Abstract
Aims
Elevated sympathetic outflow is associated with primary hypertension. However, the mechanisms involved in heightened sympathetic outflow in hypertension are unclear. The central amygdala (CeA) regulates autonomic components of emotions through projections to the brainstem. The neuronal Kv7 channel is a non-inactivating voltage-dependent K+ channel encoded by KCNQ2/3 genes involved in stabilizing the neuronal membrane potential and regulating neuronal excitability. In this study, we investigated if altered Kv7 channel activity in the CeA contributes to heightened sympathetic outflow in hypertension.
Methods and results
The mRNA and protein expression levels of Kv7.2/Kv7.3 in the CeA were significantly reduced in spontaneously hypertensive rats (SHRs) compared with Wistar–Kyoto (WKY) rats. Lowering blood pressure with coeliac ganglionectomy in SHRs did not alter Kv7.2 and Kv7.3 channel expression levels in the CeA. Fluospheres were injected into the rostral ventrolateral medulla (RVLM) to retrogradely label CeA neurons projecting to the RVLM (CeA–RVLM neurons). Kv7 channel currents recorded from CeA–RVLM neurons in brain slices were much smaller in SHRs than in WKY rats. Furthermore, the basal firing activity of CeA–RVLM neurons was significantly greater in SHRs than in WKY rats. Bath application of specific Kv7 channel blocker 10, 10-bis (4-pyridinylmethyl)-9(10H)-anthracnose (XE-991) increased the excitability of CeA–RVLM neurons in WKY rats, but not in SHRs. Microinjection of XE-991 into the CeA increased arterial blood pressure (ABP) and renal sympathetic nerve activity (RSNA), while microinjection of Kv7 channel opener QO-58 decreased ABP and RSNA, in anaesthetized WKY rats but not SHRs.
Conclusions
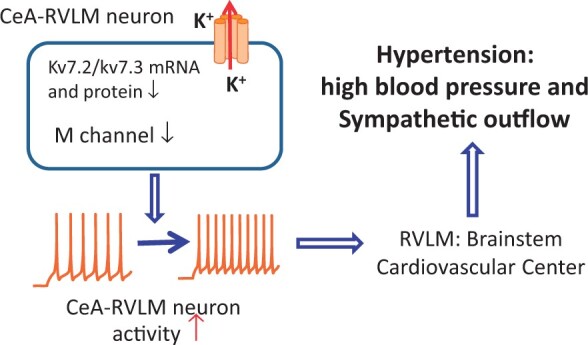
Our findings suggest that diminished Kv7 channel activity in the CeA contributes to elevated sympathetic outflow in primary hypertension. This novel information provides new mechanistic insight into the pathogenesis of neurogenic hypertension.
Keywords: Autonomic nervous system, Hypertension, Central amygdala, Sympathetic nervous system, Potassium channel
Graphical Abstract
Time for primary review: 23 days
1. Introduction
Hypertension is a highly prevalent cardiovascular disorder and a leading cause of coronary artery disease, renal failure, and stroke. The majority of hypertension cases have no clear cause and may stem from the combined effect of many factors including genetic and environmental factors.1 Although extensive studies have been carried out to elucidate the aetiology of hypertension, the mechanisms responsible for the root cause of primary hypertension remain unclear.
The elevated sympathetic outflow is one of the major mechanisms involved in the pathogenesis of hypertension.1–6 The sympathetic premotor neurons located in the rostral ventrolateral medulla (RVLM) tightly regulate sympathetic outflow through projections to sympathetic preganglionic neurons in the intermediolateral cell column in the spinal cord.7,8 In addition to receiving innervation from the hypothalamic paraventricular nucleus (PVN),9,10 the premotor neurons in the RVLM also receive projections from other brain regions including the central nucleus of amygdala (CeA).11 The CeA is an important brain region that regulates autonomic and behavioural functions related to fear, pain, and stress.12 For example, CeA neurons project to the RVLM, making synaptic contact with C1 neurons in this region.13,14 Electrolytic or chemical lesions of the CeA attenuates the development of hypertension in SHRs and stress-induced hypertension.15–17 A previous study reported that this attenuation of development of hypertension is associated with reduced food intake and weight gain in SHRs with CeA lesion.18 This proposal is premature due to the fact that the blood pressure was measured by a stressful tethered approach and the food intake and body weight were not accurately assessed. Thus, it is necessary to re-evaluate the conclusion that reduced food intake and weight gain are involved in the attenuation of hypertension in SHR with CeA lesion. On the other hand, stimulation of the CeA increases neuronal activity and c-fos expression in the RVLM.19–21 Thus, the CeA–RVLM pathway is particularly important in regulating the cardiovascular responses to stress.22 However, the role of CeA–RVLM pathway in regulating sympathetic activity and blood pressure has not been well studied. Thus, in this study, we determined the role of CeA–RVLM pathway in controlling sympathetic outflow in hypertension.
M-channel is a non-inactivating and voltage-gated K+ channel (Kv7 family encoded by KCNQ genes) that is closed by activation of muscarinic acetylcholine receptors.23–26 The M-currents are critical in stabilizing the membrane potential to resting membrane potential level because it opens more when the cell membrane depolarizes.25 In the brain, M-currents are mainly carried by a Kv7.2/Kv7.3 channel heterotetrameric encoded by KCNQ2 and KCNQ3 genes.25,27–29 Genetic knockdown or acute blocking of Kv7.2/Kv7.3 channels depolarizes neurons and increases their firing activity, whereas the opening of Kv7.2/Kv7.3 channels hyperpolarizes membrane and inhibits neuronal activity. Dysfunction of Kv7.2/Kv7.3 channels leads to several neurogenic diseases including epilepsy, pain, memory deficit/decline, and depression.30–33 It has been shown that Kv7.2/Kv7.3 channels are involved in the dysfunction of the autonomic nervous system in SHRs.34 However, the role of the Kv7.2/Kv7.3 channel in the regulation of sympathetic outflow in hypertension remains unknown. In this study, we determined the role of Kv7.2 and Kv7.3 channels in regulating CeA–RVLM neurons and sympathetic outflow in hypertension.
2. Methods
2.1 Animal
Adult (12–15 weeks old) and young (4–7 weeks old) male Wistar–Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs) (Envigo, Indianapolis, IN) were used in this study. The experimental protocols and surgical procedures were approved by the Institutional Animal Care and Use Committee of The University of Missouri School of Medicine (#9439) and conformed to the US National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
The detailed methods for the following experimental procedures including animal anaesthesia and euthanasia are described in detail in the Supplementary material online. For harvesting brain tissue for electrophysiological recording and biochemical assays including western blotting and reverse transcription polymerase chain reaction (RT–PCR), the animals were euthanized by decapitation under deep anaesthesia with isoflurane (5% in O2). The animals subjected to immunocytochemical staining were intra-cardiac perfused with 4% paraformaldehyde and 10% sucrose under deep anaesthesia (isoflurane 5% in O2). The rats used for recording of arterial blood pressure (ABP) and renal sympathetic nerve activity (RSNA) were decapitated after completion of procedures when they were still under anaesthesia by urethane and α-chloralose.
2.2 Quantitative RT–PCR
Rat brain tissue was obtained through decapitation under anaesthesia with inhalation of 5% isoflurane in O2. Total RNA was extracted from CeA tissues, quantified, and processed for RT–PCR by using primers listed in Table 1 to quantitatively analyse mRNA expression levels. The fold-change in KCNQ2/3 gene expression between WKY rats and SHRs was then calculated.
Table 1.
Kv7.2 and Kv7.3 primers
| Gene | Sense, antisense |
|---|---|
| KCNQ2 |
(+) 5′-CCACCATCAAGGAGTATGAGAAG-3′ (−) 5′-TAGCATAGAATCTCCAGGCAGAC-3′ |
| KCNQ3 |
(+) 5′-TTGGTTATGGAGACAAGACACCT-3′ (−) 5′-TCAGAGGGGTAAATAGCTTTCCT-3′ |
| GAPDH |
(+) 5′-TGACAACTTTGGCATCGTGG-3′ (−) 5′-GGGCCATCCACAGTCTTCTG-3′ |
2.3 Western immunoblotting
Rat brain was harvested through decapitation under isoflurane (5% in O2 inhalation) anaesthesia. Brain tissues including the CeA, pre-frontal cortex (PFC), hippocampus (HP), and the PVN were homogenized and treated for Western blotting with anti-Kv7.2 antibody (Cell Signaling Technology, #14752) and anti-Kv7.3 antibody (Abcam, ab113948).
2.4 Coeliac ganglionectomy (CGx) and telemetry blood pressure measurement
Under anaesthesia induced by inhalation of 2–3% isoflurane in O2, a coeliac ganglionectomy (CGx) procedure was done and a DSI blood pressure transducer was implanted. After the animal was recovered from the anaesthesia and surgery, the blood pressure was monitored by DSI telemetry system in conscious free-moving rats.
2.5 Retrograde labelling of CeA–RVLM neurons
Under 2–3% isoflurane anaesthesia, Fluospheres were injected into the RVLM in WKY rats and SHRs.
2.6 Histological identification of CeA–RVLM neurons
This procedure was used to determine if CeA–RVLM neurons were glutamatergic.
2.7 Immunohistochemical staining
Immunohistochemical staining was performed to determine the spatial distribution of Kv7 channels in the CeA–RVLM neurons by using anti-Kv7.3 (Alomone Labs) antibody. Also, we performed immunohistochemical staining on brain stem slices by using anti-tyrosine hydroxylase (TH) antibody (Cell Signaling Technology #5844S) and anti-c-fos antibodies (abcam ab208942) to determine if microinjection of Kv7 blocker into the CeA activated TH neurons in the RVLM.
2.8 Electrophysiological recordings in brain slices
Coronal brain slices at the amygdala level were prepared from the tracer injected rats. M-currents were recorded using the voltage-clamp mode and the spontaneous and evoked firing activity of labelled neurons was recorded using a current-clamp mode.
2.9 Recording of ABP and RSNA and microinjection into the CeA
Rats were anaesthetized by a bolus intraperitoneal injection of a mixture of urethane (800 mg/kg) and α-chloralose (60–75 mg/kg). The pulsatile ABP, RSNA, and heart rate (HR) triggered by pulsatile ABP were digitally recorded by LabChart Pro system (ADInstruments).
2.10 Statistical analysis
Data are presented as means ± SEM. A Student’s t-test (two-tail) was performed to compare two groups and one-way or two-way ANOVA followed by Dunnett’s post hoc test was used to compare more than two groups. Statistical analyses were performed using Prism 8 software (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered statistically significant.
3. Results
3.1 Kv7.2/Kv7.3 protein and mRNA levels were decreased in the CeA in SHRs
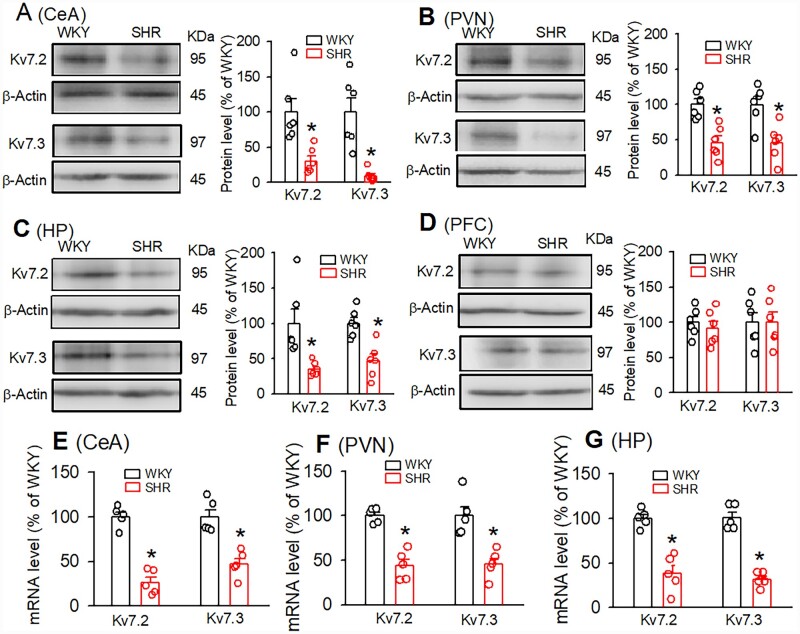
We determined Kv7.2/Kv7.3 protein expression levels in the CeA tissues obtained from WKY and SHRs. Kv7 channels are mainly composed of Kv7.2 and Kv7.3 subunits encoded by KCNQ2/3 genes in the central nervous system.29 Immunoblotting band density analysis revealed that Kv7.2 and Kv7.3 protein levels in the CeA were significantly lower in SHRs than in WKY rats (Figure 1A, n=6 rats in each group, P = 0.0028, t(10) =3.51, unpaired t-test). The degree of reduction of Kv7.2 was similar to that of Kv7.3. We also determined Kv7 protein levels in other brain tissues including the PVN, HP, and PFC. Kv7.2 and Kv7.3 protein levels in the PVN and HP were significantly lower in SHRs than in WKY rats (Figure 1). In contrast, in the PFC, Kv7.2, and Kv7.3 protein levels were not significantly different between WKY rats and SHRs (Figure 1D, n=6, P = 0.268, t(10) =0.64). In addition, the mRNA levels of Kv7.2 and Kv7.3 in the CeA, PVN, and HP were significantly lower in SHRs compared to WKY rats (CeA, Kv7.2: P < 0.001, t(8) =9.155; Kv7.3 P = 0.0011, t(8) =4.958; PVN, Kv7.2: P = 0.0001, t(8) =6.805; Kv7.3: P = 0.0026, t(8) =4.315; HP, Kv7.2: P = 0.0003, t(8) =6.069; Kv7.3: P < 0.001, t(8) =9.65, n=5 samples in each group, Figure 1).
Figure 1.
Kv7.2 and 7.3 protein and mRNA expression levels were decreased in the CeA in SHRs. (A–D) Original gel images and quantification of band density show the total protein levels of Kv7.2 and Kv7.3 in the CeA (A), PVN (B), HP (C), and PFC (D) from WKY rats and SHRs (n=6 samples in each group). In these protein assays, each sample consisting of respective tissues from one rat. (E–G) Summary of quantitative RT–PCR data show mRNA levels of Kv7.2 and Kv7.3 in the CeA (E), PVN (F), and HP (G) from WKY rats and SHRs (n=5 samples in each group). Data are presented as the mean ± SEM. *P < 0.05, compared with values in WKY rats (unpaired Student’s t-test). CeA, central amygdala; PVN, paraventricular nucleus; HP, hippocampus; PFC, pre-frontal cortex.
3.2 Reduced expression of Kv7.2/Kv7.3 protein in the CeA was independent of high blood pressure in SHRs
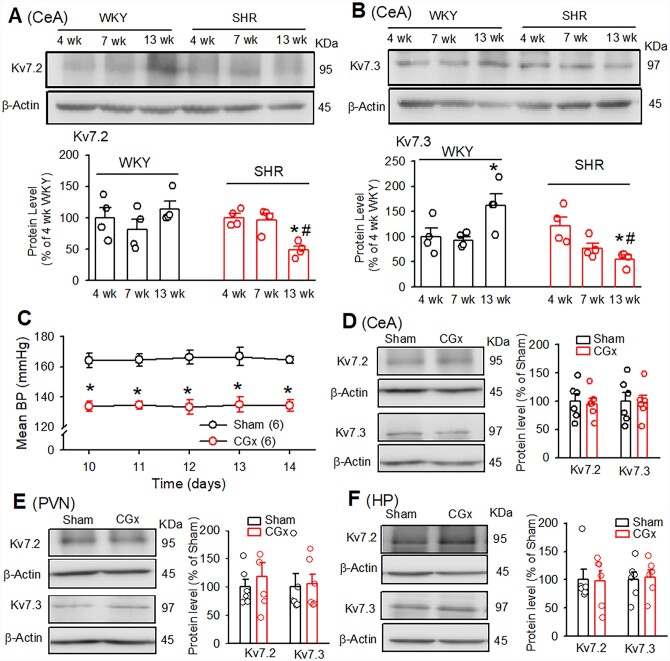
To determine if the Kv7.2/Kv7.3 levels in the CeA progressively changed in the SHR and WKY rats, we measured Kv7.2 and Kv7.3 protein levels in 4, 7, and 13 weeks old SHRs and age-matched WKY rats. The systolic blood pressure measured by the tail-cuff method in 4-week-old SHR (96.2±3.0 mmHg) did not differ from age-matched WKY rats (97.2±4.2 mmHg). The systolic blood pressure of 7 and 13 weeks old SHRs (148.3±4.6 mmHg at 7 weeks old and 191.6±4.1 mmHg at 13 weeks old) was significantly higher than age-matched WKY rats (99.8±2.8 mmHg at 7 weeks old and 99.3±3.6 mmHg at 13 weeks old). Both Kv7.2 and Kv7.3 expression levels in adult SHRs were decreased compared with age-matched WKY rats or pre-hypertensive SHRs. Kv7.2 expression levels did not alter with age in WKY rats but decreased in established hypertension in adult SHRs. Also, the Kv7.3 expression levels were decreased in adult SHRs but were increased in adult WKY rats (Figure 2A and B). These data indicate that a reduction of Kv7 channel expression in the CeA probably contributes to the maintenance of high blood pressure and elevated sympathetic output in SHRs.
Figure 2.
Reduced expression of Kv7.2/Kv7.3 in the CeA was independent of high blood pressure in SHRs. (A and B) Western blot images and quantification of band density show the total protein levels of Kv7.2 (A) and Kv7.3 (B) (normalized to β-actin) in the CeA in 4, 7, and 13 weeks old WKY rats and SHRs (n=4 rats in each group). (C) Summary data show mean ABP measured by telemetry approach in SHRs subjected to the CGx and sham surgery (n=6 rats in each group). (D–F) Western blot images and quantification of band density show the total protein levels of Kv7.2 and Kv7.3 (normalized to β-actin) in the CeA (D), the PVN (E), and HP (F) in SHRs subjected to CGx or sham surgery (n=6 rats in each group). Data are presented as the mean±SEM. *P < 0.05, compared with corresponding values in WKY rats and #P < 0.05, compared with values within SHR group, repeated-measures ANOVA with Dunnett’s post hoc test. CeA, the central amygdala; PVN, the paraventricular nucleus; HP, the hippocampus.
To determine whether the reduction of Kv7.2/Kv7.3 protein levels in the CeA of SHRs was a secondary response to high ABP in SHRs, we lowered ABP in SHRs by the CGx35,36 and then measured Kv7.2/Kv7.3 protein levels in the CeA. CGx significantly lowered ABP in SHRs, as measured by the telemetry approach in conscious free-moving SHRs, compared with SHRs with sham surgery (n=6 rats). CGx-induced reduction of ABP in SHRs started from Day 7 after CGx surgery and lasted for at least 2 weeks (Figure 2C). Immunoblotting using Kv7.2/Kv7.3 antibodies showed that reduction of ABP by the CGx did not alter significantly Kv7.2 or Kv7.3 protein levels in the CeA, the PVN, and the HP in SHRs (Figure 2D–F, n=6 rats in each group, P > 0.05). These data suggest that the down-regulation of Kv7.2 and Kv7.3 in the CeA in SHRs is independent of ABP changes.
3.3 M-currents were reduced in CeA–RVLM neurons in SHRs
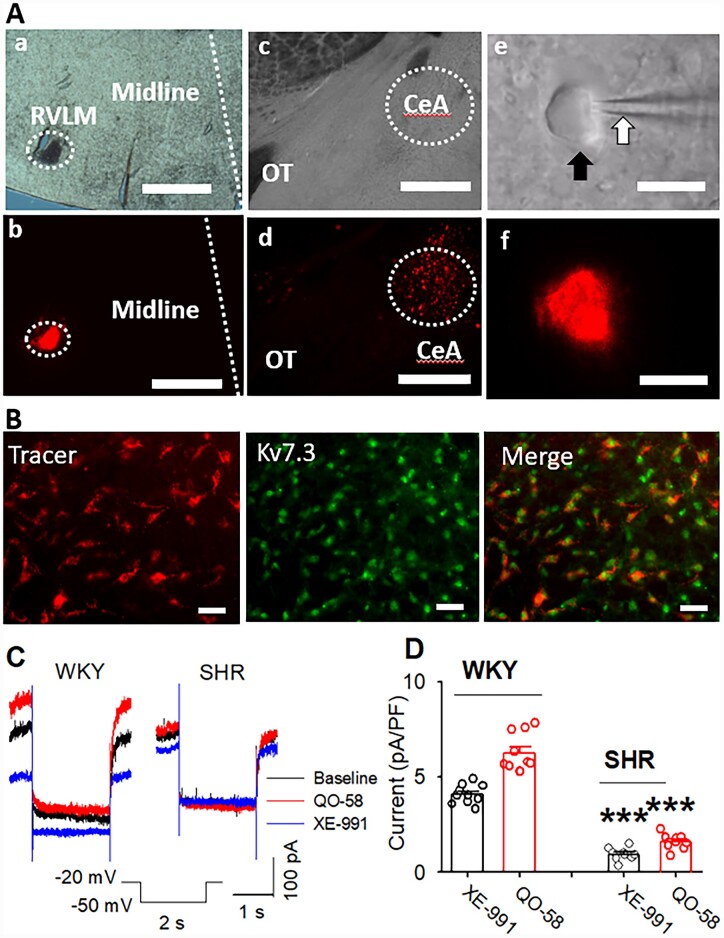
It has been shown that a population of neurons in the CeA is projected to the RVLM.13,14 We first determine if the Kv7 protein is expressed on CeA–RVLM neurons. CeA–RVLM neurons were retrogradely labelled by FluoSpheres injected into the RVLM (Figure 3A). We performed immunostaining on brain slice with labelled CeA–RVLM neurons using antibody against Kv7.3. Kv7.3 immunoreactive were expressed in the DiI-labelled CeA–RVLM neurons (Figure 3B). Then, we directly measured M-currents in retrogradely labelled CeA–RVLM neurons in brain slice. Cell membrane depolarization opens M-channels and generates M-currents.25,37 Thus, we recorded M-currents when the membrane potential was changed from −20 mV to −50 mV and being held for 1 s followed by holding at −20 mV again.31,32 Then, a highly selective Kv7 channel opener pyrazolo[1,5-a]pyrimidin-7(4H)-on (QO-58, 10 µM)38 and a specific Kv7 blocker 10, 10-bis (4-pyridinylmethyl)-9(10H)-anthracnose (XE-991, 10 µM) were successively administered via bath application. The basal or total M-currents were defined as XE-991-sensitive currents before and during the application of QO-58 (10 µM). Both basal and total M-currents were significantly decreased in labelled CeA neurons in SHRs (n=10 neurons in 4 rats) compared with WKY rats (n=9 neurons from 4 rats in each group, P < 0.0001; F(3,36) =156.56, Figure 3C and D).
Figure 3.
M-currents were decreased in CeA–RVLM neurons in SHRs. (A) Images show retrograde tracer injection sites in the RVLM viewed by light (a) and fluorescent (b) microscope and Fluosphere labelled neurons in the CeA viewed by light (c and e) and fluorescent (d and f) microscope. (B) Immunostaining images show that Kv7.3 immunoreactivities were expressed on DiI-labelled CeA neurons. (C and D) Original recordings (C) and summary data (D) show the basal and QO-58-induced M-currents, which were defined as XE-991-sensitive tail currents in basal and the presence of QO-58, were significantly diminished in retrogradely labelled CeA–RVLM neurons in SHRs (n=10 neurons in 4 rats) compared with WKY rats (n=9 neurons in 4 rats).***P < 0.0001 compared with corresponding values in WKY rats; repeated-measures ANOVA with Dunnett’s post hoc test. The scale bars in Aa and Ab indicate 0.5 mm and in Ac and Ad indicates 10.0 µm. OT, optic tract.
3.4 Kv7 channel activity is diminished in SHRs leading to an increase in excitability of CeA–RVLM neurons
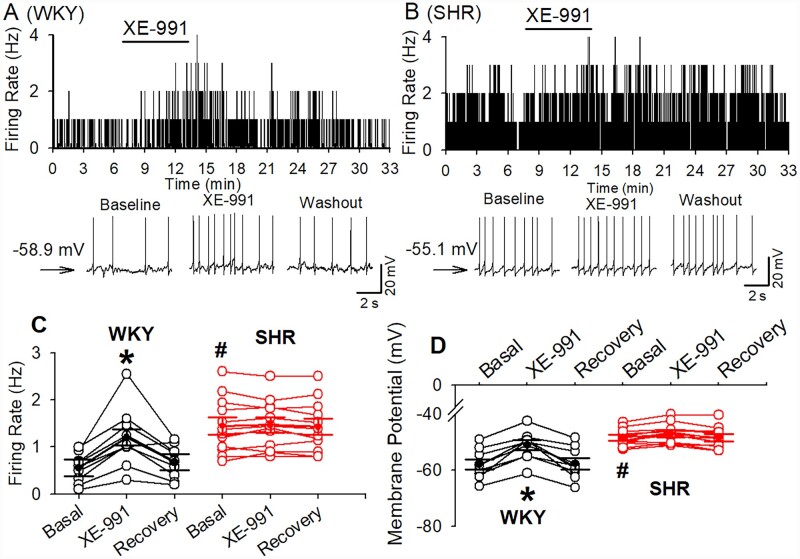
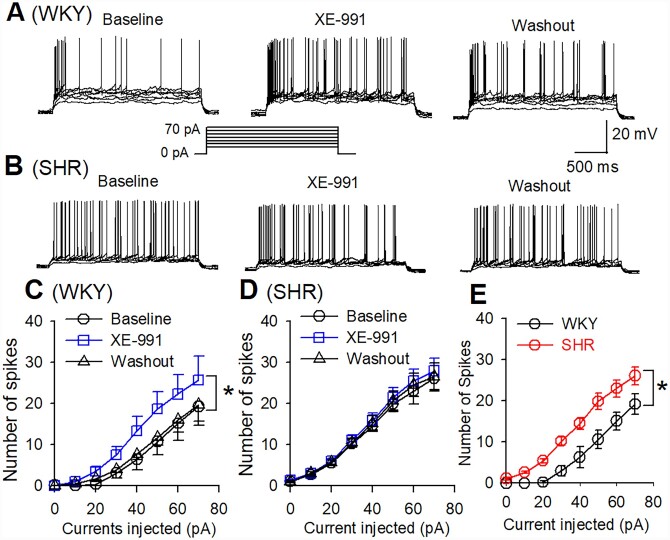
The firing activity of labelled CeA–RVLM neurons in WKY rats and SHRs was assessed in whole-cell current-clamp mode. In a total of 16 labelled CeA neurons recorded from WKY rats, 9 neurons (56.3%) displayed spontaneous activity and the remaining 7 neurons were silent. Whereas in SHRs, 12 of 19 (63.3%) neurons displayed spontaneous firing activity and the remaining 7 neurons were silent. The numbers of spontaneously firing CeA–RVLM neurons did not differ between WKY rats and SHR (χ2(1, 35)=0.1727, P = 0.67). The basal firing rate of these neurons was significantly higher in SHRs (1.6±0.2, n=12 in 4 rats) than that in WKY rats (0.6±0.1 Hz, n=9 in 4 rats, P = 0.0001, t(19) =4.857). Bath application of XE-991 (10 µM) significantly increased the firing rate from 0.6±0.1 to 1.2±0.2 Hz in WKY rats (P = 0.0001, F(2,16)=16.81, n=9 in 4 rats) and depolarized these neurons from −57.9±1.9 to −51.1±1.8 mV (P < 0.0001, F(2,16)=35.54, Figure 4). However, XE-991 (10 µM) did not significantly alter the firing rate of labelled CeA–RVLM neurons in SHRs (1.44±0.2 vs. 1.47±0.16 Hz, n=12 in 4 rats, P = 0.4065, F(2,22)=0.9377, Figure 4).
Figure 4.
Effect of Kv7 channel blocker on the firing activity of CeA–RVLM neurons in WKY rats and SHRs. (A and B) Frequency histogram (upper panel) and raw traces (lower panel) show the effect of XE-991 (10 µM) on the firing activity of labelled CeA–RVLM neurons in WKY rats (A) and SHRs (B). Please note that XE-991 increase firing activity of labelled CeA neuron in WKY rats but not in SHRs. (C and D) Summary data show that XE-991 induced an increase in firing activity (C) and a depolarization (D) in CeA–RVLM neurons (n=9) in 4 WKY rats but did not alter firing activity and membrane potentials in SHRs (n=12 neurons in 4 SHRs). *P < 0.05 compared with the baseline values in the same group; #P < 0.05 compared with the corresponding values in WKY rats; repeated-measures ANOVA with Dunnett’s post hoc test.
To determine the excitability of silent CeA–RVLM neurons, we compared the action potentials (APs) in response to a series of depolarizing currents injected into labelled CeA neurons in WKY rats and SHRs. Incremental depolarizing currents were applied onto labelled CeA neurons for a duration of 1 s (currents injected from 0 to + 60 pA, 5 s intervals). Stepwise current injection elicited firing activity in labelled CeA neurons in both WKY rats and SHRs (Figure 5). However, the number of APs elicited by current injection was significantly higher in SHRs (n=7 neurons from 4 rats) than in WKY rats (n=7 neurons from 4 rats). Also, the bath application of XE-991 (10 µM) increased the number of APs at each current injection level in WKY rats but not in SHRs (Figure 5). These data suggest that Kv7 channel activity in CeA–RVLM neurons is diminished in SHRs.
Figure 5.
Blocking Kv7 channels enhanced the response of CeA–RVLM neurons to depolarizing currents in WKY rats but not in SHRs. (A and B) Representative tracings showing that XE-991 increased the number of APs elicited by depolarizing currents (10–70 pA) in labelled CeA–RVLM neurons in WKY rats (A) but not in SHRs (B). (C and D) Summary data showing that XE-991 increased number of APs generated by injection of depolarizing currents in labelled CeA–RVLM neurons in WKY rats (C, n=7 neurons from 4 WKY rats) but did not alter the number of APs in SHRs (D, n=7 neurons from 4 SHRs). (E) Summary data showing that depolarizing currents elicited more APs of labelled CeA–RVLM neurons (n=7 neurons in each group) in 4 SHRs than in four WKY rats. *P < 0.05 compared to baseline values in (C) or values in WKY rats in (E) (repeated-measures ANOVA with Dunnett’s post hoc test).
3.5 Kv7 channel activity in the CeA was impaired in regulating ABP and sympathetic outflow in SHRs
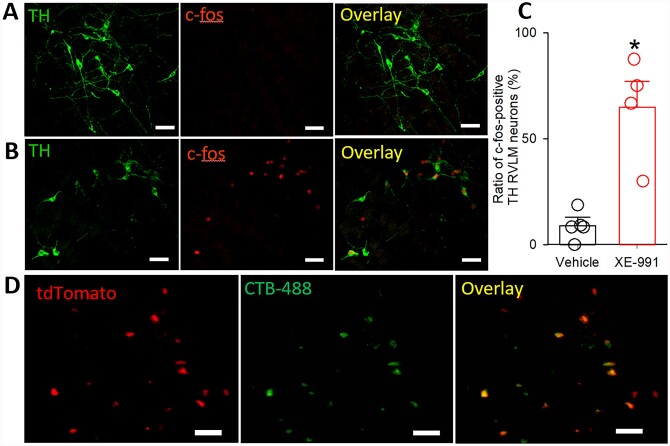
To determine if blocking Kv7 channels in the CeA activates neurons in the RVLM, we microinjected a specific Kv7 channel blocker XE-991 (10 µM)39 into the CeA and detected the c-fos expression in the RVLM. We found that blocking Kv7 channels in the CeA with XE-991 induced c-fos expression in TH-positive neurons in the RVLM. On the other hand, few RVLM TH-positive neurons displayed c-fos expression in rats with vehicle injected into the CeA (Figure 6A and B). The ratio of c-fos-positive TH neurons in the RVLM was much higher in XE-991-injected rats (64.8±12.4%, n=4) than in vehicle-injected rats (9.0±3.8%, n=5, t(7) =4.916, P=0.0017, Figure 6C).
Figure 6.
Blocking Kv7 channels in the CeA activated TH neurons in the RVLM. (A and B) Immunostaining images showing microinjection of vehicle (A) or XE-991 (B) into the CeA-induced c-fos expression in RVLM TH neurons. (C) Quantification of c-fos-positive TH neurons in the RVLM region from rats injected with vehicle or XE-991 into the CeA. (D) Images showing that CeA–RVLM neurons retrogradely labelled by CTB-Alexa Fluor™ 488 injected into the RVLM were colocalized with tdTomato-tagged VGluT2 in the CeA. *P < 0.05, compared with values in vehicle-injected rats (unpaired Student’s t-test).
To determine if CeA–-RVLM neurons are glutamatergic in nature, we injected CTB-Alexa Fluor™ 488, a retrograde tracer, into the RVLM of 3 VGluT2-Cre: tdTomatoflox/flox mice. The VGluT2-tdTomato (red)-positive neurons and CTB-Alexa Fluor™ 488 (green)-labelled neurons were scattered throughout the CeA region. All CTB-Alexa Fluor™ 488-labelled neurons were colocalized with VGluT2-tdTomato in the CeA (Figure 6D). These results are consistent with a previous report showing that RVLM-projecting CeA neurons are immunoreactive to glutamate.40
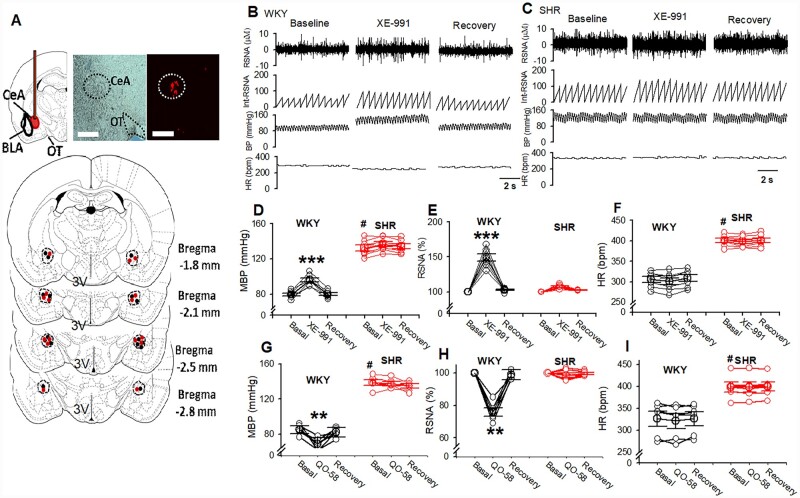
We then determined the role of Kv7 channels in the CeA in controlling sympathetic vasomotor tone outflow in WKY rats and SHRs. Bilateral microinjection of the specific Kv7 blocker XE-991 (6.7 nmol in 50 nL aCSF each side),39,41 into the CeA significantly increased mean ABP and RSNA in WKY rats (mean ABP: P < 0.0001, F(2,18)=25.1762; RSNA: P < 0.0001, F(2,18)=89.88; n=7, Figure 7). The RSNA and ABP started to increase at a 2.3±0.2 min after XE-991 injection, and the increase lasted for 26.8±3.9 min. However, the HR was not significantly altered by XE-991 microinjection (P = 0.7720, F(2,18)=0.2625, n=7). In contrast, in SHRs, microinjection of the same doses of XE-991 into the CeA failed to alter mean ABP, RSNA, or HR (mean ABP: P = 0.63, F(2,18)=0.4787; RSNA: P = 0.1901, F(2,18)=1.823, n=7, Figure 7B–F). In another group of rats, we tested the effect of microinjection of Kv7 opener QO-58 on mean ABP, RSNA, and HR in WKY and SHRs. Bilateral microinjection of Kv7 selective opener QO-58 (5 nmol in 100 nL aCSF each side) into the CeA significantly decreased mean ABP and RSNA in WKY rats (mean ABP: P<0.001, F(2,15)=18.79; RSNA: P<0.0001, F(2,15)=88.73; n=6, Figure 7). In SHRs, microinjection of the same doses of QO-58 into the CeA did not alter mean ABP, RSNA, or HR (mean ABP: P=0.1839, F(2,15)=2.14; RSNA: P=0.3727, F(2,15)=1.005; HR: P=0.2063, F(2,15)=1.867, Figure 7G–I). These data suggested that Kv7 channels in the CeA are involved in controlling sympathetic vasomotor tone, but its activity is diminished in SHRs.
Figure 7.
Blocking Kv7 channels in the CeA increased ABP and RSNA in WKY rats but not in SHRs. (A) Representative images (upper panel) and schematic drawings (lower panel) show the microinjection sites for XE-991 in the CeA in WKY rats (●) and SHRs (●). (B and C) Raw recording traces show the effect of microinjection of XE-991 into the CeA on ABP, RSNA, and HR in WKY rats (B) and SHRs (C). (D–F) Summary data show changes in mean ABP (D), RSNA (E), and HR (F) in response to microinjection of XE-991 into the CeA in WKY rats (n=8) and SHRs (n=8). (G–I) Summary data show changes in mean ABP (G), RSNA (H), and HR (I) in response to microinjection of QO-58 into the CeA in WKY rats (n=6) and SHRs (n=6). Data are presented as the mean±SEM. *P < 0.05, **P < 0.01, ***P < 0.0001, compared with the respective baseline values. #P < 0.05, compared with basal values in WKY rats (repeated-measures ANOVA with Dunnett's post hoc test). 3 V, third ventricle; CeA, central amygdala; BLA, basolateral amygdala; OT, optic track.
4. Discussion
We showed in this study that Kv7.2 and Kv7.3 protein and mRNA expression levels in the CeA were profoundly decreased in SHRs compared with WKY rats. Neuronal M-currents are mainly carried by heterotetrameric Kv7.2 and Kv7.3 subunits.28,29,42–44 The homomeric Kv7.2 subunit alone can generate M-current that is 5–10 times smaller than the current generated by heteromeric Kv7.2/Kv7.3 channels.45 It is not clear how the Kv7 expression levels in the CeA is down-regulated in SHRs. Kv7.2 mRNA levels are regulated by the lysine dimethyltransferases G9a-mediated epigenetic modification.46 Furthermore, miRNA153 reduces Kv7.4 mRNA levels in various arteries in SHRs.47 The exact epigenetic mechanism responsible for Kv7 down-regulation in the CeA in SHRs warrants further investigation.
We found that Kv7.2 and Kv7.3 expression levels were decreased in established hypertension in adult SHRs compared with pre-hypertensive SHRs. These data indicate that a reduction of Kv7 channel expression in the CeA likely contributes to the maintenance of high blood pressure and elevated sympathetic outflow in SHRs. In addition to the CeA, we found that Kv7 protein levels in the PVN and the HP tissues were significantly lower in SHRs than in WKY rats. The PVN is critically involved in the elevated sympathetic outflow in hypertension animal models, such as SHRs.4,35,48 A decrease in Kv7 expression in the PVN induced by acute stress causes hyperactivity of corticotropin-release factor neurons in the PVN.49 Thus, it is possible that the decrease in Kv7 expression levels in the PVN contributes to hyperactivity of pre-sympathetic PVN neurons and elevated sympathetic outflow in SHRs.
To determine if the decrease in expression levels of Kv7.2/Kv7.3 in the CeA is a secondary response to high blood pressure in SHRs, we measured Kv7.2/Kv7.3 protein levels after lowing blood pressure by CGx in SHRs. Because sympathetic nerve innervating the splanchnic vascular bed has particularly high outflow in SHRs,50 CGx markedly reduces ABP in SHRs.35,36 We found that although CGx significantly lowered ABP in SHRs, it did not alter Kv7.2/Kv7.3 protein levels in the CeA. These data suggest that the reduction in Kv7.2/Kv7.3 protein levels might not be a secondary response to high blood pressure in SHRs. However, CGx did not lower ABP in SHRs to normotensive level. Thus, we cannot rule out the possibility that reduced expression of Kv7 channels might be a consequence rather than a cause of high blood pressure in SHRs. The CeA is a heterogeneous nucleus containing several neuronal types including CeA–RVLM output neurons, and the changes of Kv7.2/Kv7.3 protein levels in CeA tissue may not represent the changes of Kv7.2/Kv7.3 protein levels in CeA–RVLM neurons. We showed that Kv7.3 immunoreactivities were present on retrogradely labelled CeA–RVLM neurons. Together with data showing that Kv7 channel activity was blunted in retrogradely labelled CeA–RVLM neurons, these findings suggest that Kv7 expression levels are reduced in CeA–RVLM neurons in SHRs. Because retigabine, a widely used M-channel opener, also potentiates GABAA receptor-dependent inhibitory postsynaptic currents in cultured cortical neurons,51 we used QO-58, a highly selective M-channel opener.38 Both basal and total M-currents were significantly decreased in CeA–RVLM neurons in SHRs compared with WKY rats. The diminished M-currents in CeA–RVLM neuron is likely due to a reduction of Kv7.2/Kv7.3 expression levels in the CeA in SHRs. M-currents are critically involved in the regulation of neuronal activity because inhibition of M-currents leads to a depolarization of the neuron membrane and an increase in neuronal excitability.25,26 Thus, blocking Kv7 channels with XE-991 increased the firing activity of labelled CeA–RVLM neurons in WKY rats. However, XE-991 did not alter the firing activity of CeA–RVLM neurons in SHRs, suggesting that Kv7 channel activity is diminished in hypertension. We also found that the basal firing activity of labelled CeA–RVLM neurons was significantly higher in SHRs than in WKY rats. This high basal firing activity of CeA–RVLM neurons in SHRs may result from the impaired Kv7 channel activity.
Consistent with a previous report showing that CeA–RVLM neurons are immunoreactive to glutamate,40 we found that retrogradely labelled CeA–RVLM neuron expressed VGluT2, indicating that CeA–RVLM neurons are glutamatergic. In addition, we found that blocking the Kv7 channels with XE-991 in the CeA induced c-fos expression in tyrosine hydroxylate-positive neurons in the RVLM, which are critically involved in regulating blood pressure and sympathetic outflow.52 We showed that blocking Kv7 channels in the CeA increased RSNA and ABP in WKY rats but not in SHRs. On the other hand, microinjection of QO-58 significantly decreased blood pressure and RSNA in WKY rats but not in SHRs. These data further suggest that Kv7 channel activity in the CeA was impaired in the control of sympathetic outflow in hypertension. Blocking Kv7 channels by XE-991 in the CeA in WKY rats has profound effects on the spontaneous firing rate, membrane potential, and input/output curves of CeA–RVLM neurons. However, bilateral microinjection of XE-991 into the CeA increased blood pressure from 80 to 95 mmHg in WKY rats, a value that is significantly lower than that in SHRs (>130–140 mmHg). This discrepancy between CeA–RVLM neuronal activity in the in vitro preparation and blood pressure measured in vivo is likely due to blood pressure regulatory mechanisms, such as baroreflex, which can buffer the increases in blood pressure induced by XE-991.
In addition to the central nervous system, Kv7 channels are expressed in blood vessels and play important role in regulating blood pressure during disease conditions.53 It has been shown that Kv7 opener retigabine induced dose-dependent hypotension and hindquarters vasodilatation in normotensive condition and acutely hypertensive state.54 Also, Kv7.4 expression is reduced in various arteries in SHRs, which is mediated by regulatory RNA (microRNA)55 and contributes to the hypertensive phenotype.47 Kv7 opener may be less effective to produce a depressor effect in chronic hypertension.
In summary, our study reveals a novel mechanism that diminished Kv7 channel activity in the CeA is involved in elevated sympathetic outflow in hypertension. Since blocking Kv7 channels in the CeA increases sympathetic outflow and blood pressure, it is possible that overexpression of Kv7 channels in the CeA through viral vector-mediated transfection suppresses CeA–RVLM neuronal activity and subsequently decreases sympathetic outflow and blood pressure in SHRs. However, this overexpression of Kv7 channels may non-selectively suppresses inhibitory GABAergic neuronal activity in the CeA to offset the decreases in sympathetic outflow and blood pressure. This information offers new mechanistic insight into the pathogenesis of primary hypertension and provides a rationale for developing therapeutic strategies targeting Kv7 channels in the CeA to treat neurogenic hypertension.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Author’s contributions
Z.-F.S. and D.-P.L. designed the study. Z.-F.S., H.Z., P.Z., S.C., Z.G., J.-J.Z., J.G.P., and D.-P.L. performed the experiments and analysed the data. Z.-F.S., E.T.H.Y., and D.-P.L. wrote the manuscript. H.-L.P., E.T.H.Y., and H.-M.C. revised and commented on the manuscript.
Conflict of interest: The authors declare no competing financial interests.
Funding
This study was supported by National Heart, Lung, and Blood Institute grants R01 HL142133 and R01 HL139523.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
Translational perspective
This study reveals a novel mechanistic insight into the pathogenesis of primary hypertension and provides a rationale to develop therapeutic strategies targeting Kv7 channels in the amygdala for treating hypertension. Viral vector-mediated overexpression of Kv7 channels in the amygdala is a potential approach to decrease blood pressure and inhibit sympathetic outflow in hypertension. However, this approach is not feasible to apply to hypertension patients at the current stage. Further studies to determine the molecular mechanism underlying down-regulation of Kv7 channels may identify agents that increase expression levels of Kv7 channels in hypertension.
References
- 1. Johnson AK, Xue B.. Central nervous system neuroplasticity and the sensitization of hypertension. Nat Rev Nephrol 2018;14:750–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DiBona GF. Sympathetic nervous system and hypertension. Hypertension 2013;61:556–560. [DOI] [PubMed] [Google Scholar]
- 3. Grassi G, Mark A, Esler M.. The sympathetic nervous system alterations in human hypertension. Circ Res 2015;116:976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li DP, Pan HL.. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 2007;49:916–925. [DOI] [PubMed] [Google Scholar]
- 5. Mancia G, Grassi G.. The autonomic nervous system and hypertension. Circ Res 2014;114:1804–1814. [DOI] [PubMed] [Google Scholar]
- 6. Judy WV, Watanabe AM, Henry DP, Besch HR Jr, Murphy WR, Hockel GM.. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res 1976;38:21–29. [DOI] [PubMed] [Google Scholar]
- 7. Gilbey MP, Spyer KM.. Essential organization of the sympathetic nervous system. Baillieres Clin Endocrinol Metab 1993;7:259–278. [DOI] [PubMed] [Google Scholar]
- 8. Oshima N, McMullan S, Goodchild AK, Pilowsky PM.. A monosynaptic connection between baroinhibited neurons in the RVLM and IML in Sprague-Dawley rats. Brain Res 2006;1089:153–161. [DOI] [PubMed] [Google Scholar]
- 9. Pyner S, Coote JH.. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 2000;100:549–556. [DOI] [PubMed] [Google Scholar]
- 10. Ranson RN, Motawei K, Pyner S, Coote JH.. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res 1998;120:164–172. [DOI] [PubMed] [Google Scholar]
- 11. Bowman BR, Kumar NN, Hassan SF, McMullan S, Goodchild AK.. Brain sources of inhibitory input to the rat rostral ventrolateral medulla. J Comp Neurol 2013;521:213–232. [DOI] [PubMed] [Google Scholar]
- 12. Chapp AD, Gui L, Huber MJ, Liu J, Larson RA, Zhu J, Carter JR, Chen QH.. Sympathoexcitation and pressor responses induced by ethanol in the central nucleus of amygdala involves activation of NMDA receptors in rats. Am J Physiol Heart Circ Physiol 2014;307:H701–H709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saha S, Drinkhill MJ, Moore JP, Batten TF.. Central nucleus of amygdala projections to rostral ventrolateral medulla neurones activated by decreased blood pressure. Eur J Neurosci 2005;21:1921–1930. [DOI] [PubMed] [Google Scholar]
- 14. Cassell MD, Gray TS.. The amygdala directly innervates adrenergic (C1) neurons in the ventrolateral medulla in the rat. Neurosci Lett 1989;97:163–168. [DOI] [PubMed] [Google Scholar]
- 15. Baklavadzhyan OG, Pogosyan NL, Arshakyan AV, Darbinyan AG, Khachatryan AV, Nikogosyan TG.. Studies of the role of the central nucleus of the amygdala in controlling cardiovascular functions. Neurosci Behav Physiol 2000;30:231–236. [DOI] [PubMed] [Google Scholar]
- 16. Galeno TM, Van Hoesen GW, Maixner W, Johnson AK, Brody MJ.. Contribution of the amygdala to the development of spontaneous hypertension. Brain Res 1982;246:1–6. [DOI] [PubMed] [Google Scholar]
- 17. Folkow B, Hallback-Nordlander M, Martner J, Nordborg C.. Influence of amygdala lesions on cardiovascular responses to alerting stimuli, on behaviour and on blood pressure development in spontaneously hypertensive rats. Acta Physiol Scand 1982;116:133–139. [DOI] [PubMed] [Google Scholar]
- 18. Sharma NB, Gelsema AJ.. Central nucleus of the amygdala and the development of hypertension in spontaneously hypertensive rats. Am J Physiol 1995;268:R1171–R1177. [DOI] [PubMed] [Google Scholar]
- 19. Gelsema AJ, Agarwal SK, Calaresu FR.. Cardiovascular responses and changes in neural activity in the rostral ventrolateral medulla elicited by electrical stimulation of the amygdala of the rat. J Auton Nerv Syst 1989;27:91–100. [DOI] [PubMed] [Google Scholar]
- 20. Petrov T, Krukoff TL, Jhamandas JH.. Convergent influence of the central nucleus of the amygdala and the paraventricular hypothalamic nucleus upon brainstem autonomic neurons as revealed by c-fos expression and anatomical tracing. J Neurosci Res 1995;42:835–845. [DOI] [PubMed] [Google Scholar]
- 21. Salome N, Viltart O, Leman S, Sequeira H.. Activation of ventrolateral medullary neurons projecting to spinal autonomic areas after chemical stimulation of the central nucleus of amygdala: a neuroanatomical study in the rat. Brain Res 2001;890:287–295. [DOI] [PubMed] [Google Scholar]
- 22. Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 2005;32:450–456. [DOI] [PubMed] [Google Scholar]
- 23. Marrion NV. Control of M-current. Annu Rev Physiol 1997;59:483–504. [DOI] [PubMed] [Google Scholar]
- 24. Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D.. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci 2005;8:51–60. [DOI] [PubMed] [Google Scholar]
- 25. Brown DA, Adams PR.. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 1980;283:673–676. [DOI] [PubMed] [Google Scholar]
- 26. Delmas P, Brown DA.. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 2005;6:850–862. [DOI] [PubMed] [Google Scholar]
- 27. Brown BS, Yu SP.. Modulation and genetic identification of the M channel. Prog Biophys Mol Biol 2000;73:135–166. [DOI] [PubMed] [Google Scholar]
- 28. Shah M, Mistry M, Marsh SJ, Brown DA, Delmas P.. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol 2002;544:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D.. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 1998;282:1890–1893. [DOI] [PubMed] [Google Scholar]
- 30. Cavaliere S, Malik BR, Hodge JJ.. KCNQ channels regulate age-related memory impairment. PLoS One 2013;8:e62445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qi Y, Wang J, Bomben VC, Li DP, Chen SR, Sun H, Xi Y, Reed JG, Cheng J, Pan HL, Noebels JL, Yeh ET.. Hyper-SUMOylation of the Kv7 potassium channel diminishes the M-current leading to seizures and sudden death. Neuron 2014;83:1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA.. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 2003;23:7227–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Jakubowski M, Buettner C, Kainz V, Gold M, Burstein R.. Ezogabine (KCNQ2/3 channel opener) prevents delayed activation of meningeal nociceptors if given before but not after the occurrence of cortical spreading depression. Epilepsy Behav 2013;28:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berg T. M-currents (Kv7.2-7.3/KCNQ2-KCNQ3) are responsible for dysfunctional autonomic control in hypertensive rats. Front Physiol 2016;7:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li DP, Zhou JJ, Zhang J, Pan HL.. CaMKII regulates synaptic NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Neurosci 2017;37:10690–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ye ZY, Li DP, Li L, Pan HL.. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci 2011;31:8271–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Filippov AK, Brown DA.. A mechanism for nerve cell excitation by norepinephrine via alpha-1 adrenoceptors: inhibition of potassium M-current. Cell Mol Neurobiol 2013;33:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang F, Mi Y, Qi JL, Li JW, Si M, Guan BC, Du XN, An HL, Zhang HL.. Modulation of K(v)7 potassium channels by a novel opener pyrazolo[1,5-a]pyrimidin-7(4H)-one compound QO-58. Br J Pharmacol 2013;168:1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romero M, Reboreda A, Sanchez E, Lamas JA.. Newly developed blockers of the M-current do not reduce spike frequency adaptation in cultured mouse sympathetic neurons. Eur J Neurosci 2004;19:2693–2702. [DOI] [PubMed] [Google Scholar]
- 40. Takayama K, Miura M.. Glutamate-immunoreactive neurons of the central amygdaloid nucleus projecting to the subretrofacial nucleus of SHR and WKY rats: a double-labeling study. Neurosci Lett 1991;134:62–66. [DOI] [PubMed] [Google Scholar]
- 41. Bian G, Liu J, Guo Y, Yang Y, Li L, Qiao H, Li W, Xu T, Zhang Q.. Kv7.2 subunit-containing M-type potassium channels in the lateral habenula are involved in the regulation of working memory in parkinsonian rats. Neuropharmacology 2020;168:108012. [DOI] [PubMed] [Google Scholar]
- 42. Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K.. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem 2000;275:22395–22400. [DOI] [PubMed] [Google Scholar]
- 43. Roche JP, Westenbroek R, Sorom AJ, Hille B, Mackie K, Shapiro MS.. Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels. Br J Pharmacol 2002;137:1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ.. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem 2000;275:24089–24095. [DOI] [PubMed] [Google Scholar]
- 45. Etxeberria A, Santana-Castro I, Regalado MP, Aivar P, Villarroel A.. Three mechanisms underlie KCNQ2/3 heteromeric potassium M-channel potentiation. J Neurosci 2004;24:9146–9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa JP, Pan HL.. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015;18:1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carr G, Barrese V, Stott JB, Povstyan OV, Jepps TA, Figueiredo HB, Zheng D, Jamshidi Y, Greenwood IA.. MicroRNA-153 targeting of KCNQ4 contributes to vascular dysfunction in hypertension. Cardiovasc Res 2016;112:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li DP, Zhou JJ, Pan HL.. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol 2015;593:4439–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou JJ, Gao Y, Kosten TA, Zhao Z, Li DP.. Acute stress diminishes M-current contributing to elevated activity of hypothalamic-pituitary-adrenal axis. Neuropharmacology 2017;114:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morrison SF, Whitehorn D.. Enhanced preganglionic sympathetic nerve responses in spontaneously hypertensive rats. Brain Res 1984;296:152–155. [DOI] [PubMed] [Google Scholar]
- 51. Otto JF, Kimball MM, Wilcox KS.. Effects of the anticonvulsant retigabine on cultured cortical neurons: changes in electroresponsive properties and synaptic transmission. Mol Pharmacol 2002;61:921–927. [DOI] [PubMed] [Google Scholar]
- 52. Schreihofer AM, Stornetta RL, Guyenet PG.. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol 2000;529: 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barrese V, Stott JB, Greenwood IA.. KCNQ-encoded potassium channels as therapeutic targets. Annu Rev Pharmacol Toxicol 2018;58:625–648. [DOI] [PubMed] [Google Scholar]
- 54. Fretwell LV, Woolard J.. Cardiovascular responses to retigabine in conscious rats–under normotensive and hypertensive conditions. Br J Pharmacol 2013;169:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA.. Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 2011;124:602–611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.