Abstract
Regular aerobic exercise (RAEX) elicits several positive adaptations in all organs and tissues of the body, culminating in improved health and well-being. Indeed, in over half a century, many studies have shown the benefit of RAEX on cardiovascular outcome in terms of morbidity and mortality. RAEX elicits a wide range of functional and structural adaptations in the heart and its coronary circulation, all of which are to maintain optimal myocardial oxygen and nutritional supply during increased demand. Although there is no evidence suggesting that oxidative metabolism is limited by coronary blood flow (CBF) rate in the normal heart even during maximal exercise, increased CBF and capillary exchange capacities have been reported. Adaptations of coronary macro- and microvessels include outward remodelling of epicardial coronary arteries, increased coronary arteriolar size and density, and increased capillary surface area. In addition, there are adjustments in the neural and endothelial regulation of coronary macrovascular tone. Similarly, there are several adaptations at the level of microcirculation, including enhanced (such as nitric oxide mediated) smooth muscle-dependent pressure-induced myogenic constriction and upregulated endothelium-dependent/shear-stress-induced dilation, increasing the range of diameter change. Alterations in the signalling interaction between coronary vessels and cardiac metabolism have also been described. At the molecular and cellular level, ion channels are key players in the local coronary vascular adaptations to RAEX, with enhanced activation of influx of Ca2+ contributing to the increased myogenic tone (via voltage-gated Ca2+ channels) as well as the enhanced endothelium-dependent dilation (via TRPV4 channels). Finally, RAEX elicits a number of beneficial effects on several haemorheological variables that may further improve CBF and myocardial oxygen delivery and nutrient exchange in the microcirculation by stabilizing and extending the range and further optimizing the regulation of myocardial blood flow during exercise. These adaptations also act to prevent and/or delay the development of coronary and cardiac diseases.
Keywords: Autonomic nervous system, Haemodynamic forces, Molecular signalling, Ion channels, Haemorheology
1. Introduction
Regular aerobic exercise (RAEX) elicits adaptations in all tissues and organs of the body,1,2 that culminate in improved health and well-being.3,4 Indeed, in over half a century, many studies have shown benefits of RAEX on cardiovascular outcome in terms of morbidity and mortality data.3 One important effect of RAEX is that it elicits a wide range of functional and structural adaptations in the heart and its coronary circulation,1,2 both in large and microvessels. However, the molecular and cellular mechanisms underlying these adaptations remain incompletely understood. In this article, we review the structural and functional adaptations of the coronary circulation to RAEX, with a particular focus on the cellular and molecular mechanisms underlying these adaptations.
2. Overall cardiovascular adaptations to regular exercise
During acute exercise commands from the central nervous system and exercise pressure reflexes determine the cardiovascular response.5 At the onset of exercise, heart rate increases mainly through vagal withdrawal, with sympathetic activity starting to contribute at heart rates of 100 beats/min.6 With exercise continuing, both metabolic and mechanical signals from active skeletal muscle groups provide feedback signals to the cardiovascular centres in the brain through afferent nervous fibres to balance oxygen delivery with metabolic demand.7 Contracting skeletal muscle produces vasodilator metabolites which override sympathetically mediated vasoconstriction,8,9 resulting in a decrease in local vascular resistance and increased skeletal muscle flow, while in inactive skeletal muscle and other tissues vascular resistance increases allowing the redistribution of cardiac output and maintenance or, in most cases, an increase in systemic arterial blood pressure.1,8,10
Repeated activation of the sympathetic system results in a reduction of sympathetic activity.11 Numerous studies have found that the effects of regular exercise on cardiac autonomic modulation consist of lowering sympathetic activity and greater vagal modulation.12–14 The autonomic nervous system (ANS) modulatory effects are not only observed during exercise but also immediately post-exercise, reflected in a reduction of peripheral resistance (post-exercise hypotension), more rapid heart rate recovery, and lower resting heart rate (55–60 vs. 72–74 L/min). In addition to the greater room for heart-rate (HR) increases, dilations of peripheral vessels allow to achieve a much greater cardiac output during exercise.15 For example, one of the underlying mechanisms is the upregulated nitric oxide (NO) signalling of peripheral resistance vessels as it was shown in exercised rats that the NO releasing substance acetylcholine (ACh) elicited greater systemic blood pressure reduction in exercised than in sedentary rats.16 Also, RAEX via activating the renal–adrenal function and increasing baroreflex sensitivity prepares the cardiovascular (CV) system for the greater workload in exercised individuals as compared to sedentary individuals (Figure 1).17,18
Figure 1.
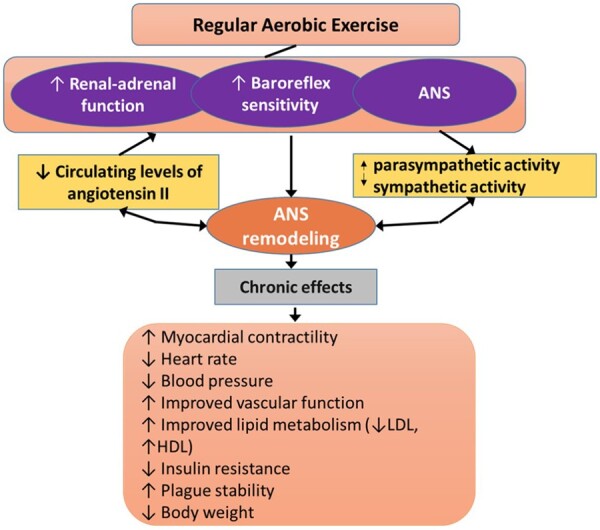
Overall effects of RAEX on the regulation of cardiovascular function by the autonomic nervous system (ANS). In addition to ANS changes, local adaptations, such as myocardial hypertrophy and vascular metabolic adaptations are also important that are not included in this figure. ANS, autonomic nervous system.
RAEX results in a wide range of structural and functional adaptations of the heart and coronary circulation.1,2 For example, RAEX results in moderate concentric cardiac hypertrophy—with an increase in left ventricular end-diastolic diameter and a similar increase in left ventricular wall thickness—that enables a greater maximal stroke volume, cardiac output and hence body oxygen delivery.1 In addition, RAEX lowers HR at rest and during submaximal exercise—through a decrease in sympathetic and an increase in parasympathetic drive—and this lower HR is accompanied by a lower myocardial oxygen demand.1
During exercise systemic blood pressure can reach 200 mmHg or even higher values, whereas cardiac output (blood flow) can increase from 5 to 25 L/min or more. In coronary arterial tree, these changes elicit substantial increases in perfusion pressure and four- to six-fold increase in coronary blood flow (CBF). In addition, coronary vascular wall is sensitive to changes in haemodynamic forces (pressure, shear stress) and translate these haemodynamic signals initially to functional and then structural changes, such as changes in the regulation of vasomotor tone and later remodelling of vascular wall and vascular network. Thus, these mechanisms play important roles in the adaptation of coronary arterial microvessels, which enable the coronary circulation to respond adequately to higher flow demands during exercise.
Indeed, RAEX also leads to a plethora of coronary vascular adaptations that act to maintain optimal myocardial oxygen supply in the presence of RAEX-induced structural and functional cardiac alterations. While there is no evidence to suggest that in the normal heart CBF capacity limits aerobic metabolism even during maximal exercise, an increase in myocardial oxygen supply following RAEX could enhance maximal performance of the heart. The blood’s oxygen transport capacity is typically maintained following RAEX.1 Myocardial oxygen extraction of the left ventricular myocardium may slightly increase following RAEX,19 but this effect is modest at best, as—even in the untrained state—oxygen extraction is already near maximal. Consequently, an increase in oxygen delivery must stem principally from an increase in blood flow. Coronary vascular adaptations in response to exercise training can be divided into functional adaptations (adaptations of vasomotor control) and structural (vascular remodelling and angiogenesis).19 Functional adaptations include changes in neurohumoral and local vascular control mechanisms, that is, myogenic, endothelial, and metabolic control of vasomotor tone.
3. Structural adaptations of the coronary circulation to RAEX
3.1 Structural adaptation of large coronary vessels to RAEX
Numerous studies have shown that RAEX increases large coronary artery diameters. Increased coronary artery size has been reported in rodents, large animals, but also in humans.19–23 These enlargements are proportional to the increase in left ventricular mass in athletes compared to healthy sedentary individuals,22–24 and evidence suggests that this outward remodelling occurs to normalize the shear stress acting on the epicardial coronary artery wall. Haskell et al.25 reported no difference in angiography measured cross-sectional areas of left main, left anterior descending, or right coronary artery between sedentary individuals vs. ultra-distance runners, under resting conditions. In contrast, the increases in coronary cross-sectional areas produced by nitroglycerin were positively correlated with aerobic exercise capacity, suggesting that the increased artery size was only apparent during nitroglycerin-induced dilation.25 Hildick-Smith et al.26 also found a larger nitroglycerin-induced dilation of the left anterior descending coronary artery in athletes compared to sedentary men, and Kozàkovà et al.20 observed that a dipyridamole also produced a greater vasodilation of the left main coronary artery in athletes compared to healthy sedentary individuals. These results are consistent with the concept that RAEX stimulates outward growth of the proximal coronary arteries that is proportional to the level of cardiac hypertrophy, and that the vasodilator capacity of epicardial coronary arteries is greater after RAEX. The simultaneous changes in vascular and cardiac morphology indicate that the most important aim is to provide sufficient blood and oxygen to working cardiac muscle.
3.2 Structural adaptations of coronary microvessels to RAEX
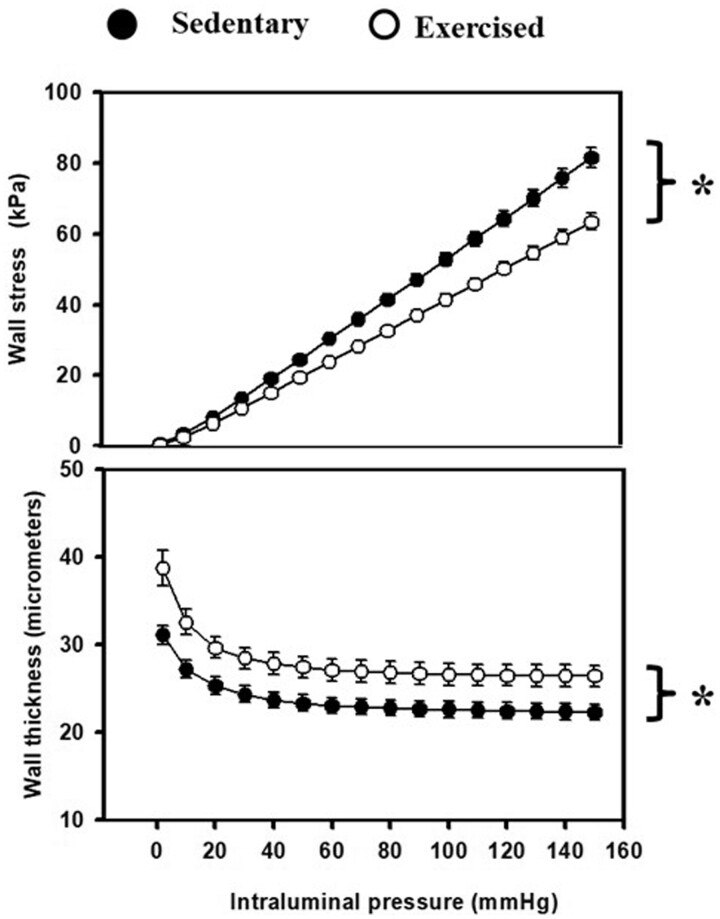
Recent studies showed that increasing intensity of 4-week treadmill RAEX program elicited structural and functional changes in isolated coronary arterioles (∼120 μm in diameter at 50 mm Hg).27 Compared to the sedentary group arterioles isolated from RAEX-animals had reduced wall stress due to thicker walls (Figure 2), accompanied by increased distensibility, and a decreased elastic modulus. Reduced wall stress allows a greater range for regulation of vasomotor tone.
Figure 2.
RAEX induces structural remodelling of rat intramural coronary arterioles resulting—among others—in reduced wall stress due to increased wall thickness (as shown in the figure), but also increased incremental distensibility, decreased incremental tangential elastic modulus. These changes allow a greater constrictor and dilator range for the coronary arterioles to operate. Mean values were used from two-way ANOVA tests with Tukey paired comparisons: *P < 0.05 (modified from ref.,27 with permission of Karger).
The coronary circulation provides ∼1 mL of blood to each gram of myocardium, every minute, 24 h a day, every day of a person’s life. During heavy exercise CBF can increase as much as four- to six-fold. Coronary transport reserve capacity consists of an ability to increase CBF (change in perfusion pressure and diameter) and capillary exchange (change in surface area and permeability) above resting levels.1,28 In normal healthy subjects RAEX increases coronary oxygen transport through an increase in capillary exchange capacity in conjunction with an increase CBF capacity.19
Extensive research with experimental animals demonstrates that RAEX leads to cardiac hypertrophy with capillary angiogenesis commensurate with the increased cardiac mass. Thus, RAEX matches angiogenesis of capillaries to cardiac hypertrophy so that capillarization is maintained in the normal range in rats,19,29 dogs,30–32 and swine.33 Evidence indicates that capillary endothelial cell division and capillary sprouting are increased in the first weeks of RAEX leading to a temporary increase in capillary densities that were no longer different from sedentary swine after 16 weeks of RAEX.34,35 Matching of capillary growth to physiological cardiac hypertrophy is in stark contrast with the capillary rarefaction reported in pathologic forms of myocardial hypertrophy.36 This maintenance of capillary density (capillary/muscle fiber ratio) in hypertrophied myocardium highlights the potential role of RAEX as a strategy to restore capillarity in pathological conditions. There is also evidence of increased myocardial arteriolarization (i.e. increased arteriolar density and cross-sectional area/g of cardiac muscle) following RAEX33–35 and that RAEX increases the compliance of coronary arterioles due to changes in the collagen/elastin ratio within the coronary arteriolar wall.37 These structural changes in the arteriolar tree may be central to increased CBF capacity after RAEX.19
Although the weight of evidence indicates that both coronary capillary diffusion and maximal CBF capacity are elevated by RAEX,1,19,28 there are a few studies reporting no change in maximal CBF capacity.33,38–44 Clinical studies of CBF capacity measured with positron emission tomography or echo-Doppler also yield a mixed view of effects of RAEX on CBF capacity with some reporting no change,45–48 and others reporting an increase in maximal CBF.20,21,24,26,49 Careful inspection of all these studies indicates that studies conducted in the presence of proven maximal vasodilation and controlled haemodynamic variables consistently report that following RAEX the CBF capacity is increased in swine,35,50 dogs,30,51 and rats.28,29,50–55
Studies in dogs and miniature swine have shown that RAEX also increases capillary exchange capacity.1,19,28 Interestingly, morphometric measurements of capillarization and capillary exchange capacity in the same hearts revealed that RAEX elevated coronary capillary exchange capacity in the absence of an increase in capillary numerical density.1,19,28,34,56 These observations suggest that RAEX produces changes in the distribution of coronary vascular resistance, likely in conjunction with a small increase in capillary diameter,35 together resulting in an increase in effective capillary surface area in the absence of a change in capillary density.19 These adaptations are likely responsible for the reported increase in myocardial oxygen extraction and lower coronary sinus oxygen content following exercise.1,19,28
In summary, coronary capillary exchange capacity and maximal CBF capacity are both increased by RAEX, acting in synergy to improve the myocardial oxygen delivery capacity and reserve. The increase in coronary transport capacity is the result of structural adaptations including enlargement of large coronary arteries and increases in arteriolar and capillary surface area, as well as functional adaptations including alterations in coronary epicardial artery, resistance artery, and arteriolar vasomotor control.
4. Functional adaptations of the coronary circulation to RAEX
4.1 Adaptations in autonomic control: large vs. microvessels
Although it is well established that RAEX increases parasympathetic and decrease sympathetic activity to the heart, RAEX appears to cause only minor alterations in autonomic control of CBF.57–59 Thus, after RAEX adrenergic tone is slightly increased or maintained in coronary resistance vessels, so that during submaximal exercise there is maintained β-adrenergic vasodilation and only slightly increased or maintained α-adrenergic constriction.19 In proximal coronary arteries, α1-adrenergic receptor stimulation was found to be blunted by RAEX in dogs60,61 and swine.62 Stehno-Bittel et al.63,64 showed that the decreased vasoconstrictor reactivity is due to RAEX-induced lowering of intracellular concentrations of calcium (Ca[i]) in coronary vascular smooth muscle (VSM) cells. In contrast, RAEX does not appear to alter vasoconstrictor responses of proximal coronary arteries to prostaglandin (PG) F2α or KCl.61,62
4.2 Adaptation of the endothelium: large vs. microvessels
In addition to morphological remodelling of resistance vessels, RAEX also changes the contribution of NO and PGs to the regulation of coronary vascular resistance (Figure 3). Thus, RAEX for 7 days, 2 h/day was reported to enhance endothelium-dependent dilation (EDD) following post-occlusion reactive hyperaemia and administration of ACh.65 In contrast, no change was seen in EDD of proximal coronary arteries after longer RAEX programs (>10 weeks) in dogs,61 swine,66 or rats.67 It appears that longer RAEX programs were accompanied by outward remodelling of the epicardial coronary arteries, resulting in a normalization of wall shear stress levels during exercise bouts, therefore endothlial-nitric-oxide-synthase (eNOS) expression returns to normal levels68,69 and—hence EDD responses—back towards baseline levels.61,66,68,70 RAEX increases EDD in coronary microcirculation as reflected in enhanced serotonin-induced increases CBF60 and increased bradykinin-induced dilation in isolated coronary arterioles (64–157 μm in diameter).71 The increased EDD is due to increased eNOS-derived NO, since eNOS expression is increased in coronary arterioles after RAEX.69 Interestingly, RAEX produces sustained augmentation of EDD in coronary microvessels, while it increases EDD only transiently in epicardial conduit arteries. Heterogeneous effects of RAEX on gene expression along the arterial tree has also been reported in skeletal muscle and diaphragma.72,73 These observations indicate that RAEX has heterogeneous effects in the various part of the vascular system, likely due to heterogeneity of haemodynamic forces they are exposed to.
Figure 3.
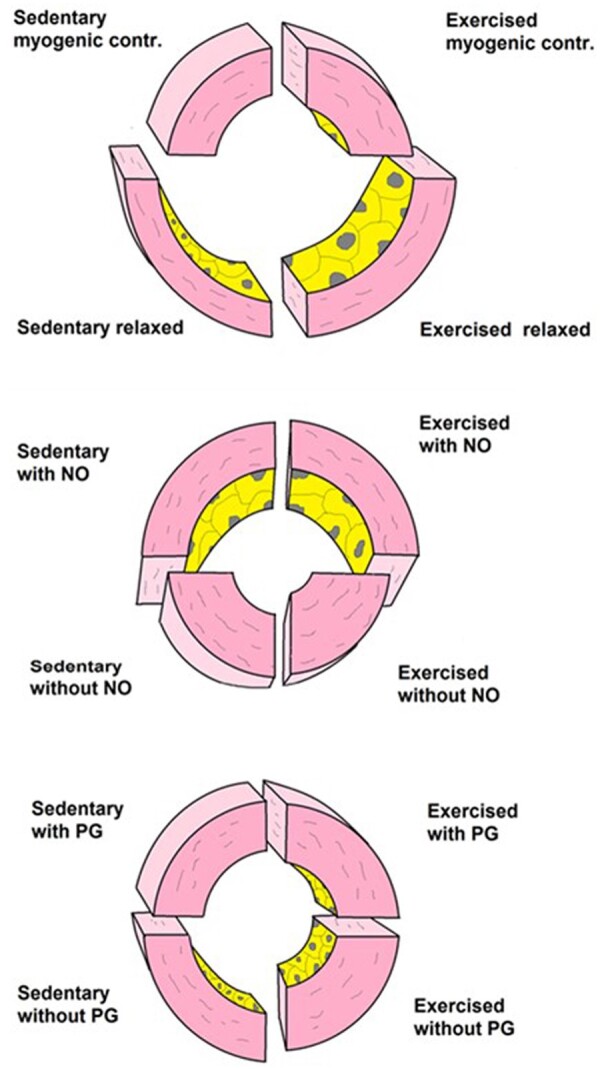
Illustration of morphological adaptations of small coronary arteries of rats to RAEX. Top panel: sedentary and exercised vessel in control condition at two intraluminal pressure values: upper half at 30 mmHg and lower half at 120 mmHg. RAEX elicits growth of vessels and thickening of vascular wall, resulting in reduced wall stress and increased elasticity of vessels. Middle panel illustrates the effect of NO, whereas the lower panel illustrates the effect of PG27 (with permission of Karger). NO, nitric oxide; PG, prostaglandin.
4.3 Adaptation of the pressure-sensitive myogenic mechanism
Systemic, thus coronary blood perfusion pressure greatly increases during exercise. Thus one would expect that the pressure-sensitive myogenic response of coronary vessels is affected as well. Indeed, RAEX increases myogenic constriction in coronary arterioles37,71 (Figure 3) perhaps resulting from altered calcium-dependent protein kinase C (PKC) signalling in coronary VSM cells74 or increased calcium currents through L-type calcium channels in VSM of large arterioles.75 The enhanced vasoconstrictor response to stretch (myogenic reactivity) is not accompanied by alterations in receptor-mediated vasoconstriction to endothelin (ET-1) or ACh, or direct stimulation of voltage-gated calcium channel activation with K+ or by the L-type calcium channel agonist Bay K8644.76 RAEX may also alter calcium control by sarcoplasmic reticulum and/or increase KCa and Kv channel activity in coronary VSM.52,77,78 Studies have shown that coronary arterioles of RAEX-swine79 and rats27 exhibited a more powerful myogenic response, i.e. the diameter of arterioles were less in the pressure range of 60–120 mmHg.
The greater constriction in this pressure range is the result of increased smooth muscle contractility and altered endothelium-derived factors. RAEX may upregulate the calcium-dependent PKC,19,74 increase activation of voltage-sensitive calcium channels and/or cGMP-sensitive calcium-dependent chloride channels, and voltage-gated calcium-dependent potassium channels leading to increased intracellular calcium oscillation.80,81
In coronary arterioles from sedentary animals an appropriate level of myogenic response is also maintained by the contribution of constrictor PGs released primarily from the endothelium, whereas in RAEX-arterioles such role for endogenous vasoconstrictor prostanoids is absent. Instead, RAEX-induced augmentation of myogenic mechanism—the increased force generation to pressure—became ‘built in’ the smooth muscle, instead of relying on endothelial constrictor factors, especially at higher pressures.
Interestingly, NO had a greater contribution to the relaxation of exercised rat coronary arterioles at lower pressures (<60 mm Hg) compared to that of sedentary vessels. One can extrapolate this finding to the regulation of coronary circulation and propose that NO-induced opening of coronary microvessels can be important during diastole when intraluminal pressure is lower and the limited diameter effect of NO at higher pressure values protects the distal part of microcirculation from high perfusion pressure. These changes contribute to the extension of the autoregulatory range in coronary vascular tree (Figure 4) important to maintain CBF in face of varying pressure.
Figure 4.
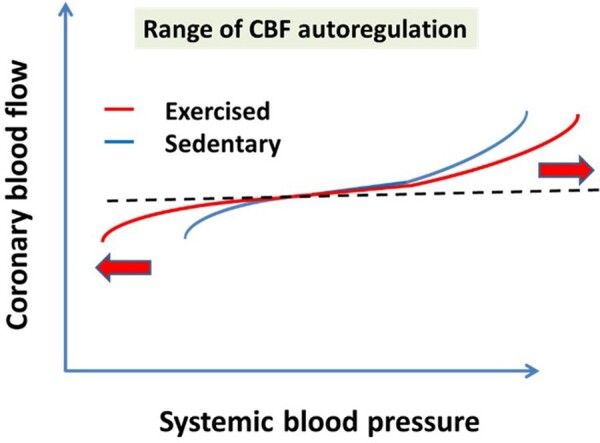
On the basis of experimental findings we propose that RAEX extends the range of CBF autoregulation resulting in a close to maintained CBF both at lower and higher pressures, as well. This is primarily due to the upregulated pressure-sensitive myogenic mechanism, a condition, which is then further modulated by local and remote vasomotor mechanisms. CBF, coronary blood flow.
4.4 Exercise-induced upregulation of flow/shear stress-induced vasodilator mechanism
During RAEX, in addition to pressure, blood flow and thus wall shear stress is also increased in coronary vessels. Around 1990s it was demonstrated in vivo that small arterioles are sensitive to changes in flow/shear stress acting on the inner surface of the endothelium and respond to this force with dilation (mechanotransduction) due to the release of various dilator factors [NO, PGs endothelium-derived-hyperpolarizing-factor (EDHF), reactive-oxygen-species (ROS)] from the endothelium.82–84 The dilation then reduces wall shear stress back to normal levels. Thus the haemodynamic force-sensitive myogenic and flow/shear stress-dependent mechanisms are importantly involved in the regulation of the coronary microcirculation, especially because in smaller vessels these forces elicits greater diameter responses.82 Thus it was logical to hypothesize that regular exercise by eliciting great increases in blood flow (which in the presence of constant diameter can only be achieved by increases in blood flow velocity resulting an in increase in wall shear stress) will result in an adaptation/upregulation of the ‘flow sensitive’ mechanism.85 Indeed, previously we have found that a short-term exercise program augmented flow/shear stress-induced dilations of arterioles (skeletal) that are mediated by simultaneous release of NO and PGs.86–89 Also an enhanced basal tone of exercised arterioles was found compared to sedentary vessels.86 The upregulation of shear stress-induced dilation contributes to enlargement of the functional dilator capacity of coronary vascular tree (Figure 5). On the basis of studies on isolated small skeletal muscle veins one can assume that during exercise coronary venular system also dilates to increases in flow/shear stress due to release of NO and PGs90 contributing to the increased blood supply of the cardiac muscle. The salient finding is that increases in shear stress produced by increases in perfusate flow velocity result in vasodilation of venules isolated from skeletal muscle, thereby keeping its level close to constant, which can have rheological consequences as well. An increase in shear stress results in the release of endothelium-derived NO and PGs, whereas in the absence of these vasodilator factors, the release of an endothelium-derived vasoconstrictor factor in response to an increase in perfusate flow was also uncovered.
Figure 5.
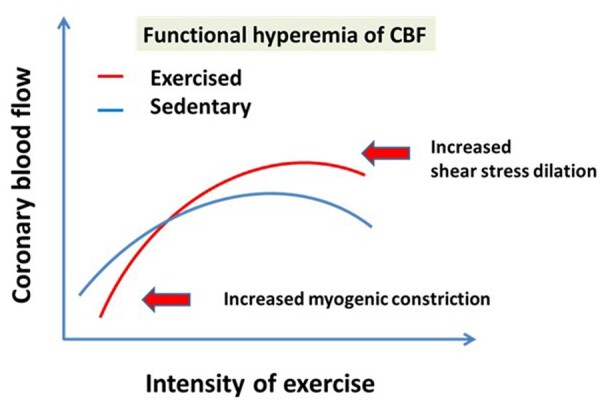
On the basis of experimental findings we propose that RAEX extends the maximum range of CBF resulting in exercise-induced great functional hyperaemia due to the upregulated pressure and flow/shear stress-sensitive vasomotor mechanisms, a basal tone which is then further modified by local and remote vasomotor mechanisms. CBF, coronary blood flow.
On the molecular level, Laughlin et al.91 observed in coronary resistance vessels isolated from exercised pigs that eNOS mRNA and protein expression were increased compared to that of sedentary pigs. The increases in eNOS and Cu/Zn SOD expression were shown to be a flow-dependent phenomenon.92 These investigators subsequently showed that RAEX-induced increases in eNOS content is not uniformly present along the coronary tree, which may be due to the type of muscle fibres they supply and/or mechanical forces exerted on their lumen.69,91–93 In addition, Yang et al.94 demonstrated that RAEX increases both endothelial and inducible NOS gene expression in rat aorta endothelial cells.
The type of exercise can be also important to activate these mechanosensitive mechanisms. For example, sudden high-intensity interval exercise results in sudden increase in intraluminal pressure and likely wall shear stress eliciting maximal stimulation of these mechanisms. For example, sudden increase in pressure leads to an increased production of ROS95 known to play crucial role in the initiation of adaptive mechanisms leading to vascular remodelling. Also, a sudden increase in wall shear stress can elicit greater release of NO (in contrast to low-intensity exercise) via activation of platelet-endothelial adhesion molecule system.96–98 The intensive effect of high temporal gradients shear stress could be incorporated in the design of exercise programs and may explain the pronounced efficacy of brief exercise at high intensity.
All in all, RAEX is accompanied with great increases in haemodynamic forces, which then elicit changes in the mechanosensitive vascular mechanisms, which are ‘translating’ physical forces to adaptive diameter changes and are balancing each other.82,89 Depending on the prevailing levels of wall shear stress and pressure, these two mechanisms together determine the basal diameter of arterioles, which is then further modulated by metabolic and neurohormonal mechanisms. In addition, these forces use similar signalling pathways, which in a long run elicit morphological vascular remodelling such as increased wall thickness and elasticity (Figure 2). These adaptations then allow a greater range for the regulation of CBF to match the needs of working cardiac muscle during exercise at various pressure and flow conditions. This has also been discussed in detail in recent reviews.19,85,99 Such remodelling of coronary arterioles is likely contributing to the optimization of CBF during exercise producing a greater range of coronary autoregulation, i.e. a greater constrictor and dilator reserve (Figure 4), and thereby more effective protection against coronary vascular diseases.
4.5 Adaptation of metabolic control
RAEX has been reported to increase the maximal adenosine-induced increase in CBF per gram of myocardial tissue in miniature swine and dogs.19 At corresponding levels of left ventricular work CBF is not changed by RAEX indicating minimal RAEX-induced changes in the coupling between myocardial metabolism and CBF.1,19,28 The interesting findings of Kuo and Chancellor100 in the porcine subepicardial coronary arterioles (50–150 microns) extend this picture in a sense that the metabolic mediator adenosine—which is released in a threshold concentration during exercise—potentiated flow/shear stress-induced dilations of coronary arterioles by activating KATP channels in the endothelium. This mechanism could be more important when oxygen supply is not matched by demands (slight hypoxia) thus more adenosine is produced from breakdown of ATP, which metabolism is limited in this conditions.
Thus, the interplay among the local mechanisms ensures an optimal supply of blood flow during and after exercise to maintain the cardiac muscle in aerobic condition as long as possible. Interestingly, animal (porcine) experiments have shown that NO-mediated dilations are impaired distal to coronary artery narrowing (stenotic or non-stenotic), which could be reversed by exercise training. This was achieved by an additional activation of a hydrogen peroxide-mediated dilator mechanism.101
4.6 Adaptation of other vasomotor factors
Interestingly, there is little if any evidence for a diminished constrictor tone in coronary microvessels following RAEX. For example, vasoconstrictor responses to ET-1, ACh, voltage-gated calcium channel activator Bay K8644, or high-dose K+ are all maintained, and myogenic tone is even enhanced, following RAEX.19 Thus, a physiological antagonism, i.e. a decrease in constrictor mechanism is not a likely explanation for the increased vasodilator influence of NO. While there is evidence that RAEX enhances bradykinin-induced endothelium-dependent dilations in porcine coronary arterioles (64–157 micron in diameter), the observation that cytosolic Cu/Zn SOD (SOD-1) is also upregulated by RAEX indeed suggests that the increased endothelium-dependent dilator responses could be, at least in part, the result of a decreased quenching of NO by superoxide. However, the vasodilator response to sodium nitroprusside is not different between sedentary and RAEX swine, suggesting that RAEX principally increases NO production rather than increasing its half-life.19 Consistent with this interpretation, the increased eNOS content in the coronary arterioles of exercised swine described by Laughlin et al.69 may likewise account for the enhanced EDD in conscious exercised dogs found by Wang et al.65
Interestingly, RAEX does not appear to increase resistance vessel sensitivity to adenosine in isolated porcine coronary resistance vessels (which is thought to be endothelium-independent), further pointing towards the unique effects of RAEX on NO production in the coronary microvasculature. On the other hand, the coronary vasodilator sensitivity to adenosine appears to be enhanced in vivo.19 An explanation for the different observations with adenosine in vitro vs. in vivo could be the flow-mediated and endothelium-(NO)-dependent dilations of upstream small arteries and larger arterioles elicited by the increased pressure drop across the arterial network due to the adenosine-induced dilation of the very small arterioles.19 Alternatively, the structural adaptations (i.e. increase in arteriolar densities and size) or the reduced extravascular compressive forces (due to RAEX-induced lowering of heart rate) may facilitate an increase in CBF in response to adenosine even in the absence of an increased sensitivity of the individual coronary arterioles.
Taken together, these findings indicate the complexity of adaptations, and while perhaps not all mechanisms are revealed, the beneficial effects of RAEX on coronary circulation—even in diseased conditions—are unquestionable.
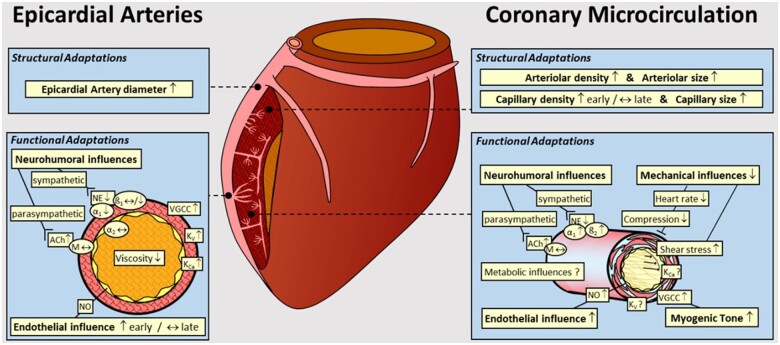
Thus as summarized in Figure 6, RAEX-induced adaptations in the coronary circulation—epicardial and microcirculation—include both structural (angiogenesis and vascular remodelling) and functional (alterations in control of vascular resistance) adaptations.19,52,53,102 Adaptations in the regulation of coronary vascular resistance are elicited by changes in neurohumoral, cardiac metabolic, and local vasomotor mechanisms and their interplay. All these beneficial modifications play important roles in the RAEX-induced increase in myocardial oxygen (and nutrition) delivery and extraction during and following exercise activities.19
Figure 6.
Graph summarizing the structural and functional coronary adaptations to RAEX in normal subjects. The coronary circulation has been divided into Epicardial Arteries and the Coronary Microcirculation, showing the structural and functional adaptations detailed for each of these coronary vascular compartments. α1, α1-adrenergic receptor; ß1, ß1-adrenergic receptor; ß2, ß2-adrenergic receptor; ACh, acetylcholine; KCa, Ca2+-dependent K channel; Kv, voltage-dependent K channel; M, muscarinic receptor; NE, norepinephrine; NO, nitric oxide; VGCC, voltage-gated Ca2+ channel. Modified from ref.19 with permission of the American Physiological Society.
4.7 Interactions among blood, coronary vessels and cardiac tissue
For the sake of simplicity it is customary to treat events taking place in blood vessels and cardiac muscle separately; however, they are—together with other tissues in the heart—form a ‘continuum’, which allow significant interactions among them. In vivo, there are no ‘hard’ borders among tissues. Molecular factors or even cells are trafficking between the myocardium, smooth muscle cells, endothelium, and blood by a variety of mechanisms, including diffusion, passive or active transport across membranes, tight junctions, etc. Many details need to be considered, but we would like to highlight this important aspect, with one particular signalling mechanism outlined in Figure 7.
Figure 7.
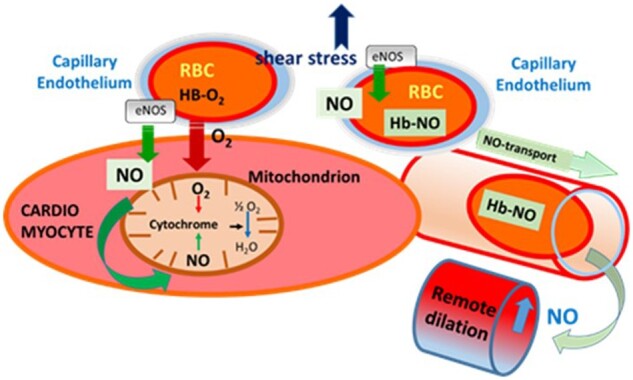
Schematic illustration of the effects of eNOS-derived NO released from capillary endothelium in response to increases in flow/wall shear stress, during exercise on mitochondrial function and remote vascular dilation. The highly diffusible NO can reach and then bind to cytochromes in the mitochondria of cardiac muscle, but also can be carried away by binding to the haemoglobin in RBC and later released eliciting dilation of remote microvessels, thereby increasing oxygen supply. eNOS, endothlial-nitric-oxide-synthase; NO, nitric oxide; RBC, red-blood-cell.
Because myocardial oxygen extraction is close to maximal, already in resting conditions, CBF must greatly increase to supply the myocardium with sufficient oxygen during exercise. Nevertheless, there is evidence that RAEX produces a small further increase in oxygen extraction capacity, likely as a result of an increase in capillary exchange capacity.19,103 Sudden increases in blood flow/wall shear stress—especially during acceleration type exercise—induces high temporal gradients,98 which greatly stimulate eNOS to release large amounts of NO, which then elicit great dilations. In addition, vascular, thus endothelial deformation during each cardiac cycle can lead to NO release—as shown previously104—contributing to coronary dilations. All these mechanisms despite continuously increased cardiac load, delay the development of hypoxia and development of anaerobic, lactic acid metabolism.
Although many of the signalling roles of NO have been appreciated (such as controlling vascular tone, platelet aggregation, adhesion, and vascular growth, etc.) two should be emphasized here, as they have a major impact on both coronary and cardiac function. First, about two decade ago Kobzik et al.105 have shown that eNOS is expressed in the capillary wall, which was later confirmed in the heart by Hintze et al.106–108 Since in the capillary wall consists exclusively of endothelial cells and is devoid of smooth muscle, it was suggested that NO may serve an additional role in this part of the microcirculation. Indeed NO as a highly diffusible molecule can reach the mitochondria underneath the cell membranes of cardiac muscle fibres and compete for the oxygen binding site at cytochrome A3.105
Early studies of Kayar and Banchero109 showed that groups of mitochondria are located very close to the wall of capillaries further supporting this concept that physiologic levels of NO can exert a tonic control of cellular respiration and metabolism108 by regulating tissue oxygen consumption through reversible inhibition of cytochrome c oxidase in the mitochondrial respiratory chain as well as substrate selection.108 Second, Stamler et al.110 and later Allen et al.111 showed that NO binds to haemoglobin in the erythrocytes to form the more stable Hb-NO, from which NO can be released under conditions of low oxygen, eliciting vasodilation and increasing blood flow in remote areas or back flow to proximally occluded network segments to prevent/reduced tissue hypoxia. This inter-tissue signalling among blood, coronary vessels, and the myocardium likely optimizes cardiac performance thereby contributing to the cardiovascular adaptations to exercise.
4.8 Adaptations in ion channel function in the vascular wall
In addition to molecular mediators, ion channels are also involved in the regulation of coronary resistance and in their adaptations to RAEX as indicated partly in Figure 6. However, the role of these channels in the adaptation to RAEX deserves a more detailed analysis.
4.8.1 Ion channels in the microcirculation
Opening and closing of ion channels, in vascular cells particularly K+ channels, modulate the electrical potential across the plasma membrane (membrane potential) which impacts on the activity of (other) voltage-gated ion channels thereby feeding back to membrane potential or altering cytosolic ion concentrations.112 The latter effect is specifically important for Ca2+ because cytosolic Ca2+ concentrations are very low. In smooth muscle cells, Ca2+ activates the contractile machinery and thereby significantly determines arteriolar tone. Moreover, Ca2+ is also an important activator of specific K+ channels (Ca2+-dependent K+ channels), which are expressed mainly in endothelial cells, and in this setting localized Ca2+ increases are sufficient to induce opening of such channels. As the opening of K+ channels drives the membrane potential from the physiological range of −45 to −30 mV towards the K+ equilibrium potential of about −90 mV (hyperpolarization) it prevents the opening of voltage-gated Ca2+ channels (VGCC) in smooth muscle cells and thereby promotes arteriolar dilation.113 The activation of endothelial K+ channels likewise induces hyperpolarization which initiates a similarly directed membrane potential change in smooth muscle either through gap junctional-mediated coupling or by the transfer of a factor that induces the hyperpolarization in these adjacent cells (endothelium-derived hyperpolarization, EDH).114,115
The most important modulators are K+ channels and they consist in vascular cells of four (or more) classes, namely voltage-gated (KV), Ca2+-dependent (KCa), inwardly rectifying (KIR), and ATP-dependent (KATP). K+ channels have been implicated in functional dilation116,117 (specifically in the coronary vascular bed KV1.5 and KV1.3)118,119 and are therefore also likely targets of exercise training. The focus in this section will therefore be on K+ channels and the main effector in smooth muscle, i.e. VGCC.
4.8.2 Ion channels in smooth muscle cells affected by RAEX
An enhanced vascular tone is frequently observed in exercised animals19 most likely due to a selectively exaggerated myogenic tone (see above) specifically in resistance vessels.76,79 Myogenic responses are well known to rely on depolarization and subsequent opening of VGCC120 and, in fact, smooth muscle cells isolated from coronary vessels of different sizes of exercise-trained animals exhibited two-fold Ca2+ current densities through L-type VGCC.75 Whether this phenomenon is due to enhanced channel expression or related to a phosphorylation of the channel with enhanced conductance remains to be determined. Concomitantly, constrictions upon non-selective K+ channel blockade were enhanced in such vessels indicating increased negative feedback through K+ channels thereby limiting further constrictions. However, the enhanced K+ channel activity was not observed in isolated smooth muscle cells suggesting that the density of K+ channels and their conductance remained unchanged.121 Similar results were obtained in a follow-up study for overall K+ currents or currents through KCa channels (KCa1.1) in isolated smooth muscle cells from exercised animals.122
This suggests that these channels are only more active in intact pressurized vessels and pressure (and stretch) may then provide the appropriate stimulus which is either a stronger depolarization (KV channels)120 or subsequent enhanced Ca2+ influx (KCa channels).123,124 Despite enhanced Ca2+ currents and higher rates of cytosolic Ca2+ increases the net accumulation of free Ca2+ was not increased suggesting a compensatory Ca2+ extrusion mechanism (distinct from SERCA pump and sodium-calcium exchange) which prevents overshooting Ca2+ levels in vessels of exercised animals.77 Taken together, modulation of ion channel function, mainly VGCC, in smooth muscle supports enhanced arteriolar tone in the resting state in exercise-trained animals.125
4.8.3 Ion channels in endothelial cells potentially targeted by regular exercise
Exercise-induced flow increases may increase shear stress acting on endothelial cells85,126 which is a major factor contributing to augmented NO-mediated responses in the coronary circulation as discussed above. It is also well established that endothelial K+ channels are important players in EDD, particularly in arterioles.115,127 Although some K+ channels are likewise activated in a flow-dependent manner, little is known about exercise-induced changes of the function of these endothelial K+ channels in coronary arterioles. Only rarely EDH-type dilations are investigated although a beneficial effect of regular exercise was reported more than 20 years ago.128 In the endothelium two types of KCa channels (KCa3.1/IKCa and KCa2.3/SKCa) and the inwardly rectifying K+ channel (KIR) are functionally dominant.129,130 Specifically, KCa are activators of the EDH-type dilation.127,131,132 SKCa channels are also activated by shear stress132 and support active hyperaemia133 (Figure 8).
Figure 8.
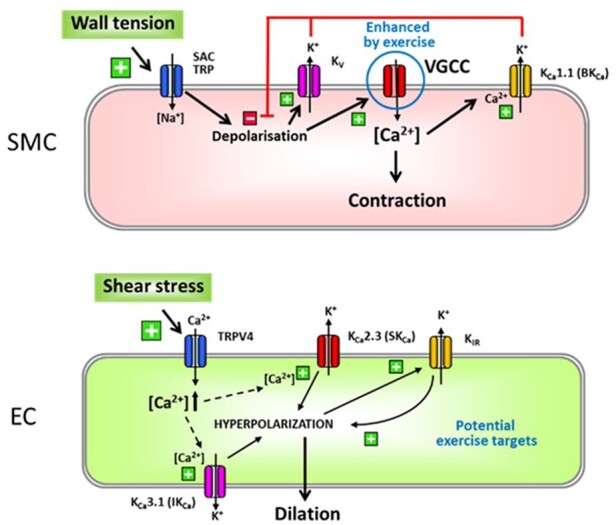
Regular exercise may affect multiple ion channels in SMC (upper panel) or EC (bottom panel). However, experimental data have shown up to now only enhanced Ca2+ currents through VGCC (encircled in blue) in SMC after RAEX. This is either due to changes in conductance upon channel phosphorylation or due to an upregulation at the protein level. VGCC are involved in myogenic responses initiated by a depolarization through activation of SAC or TRP channels. The increased activity of K+ channels (BKCa and KV) found in exercised animals is most likely attributable to the initial enhanced VGCC activity because they act in negative feedback manner limiting depolarization. The ion channels involved in shear stress-induced endothelium-dependent responses are shown for EC. TRPV4 channels enable Ca2+ influx leading to activation of SKCa and IKCa and subsequent EDH-type dilation, while endothelial KIR acts as an amplifier of hyperpolarizing signals. All channels may be functionally modulated by RAEX. However, up to date this was not verified at the molecular level although RAEX enhances EDDs including EDH-type dilation. For further details see text. EC, endothelial cells; SAC, stretch-activated channels; SMC, smooth muscle cells; TRP, transient receptor potential channels; VGCC, voltage-gated Ca2+ channel.
Endothelial Ca2+ increases induced by agonists or wall shear stress may not only enhance NO production but also activate KCa channels. In this setting, the transient receptor potential channel TRPV4134 is reported to contribute by enabling Ca2+ influx135,136 generating localized Ca2+ increases that activate KCa channels137,138 or release mitochondrial reactive oxygen species to mediate flow/shear stress-induced dilations in coronary arterioles.139 In endothelial cells in vitro, TRPV4 channels are redistributed upon mechanical stimulation140 which suggests that exercise may also have an impact on these signalling cascades in coronary endothelium (Figure 8).
Future work will possibly elucidate these pathways further and hopefully define a more precise picture of the phenomenon which is nowadays briefly summarized as an enhancement of EDD, specifically pronounced in cardiac diseased conditions141 or during physiologic ageing.142,143
5. Effects of exercise on ‘haemorheological’ resistance
From the Hagen–Poiseuille equation it is clear that viscosity also an important factor determining resistance to flow. Thus although it is not specific to coronary circulation, changes in the properties of blood in response to RAEX can greatly affect the blood delivery function of coronary vascular tree as well. At present, however, there are no means to specifically determine rheological changes only in the coronary lumens, yet they can be very important. Because in smaller vessels blood behaves as a non-Newtonian fluid, its viscosity varies with haemodynamic conditions and shear rate. Blood becomes less viscous at high shear rates, which occur with increased flow (velocity), such as during exercise or in peak-systole. Contrarily, blood viscosity increases when shear rate goes down as with increased vessel diameters or with low flow, such as downstream from an obstruction or during diastole.144 One of the most important determinant of plasma viscosity is fibrinogen concentration, a haemostatic protein, which level could be affected by exercise. Changes in the balance between haemorheological and haemostatic determinants can lead to thrombotic complications.145 Exercise affects haemorheology and haemostasis but results are equivocal, likely due to confounding factors, such as age, exercise intensity and duration, or exercise conditions of the individual.146
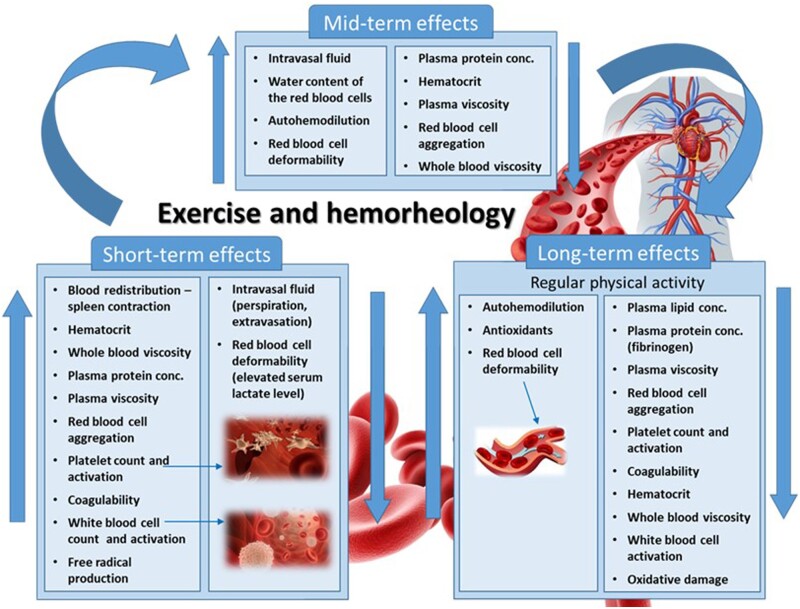
Acute exercise is associated with a rise in haematocrit, decreased red blood cell deformability, increased red blood cell aggregation, and increased fibrinogen level and therefore with high plasma and whole blood viscosity. These haemorheological changes result in reduced capillary circulation—acting against haemodynamic changes—and thus can diminish tissue perfusion. In contrast, RAEX has a beneficial effect on all of these haemorheological parameters (called ‘haemorheological fitness’) reducing cardiovascular risk and improving capillary blood flow even in patients with ischaemic heart disease (Figure 9).
Figure 9.
Illustration of short-, medium-, and long-term effects of RAEX on the rheological behaviours of constituents and cellular elements blood achieving rheological fitness further supporting the blood to pass the extremely complex microvascular network of coronary circulation.
A large number of studies in healthy humans, considering different RAEX programs, show a direct association between intensity of aerobic physical activity (endurance and resistance exercise) and transient pro-thrombotic states,147–151 including increased platelet counts, higher levels of coagulation factors (i.e. FVIII) and/or reduced fibrinolytic factors and stimulated coagulation activity mainly through activation of the intrinsic-pathway resulting in increased levels of fibrinopeptide A and D-dimers, as markers of fibrin formation.151,152 Current evidence supports that exercise intensity rather than duration is the main factor modulating the haemostatic parameters. Regular exercise returns the initial activation of pro-thrombotic variables to baseline153,154 preventing thrombosis. It is of note that acute exercise (in sedentary individuals) can induce hyperviscosity (mostly due to haemoconcentration and alterations of red blood cell properties), whereas regular exercise improves all haemorheological parameters, thus improving haemorheological fitness (Figure 9).
6. Sex, age, and cold environment modulate cardiovascular responses to exercise
6.1 Influence of sex
Acute exercise increases sympathetic tone, which results in positive chronotropic and inotropic responses, thereby increasing myocardial oxygen demand. High levels of sympathetic drive also stimulate the endothelial cells to produce vasodilator molecules, such as NO. Several studies have shown sex differences with respect to autonomic nervous control of cardiac function in response to RAEX. Despite higher heart rates, women exhibit lower sympathetic activity, greater vagal modulation, and reactivation following maximal exercise, while men display elevated resting sympathetic tone.155–157 Sex differences in cardiac autonomic modulation are largely described in premenopausal women as compared to age-matched men.158–161 Oestrogens have been shown to prevent parasympathetic/sympathetic imbalance and improve baroreflex sensitivity.162 Sex-specific differences are mitigated progressively after the menopause and the sex-related difference of heart rate variability (HRV) also decreases with ageing.161 Additionally, oestrogen may improve vasomotor tone though the vasodilator effect of β‐adrenergic activation, which also activates NO mechanism and lowers blood pressure.162,163 It was also found that the presence of oestrogen via an increased NO release and antioxidant activity result in a greater flow-dependent dilation.164–166 Recently, after 12 weeks of swimming exercise program Török et al.167 found many similar adaptation in rat coronary vessels isolated from both sexes compared to that of sedentary controls, but they also found differences related to sex, such an increased spontaneous and TxA2 agonist-induced tone in coronaries of exercised females and a more effective endothelium-dependent relaxation in coronaries of exercised males compared to that of sedentary groups.167 At the moment, the clinical consequences of these experimental findings are not yet clear and highlight the existence of still somewhat conflicting findings in sex-related adaptation of coronary vascular tree and other vascular territories.
6.2 Influence of age
Ageing is associated with sympathetic dysregulation and a decrease in heart rate variability (HRV) as well as endothelial dysfunction, which are associated with abnormal regulation of CBF.168 Elderly healthy individuals have a reduced exercise tolerance and a decreased left ventricular inotropic reserve related to increased afterload, physical deconditioning, and impaired autonomic regulation resulting—in part—from ‘beta-adrenergic desensitization’.161,169,170 Beta-adrenergic receptor density reduction may lower intracellular Ca2+ transients with subsequent impaired inotropic and chronotropic responses to adrenergic stimuli.161,170–172 A recent study in old individuals showed that 5-year leisure-time activity and walking were associated with higher global HRV and vagal-related indexes indicative of improved circadian fluctuations, which suggests that RAEX may counterbalance the age-related decline in cardiac autonomic control.173 According to the above-mentioned hypothesis, increased vagal tone appears to induce an anti-inflammatory milieu: HRV is inversely related to the production of many inflammatory markers such as interleukin-6, C-reactive protein, and fibrinogen in healthy individuals as well as in older adults especially in cardiovascular disease.174,175 In sum, these observations suggest that preserved vagal tone is crucial to cardiovascular health in ageing and RAEX can potentially modulate cardiac ageing phenotypes.
6.3 Influence of ambient temperature
A cold environment can have an effect on the adaptation of cardiovascular system to regular exercise by its impact on the parasympathetic/sympathetic balance. Exposure to cold leads to various parasympathetic/sympathetic responses such as an increase in heart rate probably due to parasympathetic withdrawal including, an increase in systemic vascular resistance, systolic and diastolic blood pressure.163,176–179 Initially cold exposure increases sympathetic activity, which is blunted, whereas parasympathetic activity is enhanced after cold acclimation. In sum, one can assert that cold adaptation lowers sympathetic activation and causes a shift towards increased parasympathetic activity,180 with the consequent changes on CBF.
More importantly an underlying cardiovascular disease (CAD) may modify the relationship between exercise and acute or prolonged cold exposure. Cold exposure reduces myocardial oxygen supply in CAD, which may lead to more severe ischaemia. Exercise in cold augments cardiac workload in patients with CAD more than when it is performed in regular temperature/condition. At the same time, exercise may reduce myocardial perfusion, due to endothelial dysfunction or flow-limiting stenosis, leading to early ischaemia, angina, impaired performance, and release of pro-arrhythmic substrates.18,163,179 Furthermore, in the setting of CAD, autoregulation may lead to uneven distribution of microvascular resistance and thus flow among different coronary territories. In case of increased myocardial oxygen demand/supply, such as cold exposure the accentuation of this pathophysiological condition can lead to haemodynamic interactions between a collateral receiving and collateral supplying vascular bed in the form of coronary steal, defined as a drop of coronary flow below resting levels in the affected area. The phenomenon of coronary steal is clinically relevant due to a prevalence of 10–20% in non-obstructive CAD.181–184
Another interesting issue of relevance is the exercise in cold water. It has been hypothesized that submersion in cold water and the release of breath holding promotes an ‘autonomic conflict’ due to activation of two opposing reflexes ‘cold shock reflex’ and the ‘diving reflex’.185 The ‘cold shock reflex’ driven by cutaneous cold thermoreceptors induces the activation of a sympathetically driven tachycardia, hyperventilation, peripheral vasoconstriction, and hypertension. The ‘diving reflex’ driven by activation of facial trigeminal receptors during facial immersion promotes an acute bradycardia mediated by the vagus and an expiratory apnoea, which in turn leads to arterial hypoxaemia and hypercapnia producing further vasoconstriction.185 This ‘autonomic conflict’ may be responsible for sudden alteration in coronary circulation, arrhythmias, particularly at release of breath holding, which increases vagal tone that varies with respiration.179,185 Such disarrangement in the ANS can lead to dysregulation of cardiac and coronary function especially in extreme cold, CAD, myocardial hypertrophy, and channelopathies.185,186 Further studies are needed, however, to uncover the underlying coronary vasomotor mechanisms.
7. Important areas for future research
There are insufficient number of studies elucidating the RAEX-induced differences in the adaptation of human coronary circulation in both sexes, as a function of age and temperatures (heat and cold) in sedentary people, athletes, and elite athletes or after retirement of elite athletes.187 All of which has great impact not only on the life of individuals but also on the ‘fitness’ of societies. Moreover for personalized prescription of exercise as medicine necessitate the delineation of these differences and the uncovering of the underlying mechanisms.
8. Clinical perspective and concluding remarks
There is ample evidence that RAEX elicits beneficial cardiovascular adaptations in healthy subjects as well as in individuals affected by cardiovascular diseases—including heart failure and ischaemic heart disease—and their comorbidities—including hypertension, obesity, type II diabetes, hypercholesterolaemia, and chronic kidney disease.4,161,171 RAEX—likely, in part, due to haemodynamic force stimulation—induces anti-atherogenic adaptations in endothelial function and vascular structure, regardless of traditional and novel risk factors cardiovascular-disease (CVD) risk factors. For the same reasons and due to intermittent hypoxia RAEX may improve angiogenesis of coronary capillaries and myocardial arteriolarization, thus promoting coronary collateral circulation growth, which in turn may improve CBF, increase ischaemic threshold, limit infarct size and provide protection against myocardial ischaemia–reperfusion injury. Furthermore, the mechanisms activated by RAEX by releasing NO, PGs and hyperpolarization factors may reduce pro-thrombotic risk, by improving haemorheological parameters. Another benefit of regular exercise is that it promotes anti-inflammatory milieu mediated by myokines.
Taken together, there is ample evidence that RAEX, such as running, cycling, swimming, or walking is associated with improved functional capacity, better quality of life, and prolonged survival in health and disease.4 RAEX also improves mental health, e.g. it promotes positive self-esteem and mitigates depression, effects that may also contribute to the beneficial effects of RAEX on the pathogenesis and progression of cardiovascular disease.188 For example, these adaptations can be useful in the treatment of early hypertension highlighting the potential mechanism(s) by which exercise can be used as an antihypertensive medicine. Also, greater range for the regulation of coronary function would extend the range for aerobic metabolism, delaying lactate production, and contribute to the benefit of RAEX in the setting of coronary artery disease. Regular exercise-induced coronary dilations are primarily due to activation of factors intrinsic to the vascular wall by the changes in haemodynamic forces and cardiac metabolism, all of which interact with each other at several levels. Finally, it should be noted that the mechanisms described in this review provide mechanistic underpinning for the recommendations regarding the frequency, intensity, time, and type and mode of exercise regimen in cardiovascular diseases, an approach summarized by the 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular diseases,187,189 This Guidelines are giving substantial help to make decisions regarding the above-mentioned aspects, although most of them are based on Level of Evidence C, necessitating further research.
In conclusion, in this review we summarized the physiological, cellular, ion channel, and molecular mechanisms as well as haemorheological mechanisms activated by RAEX eliciting adaptations of the coronary circulation and some of the human and clinical aspects. All these adaptations serve to provide a greater range for the regulation of vascular and haemorheological resistance, allowing an enhanced blood supply and exchange capacity during the increased workload of heart during aerobic exercise. These adaptations enable an increased oxygen supply to the heart to match the needs of increased cardiac workload during exercise. Future experimental studies should reveal so far unknown molecular mechanisms activated by different types of exercise regimens, in both sexes, which also contribute to the coronary vascular adaptations in health and disease conditions promoting thereby the specific, personalized use of various exercise regimens in prevention and rehabilitation of cardiovascular disease as described in the 2020 ESC Guidelines on sports cardiology and physical activity in patients with CVD: implications for practice, so we can base on mechanisms the statement: exercise is medicine.187
Acknowledgements
The authors would like to dedicate this paper to professor Thomas H. Hintze, PhD, who made tremendous contribution to the field of exercise and cardiovascular system. Dr Hintze passed away on 19 March 2021, was a faculty member at New York Medical College for 37 years, and served as Chair of the Department of Physiology for more than a decade. He was only 71 years old.
Conflict of interest: L.B. received a research grant from AstraZeneca; hold advisory board work for Sanofi, Bayer, and AstraZeneca; is founder and shareholder of Glycardial Diagnosis SL and received speaker fees from Lilly, MSD-Boehringer, and AstraZeneca (all outside of this work). All other authors declared no conflict of interest.
Funding
The author’s work was supported by several grants: A.K.: Scientific Excellence Program NKFIH-1281-2/2020 TKP2020-NKA-17 at the University of Physical Education, Innovation and Technology Ministry, Hungary, National Research, Development and Innovation Fund, OTKA K108444, K116954, K132596, Innovation and Technology Ministry and Higher Education Institutional Excellence Program at Semmelweis University, Hungary, FP7 Marie Sklodowska Curie projects—Small Artery Remodeling (SmART and SmArter), and the National Heart, Lung, and Blood Institute Grants NIH PO1 HL-43023 and NIH HL-46813; M.H.L.: the National Heart, Lung, and Blood Institute Grants HL-52490, HL-35088, and HL112998; L.B.: Spanish Ministry of Economy and Competitiveness of Science (PNS2016-76819-R) and the Institute of Health Carlos III, ISCIII (Red Terapia Celular TerCel—RD16/0011/0018 to L.B.) cofounded by FEDER ‘Una Manera de Hacer Europa’; T.P.: Institute of Health Carlos III, ISCIII (FIS PI19/01687) cofounded by FEDER ‘Una Manera de Hacer Europa’; K.T.: National Research, Development and Innovation Fund, Scientific Excellence Program 2019, at the University of Pecs; and D.J.D.: The Netherlands Cardiovascular Research Initiative, an initiative with the financial support of the Dutch Heart Foundation (CVON2014-11; RECONNECT).
Contributor Information
Akos Koller, Department of Translational Medicine, Semmelweis University, Budapest, Hungary; Research Center for Sports Physiology, University of Physical Education, Budapest, Hungary; Department of Physiology, New York Medical College, Valhalla, NY 10595, USA.
M Harold Laughlin, Department of Biomedical Sciences, University of Missouri, Columbia, MO 65211, USA.
Edina Cenko, Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, Italy.
Cor de Wit, Institut für Physiologie, Universitat zu Lübeck, Lübeck, Germany; DZHK (German Center for Cardiovascular Research), partner site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Kálmán Tóth, Division of Cardiology, 1st Department of Medicine, Medical School, University of Pécs, Pécs, Hungary.
Raffaele Bugiardini, Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, Italy.
Danijela Trifunovits, Cardiology Department, Clinical Centre of Serbia and Faculty of Medicine University of Belgrade, Belgrade, Serbia.
Marija Vavlukis, University Clinic for Cardiology, Medical Faculty, Ss’ Cyril and Methodius University, Skopje, Republic of Macedonia.
Olivia Manfrini, Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, Italy.
Adam Lelbach, Departmental Group of Geriatrics, Department of Internal Medicine and Oncology, Faculty of Medicine, Semmelweis University, Budapest, Dr. Rose Private Hospital, Budapest, Hungary.
Gabriella Dornyei, Department of Morphology and Physiology, Faculty of Health Sciences, Semmelweis University, Budapest, Hungary.
Teresa Padro, Cardiovascular Program-ICCC, Research Institute Hospital Santa Creu i Sant Pau, IIB-Sant Pau, CiberCV-Institute Carlos III, Barcelona, Spain.
Lina Badimon, Cardiovascular Program-ICCC, Research Institute Hospital Santa Creu i Sant Pau, IIB-Sant Pau, CiberCV-Institute Carlos III, Barcelona, Spain.
Dimitris Tousoulis, First Department of Cardiology, Hippokration Hospital, University of Athens Medical School, Athens, Greece.
Stephan Gielen, Department of Cardiology, Angiology, and Intensive Care Medicine, Klinikum Lippe, Detmold, Germany.
Dirk J Duncker, Division of Experimental Cardiology, Department of Cardiology, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
References
- 1. Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2012;2:321–447. [DOI] [PubMed] [Google Scholar]
- 2. Heinonen I, Kalliokoski KK, Hannukainen JC, Duncker DJ, Nuutila P, Knuuti J. Organ-specific physiological responses to acute physical exercise and long-term training in humans. Physiology (Bethesda) 2014;29:421–436. [DOI] [PubMed] [Google Scholar]
- 3. Gielen S, Laughlin MH, O'Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis 2015;57:347–355. [DOI] [PubMed] [Google Scholar]
- 4. Sharma S, Merghani A, Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J 2015;36:1445–1453. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc 1990;22:141–154. [PubMed] [Google Scholar]
- 6. Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to static leg exercise. J Appl Physiol (1985) 1992;73:1523–1529. [DOI] [PubMed] [Google Scholar]
- 7. Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol 2009;94:947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keller DM, Ogoh S, Greene S, Olivencia-Yurvati A, Raven PB. Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol 2004;561:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 2003;553:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joyner MJ, Thomas GD. Having it both ways? Vasoconstriction in contracting muscles. J Physiol 2003;550:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrão CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail 2007;9:630–636. [DOI] [PubMed] [Google Scholar]
- 12. Galbreath MM, Shibata S, VanGundy TB, Okazaki K, Fu Q, Levine BD. Effects of exercise training on arterial-cardiac baroreflex function in POTS. Clin Auton Res 2011;21:73–80. [DOI] [PubMed] [Google Scholar]
- 13. Iwasaki K, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol (1985) 2003;95:1575–1583. [DOI] [PubMed] [Google Scholar]
- 14. Levy WC, Cerqueira MD, Harp GD, Johannessen KA, Abrass IB, Schwartz RS, Stratton JR. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol 1998;82:1236–1241. [DOI] [PubMed] [Google Scholar]
- 15. Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol 1991;260:H862–H868. [DOI] [PubMed] [Google Scholar]
- 16. Dornyei G, Monos E, Kaley G, Koller A. Regular exercise enhances blood pressure lowering effect of acetylcholine by increased contribution of nitric oxide. Acta Physiol Hung 2000;87:127–138. [PubMed] [Google Scholar]
- 17. Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin 2001;19:369–387. [DOI] [PubMed] [Google Scholar]
- 18. Manfrini O, Pizzi C, Trerè D, Fontana F, Bugiardini R. Parasympathetic failure and risk of subsequent coronary events in unstable angina and non-ST-segment elevation myocardial infarction. Eur Heart J 2003;24:1560–1566. [DOI] [PubMed] [Google Scholar]
- 19. Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 2012;302:H10–H23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kozàkovà M, Galetta F, Gregorini L, Bigalli G, Franzoni F, Giusti C, Palombo C, Coronary vasodilator capacity and epicardial vessel remodeling in physiological and hypertensive hypertrophy. Hypertension 2000;36:343–349. [DOI] [PubMed] [Google Scholar]
- 21. Kozakova M, Paterni M, Bartolomucci F, Morizzo C, Rossi G, Galetta F, Palombo C. Epicardial coronary artery size in hypertensive and physiologic left ventricular hypertrophy. Am J Hypertens 2007;20:279–284. [DOI] [PubMed] [Google Scholar]
- 22. Pelliccia A, Spataro A, Granata M, Biffi A, Caselli G, Alabiso A. Coronary arteries in physiological hypertrophy: echocardiographic evidence of increased proximal size in elite athletes. Int J Sports Med 1990;11:120–126. [DOI] [PubMed] [Google Scholar]
- 23. Zandrino F, Molinari G, Smeraldi A, Odaglia G, Masperone MA, Sardanelli F. Magnetic resonance imaging of athlete's heart: myocardial mass, left ventricular function, and cross-sectional area of the coronary arteries. Eur Radiol 2000;10:319–325. [DOI] [PubMed] [Google Scholar]
- 24. Windecker S, Allemann Y, Billinger M, Pohl T, Hutter D, Orsucci T, Blaga L, Meier B, Seiler C. Effect of endurance training on coronary artery size and function in healthy men: an invasive followup study. Am J Physiol Heart Circ Physiol 2002;282:H2216–H2223. [DOI] [PubMed] [Google Scholar]
- 25. Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation 1993;87:1076–1082. [DOI] [PubMed] [Google Scholar]
- 26. Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, Shapiro LM. Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracic echocardiographic study. Heart 2000;84:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szekeres M, Nádasy GL, Dörnyei G, Szénási A, Koller A. Remodeling of wall mechanics and the myogenic mechanism of rat intramural coronary arterioles in response to a short-term daily exercise program: role of endothelial factors. J Vasc Res 2018;55:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laughlin MK, Duncker DJ, Bache RJ. Chapter 16. Control of Blood Flow to Cardiac and Skeletal Muscle during Exercise. New York, NY: American Physiological Society and Oxford University Press; 1996. [Google Scholar]
- 29. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 2008;88:1009–1086. [DOI] [PubMed] [Google Scholar]
- 30. Laughlin MH, Tomanek RJ. Myocardial capillarity and maximal capillary diffusion capacity in exercise-trained dogs. J Appl Physiol (1985) 1987;63:1481–1486. [DOI] [PubMed] [Google Scholar]
- 31. Wyatt HL, Mitchell JH. Influences of physical training on the heart of dogs. Circ Res 1974;35:883–889. [DOI] [PubMed] [Google Scholar]
- 32. Wyatt HL, Mitchell J. Influences of physical conditioning and deconditioning on coronary vasculature of dogs. J Appl Physiol Respir Environ Exerc Physiol 1978;45:619–625. [DOI] [PubMed] [Google Scholar]
- 33. Breisch EA, White FC, Nimmo LE, McKirnan MD, Bloor CM. Exercise-induced cardiac hypertrophy: a correlation of blood flow and microvasculature. J Appl Physiol (1985) 1986;60:1259–1267. [DOI] [PubMed] [Google Scholar]
- 34. White FC, McKirnan MD, Breisch EA, Guth BD, Liu YM, Bloor CM. Adaptation of the left ventricle to exercise-induced hypertrophy. J Appl Physiol (1985) 1987;62:1097–1110. [DOI] [PubMed] [Google Scholar]
- 35. White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol (1985) 1998;85:1160–1168. [DOI] [PubMed] [Google Scholar]
- 36. Bache RJ. Effects of hypertrophy on the coronary circulation. Prog Cardiovasc Dis 1988;30:403–440. [DOI] [PubMed] [Google Scholar]
- 37. Hanna MA, Taylor CR, Chen B, La HS, Maraj JJ, Kilar CR, Behnke BJ, Delp MD, Muller-Delp JM. Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol (1985) 2014;117:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barnard RJ, Duncan HW, Baldwin KM, Grimditch G, Buckberg GD. Effects of intensive exercise training on myocardial performance and coronary blood flow. J Appl Physiol Respir Environ Exerc Physiol 1980;49:444–449. [DOI] [PubMed] [Google Scholar]
- 39. Bove AA, Hultgren PB, Ritzer TF, Carey RA. Myocardial blood flow and hemodynamic responses to exercise training in dogs. J Appl Physiol Respir Environ Exerc Physiol 1979;46:571–578. [DOI] [PubMed] [Google Scholar]
- 40. Carey RA, Santamore WP, Michele JJ, Bove AA. Effects of endurance training on coronary resistance in dogs. Med Sci Sports Exerc 1983;15:355–359. [PubMed] [Google Scholar]
- 41. Cohen MV. Coronary vascular reserve in the greyhound with left ventricular hypertrophy. Cardiovasc Res 1986;20:182–194. [DOI] [PubMed] [Google Scholar]
- 42. Liang IY, Hamra M, Stone HL. Maximum coronary blood flow and minimum coronary resistance in exercise-trained dogs. J Appl Physiol Respir Environ Exerc Physiol 1984;56:641–647. [DOI] [PubMed] [Google Scholar]
- 43. Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res 1981;48:523–530. [DOI] [PubMed] [Google Scholar]
- 44. Stone HL. Coronary flow, myocardial oxygen consumption, and exercise training in dogs. J Appl Physiol Respir Environ Exerc Physiol 1980;49:759–768. [DOI] [PubMed] [Google Scholar]
- 45. Radvan J, Choudhury L, Sheridan DJ, Camici PG. Comparison of coronary vasodilator reserve in elite rowing athletes versus hypertrophic cardiomyopathy. Am J Cardiol 1997;80:1621–1623. [DOI] [PubMed] [Google Scholar]
- 46. Hannukainen JC, Janatuinen T, Toikka JO, Järvisalo MJ, Heinonen OJ, Kapanen J, Någren K, Nuutila P, Kujala UM, Kaprio J, Knuuti J, Kalliokoski KK. Myocardial and peripheral vascular functional adaptation to exercise training. Scand J Med Sci Sports 2007;17:139–147. [DOI] [PubMed] [Google Scholar]
- 47. Kjaer A, Meyer C, Wachtell K, Olsen MH, Ibsen H, Opie L, Holm S, Hesse B. Positron emission tomographic evaluation of regulation of myocardial perfusion in physiological (elite athletes) and pathological (systemic hypertension) left ventricular hypertrophy. Am J Cardiol 2005;96:1692–1698. [DOI] [PubMed] [Google Scholar]
- 48. Heinonen I, Nesterov SV, Liukko K, Kemppainen J, Någren K, Luotolahti M, Virsu P, Oikonen V, Nuutila P, Kujala UM, Kainulainen H, Boushel R, Knuuti J, Kalliokoski KK. Myocardial blood flow and adenosine A2A receptor density in endurance athletes and untrained men. J Physiol 2008;586:5193–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hägg U, Wandt B, Bergström G, Volkmann R, Gan LM. Physical exercise capacity is associated with coronary and peripheral vascular function in healthy young adults. Am J Physiol Heart Circ Physiol 2005;289:H1627–H1634. [DOI] [PubMed] [Google Scholar]
- 50. Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol (1985) 1989;67:1140–1149. [DOI] [PubMed] [Google Scholar]
- 51. Laughlin MH. Effects of exercise training on coronary transport capacity. J Appl Physiol (1985) 1985;58:468–476. [DOI] [PubMed] [Google Scholar]
- 52. Laughlin MH, Joseph B. Wolfe Memorial lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc 2004;36:352–362. [DOI] [PubMed] [Google Scholar]
- 53. Laughlin MH. Coronary transport reserve in normal dogs. J Appl Physiol Respir Environ Exerc Physiol 1984;57:551–561. [DOI] [PubMed] [Google Scholar]
- 54. Buttrick PM, Levite HA, Schaible TF, Ciambrone G, Scheuer J. Early increases in coronary vascular reserve in exercised rats are independent of cardiac hypertrophy. J Appl Physiol (1985) 1985;59:1861–1865. [DOI] [PubMed] [Google Scholar]
- 55. Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol 2010;105:1–5. [DOI] [PubMed] [Google Scholar]
- 56. Overholser KA, Bhatte MJ, Laughlin MH. Modeling the effect of flow heterogeneity on coronary permeability-surface area. J Appl Physiol (1985) 1991;71:758–769. [DOI] [PubMed] [Google Scholar]
- 57. Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol 1983;45:169–189. [DOI] [PubMed] [Google Scholar]
- 58. Raven PB, Rohm-Young D, Blomqvist CG. Physical fitness and cardiovascular response to lower body negative pressure. J Appl Physiol Respir Environ Exerc Physiol 1984;56:138–144. [DOI] [PubMed] [Google Scholar]
- 59. Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol 1977;39:221–251. [DOI] [PubMed] [Google Scholar]
- 60. Bove AA, Dewey JD. Proximal coronary vasomotor reactivity after exercise training in dogs. Circulation 1985;71:620–625. [DOI] [PubMed] [Google Scholar]
- 61. Rogers PJ, Miller TD, Bauer BA, Brum JM, Bove AA, Vanhoutte PM. Exercise training and responsiveness of isolated coronary arteries. J Appl Physiol (1985) 1991;71:2346–2351. [DOI] [PubMed] [Google Scholar]
- 62. Oltman CL, Parker JL, Adams HR, Laughlin MH. Effects of exercise training on vasomotor reactivity of porcine coronary arteries. Am J Physiol 1992;263:H372–H382. [DOI] [PubMed] [Google Scholar]
- 63. Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training alters Ca release from coronary smooth muscle sarcoplasmic reticulum. Am J Physiol 1990;259:H643–H647. [DOI] [PubMed] [Google Scholar]
- 64. Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training depletes sarcoplasmic reticulum calcium in coronary smooth muscle. J Appl Physiol (1985) 1991;71:1764–1773. [DOI] [PubMed] [Google Scholar]
- 65. Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res 1993;73:829–838. [DOI] [PubMed] [Google Scholar]
- 66. Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. J Appl Physiol (1985) 1995;79:33–40. [DOI] [PubMed] [Google Scholar]
- 67. Parker JL, Mattox ML, Laughlin MH. Contractile responsiveness of coronary arteries from exercise-trained rats. J Appl Physiol (1985) 1997;83:434–443. [DOI] [PubMed] [Google Scholar]
- 68. Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc 1995;27:1135–1144. [PubMed] [Google Scholar]
- 69. Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol (1985) 2001;90:501–510. [DOI] [PubMed] [Google Scholar]
- 70. Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol 2003;284:H1307–H1312. [DOI] [PubMed] [Google Scholar]
- 71. Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation 1994;89:2308–2314. [DOI] [PubMed] [Google Scholar]
- 72. Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise training causes differential changes in gene expression in diaphragm arteries and 2A arterioles of obese rats. J Appl Physiol (1985) 2015;119:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise-induced differential changes in gene expression among arterioles of skeletal muscles of obese rats. J Appl Physiol (1985) 2015;119:583–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol (1985) 2004;96:1425–1432. [DOI] [PubMed] [Google Scholar]
- 75. Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol 1998;275:H2159–H2169. [DOI] [PubMed] [Google Scholar]
- 76. Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol (1985) 1998;84:884–889. [DOI] [PubMed] [Google Scholar]
- 77. Heaps CL, Bowles DK, Sturek M, Laughlin MH, Parker JL. Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation. J Physiol 2000;528:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bowles DK, Wamhoff BR. Coronary smooth muscle adaptation to exercise: does it play a role in cardioprotection? Acta Physiol Scand 2003;178:117–121. [DOI] [PubMed] [Google Scholar]
- 79. Muller JM, Myers PR, Laughlin MH. Exercise training alters myogenic responses in porcine coronary resistance arteries. J Appl Physiol (1985) 1993;75:2677–2682. [DOI] [PubMed] [Google Scholar]
- 80. Jacobsen JC, Aalkjaer C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH. A model of smooth muscle cell synchronization in the arterial wall. Am J Physiol Heart Circ Physiol 2007;293:H229–H237. [DOI] [PubMed] [Google Scholar]
- 81. Li Z, Lu N, Shi L. Exercise training reverses alterations in Kv and BKCa channel molecular expression in thoracic aorta smooth muscle cells from spontaneously hypertensive rats. J Vasc Res 2014;51:447–457. [DOI] [PubMed] [Google Scholar]
- 82. Jones CJ, Kuo L, Davis MJ, Chilian WM. Myogenic and flow-dependent control mechanisms in the coronary microcirculation. Basic Res Cardiol 1993;88:2–10. [DOI] [PubMed] [Google Scholar]
- 83. Koller A, Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol 1990;258:H916–H920. [DOI] [PubMed] [Google Scholar]
- 84. Koller A, Kaley G. Role of endothelium in reactive dilation of skeletal muscle arterioles. Am J Physiol 1990;259:H1313–H1316. [DOI] [PubMed] [Google Scholar]
- 85. Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 2017;97:495–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Role of endothelial nitric oxide and prostaglandins. Circ Res 1995;76:544–550. [DOI] [PubMed] [Google Scholar]
- 87. Sun D, Huang A, Koller A, Kaley G. Enhanced NO-mediated dilations in skeletal muscle arterioles of chronically exercised rats. Microvasc Res 2002;64:491–496. [DOI] [PubMed] [Google Scholar]
- 88. Sun D, Huang A, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol (1985) 1994;76:2241–2247. [DOI] [PubMed] [Google Scholar]
- 89. Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res 1998;56:54–61. [DOI] [PubMed] [Google Scholar]
- 90. Koller A, Dornyei G, Kaley G. Flow-induced responses in skeletal muscle venules: modulation by nitric oxide and prostaglandins. Am J Physiol 1998;275:H831–H836. [DOI] [PubMed] [Google Scholar]
- 91. Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol 1997;273:H2575–H2579. [DOI] [PubMed] [Google Scholar]
- 92. Woodman CR, Muller JM, Rush JW, Laughlin MH, Price EM. Flow regulation of ecNOS and Cu/Zn SOD mRNA expression in porcine coronary arterioles. Am J Physiol 1999;276:H1058–H1063. [DOI] [PubMed] [Google Scholar]
- 93. McAllister RM, Laughlin MH. Vascular nitric oxide: effects of physical activity, importance for health. Essays Biochem 2006;42:119–131. [DOI] [PubMed] [Google Scholar]
- 94. Yang AL, Tsai SJ, Jiang MJ, Jen CJ, Chen HI. Chronic exercise increases both inducible and endothelial nitric oxide synthase gene expression in endothelial cells of rat aorta. J Biomed Sci 2002;9:149–155. [DOI] [PubMed] [Google Scholar]
- 95. Ungvari Z, Pacher P, Rischák K, Szollár L, Koller A, Dysfunction of nitric oxide mediation in isolated rat arterioles with methionine diet-induced hyperhomocysteinemia. Arterioscler Thromb Vasc Biol 1999;19:1899–1904. [DOI] [PubMed] [Google Scholar]
- 96. Bagi Z, Ungvari Z, Szollár L, Koller A. Flow-induced constriction in arterioles of hyperhomocysteinemic rats is due to impaired nitric oxide and enhanced thromboxane A(2) mediation. Arterioscler Thromb Vasc Biol 2001;21:233–237. [DOI] [PubMed] [Google Scholar]
- 97. Bagi Z, Ungvari Z, Koller A. Xanthine oxidase-derived reactive oxygen species convert flow-induced arteriolar dilation to constriction in hyperhomocysteinemia: possible role of peroxynitrite. Arterioscler Thromb Vasc Biol 2002;22:28–33. [DOI] [PubMed] [Google Scholar]
- 98. Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol 2005;25:1590–1595. [DOI] [PubMed] [Google Scholar]
- 99. Duncker DJ, Bache RJ, Merkus D. Regulation of coronary resistance vessel tone in response to exercise. J Mol Cell Cardiol 2012;52:802–813. [DOI] [PubMed] [Google Scholar]
- 100. Kuo L, Chancellor JD. Adenosine potentiates flow-induced dilation of coronary arterioles by activating KATP channels in endothelium. Am J Physiol 1995;269:H541–H549. [DOI] [PubMed] [Google Scholar]
- 101. Thengchaisri N, Shipley R, Ren Y, Parker J, Kuo L. Exercise training restores coronary arteriolar dilation to NOS activation distal to coronary artery occlusion: role of hydrogen peroxide. Arterioscler Thromb Vasc Biol 2007;27:791–798. [DOI] [PubMed] [Google Scholar]
- 102. Brown MD. Exercise and coronary vascular remodelling in the healthy heart. Exp Physiol 2003;88:645–658. [DOI] [PubMed] [Google Scholar]
- 103. Duncker DJ, Bache RJ, Merkus D, Laughlin MH. Chapter 22—Exercise and the coronary circulation. In Zoladz JA (ed). Muscle and Exercise Physiology. Cambridge, Massachusetts,-USA: Academic Press; 2019. p.467–503. [Google Scholar]
- 104. Sun D, Huang A, Recchia FA, Cui Y, Messina EJ, Koller A, Kaley G. Nitric oxide-mediated arteriolar dilation after endothelial deformation. Am J Physiol Heart Circ Physiol 2001;280:H714–H721. [DOI] [PubMed] [Google Scholar]
- 105. Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun 1995;211:375–381. [DOI] [PubMed] [Google Scholar]
- 106. Forfia PR, Zhang X, Knight DR, Smith AH, Doe CP, Wolfgang EA, Flynn DM, Wolin MS, Hintze TH. NO modulates myocardial O2 consumption in the nonhuman primate: an additional mechanism of action of amlodipine. Am J Physiol 1999;276:H2069–H2075. [DOI] [PubMed] [Google Scholar]
- 107. Shen W, Xu X, Ochoa M, Zhao G, Wolin MS, Hintze TH. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res 1994;75:1086–1095. [DOI] [PubMed] [Google Scholar]
- 108. Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circ Res 2000;87:1108–1117. [DOI] [PubMed] [Google Scholar]
- 109. Kayar SR, Banchero N. Volume density and distribution of mitochondria in myocardial growth and hypertrophy. Respir Physiol 1987;70:275–286. [DOI] [PubMed] [Google Scholar]
- 110. Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 1997;276:2034–2037. [DOI] [PubMed] [Google Scholar]
- 111. Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 2009;15:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol 1990;259:C3–C18. [DOI] [PubMed] [Google Scholar]
- 113. Jackson WF. KV channels and the regulation of vascular smooth muscle tone. Microcirculation 2018;25: Doi: 10.1111/micc.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch 2010;459:897–914. [DOI] [PubMed] [Google Scholar]
- 115. Garland CJ, Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol (Oxf) 2017;219:152–161. [DOI] [PubMed] [Google Scholar]
- 116. Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K+ channels to metabolic control of coronary blood flow. J Mol Cell Cardiol 2012;52:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol 2006;291:H2090–H2097. [DOI] [PubMed] [Google Scholar]
- 118. Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite role of Kv1.5 channels in coronary metabolic dilation. Circ Res 2015;117:612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Luli J, Graham K, Khayata M, Logan S, Kmetz J, Chilian WM. Kv1.3 channels facilitate the connection between metabolism and blood flow in the heart. Microcirculation 2017;24:Doi: 10.1111/micc.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 1999;79:387–423. [DOI] [PubMed] [Google Scholar]
- 121. Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+-channel contribution to regulation of coronary arterial tone. J Appl Physiol (1985) 1998;84:1225–1233. [DOI] [PubMed] [Google Scholar]
- 122. Deer RR, Heaps CL. Exercise training enhances multiple mechanisms of relaxation in coronary arteries from ischemic hearts. Am J Physiol Heart Circ Physiol 2013;305:H1321–H1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 1992;256:532–535. [DOI] [PubMed] [Google Scholar]
- 124. Guia A, Wan X, Courtemanche M, Leblanc N. Local Ca2+ entry through L-type Ca2+ channels activates Ca2+-dependent K+ channels in rabbit coronary myocytes. Circ Res 1999;84:1032–1042. [DOI] [PubMed] [Google Scholar]
- 125. Bowles DK. Adaptation of ion channels in the microcirculation to exercise training. Microcirculation 2000;7:25–40. [PubMed] [Google Scholar]
- 126. Whyte JJ, Laughlin MH. The effects of acute and chronic exercise on the vasculature. Acta Physiol (Oxf) 2010;199:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ellinsworth DC, Sandow SL, Shukla N, Liu Y, Jeremy JY, Gutterman DD. Endothelium-derived hyperpolarization and coronary vasodilation: diverse and integrated roles of epoxyeicosatrienoic acids, hydrogen peroxide, and gap junctions. Microcirculation 2016;23:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mombouli JV, Nakashima M, Hamra M, Vanhoutte PM. Endothelium-dependent relaxation and hyperpolarization evoked by bradykinin in canine coronary arteries: enhancement by exercise-training. Br J Pharmacol 1996;117:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Grgic I, Kaistha BP, Hoyer J, Köhler R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses—relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol 2009;157:509–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jackson WF. Boosting the signal: endothelial inward rectifier K+ channels. Microcirculation 2017;24:Doi: 10.1111/micc.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Köhler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res 2006;99:537–544. [DOI] [PubMed] [Google Scholar]
- 132. Brähler S, Kaistha A, Schmidt VJ, Wölfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Köhler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 2009;119:2323–2332. [DOI] [PubMed] [Google Scholar]
- 133. Milkau M, Köhler R, de Wit C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J 2010;24:3572–3579. [DOI] [PubMed] [Google Scholar]
- 134. Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol 2004;286:C195–C205. [DOI] [PubMed] [Google Scholar]
- 135. Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol 2013;61:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 2010;298:H466–H476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2007;2:e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012;336:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol 2012;302:H634–H642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Baratchi S, Knoerzer M, Khoshmanesh K, Mitchell A, McIntyre P. Shear stress regulates TRPV4 channel clustering and translocation from adherens junctions to the basal membrane. Sci Rep 2017;7:15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, Earnest CP, Church TS, O'Keefe JH, Milani RV, Blair SN. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res 2015;117:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Roh J, Rhee J, Chaudhari V, Rosenzweig A. The role of exercise in cardiac aging: from physiology to molecular mechanisms. Circ Res 2016;118:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Viña J, Rodriguez-Mañas L, Salvador-Pascual A, Tarazona-Santabalbina FJ, Gomez-Cabrera MC. Exercise: the lifelong supplement for healthy ageing and slowing down the onset of frailty. J Physiol 2016;594:1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Toth K, Kesmarky G, Alexy T. Clinical Significance of Hemorheological Alterations. Amsterdam, Netherlands: IOS Press; 2007. [Google Scholar]
- 145. Connes P, Simmonds MJ, Brun JF, Baskurt OK. Exercise hemorheology: classical data, recent findings and unresolved issues. Clin Hemorheol Microcirc 2013;53:187–199. [DOI] [PubMed] [Google Scholar]
- 146. Romain AJ, Brun JF, Varlet-Marie E, Raynaud de Mauverger E. Effects of exercise training on blood rheology: a meta-analysis. Clin Hemorheol Microcirc 2011;49:199–205. [DOI] [PubMed] [Google Scholar]
- 147. Haynes A, Linden MD, Robey E, Watts GF, Barrett H, Naylor LH, Green DJ. Impact of commonly prescribed exercise interventions on platelet activation in physically inactive and overweight men. Physiol Rep 2016;4:e12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Bakovic D, Pivac N, Zubin Maslov P, Breskovic T, Damonja G, Dujic Z. Spleen volume changes during adrenergic stimulation with low doses of epinephrine. J Physiol Pharmacol 2013;64:649–655. [PubMed] [Google Scholar]
- 149. Gram AS, Petersen MB, Quist JS, Rosenkilde M, Stallknecht B, Bladbjerg EM. Effects of 6 months of active commuting and leisure-time exercise on fibrin turnover in sedentary individuals with overweight and obesity: a randomised controlled trial. J Obes 2018;2018:7140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Whittaker JP, Linden MD, Coffey VG. Effect of aerobic interval training and caffeine on blood platelet function. Med Sci Sports Exerc 2013;45:342–350. [DOI] [PubMed] [Google Scholar]
- 151. Menzel K, Hilberg T. Coagulation and fibrinolysis are in balance after moderate exercise in middle-aged participants. Clin Appl Thromb Hemost 2009;15:348–355. [DOI] [PubMed] [Google Scholar]
- 152. Posthuma JJ, van der Meijden PE, Ten Cate H, Spronk HM. Short- and Long-term exercise induced alterations in haemostasis: a review of the literature. Blood Rev 2015;29:171–178. [DOI] [PubMed] [Google Scholar]
- 153. Mongirdienė A, Kubilius R. Effect of physical training on indices of platelet aggregation and fibrinogen concentration in patients with chronic heart failure. Medicina (Kaunas) 2015;51:343–350. [DOI] [PubMed] [Google Scholar]
- 154. van der Vorm LN, Huskens D, Kicken CH, Remijn JA, Roest M, de Laat B, Miszta A. Effects of repeated bouts of exercise on the hemostatic system. Semin Thromb Hemost 2018;44:710–722. [DOI] [PubMed] [Google Scholar]
- 155. Kappus RM, Ranadive SM, Yan H, Lane-Cordova AD, Cook MD, Sun P, Harvey IS, Wilund KR, Woods JA, Fernhall B. Sex differences in autonomic function following maximal exercise. Biol Sex Differ 2015;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev 2016;64:288–310. [DOI] [PubMed] [Google Scholar]
- 157. Manfrini O, Morgagni G, Pizzi C, Fontana F, Bugiardini R. Changes in autonomic nervous system activity: spontaneous versus balloon-induced myocardial ischaemia. Eur Heart J 2004;25:1502–1508. [DOI] [PubMed] [Google Scholar]
- 158. Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, Katus HA. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol 2003;14:791–799. [DOI] [PubMed] [Google Scholar]
- 159. Stein PK, Kleiger RE, Rottman JN. Differing effects of age on heart rate variability in men and women. Am J Cardiol 1997;80:302–305. [DOI] [PubMed] [Google Scholar]
- 160. Ramaekers D, Ector H, Aubert AE, Rubens A, Van de Werf F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur Heart J 1998;19:1334–1341. [DOI] [PubMed] [Google Scholar]
- 161. Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Prog Cardiovasc Dis 2012;55:321–331. [DOI] [PubMed] [Google Scholar]
- 162. Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R; Working Group on Coronary Pathophysiology and Microcirculation. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res 2011;90:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Crea F, Davies G, Chierchia S, Romeo F, Bugiardini R, Kaski JC, Freedman B, Maseri A. Different susceptibility to myocardial ischemia provoked by hyperventilation and cold pressor test in exertional and variant angina pectoris. Am J Cardiol 1985;56:18–22. [DOI] [PubMed] [Google Scholar]
- 164. Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 2001;280:H2462–H2469. [DOI] [PubMed] [Google Scholar]
- 165. Huang A, Sun D, Koller A, Kaley G. Gender difference in flow-induced dilation and regulation of shear stress: role of estrogen and nitric oxide. Am J Physiol 1998;275:R1571–R1577. [DOI] [PubMed] [Google Scholar]
- 166. Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol 2001;280:H2456–H2461. [DOI] [PubMed] [Google Scholar]
- 167. Török M, Monori-Kiss A, Pál É, Horváth E, Jósvai A, Merkely P, Barta BA, Mátyás C, Oláh A, Radovits T, Merkely B, Ács N, Nádasy GL, Várbíró S. Long-term exercise results in morphological and biomechanical changes in coronary resistance arterioles in male and female rats. Biol Sex Differ 2020;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 2015;5:475–512. [DOI] [PubMed] [Google Scholar]
- 169. Michalis M, Finn KJ, Podstawski R, Gabnai S, Koller A, Cziraki A, Szanto M, Alfoldi Z, Ihasz F. Differences in cardiorespiratory responses of young and senior male endurance athletes to maximal graded exercise test. Physiol Int 2020;107:444–454. [DOI] [PubMed] [Google Scholar]
- 170. Davies CH, Ferrara N, Harding SE. Beta-adrenoceptor function changes with age of subject in myocytes from non-failing human ventricle. Cardiovasc Res 1996;31:152–156. [PubMed] [Google Scholar]
- 171. Manfrini O, Pizzi C, Viecca M, Bugiardini R. Abnormalities of cardiac autonomic nervous activity correlate with expansive coronary artery remodeling. Atherosclerosis 2008;197:183–189. [DOI] [PubMed] [Google Scholar]
- 172. Shah AS, Lampert R, Goldberg J, Bremner JD, Li L, Thames MD, Vaccarino V, Shah AJ. Alterations in heart rate variability are associated with abnormal myocardial perfusion. Int J Cardiol 2020;305:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Soares-Miranda L, Sattelmair J, Chaves P, Duncan GE, Siscovick DS, Stein PK, Mozaffarian D. Physical activity and heart rate variability in older adults: the Cardiovascular Health Study. Circulation 2014;129:2100–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 2008;33:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Stein PK, Barzilay JI, Chaves PH, Traber J, Domitrovich PP, Heckbert SR, Gottdiener JS. Higher levels of inflammation factors and greater insulin resistance are independently associated with higher heart rate and lower heart rate variability in normoglycemic older individuals: the Cardiovascular Health Study. J Am Geriatr Soc 2008;56:315–321. [DOI] [PubMed] [Google Scholar]
- 176. Bugiardini R, Pozzati A, Ottani F, Morgagni GL, Puddu P. Vasotonic angina: a spectrum of ischemic syndromes involving functional abnormalities of the epicardial and microvascular coronary circulation. J Am Coll Cardiol 1993;22:417–425. [DOI] [PubMed] [Google Scholar]
- 177. Heindl S, Struck J, Wellhoner P, Sayk F, Dodt C. Effect of facial cooling and cold air inhalation on sympathetic nerve activity in men. Respir Physiol Neurobiol 2004;142:69–80. [DOI] [PubMed] [Google Scholar]
- 178. Prior JO, Schindler TH, Facta AD, Hernandez-Pampaloni M, Campisi R, Dahlbom M, Schelbert HR. Determinants of myocardial blood flow response to cold pressor testing and pharmacologic vasodilation in healthy humans. Eur J Nucl Med Mol Imaging 2007;34:20–27. [DOI] [PubMed] [Google Scholar]
- 179. Manou-Stathopoulou V, Goodwin CD, Patterson T, Redwood SR, Marber MS, Williams RP. The effects of cold and exercise on the cardiovascular system. Heart 2015;101:808–820. [DOI] [PubMed] [Google Scholar]
- 180. Makinen TM, Mantysaari M, Paakkonen T, Jokelainen J, Palinkas LA, Hassi J, Leppaluoto J, Tahvanainen K, Rintamaki H. Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Aviat Space Environ Med 2008;79:875–882. [DOI] [PubMed] [Google Scholar]
- 181. Bugiardini R. Normal coronary arteries: clinical implications and further classification. Herz 2005;30:3–7. [DOI] [PubMed] [Google Scholar]
- 182. Bugiardini R, Galvani M, Ferrini D, Gridelli C, Mari L, Puddu P, Lenzi S. Effects of iloprost, a stable prostacyclin analog, on exercise capacity and platelet aggregation in stable angina pectoris. Am J Cardiol 1986;58:453–459. [DOI] [PubMed] [Google Scholar]
- 183. Bugiardini R, Galvani M, Ferrini D, Gridelli C, Tollemeto D, Mari L, Puddu P, Lenzi S. Myocardial ischemia induced by prostacyclin and iloprost. Clin Pharmacol Ther 1985;38:101–108. [DOI] [PubMed] [Google Scholar]
- 184. Bugiardini R, Galvani M, Ferrini D, Gridelli C, Tollemeto D, Macri N, Puddu P, Lenzi S. Myocardial ischemia during intravenous prostacyclin administration: hemodynamic findings and precautionary measures. Am Heart J 1987;113:234–240. [DOI] [PubMed] [Google Scholar]
- 185. Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol 2012;590:3219–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Pozzati A, Pancaldi LG, Di Pasquale G, Pinelli G, Bugiardini R. Transient sympathovagal imbalance triggers “ischemic” sudden death in patients undergoing electrocardiographic Holter monitoring. J Am Coll Cardiol 1996;27:847–852. [DOI] [PubMed] [Google Scholar]
- 187. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M; ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 188. Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, Duncker DJ, Koller A, Manfrini O, Milicic D, Padro T, Pries AR, Quyyumi AA, Tousoulis D, Trifunovic D, Vasiljevic Z, de Wit C, Bugiardini R; ESC Scientific Document Group Reviewers. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J 2020;41:1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Koller A, Takács J. Translation of scientific evidence into cardiovascular guidelines. JBI Evidence Implementation 2020; doi:10.1097/XEB.0000000000000266. [Google Scholar]