This article presents the final updated recommendations from the American College of Physicians on the use of SARS-CoV-2 antibody tests for diagnosis and to predict the degree or duration of natural immunity.
Abstract
Description:
The Scientific Medical Policy Committee (SMPC) of the American College of Physicians (ACP) developed these living, rapid practice points to summarize the current best available evidence on the antibody response to SARS-CoV-2 infection and protection against reinfection with SARS-CoV-2. This is version 2 of the ACP practice points, which serves to update version 1, published on 16 March 2021. These practice points do not evaluate vaccine-acquired immunity or cellular immunity.
Methods:
The SMPC developed this version of the living, rapid practice points based on an updated living, rapid, systematic review conducted by the Portland VA Research Foundation and funded by the Agency for Healthcare Research and Quality.
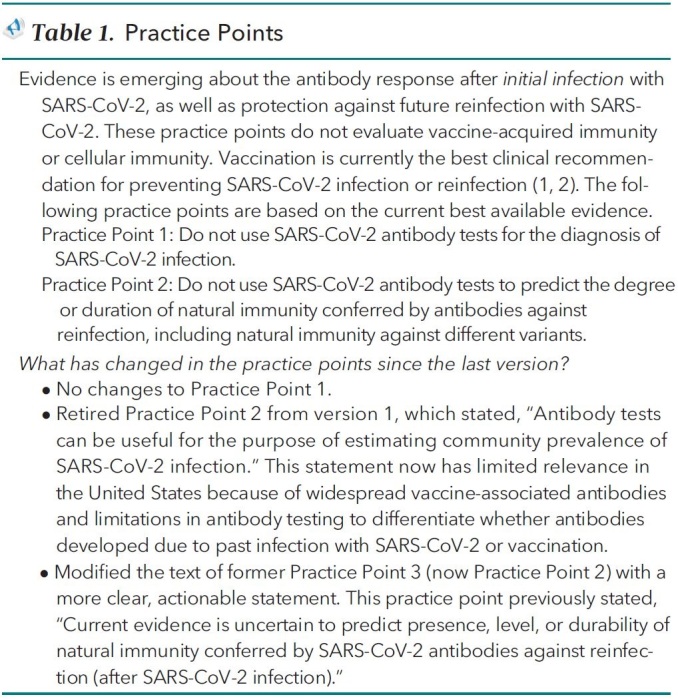
Practice Point 1:
Do not use SARS-CoV-2 antibody tests for the diagnosis of SARS-CoV-2 infection.
Practice Point 2:
Do not use SARS-CoV-2 antibody tests to predict the degree or duration of natural immunity conferred by antibodies against reinfection, including natural immunity against different variants.
Retirement From Living Status:
Although natural immunity remains a topic of scientific interest, this topic is being retired from living status given the availability of effective vaccines for SARS-CoV-2 and widespread recommendations for and prevalence of their use. Currently, vaccination is the best clinical recommendation for preventing infection, reinfection, and serious illness from SARS-CoV-2 and its variants.
The Scientific Medical Policy Committee (SMPC) of the American College of Physicians (ACP) has been maintaining these living, rapid practice points to summarize the current best available evidence on the antibody response to SARS-CoV-2 infection and protection against reinfection with SARS-CoV-2 (Table 1). This is version 2 of the ACP practice points, which serves to update version 1, published on 16 March 2021 (3, 4). It is based on a focused update of a living, rapid, systematic review conducted by the Portland VA Research Foundation and funded by the Agency for Healthcare Research and Quality (5, 6). The SMPC developed these practice points according to ACP's practice points development process, details of which can be found in ACP's methods paper (7).
Table 1.
Practice Points
The intended audience for these practice points includes clinicians, patients, the public, and public health officials. The population includes adults who have been previously infected with SARS-CoV-2.
This version was approved by the ACP Executive Committee of the Board of Regents on behalf of the Board of Regents on 9 August 2021 and was submitted to Annals of Internal Medicine on 6 August 2021.
Although vaccine-acquired immunity and cellular immunity are important areas of research, this article does not evaluate them.
Key Questions Addressed in the Living and Rapid Systematic Review
Key Question 1 (not updated): What are the prevalence, level, and duration of detectable anti–SARS-CoV-2 antibodies among patients infected with or recovered from reverse transcriptase polymerase chain reaction (RT-PCR)–diagnosed SARS-CoV-2 infection?
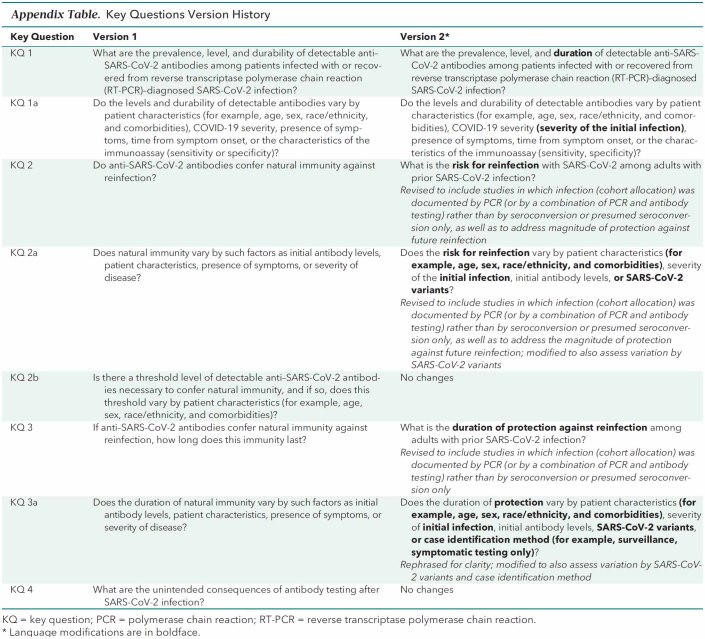
Key Question 1a (not updated): Do the levels and durability of detectable antibodies vary by patient characteristics (for example, age, sex, race/ethnicity, and comorbidities), COVID-19 severity (severity of the initial infection), presence of symptoms, time from symptom onset, or the characteristics of the immunoassay (sensitivity, specificity)?
Key Question 2 (updated): What is the risk for reinfection with SARS-CoV-2 among adults with prior SARS-CoV-2 infection?
Key Question 2a (updated): Does the risk for reinfection vary by patient characteristics (for example, age, sex, race/ethnicity, and comorbidities), severity of the initial infection, initial antibody levels, or SARS-CoV-2 variants?
Key Question 2b (updated): Is there a threshold level of detectable anti–SARS-CoV-2 antibodies necessary to confer natural immunity, and if so, does this threshold vary by patient characteristics (for example, age, sex, race/ethnicity, and comorbidities)?
Key Question 3 (updated): What is the duration of protection against reinfection among adults with prior SARS-CoV-2 infection?
Key Question 3a (updated): Does the duration of protection vary by patient characteristics (for example, age, sex, race/ethnicity, and comorbidities), severity of initial infection, initial antibody levels, SARS-CoV-2 variants, or case identification method (for example, surveillance, symptomatic testing only)?
Key Question 4 (not updated): What are the unintended consequences of antibody testing after SARS-CoV-2 infection?
Key Questions: Rationale for a Focused Update to the Living and Rapid Systematic Review
Updates to key questions in the living, rapid, systematic review are prioritized on the basis of identification of new evidence from literature surveillance that will likely substantially modify the conclusions or the certainty of evidence. Based on literature surveillance, the Portland VA Research Foundation and the SMPC determined that there was a signal to perform a focused update of key questions 2, 2a, 2b, 3, and 3a (large population-based studies that included uninfected comparison groups were published) and that the evidence for key questions 1, 1a, and 4 had not matured enough to evaluate the long-term persistence of antibodies, which would substantially modify the conclusions or certainty of evidence in the previous version. Consistent with methods for living systematic reviews and our living, rapid practice points (7), the inclusion criteria were modified to include large longitudinal studies with control groups to evaluate the risk for reinfection, and key questions were modified for clarity (Appendix Table).
Appendix Table.
Key Questions Version History
Overview of New Evidence
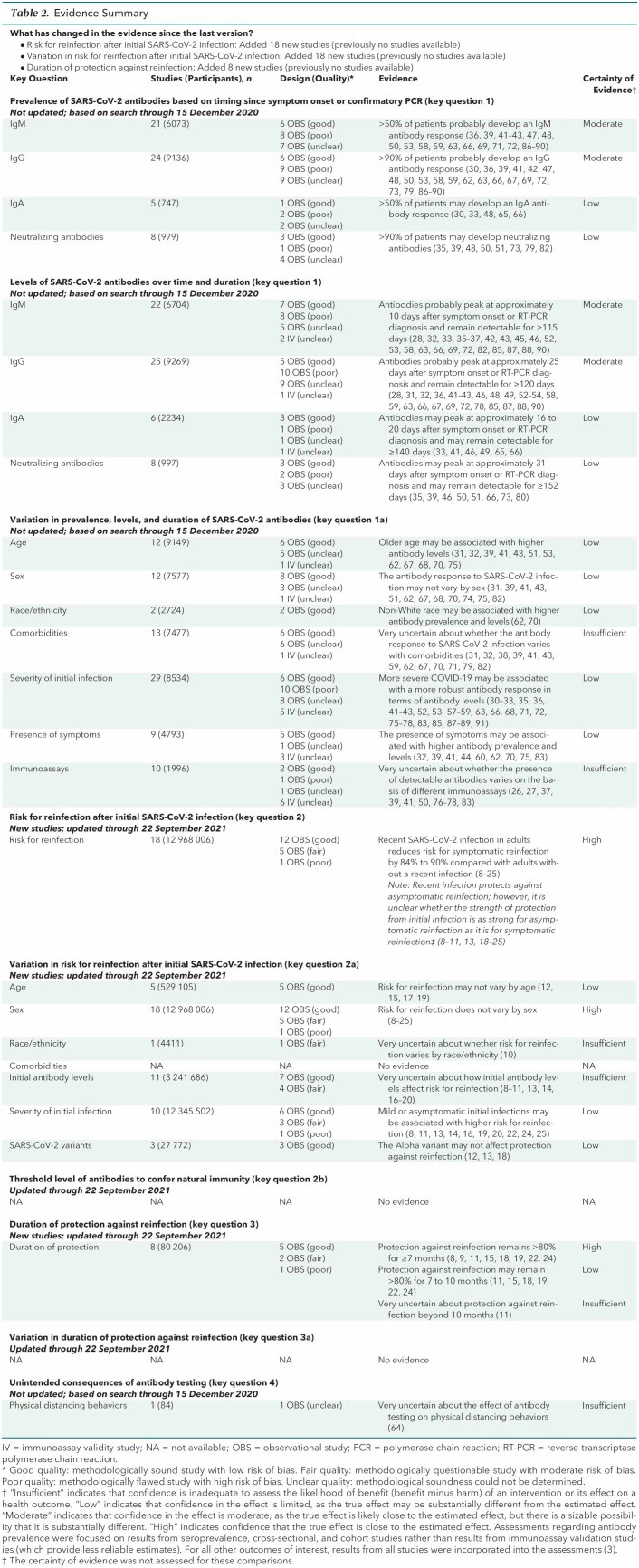
The evidence update (5, 6) identified 18 new studies (8–25) informing key questions 2, 2a, and 3, for which there were previously no studies that met the inclusion criteria in version 1 (3). These studies were initiated before the emergence of the Delta and Omicron variants and before the U.S. Food and Drug Administration's emergency use authorization of vaccines late in 2020 (5, 6). The new studies compared the risk for symptomatic reinfection (as a primary outcome) among adults with a recent SARS-CoV-2 infection with the risk for infection among adults without a recent infection, with “recent” defined as within 7 months of initial SARS-CoV-2 infection. These studies were designed to evaluate risk for symptomatic reinfection, with risk for asymptomatic reinfection as a secondary outcome. The new studies showed that patients with a recent SARS-CoV-2 infection have a substantially reduced risk for symptomatic reinfection (88% in the general population and 87% in health care workers) compared with those without a recent infection (key question 2) over follow-up of 4 to 13 months. There is also protection for asymptomatic reinfections, but the evidence is unclear about whether the degree of protection for asymptomatic reinfections is as high as it is for symptomatic reinfections. No evidence was identified on threshold levels of antibodies needed to confer protection from reinfection or the contribution of the antibody response to this protection (key question 2b). The systematic review update did not identify evidence from included studies on whether risk for reinfection varied by patient comorbidities (including immunosuppression) or by viral variants other than the Alpha variant (including the Delta and Omicron variants) (key question 2a), or whether the variation in the duration of protection varies by patient or clinical characteristics (key question 3a).
Updated Practice Points and Rationales (Version 2)
Evidence continues to emerge about the antibody response to SARS-CoV-2 infection and protection against future reinfection. The following practice points are based on the current best available evidence. The Figure, Table 2, and the accompanying systematic review (5, 6) summarize changes in the findings. Table 3 presents clinical considerations, and Table 4 identifies evidence gaps.
Figure. Evidence description.
The evidence search and assessment were conducted by the Portland VA Research Foundation (3, 5, 6). The evidence search was updated through 22 September 2021. PCR = polymerase chain reaction.
* Observational studies include studies estimating seroprevalence among a given population that includes a small subpopulation known to have SARS-CoV-2 infection; cross-sectional or cohort studies characterizing the antibody response among adults with SARS-CoV-2 infection; and large, population-based observational (cohort, case–control) studies comparing risk for reinfection in adults with and without recent SARS-CoV-2 infection (3,5,6). Immunoassay validation studies include those validating the diagnostic performance of 1 or more immunoassays (3).
Table 2.
Evidence Summary
Table 3.
Clinical Considerations

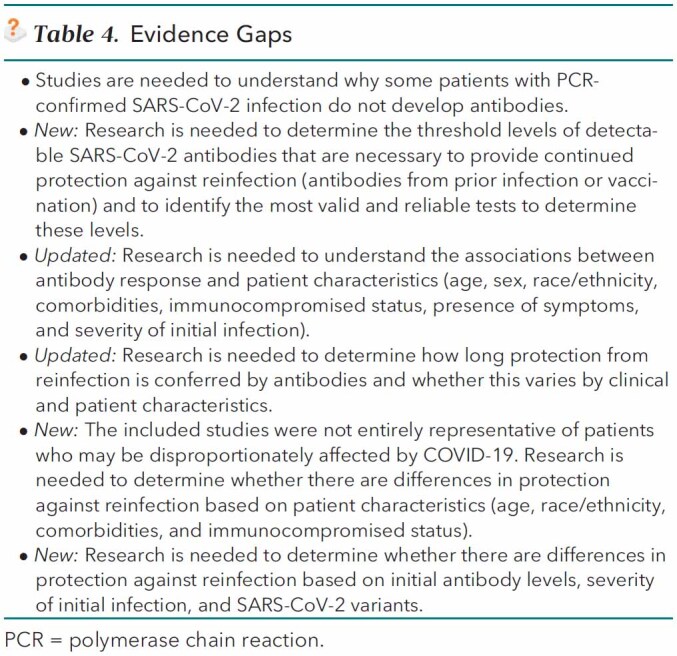
Table 4.
Evidence Gaps
We have retired Practice Point 2 from version 1, which stated, “Antibody tests can be useful for the purpose of estimating community prevalence of SARS-CoV-2 infection.” The relevance of this statement is now limited given the increase in vaccinations in the United States and because antibody tests cannot differentiate antibodies that develop due to past SARS-CoV-2 infection from those that develop due to vaccination.
Practice Point 1: Do not use SARS-CoV-2 antibody tests for the diagnosis of SARS-CoV-2 infection
Reaffirmed Rationale
Studies included in the version 1 systematic review evaluated the prevalence, levels, and duration of different types of antibodies after symptom onset or confirmation of SARS-CoV-2 infection with a positive RT-PCR result (3). These studies showed that most patients develop detectable antibodies after SARS-CoV-2 infection; however, the timing of when different antibodies peak and how long they remain detectable may vary (low to moderate certainty). Furthermore, the antibody response may vary by age, sex, race/ethnicity, and the severity of the initial infection (low certainty), and the evidence is very uncertain (insufficient) as to whether the response varies by comorbidities or type of immunoassay. In addition, the diagnostic test characteristics (for example, sensitivity, specificity, and accuracy) vary substantially across the antibody tests used in the included studies (3–6), contributing to differing risks for false-negative and false-positive results (94, 95). For these reasons, based on the studies included in version 1, antibody tests should not be used for the diagnosis of SARS-CoV-2 infection.
Practice Point 2: Do not use SARS-CoV-2 antibody tests to predict the degree or duration of natural immunity conferred by antibodies against reinfection, including natural immunity against different variants
Updated Rationale
Because measuring antibodies is an approach for evaluating the immune response, questions arise about the role of antibody testing in assessing natural immunity and protection from reinfection after SARS-CoV-2 infection. Although new evidence (18 new studies) has emerged addressing the risk for reinfection among adults with recent SARS-CoV-2 infection, several important evidence gaps remain in the new body of evidence that limit the clinical role of antibody testing (Table 4).
Low- to moderate-certainty evidence showed that patients with asymptomatic and symptomatic initial infections develop detectable antibodies (3), and high-certainty evidence from new studies showed that recent initial SARS-CoV-2 infection reduced the risk for symptomatic reinfection by 84% to 90% in adults over follow-up ranging from 4 to 13 months. This degree of protection may be similar across age groups (low certainty), with the Alpha variant (low certainty), in persons in the general population and health care workers, and does not vary according to sex (high certainty). However, these studies do not establish that antibodies are primarily responsible for the observed natural immunity because none of the new studies examined the relationship between antibody levels and degree of natural immunity, including threshold levels of detectable SARS-CoV-2 antibodies necessary to confer natural immunity. Furthermore, the included studies were conducted before the Delta and Omicron variants became the dominant circulating strains. However, the systematic review identified 3 studies that were not yet fully reported (96) or were longitudinal uncontrolled studies (97, 98) and thus did not meet the inclusion criteria; these studies suggest that recent SARS-CoV-2 infection reduced risk for reinfection in adults after the Delta variant became the dominant strain.
It is important to note that none of the new included studies reported on the variation in risk for reinfection in patients who are immunocompromised or have other comorbidities, and evidence is very uncertain (insufficient) about other factors that may modify risk for reinfection, including initial antibody levels and race/ethnicity. Evidence is also conflicting about risk for reinfection in patients who had an asymptomatic initial infection (5, 6); studies show that risk for reinfection may be higher for patients who had a mild or asymptomatic initial infection compared with those who had a symptomatic initial infection (low certainty). Although evidence suggests a high degree of protection (>80%) against symptomatic SARS-CoV-2 reinfection in the short term (high certainty for up to 7 months and low certainty for 7 to 10 months), the duration of protection beyond 10 months is very uncertain (insufficient), and follow-up in the included studies is constrained by time elapsed since the beginning of the pandemic. Finally, none of the included studies reported on how the duration of protection might vary by such factors as variant strains, initial antibody levels, and patient characteristics.
Despite evidence that patients develop detectable antibodies (3) and have reduced risk for reinfection after initial SARS-CoV-2 infection, knowledge about the direct association of the antibody response and the degree of natural immunity to SARS-CoV-2 is still limited. In light of these evidence gaps, and considering previously reported insufficient (very uncertain) evidence (3) about the unintended consequences of antibody testing, we advise against antibody testing to evaluate for natural immunity. Patients with current or previous SARS-CoV-2 infection should continue to follow recommended infection prevention and control procedures to slow and reduce transmission of the virus (92, 93, 99).
Retirement From Living Status
The SMPC is retiring the ACP living, rapid practice points on the antibody response to SARS-CoV-2 infection and protection against reinfection with SARS-CoV-2 from living status (7), given the widespread availability and use of effective vaccines against SARS-CoV-2 infection in the United States. Vaccination is currently the best clinical recommendation for prevention of SARS-CoV-2 infection and reinfection, including from currently circulating viral variants (1, 2).
Footnotes
This article was published at Annals.org on 25 January 2022.
* This paper, written by Amir Qaseem, MD, PhD, MHA; Jennifer Yost, RN, PhD; Itziar Etxeandia-Ikobaltzeta, PharmD, PhD; Mary Ann Forciea, MD; George M. Abraham, MD, MPH; Matthew C. Miller, MD; Adam J. Obley, MD; and Linda L. Humphrey, MD, MPH, was developed for the Scientific Medical Policy Committee of the American College of Physicians. Individuals who served on the Scientific Medical Policy Committee from initiation of the project until its approval were Linda L. Humphrey, MD, MPH† (Chair); Adam J. Obley, MD† (Vice Chair); Robert M. Centor, MD‡ (Immediate Past Vice Chair); Elie A. Akl, MD, MPH, PhD†; Rebecca Andrews, MS, MD†; Thomas A. Bledsoe, MD‡; Andrew Dunn, MD, MPH†; Mary Ann Forciea, MD†; Ray Haeme†§; Janet A. Jokela, MD, MPH‡; Devan L. Kansagara, MD, MCR†; Maura Marcucci, MD, MSc‡; Matthew C. Miller, MD†; and CDR Mark P. Tschanz, DO†. Kate Carroll, MPH, and Shannon Merillat, MPH, MLIS, were nonauthor contributors from ACP staff. Approved by the ACP Executive Committee of the Board of Regents on behalf of the Board of Regents on 9 August 2021.
† Author.
‡ Nonauthor contributor.
§ Nonphysician public representative.
Update Alerts: The literature update end date is 22 September 2021. No further updates for this topic are planned at this time.
Contributor Information
Collaborators: Mary Ann Forciea, Matthew C. Miller, Adam J. Obley, Linda L. Humphrey, Robert M. Centor, Thomas A. Bledsoe, Janet A. Jokela, and Maura Marcucci
References
- 1. Advisory Committee on Immunization Practices. COVID-19 ACIP Vaccine Recommendations. Accessed at www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html on 1 December 2021.
- 2. World Health Organization. COVID-19 advice for the public: Getting vaccinated. Accessed at www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice on 1 December 2021.
- 3. Arkhipova-Jenkins I, Helfand M, Armstrong C, et al. Antibody response after SARS-CoV-2 infection and implications for immunity: a rapid living review. Ann Intern Med. 2021;174:811-21. [PMID: ] doi: 10.7326/M20-7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qaseem A, Yost J, Etxeandia-Ikobaltzeta I, et al; Scientific Medical Policy Committee of the American College of Physicians. What is the antibody response and role in conferring natural immunity after SARS-CoV-2 infection? Rapid, living practice points from the American College of Physicians (version 1). Ann Intern Med. 2021;174:828-35. [PMID: ] doi: 10.7326/M20-7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helfand M, Fiordalisi C, Wiedrick J, et al. Risk of reinfection after SARS-CoV-2. Living rapid review for ACP practice points on the role of the antibody response in conferring immunity following SARS-CoV-2 infection. Ann Intern Med. 25 January 2022. [Epub ahead of print]. doi:10.7326/M21-4245 [DOI] [PMC free article] [PubMed]
- 6. Helfand M, Fiordalisi C, Wiedrick J, et al. Risk of Reinfection From SARS-CoV-2 – An Update of an Antibody Response Following SARS-CoV-2 Infection and Implications for Immunity: A Living Rapid Review. (Prepared by the Scientific Resource Center under contract no. 290-2017-0003.) AHRQ publication no. 21-EHC034. Agency for Healthcare Research and Quality; January 2022. Posted final reports are located on the Effective Health Care Program search page. doi: 10.23970/AHRQEPCCOVIDIMMUNITY2 [DOI]
- 7. Qaseem A, Yost J, Forciea MA, et al; Scientific Medical Policy Committee of the American College of Physicians. The development of living, rapid practice points: summary of methods from the Scientific Medical Policy Committee of the American College of Physicians. Ann Intern Med. 2021;174:1126-32. [PMID: ] doi: 10.7326/M20-7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abo-Leyah H, Gallant S, Cassidy D, et al. The protective effect of SARS-CoV-2 antibodies in Scottish healthcare workers. ERJ Open Res. 2021;7. [PMID: ] doi: 10.1183/23120541.00080-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine. 2021;35:100861. [PMID: ] doi: 10.1016/j.eclinm.2021.100861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finch E, Lowe R, Fischinger S, et al. SARS-CoV-2 infection and reinfection in a seroepidemiological workplace cohort in the United States. medRxiv. Preprint posted online 6 May 2021. doi:10.1101/2021.05.04.21256609
- 11. Gallais F, Gantner P, Bruel T, et al. Anti-SARS-CoV-2 antibodies persist for up to 13 months and reduce risk of reinfection. medRxiv. Preprint posted online 17 May 2021. doi:10.1101/2021.05.07.21256823
- 12. Goldberg Y, Mandel M, Woodbridge Y, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: a three-month nationwide experience from Israel. medRxiv. Preprint posted online 24 April 2021. doi:10.1101/2021.04.20.21255670
- 13. Hall VJ, Foulkes S, Charlett A, et al; SIREN Study Group. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397:1459-69. [PMID: ] doi: 10.1016/S0140-6736(21)00675-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanrath AT, Payne BAI, Duncan CJA. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection [Letter]. J Infect. 2021;82:e29-e30. [PMID: ] doi: 10.1016/j.jinf.2020.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen CH, Michlmayr D, Gubbels SM, et al. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204-12. [PMID: ] doi: 10.1016/S0140-6736(21)00575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harvey RA, Rassen JA, Kabelac CA, et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021;181:672-9. [PMID: ] doi: 10.1001/jamainternmed.2021.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeffery-Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021;26. [PMID: ] doi: 10.2807/1560-7917.ES.2021.26.5.2100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krutikov M, Palmer T, Tut G, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long term care facilities (VIVALDI study). medRxiv. Preprint posted online 10 March 2021. doi:10.1101/2021.03.08.21253110 [DOI] [PMC free article] [PubMed]
- 19. Leidi A, Koegler F, Dumont R, et al; SEROCoV-POP study group. Risk of reinfection after seroconversion to SARS-CoV-2: a population-based propensity-score matched cohort study. Clin Infect Dis. 2021. [PMID: ] doi: 10.1093/cid/ciab495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lumley SF, O’Donnell D, Stoesser NE, et al; Oxford University Hospitals Staff Testing Group. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533-40. [PMID: ] doi: 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manica M, Pancheri S, Poletti P, et al. The risk of symptomatic reinfection during the second COVID-19 wave in individuals previously exposed to SARS-CoV-2. medRxiv. Preprint posted online 20 April 2021. doi:10.1101/2021.04.14.21255502
- 22. Pilz S, Chakeri A, Ioannidis JP, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51:e13520. [PMID: ] doi: 10.1111/eci.13520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rennert L, McMahan C. Risk of SARS-CoV-2 reinfection in a university student population. Clin Infect Dis. 2021. [PMID: ] doi: 10.1093/cid/ciab454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheehan MM, Reddy AJ, Rothberg MB. Reinfection rates among patients who previously tested positive for coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis. 2021;73:1882-6. [PMID: ] doi: 10.1093/cid/ciab234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vitale J, Mumoli N, Clerici P, et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy [Letter]. JAMA Intern Med. 2021;181:1407-8. [PMID: ] doi: 10.1001/jamainternmed.2021.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andrey DO, Cohen P, Meyer B, et al. Head-to-head accuracy comparison of three commercial COVID-19 IgM/IgG serology rapid tests. J Clin Med. 2020;9. [PMID: ] doi: 10.3390/jcm9082369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andrey DO, Cohen P, Meyer B, et al; Geneva Centre for Emerging Viral Diseases. Diagnostic accuracy of Augurix COVID-19 IgG serology rapid test. Eur J Clin Invest. 2020;50:e13357. [PMID: ] doi: 10.1111/eci.13357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bao Y, Ling Y, Chen YY, et al. Dynamic anti-spike protein antibody profiles in COVID-19 patients. Int J Infect Dis. 2021;103:540-8. [PMID: ] doi: 10.1016/j.ijid.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test-retest strategy in residents and health care personnel of a nursing home facing a COVID-19 outbreak. J Am Med Dir Assoc. 2020;21:933-6. [PMID: ] doi: 10.1016/j.jamda.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruni M, Cecatiello V, Diaz-Basabe A, et al. Persistence of anti-SARS-CoV-2 antibodies in non-hospitalized COVID-19 convalescent health care workers. J Clin Med. 2020;9. [PMID: ] doi: 10.3390/jcm9103188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y, Ke Y, Liu X, et al. Clinical features and antibody response of patients from a COVID-19 treatment hospital in Wuhan, China. J Med Virol. 2021;93:2782-9. [PMID: ] doi: 10.1002/jmv.26617 [DOI] [PubMed] [Google Scholar]
- 32. Chen Y, Zuiani A, Fischinger S, et al. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell. 2020;183:1496-1507.e16. [PMID: ] doi: 10.1016/j.cell.2020.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chirathaworn C, Sripramote M, Chalongviriyalert P, et al. SARS-CoV-2 RNA shedding in recovered COVID-19 cases and the presence of antibodies against SARS-CoV-2 in recovered COVID-19 cases and close contacts, Thailand, April–June 2020. PLoS One. 2020;15:e0236905. [PMID: ] doi: 10.1371/journal.pone.0236905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choe JY, Kim JW, Kwon HH, et al. Diagnostic performance of immunochromatography assay for rapid detection of IgM and IgG in coronavirus disease 2019. J Med Virol. 2020;92:2567-72. [PMID: ] doi: 10.1002/jmv.26060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;223:197-205. [PMID: ] doi: 10.1093/infdis/jiaa618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dave M, Poswal L, Bedi V, et al. Study of antibody-based rapid card test in COVID-19 patients admitted in a tertiary care COVID hospital in Southern Rajasthan. Journal, Indian Academy of Clinical Medicine. 2020;21:7-11.
- 37. de la Iglesia J, Fernández-Villa T, Fegeneda-Grandes JM, et al. Concordance between two rapid diagnostic tests for the detection of antibodies against SARS-CoV-2. Semergen. 2020;46 Suppl 1:21-25. [PMID: ] doi: 10.1016/j.semerg.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dellière S, Salmona M, Minier M, et al; Saint-Louis CORE (COvid REsearch) group. Evaluation of the COVID-19 IgG/IgM rapid test from Orient Gene Biotech. J Clin Microbiol. 2020;58. [PMID: ] doi: 10.1128/JCM.01233-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fafi-Kremer S, Bruel T, Madec Y, et al. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. EBioMedicine. 2020;59:102915. [PMID: ] doi: 10.1016/j.ebiom.2020.102915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flannery DD, Gouma S, Dhudasia MB, et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5. [PMID: ] doi: 10.1126/sciimmunol.abd5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724-34. [PMID: ] doi: 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hou H, Wang T, Zhang B, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunology. 2020;9:e01136. [PMID: ] doi: 10.1002/cti2.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang M, Lu QB, Zhao H, et al. Temporal antibody responses to SARS-CoV-2 in patients of coronavirus disease 2019 [Letter]. Cell Discov. 2020;6:64. [PMID: ] doi: 10.1038/s41421-020-00209-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imai K, Tabata S, Ikeda M, et al. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J Clin Virol. 2020;128:104393. [PMID: ] doi: 10.1016/j.jcv.2020.104393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Infantino M, Grossi V, Lari B, et al. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: an Italian experience. J Med Virol. 2020;92:1671-5. [PMID: ] doi: 10.1002/jmv.25932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5. [PMID: ] doi: 10.1126/sciimmunol.abe5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401-8. [PMID: ] doi: 10.1016/S1473-3099(20)30589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5. [PMID: ] doi: 10.1126/sciimmunol.abe0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jääskeläinen AJ, Kekäläinen E, Kallio-Kokko H, et al. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Euro Surveill. 2020;25. [PMID: ] doi: 10.2807/1560-7917.ES.2020.25.18.2000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ko JH, Joo EJ, Park SJ, et al. Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients. J Clin Med. 2020;9. [PMID: ] doi: 10.3390/jcm9072268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koblischke M, Traugott MT, Medits I, et al. Dynamics of CD4 T cell and antibody responses in COVID-19 patients with different disease severity. Front Med (Lausanne). 2020;7:592629. [PMID: ] doi: 10.3389/fmed.2020.592629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kwon JS, Kim JY, Kim MC, et al. Factors of severity in patients with COVID-19: cytokine/chemokine concentrations, viral load, and antibody responses. Am J Trop Med Hyg. 2020;103:2412-8. [PMID: ] doi: 10.4269/ajtmh.20-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li K, Huang B, Wu M, et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun. 2020;11:6044. [PMID: ] doi: 10.1038/s41467-020-19943-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu J, Guo J, Xu Q, et al. Detection of IgG antibody during the follow-up in patients with COVID-19 infection [Letter]. Crit Care. 2020;24:448. [PMID: ] doi: 10.1186/s13054-020-03138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu J, Lian R, Zhang G, et al. Changes in serum virus-specific IgM/IgG antibody in asymptomatic and discharged patients with reoccurring positive COVID-19 nucleic acid test (RPNAT). Ann Med. 2021;53:34-42. [PMID: ] doi: 10.1080/07853890.2020.1811887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu L, Liu W, Zheng Y, et al. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect. 2020;22:206-11. [PMID: ] doi: 10.1016/j.micinf.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu R, Liu X, Yuan L, et al. Analysis of adjunctive serological detection to nucleic acid test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection diagnosis. Int Immunopharmacol. 2020;86:106746. [PMID: ] doi: 10.1016/j.intimp.2020.106746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu X, Wang J, Xu X, et al. Patterns of IgG and IgM antibody response in COVID-19 patients [Letter]. Emerg Microbes Infect. 2020;9:1269-74. [PMID: ] doi: 10.1080/22221751.2020.1773324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lynch KL, Whitman JD, Lacanienta NP, et al. Magnitude and kinetics of anti-severe acute respiratory syndrome coronavirus 2 antibody responses and their relationship to disease severity. Clin Infect Dis. 2021;72:301-8. [PMID: ] doi: 10.1093/cid/ciaa979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pancrazzi A, Magliocca P, Lorubbio M, et al. Comparison of serologic and molecular SARS-CoV 2 results in a large cohort in Southern Tuscany demonstrates a role for serologic testing to increase diagnostic sensitivity. Clin Biochem. 2020;84:87-92. [PMID: ] doi: 10.1016/j.clinbiochem.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Payne DC, Smith-Jeffcoat SE, Nowak G, et al; CDC COVID-19 Surge Laboratory Group. SARS-CoV-2 infections and serologic responses from a sample of U.S. Navy service members—USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:714-21. [PMID: ] doi: 10.15585/mmwr.mm6923e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Petersen LR, Sami S, Vuong N, et al. Lack of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a large cohort of previously infected persons. Clin Infect Dis. 2021;73:e3066-e3073. [PMID: ] doi: 10.1093/cid/ciaa1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qu J, Wu C, Li X, et al. Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:2255-8. [PMID: ] doi: 10.1093/cid/ciaa489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robbins T, Kyrou I, Laird S, et al. Healthcare staff perceptions and misconceptions regarding antibody testing in the United Kingdom: implications for the next steps for antibody screening. J Hosp Infect. 2021;111:102-6. [PMID: ] doi: 10.1016/j.jhin.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schaffner A, Risch L, Weber M, et al. Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: a population-based study [Letter]. Clin Chem Lab Med. 2020;59:e49-e51. [PMID: ] doi: 10.1515/cclm-2020-1347 [DOI] [PubMed] [Google Scholar]
- 66. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598-607. [PMID: ] doi: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shang Y, Liu T, Li J, et al. Factors affecting antibody response to SARS-CoV-2 in patients with severe COVID-19 [Letter]. J Med Virol. 2021;93:612-4. [PMID: ] doi: 10.1002/jmv.26379 [DOI] [PubMed] [Google Scholar]
- 68. Shen B, Zheng Y, Zhang X, et al. Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG. Am J Transl Res. 2020;12:1348-54. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- 69. Shu H, Wang S, Ruan S, et al. Dynamic changes of antibodies to SARS-CoV-2 in COVID-19 patients at early stage of outbreak. Virol Sin. 2020;35:744-51. [PMID: ] doi: 10.1007/s12250-020-00268-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Staines HM, Kirwan DE, Clark DJ, et al. IgG seroconversion and pathophysiology in severe acute respiratory syndrome coronavirus 2 infection. Emerg Infect Dis. 2021;27. [PMID: ] doi: 10.3201/eid2701.203074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stock da Cunha T, Gomá-Garcés E, Avello A, et al. The spectrum of clinical and serological features of COVID-19 in urban hemodialysis patients. J Clin Med. 2020;9. [PMID: ] doi: 10.3390/jcm9072264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun B, Feng Y, Mo X, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940-8. [PMID: ] doi: 10.1080/22221751.2020.1762515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suthar MS, Zimmerman MG, Kauffman RC, et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1:100040. [PMID: ] doi: 10.1016/j.xcrm.2020.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takahashi T, Wong P, Ellingson MK, et al; Yale IMPACT research team. Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes. med. Rxiv. 2020. [PMID: ] doi: 10.1101/2020.06.06.20123414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Terpos E, Politou M, Sergentanis TN, et al. Anti-SARS-CoV-2 antibody responses in convalescent plasma donors are increased in hospitalized patients; subanalyses of a phase 2 clinical study. Microorganisms. 2020;8. [PMID: ] doi: 10.3390/microorganisms8121885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Theel ES, Harring J, Hilgart H, et al. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58. [PMID: ] doi: 10.1128/JCM.01243-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Traugott M, Aberle SW, Aberle JH, et al. Performance of severe acute respiratory syndrome coronavirus 2 antibody assays in different stages of infection: comparison of commercial enzyme-linked immunosorbent assays and rapid tests. J Infect Dis. 2020;222:362-6. [PMID: ] doi: 10.1093/infdis/jiaa305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van Elslande J, Decru B, Jonckheere S, et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin Microbiol Infect. 2020;26:1557.e1-1557.e7. [PMID: ] doi: 10.1016/j.cmi.2020.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang B, Van Oekelen O, Mouhieddine TH, et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. medRxiv. Preprint posted online 29 June 2020. doi:10.1101/2020.06.04.20122846 [DOI] [PMC free article] [PubMed]
- 80. Wang K, Long QX, Deng HJ, et al. Longitudinal dynamics of the neutralizing antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Clin Infect Dis. 2021;73:e531-e539. [PMID: ] doi: 10.1093/cid/ciaa1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang P. Combination of serological total antibody and RT-PCR test for detection of SARS-COV-2 infections. J Virol Methods. 2020;283:113919. [PMID: ] doi: 10.1016/j.jviromet.2020.113919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wendel S, Kutner JM, Fontao-Wendel R, et al. Screening for SARS-CoV-2 antibodies in convalescent plasma (CCP) in Brazil: results from a voluntary convalescent donor program. Transfusion. 2020;60:296A. [DOI] [PMC free article] [PubMed]
- 83. Wolff F, Dahma H, Duterme C, et al. Monitoring antibody response following SARS-CoV-2 infection: diagnostic efficiency of 4 automated immunoassays. Diagn Microbiol Infect Dis. 2020;98:115140. [PMID: ] doi: 10.1016/j.diagmicrobio.2020.115140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1930-4. [PMID: ] doi: 10.1093/cid/ciaa461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xie L, Wu Q, Lin Q, et al. Dysfunction of adaptive immunity is related to severity of COVID-19: a retrospective study. Ther Adv Respir Dis. 2020;14:1753466620942129. [PMID: ] doi: 10.1177/1753466620942129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu X, Sun J, Nie S, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193-5. [PMID: ] doi: 10.1038/s41591-020-0949-6 [DOI] [PubMed] [Google Scholar]
- 87. Young BE, Ong SWX, Ng LFP, et al; Singapore 2019 Novel Coronavirus Outbreak Research Team. Viral dynamics and immune correlates of coronavirus disease 2019 (COVID-19) severity. Clin Infect Dis. 2021;73:e2932-e2942. [PMID: ] doi: 10.1093/cid/ciaa1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. [PMID: ] doi: 10.3389/fmolb.2020.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao G, Su Y, Sun X, et al. A comparative study of the laboratory features of COVID-19 and other viral pneumonias in the recovery stage. J Clin Lab Anal. 2020;34:e23483. [PMID: ] doi: 10.1002/jcla.23483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027-34. [PMID: ] doi: 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zheng Y, Yan M, Wang L, et al. Analysis of the application value of serum antibody detection for staging of COVID-19 infection. J Med Virol. 2021;93:899-906. [PMID: ] doi: 10.1002/jmv.26330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. World Health Organization. Advice on the use of masks in the context of COVID-19: interim guidance, 5 June 2020. Accessed at https://apps.who.int/iris/handle/10665/332293 on 1 December 2021.
- 93. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. Accessed at www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html on 1 December 2021.
- 94. Centers for Disease Control and Prevention. Using Antibody Tests for COVID-19. Accessed at www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests.html on 1 December 2021.
- 95. Watson J, Richter A, Deeks J. Testing for SARS-CoV-2 antibodies. BMJ. 2020;370:m3325. [PMID: ] doi: 10.1136/bmj.m3325 [DOI] [PubMed] [Google Scholar]
- 96. Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv. Preprint posted online 25 August 2021. doi:10.1101/2021.08.24.21262415
- 97. Cavanaugh AM, Spicer KB, Thoroughman D, et al. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination—Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081-3. [PMID: ] doi: 10.15585/mmwr.mm7032e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hagan LM, McCormick DW, Lee C, et al. Outbreak of SARS-CoV-2 B.1.617.2 (Delta) variant infections among incarcerated persons in a federal prison—Texas, July–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1349-54. [PMID: ] doi: 10.15585/mmwr.mm7038e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Centers for Disease Control and Prevention. How to Protect Yourself & Others. Accessed at www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html on 1 December 2021.