Abstract
Atrial fibrillation (AF) is an important clinical problem. Chronic pressure/volume overload of the atria promotes AF, particularly via enhanced extracellular matrix (ECM) accumulation manifested as tissue fibrosis. Loading of cardiac cells causes cell stretch that is generally considered to promote fibrosis by directly activating fibroblasts, the key cell type responsible for ECM production. The primary purpose of this article is to review the evidence regarding direct effects of stretch on cardiac fibroblasts, specifically: (i) the similarities and differences among studies in observed effects of stretch on cardiac fibroblast function; (ii) the signalling pathways implicated; and (iii) the factors that affect stretch-related phenotypes. Our review summarizes the most important findings and limitations in this area and gives an overview of clinical data and animal models related to cardiac stretch, with particular emphasis on the atria. We suggest that the evidence regarding direct fibroblast activation by stretch is weak and inconsistent, in part because of variability among studies in key experimental conditions that govern the results. Further work is needed to clarify whether, in fact, stretch induces direct activation of cardiac fibroblasts and if so, to elucidate the determining factors to ensure reproducible results. If mechanical load on fibroblasts proves not to be clearly profibrotic by direct actions, other mechanisms like paracrine influences, the effects of systemic mediators and/or the direct consequences of myocardial injury or death, might account for the link between cardiac stretch and fibrosis. Clarity in this area is needed to improve our understanding of AF pathophysiology and assist in therapeutic development.
Keywords: Pressure overload, Stretch, Mechanical strain, Atrial fibrillation, Fibrosis, Cardiac fibroblast
Graphical Abstract
1. Introduction
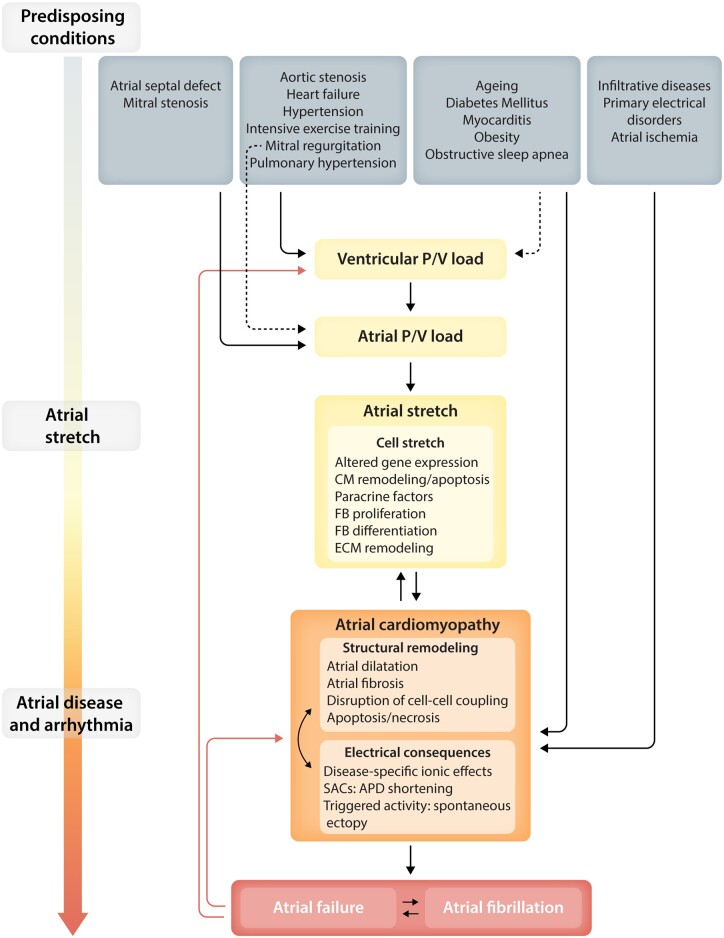
Mechanical stretch of the heart, typically associated with pressure and/or volume overload, is believed to be an important contributor to the development of cardiac fibrosis. Atrial fibrosis promotes the occurrence of atrial fibrillation (AF), the most common cardiac arrhythmia, and its associated risk of stroke, heart failure, and mortality.1 The lifetime risk of AF is estimated to be 22–26%, meaning that 1 in 4 people will be affected over the course of their lives.1 AF is the single most important risk factor for stroke in the elderly and is believed to be a potentially important contributor to cognitive decline and dementia.1 Increased atrial pressure alters atrial electrophysiology and is associated with atrial fibrosis, thereby favouring AF occurrence.2–5 Moreover, AF per se impairs atrial mechanical function, establishing a positive feedback loop that exacerbates the situation (Figure 1).6,7 Animal models that recapitulate cardiac pressure overload show atrial remodelling that is characterized by conduction slowing, cellular calcium overload, fibrosis, fibroblast proliferation, and alterations in collagen degradation.8 How mechanical stimuli are transduced at a cellular level and trigger fibrosis is still a matter of active investigation. Stretch and hemodynamic load modulate the function of many mechanosensitive ion channels and transmembrane proteins that, in tight interplay with the extracellular matrix (ECM), activate a range of signalling pathways to modify cellular function.9–11
Figure 1.
Schematic of processes believed to be involved in AF-promoting responses to stretch. Conditions leading to atrial stretch and its consequences are shown at the top. These lead to atrial stretch, either directly via altered atrial load (primary or secondary to ventricular overload) or indirectly by affecting atrial function and causing atrial cardiomyopathy. Atrial stretch in itself causes cellular consequences that lead to atrial cardiomyopathy. Atrial cardiomyopathy leads to AF and can impair atrial function sufficiently to lead to atrial failure. CM, cardiomyocyte; ECM, extracellular matrix; FB, fibroblast; P/V, pressure/volume; SAC, stretch-activated channel.
Cardiac fibroblasts are widely distributed and are the primary cellular controllers of ECM homeostasis. Sustained hemodynamic loading is believed to cause fibroblasts to proliferate, migrate, and differentiate into myofibroblasts, which abundantly secrete ECM proteins. This process causes fibrosis that prompts the progression of many cardiac disorders by hampering myocardial excitation–contraction coupling and by disturbing impulse propagation and ECM-dependent signalling pathways.12 The general molecular mechanisms and signalling pathways involved in fibroblast dysregulation and cellular mechanotransduction have been described extensively elsewhere.13 Mechanical forces can promote fibroblast activation via a number of processes, including changes in the neurohumoral environment, the release of paracrine factors from cardiomyocytes, interactions with leucocytes and cytokines, and direct effects of mechanical stretch on fibroblasts. A direct profibrotic effect of fibroblast stretch is often invoked in the literature and has been studied extensively. Here, we will briefly review in vivo evidence from patients and experimental models and then focus on the results of studies examining the direct response of cardiac fibroblasts to stretch. The primary issues that we aim to address are as follows: (i) how cardiac fibroblasts respond to stretch, (ii) whether the profibrotic effect of hemodynamic load in vivo can be attributed to direct fibroblast activation, and if so, (iii) what molecular pathways are responsible for such activation.
2. Clinical evidence regarding electrophysiological consequences of atrial stretch
Atrial overload is manifested as increased atrial pressure and/or volume. Although acute stretch caused by acute atrial overload might have substantial short-term consequences, the clinical occurrence is quite limited. Acute mitral regurgitation due to tear or rupture of part of the mitral valve apparatus results in immediate increases in left atrial (LA) pressure and volume. Substantial valve disruption (e.g. due to papillary muscle rupture) is usually rapidly fatal. Less severe dysfunction (e.g. due to a ruptured corda tendinea) is often compensated by adaptive responses, which are followed by changes typical of chronic stretch. Extensive acute myocardial infarction or myocarditis can result in rapid increases in atrial stretch, but the severe associated ventricular dysfunction usually overshadows the atrial changes for which the main clinical manifestation is usually AF.
Chronic atrial overload inducing sustained increases in atrial stretch is much more frequent, and therefore relevant, in clinical practice. Chronic atrial selective stretch is present in conditions generating primarily atrial overload, such as mitral stenosis, the classical paradigm of atrial pressure overload,14 or congenital heart defects causing atrial volume overload like atrial septal defects.15 More commonly, chronic atrial stretch develops in response to primary ventricular overload, either pressure- or volume-related, in which, despite initial adaptive responses at the ventricular level, the haemodynamic load is eventually transmitted retrogradely to the atria. Typical settings include hypertension, heart failure, and valve disease other than mitral stenosis (Figure 1). Patients with hypertension often show features of diastolic dysfunction including increased LA pressure and atrial dilation,16 a common surrogate of atrial stretch, and inadequate blood pressure control is associated with further atrial dilation.17 Atrial overload is particularly important in heart failure, both with reduced and preserved ejection fraction, and in valvular heart diseases such as mitral regurgitation and aortic stenosis, where LA enlargement is common and portends a greater risk of ventricular decompensation and clinical morbidity/mortality.18–21 Other clinical conditions like intensive exercise training,22 obstructive sleep apnea,23 and pulmonary hypertension24 have also been associated with atrial dilation.
The clinical consequences of atrial stretch are very important. As shown in Figure 1, a variety of common clinical conditions lead to atrial stretch, either directly via altered atrial load (primary or secondary to ventricular overload) or indirectly by affecting atrial function and causing atrial cardiomyopathy. Atrial stretch itself produces remodelling that causes structural and electrical changes in the atria that, with time, lead to the development of atrial cardiomyopathy.25 Atrial cardiomyopathy produces two principal clinically relevant manifestations, AF and the newly recognized entity of ‘atrial failure’.26,27 Atrial failure is characterized by cardiac dysfunction due to primary abnormalities in atrial function, in the absence of significant ventricular or valvular dysfunction.27 AF and atrial failure are intrinsically related to each other (Figure 1), and both conditions feed back to each other and to atrial cardiomyopathy, directly and by inducing further ventricular overload. Atrial failure and AF have significant prognostic consequences, since both have potential deleterious effects on ventricular function and may facilitate the progression to heart failure, and both are believed to be associated with blood stasis and endothelial dysfunction, predisposing to thrombus formation and thromboembolic events.26,27
Clinical observations on the atrial remodelling consequences of atrial stretch are limited. Atrial pressure increases induced by ventricular pacing or initiation of AV node re-entrant tachycardia are associated with acute decreases in atrial effective refractory period (ERP) and monophasic action potential duration.28 Acute atrial loading with atrial pacing causes exaggerated atrial responses among AF patients compared to sinus rhythm (SR) controls in terms of increased LA wall tension and decreased ERP, despite comparable increases in LA pressure.29 In contrast with the direct effects of acute atrial stretch, patients with chronic atrial volume loading due to atrial septal defects show increased low-atrial ERP, P-wave duration, and conduction delay across the crista terminalis.15 Inherited atrial cardiomyopathy due to mutation in the atrial natriuretic peptide gene causes extensive atrial fibrosis in association with atrial dilation and contractility impairment.30
3. Experimental observations relating to cardiac stretch in animal models
Common findings in pressure-overloaded hearts include the induction of the immediate early genes (IEG), c-Myc, c-Fos, and Fra-1 as the earliest response.31 This is followed by increases in α-smooth muscle actin (αSMA) in the myocardium and fibroblasts from pressure-overloaded hearts,32–34 although some studies found that the changes are time variant, first increasing and then declining slowly after several days.35 Fibroblast activation and increased transforming growth factor-β (TGFβ) expression also occur in pressure-overloaded rat hearts,36–38 followed by increased deposition of collagen and fibronectin, and reduced collagen degradation.38–40 A study of systemic hypertension also showed early collagen remodelling, with collagen type3/type1 ratio increasing during the progression of systemic hypertension (4 weeks) while returning to basal levels thereafter (35–88 weeks).41 However, another study reported unchanged collagen expression 1 week after the establishment of the model and decreased collagen type3/type1 ratios after 8 weeks because of an increase in the synthesis of collagen I.42 Lipoma-preferred partner (LPP), a nucleo-cytoplasmic shuttling adaptor protein that is a mechanosensitive protein highly expressed in cardiac fibroblasts, is up-regulated in hearts from pressure-overloaded rats but unchanged in hearts from myocardial infarction rats.43 The expression of chymase, an enzyme capable of converting angiotensin I to II as well as affecting a variety of ECM-localized enzyme systems, is increased in cardiac fibroblasts isolated from a pure volume-overload rat model.44
Enhanced collagen degradation and increased MMP levels are seen in patients with congestive heart failure (CHF).45 Similarly, in the LA and left ventricle (LV) of dogs with tachypacing-induced CHF, apoptosis and white-cell infiltration are transiently increased after the imposition of tachypacing, mitogen-activated protein kinases (MAPKs) are activated, and active TGFβ1 monomer and angiotensin II expression are increased compared to control dogs. In the same study, LA fibrous-tissue content was increased 20-fold with CHF.46 Burstein et al. also reported much greater profibrotic responses in the LA vs. LV of CHF dogs, including increased fibroblast proliferation and activation, increased ECM gene expression, and induction of platelet-derived growth factor (PDGF) and its receptor.47 A significant interaction between chamber (atrium vs. ventricle) and condition (CHF vs. control) indicates that these responses are chamber specific.47 Dawson et al. also reported that atrial tissues and freshly isolated atrial fibroblasts from CHF dogs displayed significantly greater mRNA levels of ECM genes.48 Atrial fibrosis is also produced in a pig model of mitral regurgitation and promotes AF development.49 On the other hand, in a goat model of chronic atrial volume loading due to an aortic LA shunt, while AF susceptibility was increased, neither LA ERP nor collagen content was changed.50 Work in an LV-LA shunt dog model also showed AF promotion, but with slightly decreased LA ERP, increased conduction heterogeneity, and fibrosis localized to the inferior pulmonary vein region.51
Thus, both clinical and experimental data indicate that cardiac fibrosis occurs in pressure/volume-overloaded contexts, along with characteristic molecular changes, and that the atria are particularly susceptible to fibrosis development.
4. Fibroblasts and the ECM
Cardiac fibroblasts control the ECM framework that creates the functional scaffold for the myocardium.52–54 Far from a passive skeleton, the ECM in turn acts as a reservoir for multiple growth factors, chemokines, enzymes, and matricellular proteins that regulate fibroblast phenotype and function.55–57 Cardiac fibrosis appears as a result of a series of phenotypic changes that include fibroblast proliferation, differentiation, and ECM remodelling. Fibrosis affects cardiac structure and electrical function in complex ways. In addition to direct disturbance of conduction pathways by interrupting muscle bundles,58 cardiomyocyte electrical activity can be affected by electrical coupling between fibroblasts and cardiomyocytes,59,60 although the extent to which this occurs in vivo remains controversial. Upon cardiac injury, quiescent fibroblasts get activated by growth factors, cytokines, and other stimuli and differentiate into myofibroblasts.61 Myofibroblasts are normally eliminated by programmed cell death after completing wound healing or tissue remodelling.62 However, under conditions like prolonged cardiac injury, inflammation, or aging, myofibroblasts become resistant to apoptosis, accumulate in fibrotic regions, and lay down large amounts of ECM.63
In comparison with fibroblasts, myofibroblasts possess greater ability to synthesize collagen, deposit and degrade ECM, recruit inflammatory cells, and promote inflammatory cell infiltration.64–67 They also express larger quantities of αSMA that confers contractile capacity involved in wound retraction68–70 and extra domain-A (ED-A) fibronectin, the biologically active splice variant that plays a key role in activation.69,71 Myofibroblasts secrete collagen I and collagen III, which constitute 80% and 10% of the normal structural ECM, respectively.72 The metabolism of these proteins is determined by the balance between deposition of newly synthesized molecules, degradation of existing collagen by matrix metalloproteinases (MMPs), and inhibition of MMPs by the tissue inhibitors of metalloproteinases (TIMPs).72 Collagen strand assembly in mature fibres, which is controlled by the lysyl oxidase enzyme (LOX) family members that cross-link collagen fibrils, is also a determinant step.73,74 The ECM is a highly dynamic structure, with constant turnover of collagens and other ECM proteins.
At the cellular level, αSMA, vimentin, and other filaments form cellular stress fibres that in consort with the ECM, membrane receptors, integrins, focal adhesions, and other membrane proteins, participate in transducing external mechanical forces into subcellular structures, particularly the nucleus. In turn, changes in gene expression induce ECM remodelling and release of growth factors and other biologically active molecules that contribute to the maintenance of cardiac mechanotransduction.13,55,75,76 This system constitutes a feed-forward loop that can result in continuous myofibroblast activation and contribute to pathological remodelling of the heart. It is widely assumed that the occurrence of fibrosis in conditions inducing cardiac pressure/volume loading is due to direct fibroblast activation by stretch, as described for non-cardiac fibroblasts.77 In the following section, we review evidence from studies examining the response of isolated cardiac fibroblasts to stretch to evaluate (i) the nature of their response and (ii) the mechanisms involved.
5. Stretch studies in cultured cardiac fibroblasts
For over 30 years, there has been an increasing interest in studying how mechanical forces act on cardiac cells.78 It is difficult to investigate the effects of stretch per se in vivo, because most manipulations that alter stretch also affect many other functions, for example autonomic tone, neurohormone concentrations, cytokine release, etc. Therefore, the only way to study pure and isolated stretch effects on fibroblasts is to evaluate them with in vitro systems. Cell-stretching devices have been used extensively to generate in vitro mechanical strain in many isolated cell types, particularly fibroblasts, in order to determine the consequences of cellular mechanotransduction.79–85 One of the first in vitro stretch studies performed on cardiac cells was performed by Terracio et al., who applied cyclic uniaxial stretch to neonatal rat cardiac fibroblasts.78 They demonstrated that stretched fibroblasts elongate and orient perpendicular to the direction of stretch, with vimentin intermediate filaments reorganizing parallel to the long axis of the cells. This was the first study showing that mechanical stretch has a direct effect on cardiac fibroblasts. Since then, many other researchers have investigated cardiac fibroblast mechanotransduction by using similar stretch paradigms. Relatively few studies have specifically investigated atrial fibroblasts. The discussion below addresses findings for cardiac fibroblasts in general and considers the issue of the specific considerations that apply to atrial fibroblasts.
5.1 Stretch-induced phenotypic changes in cardiac fibroblasts
5.1.1 Proliferation, apoptosis, and morphology
There is no clear consensus on whether stretch increases, decreases, or has no real effect on fibroblast proliferation (Table 1). Some investigators reported a decrease in fibroblast proliferation under mechanical loading,86–90 while others have seen an increase.87,90,91 Moreover, many of those studies showed that proliferation of the stretched fibroblasts could be modified by changing other parameters in the experimental system, such as oxygen content, serum, or surface rigidity, suggesting that if stretch influences fibroblast proliferation, it is not in a straightforward manner and may be modulated by the specific conditions existing at the time of stretch.
Table 1.
Stretch-induced changes in fibroblast proliferation, apoptosis and morphology
| Response | Main findings | Cell type | Coating and plate | Stretch machine | Stretch pattern | Stretch duration | Serum | Reference |
|---|---|---|---|---|---|---|---|---|
| Increased proliferation | Small proliferation increase with stretch | NRVF | Collagen-coated silicone membrane | Homemade stretch device | 20% uniaxial | 24 h | Serum-free | Sadoshima et al., (1992)132 |
| Proliferation increased by stretch | NRVF | Collagen-coated silicone membrane plate | Flexcell∗ | 1-Hz, 30% | 24 h and 48 h | Serum-free | Cao and Gardner, (1995)91 | |
| Increased fibroblast proliferation with low stretch (2%) for 24 or 72 h. In high-stretch conditions (8%), increased only at 24 h | Human atrial fibroblast | Fibronectin | Homemade stretch device | 1-Hz, 2% or 8% uniaxial | 24 h and 72 h | NS | Ugolini et al., (2016)92 | |
| Increase in proliferation of stretched fibroblasts seeded on low stiffness substrate (3 kPa), and in fibroblasts cultured with medium from stretched cardiomyocytes | Adult mouse ventricular fibroblast | Collagen I-coated polyacrylamide gel on PDMS membrane | Homemade stretch device | Static, 3% or 6% uniaxial | 24 h and 48 h | 10%-FBS | Herum et al., (2017)90 | |
| Increased proliferation with low stretch+hypoxia (1%-O2), no stretch+hypoxia and low stretch+physoxia (6%-O2) | Human ventricular fibroblast | Fibronectin | Homemade stretch device | 1-Hz, 2% or 8% uniaxial | 24 h | NS | Ugolini et al., (2017)87 | |
| No change or decreased proliferation | Decreased proliferation after 12-h stretch in 10% FBS-containing medium. No change with longer stretch or 1% FBS | Foetal RCF | Elastin-coated plate | Flexercell∗ FX-2000 | 1.5-Hz, 20% equibiaxial | 8–88 h | 0%, 1%, or 10% FBS | Butt and Bishop, (1997)89 |
| Decreased proliferation after stretch. Fibroblasts stopped at G2/M; increased p21, decreased cyclin-B1 | Neonatal RCF | Collagen-coated silicone membrane | Modified from Lee et al., 1996131 | 20% sustained stretch | 1, 4, 12, 24 h | 10% FBS | Liao et al., (2004)88 | |
| Decreased proliferation by 12-h stretch regardless of ECM substrate. Decrease most marked on randomly organized collagen | Neonatal RCF | Fibronectin-, laminin-, collagen-coated and uncoated charged silicone elastic membrane | Modified from Yost et al., 2000101 | 0.08 Hz, 5% uniaxial | 12 h | 10% NBS + 5% FBS | Atance et al., (2004)86 | |
| Decreased proliferation with high strain (8% stretch) for 72 h | Human atrial fibroblast | Fibronectin | Homemade stretch device | 1 Hz, 2% or 8% uniaxial | 24 h and 72 h | NS | Ugolini et al., (2016)92 | |
| Decreased proliferation on high substrate stiffness (8 kPa) | Adult mouse ventricular fibroblast | Collagen I-coated polyacrylamide gel, polydimethylsiloxane membrane | Homemade stretch device | Static, 3% or 6% uniaxial | 24 h and 48 h | 10% FBS | Herum et al., (2017)90 | |
| No change with no-stretch+physoxia (6%-O2) or high-strain stretch (8%)+physoxia | Human ventricular fibroblast | Fibronectin | Homemade device | 1 Hz, 2% or 8% uniaxial | 24 h | NS | Ugolini et al., (2017)87 | |
| Apoptosis | No cell death or apoptosis | NRVF | Collagen I | Flexercell FX-2000 | 1 Hz, 20% equiaxial | 24, 48, 96 h | 2.5% FBS | Persoon-Rothert et al., (2002)98 |
| No apoptosis | Neonatal RCF | Rat tail collagen-coated silicone membrane | Modified from Lee et al., 1996131 | 20% sustained stretch | 1, 4, 12, 24 h | 10% FBS | Liao et al., (2004)88 | |
| Few apoptotic genes differentially expressed in stretched fibroblasts; >15% apoptotic genes increased in myocyte-enriched fractions | NRVF | Collagen I | Flexercell FX-2000 | 1 Hz, 20% equiaxial | 24 h | 2.5% FBS | Boerma et al., (2005)99 | |
| Morphology | Cells elongated, oriented, perpendicular to the direction of stretch | Neonatal RCF | Laminin-coated silicone elastomer membranes | Homemade stretch device | 10 cycles/min, 10% uniaxial stretch | 72 h | NS | Terracio et al., (1988)78 |
| Cells rotated to align more perpendicular to the direction of stretch | Neonatal RCF | Aligned collagen-coated silicone membrane | Homemade stretch device | 3%, 6%, 12% uniaxial; 0, 5, 10 cycles/min | 12 h | 5% FBS + 10% NBS | Yost et al., (2000)101 | |
| Cells surface area increased on all ECM substrates | Neonatal RCF | Fibronectin-, laminin-, collagen-coated; uncoated charged silicone elastic membrane | Modified from Yost et al., 2000101 | 0.08 Hz, 5% uniaxial | 1, 5, and 10 min | 10% NBS + 5% FBS | Atance et al., (2004)86 | |
| Cell margin ruffling, disorganization of long actin stress fibres, retraction of cortical cytoplasm. Effects that are immediate, persisted over time | Neonatal RCF | Laminin-coated silastic membrane | Modified from MacKenna et al., 200076 | 5% equibiaxial | 0, 1.25, 2.5, 5, 10, 20, and 40 min | 10% FBS + 5% NBS | Fuseler et al., (2007)100 |
ECM, extracellular matrix; FBS, foetal bovine serum; NBS, newborn bovine serum; NRVF, neonatal rat ventricular fibroblasts; NS, not stated; RCF, rat cardiac fibroblasts.
Please note that this company has used both Flexcell and Flexercell as names; we have retained the usage in each paper cited.
Ugolini et al. reported an increase in proliferation of fibroblasts stretched under hypoxic conditions and their data suggest that proliferation may be more dependent on oxygen levels than stretch.87 Moreover, in physiological conditions, described by the authors as 6% oxygen, stretch only increased proliferation under low strain levels.92 Herum et al. reported that stretch-induced proliferation depended on the stiffness of the substrate for cell seeding, and although the profibrotic markers collagen and fibronectin were up-regulated after stretch regardless of the substrate, they saw increased proliferation when fibroblasts were seeded on low-stiffness membranes, and decreased proliferation upon seeding on high-stiffness membranes.90 These authors observed paracrine signalling from stretched cardiomyocytes on fibroblast proliferation. Other studies have also suggested that paracrine factors are important in stretch-related cellular signalling,55,93,94 which reinforces the idea that a proliferative response of fibroblasts in stretched myocardium may be dependent on cardiomyocyte-released factors rather than, or in addition to, the direct effects of stretch on fibroblasts themselves. Studies on human samples have shown a reduction in fibroblast proliferation and migration (but an increase in differentiation) in cultured atrial fibroblasts isolated from AF patients vs. SR controls.95 In the scenario of atrial fibrosis, which is a common feature of AF patients,96 an increase in the atrial content of secretory myofibroblasts is expected and might explain the reduction in overall proliferation.
Results are much more consistent in terms of apoptosis. Although it is well established that cardiomyocytes enter apoptosis when stretched or when exposed to pressure overload,46,97 cardiac fibroblasts did not show significant signs of cell necrosis or apoptosis even after 96 h of high-strain stretch.98 Boerma et al. reported that after stretch, up to 15% of the cardiomyocyte apoptotic genes were differentially regulated compared to non-stretched controls, while fibroblasts had very few genes differentially expressed.99 These studies suggest that cultured cardiac fibroblasts are more resilient to stretch-induced apoptosis than cardiomyocytes.
Fibroblasts do change morphology and orientation when stretched, although these changes can also be modulated by ECM substrates.86 Fuseler et al. observed ruffling of cell edges, disorganization of actin fibres, and cell shortening in all directions when fibroblasts were subjected to static equibiaxial stretch with 5% elongation.100 In response to long-term uniaxial stretch, cardiac fibroblasts and vimentin filaments were elongated, polarized, and oriented perpendicular to the direction of stretch.78,101 These studies suggest that fibroblast shape is sensitive to stretch but that change in cytoskeleton, cell morphology, and cell polarity depend on time and direction of the applied stretch, as well as on culture conditions, particularly the matrix.
5.1.2 Short-term responses at a molecular level
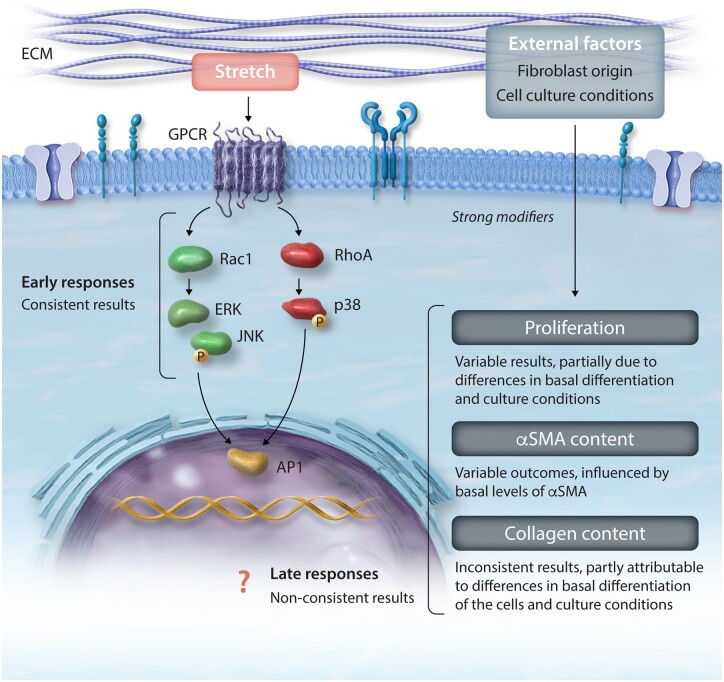
The initial responses of cultured fibroblasts to stretch are primarily the activation of the immediate early gene (IEG) pathways (Figure 2, Table 2). Activation of G proteins and kinases activates ERK2 and JNK1 and leads to the phosphorylation of the early response genes c-Jun, c-Fos, and Fra-1.102,103 These enhance the transcriptional activity of active dimer activator protein-1 (AP1)-dependent genes,102,103 which orchestrate longer-term responses.104 In contrast to the unclear effects of stretch on proliferation, there seems to be a consensus that the phosphorylation and nuclear translocation of IEG are the first responses in stretched fibroblasts. MacKenna et al. demonstrated a rapid, matrix-dependent activation of the ERK pathway and the JNK pathway in response to 4% static stretch, which could also be induced, although to a lesser extent, by stretch-conditioned medium.55 Rapid activation of JNK and p38 was confirmed by Papakrivopoulou et al., who showed phosphorylation after 10 min of cyclical stretch.105 Lal et al. demonstrated that the phosphorylation of JNK1 was fast and transient, appearing after 5 min of stretch, peaking at 15 min and disappearing immediately thereafter. In contrast, p38 also peaked at 15 min and returned to baseline but was phosphorylated again after 4 h of stretch and remained phosphorylated after 24 h.106 Atance et al. also reported significant MAPK activation 1 min after starting cyclic stretch,86 and G proteins were also activated in cells subjected to 1 min of equibiaxial stretch.106 Similar increases in c-Jun and Fra-1 gene expression were also observed when non-stretched fibroblasts were cultured with medium obtained from stretched fibroblasts or cardiomyocytes.93
Figure 2.
Schematic of biochemical and functional changes in response to stretch and modifying factors. αSMA, alpha-smooth muscle actin; AP1, activator protein 1; ECM, extracellular matrix; ERK, extracellular signal-regulated kinase; GPCR, G-protein-coupled receptor; JNK, c-Jun N-terminal kinase; p38, p38 mitogen-activated protein kinase; Rac1, Ras-related C3 botulinum toxin substrate 1; RhoA, Ras homolog family member A.
Table 2.
Immediate responses of signalling molecules in fibroblasts to stretch
| Response | Main findings | Cell type | Coating and plate | Stretch machine | Stretch pattern | Stretch duration | Serum | Reference |
|---|---|---|---|---|---|---|---|---|
| Immediate early gene activation | Quick and transient phosphorylation of c-fos | NRVF | Collagen-coated silicone membrane | Homemade stretch device | 20% uniaxial | 24 h | Serum-free | Sadoshima et al., (1992)132 |
| ERK2 and JNK1 activated by stretch in matrix-dependent manner. Stretch-conditioned medium also activated | ARVF | Collagen, laminin, vitronectin, and fibronectin | Modified from Lee et al., 1996131 | 4% static equibiaxial | 1, 5, 10 min | NS | MacKenna et al., (1998)55 | |
| G-proteins activated by 1 min of stretch, several GTP-binding response depending on stretch pattern | Adult male RCF | Collagen I-coated silicone elastic membrane | Modified from Lee et al., 1996131 | 5-s, 10-s, or 60-s stretch, 3% or 6% equibiaxial | 5–60 s | 10% FBS | Gudi et al., (1998)106 | |
| C-fos and fra-1 mRNA increased. C-Jun mRNA unchanged | NRVF | Collagen I-coated flexible plate | Flexercell | 15% biaxial static stretch | 0–60 min | Serum-free | Van Wamel et al., (2000)93 | |
| P38-phosphorylation increased | ARVF | Collagen I, plasma fibronectin, or fibronectin ED-A domain peptide-coated magnetite beads | Ceramic permanent magnet | Static force perpendicular to cell surface | 15 min to 8 h | Serum-free | Wang et al., (2000)112 | |
| ERK and p38 phosphorylation increased | NRVF | Collagen I-coated magnetite beads | Ceramic permanent magnet | Static force perpendicular to cell surface | 0.5, 1, 2, and 4 h | Serum-free | Wang et al., (2003)68 | |
| MAPK activated in time-dependent and coating-dependent manner; greater and faster on collagen and charged membrane | Neonatal RCF | Fibronectin-, laminin-, collagen-coated; uncoated charged silicone elastic membrane | Modified from Yost et al., 2000101 | 0.08 Hz, 5% uniaxial | 1, 5, and 10 min | 10% NBS + 5% FBS | Atance et al., (2004)86 | |
| ERK1/2 and p38 phosphorylation increased | Foetal RCF | Elastin-coated Flex-I plate | Flexercell FX-3000 | 1.5 Hz, 20% | From 10 min to 24 h | 10% FBS | Papakrivopoulou et al., (2004)105 | |
|
Rapid and transient phosphorylation of JNK. Long-term stable phosphorylation of p38 (4–24 h). p38α increased angiotensinogen expression; JNK1/2 phosphorylation decreased it |
NRVF | Collagen IV-coated BioFlex plate | Flexercell FX-3000 | 20% equiaxial static stretch | 2–30 min and 1–24 h | Serum-free | Lal et al., (2008)115 | |
| Stretch activated Rac1 and RhoA within 5 min. Rac1 activity returned to control after 4 h; RhoA remained high throughout 24 h of stretch. Rac1 inhibited angiotensin expression through JNK-dependent and independent mechanisms; RhoA stimulated it through p38-dependent mechanism | NRVF | Collagen IV-coated BioFlex plate | Flexercell FX-3000 | 20% equiaxial static stretch | From 2 min to 24 h | Serum-free | Verma et al., (2011)116 |
ARVF, adult rat ventricular fibroblasts; ERK, extracellular signal-regulated kinase; FBS, foetal bovine serum; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; NBS, newborn bovine serum; NRVF, neonatal rat ventricular fibroblasts; NS, not stated; p38, p38 mitogen-activated protein kinase; Rac1, Ras-related C3 botulinum-toxin substrate-1; RCF, rat cardiac fibroblasts; RhoA, Ras homolog family member A.
5.1.3 Long-term responses at a molecular level
Despite the consensus about the short-term responses being mediated by the activation of the IEG, the downstream long-term outcomes are much more variable among studies and seem to depend largely on the type of stretch and culture conditions. The transition to myofibroblast phenotype generally involves activation of the TGFβ pathway and is associated with up-regulation of αSMA and collagen production.84,107
Collagen metabolism is a complex process that is tightly regulated by many enzymes. Both increases in collagen secretion in pressure-overloaded hearts and in fibrotic cardiac regions, and enhanced collagen degradation, have been described in patients with CHF.45 As seen in studies with cultured cardiac fibroblasts from patients and experimental animals, the responses of collagen expression/secretion to stretch are diverse (Table 3). Stretch significantly increased collagen I expression and/or secretion in some studies86,89,105,108,109 and decreased it in others,43,89,107,110 with some studies showing both increases and decreases depending on the conditions. As well, collagen III levels were increased after stretch in some papers107,108,110,111 and decreased in others.86,110 Differential behaviours in collagen expression occurred with variations in cell culture parameters like serum, membrane-coating, etc.68,84,89,107,110,112 Furthermore, the relative myofibroblast vs. fibroblast content is likely important, given the different phenotypes they express.
Table 3.
Stretch-induced changes in fibroblast collagen synthesis
| Response | Main findings | Cell type | Coating and Plate | Stretch Machine | Stretch Pattern | Stretch Duration | Serum | Reference |
|---|---|---|---|---|---|---|---|---|
| Increase | Collagen-1A1 promoter activated by stretch | Foetal RCF | Collagen I | Flexercell∗ FX-3000 | 1 Hz, 10% | 24–48 h | Serum-free | Lindahl et al., (2002)114 |
| Procollagen-1A1 mRNA increased in ERK1/2- and p38-phosphorylation-dependent manner | Foetal RCF | Elastin-coated Flex I plate | Flexercell FX-3000 | 1.5 Hz, 20% | 10 min to 24 h | 10% FBS | Papakrivopoulou et al., (2004)105 | |
| Collagen-1A1 mRNA increased | Human cardiac fibroblasts | BioFlex plate | Flexcell∗ strain-unit | 1 Hz, 5% | 24 h | 10% FBS | Prante et al., (2007)130 | |
| No change or dual modulation | Collagen III/I-ratio increased; mRNA-level of collagen III increased, not collagen I | Neonatal RCF | Laminin-coated silicone elastomer membranes | Modified from Terracio et al., 198878 | 5% static uniaxial; 0.33 Hz, 5% cyclic uniaxial | 6, 12, and 24 h | 5% FBS + 10% NBS | Carver et al., (1991)111 |
| Collagen protein increased with 48-h stretch in 10% FBS, unchanged at 24 h or after 48-h stretch in 1% FBS | RCF | Elastin-coated flexible or rigid plate | Flexercell strain-unit | 1.5 Hz, 20% | 24 h and 48 h | 1% or 10% FBS | Butt et al., (1995)109 | |
| Procollagen-1A1 mRNA, protein synthesis and degradation increased after stretch in 10% FBS. Procollagen synthesis reduced in 0% FBS | Foetal RCF | Elastin-coated plate | Flexercell FX-2000 | 1.5 Hz, 20% equibiaxial | 8–88 h | 0%, 1%, or 10% FBS | Butt and Bishop, (1997)89 | |
|
Fibronectin, collagen I, collagen III mRNA increased by 10% uniaxial strain and 3% equibiaxial strain Decrease or no change with 20% uniaxial or 6% equibiaxial strain. |
ARVF | Collagen I-coated silicone elastic membrane | Homemade uniaxial and equibiaxial stretchers | 10%, 20% uniaxial; −6%, −3%, 3%, 6% equibiaxial | 24 h | NS | Lee et al., (1999)84 | |
|
Collagen I mRNA and protein secretion increased on all substrates, particularly on collagen substrate Collagen III unchanged |
Neonatal RCF | Fibronectin-, laminin-, collagen-coated; uncoated charged silicone elastic membrane | Modified from Yost et al., 2000101 | 0.08 Hz, 5% uniaxial | 1, 5, 10 min, and 12 h | 5% FBS + 10% NBS | Atance et al., (2004)86 | |
| Collagen I and Collagen III mRNA increased after 24-h stretch in 0% FBS, decreased with stretched in 10% FBS | Male ARVF | Collagen I-coated Bioflex culture plate | Flexercell | 0.33 Hz, 3%, 6%, 9% equibiaxial | 24 h | 0%, 0.5%, 5%, or 10% FBS | Husse et al., (2007)110 | |
| Stretch reduced TGFβ-induced up-regulation of collagen I mRNA, enhanced TGFβ-induction of collagen III expression | HVF | Fibronectin-coated BioFlex plate | Flexercell FX-4000 | 1 Hz, 10% | 72 h | Serum-free | Watson et al., (2012)107 | |
| Collagen-1A1 and -3A1 mRNA increased by stretch on collagen-VI and laminin matrix. No change on collagen IV matrix or collagen I matrix | HVF | BioFlex plate coated with collagens I, IV, VI, or laminin | Flexercell FX-4000T | 1 Hz, 10% equibiaxial | 72 h | NS | Watson et al., (2014)108 | |
| Collagen protein increased in all hypoxic (1% O2) conditions regardless of stretch. Under physoxia (6% O2), collagen protein increased only with high-strain stretch (8%) | HVF | Fibronectin-coated | Homemade device | 1 Hz, 2% or 8% uniaxial | 24 h | NS | Ugolini et al., (2017)87 | |
| Collagen-1A1, -1A2, -3A1, and fibronectin mRNA increased but only statistically significant on high-stiffness matrix | Adult mouse ventricular fibroblast | Collagen I-coated polyacrylamide gel on polydimethylsiloxane membrane | Homemade stretch device | Static, 3% or 6% uniaxial | 24 and 48 h | 10% FBS | Herum et al., (2017)90 | |
| Decrease | Procollagen protein decreased by long-term cyclic stretch | NRVF | NS | Flexcell FX-4000 | Short term: 1 Hz, 10%, 2 h. Long term: 1 Hz, 5% | 2 and 48 h | Serum-free | Hooper et al., (2012)43 |
ARVF, adult rat ventricular fibroblasts; ERK, extracellular signal-regulated kinase; FBS, foetal bovine serum; HVF, human ventricular fibroblasts; NBS, newborn bovine serum; NRVF, neonatal rat ventricular fibroblasts; NS, not stated; p38, p38 mitogen-activated protein kinase; RCF, rat cardiac fibroblasts; TGFβ, transforming growth-factor β.
Please note that this company has used both Flexcell and Flexercell as names; we have retained the usage in each paper cited.
Several studies have reported changes in αSMA expression in cardiac fibroblasts exposed to sustained mechanical forces (Table 4). Some studies suggest that stretch has opposite effects on αSMA depending on the basal level of αSMA expression, enhancing expression when basal levels were low and decreasing expression in fibroblasts with high basal levels.68,112 However, this finding is not universal.90,108 Moreover, levels of αSMA have also been seen to fluctuate in an in vivo pressure-overloaded model, first increasing and then declining slowly after abdominal aortic constriction.35
Table 4.
Stretch-induced changes in fibroblast alpha-smooth muscle actin expression
| Response | Main findings | Cell type | Coating and plate | Stretch machine | Stretch pattern | Stretch duration | Serum | Reference |
|---|---|---|---|---|---|---|---|---|
| Increase | αSMA mRNA and protein increased with stretch of one-day cultured fibroblasts (low basal αSMA level) | NRVF | Collagen I-coated magnetite beads | Ceramic permanent magnet | Static force perpendicular to cell surface | 0.5, 1, 2, and 4 h | Serum-free | Wang et al., (2003)68 |
| αSMA-mRNA increased on collagen-VI, collagen IV, and laminin matrix | AHVF | BioFlex plate coated with collagen I, IV, VI, or laminin | Flexcell∗ FX-4000T | 1 Hz, 10% equibiaxial | 72 h | NS | Watson et al., (2014)108 | |
| αSMA-mRNA increased by stretch. αSMA fibre formation with matrix stiffening | Adult mouse ventricular fibroblast | Collagen I-coated polyacrylamide gel adherent on PDMS membrane | Modified homemade stretch device | Static, 3% or 6% uniaxial | 24 h and 48 h | 10% FBS | Herum et al., (2017)90 | |
| αSMA-positive cells increased after 48-h stretch at 0.5 and 1 Hz; not observed in p38-deficient fibroblasts | Mouse embryonic fibroblasts | NS | Flexcell | 0.5 and 1.0 Hz | 48 h | 10% FBS | Molkentin et al. (2017)113 | |
| No change | αSMA mRNA unchanged with stretch on collagen I matrix | AHVF | BioFlex plate coated with collagen I, IV, VI, or laminin | Flexcell FX-4000T | 1 Hz, 10% equibiaxial | 72 h | NS | Watson et al., (2014)108 |
| Decrease | αSMA mRNA and protein decreased with stretch on collagen I matrix, p38 dependent | ARVF | Collagen I, fibronectin, or fibronectin ED-A domain peptide-coated magnetite beads | Ceramic permanent magnet | Static force perpendicular to cell surface | 15 min to 8 h | Serum-free | Wang et al., (2000)112 |
| αSMA mRNA and protein decreased with stretch of three-day cultured fibroblasts (high basal αSMA level), p38-dependent | NRVF | Collagen I-coated magnetite beads | Ceramic permanent magnet | Static force perpendicular to cell surface | 0.5, 1, 2, and 4 h | Serum-free | Wang et al., (2003)68 | |
| αSMA protein decreased by long-term stretch | NRVF | NS | Flexcell 4000 | Short term: 1 Hz, 10%. Long term: 1 Hz, 5%. | 2 h and 48 h | Serum-free | Hooper et al., (2012)43 | |
| TGFβ-induced up-regulation of αSMA mRNA and protein. Stretch reduced TGFβ induction of αSMA | AHVF | Fibronectin-coated BioFlex plate | Flexercell∗ FX-4000 T | 1 Hz, 10% | 72 h | Serum-free | Watson et al., (2012)107 |
AHVF, adult human ventricular fibroblasts; ARVF, adult rat ventricular fibroblasts; αSMA, alpha-smooth muscle actin; FBS, foetal bovine serum; NRVF, neonatal rat ventricular fibroblasts; NS, not stated; p38, p38 mitogen-activated protein kinase; TGFβ, transforming growth-factor β.
Please note that this company has used both Flexcell and Flexercell as names; we have retained the usage in each paper cited.
There are also discrepancies in upstream regulation of αSMA. Wang et al. and Papakrivopoulou et al. reported that the stretch-induced increase in αSMA and procollagen 1A1 mRNA expression were dependent on ERK phosphorylation, while their reduction was dependent on p38;68,105,112 another study reported the opposite, showing that the stretch-induced increase in αSMA expression was prevented in p38-deficient cells.113
Similarly, TGFβ, the most known driver of fibroblast differentiation, has been shown to act differently in several stretch studies (Table 5). Some studies reported that stretch induced an increase in the expression and activity of TGFβ1,84,107 depending on the type of stretch applied. Exposing fibroblasts to exogenous TGFβ exerted a profibrotic effect by inducing collagen and αSMA expression, although interestingly this effect was attenuated in the presence of stretch.93,112,113 Further studies also demonstrate that promoter activity of collagen 1A1 was dependent on TGFβ.114 Although many studies have focussed on the role of TGFβ1, not all have seen an increase in its activity with stretch84 and little is known on the effects on cardiac fibroblasts of other TGFβ cytokines like TGFβ2 and β3.
Table 5.
Stretch-induced changes in fibroblast cytokine-regulation and other molecular effects
| Response | Main findings | Cell type | Coating and plate | Stretch machine | Stretch pattern | Stretch duration | Serum | Reference |
|---|---|---|---|---|---|---|---|---|
| Cytokine regulation | Uniaxial stretch stimulated TGFβ1 activity, effect dependent on strain magnitude | ARVF | Collagen I-coated silicone elastic membrane | Homemade uniaxial and equibiaxial stretchers | 10%, 20% uniaxial; −6%, −3%, 3%, 6% equibiaxial | 24 h | NS | Lee et al., (1999)84 |
| Stretch increased TGFβ mRNA, BNP protein, and receptor. Production and secretion of TGFβ protein, TGFβ receptor expression unchanged. Profibrotic effects of TGFβ attenuated | AHVF | Fibronectin-coated BioFlex plate | Flexercell∗ FX-4000 T | 1 Hz, 10% | 72 h | Serum-free | Watson et al., (2012)107 | |
| General gene expression | Protein synthesis enhanced at 2 h of stretch, persisted to 24-h stretch. Increased protein/DNA ratio in cardiomyocytes incubated with conditioned medium from stretched fibroblasts | NRVF | Collagen I-coated plate | Flexercell | 1 Hz, 20% | 2, 4, 6, 18, and 24 h | Serum-free | Ruwhof et al., (2001)94 |
| Other | MMP increased; tissue plasminogen activator secretion increased | AHVF | Collagen I-coated silicone membrane | Flexcell∗ 3000 | 0.42 Hz, 25–35% | 24 h | Serum-free and 5% FBS | Tyagi et al., (1998)120 |
| TNF production increased by stretch in fibroblasts but not in cardiomyocytes | NRVF | Collagen-coated silicone membrane | Flexcell | 0.1 Hz, elongation < 18% | 6 h | NS | Yokoyama et al., (1999)118 | |
| Dual regulation of integrin β1-protein: expression increased under 3%, and decreased under 6% and 12% stretch | NRVF | Collagen-coated silicone membrane | Homemade stretcher | 3%, 6%, 12% uniaxial at 0, 5, and 10 cycles/min | 12 h | 5% FBS + 10% NBS | Yost et al., (2000)101 | |
| XT-I mRNA and activity, total CS-GAG content increased by stretch. Stretch-induced XT-I expression reduced with anti-TGFβ1 antibody | AHVF | BioFlex plate | Flexcell | 1 Hz, 5% | 24 h | 10% FBS | Prante et al., (2007)130 | |
| Angiotensinogen mRNA decreased after 4-h stretch, JNK-phosphorylation-dependent; p38-dependent increase after 8–24 h of stretch | NRVF | Collagen-IV-coated BioFlex plate | Flexercell FX-3000 | Static, 20% equiaxial | From 2 min to 24 h | Serum-free | Lal et al., (2008)115 | |
| Rac1 controlled JNK-dependent decrease of angiotensin genes with stretch, RhoA acted upstream of p38 and mediated its increase | NRVF | Collagen-IV-coated BioFlex plate | Flexercell FX-3000 | Static, 20% equiaxial | From 2 min to 24 h | Serum-free | Verma et al., (2011)116 | |
| Membrane LPP protein decreased by short-term, increased by long-term, cyclic stretch | NRVF | NS | Flexcell 4000 | Short term: 1 Hz, 10%; Long term: 1 Hz, 5%. | 2 and 48 h | Serum-free | Hooper et al., (2012)43 | |
| SGK1 mRNA increased in time-dependent manner. Phospho-SGK1 and total SGK1 proteins increased in stretch elongation-dependent manner. SGK1-dependent chemokine release controlled by NF-κB pathway promoted macrophage migration. Effects lost with SGK1 knockout | Ventricular fibroblasts from adult WT and SGK1 knockout mice | Silicone elastomer-bottomed plate | Flexcell FX-5000 | 1 Hz, 5% or 18% | 1, 4, 6, 12, 24 h | NS | Gan et al., (2018)119 |
AHVF, adult human ventricular fibroblasts; ARVF, adult rat ventricular fibroblasts; CS-GAG, chondroitin sulphate-glycosaminoglycan; FBS, foetal bovine serum; JNK, c-Jun N-terminal kinase; LPP, lipoma-preferred partner; MMP, matrix metalloproteinase; NBS, newborn bovine serum; NF-κB, nuclear factor kappa light-chain enhancer of activated B-cells; NRVF, neonatal rat ventricular fibroblasts; NS, not stated; p38, p38 mitogen-activated protein kinase; Rac1, Ras-related C3 botulinum-toxin substrate-1; RhoA, Ras homolog family member A; SGK1, serum-glucocorticoid-regulated kinase 1; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor; XT-I, xylosyltransferase-I.
*Please note that this company has used both Flexcell and Flexercell as names; we have retained the usage in each paper cited.
Several papers have reported that stretch affects the expression of angiotensin genes or tumour necrosis factor (TNF). Lal et al. observed that p38 phosphorylation increased angiotensinogen expression while JNK1/2 decreased it; they noted time-dependent regulation of angiotensin expression by static stretch, decreasing it at 4 h and increasing it after 8 h.115 Another study from the same group characterized the upstream regulators of these events and saw that stretch activated Rac1 and RhoA within 5 min. Rac1 activity returned to control levels after 4 h, whereas RhoA remained high throughout the whole period of stretch (24 h). Rac1 inhibited the expression of angiotensin through both JNK-dependent and JNK-independent mechanisms, while RhoA stimulated it through a p38-dependent mechanism.116 Exposing cardiac fibroblasts to exogenous angiotensin increased collagen I and αSMA expression, indicating a profibrotic effect.112,117 In fact, angiotensin exposure also increased TNF to a similar extent as stretch alone.118
In this section, we have tried to summarize key observations regarding the main effects of stretch on cardiac fibroblasts. A great deal of variability in responses was seen among studies; in the section below, we identify some of the technical factors that might explain this apparent lack of consistency.
5.2 Factors that govern the response of fibroblast to stretch
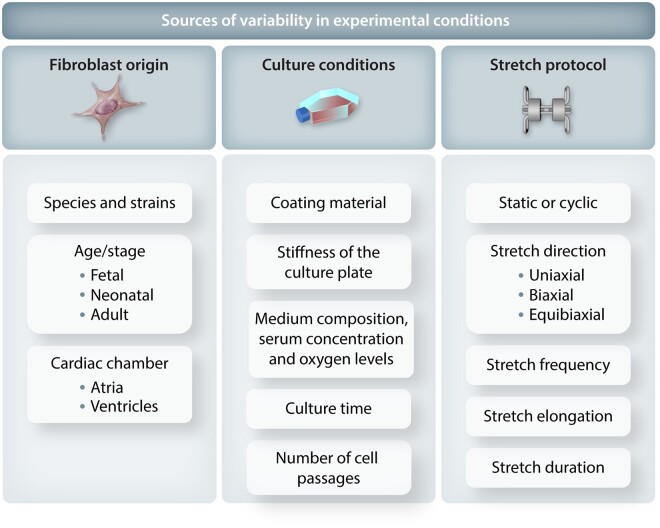
There is substantial variability in the published results of studies evaluation in vitro stretch on cardiac fibroblasts. Many factors are likely involved but differences in experimental conditions likely play a major role (Figure 3). The key factors can be classified into: (i) differences in fibroblast origin, (ii) culture conditions, and (iii) type of stretch.
Figure 3.
Factors that can affect the results of cardiac fibroblast stretch studies.
5.2.1 Fibroblast origin
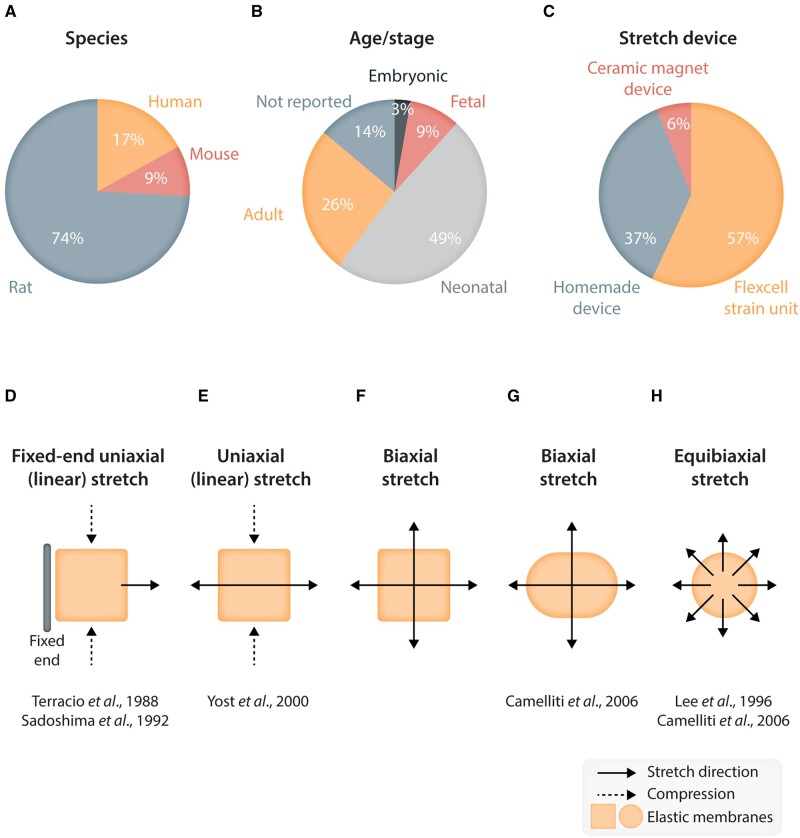
An important source of variability between studies is the origin of cardiac fibroblasts. Three central factors have to be considered: species, age of the animal, and cardiac chamber of origin. The most commonly used species is the rat, although stretch studies have been also reported using mouse90,119 and human107,120 fibroblasts (Figure 4A). In terms of subject age, cells can be isolated from foetal, neonatal, or adult animals (Figures 3 and 4B). Whether stretch responses can be affected by age remains unknown, but as with cardiomyocytes that present greater surface adherence and reproductive abilities at the foetal and neonatal stage,121 fibroblasts present stage/age-related phenotypic differences that could condition their behaviour towards stretch. For example, foetal human cardiac fibroblasts are smaller and proliferate faster than adult and differently influence neighbouring cardiomyocytes.122,123 Foetal fibroblasts also have distinct epigenetic and transcriptomic features including chromatin accessibility, histone marks, motifs, and corresponding transcription factors, suggesting that their responses to stretch or other stimuli could differ.122
Figure 4.
Schematic representing (top) the relative percentage use of different key conditions (species, age/stage and stretch device) across different studies reviewed in this paper; and (bottom) different types of stretch pattern used in research on stretch effects on fibroblasts. (A–C) Pie charts indicating the percentage of various species (A), age/stage of fibroblasts (B), and type of stretch devices (C) that were used in the in vitro stretch studies reviewed in the present paper. (D–H) Schematics showing the types of stretch patterns used for in vitro studies of stretch effects on fibroblasts. The solid arrows indicate the direction of applied stretch. (D, E) Uniaxial (linear) stretch. (F, G) Biaxial stretch. (H) Isotropic, equibiaxial stretch.
Another crucial consideration is the heart chamber from which fibroblasts are isolated. Most stretch studies use either isolated ventricular fibroblasts or pool atrial and ventricular fibroblasts together. The latter approach introduces additional variability, since atria and ventricle fibroblasts have different phenotypes124 and respond differently to external stimuli.47 While stretch responses of atrial fibroblasts have not been extensively investigated in vitro, it is known that atria and atrial fibroblasts exposed to pressure overload exhibit greater fibrotic responses compared to ventricular fibroblasts.35,68,84,125,126 Moreover, atrial fibroblasts from patients with chronic AF showed significant changes in gene expression, proliferation, migration, and myofibroblast differentiation, suggesting they might respond differently to external stimuli compared to ventricles.95
5.2.2 Coating materials and membrane stiffness
Silicone membranes are often used in stretch devices because they are flexible, transparent, and non-deformable. However, they are hydrophobic and unsuitable for cell culture if not treated.127 Researchers generally coat the membranes with ECM proteins, as they improve cell adhesion and mimic the in vivo environment around cardiac fibroblasts. Laminin, collagen, and fibronectin are the most common coating materials, followed by elastin and vitronectin. As an alternative, Lateef et al. used arginylglycylaspartic acid peptide, the binding sequence for integrins found in most ECM proteins, and also showed stable cell adhesion throughout the stretch protocol.127
Although the coating step might seem trivial, the ECM component used for coating is a critical determinant of the cellular responses induced by mechanical loading. Identical stretch protocols applied to fibroblasts seeded on different coatings induce differential responses in cell morphology, cell proliferation, signalling pathways, and gene expression.17,18,98,108 The most plausible explanation is that different coatings differentially affect different cell contact proteins like integrins and produce differential activation of signalling pathways.55 For example, Watson et al. reported that fibroblasts cultured on fibronectin-coated membranes decreased collagen I and increased collagen III expression under stretch conditions.107 The same stretch protocol applied to cells cultured on collagen VI or laminin increased both collagen I and collagen III expressions, and when cells were seeded on collagen IV or I, there was no change.108 The αSMA expression in those studies was also different depending on the coating: decreased by stretch on fibronectin coating but increased on collagen VI or laminin matrix.108 Another study reported that stretch decreased αSMA expression when cells were cultured on collagen I matrix but not on fibronectin or fibronectin ED-A domain polypeptide.112
These observations highlight the crucial role of ECM components in mechanotransduction and suggest that stretch-induced effects might be ligand specific. The ECM is a complex structure containing and regulated by many proteins and molecules. Which component reproduces the ‘correct’ in vivo response is unclear and it is possible that in vivo responses result from a complex blend of ECM protein regulated effects that may be very difficult to reconstitute in an in vitro system.
Substrate stiffness can also influence the basal expression levels of profibrotic genes and cell proliferation.90,107 Herum et al. reported that as substrate stiffness increased, more profibrotic responses were seen in the seeded fibroblasts and suggested 8 kPa as the suitable stiffness to avoid differentiation. This is considered the stiffness of a healthy myocardium, as opposed to 20–100 kPa values in fibrotic cardiac tissue.90
5.2.3 Culture time and cell passage
It is well known that cell culture and passage affect fibroblast properties, fostering their differentiation into myofibroblasts.48,128 The state of fibroblast differentiation can have important consequences on the outcome of a stretch experiment and must be considered when comparing studies.68 For example, freshly isolated fibroblasts from CHF dog atria differed from fibroblasts isolated from control animals in cell morphology and ECM gene expression (reflecting the in vivo profibrotic state), while after two days in culture these differences disappeared because control fibroblasts increased their collagen and fibronectin expression levels to a greater extent through the response to culture.129 These culture/passage-dependent effects, seen in both neonatal and adult fibroblasts, modify collagen deposition, growth factor production, membrane receptor expression, focal adhesion proteins, and key myofibroblast marker proteins including vimentin, αSMA, and smooth muscle myosin heavy chain. In some studies, primary cardiac fibroblasts were stretched after short periods of culture,110 while in others fibroblasts underwent up to more than 10 passages before stretch;108 clearly these differences can importantly affect outcomes. Differences in basal levels of profibrotic genes due to culture conditions might contribute to discrepancies between studies. As mentioned above, stretch increased αSMA expression in cells cultured for only 1 day before stretch, while decreasing αSMA expression when cells were cultured for 3 days pre-stretch and had higher basal αSMA expression.68,112 To avoid excessive cell differentiation because of culture, cells should not be cultured for prolonged periods and should be kept at very low passages.
5.2.4 Serum concentration
Serum is used in fibroblast culture and serum concentration plays a critical role in regulating fibroblast responses in general, but its specific effect on stretch is not clear. Two studies by Butt et al. demonstrated a synergistic effect on collagen expression between serum concentrations and stretch. They reported that cells stretched over 48 h and cultured in 10% FBS increased procollagen expression and procollagen degradation, while when cultured in 1% FBS, collagen production did not change,109 and in serum-free medium, procollagen synthesis decreased.89 However, Husse et al. reported opposite results: a decrease in collagen I and III in 10% FBS medium, and an increase when cells were stretched in serum-free medium.110 Although both teams used the same stretch device, they applied different stretch amplitudes and coating materials, which could explain the discrepancies. Other studies reported similar results to Butt et al.,43,105,130 while Lindahl et al. reported a result similar to Husse et al.114 These apparent inconsistencies reinforce the complexity and limitations of these stretch studies and the need to clarify their basis.
5.2.5 Stretch protocol
One of the main constraints in studying mechanotransduction in a monolayer system is the difficulty in mimicking the complex and dynamic forces that mechanical stimuli exert in the intact heart. Static stretch mimics a chronic hemodynamic overload such as an increase in heart volume,84,90,131–133 while cyclical stretch reflects regular cardiac contraction better.92,101,131,134 However, performing in vitro stretch over extended periods at the frequencies of heart rates of a rat (∼6 Hz) or a mouse (∼10 Hz) is technically difficult and hard on the cells.
The strain generated by stretch devices can be classified as uniaxial, biaxial, and equibiaxial (Figures 3 and 4D–H). Uniaxial strain distribution is anisotropic and non-homogeneous on the elastic membrane;135 the devices generate concomitant compression perpendicular to the axis of stretch and shear forces near membrane edges.92,134 In contrast, biaxial stretch allows cells to be stretched without shear stress,101 and equibiaxial stretch generates homogeneous and isotropic strains to the cell cultures.133,136 Ugolini et al. designed a microdevice with a cell culture area of 5 mm2, allowing the seeding of 1,000 cells, which generates uniform strain fields in a high-throughput fashion.87,92 However, these small numbers of cells are often not compatible with expression assays like western blot. Another system designed by Wang et al. applies continuous static perpendicular forces to cardiac fibroblasts cultured on magnetic beads, but strain is not homogeneously distributed68,112 (Figure 4C). Mechanical strain in intact hearts are multiaxial and non-uniform, so even the most sophisticated devices fail to fully recapitulate what happens in an intact heart.84
In principle, the elongation and frequency of stretch should mimic the natural mechanical forces exerted in the heart, which are very hard to quantify. Researchers have applied levels of stretch sufficient to produce 4% to 20% elongation. In their natural environment, fibroblasts stretch because of the contraction and relaxation of adjacent cardiomyocytes; therefore, myocyte stretch has been taken as a reference. Sadoshima et al. claimed that in the intact heart, a 20% increase in cell length is within the physiological range and suggested that cardiomyocytes were not injured or detached during such stretch.132 Wang et al. also used 10% and 20% uniaxial stretch to mimic myocardial hypertrophy,137 and MacKenna et al. claimed that the area change generated by 4% equibiaxial stretch was comparable to the changes observed in vivo.55 Gan et al. also reported that equibiaxial stretch with a 5% elongation could simulate the physiological in vivo condition but that 18% equibiaxial stretch was deleterious.119 Lee et al. showed that changing elongation extent caused collagen expression to change in different directions, although these findings were not reproduced by others.84 When 10% uniaxial or 3% equibiaxial stretch was applied, collagen mRNA and fibronectin mRNA expression increased, but when the elongation was increased to 20% and 6% respectively, they decreased.84 This observation implies that cardiac fibroblasts are able to distinguish and react differently to different types of mechanical forces. The range between physiological and pathological stretch is still a matter of debate. Overall, it is generally assumed that a uniaxial stretch between 10% and 20% cell elongation and an equibiaxial stretch of 3%–6% are within the physiological range of both cardiomyocytes and fibroblasts.
In terms of frequency, 1 Hz is most commonly used, although studies have used frequencies between 0.1 Hz and 1.5 Hz. Rodent cardiac fibroblasts in an intact heart are exposed to rates 6 to 10 times higher than the frequencies recapitulated by the stretch devices. There is no information available regarding differential cellular responses due to changes in frequencies.
The duration of stretch may also condition outcomes. Stretch-induced responses occur in a specific temporal sequence. Stretch protocols in the literature have been applied for periods varying from 5 s to 96 h.98,106 The earliest response to stretch reported is the activation of the IEG and MAPKs and this does not seem to be affected by different experimental conditions (Table 2). However, changes in profibrotic genes like αSMA and collagen are only observed after long-term stretch (of at least 2 h for αSMA and at least 12 h for collagen)68,111 and the reported responses vary widely (Tables 3 and 4). For example, angiotensinogen gene expression changes were biphasic, with a decrease after 4 h of static stretch and an increase between 8 h and 24 h of static stretch.115 Another example is LPP, for which 2 h of cyclical stretch at 1 Hz with 10% elongation only affected subcellular localization, but a 48-h stretch period at 1 Hz with 5% elongation up-regulated protein expression.43 The expression of serum-glucocorticoid-regulated kinase 1 (SGK1), a kinase that contributes to cardiac remodelling and the development of heart failure, is similarly regulated by stretch in a time-dependent fashion.119
The influence of the wide range of technical factors detailed above makes this literature particularly difficult to analyse. It is rare for different studies not to vary in important technical determinants and therefore when discrepant results are obtained, it becomes very difficult to know whether the results are truly in conflict or differ because of technical determinants, and if so which (if any) of the obvious technical differences are responsible for the differing outcomes. There seems to be a clear need to obtain a clearer consensus about the effects of stretch on fibroblast function and to understand better the determinants of the response.
6. Conclusions
It is clear that fibrosis occurs in many paradigms of cardiac pressure and/or volume overload. In atria subjected to chronic pressure/volume loading conditions, fibrosis is common and appears to play an important role in AF. However, contrary to widespread assumptions, the evidence regarding direct cardiac fibroblast activation by mechanical forces is weak and unclear, with key responses like fibroblast proliferation, collagen production, and indices of differentiation to myofibroblasts showing divergent and sometimes directionally opposite changes among studies in response to stretch. While the differences in response may be due to technical differences in terms of fibroblast origin, state of differentiation, degree of stretch, seeding medium, etc., it is not possible to identify clear patterns in the published work. Further research is needed to obtain reproducible results that answer the question of whether, and if so how, mechanical stretch directly activates fibroblasts.
There is great interest in developing novel therapeutic approaches that prevent fibrosis development in the treatment of heart disease, particularly AF.138 In order to accomplish this goal, the key mechanistic determinants need to be understood. It is therefore important to determine definitively whether the association between pressure/volume loading and cardiac fibrosis is due to a direct activating effect on fibroblasts, to profibrotic substances produced by cardiomyocytes, leucocytes, or neurohormonal responses, or to some combination of these factors. Important new tools have become available to help in this effort. For example, single-cell RNA sequencing allows for the identification of changes in cell populations identified by their transcriptomic signature, rather than by classical, somewhat arbitrary criteria. This approach has recently been used to evaluate changes in specific fibroblast cell populations associated with AF.139 Clarifying these issues may provide the key to developing new and more effective therapies to prevent the development and progression of atrial fibrosis and positively influence the natural history of AF.
Acknowledgements
The authors thank Lucie Lefebvre for expert secretarial assistance.
Conflict of interest: none declared.
Funding
This work was supported by the Canadian Institutes of Health Research (SN, Foundation Grant—148401) and the Heart and Stroke Foundation of Canada (18-0022032). AGE was supported by the 2018 Research Grant of the Spanish Fundación Alfonso Martín Escudero.
References
- 1. Andrade J, Khairy P, Dobrev D, Nattel S.. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 2. Satoh T, Zipes DP.. Unequal atrial stretch in dogs increases dispersion of refractoriness conducive to developing atrial fibrillation. J Cardiovasc Electrophysiol 1996;7:833–842. [DOI] [PubMed] [Google Scholar]
- 3. Sideris DA, Toumanidis ST, Thodorakis M, Kostopoulos K, Tselepatiotis E, Langoura C, Stringli T, Moulopoulos SD.. Some observations on the mechanism of pressure related atrial fibrillation. Eur Heart J 1994;15:1585–1589. [DOI] [PubMed] [Google Scholar]
- 4. Pellman J, Lyon RC, Sheikh F.. Extracellular matrix remodeling in atrial fibrosis: mechanisms and implications in atrial fibrillation. J Mol Cell Cardiol 2010;48:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gottdiener JS, Seliger S, deFilippi C, Christenson R, Baldridge AS, Kizer JR, Psaty BM, Shah SJ.. Relation of biomarkers of cardiac injury, stress, and fibrosis with cardiac mechanics in patients ≥ 65 years of age. Am J Cardiol Elsevier 2020;136:156–163. [DOI] [PubMed] [Google Scholar]
- 6. White CW, Kerber RE, Weiss HR, Marcus ML.. The effects of atrial fibrillation on atrial pressure—volume and flow relationships. Circ Res 1982;51:205–215. [DOI] [PubMed] [Google Scholar]
- 7. Leistad E, Christensen G, Ilebekk A.. Effects of atrial fibrillation on left and right atrial dimensions, pressures, and compliances. Am J Physiol Heart Circ Physiol 1993;264:H1093–H1097. [DOI] [PubMed] [Google Scholar]
- 8. Schüttler D, Bapat A, Kääb S, Lee K, Tomsits P, Clauss S, Hucker WJ.. Animal models of atrial fibrillation. Circ Res 2020;127:91–110. [DOI] [PubMed] [Google Scholar]
- 9. von Lewinski D, Stumme B, Fialka F, Luers C, Pieske B.. Functional relevance of the stretch-dependent slow force response in failing human myocardium. Circ Res 2004;94:1392–1398. [DOI] [PubMed] [Google Scholar]
- 10. Grossman W, Jones D, McLaurin LP.. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975;56:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toischer K, Rokita AG, UnsöLd B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuß L, Gupta SN, Schmidt K, Lehnart SE, KrüGer M, Linke WA, Backs J, Regitz-Zagrosek V, SchäFer K, Field LJ, Maier LS, Hasenfuss G.. Differential cardiac remodeling in preload versus afterload. Circulation 2010;122:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frangogiannis NG. The extracellular matrix in ischemic and nonischemic heart failure. Circ Res 2019;125:117–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herum KM, Lunde IG, McCulloch AD, Christensen G.. The soft- and hard-heartedness of cardiac fibroblasts: mechanotransduction signaling pathways in fibrosis of the heart. JCM 2017;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. John B, Stiles MK, Kuklik P, Chandy ST, Young GD, Mackenzie L, Szumowski L, Joseph G, Jose J, Worthley SG, Kalman JM, Sanders P.. Electrical remodelling of the left and right atria due to rheumatic mitral stenosis. Eur Heart J 2008;29:2234–2243. [DOI] [PubMed] [Google Scholar]
- 15. Morton JB, Sanders P, Vohra JK, Sparks PB, Morgan JG, Spence SJ, Grigg LE, Kalman JM.. Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation 2003;107:1775–1782. [DOI] [PubMed] [Google Scholar]
- 16. Healey JS, Connolly SJ.. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol 2003;91:9–14. [DOI] [PubMed] [Google Scholar]
- 17. Kamioka M, Hijioka N, Matsumoto Y, Nodera M, Kaneshiro T, Suzuki H, Takeishi Y.. Uncontrolled blood pressure affects atrial remodeling and adverse clinical outcome in paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2018;41:402–410. [DOI] [PubMed] [Google Scholar]
- 18. Trulock KM, Narayan SM, Piccini JP.. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014;64:710–721. [DOI] [PubMed] [Google Scholar]
- 19. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O’Meara E, Ryan JJ, Shah SJ, Mullens W, Zile MR, Lam CSP, McMurray JJV, Solomon SD.. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2019;74:2858–2873. [DOI] [PubMed] [Google Scholar]
- 20. Essayagh B, Antoine C, Benfari G, Messika-Zeitoun D, Michelena H, Tourneau T, Le Mankad S, Tribouilloy CM, Thapa P, Enriquez-Sarano M.. Prognostic implications of left atrial enlargement in degenerative mitral regurgitation. J Am Coll Cardiol 2019;74:858–870. [DOI] [PubMed] [Google Scholar]
- 21. Marques-Alves P, Marinho AV, Teixeira R, Baptista R, Castro G, Martins R, Gonçalves L.. Going beyond classic echo in aortic stenosis: left atrial mechanics, a new marker of severity. BMC Cardiovasc Disord 2019;19:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pelliccia A, Maron BJ, Paolo FM, Di Biffi A, Quattrini FM, Pisicchio C, Roselli A, Caselli S, Culasso F.. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol 2005;46:690–696. [DOI] [PubMed] [Google Scholar]
- 23. Butt M, Dwivedi G, Shantsila A, Khair OA, Lip GYH.. Left ventricular systolic and diastolic function in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Circ Heart Fail 2012;5:226–233. [DOI] [PubMed] [Google Scholar]
- 24. Shelburne NJ, Parikh KS, Chiswell K, Shaw LK, Sivak J, Arges K, Tomfohr J, Velazquez EJ, Kisslo J, Samad Z, Rajagopal S.. Echocardiographic assessment of right ventricular function and response to therapy in pulmonary arterial hypertension. Am J Cardiol 2019;124:1298–1304. [DOI] [PubMed] [Google Scholar]
- 25. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GYH, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, van Wagoner DR, Nattel S.. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrhythm 2016;32:247–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guichard JB, Nattel S.. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol 2017;70:756–765. [DOI] [PubMed] [Google Scholar]
- 27. Bisbal F, Baranchuk A, Braunwald E, Bayés de Luna A, Bayés-Genís A.. Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol 2020;75:222–232. [DOI] [PubMed] [Google Scholar]
- 28. Manios EG, Mavrakis HE, Kanoupakis EM, Kallergis EM, Kafarakis PK, Vardas PE.. Evidence of mechanoelectric feedback in the atria of patients with atrioventricular nodal reentrant tachycardia. J Interv Card Electrophysiol 2006;16:51–57. [DOI] [PubMed] [Google Scholar]
- 29. Ágoston G, Szilágyi J, Bencsik G, Tutuianu C, Klausz G, Sághy L, Varga A, Forster T, Pap R.. Impaired adaptation to left atrial pressure increase in patients with atrial fibrillation. J Interv Card Electrophysiol 2015;44:113–118. [DOI] [PubMed] [Google Scholar]
- 30. Disertori M, Masè M, Marini M, Mazzola S, Cristoforetti A, Greco MD, Kottkamp H, Arbustini E, Ravelli F.. Electroanatomic mapping and late gadolinium enhancement MRI in a genetic model of arrhythmogenic atrial cardiomyopathy. J Cardiovasc Electrophysiol 2014;25:964–970. [DOI] [PubMed] [Google Scholar]
- 31. Ruwhof C. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 2000;47:23–37. [DOI] [PubMed] [Google Scholar]
- 32. Leslie KO, Taatjes DJ, Schwarz J, von Turkovich M, Low RB.. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol 1991;139:207–216. [PMC free article] [PubMed] [Google Scholar]
- 33. Schwartz K, Carrier L, Lompré AM, Mercadier JJ, Boheler KR.. Contractile proteins and sarcoplasmic reticulum calcium-ATPase gene expression in the hypertrophied and failing heart. Basic Res Cardiol 1992;87(Suppl 1):285–290. [DOI] [PubMed] [Google Scholar]
- 34. Simpson PC, Long CS, Waspe LE, Henrich CJ, Ordahl CP.. Transcription of early developmental isogenes in cardiac myocyte hypertrophy. J Mol Cell Cardiol 1989;21:79–89. [DOI] [PubMed] [Google Scholar]
- 35. Schwartz K, de la Bastie D, Bouveret P, Oliviéro P, Alonso S, Buckingham M.. Alpha-skeletal muscle actin mRNA’s accumulate in hypertrophied adult rat hearts. Circ Res 1986;59:551–555. [DOI] [PubMed] [Google Scholar]
- 36. Komuro I, Katoh Y, Hoh E, Takaku F, Yazaki Y.. Mechanisms of cardiac hypertrophy and injury–possible role of protein kinase c activation. Jpn Circ J 1991;55:1149–1157. [DOI] [PubMed] [Google Scholar]
- 37. Takahashi N, Calderone A, Izzo NJ, Mäki TM, Marsh JD, Colucci WS.. Hypertrophic stimuli induce transforming growth factor-beta 1 expression in rat ventricular myocytes. J Clin Invest 1994;94:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Villarreal FJ, Dillmann WH.. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol 1992;262:H1861–H1866. [DOI] [PubMed] [Google Scholar]
- 39. Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS.. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 1994;28:1581–1585. [DOI] [PubMed] [Google Scholar]
- 40. Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T.. Transforming growth factor-β function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 2002;106:130–135. [DOI] [PubMed] [Google Scholar]
- 41. Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI.. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res 1988;62:757–765. [DOI] [PubMed] [Google Scholar]
- 42. Chapman D, Weber KT, Eghbali M.. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res 1990;67:787–794. [DOI] [PubMed] [Google Scholar]
- 43. Hooper CL, Dash PR, Boateng SY.. Lipoma preferred partner is a mechanosensitive protein regulated by nitric oxide in the heart. FEBS Open Bio 2012;2:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fu L, Wei C-C, Powell PC, Bradley WE, Ahmad S, Ferrario CM, Collawn JF, Dell'Italia LJ.. Increased fibroblast chymase production mediates procollagen autophagic digestion in volume overload. J Mol Cell Cardiol 2016;92:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spinale FG. Matrix metalloproteinases. Circ Res 2002;90:520–530. [DOI] [PubMed] [Google Scholar]
- 46. Hanna N, Cardin S, Leung T-K, Nattel S.. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res 2004;63:236–244. [DOI] [PubMed] [Google Scholar]
- 47. Burstein B, Libby E, Calderone A, Nattel S.. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 2008;117:1630–1641. [DOI] [PubMed] [Google Scholar]
- 48. Dawson K, Wu C-T, Qi XY, Nattel S.. Congestive heart failure effects on atrial fibroblast phenotype: differences between freshly-isolated and cultured cells. PLoS ONE 2012;7:e52032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li B, Luo F, Luo X, Li B, Qi L, Zhang D, Tang Y.. Effects of atrial fibrosis induced by mitral regurgitation on atrial electrophysiology and susceptibility to atrial fibrillation in pigs. Cardiovasc Pathol 2019;40:32–40. [DOI] [PubMed] [Google Scholar]
- 50. Remes J, van Brakel TJ, Bolotin G, Garber C, de Jong MM, van der Veen FH, Maessen JG.. Persistent atrial fibrillation in a goat model of chronic left atrial overload. J Thorac Cardiovasc Surg 2008;136:1005–1011. [DOI] [PubMed] [Google Scholar]
- 51. Ruaengsri C, Schill MR, Lancaster TS, Khiabani AJ, Manghelli JL, Carter DI, Greenberg JW, Melby SJ, Schuessler RB, Damiano RJ.. The hemodynamic and atrial electrophysiologic consequences of chronic left atrial volume overload in a controllable canine model. J Thorac Cardiovasc Surg 2018;156:1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Furtado MB, Nim HT, Boyd SE, Rosenthal NA.. View from the heart: cardiac fibroblasts in development, scarring and regeneration. Development 2016;143:387–397. [DOI] [PubMed] [Google Scholar]
- 53. Hara H, Takeda N, Komuro I.. Pathophysiology and therapeutic potential of cardiac fibrosis. Inflamm Regen 2017;37: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pedrotty DM, Klinger RY, Kirkton RD, Bursac N.. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res 2009;83:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. MacKenna DA, Dolfi F, Vuori K, Ruoslahti E.. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest 1998;101:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, Folkesson HG, Horne WI, Doane KJ, Meszaros JG.. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol 2006;290:H323–H330. [DOI] [PubMed] [Google Scholar]
- 57. Nicoletti A, Michel J-B.. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res 1999;41:532–543. [DOI] [PubMed] [Google Scholar]
- 58. Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh Y-H, Nattel S.. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res 2009;105:1213–1222. [DOI] [PubMed] [Google Scholar]
- 59. Chilton L, Giles WR, Smith GL.. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol 2007;583:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gaudesius G, Miragoli M, Thomas SP, Rohr S.. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res 2003;93:421–428. [DOI] [PubMed] [Google Scholar]
- 61. Ivey MJ, Tallquist MD.. Defining the cardiac fibroblast. Circ J 2016;80:2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Desmoulière A, Redard M, Darby I, Gabbiani G.. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 63. Kis K, Liu X, Hagood JS.. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med 2011;13:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petrov VV, Fagard RH, Lijnen PJ.. Stimulation of collagen production by transforming growth factor-β 1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002;39:258–263. [DOI] [PubMed] [Google Scholar]
- 65. Schürch W, Seemayer TA, Gabbiani G.. The myofibroblast: a quarter century after its discovery. Am J Surg Pathol 1998;22:141–147. [DOI] [PubMed] [Google Scholar]
- 66. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB.. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 1999;277:C1–C9. [DOI] [PubMed] [Google Scholar]
- 67. Berk BC, Fujiwara K, Lehoux S.. ECM remodeling in hypertensive heart disease. J Clin Invest 2007;117:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang J, Chen H, Seth A, McCulloch CA.. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 2003;285:H1871–H1881. [DOI] [PubMed] [Google Scholar]
- 69. Serini G, Bochaton-Piallat M-L, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G.. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol 1998;142:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C.. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001;12:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Klingberg F, Chau G, Walraven M, Boo S, Koehler A, Chow ML, Olsen AL, Im M, Lodyga M, Wells RG, White ES, Hinz B.. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J Cell Sci 2018;131:jcs201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bosman FT, Stamenkovic I.. Functional structure and composition of the extracellular matrix. J Pathol 2003;200:423–428. [DOI] [PubMed] [Google Scholar]
- 73. Sibilla S, Godfrey M, Brewer S, Budh-Raja A, Genovese L.. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: scientific background and clinical studies. Open Nutraceuticals J 2015;8:29–42. [Google Scholar]
- 74. Wu M, Crane JS.. Biochemistry, Collagen Synthesis. StatPearls Treasure Island (FL: ): StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 75. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA.. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363. [DOI] [PubMed] [Google Scholar]
- 76. MacKenna D, Summerour SR, Villarreal FJ.. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res 2000;46:257–263. [DOI] [PubMed] [Google Scholar]
- 77. Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol 1999;18:417–426. [DOI] [PubMed] [Google Scholar]
- 78. Terracio L, Miller B, Borg TK.. Effects of cyclic mechanical stimulation of the cellular components of the heart: in vitro. In Vitro Cell Dev Biol 1988;24:53–58. [DOI] [PubMed] [Google Scholar]
- 79. Blaauboer ME, Smit TH, Hanemaaijer R, Stoop R, Everts V.. Cyclic mechanical stretch reduces myofibroblast differentiation of primary lung fibroblasts. Biochem Biophys Res Commun 2011;404:23–27. [DOI] [PubMed] [Google Scholar]
- 80. Kato T, Ishiguro N, Iwata H, Kojima T, Ito T, Naruse K.. Up-regulation of COX2 expression by uni-axial cyclic stretch in human lung fibroblast cells. Biochem Biophys Res Commun 1998;244:615–619. [DOI] [PubMed] [Google Scholar]
- 81. Kaneko D, Sasazaki Y, Kikuchi T, Ono T, Nemoto K, Matsumoto H, Toyama Y.. Temporal effects of cyclic stretching on distribution and gene expression of integrin and cytoskeleton by ligament fibroblasts in vitro. Connect Tissue Res 2009;50:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kushida N, Yamaguchi O, Kawashima Y, Akaihata H, Hata J, Ishibashi K, Aikawa K, Kojima Y.. Uni-axial stretch induces actin stress fiber reorganization and activates c-Jun NH2 terminal kinase via Rhoa and Rho kinase in human bladder smooth muscle cells. BMC Urol 2016;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huycke TR, Miller BM, Gill HK, Nerurkar NL, Sprinzak D, Mahadevan L, Tabin CJ.. Genetic and mechanical regulation of intestinal smooth muscle development. Cell 2019;179:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee AA, Delhaas T, McCulloch AD, Villarreal FJ.. Differential responses of adult cardiac fibroblasts to in vitro biaxial strain patterns. J Mol Cell Cardiol 1999;31:1833–1843. [DOI] [PubMed] [Google Scholar]
- 85. Li M, Zhang C, Yang Y.. Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts. Bone Joint Res 2019;8:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Atance J, Yost MJ, Carver W.. Influence of the extracellular matrix on the regulation of cardiac fibroblast behavior by mechanical stretch. J Cell Physiol 2004;200:377–386. [DOI] [PubMed] [Google Scholar]
- 87. Ugolini GS, Pavesi A, Rasponi M, Fiore GB, Kamm R, Soncini M.. Human cardiac fibroblasts adaptive responses to controlled combined mechanical strain and oxygen changes in vitro. eLife 2017;6:e22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liao XD, Wang XH, Jin HJ, Chen LY, Quan C.. Mechanical stretch induces mitochondria-dependent apoptosis in neonatal rat cardiomyocytes and G 2/M accumulation in cardiac fibroblasts. Cell Res 2004;14:16–26. [DOI] [PubMed] [Google Scholar]
- 89. Butt RP, Bishop JE.. Mechanical load enhances the stimulatory effect of serum growth factors on cardiac fibroblast procollagen synthesis. J Mol Cell Cardiol 1997;29:1141–1151. [DOI] [PubMed] [Google Scholar]
- 90. Herum KM, Choppe J, Kumar A, Engler AJ, McCulloch AD.. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol Biol Cell 2017;28:1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cao L, Gardner DG.. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 1995;25:227–234. [DOI] [PubMed] [Google Scholar]
- 92. Ugolini GS, Rasponi M, Pavesi A, Santoro R, Kamm R, Fiore GB, Pesce M, Soncini M.. On-chip assessment of human primary cardiac fibroblasts proliferative responses to uniaxial cyclic mechanical strain. Biotechnol Bioeng 2016;113:859–869. [DOI] [PubMed] [Google Scholar]
- 93. van Wamel JE, Ruwhof C, van der Valk-Kokshoorn EJ, Schrier PI, van der Laarse A.. Rapid gene transcription induced by stretch in cardiac myocytes and fibroblasts and their paracrine influence on stationary myocytes and fibroblasts. Pflugers Arch Eur J Physiol 2000;439:781–788. [DOI] [PubMed] [Google Scholar]
- 94. Ruwhof C, van Wamel AET, van der Valk LJM, Schrier PI, van der Laarse A.. Direct, autocrine and paracrine effects of cyclic stretch on growth of myocytes and fibroblasts isolated from neonatal rat ventricles. Arch Physiol Biochem 2001;109:10–18. [DOI] [PubMed] [Google Scholar]
- 95. Poulet C, Künzel S, Büttner E, Lindner D, Westermann D, Ravens U.. Altered physiological functions and ion currents in atrial fibroblasts from patients with chronic atrial fibrillation. Physiol Rep 2016;4:e12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Burstein B, Nattel S.. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 2008;51:802–809. [DOI] [PubMed] [Google Scholar]
- 97. Aimé-Sempé C, Folliguet T, Rücker-Martin C, Krajewska M, Krajewski S, Heimburger M, Aubier M, Mercadier J-J, Reed JC, Hatem SN.. Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol 1999;34:1577–1586. [DOI] [PubMed] [Google Scholar]
- 98. Persoon-Rothert M, van der Wees KGC, van der Laarse A.. Mechanical overload-induced apoptosis: a study in cultured neonatal ventricular myocytes and fibroblasts. Mol Cell Biochem 2002;241:115–124. [DOI] [PubMed] [Google Scholar]
- 99. Boerma M, van der Wees CGC, Vrieling H, Svensson JP, Wondergem J, van der Laarse A, Mullenders LHF, van Zeeland AA.. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics 2005;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fuseler JW, Millette CF, Davis JM, Carver W.. Fractal and image analysis of morphological changes in the actin cytoskeleton of neonatal cardiac fibroblasts in response to mechanical stretch. Microsc Microanal 2007;13:133–143. [DOI] [PubMed] [Google Scholar]
- 101. Yost MJ, Simpson D, Wrona K, Ridley S, Ploehn HJ, Borg TK, Terracio L.. Design and construction of a uniaxial cell stretcher. Am J Physiol Heart Circ Physiol 2000;279:H3124–H3130. [DOI] [PubMed] [Google Scholar]
- 102. Bahrami S, Drabløs F.. Gene regulation in the immediate-early response process. Adv Biol Regul 2016;62:37–49. [DOI] [PubMed] [Google Scholar]
- 103. O'Donnell A, Odrowaz Z, Sharrocks AD.. Immediate-early gene activation by the mapk pathways: what do and don’t we know? Biochem Soc Trans 2012;40:58–66. [DOI] [PubMed] [Google Scholar]
- 104. Kimura R, Ishikawa C, Rokkaku T, Janknecht R, Mori N.. Phosphorylated c-jJun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim Biophys Acta 2011;1813:1543–1553. [DOI] [PubMed] [Google Scholar]
- 105. Papakrivopoulou J, Lindahl GE, Bishop JE, Laurent GJ.. Differential roles of extracellular signal-regulated kinase 1/2 and p38MAPK in mechanical load-induced procollagen α1(I) gene expression in cardiac fibroblasts. Cardiovasc Res 2004;61:736–744. [DOI] [PubMed] [Google Scholar]
- 106. Gudi SRP, Lee AA, Clark CB, Frangos JA.. Equibiaxial strain and strain rate stimulate early activation of G proteins in cardiac fibroblasts. Am J Physiol 1998;274:C1424–C1428. [DOI] [PubMed] [Google Scholar]
- 107. Watson CJ, Phelan D, Xu M, Collier P, Neary R, Smolenski A, Ledwidge M, McDonald K, Baugh J.. Mechanical stretch up-regulates the B-type natriuretic peptide system in human cardiac fibroblasts: a possible defense against transforming growth factor-β mediated fibrosis. Fibrogenesis Tissue Repair 2012;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Watson CJ, Phelan D, Collier P, Horgan S, Glezeva N, Cooke G, Xu M, Ledwidge M, McDonald K, Baugh JA.. Extracellular matrix sub-types and mechanical stretch impact human cardiac fibroblast responses to transforming growth factor beta. Connect Tissue Res 2014;55:248–256. [DOI] [PubMed] [Google Scholar]
- 109. Butt RP, Laurent GJ, Bishop JE.. Mechanical load and polypeptide growth factors stimulate cardiac fibroblast activity. Ann NY Acad Sci 1995;752:387–393. [DOI] [PubMed] [Google Scholar]
- 110. Husse B, Briest W, Homagk L, Isenberg G, Gekle M.. Cyclical mechanical stretch modulates expression of collagen I and collagen III by PKC and tyrosine kinase in cardiac fibroblasts. Am J Physiol Regul Integr Comp Physiol 2007;293:R1898–R1907. [DOI] [PubMed] [Google Scholar]
- 111. Carver W, Nagpal ML, Nachtigal M, Borg TK, Terracio L.. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ Res 1991;69:116–122. [DOI] [PubMed] [Google Scholar]
- 112. Wang J, Seth A, McCulloch CAG.. Force regulates smooth muscle actin in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 2000;279:H2776–H2785. [DOI] [PubMed] [Google Scholar]
- 113. Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, Gunaje J, Otsu K, Davis J.. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation 2017;136:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lindahl GE, Chambers RC, Papakrivopoulou J, Dawson SJ, Jacobsen MC, Bishop JE, Laurent GJ.. Activation of fibroblast procollagen α1(I) transcription by mechanical strain is transforming growth factor-β-dependent and involves increased binding of CCAAT-binding factor (CBF/NF-Y) at the proximal promoter. J Biol Chem 2002;277:6153–6161. [DOI] [PubMed] [Google Scholar]
- 115. Lal H, Verma SK, Golden HB, Foster DM, Smith M, Dostal DE.. Stretch-induced regulation of angiotensinogen gene expression in cardiac myocytes and fibroblasts: opposing roles of JNK1/2 and p38alpha MAP kinases. J Mol Cell Cardiol 2008;45:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Verma SK, Lal H, Golden HB, Gerilechaogetu F, Smith M, Guleria RS, Foster DM, Lu G, Dostal DE.. Rac1 and Rhoa differentially regulate angiotensinogen gene expression in stretched cardiac fibroblasts. Cardiovasc Res 2011;90:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chen K, Chen J, Li D, Zhang X, Mehta JL.. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts. Hypertension 2004;44:655–661. [DOI] [PubMed] [Google Scholar]
- 118. Yokoyama T, Sekiguchi K, Tanaka T, Tomaru K, Arai M, Suzuki T, Nagai R.. Angiotensin II and mechanical stretch induce production of tumor necrosis factor in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 1999;276:H1968–H1976. [DOI] [PubMed] [Google Scholar]
- 119. Gan W, Li T, Ren J, Li C, Liu Z, Yang M.. Serum–glucocorticoid-regulated kinase 1 contributes to mechanical stretch-induced inflammatory responses in cardiac fibroblasts. Mol Cell Biochem 2018;445:67–78. [DOI] [PubMed] [Google Scholar]
- 120. Tyagi SC, Lewis K, Pikes D, Marcello A, Mujumdar VS, Smiley LM, Moore CK.. Stretch-induced membrane type matrix metalloproteinase and tissue plasminogen activator in cardiac fibroblast cells. J Cell Physiol 1998;176:374–382. [DOI] [PubMed] [Google Scholar]
- 121. Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK.. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res 1991;68:734–744. [DOI] [PubMed] [Google Scholar]
- 122. Jonsson MKB, Hartman RJG, Ackers-Johnson M, Tan WLW, Lim B, van Veen TAB, Foo RA.. Transcriptomic and epigenomic comparison of fetal and adult human cardiac fibroblasts reveals novel key transcription factors in adult cardiac fibroblasts. JACC Basic Transl Sci 2016;1:590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong T-T, Shaw RM, Srivastava D.. Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev Cell 2009;16:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tucker NR, Chaffin M, Fleming SJ, Hall AW, Parsons VA, Bedi KC, Akkad A-D, Herndon CN, Arduini A, Papangeli I, Roselli C, Aguet F, Choi SH, Ardlie KG, Babadi M, Margulies KB, Stegmann CM, Ellinor PT.. Transcriptional and cellular diversity of the human heart. Circulation 2020;142:466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Boluyt MO, O'Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH, Crow MT, Lakatta EG.. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure: marked upregulation of genes encoding extracellular matrix components. Circ Res 1994;75:23–32. [DOI] [PubMed] [Google Scholar]
- 126. Boluyt MO, Bing OHL.. Matrix gene expression and decompensated heart failure: the aged SHR model. Cardiovasc Res 2000;46:239–249. [DOI] [PubMed] [Google Scholar]
- 127. Lateef SS, Boateng S, Hartman TJ, Crot CA, Russell B, Hanley L.. GRGDSP peptide-bound silicone membranes withstand mechanical flexing in vitro and display enhanced fibroblast adhesion. Biomaterials 2002;23:3159–3168. [DOI] [PubMed] [Google Scholar]
- 128. Tadevosyan A, Xiao J, Surinkaew S, Naud P, Merlen C, Harada M, Qi X, Chatenet D, Fournier A, Allen BG, Nattel S.. Intracellular angiotensin-II interacts with nuclear angiotensin receptors in cardiac fibroblasts and regulates RNA synthesis, cell proliferation, and collagen secretion. J Am Heart Assoc 2017;6:e004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Santiago J-J, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E, Dixon IMC.. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn 2010;239:1573–1584. [DOI] [PubMed] [Google Scholar]
- 130. Prante C, Milting H, Kassner A, Farr M, Ambrosius M, Schön S, Seidler DG, Banayosy AE, Körfer R, Kuhn J, Kleesiek K, Götting C.. Transforming growth factor beta1-regulated xylosyltransferase I activity in human cardiac fibroblasts and its impact for myocardial remodeling. J Biol Chem 2007;282:26441–26449. [DOI] [PubMed] [Google Scholar]
- 131. Lee AA, Delhaas T, Waldman LK, MacKenna DA, Villarreal FJ, McCulloch AD.. An equibiaxial strain system for cultured cells. Am J Physiol 1996;271:C1400–C1408. [DOI] [PubMed] [Google Scholar]
- 132. Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S.. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells: an in vitro model of load-induced cardiac hypertrophy. J Biol Chem 1992;267:10551–10560. [PubMed] [Google Scholar]
- 133. Camelliti P, Gallagher JO, Kohl P, McCulloch AD.. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat Protoc 2006;1:1379–1391. [DOI] [PubMed] [Google Scholar]
- 134. Terracio L, Tingström A, Peters WH, Borg TK.. A potential role for mechanical stimulation in cardiac development. Ann NY Acad Sci 1990;588:48–60. [DOI] [PubMed] [Google Scholar]
- 135. Vande Geest JP, Di Martino ES, Vorp DA.. An analysis of the complete strain field within flexercell membranes. J Biomech 2004;37:1923–1928. [DOI] [PubMed] [Google Scholar]
- 136. Colombo A, Cahill PA, Lally C.. An analysis of the strain field in biaxial flexcell membranes for different waveforms and frequencies. Proc Inst Mech Eng H 2008;222:1235–1245. [DOI] [PubMed] [Google Scholar]
- 137. Wang B-W, Wu G-J, Cheng W-P, Shyu K-G.. Mechanical stretch via transforming growth factor-β1 activates microRNA-208a to regulate hypertrophy in cultured rat cardiac myocytes. J Formos Med Assoc 2013;112:635–643. [DOI] [PubMed] [Google Scholar]
- 138. Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol 2017;3:425–435. [DOI] [PubMed] [Google Scholar]
- 139. Moreira LM, Takawale A, Hulsurkar M, Menassa DA, Antanaviciute A, Lahiri SK, Mehta N, Evans N, Psarros C, Robinson P, Sparrow AJ, Gillis MA, Ashley N, Naud P, Barallobre-Barreiro J, Theofilatos K, Lee A, Norris M, Clarke MV, Russell PK, Casadei B, Bhattacharya S, Zajac JD, Davey RA, Sirois M, Mead A, Simmons A, Mayr M, Sayeed R, Krasopoulos G, Redwood C, Channon KM, Tardif JC, Wehrens XHT, Nattel S, Reilly S.. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 2020;587:460–465. [DOI] [PubMed] [Google Scholar]