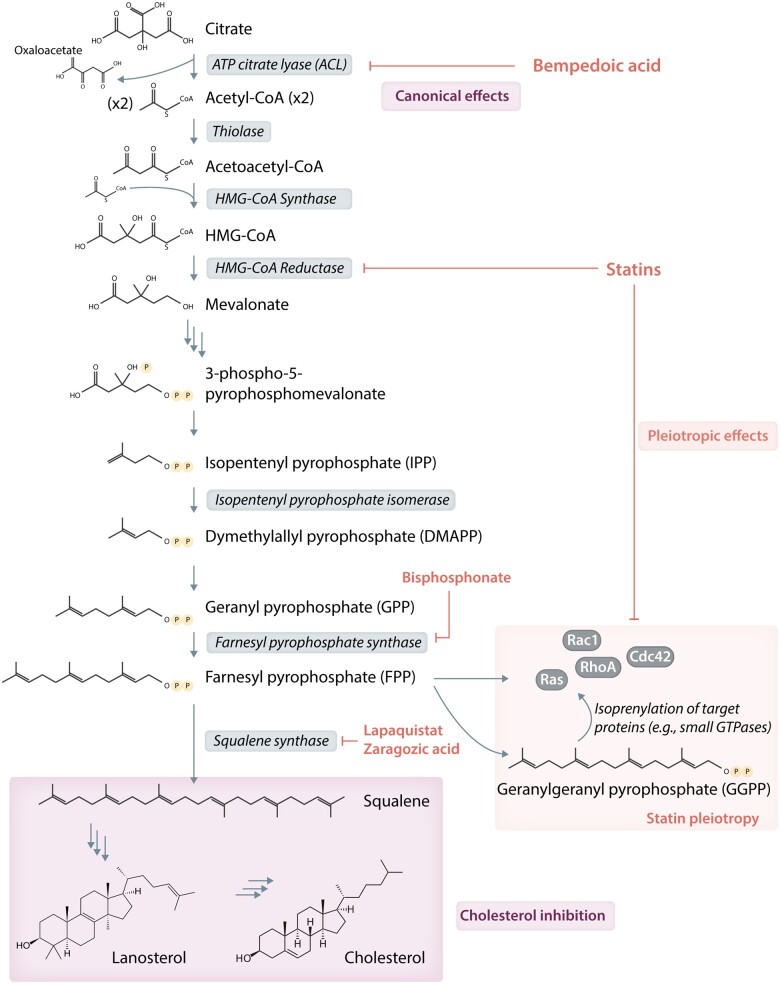
The first statin, mevastatin (Compactin; ML-236B), was isolated from fungal species Penicillium citrinum by Endo et al.5 in 1976. This was followed by isolation of mevinolin from Aspergillus terreus6 and its clinical and commercial success as lovastatin. As of 2020, at least nine statins with a range of structures and pharmacologic parameters have been developed, of which seven are currently approved in the United States (Figure 17) while one (cerivastatin) was withdrawn from market.8 All statins competitively bind to HMG-CoA reductase’s enzymatic site, and the Ki of statins are generally in the nanomolar range.9 This inhibits the rate-limiting step of cholesterol biosynthesis, the conversion of HMG-CoA to mevalonate (Figure 2), and leads to decreased hepatic cholesterol production, upregulation of LDL receptor density on hepatocyte, and increased serum LDL-C clearance. In subsequent clinical trials, the LDL-lowering effects of statins have been shown to reduce cardiovascular events.
Figure 1.
Chemical structures of statins of major historical and clinical significance and their classifications by marketing generation and key chemical characteristics.
Figure 2.
Flow chart of cholesterol biosynthesis and key enzymatic steps that are targeted by key classes of lipid-lowering drugs, with the statins’ target sites broken down by canonical lipid-lowering and pleiotropic effects.
2. Early clinical evidence suggesting statin pleiotropy
Several interesting observations from statin trials led to speculations that statins might act beyond LDL-lowering in achieving their clinical efficacy, a phenomenon later termed ‘statin pleiotropy’. First, the magnitude of CHD reduction (30–40% in all observed MIs) was somewhat greater than what could be anticipated by statins’ LDL lowering (25% in WOSCOPS and AFCAPS/Tex-CAPS trials). The time to benefit was also noted to be more rapid compared to other classes of lipid-lowering agents available at the time (e.g. fibrates in VA-HIT trial,10 niacin in CDP trial,11 and cholestyramine in LRC trial12), with the Kaplan–Meier curves starting to diverge by 1 year in the WOSCOPS, AFCAPS/Tex-CAPS, HPS, and TNT trials.13–19 In PROVE-IT TIMI-22 trial, the benefit of high-dose atorvastatin emerged as early as 30 days20 and in the MIRACL trial that enrolled 3086 ACS patients, aggressive atorvastatin therapy led to measurable clinical benefit as early as at 16 weeks.21 In addition, statins were consistently shown to be effective in the primary prevention of strokes across all major trials (37% in 4S trial,17 31% in CARE trial,18 25% in TNT trial,16 and 25% in HPS trial15). This is a notable finding given that large observational studies failed to show convincing association between ischemic stroke and elevated LDL-C levels.22–24 Finally, in the secondary prevention SPARCL trial, which enrolled 4731 patients with recent strokes and TIAs but no known CHD, atorvastatin 80 mg daily decreased LDL-C by 53% and resulted in a 16% decrease in total stroke at 5 years.25 Statin’s quicker and disproportionally greater cardiovascular effect, coupled with its unexpectedly strong effect on cerebrovascular outcomes, provided the initial clinical suggestion of statin pleiotropy.
3. Potential mechanisms of statin pleiotropy: Plaque stability and vascular inflammation
Several potential mechanisms were initially proposed to explain statins’ apparent pleiotropic effects. One early hypothesis was that statins stabilized atherosclerotic plaque and disproportionally reduced the incidence of clinical ischemic events. This was first investigated by direct imaging of coronary vessels with intravascular ultrasound (IVUS) in the REVERSAL, ASTEROID, and SATURN trials, which demonstrated slowing and regression of atherosclerotic plaques.26–28 The salutary changes in plaque burden occurred by 18–24 months, in line with the observed time to efficacy in most statin outcome trials, but the degree of regression was rather small at around 1% as measured by percent atheroma volume on IVUS. This suggests that in the high-risk patient cohort with the most derivable benefit, plaque burden alone was inadequate to account for changes in the incidence of clinical cardiovascular events. Subsequent studies using newer imaging technologies confirmed that atherosclerotic plaque composition likely play a much bigger role in determining plaque vulnerability. For example, the EASY-FIT study employed optical coherence tomography to show that patients on higher intensity atorvastatin led to thicker fibrous cap in coronary plaques,29 while the much larger multinational PARADIGM study followed 1255 patients longitudinally with serial coronary computed tomography angiography and showed that statin therapy resulted in not only slower progression of atherosclerosis volume but also concomitant increased plaque calcification and reduction in high-risk plaque features.30 Such findings have been coupled with animal studies that statin can alter smooth muscle and collagen content of atherosclerotic plaques,31 increase plaque calcification,32 and reduce matrix metalloproteinase production and cap degradation33,34 by mechanisms that are independent of cholesterol lowering.
As it became increasingly evident that statin therapy led to plaque stabilization and regression,35,36 vascular inflammation became the next focus of investigation. Hyperactivation of the cellular and humoral immune systems with increased reactive oxygen species generation can lead to a pro-inflammatory cascade, including cytokine release and T lymphocyte and macrophage recruitment and activation, which together may predispose to accelerated atherosclerosis and plaque vulnerability.37 A number of studies showed association between the cardinal inflammatory markers C-reactive protein (CRP) and increased cardiovascular risk.38,39 Interestingly, statins reduced both short-term and long-term CRP levels by 14% in the PRINCE trial at 12 weeks,40 by 34% in the MIRACL trial at 16 weeks,41 and by 38% in the CARE trial at 5 years.42 The significance of modulating vascular inflammation was highlighted in the JUPITER trial, in which rosuvastatin 20 mg daily reduced LDL by 50% and CRP by 37% at 1.9 years of follow-up and resulted in a decrease of 44% in primary endpoints (occurrence of a first major cardiovascular event), 54% in all MI, and 20% in all-cause mortality.43 The degree of cardiovascular benefit exceeded projections from earlier trials based on LDL-C lowering alone,44 suggesting that statin’s anti-inflammatory effects might account for the difference in efficacy. This hypothesis is corroborated by studies showing that statins can inhibit in vitro activation of several pro-inflammatory transcription factors including NFκB, AP-1, and HIF-1α45 and to alter the balance of T-cell differentiation by blunting proinflammatory IL-17 helper T cells while promoting the FoxP3-expressing regulatory T cells (Tregs) that induce immune tolerance.46 Furthermore, several adhesion molecules including integrins, selectins, PECAM-1, ICAM-1, and VCAM-1 were shown to be affected by statin treatment in mediating leukocyte-endothelium adhesion and transmigration.47,48
Recently, three large trials (CANTOS, COLCOT, and CIRT) studied the clinical outcome of reducing inflammation without altering LDL-C level. In CANTOS, treatment with canakinumab (an interleukin-1β antibody) reduced CRP by 26–41% and the composite primary endpoint by 15%, MI by 24%, and stroke by 26%. In COLCOT, colchicine lowered the primary endpoint by 23% and cardiovascular death by 16%.49 In CIRT, treatment with low-dose methotrexate failed to lower either CRP or the composite primary cardiovascular endpoint.50 While these trials suggest that inflammation modulation influenced cardiovascular outcomes and might theoretically contribute to statin pleiotropy, it should be noted that Mendelian randomization models showed CRP not to be a direct causal factor,51,52 and a meta-analysis of 24 trials failed to show correlation of magnitude of CRP reduction to cardiovascular risk reduction.53 Therefore, it remains unclear whether statins’ anti-inflammatory effects are independent mediators vs. confounding factors in cardiovascular event reduction. In addition, even assuming vascular inflammation to be an independent causal factor of cardiovascular events, it remains debatable whether statins reduce inflammation independent of LDL-C, which itself could lower oxidized LDL (oxLDL) in the atherosclerotic plaque, reduce macrophage and platelet activation,54 and ultimately contribute to reduction in inflammation.
4. Bedside to bench side: Statins’ effect on prenylation and beyond
An important reason for the growing interest in statin pleiotropy is the experimental data supporting statins’ lipid-independent cellular effects. One proposed mechanism is thought to be due to statin’s effect on modulating protein prenylation. Mevalonic acid, the intermediate metabolite targeted by statins, is the shared precursor for biosynthesis of isoprenoids, which are essential for the production of 15-carbon farnesyl pyrophosphate (FFP) and 20-carbon geranylgeranyl pyrophosphate (GGPP) (Figure 2). FFP and GGPP in turn are substrates for post-translational modification of Rho superfamily of small GTPases (e.g. Ras, Rho, Rab, and Cdc42), which facilitate their cell membrane trafficking, localization, and signalling.55 Statins have been shown to inhibit Rho-family protein signalling in animal models and humans.56–61 In particular, inhibition of Rho by statins leads to the upregulation of eNOS,62 inhibition of vascular reactivity,63 attenuation of leukocyte adhesion,64 mobilization of endothelial progenitor cells from bone marrow, and reendothelialization after vascular injury.65–67 In addition to endothelial cells, statins can target other cell lineages by promoting cell-cycle arrest in fibroblast,68 attenuating vascular smooth muscle cell proliferation,69,70 and decreasing platelet reactivity.71,72 The proposed molecular mechanisms of these effects include both Rho/ROCK signalling and a variety of additional pathways such as inhibition of thromboxane biosynthesis,73,74 modulation of cytosolic calcium concentration,75 PECAM-1-mediated PI3K signalling,76 and PPAR signalling.77,78 The observed regulatory effects on smooth-muscle proliferation by various statins79,80 are of particular importance in preventing cardiac allograft vasculopathy in post-transplant patients.81,82
5. Challenging the statin pleiotropy hypothesis with ezetimibe and PCSK9 inhibitor trials
Despite the myriad of experimental data supporting statin pleiotropy, it remains difficult to definitively prove that these in vitro findings translate to clinical significance in humans. Importantly, it is nearly impossible to prove that statin’s observed effects on plaque stability, endothelial dysfunction, and vascular inflammation occur independently of lipid-lowering instead of simply being its secondary downstream effects. One historic argument pointed to the relatively poor cardiovascular efficacy displayed by older, non-statin lipid-lowering agents to highlight the mechanistic uniqueness of statins. This paradigm was first challenged by the IMPROVE-IT trial, in which ezetimibe 10 mg on top of simvastatin 40 mg resulted in 17% additional LDL-C lowering compared to simvastatin 40 mg plus placebo and resulted in 6.4% reduction in the composite endpoint and 13% reduction in all MI at a follow-up of 7 years.83 While the lipid-lowering effect of ezetimibe was modest, it nonetheless illustrated that improvement in cardiovascular endpoints was achievable by a non-statin lipid-lowering agent.
The advent of PCSK9 inhibitors, by far, the most potent non-statin lipid-lowering drug, provided another opportunity to test statin pleiotropy. To date, four large outcome trials using PCSK9 monoclonal antibodies have been published. The pair of studies using bococizumab (SPIRE-1 and SPIRE-2, with different LDL-C entry levels) was plagued by the development of high titres of anti-drug antibodies, ultimately leading to premature study termination. Nonetheless, they still showed 56% LDL-C reduction at 14 weeks on the background of 93% statin use and resulted in decrease of 21% in composite primary endpoints and 24% in non-fatal MI at 12 months in the higher-LDL, longer-duration SPIRE-2 (LDL-C ≥ 100 mg/dL).84 In the FOURIER trial that enrolled 27 564 patients with CAD and LDL-C ≥ 70 mg/dL on maximal dose of statin, evolocumab led to 59% lower LDL-C compared to placebo and resulted in a decrease of 15% in composite primary endpoint and 27% in myocardial infarction at 2.2 years.85 In the ODYSSEY OUTCOMES trial that enrolled 18 924 patients who had ACS within the preceding 1–12 months and one of several elevated lipid parameters while on maximal dose of statin, alirocumab led to 63% initial relative lowering of LDL-C vs. placebo and resulted in a decrease of 15% in the composite primary endpoint, 12% in any coronary events, and 15% in all-cause mortality at 2.8 years.86
The results from the PCSK9 inhibitor trials rekindled debates over the importance of statin pleiotropy: substantial LDL-lowering beyond that of maximal intensity statin was shown to be achievable and correlated with further cardiovascular event reduction. Additionally, several early features of statin efficacy underlying the conception of statin pleiotropy (time to benefit, plaque regression, and stroke reduction) were replicated by PCSK9 inhibitors. The time to benefit was about 1 year in the FOURIER trial and 2 years in the ODYSSEY OUTCOMES trial, comparable to these observed in the WOSCOP and AFCAPS/Tex-CAPS trials. The burden of atherosclerotic plaques was assessed in the GLAGOV trial, in which evolocumab lowered LDL-C by 61% and induced greater reduction of atheroma volume than statin alone on serial IVUS.87 Finally, the incidence of ischemic stroke was reduced by 21 and 27% with PCSK9 inhibition in the FOURIER trial and the ODYSSEY Outcomes trials, respectively, comparable to the degree of stroke reduction in most statin trials. Together, these findings demonstrate that the several clinical characteristics suggestive of pleiotropy are no longer unique to statins. One meta-regression analysis with data from statin and non-statin trials including PCSK9 inhibitors was able to model changes in stroke incidence entirely from total cholesterol changes, leading the authors to claim that there is ‘no longer room for pleiotropic effects of statin’.88 However, it should be pointed out that many of the outcome results of PCSK9 inhibitors were achieved on top of statin therapy, and it is possible that PCSK9 inhibitors or further lipid lowering could potentiate or augment statin pleiotropy.
6. Pleiotropic effects or confounders?
A major challenge in assessing statin pleiotropy is the potential presence of multiple confounding factors. Mendelian randomization studies using genome-wide lipid-associated single-nucleotide polymorphisms allowed re-analysis of composite large randomized control trial data for direct association between lipid-lowering and cardiovascular outcomes. Using an adapted Egger technique, Labos et al.89 showed that each 1 mmol/L of LDL-C change from statin therapy was associated with a hazard ratio of 0.77 in cardiovascular endpoints, with an intercept indistinguishable from zero, which suggested that statins’ cardiovascular benefits were entirely derived from LDL-C lowering. Similar analysis on stroke outcomes revealed more heterogenous findings, with genetically mediated LDL-elevation associated with increased risk of ischemic and large artery atherosclerotic strokes but not with small artery occlusion or cardioembolic strokes.90 Another strike against statin pleiotropy is a Mendelian randomization analysis that suggested that CRP was not a direct causal factor in cardiovascular risk reduction.51
7. Comparative statin pleiotropy beyond traditional cardiovascular events
The well-established epidemiologic association between LDL-C and atherosclerotic cardiovascular events makes it difficult to untangle statin’s LDL-lowering and pleiotropic effects. Venous thromboembolism (VTE), a non-classic cardiovascular event, provides an intriguing vantage point. In the JUPITER trial, the incidence of VTE was a pre-specified secondary endpoint and shown to be reduced by 43% in the rosuvastatin arm compared to placebo.91 In the FOURIER trial, treatment with evolocumab also reduced incidence of VTE by 29%, and the reduction remains statistically significant at 31% when results from FOURIER and ODYSSEY Outcomes trials are combined.92 These findings are somewhat surprising, given the lack of clear epidemiologic associations between VTE incidence and traditional lipid parameters such as LDL-C and HDL-C.93–96 Analysis of secondary biomarkers revealed several interesting distinctions: while rosuvastatin lowered inflammatory marker CRP by 37% in the JUPITER trial, PCSK9 inhibitors had no effect on CRP in the trials to date;87,97,98 on the other hand, in the FOURIER trial, evolocumab ameliorated several non-traditional lipid parameters including lipoprotein(a) (Lp(a)),85,99 while in the JUPITER trial, rosuvastatin had no effect on the median Lp(a) level.100 These findings suggest that while both statins and PCSK9 inhibitors can achieve profound LDL-C lowering, they exhibit differential effects towards the spectrum of secondary pro-atherosclerotic, pro-thrombotic serum markers. While nuanced, such differences may nonetheless carry physiologic significance and determine pleiotropic effects of statins vs. PCSK9 inhibitors. The exact mechanism behind this divergence is unclear but may be related to their distinct sites of action: statin targets cholesterol synthesis and could directly deplete intracellular cholesterol storage, while PCSK9 inhibitors increase cell-surface LDL receptor density and clear serum LDL particles without disrupting intracellular cholesterol synthesis or sterol flux, a process that has been linked to regulation of inflammatory and immune responses.101 Inclisiran, an siRNA-based drug that inhibits PCSK9 synthesis,102 and bempedoic acid, a small molecule inhibitor of ATP-citrate lyase (ACL) upstream of HMG-CoA synthetase in the cholesterol biosynthetic pathway,103,104 are novel players in the lipid-lowering arena; results from their anticipated outcome trials could in turn shed important insight on whether VTE reduction and CRP/Lp(a) alterations are drug-specific effects vs. class effects of lipid-lowering.
8. Comparison of CV event reduction between statins and non-statins
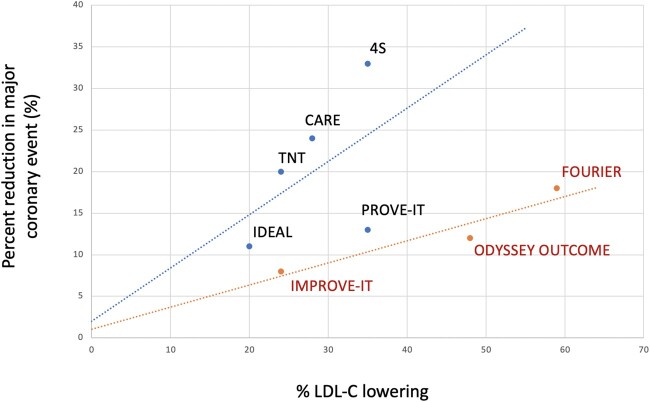
The percentage outcome reduction (∼15%) achieved by PCSK9 inhibitors was relatively modest compared to their potent LDL-C reduction (∼40–60%), while the early statin trials showed at least proportional, if not supra-proportional reduction in cardiovascular events relative to their LDL-C lowering effects. Given difficulty in head-to-head comparison between trials due to different and evolving primary endpoints, an attempt is made here to calculate the ratio of % difference in major coronary event rate per absolute LDL-C difference in major secondary prevention trials (Table 1). Using this mathematical estimate, the average reduction of events for statins is 23% per 40 mg/dL LDL-C lowering, higher than ezetimibe (19%) and PCSK9 inhibitors (12%). A summary of statin vs. non-statin trials is further summarized in Table 2. In addition, when the percent reduction in major coronary event rate is plotted against percent LDL-C lowering, the statin versus non-statin trials do not fall along the same line, but instead form two distinct lines (Figure 3). Indeed, the ezetimibe/PCSK9 trials fall on a much less sharp incline than statin trials due to relatively less event reduction compared to their lipid-lowering potency. Again, these estimates are crude at best, require multiple assumptions, and do not account for difference in follow-up time (∼5 years for statins, 7 years for ezetimibe, and 2–3 years for PCSK9 inhibitors). Furthermore, the interpretation should be cautioned that PCSK9 inhibitor therapy was instituted on the background of maximally tolerated statin therapy, and it is possible that a different linear relationship occurs in the hyper-LDL-C depletion range or with combination therapy. Nonetheless, such analysis suggests that statins may improve clinical outcomes more substantially than non-statin agents per unit of LDL-C lowering. The pending longer-term outcome results of PCSK9 inhibitor trials will provide a better comparison with respect to the same unit of time.
Table 1.
Comparison of major coronary event rates across secondary prevention trials with statins vs. non-statins
| Statin vs. placebo |
High vs. low intensity statin |
Statin/ ezetimibe vs. statin | Statin/PCSK9 inhibitor vs. statin |
|||||
|---|---|---|---|---|---|---|---|---|
| 4S | CARE | TNT | IDEAL | PROVE-IT | IMPROVE-IT | FOURIER | ODYSSEY OUTCOMES | |
| Follow-up period (year) | 5.4 | 5.0 | 4.9 | 4.8 | 2.0 | 7.0 | 2.2 | 2.8 |
| Mean LDL-C differencea (mg/dL) | 65 | 38 | 24 | 21 | 33 | 17 | 56 | 46 d |
| Mean % LDL -C difference | 35% | 28% | 24% | 20% | 35% | 24% | 59% | 48% |
| Absolute reduction in major coronary eventsb (%) | 10% | 3% | 1.6% | 1.1% | 1.1% | 1.7% | 1.0% c | 1.1% |
| % difference in major coronary events | 33% | 24% | 20% | 11% | 13% | 8% | 18% | 12% |
| % reduction in CV event/40 mg/dL of LDL-C reduction | 20% | 25% | 33% | 21% | 16% | 19% | 13% | 10% |
| Average by category | 23% (statin trials) | 19% (ezetimibe trials) | 12% (PCSK9 trials) | |||||
Measures difference in LDL between treatment and placebo arms at follow-up, NOT reduction at follow-up vs. baseline.
As directly reported by trial, or if not directly reported, calculated as sum of CHD death and non-fatal MI unless otherwise described.
Not directly reported. Calculated retrospectively as sum of (cardiovascular death—stroke death) + (total MI—fatal MI).
Averaged from reported values at 4, 12, and 48 months (LDL-C values at other time points not reported).
Table 2.
Comparison of major cardiovascular clinical trials by therapy category
| Statins | Ezetimibe | PCSK9 monoclonal Ab | Inflammatory modulators | Bempedoic acid | |
|---|---|---|---|---|---|
| LDL-C lowering | ↓↓ to ↓↓↓ (depending on intensity) | ↓ | ↓↓↓ | – | ↓ |
| Primary vs. secondary prevention | Primary and secondary | Secondary | Secondary | Secondary | Pendinga |
| Compared vs. no statin? | Yes available | No | No | No | No |
| CRP reduction? | Yes | Yes | No | Yesb | Yes |
| Plaque reduction on IVUS? | Yes | N/A | Yes | N/A | N/A |
| Endothelial function improvement? | Yes | No | N/A | N/A | N/A |
| Stroke reduction? | Yes | Yes | Yes | Yes/Noc | Pending |
| Years to first CV endpoint improvement | 1–2 | 7 | 2–3 | ∼2 | Pending |
Outcome trial (CLEAR Outcome) pending.
Reduced in CANTOS and COLCOT trials but not in CIRT trial.
Reduced in COLCOT but not CANTOS or CIRT trials.
Figure 3.
Graphic correlation of percent reduction in major coronary events to percent LDL-C lowering based on published data from major secondary prevention trials. Notably, statin trials are distributed along a steeper line than non-statin trials, suggesting contribution from statins’ pleiotropic effects on CV outcomes beyond pure LDL-C lowering.
9. Re-purposing of statin for other systemic inflammatory diseases
While it remains under debate whether part of statin’s cardiovascular benefit is derived from its non-lipid-lowering actions, substantial evidence has emerged to support its re-purposed use in certain non-cardiovascular diseases with no apparent link to hypercholesterolemia. In particular, given statins’ documented anti-inflammatory actions both in vitro and in clinical trials, they were explored as immune modulatory agents in systemic inflammatory diseases.105 Two meta-analyses showed statins to attenuate disease activity of rheumatoid arthritis (RA) by lowering of serum inflammatory markers and symptom improvement,106,107 while a large population-based nested case–control study showed reduced risk of RA with statin use.108 Similarly, statins have been reported to confer beneficial effects in systemic lupus erythematosus,109 periodontitis,110,111 primary sclerosing cholangitis,112 inflammatory bowel diseases,113 cognitive function/dementia,114,115 and psychological well-being.116 The successful secondary application of statins in non-cardiovascular fields lends credence from a different dimension to their pleiotropic effects.
A perhaps timely re-application of statin therapy is in pulmonary medicine, in particular for re-purposed use during the current COVID-19 pandemic, which has infected over 48 million people globally and resulted in 1.2 million deaths as of 1 November 2020. In patients infected with SARS-CoV-2 virus, critical illness developed as a result of acute respiratory distress syndrome coupled with cytokine storm, leading to unchecked systemic hyperactivation of immune response that often prove fatal. In this context, statins are actively being explored for re-purposed application as an anti-inflammatory, cardiopulmonary protective agent in the fight against COVID-19.117–119 We can extrapolate statin’s potential therapeutic effects against SARS-CoV-2 by prior reports of its clinical benefit in asthma120 and inflammatory lung diseases121 and of statin’s direct interaction with SAR-CoV2 main protease in silico.122 While randomized clinical trials are needed to establish statin’s efficacy in this arena, given the present lack of approved targeted treatment, statins may be a safe, readily available added option in the globally fight against COVID-19.
10. Current and future perspectives regarding statin pleiotropy
Studies into statin’s expanded clinical use outside of cardiovascular diseases provided fresh evidence for statin pleiotropy. Nevertheless, the debate over its legitimacy in the realm of cardiovascular diseases still continues given conflicting data and competing interpretations from existing clinical trials. To help settle this debate, we propose three investigative approaches: (1) developing a ‘neo-statin’ that inhibits cholesterol synthesis without affecting its other cellular pathways such as prenylation; (2) comparing head-to-head benefits between statin and ezetimibe and/or PCSK9 inhibitors in a large clinical trial; and (3) creating tissue-specific HMG CoA reductase (HMGCR) knockout animal models and studying their cardiovascular outcomes.
Regarding the first approach, we would need a molecule that acts at later steps in the cholesterol biosynthetic pathways such as squalene synthetase inhibitor that spares the isoprenoid synthesis branch,55 thereby dissecting out reduced prenylation from the drug effect. The squalene synthase inhibitors fit such requirements (see Figure 2), and one member lapaquistat acetate progressed as far as phase III studies but unfortunately was abandoned due to mediocre potency in LDL-C lowering (18–23%) and concerns over hepatotoxicity.123 While other enzyme targets exist at more distal steps of cholesterol synthesis pathway, the effort at developing their inhibitors has remained largely academic, and there is currently no known industrial effort at their pharmaceutical adaptation. Of note, bempedoic acid is an upstream inhibitor in the cholesterol biosynthetic pathway upon which hepatic activation can inhibit ACL, the enzyme that generates acetyl-CoA (Figure 2). Four clinical trials (CLEAR Serenity, CLEAR Tranquility, CLEAR Harmony, and CLEAR Wisdom) have shown its efficacy in further reduction of LDL-C by 15–20% on the background of maximally tolerated statin treatment while also reducing hs-CRP level.103,124–126 The bempedoic acid/ezetimibe combination (Nexlizet) was just approved by FDA for use in adults with heterozygous familial hypercholesterolemia or established ASCVD requiring further LDL-C lowering, and the CLEAR Outcomes trial is expected to report its cardiovascular outcome results in 2022.104 While bempedoic acid acts above the FFP bifurcation point and therefore unable to mechanistically distinguish cholesterol synthesis from prenylation, it would be very interesting to observe whether it replicates some of statins’ efficacy on cardiovascular risk reduction and is more superior than ezetimibe when added to statins with equivalent LDL lowering.
Regarding the second approach, an ideal ‘match’ would be to randomize patients to statin vs. PCSK9 inhibitors to achieve identical LDL-C lowering and assess if there is a difference in clinical outcomes. While conceptually simple, blinding would be difficult due to different routes of drug administration; furthermore, the cost of PCSK9 inhibitor and the need to withhold a guideline-indicated treatment in statin pose financial and ethical dilemmas. One unique scenario would be to study patients with statin intolerance. Indeed, at least five trials have been conducted (GAUSS 1–4 and ODYSSEY ALTERNATIVE) to that effect, and in fact, one trial (ODYSSEY ALTERNATIVE) was able to re-challenge one study arm with atorvastatin under blinded conditions with high adherence rate.127 Unfortunately, the duration of follow-up was short (24 weeks), and the majority of the enrolled patients was switched to alirocumab during the open-label follow-up phase, precluding a precious chance at comparing cardiovascular outcomes between alirocumab and atorvastatin monotherapies at otherwise grossly comparable LDL-C-lowering efficacies (45% vs. 32%). Nonetheless, a repeat effort with a similar trial design but large enrolment size and longer follow-up to allow outcome comparison would hold immense value. Another trial design would be to use low intensity statin/PCSK9 inhibitor combination vs. high intensity statin to lower LDL-C by identical degrees and investigate any differences in clinical outcomes. On this aspect, we may derive some insight from a small prospective study in Taiwan of 60 patients that compared simvastatin 40 mg to simvastatin 10 mg/ezetimibe 10 mg and showed improved vasoreactivity at 28 days despite identical lipid-lowering by both treatment groups when compared to placebo.128
Regarding the third approach, the aim is to fundamentally dissect out the role of LDL-C lowering on different cell lineages and tissue components of the cardiovascular system. For instance, if the statins’ effect on endothelial functions (e.g. via small GTPase prenylation and eNOS modulation) carries physiologic significance independent of atherosclerotic plaque formation, an endothelial-cell-specific HMGCR knockout animal model (homozygous or heterozygous) should in theory translate into improved cardiovascular outcomes without perturbing the systemic lipid profiles. HMGCR has been successfully knocked out in the liver, skeletal muscle cells, and myeloid cells, which have served as powerful genetic tools in study statin-associated hepatotoxicity, myopathy, and effects of macrophage migration on atherosclerosis, respectively.129–131 Similar targeted deletions in endothelial cells, smooth muscle cells, and platelets could yield important insight into statin pleiotropy. Given embryonic lethality of homozygous HMGCR knockout in mice,132 an inducible model of genetic ablation may be preferable and better reproduce physiologic effect of adult-stage statin therapy.
11. Conclusions
In an era of rising cardiovascular disease burden, statins served as a textbook example of successful hypothesis-driven drug development. To fully account for statin’s clinical impact on cardiovascular disease, the concept of statin pleiotropy emerged and has led to a substantial body of research evaluating its significance. While growing scientific work has provided provocative rationale for statin’s pleiotropic effects and encouraged statin’s expanded application in non-cardiovascular fields, an equally expanding body of clinical data pointed to a more nuanced interpretation of the statin pleiotropy. Indeed, results from ezetimibe and PCSK9 inhibitor trials, coupled with Mendelian association analyses, have provided strong arguments as to whether statin pleiotropy is clinically meaningful. Taken together, statins likely confer the majority of cardiovascular benefits through LDL-C lowering, which itself may affect multiple molecular pathways beyond atherosclerotic plaque formation. We expect that newer studies with novel lipid-lowering agents, targeted experimental approaches, and creative trial designs will more effectively address the questions of statin pleiotropy, which, despite a substantial body of literature, still remains unanswered.
Acknowledgements
We would like to thank A.O. and M.S. for their valuable time and input during the composition and revision of this review.
Conflict of interest: none declared.
Funding
Supported in part by funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL136962).
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: a Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Gordon T, Kannel WB.. Premature mortality from coronary heart disease. The Framingham study. JAMA 1971;215:1617–1625. [PubMed] [Google Scholar]
- 3. Stamler J, Wentworth D, Neaton JD.. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986;256:2823–2828. [PubMed] [Google Scholar]
- 4. Verschuren WM, Jacobs DR, Bloemberg BP, Kromhout D, Menotti A, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F.. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA 1995;274:131–136. [PubMed] [Google Scholar]
- 5. Endo A, Kuroda M, Tsujita Y.. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot 1976;29:1346–1348. [DOI] [PubMed] [Google Scholar]
- 6. Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J.. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA 1980;77:3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sirtori CR. The pharmacology of statins. Pharmacol Res 2014;88:3–11. [DOI] [PubMed] [Google Scholar]
- 8. Istvan ES, Deisenhofer J.. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001;292:1160–1164. [DOI] [PubMed] [Google Scholar]
- 9. Endo A. Drugs inhibiting HMG-CoA reductase. Pharmacol Ther 1985;31:257–267. [DOI] [PubMed] [Google Scholar]
- 10. Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J.. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–418. [DOI] [PubMed] [Google Scholar]
- 11. Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W.. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–1255. [DOI] [PubMed] [Google Scholar]
- 12.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–364. [DOI] [PubMed] [Google Scholar]
- 13. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr.. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 14. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ.. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995;333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 15.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22. [DOI] [PubMed] [Google Scholar]
- 16. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK.. Treating to New Targets I. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 17. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–1389. [PubMed] [Google Scholar]
- 18. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E.. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 19.Long-Term Intervention with Pravastatin in Ischaemic Disease Study G. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 20. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM.. Pravastatin or atorvastatin E, infection therapy-thrombolysis in myocardial infarction I. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T.. Myocardial ischemia reduction with aggressive cholesterol lowering study I. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711–1718. [DOI] [PubMed] [Google Scholar]
- 22. Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR.. Atherosclerosis risk in communities S. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2003;34:623–631. [DOI] [PubMed] [Google Scholar]
- 23. Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE.. Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health 2002;56: i19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LeppäLä JM, Virtamo J, Fogelholm R, Albanes D, Heinonen OP, Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke 1999;30:2535–2540. [DOI] [PubMed] [Google Scholar]
- 25. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA, Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. Stroke prevention by aggressive reduction in cholesterol levels I. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 26. Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria ANREVERSAL Investigators.. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071–1080. [DOI] [PubMed] [Google Scholar]
- 27. Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif J-C, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EMAsteroid Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556–1565. [DOI] [PubMed] [Google Scholar]
- 28. Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, Nissen SE.. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078–2087. [DOI] [PubMed] [Google Scholar]
- 29. Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y, Takarada S, Okumoto Y, Shiono Y, Orii M, Shimamura K, Ueno S, Yamano T, Tanimoto T, Ino Y, Yamaguchi T, Kumiko H, Tanaka A, Imanishi T, Akagi H, Akasaka T.. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol 2014;64:2207–2217. [DOI] [PubMed] [Google Scholar]
- 30. Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Lee BK, Chun EJ, Cademartiri F, Maffei E, Marques H, Leipsic JA, Shin S, Choi JH, Chinnaiyan K, Raff G, Virmani R, Samady H, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK.. Effects of statins on coronary atherosclerotic plaques: the PARADIGM Study. JACC Cardiovasc Imag 2018;11:1475–1484. [DOI] [PubMed] [Google Scholar]
- 31. Fukumoto Y, Libby P, Rabkin E, Hill CC, Enomoto M, Hirouchi Y, Shiomi M, Aikawa M.. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation 2001;103:993–999. [DOI] [PubMed] [Google Scholar]
- 32. Healy A, Berus JM, Christensen JL, Lee C, Mantsounga C, Dong W, Watts JP Jr., Assali M, Ceneri N, Nilson R, Neverson J, Wu WC, Choudhary G, Morrison AR.. Statins disrupt macrophage rac1 regulation leading to increased atherosclerotic plaque calcification. ATVB 2020;40:714–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P.. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001;103:276–283. [DOI] [PubMed] [Google Scholar]
- 34. Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J.. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 2001;103:926–933. [DOI] [PubMed] [Google Scholar]
- 35. Almeida SO, Budoff M.. Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med 2019;29:451–455. [DOI] [PubMed] [Google Scholar]
- 36. Sakellarios AI, Fotiadis DI.. Editorial commentary: the pleiotropic effect of statins on the atherosclerotic plaque and coronary heart disease. Trends Cardiovasc Med 2019;29:456–457. [DOI] [PubMed] [Google Scholar]
- 37. Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol 2001;88:3–6J. [DOI] [PubMed] [Google Scholar]
- 38. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR.. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347:1557–1565. [DOI] [PubMed] [Google Scholar]
- 39. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB.. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599–2610. [DOI] [PubMed] [Google Scholar]
- 40. Albert MA, Danielson E, Rifai N, Ridker PM, PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 41. Kinlay S, Schwartz GG, Olsson AG, Rifai N, Leslie SJ, Sasiela WJ, Szarek M, Libby P, Ganz P.. Myocardial ischemia reduction with aggressive cholesterol lowering study I. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation 2003;108:1560–1566. [DOI] [PubMed] [Google Scholar]
- 42. Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E.. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999;100:230–235. [DOI] [PubMed] [Google Scholar]
- 43. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, Group JSRosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 44. Oesterle A, Laufs U, Liao JK.. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dichtl W, Dulak J, Frick M, Alber HF, Schwarzacher SP, Ares MP, Nilsson J, Pachinger O, Weidinger F.. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. ATVB 2003;23:58–63. [DOI] [PubMed] [Google Scholar]
- 46. Kagami S, Owada T, Kanari H, Saito Y, Suto A, Ikeda K, Hirose K, Watanabe N, Iwamoto I, Nakajima H.. Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. Int Immunol 2009;21:679–689. [DOI] [PubMed] [Google Scholar]
- 47. Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM Jr., Boerwinkle E.. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997;96:4219–4225. [DOI] [PubMed] [Google Scholar]
- 48. Wang S, Dangerfield JP, Young RE, Nourshargh S.. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J Cell Sci 2005;118:2067–2076. [DOI] [PubMed] [Google Scholar]
- 49. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F.. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 50. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ, CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, Engert JC, Clarke R, Davey-Smith G, Nordestgaard BG, Saleheen D, Samani NJ, Sandhu M, Anand S, Pepys MB, Smeeth L, Whittaker J, Casas JP, Thompson SG, Hingorani AD, Danesh J, Collaboration CRPCHDG. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Catapano AL, Pirillo A, Norata GD.. Vascular inflammation and low-density lipoproteins: is cholesterol the link? A lesson from the clinical trials. Br J Pharmacol 2017;174:3973–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang XL, Lan RF, Zhang XW, Xu W, Wang L, Kang LN, Xu B.. Association between baseline, achieved, and reduction of CRP and cardiovascular outcomes after LDL cholesterol lowering with statins or ezetimibe: a systematic review and meta-analysis. J Am Heart Assoc 2019;8:e012428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Linton MRF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, Doran AC, Vickers KC.. The Role of Lipids and Lipoproteins in Atherosclerosis. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A (eds). Endotext. South Dartmouth, MA, 2019, pp.2000–2021. [Google Scholar]
- 55. Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest 2002;110:285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kozai T, Eto M, Yang Z, Shimokawa H, Luscher TF.. Statins prevent pulsatile stretch-induced proliferation of human saphenous vein smooth muscle cells via inhibition of Rho/Rho-kinase pathway. Cardiovasc Res 2005;68:475–482. [DOI] [PubMed] [Google Scholar]
- 57. Yamanouchi D, Banno H, Nakayama M, Sugimoto M, Fujita H, Kobayashi M, Kuwano H, Komori K.. Hydrophilic statin suppresses vein graft intimal hyperplasia via endothelial cell-tropic Rho-kinase inhibition. J Vasc Surg 2005;42:757–764. [DOI] [PubMed] [Google Scholar]
- 58. Rawlings R, Nohria A, Liu PY, Donnelly J, Creager MA, Ganz P, Selwyn A, Liao JK.. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on rho kinase activity in Caucasian men with a previous atherosclerotic event. Am J Cardiol 2009;103:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nohria A, Prsic A, Liu PY, Okamoto R, Creager MA, Selwyn A, Liao JK, Ganz P.. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis 2009;205:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ma MM, Li SY, Wang M, Guan YY.. Simvastatin attenuated cerebrovascular cell proliferation in the development of hypertension through Rho/Rho-kinase pathway. J Cardiovasc Pharmacol 2012;59:576–582. [DOI] [PubMed] [Google Scholar]
- 61. Ronzier E, Parks XX, Qudsi H, Lopes CM.. Statin-specific inhibition of Rab-GTPase regulates cPKC-mediated IKs internalization. Sci Rep 2019;9:17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Laufs U, La Fata V, Plutzky J, Liao JK.. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998;97:1129–1135. [DOI] [PubMed] [Google Scholar]
- 63. Yildirim FİA, Durman DK, Aypar E, Ark M, Özdemir O, Doğan BSU.. Atorvastatin acutely reduces the reactivity to spasmogens in rat aorta: implication of the inhibition of geranylgeranylation and MYPT-1 phosphorylation. Fundam Clin Pharmacol 2016;30:96–106. [DOI] [PubMed] [Google Scholar]
- 64. Silveira AAA, Dominical VM, Almeida CB, Chweih H, Ferreira WA Jr., Vicente CP, Costa FTM, Werneck CC, Costa FF, Conran N.. TNF induces neutrophil adhesion via formin-dependent cytoskeletal reorganization and activation of beta-integrin function. J Leukoc Biol 2018;103:87–98. [DOI] [PubMed] [Google Scholar]
- 65. Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S.. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 2001;103:2885–2890. [DOI] [PubMed] [Google Scholar]
- 66. Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T.. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest 2001;108:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM.. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002;105:3017–3024. [DOI] [PubMed] [Google Scholar]
- 68. Jakobisiak M, Bruno S, Skierski JS, Darzynkiewicz Z.. Cell cycle-specific effects of lovastatin. Proc Natl Acad Sci USA 1991;88:3628–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Laufs U, Marra D, Node K, Liao JK.. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1). J Biol Chem 1999;274:21926–21931. [DOI] [PubMed] [Google Scholar]
- 70. Tang FC, Wang HY, Ma MM, Guan TW, Pan L, Yao DC, Chen YL, Li SJ, Yang H, Zhu XQ, Tu YS.. Simvastatin attenuated rat thoracic aorta remodeling by decreasing ROCK2mediated CyPA secretion and CD147ERK1/2cyclin pathway. Mol Med Rep 2017;16:8123–8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huhle G, Abletshauser C, Mayer N, Weidinger G, Harenberg J, Heene DL.. Reduction of platelet activity markers in type II hypercholesterolemic patients by a HMG-CoA-reductase inhibitor. Thromb Res 1999;95:229–234. [DOI] [PubMed] [Google Scholar]
- 72. Pawelczyk M, Chmielewski H, Kaczorowska B, Przybyła M, Baj Z.. The influence of statin therapy on platelet activity markers in hyperlipidemic patients after ischemic stroke. aoms 2015;1:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Notarbartolo A, Davi G, Averna M, Barbagallo CM, Ganci A, Giammarresi C, La Placa FP, Patrono C.. Inhibition of thromboxane biosynthesis and platelet function by simvastatin in type IIa hypercholesterolemia. ATVB 1995;15:247–251. [DOI] [PubMed] [Google Scholar]
- 74. Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, Ferrante E, Ciabattoni G, Scarpini F, Ghezzi A, Auteri A, Davi G.. Effects of atorvastatin and rosuvastatin on thromboxane-dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis 2011;214:122–128. [DOI] [PubMed] [Google Scholar]
- 75. Le Quan Sang KH, Levenson J, Megnien JL, Simon A, Devynck MA.. Platelet cytosolic Ca2+ and membrane dynamics in patients with primary hypercholesterolemia. Effects of pravastatin. ATVB 1995;15:759–764. [DOI] [PubMed] [Google Scholar]
- 76. Moraes LA, Vaiyapuri S, Sasikumar P, Ali MS, Kriek N, Sage T, Gibbins JM.. Antithrombotic actions of statins involve PECAM-1 signaling. Blood 2013;122:3188–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ali FY, Armstrong PC, Dhanji AR, Tucker AT, Paul-Clark MJ, Mitchell JA, Warner TD.. Antiplatelet actions of statins and fibrates are mediated by PPARs. ATVB 2009;29:706–711. [DOI] [PubMed] [Google Scholar]
- 78. Du H, Hu H, Zheng H, Hao J, Yang J, Cui W.. Effects of peroxisome proliferator-activated receptor gamma in simvastatin antiplatelet activity: influences on cAMP and mitogen-activated protein kinases. Thromb Res 2014;134:111–120. [DOI] [PubMed] [Google Scholar]
- 79. Chen Z, Fukutomi T, Zago AC, Ehlers R, Detmers PA, Wright SD, Rogers C, Simon DI.. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation 2002;106:20–23. [DOI] [PubMed] [Google Scholar]
- 80. Desai P, Helkin A, Odugbesi A, Stein J, Bruch D, Lawler J, Maier KG, Gahtan V.. Fluvastatin inhibits intimal hyperplasia in wild-type but not Thbs1-null mice. J Surg Res 2017;210:1–7. [DOI] [PubMed] [Google Scholar]
- 81. Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, Trosian K, Hamilton MA, Moriguchi JD, Kawata N, Hage A, Drinkwater DC, Stevenson LW.. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995;333:621–627. [DOI] [PubMed] [Google Scholar]
- 82. Som R, Morris PJ, Knight SR.. Graft vessel disease following heart transplantation: a systematic review of the role of statin therapy. World J Surg 2014;38:2324–2334. [DOI] [PubMed] [Google Scholar]
- 83. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, IMPROVE IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 84. Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL, Tardif JC, SPIRE Cardiovascular Outcome Investigators. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017;376:1527–1539. [DOI] [PubMed] [Google Scholar]
- 85. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 86. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM, ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 87. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE.. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV Randomized Clinical Trial. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 88. Salvatore T, Morganti R, Marchioli R, De Caterina R.. Cholesterol lowering and stroke: no longer room for pleiotropic effects of statins—confirmation from PCSK9 inhibitor studies. Am J Med 2020;133:95–99.e96. [DOI] [PubMed] [Google Scholar]
- 89. Labos C, Brophy JM, Smith GD, Sniderman AD, Thanassoulis G.. Evaluation of the pleiotropic effects of statins: a reanalysis of the randomized trial evidence using egger regression-brief report. Arterioscler Thromb Vasc Biol 2018;38:262–265. [DOI] [PubMed] [Google Scholar]
- 90. Hindy G, Engstrom G, Larsson SC, Traylor M, Markus HS, Melander O, Orho-Melander M, Stroke Genetics N. Role of blood lipids in the development of ischemic stroke and its subtypes: a Mendelian Randomization Study. Stroke 2018;49:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM.. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009;360:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marston NA, Gurmu Y, Melloni GEM, Bonaca M, Gencer B, Sever PS, Pedersen TR, Keech AC, Roselli C, Lubitz SA, Ellinor PT, O’Donoghue ML, Giugliano RP, Ruff CT, Sabatine MS.. The Effect of PCSK9 (proprotein convertase subtilisin/Kexin Type 9) inhibition on the risk of venous thromboembolism. Circulation 2020;141:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chamberlain AM, Folsom AR, Heckbert SR, Rosamond WD, Cushman M.. High-density lipoprotein cholesterol and venous thromboembolism in the Longitudinal Investigation of Thromboembolism Etiology (LITE). Blood 2008;112:2675–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van Schouwenburg IM, Mahmoodi BK, Gansevoort RT, Muntinghe FL, Dullaart RP, Kluin-Nelemans HC, Veeger NJ, Meijer K.. Lipid levels do not influence the risk of venous thromboembolism. Results of a population-based cohort study. Thromb Haemost 2012;108:923–929. [DOI] [PubMed] [Google Scholar]
- 95. Morelli VM, Lijfering WM, Bos MHA, Rosendaal FR, Cannegieter SC.. Lipid levels and risk of venous thrombosis: results from the MEGA-study. Eur J Epidemiol 2017;32:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Everett BM, Glynn RJ, Buring JE, Ridker PM.. Lipid biomarkers, hormone therapy and the risk of venous thromboembolism in women. J Thromb Haemost 2009;7:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, Lira Pineda A, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS.. Inflammatory and cholesterol risk in the FOURIER Trial. Circulation 2018;138:131–140. [DOI] [PubMed] [Google Scholar]
- 98. Cao YX, Li S, Liu HH, Li JJ.. Impact of PCSK9 monoclonal antibodies on circulating hs-CRP levels: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2018;8:e022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, Harrington RA, Jukema JW, Loizeau V, Moriarty PM, Moryusef A, Pordy R, Roe MT, Sinnaeve P, Tsimikas S, Vogel R, White HD, Zahger D, Zeiher AM, Steg PG, Schwartz GG, ODYSSEY OUTCOMES Committees and Investigators. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol 2020;75:133–144. [DOI] [PubMed] [Google Scholar]
- 100. Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S.. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation 2014;129:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fessler MB. The intracellular cholesterol landscape: dynamic integrator of the immune response. Trends Immunol 2016;37:819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, Wijngaard P, Wright RS, Kastelein JJ.. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017;376:1430–1440. [DOI] [PubMed] [Google Scholar]
- 103. Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, Kelly S, Stroes ESG.. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019;8:e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Honigberg MC, Natarajan P.. Bempedoic acid for lowering LDL cholesterol. JAMA 2019;322:1769–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zeiser R. Immune modulatory effects of statins. Immunology 2018;154:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lv S, Liu Y, Zou Z, Li F, Zhao S, Shi R, Bian R, Tian H.. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol 2015;33:69–76. [PubMed] [Google Scholar]
- 107. Li GM, Zhao J, Li B, Zhang XF, Ma JX, Ma XL, Liu J.. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: a systemic review and meta-analysis of 15 randomized controlled trials. Autoimmun Rev 2018;17:215–225. [DOI] [PubMed] [Google Scholar]
- 108. Tascilar K, Dell'Aniello S, Hudson M, Suissa S.. Statins and risk of rheumatoid arthritis: a nested case-control study. Arthritis Rheumatol 2016;68:2603–2611. [DOI] [PubMed] [Google Scholar]
- 109. Tu H, Li Q, Xiang S, Jiang H, Mao Y, Shou Z, Chen J.. Dual effects of statins therapy in systemic lupus erythematosus and SLE-related atherosclerosis: the potential role for regulatory T cells. Atherosclerosis 2012;222:29–33. [DOI] [PubMed] [Google Scholar]
- 110. Estanislau IMG, Terceiro IRC, Lisboa MRP, Teles PDB, Carvalho RDS, Martins RS, Moreira MMSM.. Pleiotropic effects of statins on the treatment of chronic periodontitis—a systematic review. Br J Clin Pharmacol 2015;79:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Petit C, Batool F, Bugueno IM, Schwinte P, Benkirane-Jessel N, Huck O.. Contribution of statins towards periodontal treatment: a review. Mediators Inflamm 2019;2019:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stokkeland K, Höijer J, Bottai M, Söderberg-Löfdal K, Bergquist A.. Statin use is associated with improved outcomes of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2019;17:1860–1866.e1861. [DOI] [PubMed] [Google Scholar]
- 113. Ungaro R, Chang HL, Cote-Daigneault J, Mehandru S, Atreja A, Colombel JF.. Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol 2016;111:1416–1423. [DOI] [PubMed] [Google Scholar]
- 114. Chu CS, Tseng PT, Stubbs B, Chen TY, Tang CH, Li DJ, Yang WC, Chen YW, Wu CK, Veronese N, Carvalho AF, Fernandes BS, Herrmann N, Lin PY.. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci Rep 2018;8:5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schultz BG, Patten DK, Berlau DJ.. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl Neurodegener 2018;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM.. Long-term statin use and psychological well-being. J Am Coll Cardiol 2003;42:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dashti-Khavidaki S, Khalili H.. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy 2020;40:484–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Phadke M, Saunik S.. COVID-19 treatment by repurposing drugs until the vaccine is in sight. Drug Dev Res 2020;81:541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Castiglione V, Chiriaco M, Emdin M, Taddei S, Vergaro G.. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother 2020;6:258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tse SM, Li L, Butler MG, Fung V, Kharbanda EO, Larkin EK, Vollmer WM, Miroshnik I, Rusinak D, Weiss ST, Lieu T, Wu AC.. Statin exposure is associated with decreased asthma-related emergency department visits and oral corticosteroid use. Am J Respir Crit Care Med 2013;188:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bradbury P, Traini D, Ammit AJ, Young PM, Ong HX.. Repurposing of statins via inhalation to treat lung inflammatory conditions. Adv Drug Deliv Rev 2018;133:93–106. [DOI] [PubMed] [Google Scholar]
- 122. Reiner Z, Hatamipour M, Banach M, Pirro M, Al-Rasadi K, Jamialahmadi T, Radenkovic D, Montecucco F, Sahebkar A.. Statins and the COVID-19 main protease: in silico evidence on direct interaction. AOMS 2020;16:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liao JK. Squalene synthase inhibitor lapaquistat acetate: could anything be better than statins? Circulation 2011;123:1925–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, Stroes ES, MacDougall D, Zhao X, Catapano AL.. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2019;27:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM CLEAR Harmony Trial. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 126. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, Lalwani ND, Patel PM, Zhao X, Duell PB.. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom Randomized Clinical Trial. JAMA 2019;322:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, Jacobson TA, Kopecky SL, Baccara-Dinet MT, Du Y, Pordy R, Gipe DA, ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 128. Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK.. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation 2009;119:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Osaki Y, Nakagawa Y, Miyahara S, Iwasaki H, Ishii A, Matsuzaka T, Kobayashi K, Yatoh S, Takahashi A, Yahagi N, Suzuki H, Sone H, Ohashi K, Ishibashi S, Yamada N, Shimano H.. Skeletal muscle-specific HMG-CoA reductase knockout mice exhibit rhabdomyolysis: a model for statin-induced myopathy. Biochem Biophys Res Commun 2015;466:536–540. [DOI] [PubMed] [Google Scholar]
- 130. Nagashima S, Yagyu H, Ohashi K, Tazoe F, Takahashi M, Ohshiro T, Bayasgalan T, Okada K, Sekiya M, Osuga J, Ishibashi S.. Liver-specific deletion of 3-hydroxy-3-methylglutaryl coenzyme A reductase causes hepatic steatosis and death. Arterioscler Thromb Vasc Biol 2012;32:1824–1831. [DOI] [PubMed] [Google Scholar]
- 131. Sakai K, Nagashima S, Wakabayashi T, Tumenbayar B, Hayakawa H, Hayakawa M, Karasawa T, Ohashi K, Yamazaki H, Takei A, Takei S, Yamamuro D, Takahashi M, Yagyu H, Osuga JI, Takahashi M, Tominaga SI, Ishibashi S.. Myeloid HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase determines atherosclerosis by modulating migration of macrophages. Arterioscler Thromb Vasc Biol 2018;38:2590–2600. [DOI] [PubMed] [Google Scholar]
- 132. Ohashi K, Osuga J, Tozawa R, Kitamine T, Yagyu H, Sekiya M, Tomita S, Okazaki H, Tamura Y, Yahagi N, Iizuka Y, Harada K, Gotoda T, Shimano H, Yamada N, Ishibashi S.. Early embryonic lethality caused by targeted disruption of the 3-hydroxy-3-methylglutaryl-CoA reductase gene. J Biol Chem 2003;278:42936–42941. [DOI] [PubMed] [Google Scholar]