Introduction
Colorectal cancer is the fourth most common cancer and second most common cause of cancer death in the United States, and nearly 30% of all newly diagnosed colorectal cancers are located in the rectum [1]. Oncological outcomes of locally advanced rectal cancer (LARC) have improved over the last three decades due to multidisciplinary care and standardization of diagnostic imaging, surgical technique, surgical pathology, and perioperative multimodal treatment. Accepted standard treatment for LARC includes preoperative chemoradiotherapy (CRT), total mesorectal excision (TME), and postoperative systemic chemotherapy [2]. Intensive multimodal treatment is associated with excellent local control but inadequate systemic control, with about one-third of patients succumbing to metastatic disease [3]. Additionally, multimodal treatment is detrimental to patients’ quality of life (QoL) due to surgical morbidity; risk of permanent stoma; and bowel, bladder, and sexual dysfunction. Two key challenges are improving survival by preventing metastases and improving patients’ QoL by preventing functional impairments.
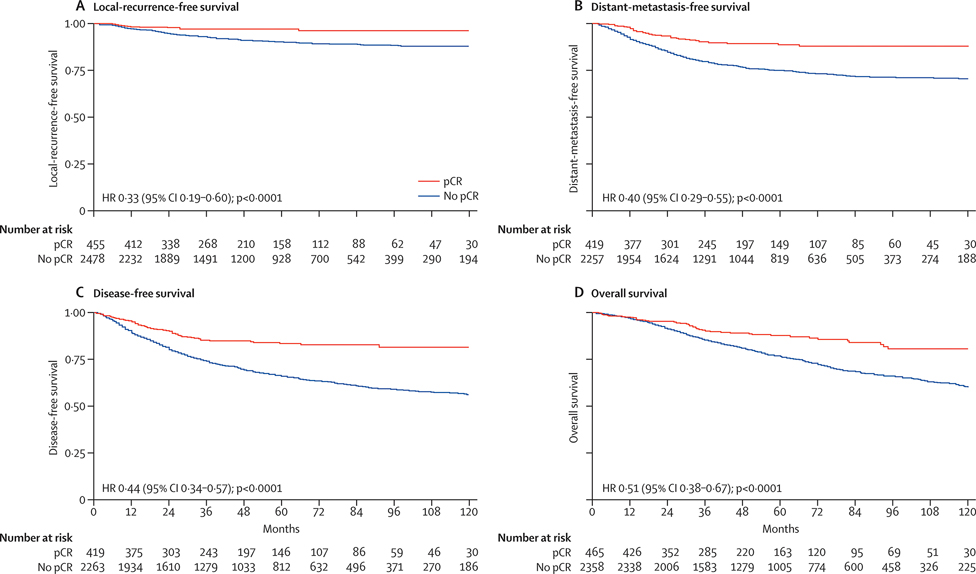
The rate of pathologic complete response (pCR) is approximately 20% in patients who undergo CRT alone and can reach nearly 40% in patients who undergo preoperative CRT in combination with systemic chemotherapy [4]. Patients with pCR have a 5-year survival rate of 95% and a local-recurrence rate of around 1% [5] (Fig. 1). In view of these outcomes, surgical removal of the rectum in patients with pCR may constitute overtreatment, and the risk of sequelae from surgery may outweigh the risk of tumor progression during observation. In this context, avoiding surgery may provide important benefits such as lower morbidity, lower health care costs, and better QoL.
Fig. 1.
Kaplan-Meier survival curves for patients with or without pCR. p values were determined by the log-rank test. HR, hazard ratio. (From Maas et al. 2010, Lancet Oncol. 11:835–44; with permission.)
Acceptance is growing for watch-and-wait (WW) as an alternative nonstandard strategy aimed at preserving the rectum in LARC patients with clinical complete response (cCR), based on favorable retrospective studies conducted at specialized cancer centers. Prospective data from the phase II trial Organ Preservation in Rectal Adenocarcinoma (OPRA; NCT02008656)— which is evaluating 3-year disease-free survival in patients who receive CRT plus induction or consolidation chemotherapy and then undergo WW management or TME depending on response—has been collected [6] and will soon be reported.
Terminology
The nonstandard approach of WW also has some nonstandard language. Whereas a pCR is an objective determination that no tumor cells are present in the surgical specimen, a cCR is a subjective assessment by treating physicians that no viable tumor remains in the rectum following neoadjuvant therapy (NAT). Because the decision to proceed with WW is based on this subjective assessment, some patients with cCR may have clinically obscure remnants of the tumor that will eventually become clinically evident.
WW is a management strategy for patients with cCR following NAT, while organ preservation is the desired outcome of this approach. The term nonoperative management is often used interchangeably with WW, but because operative local excision can be a part of a strategy for organ preservation, the use of this term is potentially confusing.
Reappearance of neoplasia at the site of a treated tumor is called regrowth. Regrowth is considered salvageable when it can be cured by TME. Tumor recurrence in the context of WW is nonsalvageable regrowth or presence of cancer in the pelvis following a curative resection. The term recurrence should not be used for the presence of salvageable regrowth in patients with cCR.
Neoadjuvant therapy is administered before surgery and adjuvant therapy is administered after surgery. Systemic chemotherapy can be added to CRT in the neoadjuvant period. When chemotherapy is administered before CRT, it is called induction chemotherapy; when it is administered after CRT (but before surgery) it is called consolidation chemotherapy. A full course of induction or consolidation chemotherapy along with CRT is called total neoadjuvant therapy (TNT).
History of WW
Although reports of radiotherapy curing rectal cancer without surgery appeared as early as 1920 [7], A. Habr-Gama’s group pioneered WW of rectal cancer in modern times, as described in their report on 265 patients with surgically curable rectal cancer assessed for response 8 weeks after CRT [8]. In that study, patients with incomplete response underwent surgical resection, and 71 patients with cCR (27%) entered a strict surveillance program.
With a mean follow-up of 57.3 months for patients with a cCR, three patients developed distant metastases. Two patients developed regrowth, which was salvaged with local therapy. Overall survival and disease-free survival did not differ significantly between patients with cCR and patients with pCR. These results demonstrated that with structured surveillance, deferral of surgery is safe, regrowth can be effectively treated, and sustained cCR can be comparable to a cure. Additional reports from other specialized cancer centers have provided support of the efficacy and safety of WW [9–16]. However, many surgeons are reluctant to adopt WW [17], because of the lack of standardization in response assessment and the lack of randomized prospective data.
Patient Perspectives on WW
Patients are interested in treatment strategies that preserve the rectum, and they are willing to make oncological compromises for this goal. In a study by Gani et al. [18], in which questionnaires were collected from patients awaiting multimodal treatment, 83% of the patients said that they would consider WW in case of cCR; 94% were willing to accept a 2-year regrowth rate of 25%, and 95% were willing to accept an intensive follow-up protocol. Although 55% of the patients would enter WW only if this approach was associated with equivalent cure rates, 30% of patients were willing to accept a 2% absolute reduction in the long-term cure rate, and 11% were willing to accept a 10% reduction. Another study [19], in which questionnaires were collected from LARC patients following multimodal treatment, found that the median respondent would accept an absolute increase of 20% in recurrence risk with WW and would need an additional 2 years of life and an absolute increase of 10% in the likelihood of surviving 7 years to prefer TME.
Patient Evaluation
Workup
The decision to proceed with WW is made during response assessment following NAT, and all patients who are candidates for NAT are also potential candidates for organ preservation. The initial workup should be the standard rectal cancer workup based on the guidelines of the National Comprehensive Cancer Network. Documentation of the endoscopic characteristics of the tumor at baseline, ideally with endoscopic images, is important for comparison in subsequent response evaluations. Following NAT, patients with a cCR (or with a significant response that does not meet all criteria of cCR) can enter a WW protocol after the patient, the surgeon, and the disease management team agree to proceed with this nonstandard approach.
Selection of patients based on tumor stage
Several studies on WW have reported outcomes for LARC patients who received multimodal NAT as part of standard care. WW offers considerable potential benefit for LARC patients, given the unsatisfactory QoL for these patients after NAT and TME. The majority of LARC patients treated by both modalities have long term bowel dysfunction, also known as low anterior resection syndrome [20]. A potential disadvantage of WW for LARC patients is withholding a treatment modality when the risk of disease progression is not negligible. Characteristics that have been associated with risk of local recurrence include ≤1-mm distance of the tumor from the mesorectal fascia, extramural venous invasion, and extensive nodal disease or lateral pelvic nodal metastasis [21, 22]. While the presence of these features is not a contraindication for WW, a cCR in patients with advanced tumors is less probable, and assessment of response may be challenging. Ulcerated circumferential tumors may scar in a way that narrows the rectal lumen and prevents proper endoscopic evaluation. Patients with these tumors may not be appropriate candidates for WW.
Some studies have focused on WW for patients with early-stage (cT2N0M0) low rectal cancer [23, 24], for which TME without NAT is the standard of care. A distal cT2N0M0 tumor can be adequately resected only by abdominoperineal resection, and the prospect of avoiding a permanent stoma is highly desirable for patients. In addition, early stage tumors are more sensitive to NAT than more advanced tumors and rates of organ preservation of cT2N0M0 tumors following NAT can reach 67% at 5 years[23]. However, for patients who end up with an incomplete clinical response (iCR) to NAT, NAT carries the potential disadvantage of the synergistic sequelae of radiation and surgery, compared with the option of surgery alone.
Selection of patients based on tumor location
Patients receiving NAT for rectal cancer in any location are potential candidates for WW, but patients with distal tumors requiring a low anastomosis or abdominoperineal resection are likely to benefit the most. In addition to the prospect of avoiding a permanent stoma and poor QoL (as a consequence of a very low anastomosis in a previously irradiated rectum), patients with a distal tumor who undergo WW can benefit from the fact that distal tumors are easily evaluable by a digital rectal exam (DRE) and respond better to NAT than proximal tumors [25].
Baseline characteristics associated with response to NAT
A meta-analysis of nearly 1200 patients found that characteristics associated with pCR include older age, smaller tumor size, shorter distance from the anal verge, and negative lymph node status [26]. A large retrospective study demonstrated that response is inversely associated with the circumferential extent of the tumor, level of carcinoembryonic antigen, and distance from the anal verge [25].
Response to NAT is also associated with certain common genomic alterations. Mutations in p53 and KRAS, found in about 70% and 40% of rectal tumors, respectively [27, 28], are associated with poor response to NAT. Conversely, mismatch repair deficiency and microsatellite instability, found in <5% of rectal tumors, are associated with good response to radiation[29].
Radiotherapy Regimens
The ability of preoperative radiotherapy to prevent locoregional recurrence in patients with LARC has been well established for both short-course radiation therapy (SCRT), consisting of five fractions of 5 Gy (total, 25 Gy), and long-course CRT combined with a sensitizing fluoropyrimidine, consisting of multiple fractions of 1.8 to 2 Gy (total, 40 to 50.4 Gy) [2, 30]. The interval to surgery after CRT is usually 6 to 8 weeks, whereas the interval to surgery after SCRT can be short (1 week) or extended (4 to 8 weeks) [2, 4]. CRT is the approach most commonly utilized in WW protocols [8–13, 15, 16].
Although data on the use of SCRT in WW is limited, the incorporation of SCRT into TNT has shown promising results [31–35] and has been described in the context of WW [31, 36]. Additionally, SCRT has been increasingly utilized during the COVID-19 pandemic because of its efficiency in resource utilization and relatively few patient visits needed [37, 38]. This change in practice may help elucidate the effectiveness of SCRT-based TNT in organ preservation strategy.
Results of the RAPIDO trial (NCT01558921) [39]—in which patients are randomized to standard CRT followed by TME and optional adjuvant chemotherapy or to SCRT and six cycles of capecitabine-oxaliplatin consolidation therapy followed by TME—may also provide indirect data on the value of SCRT in WW. Early reports from the trial indicate a higher pCR rate (28% vs 14%, p<0.001) with SCRT–consolidation chemotherapy and lower rates of distant metastases [40], but raise two questions: (i) is SCRT-consolidation more effective than CRT-consolidation and (ii) how toxic is SCRT-consolidation if the rectum is not removed?
Enhancing the Response to NAT
Response to NAT can be enhanced by (i) increasing the time interval to assessment, (ii) increasing the radiation dose, (iii) adding systemic chemotherapy to radiotherapy in the neoadjuvant setting, and/or (iv) administering consolidation chemotherapy rather than induction chemotherapy.
Interval to assessment
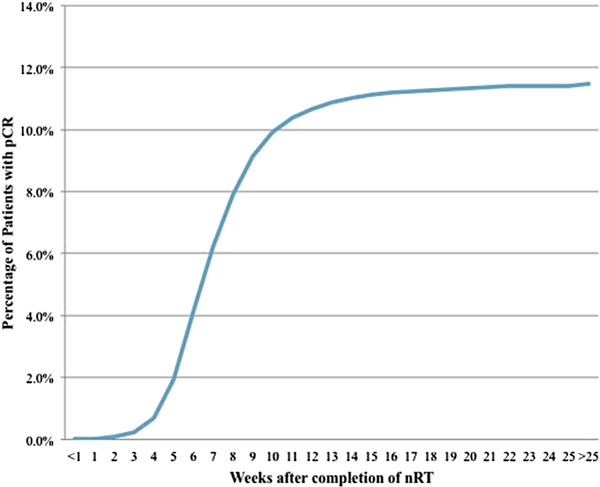
Tumor regression following radiation therapy is time dependent, and maximal tumor response can take months. A retrospective analysis of National Cancer Database data from more than 17,000 patients who underwent neoadjuvant CRT and TME found that intervals of more than 8 weeks from CRT to resection were associated with higher rates of pCR [41]. The rates of pCR were around 0% at 1 week and around 11% at 14 weeks, after which they remained nearly constant (Fig. 2). Longer intervals were also associated with lower rates of readmission after surgery.
Fig. 2.
Cumulative percentage of patients with pCR at different time points after completion of neoadjuvant radiotherapy (nRT). (From Probst et al. 2015, J. Am. Coll. Surg. 221:430–40; with permission.)
In the Stockholm III trial (NCT00904813), patients who underwent SCRT with delayed surgery had a higher rate of pCR and tumor regression grade than patients who underwent surgery immediately after SCRT [42, 43]. Survival did not differ between the two groups, but SCRT with delayed surgery was associated with fewer postoperative complications. In another trial [44] (NCT03287843), patients were randomized to TME either within 8 weeks after CRT or more than 8 weeks after CRT. The rate of pCR was significantly higher in the latter group (18.6% vs. 10%; p = 0.03), with no difference in surgical morbidity or in the quality of resection.
Radiation dose
Higher radiation doses have been reported to be associated with higher rates of tumor response. Patients with early-stage rectal cancer treated with 54 Gy have higher rates of initial cCR and higher rates of organ preservation at 5 years than patients treated with 50.4 Gy [23]. One study found histological evidence of a dose-response relationship in vitro with radiation doses of 50.4 to 70 Gy [45]. This range is higher than what is commonly used for LARC, and the authors of that study concluded that the higher doses may have potential for use in WW protocols. A subsequent study by the same group investigated high-dose CRT in patients with T2-T3 N0-N1 disease considered for WW [12]. At 6 weeks following treatment, a remarkable 78% of patients had a cCR. Approximately a third of the patients had early-stage rectal cancer, which may have contributed to the high response rate.
Addition of neoadjuvant systemic chemotherapy
Adding neoadjuvant systemic chemotherapy either before or after CRT can improve response rates. In a retrospective study with 628 patients [46], complete response was observed in 37% of patients who received TNT and only 21% of patients who received CRT. In a nonrandomized clinical trial [4] comparing the rate of pCR between four sequential groups of patients who received CRT alone or in combination with two, four, or six cycles of FOLFOX (leucovorin, fluorouracil, oxaliplatin), the pCR rate increased with each addition of two cycles of FOLFOX: no FOLFOX, 18%; two cycles, 25%; four cycles, 30%; six cycles, 38%. The rate of serious adverse events did not differ between the groups. Extension of the time interval to resection with each addition of FOLFOX cycles may have contributed to the higher pCR rates. In the PRODIGE 23 phase III randomized trial (NCT01804790), patients who received six cycles of FOLFORINOX (leucovorin, fluorouracil, irinotecan, oxaliplatin) followed by CRT, TME, and 3 months of adjuvant systemic chemotherapy had a significantly higher pCR rate (28% vs. 12%, p < 0.001) and higher 3-year rates of disease-free and metastasis-free survival than patients who underwent CRT, TME, and 6 months of adjuvant systemic chemotherapy [47].
Induction vs. consolidation chemotherapy in TNT
TNT employing consolidation chemotherapy is associated with higher rates of response and organ preservation than TNT employing induction chemotherapy. Fokas et al. [48], who randomized 311 patients with stage II or III rectal cancer to either induction or consolidation TNT followed by TME, found that consolidation was associated with a higher rate of pCR (25% vs. 17%; p < 0.001). The preliminary results of the OPRA trial (NCT02008656), in which patients were randomized to induction or consolidation TNT and then proceeded to surgery or WW depending on response, demonstrated higher rates of organ preservation in the consolidation arm (58% vs 43%, p=0.01), with no difference in disease-free survival or distant-metastasis-free survival [6]. Based on the results of these two trials, LARC patients who are candidates for TNT and potentially for WW may benefit more from consolidation-based TNT than from induction-based TNT.
Complications of NAT
Although NAT helps lower the likelihood of local recurrence and facilitates organ preservation, it is associated with severe acute and chronic adverse events, including death. In the seminal randomized control trial comparing pre- and postoperative CRT for LARC, preoperative CRT was associated with much lower toxicity than postoperative CRT, but acute grade 3–4 toxic effects were still seen in 27% of patients who received preoperative CRT (27% vs. 40%, p < 0.001) [2]. Higher rates of toxicities can be observed with the addition of systemic chemotherapy to CRT, without a benefit in survival; grade 3–5 toxicities can be seen in as many as 42% of patients and deaths attributable to NAT can be seen in as many as 1.5% of patients[49]. These rates may be even higher in elderly patients, who should receive individualized treatment, based on their overall health and frailty assessment.
Assessment of Response
Selection of patients for WW remains a challenge. Ideally, patients for whom a pCR would likely be identified were they to undergo resection should be selected for WW. However, the decision to manage a patient’s rectal cancer with WW must be based on clinical findings, even though the correlation between clinical assessment of response and pathologic assessment of response is imperfect. In the first 2 years following NAT, regrowth occurs in up to 25% of patients with a cCR [50]. Conversely, up to 15% of patients with an iCR who undergo TME end up having a pCR [8, 51]. Overly strict criteria of cCR will result in some patients undergoing surgery needlessly, and overly loose criteria will result in missing some cases of residual tumor.
Three modalities are routinely used to assess response: DRE, MRI, and endoscopy. Each is imperfect, but combining modalities increases accuracy. The accuracy is 98% when all three modalities indicate that the tumor is gone [51].
Classic endoscopic features of cCR include a flat white scar, telangiectasia, and absence of both ulcer and nodularity. Typical MRI features of cCR include a scar not thicker than the rectal wall, only dark T2 signal, no visible lymph nodes, no restricted diffusion, and lack of or low signal on ADC map. Optimally a DRE of a cCR should be normal but sometimes minor mucosal abnormalities can be palpated.
One of the hurdles to widespread implementation of WW is the lack of uniform and reproducible criteria for tumor response and patient selection that also take into account borderline cases. Patients with a significant response that does not meet all criteria of cCR— called near complete clinical response (ncCR)—can enter WW if the tumor continues showing signs of response until it meets the full criteria of cCR. In some atypical cases, a cCR can take up to a year. A three-tiered response assessment schema, which includes the gray area of ncCR, is currently being tested in the OPRA trial (Table 1 and Fig. 3). Patients with iCR undergo immediate TME, whereas patients with cCR or ncCR enter WW management.
Table 1.
Criteria for clinical assessment of response to neoadjuvant therapy in the OPRA triala
| Modality | Criteria |
||
|---|---|---|---|
| Complete response | Near complete response | Incomplete response | |
| Endoscopy | Flat white scar, telangiectasia, no ulcer, no nodularity | Irregular mucosa, small mucosal nodules or minor mucosal abnormality, superficial ulceration, mild persisting erythema of scar | Visible tumor |
| Digital rectal exam | Normal | Smooth induration or minor mucosal abnormalities | Palpable tumor nodules |
| MRI | |||
| T2 weighted | Only dark T2 signal, no intermediate T2 signal, no visible lymph nodes | Mostly dark T2 signal, some remaining intermediate signal, or partial regression of lymph nodes | More intermediate than dark T2 signal, no T2 scar, or no regression of lymph nodes |
| Diffusion weighted | No visible tumor on B800-B1000 signal or Lack of or low signal on ADC mapb |
Significant regression of B800-B1000 signal or minimal residual signal on ADC map | Insignificant regression of B800-B1000 signal or obvious low signal on ADC map |
From Yuval et al. 2020, J. Gastrointest. Surg. 24:1880–1888; with permission.
Uniform linear signal in wall above the tumor is acceptable. ADC, apparent diffusion coefficient.
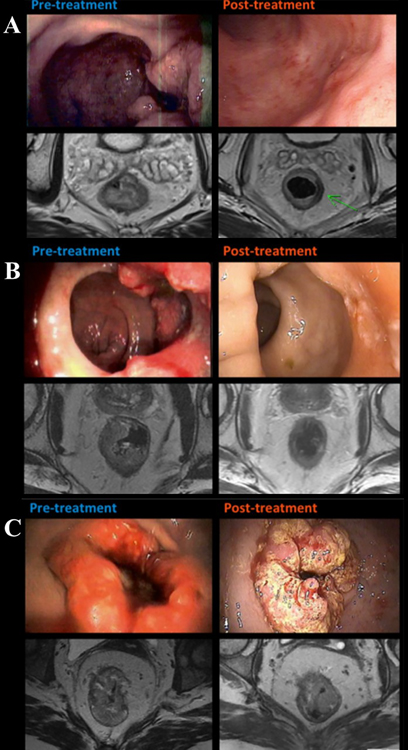
Fig. 3.
Endoscopic and T2-weighted MRI examples of cCR (A), ncCR (B) and iCR (C). (Adapted from Smith et al. 2015, BMC Cancer 15:767; with permission.)
Long-Term Monitoring of Response
Surveillance protocols for patients entering WW vary but typically include measurement of the level of carcinoembryonic antigen in serum; clinical examination; DRE, endoscopy, and MRI for assessment of local recurrence; and CT of the chest, abdomen, and pelvis for assessment of distant metastases. Surveillance should be most intensive in the first 2 years after treatment, because that’s when most cases of regrowth occur. In years 3–5 following treatment, monitoring can be less frequent; after year 5, patients can enter survivorship surveillance [52]. The long-term surveillance protocol used in the OPRA trial is shown in Table 2.
Table 2.
Long-term surveillance in the OPRA triala
| Exam or measureb | Months after treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3–6 | 9–12 | 15–18 | 21–24 | 30c | 36c | 42c | 48c | 54c | 60c | |
| History and physical | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Endoscopy | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| MRI rectum | √ | √ | √ | √ | √ | √ | ||||
| CT CAP | √ | √ | √ | √ | √ | |||||
| CEA | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
From Yuval et al. 2020, J. Gastrointest. Surg. 24:1880–1888; with permission.
CAP, chest-abdomen-pelvis; CEA, carcinoembryonic antigen.
±30 days.
Long-Term Outcomes
Oncological outcomes
Oncological outcomes in WW patients are similar to outcomes in TME patients, with the exception of regrowth and rectum preservation. The data for WW are largely retrospective, and interpreting oncological outcomes based on retrospective series can be tricky because of differences in treatment regimens and indications and lack of uniform criteria for staging and patient selection. The interval from completion of radiotherapy to assessment can also vary, and the actual denominator is often unclear because studies begin with a cohort of patients with cCR and not with a cohort of patients starting NAT.
In the only prospective study reported to date, which looked at 40 patients with cCR in Denmark, the rate of regrowth was 25% at 2 and 3 years and 31% at 5 years following NAT [12,53]. All but one case of regrowth were salvaged by TME (92%). Overall survival at 2, 3, and 5 years was 100%, 95%, and 85%, respectively, and distant-metastasis-free survival at 5 years was 83%.
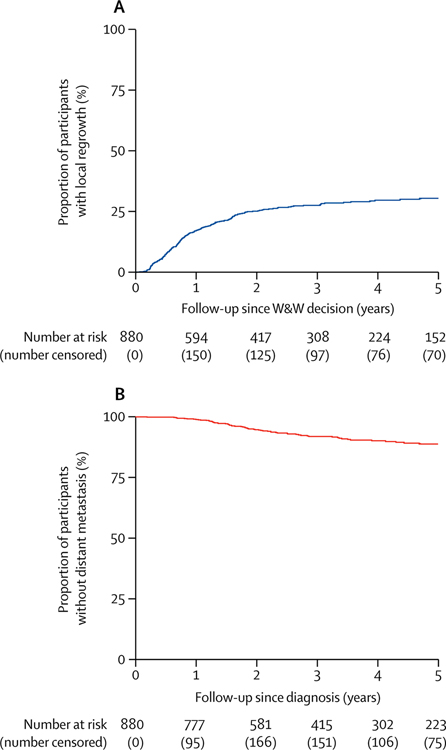
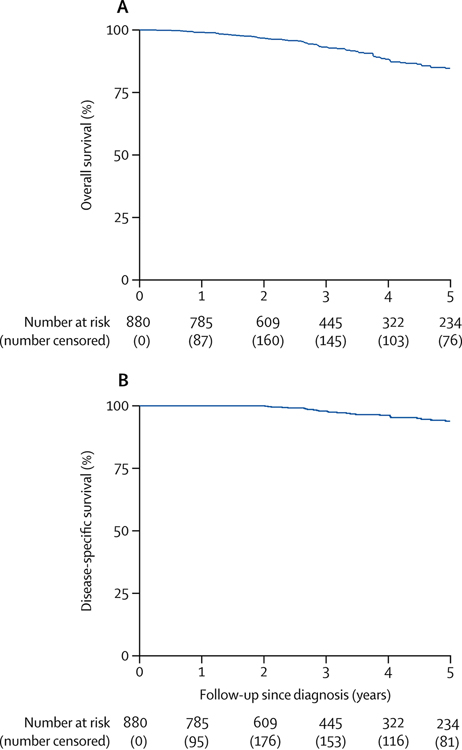
In a 2018 analysis of International Watch & Wait database data from 880 patients with cCR who underwent WW at 47 medical centers in various countries [50], the rate of regrowth at 2 years was 25% (Fig. 4A); 77% of the regrowth cases were salvaged by TME. Distant metastases developed in 8% of patients (Fig. 4B). Five-year overall survival and disease-specific survival were 85% and 94%, respectively (Fig. 5).
Fig. 4.
Local tumor regrowth and distant-metastasis-free survival in patients with cCR. (From van der Valk et al. 2018, Lancet 391:2537–45; with permission.)
Fig. 5.
Five-year overall survival and disease-specific survival in patients with cCR. (From van der Valk et al. 2018, Lancet 391:2537–45; with permission.)
Rates of organ preservation
The rate of rectum preservation in patients with cCR who enter WW ranges between 70% and 82% [11, 12, 15, 16]. However, this rate can be misleading because it misses the true denominator of patients by excluding those with an iCR. The rate of rectum preservation for all LARC patients undergoing TNT with selective organ preservation in the OPRA trial (median follow-up, 2.1 years) was 43% in the induction chemotherapy arm and 58% in the consolidation chemotherapy arm [6].
Functional outcomes
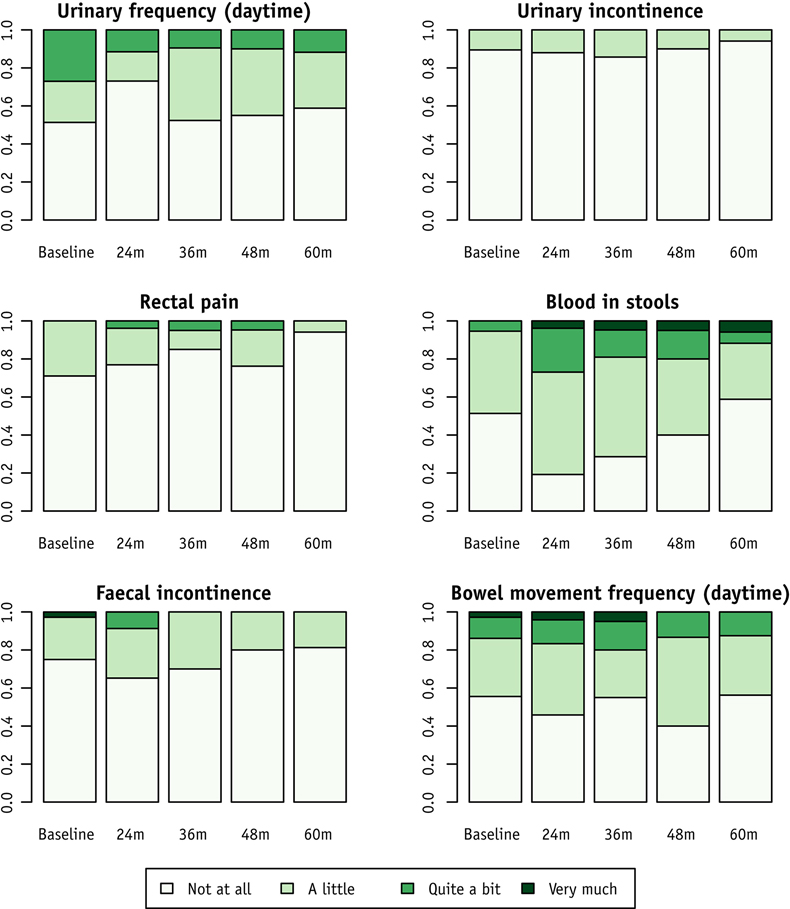
A few studies have investigated patient-reported functional outcomes in WW patients without a comparison to patients who underwent TME. Appelt et al. [12] reported good functional outcomes in WW patients 2 years after NAT, with 69% of patients reporting no fecal incontinence (median Jorge-Wexner score, 0). According to a subsequent report by the same group [53], no patients had symptoms of incontinence at 4 and 5 years following NAT. QoL scores showed little variation over time, but the overall score at 5 years was better than at baseline. The rates of selected patient- reported symptoms in that study are shown in Fig. 6.
Fig. 6.
Selected patient-reported symptoms at baseline and during follow-up. The data are proportions of WW patients with reports available at each time point. (From Dizdarevic et al. 2020, Int. J. Radiat. Oncol. Biol. Phys. 106:556–63; with permission.)
Footnote: This review is based in part on Yuval et al. 2020, J. Gastrointest. Surg. 24:1880–1888
In another study, Martens et al. [11] reported that at 3 years after NAT, 14% of patients who underwent WW management reported major incontinence. A subset of patients with iCR at restaging underwent local excision. These patients had worse functional outcomes, with 43% experiencing major incontinence, in contrast to 4.5% of patients who did not require local excision.
In a 2020 study, Quezada-Diaz et al. retrospectively compared patient-reported bowel function between patients who underwent WW and a matched cohort of patients who underwent sphincter-preserving TME [54]. With median follow-up of 5 months, WW patients had better functional scores for diet, urgency/soilage, frequency, and overall bowel function than TME patients. Although intriguing, the findings should be reproduced in a larger prospective study with longer follow up.
Summary, Discussion and Future Directions
Patients with a complete response to NAT may not benefit from TME, but identifying true responders by clinical assessment remains a challenge. Evidence supporting WW is largely retrospective, and prospectively collected data will be of great value. Since WW is acceptable to rectal cancer patients who are even willing to make an oncological compromise for the prospect of organ preservation, WW should be part of the treatment discussion. Patients entering WW must have a cCR or ncCR, be well-informed, and be willing to undergo intensive surveillance. Currently, the best setting for patients to undergo WW may be a prospective trial with a strict protocol and objective assessment standards. Uniform and reproducible criteria for response assessment will help WW gain wider acceptance.
Key points.
Neoadjuvant treatment can eliminate all tumor cells in some patients with rectal cancer, but with standard management these patients are recognized only after rectal resection, when their specimen shows pathologic complete response.
Patients with pathologic complete response have excellent long-term oncological outcomes, and rectal resection in these patients may constitute overtreatment, causing functional impairments deleterious to patients’ quality of life.
The idea behind watch-and-wait is to correctly identify by the best preoperative diagnostic methods those patients whose tumor has responded completely to neoadjuvant therapy and to substitute upfront resection with strict surveillance.
Because clinical assessment of response is not as accurate as pathologic assessment of response, strict surveillance is necessary to recognize tumor regrowth in a timely manner, when the tumor is still curable by resection.
Although promising, most published data on watch-and-wait are from retrospective series. Prospectively collected data from randomized trials will soon be available.
Synopsis.
Watch and wait management for rectal cancer following complete response has emerged as non-standard approach in which upfront surgery is substituted by strict surveillance. The desired outcome of this approach is organ preservation and its associated improvement in patients’ quality of life. Herein we will review key considerations and the fundamentals of this approach including terminology, the history of watch and wait, patients’ perspectives, patient evaluation, possible treatment regimens, assessment of response, long-term surveillance, and long-term functional and oncological outcomes.
CLINICS CARE POINTS.
Only patients with a cCR or ncCR can be managed by WW; all other patients should undergo treatment according to standard management guidelines.
WW is suitable for patients who are willing to undergo strict surveillance, can manage uncertainties regarding their disease course, and have streamlined access to appropriate healthcare services.
Documentation of tumors by endoscopic images at different time points helps clinicians assess response and detect regrowth.
NAT is associated with adverse events, toxicities, and even death; management of LARC should be tailored to the patient’s health and functional status.
TNT with consolidation systemic chemotherapy may offer the best chance of rectum preservation for patients with LARC.
Tumor response to NAT is time dependent and can take months. Patients with ncCR can proceed with WW as long as tumor response continues to improve.
Regrowth appears in the first 2 years following NAT in about 25% of WW patients; surveillance should be most intensive during this period.
Five-year overall survival for patients with cCR or ncCR entering WW is approximately 85%, which is comparable to 5-year overall survival for patients who undergo standard treatment including TME and who have similar tumor biology.
Many patients are willing to make survival compromises for the potential improvement in QoL associated with organ preservation.
Financial support
Research at Memorial Sloan Kettering is funded in part by grant P30 CA008748 from the National Cancer Institute. J. B. Yuval’s research fellowship at Memorial Sloan Kettering was funded in part by grant T32 CA009501 from the National Cancer Institute.
Disclosure:
Julio Garcia-Aguilar has received honoraria from Intuitive Inc., Medtronic, and Johnson & Johnson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Goding Sauer A et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70: 145–164. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Atkin W, Lenz HJ et al. Colorectal cancer. Lancet 2010; 375: 1030–1047. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Aguilar J, Chow OS, Smith DD et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015; 16: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas M, Nelemans PJ, Valentini V et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010; 11: 835–844. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Aguilar J, Patil S, Kim JK et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. Journal of Clinical Oncology 2020; 38: 4008–4008. [Google Scholar]
- 7.Janeway H Treatment of cancer, particularly of the tongue, tonsil and rectum, by buried emanation. Am J Roentgenol 1920; 7: 92. [Google Scholar]
- 8.Habr-Gama A, Perez RO, Nadalin W et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004; 240: 711–717; discussion 717–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maas M, Beets-Tan RG, Lambregts DM et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011; 29: 4633–4640. [DOI] [PubMed] [Google Scholar]
- 10.Araujo RO, Valadao M, Borges D et al. Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study. Eur J Surg Oncol 2015; 41: 1456–1463. [DOI] [PubMed] [Google Scholar]
- 11.Martens MH, Maas M, Heijnen LA et al. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst 2016; 108. [DOI] [PubMed] [Google Scholar]
- 12.Appelt AL, Ploen J, Harling H et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015; 16: 919–927. [DOI] [PubMed] [Google Scholar]
- 13.Smith JD, Ruby JA, Goodman KA et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 2012; 256: 965–972. [DOI] [PubMed] [Google Scholar]
- 14.Ayloor Seshadri R, Kondaveeti SS, Jayanand SB et al. Complete clinical response to neoadjuvant chemoradiation in rectal cancers: can surgery be avoided? Hepatogastroenterology 2013; 60: 410–414. [DOI] [PubMed] [Google Scholar]
- 15.Renehan AG, Malcomson L, Emsley R et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016; 17: 174–183. [DOI] [PubMed] [Google Scholar]
- 16.Smith JJ, Strombom P, Chow OS et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol 2019; 5: e185896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caycedo-Marulanda A, Patel SV, Verschoor CP et al. A Snapshot of the International Views of the Treatment of Rectal Cancer Patients, a Multi-regional Survey: International Tendencies in Rectal Cancer. World J Surg 2020. [DOI] [PubMed] [Google Scholar]
- 18.Gani C, Gani N, Zschaeck S et al. Organ Preservation in Rectal Cancer: The Patients’ Perspective. Front Oncol 2019; 9: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunjur A, Chazan G, Newnham G, McLachlan SA. Pilot Study of Patients’ Preferences for Immediate Resection Versus a Watch and Wait Approach After Neoadjuvant Chemoradiation for Locally Advanced Rectal Cancer. JCO Oncol Pract 2020; OP2000158. [DOI] [PubMed] [Google Scholar]
- 20.Chen TY, Wiltink LM, Nout RA et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer 2015; 14: 106–114. [DOI] [PubMed] [Google Scholar]
- 21.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol 2008; 47: 20–31. [DOI] [PubMed] [Google Scholar]
- 22.Kim YI, Jang JK, Park IJ et al. Lateral lymph node and its association with distant recurrence in rectal cancer: A clue of systemic disease. Surg Oncol 2020; 35: 174–181. [DOI] [PubMed] [Google Scholar]
- 23.Habr-Gama A, Sao Juliao GP, Vailati BB et al. Organ Preservation in cT2N0 Rectal Cancer After Neoadjuvant Chemoradiation Therapy: The Impact of Radiation Therapy Dose-escalation and Consolidation Chemotherapy. Ann Surg 2019; 269: 102–107. [DOI] [PubMed] [Google Scholar]
- 24.Lezoche E, Baldarelli M, Lezoche G et al. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 2012; 99: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 25.Das P, Skibber JM, Rodriguez-Bigas MA et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 2007; 109: 1750–1755. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Lee D, Young C. Predictors for complete pathological response for stage II and III rectal cancer following neoadjuvant therapy - A systematic review and meta-analysis. Am J Surg 2020. [DOI] [PubMed] [Google Scholar]
- 27.Chen MB, Wu XY, Yu R et al. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: a meta-analysis in rectal cancer. PLoS One 2012; 7: e45388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Aguilar J, Chen Z, Smith DD et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg 2011; 254: 486–492; discussion 492–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Rosa N, Rodriguez-Bigas MA, Chang GJ et al. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J Clin Oncol 2016; 34: 3039–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swedish Rectal Cancer T, Cedermark B, Dahlberg M et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997; 336: 980–987. [DOI] [PubMed] [Google Scholar]
- 31.Jia AY, Narang A, Safar B et al. Sequential short-course radiation therapy and chemotherapy in the neoadjuvant treatment of rectal adenocarcinoma. Radiat Oncol 2019; 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bujko K, Nowacki MP, Nasierowska-Guttmejer A et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 33.Markovina S, Youssef F, Roy A et al. Improved Metastasis- and Disease-Free Survival With Preoperative Sequential Short-Course Radiation Therapy and FOLFOX Chemotherapy for Rectal Cancer Compared With Neoadjuvant Long-Course Chemoradiotherapy: Results of a Matched Pair Analysis. Int J Radiat Oncol Biol Phys 2017; 99: 417–426. [DOI] [PubMed] [Google Scholar]
- 34.Myerson RJ, Tan B, Hunt S et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys 2014; 88: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Valk MJM, Marijnen CAM, van Etten B et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer - Results of the international randomized RAPIDO-trial. Radiother Oncol 2020; 147: 75–83. [DOI] [PubMed] [Google Scholar]
- 36.Jin J, Liu S, Zhu Y et al. The Updated Results for the Phase 3 Study of 5×5 Gy Followed By Chemotherapy in Locally Advanced Rectal Cancer (STELLAR trial). International Journal of Radiation Oncology*Biology*Physics 2017; 99: E157. [Google Scholar]
- 37.Romesser PB, Wu AJ, Cercek A et al. Management of Locally Advanced Rectal Cancer During The COVID-19 Pandemic: A Necessary Paradigm Change at Memorial Sloan Kettering Cancer Center. Adv Radiat Oncol 2020; 5: 687–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raj Kumar B, Pandey D, Rohila J et al. An observational study of the demographic and treatment changes in a tertiary colorectal cancer center during the COVID-19 pandemic. J Surg Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson PJ, van Etten B, Hospers GA et al. Short-course radiotherapy followed by neoadjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer 2013; 13: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hospers G, Bahadoer RR, Dijkstra EA et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. Journal of Clinical Oncology 2020; 38: 4006–4006. [Google Scholar]
- 41.Probst CP, Becerra AZ, Aquina CT et al. Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg 2015; 221: 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erlandsson J, Holm T, Pettersson D et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017; 18: 336–346. [DOI] [PubMed] [Google Scholar]
- 43.Pettersson D, Lorinc E, Holm T et al. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg 2015; 102: 972–978; discussion 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akgun E, Caliskan C, Bozbiyik O et al. Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2018; 105: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 45.Appelt AL, Ploen J, Vogelius IR et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013; 85: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cercek A, Roxburgh CSD, Strombom P et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol 2018; 4: e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conroy T, Lamfichekh N, Etienne P-L et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. Journal of Clinical Oncology 2020; 38: 4007–4007. [Google Scholar]
- 48.Fokas E, Allgauer M, Polat B et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol 2019; 37: 3212–3222. [DOI] [PubMed] [Google Scholar]
- 49.Allegra CJ, Yothers G, O’Connell MJ et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J Natl Cancer Inst 2015; 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Valk MJM, Hilling DE, Bastiaannet E et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018; 391: 2537–2545. [DOI] [PubMed] [Google Scholar]
- 51.Maas M, Lambregts DM, Nelemans PJ et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol 2015; 22: 3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuval JB, Thompson HM, Garcia-Aguilar J. Organ Preservation in Rectal Cancer. J Gastrointest Surg 2020; 24: 1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dizdarevic E, Frostrup Hansen T, Ploen J et al. Long-Term Patient-Reported Outcomes After High-Dose Chemoradiation Therapy for Nonsurgical Management of Distal Rectal Cancer. Int J Radiat Oncol Biol Phys 2020; 106: 556–563. [DOI] [PubMed] [Google Scholar]
- 54.Quezada-Diaz FF, Smith JJ, Jimenez-Rodriguez RM et al. Patient-Reported Bowel Function in Patients With Rectal Cancer Managed by a Watch-and-Wait Strategy After Neoadjuvant Therapy: A Case-Control Study. Dis Colon Rectum 2020; 63: 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]