Summary
Neuroimaging and genetics studies have advanced our understanding of the neurobiology of sleep and its disorders. However, individual studies usually have limitations to identifying consistent and reproducible effects, including modest sample sizes, heterogeneous clinical characteristics and varied methodologies. These issues call for a large-scale multi-centre effort in sleep research, in order to increase the number of samples, and harmonize the methods of data collection, preprocessing and analysis using pre-registered well-established protocols. The Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) consortium provides a powerful collaborative framework for combining datasets across individual sites. Recently, we have launched the ENIGMA-Sleep working group with the collaboration of several institutes from 15 countries to perform large-scale worldwide neuroimaging and genetics studies for better understanding the neurobiology of impaired sleep quality in population-based healthy individuals, the neural consequences of sleep deprivation, pathophysiology of sleep disorders, as well as neural correlates of sleep disturbances across various neuropsychiatric disorders. In this introductory review, we describe the details of our currently available datasets and our ongoing projects in the ENIGMA-Sleep group, and discuss both the potential challenges and opportunities of a collaborative initiative in sleep medicine.
Keywords: ENIGMA consortium, large-scale collaboration, neurogenetics, neuroimaging, sleep
Everyone sleeps, except lovers, who stay awake, telling stories to God
(Rumi)
1 ∣. INTRODUCTION
The role of sleep in human evolution remains mysterious (Nunn et al., 2016), but it is well known that sleep plays a pivotal role in synaptic plasticity, memory consolidation, metabolite and hormonal regulation, adaptive cognitive and emotional functions, individual performance, and overall well-being and health (Leproult & Van Cauter, 2010; Shattuck et al., 2019; Stickgold, 2005; Walker, 2009; Xie et al., 2013). Nowadays, a substantial number of people suffer from inadequate sleep and various sleep disorders which, in addition to the genetics, psycho-social and developmental factors, can be attributed to excessive stressful life-events, the 24/7 rhythm of the modern world, shift-work, and a significant amount of entertainment and media consumption before sleep (Grandner, 2017). Sleep disturbances are associated with diverse physical health issues and medical conditions, such as hypertension, obesity, cardiovascular diseases, metabolic diseases, motor vehicle collisions and low quality of life (Cappuccio & Miller, 2017; Medic et al., 2017; Ohayon et al., 2002). In addition, sleep disturbances have a robust link with cognitive and emotional impairment in healthy subjects, and are common in patients with various neuropsychiatric disorders including dementia, Parkinson’s disease, major depressive disorder (MDD), bipolar disorder, post-traumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), schizophrenia, or anxiety disorders (Baglioni et al., 2016; Bucks et al., 2017; Durmer & Dinges, 2005; Dyken et al., 2012; Freeman et al., 2020; Harvey et al., 2011; Macedo et al., 2017; Morin et al., 2015; Nedergaard & Goldman, 2020; Poudel et al., 2013; Rosenzweig et al., 2015; Tahmasian et al., 2020). However, despite a high prevalence of sleep problems (e.g. insomnia symptoms in 30%–35% and insomnia disorder in 10% of the general population) and their high socioeconomic burden (Morin et al., 2015), the underlying neural mechanisms of sleep disorders, neurobiological consequences of sleep deprivation, as well as the link between sleep disturbances and a variety of neuropsychiatric disorders are poorly understood.
We recently launched a sleep working group in the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) consortium, in order to perform coordinated multi-centre neuroimaging and genetics studies on the field of sleep medicine. In the present article, we will discuss the challenges and opportunities in this field, and highlight the need for such a worldwide collaborative approach to tackle them in the ENIGMA-Sleep working group framework.
2 ∣. WHY IS AN INTERNATIONAL COLLABORATION NEEDED?
Sleep has been the subject of numerous studies utilizing state-of-the-art genetic, behavioural and brain imaging approaches in the general population, healthy subjects with experimental sleep deprivation, or patients with sleep disorders (Freeman et al., 2020; Jansen et al., 2019; Krause et al., 2017; Poudel et al., 2018; Tahmasian et al., 2020). One of the promising goals of these studies is to improve our understanding of the neurobiological underpinnings of these sleep disturbances. However, historically, most of the studies have had limited statistical power and were poorly replicated, predominantly due to small sample sizes, diversity of imaging acquisition, preprocessing and analytic methods used, as well as due to heterogeneous, and often not so well-defined, clinical populations (Button et al., 2013). Most importantly, due to the finite funding, resources and availability of volunteers, many of the individual studies have small sample sizes (e.g. see the included studies in some recent sleep-related neuroimaging meta-analyses; Javaheripour et al., 2019; Tahmasian, Noori, et al., 2018). As a consequence of low power, small and subtle effects are less likely to be identified, resulting in a lower positive predictive value and higher probability of reporting false-positive results (Ioannidis, 2005). On the other hand, it is difficult to publish non-significant results, contributing to the publication bias and creating further problems for interpreting the results of individual studies (Franco et al., 2014). In addition, due to the higher demand for publishing significant findings as much/soon as possible, when researchers get non-significant results, they are sometimes incentivized to apply more liberal analytic methods, or try post hoc modifications of pre-planned methods that may identify statistical significance (Poldrack et al., 2017).
Coordinate- or image-based neuroimaging meta-analyses (CBMA, IBMA) have become increasingly popular approaches that can transcend the limitations of individual single studies by retrospectively synthesizing the findings of the previously published literature (Laird et al., 2009; Müller et al., 2018; Salimi-Khorshidi et al., 2009; Tahmasian et al., 2019). Recently, several coordinate-based meta-analyses on sleep deprivation (Javaheripour et al., 2019; Ma et al., 2015; Saletin et al., 2019), insomnia disorder (Jiang et al., 2020; Tahmasian, Noori, et al., 2018; Wu et al., 2020), obstructive sleep apnea (OSA; Huang et al., 2019; Shi et al., 2017; Tahmasian et al., 2016; Weng et al., 2014), narcolepsy (Weng et al., 2015) and restless legs syndrome (RLS; Sheng et al., 2020) reported converging regional brain abnormalities across available original studies. However, the findings of these meta-analyses are also divergent because of their heterogeneity in search strategies, included samples and analysis flexibility (Müller et al., 2018; Tahmasian et al., 2019). In particular, unlike the ENIGMA’s prospective meta-analyses (c.f. Section 3), the conventional retrospective meta-analyses often include studies with diverse methods/populations, and need to weigh-up the importance of homogeneity on the one hand and the number of their included studies on the other (Tahmasian, Zarei, et al., 2018). Furthermore, as mentioned, non-significant findings are less likely to get published and are therefore not included in many CBMAs, which leads to over-representation of positive findings in the results of available meta-analyses. These limitations of both individual and retrospective, literature-based meta-analytic studies and notable divergence in their findings substantially hinder our understanding of the neurobiology of sleep. There is a need to form collaborations and, together, perform high-powered studies. In the absence of expensive large-scale coordinated multi-site data collection studies, which are planned prospectively, a coordinated effort to pool retrospectively collected information across studies is promising and feasible. By harmonizing existing data and methodologies, we can determine the source of the divergent findings in the literature, and present a common brain signature for the sleep conditions/disorders across the heterogeneous populations. The initial strides towards this goal may be accomplished by recruiting internationally representative clinical populations, pre-registration of analysis plans, and standardization of processing pipelines, which altogether call for large-scale worldwide collaborations in sleep and neuroimaging/genetics field.
Accordingly, we need a consensus approach that has not previously been established in sleep research. Of note, there have been a few large-scale studies on neuroimaging/genetics of sleep, owing to big data initiatives such as Lifebrain, UK Biobank, Enhanced Nathan Kline Institute (eNKI), or the Human Connectome Project (HCP). These datasets usually contain subjective information about the sleep quality and quantity from their participants. For examples, see Cheng et al. (2018); Fjell et al. (2020); Jansen et al. (2019); Toschi et al. (2020). However, these large neuroimaging data initiatives are multipurpose and often lack specific sleep assessments using, for example, well-established sleep questionnaires, actigraphy, and polysomnography (PSG). They usually have not included diagnosed patients with sleep disorders based on psychiatric interviews using Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Sleep Disorders (ICSD).
3 ∣. ENIGMA CONSORTIUM
The ENIGMA consortium is a worldwide collaboration of more than 1,400 scientists from more than 200 institutes, across 45 countries, which was started in 2009 and to date have performed many large-scale neuroimaging and genetics mega- and meta-analyses by pooling data across the whole world (Thompson et al., 2020). Currently, the ENIGMA consortium has more than 50 working groups consisting of clinicians, neuroscientists, neuroimaging methodologists and other worldwide experts to study healthy brain variations, various neuropsychiatric disorders or to develop imaging techniques. For example, see (Bas-Hoogendam et al., 2020; Dennis et al., 2020; Schmaal et al., 2020;Thompson et al., 2020; van den Heuvel et al., 2020). Meta- and mega-analyses are the two common types of studies performed by the ENIGMA groups. In prospective meta-analyses, identical and standardized experimental/analytical protocols are used in each institute/centre, and the resulting group-level statistics are shared and pooled across them. This approach is analytically quite similar to the conventional literature-based retrospective meta-analyses, but it reduces the effects of heterogeneity and publication bias, which are common in conventional meta-analyses, by virtue of using standardized protocols and being prospective. The ENIGMA’s meta-analytic studies allow for an increased number of participating sites by eliminating the need for sharing individual raw data, which might be subject to legislative, ethical or personal prohibitions. In the ENIGMA's mega-analyses, the raw or processed (e.g. cortical thickness of brain areas) individual data are shared and pooled across the sites, leading to a potentially higher power and greater flexibility (e.g. by enabling us to apply machine learning methods that require individual data), at the cost of lower number of participating sites, and more computational/storage requirements at the pooling site (Zugman et al., 2020). The sites which are not able to share individual-level measures can then participate as part of replication attempts.
4 ∣. LAUNCHING ENIGMA-SLEEP WORKING GROUP
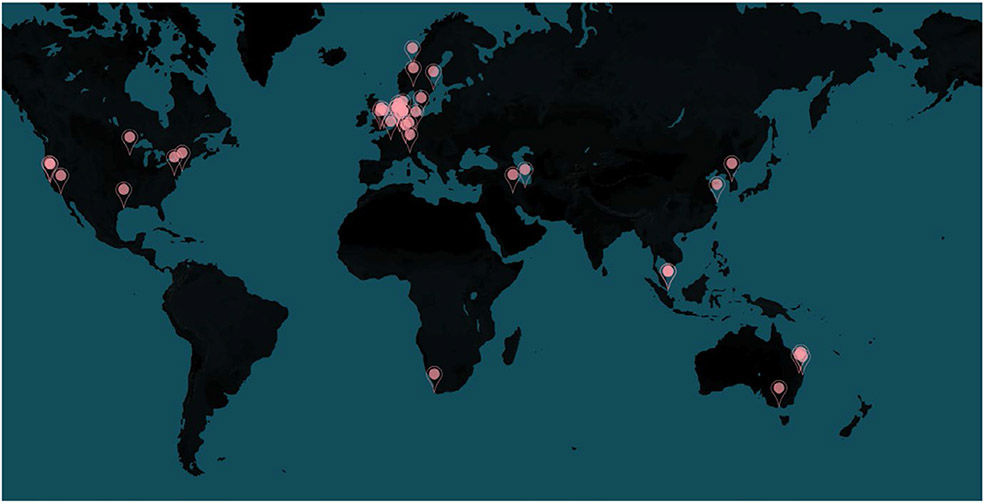
Recently, the ENIGMA-Sleep working group (http://enigma.ini.usc.edu/ongoing/enigma-sleep/) was formed by a collaboration of several scientists from different institutes across 15 countries (Figure 1), as the largest neuroimaging collaboration focusing on neuroimaging/genetics of sleep conditions in healthy subjects and sleep disorders. Here, we will discuss the road map, open questions, challenges and opportunities of the ENIGMA-Sleep working group in the field of sleep research. Currently, the ENIGMA-Sleep working group focuses on assessing neural correlates of subjective and objective sleep quality/quantity in healthy subjects, adaptive/maladaptive brain reorganization due to experimental sleep deprivation or sleep disturbances, sleep patterns and their consequences during lifespan, as well as identifying structural and functional alterations in sleep disorders using multimodal neuroimaging and genetics data that are pooled across centres via meta- and mega-analytical approaches (Figure 2).
FIGURE 1.
Map of participating institutes in the ENIGMA-Sleep working group across 15 countries
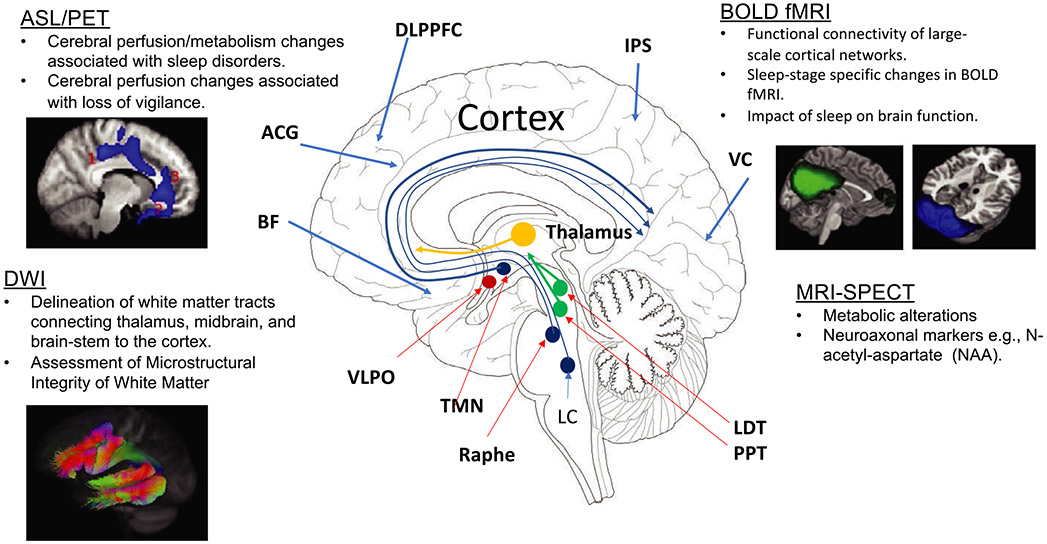
FIGURE 2.
An overview of neuroimaging tools for assessing the functional and structural pathways underpinning sleep physiology. The primary sleep circuit is located in the sub-cortical regions within the brainstem and thalamus. The cortical regions, including dorsolateral prefrontal cortex (DLPFC), basal forebrain (BF), anterior cingulate gyrus (ACG), intraparietal sulcus (IPS) and visual cortex, and direct afferent/efferent connections to sub-cortical sleep regions may be important for cortical regulation of sleep physiology
The ENIGMA working group’s chairs are usually composed of a selection of principal investigators (PIs) participating in that working group, who are also experts in the field. The chairs can be nominated or self-nominated, and the ENIGMA director and central team discuss and approve such requests. In this case, the chairs of the ENIGMA-Sleep group have actively sought potential collaborators willing to contribute their data to this initiative, and the majority of recruitment has been achieved via pre-existing personal contacts and collaborations, as well as by reaching out to the members of sleep societies and other ENIGMA working groups. However, any interested researcher with a fitting dataset is welcome to join and may do so by contacting the consortium or the working group's chairs. The contributing PIs signed the Memorandum of Understanding (MOU) accepting the terms and policies of the ENIGMA-Sleep working group for data sharing, research proposals, analysis, and publications. In addition, they filled in a form to report the types and quantity of their available neuroimaging/genetics and sleep measured data, as well as demographic characteristics of their samples. Each PI in the ENIGMA-Sleep working group can suggest a new project by filling out the proposal form that would be initially reviewed by the chairs and then distributed among PIs for discussion in a virtual meeting. Once the proposal is approved, the project leader will get access to the relevant raw data or primary results from each site, with permission from the group members who are willing to participate in that project. The analyses can either be based on the established ENIGMA protocols (c.f. http://enigma.ini.usc.edu/protocols/ and Lariviere et al., 2020), or can be done using the custom protocols specific to the research question.
Currently, the available data across sites include 20,554 subjects from various sites with sleep diary and different questionnaires such as Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989), Insomnia Severity Index (ISI; Bastien et al., 2001), Epworth Sleepiness Scale (ESS; Johns, 1991), Global Sleep Assessment Questionnaire (GSAQ; Roach et al., 2020), Berlin questionnaire (Netzer et al., 1999), Adolescent Sleep-Wake Scale (ASWS; LeBourgeois et al., 2005), Composite Scale of Morningness (CSM; Smith et al., 1989), Evaluation List Insomnia Therapy (ELIT; Kerkhof, 1999), Holland Sleep Disorders Questionnaire (HSDQ; Kerkhof et al., 2013), Morningness-Eveningness questionnaire (MEQ; Horne & Ostberg, 1976), Johns Hopkins Restless Legs Severity Scale (JHRLSS; Allen & Earley, 2001), Restless Legs Syndrome-Diagnostic Index (RLS-DI; Benes & Kohnen, 2009), Karolinska Sleep Questionnaire (KSQ; Åkerstedt et al., 2002), Karolinska Sleepiness Scale (KSNS; Åkerstedt & Gillberg, 1990), Diurnal Type Scale (DTS; Torsvall & Akerstedt, 1980). The dataset includes 4,076 actigraphy and 4,288 PSG recordings. In addition, we have 1,187 healthy control subjects and 655 healthy subjects with experimental sleep deprivation, 1,575 patients with insomnia disorder, 1,123 patients with OSA, 2,242 patients with RLS, 132 patients with ADHD disorder, 269 patients with bipolar disorder, 226 patients with MDD, and 15,486 participants including population-based samples. The overall neuroimaging data include 10,072 subjects with T1-weighted images, 6,396 with functional magnetic resonance imaging (fMRI) including 4,951 with resting-state and 1,445 with task-based fMRI data, and 8,006 with diffusion tensor imaging (DTI) data. Details on the sleep and neuroimaging data of the subjects, within the participating cohorts within the ENIGMA-Sleep, are summarized in Table 1. Of note, data collection is still ongoing in the several mentioned cohorts.
TABLE 1.
ENIGMA-Sleep samples characteristics and the available imaging, genetics and sleep data
| General sample information |
Age range (y) |
MRI information |
Type of MRI data |
Objective sleep assessment |
Sleep questionnaires and genetic information |
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample # |
Country | Institute | Disorder | F/M ratio (~) |
# Number of subjects |
Children (<11) |
Adolescents (11-18) |
Younger Adults (18-55) |
Older Adults (>55) |
Field strength (T) |
T1-w | DTI | rs-fMRI | MRS | Task fmri |
Acti- graphy |
Poly- somno- graphy |
GWAS | PSQI | sleep diary |
AHI | ISI | BERLIN | ASWS | CSM | ELIT | GSAQ | HSDQ | MEQ | JHRLSS | RLS- DI |
KSQ | KSNS | DTS | Other |
| 1 | Kermanshah, iran | KUMS | Insomnia | 1.660 | 57 | --- | --- | 57 | 11 | 1.5 | 76 | 43 | 57 | 0 | 0 | 0 | 57 | 0 | 76 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ESS, HAM-A, HAM-D, BAI, BDI |
| Kermanshah, iran | KUMS | OSA | 0.272 | 14 | --- | --- | 7 | 7 | 1.5 | 14 | 14 | 14 | 0 | 0 | 0 | 14 | 0 | 14 | 0 | 14 | 0 | 14 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ESS | |
| Kermanshah, iran | KUMS | Healthy controls | 1.290 | 53 | --- | --- | 67 | 11 | 1.5 | 72 | 48 | 53 | 0 | 0 | 0 | 0 | 0 | 72 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 53 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ESS, HAM-A, HAM-D, BAI, BDI | |
| 2 | Milano, Italy | IRCCS | Bipolar | 0.620 | 142 | --- | --- | 105 | 37 | 3 | 75 | 75 | 75 | 0 | 50 | 142 | 0 | 0 | 70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 142 | 0 | 0 | 0 | 0 | 0 | HAM-D, DSM-V |
| Milano, Italy | IRCCS | MDD | 0.860 | 49 | --- | --- | 35 | 14 | 3 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 0 | 0 | 0 | HAM-D, DSM-V | |
| Milano, Italy | IRCCS | Healthy controls | 0.650 | 20 | --- | --- | 14 | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | HAM-D, DSM-V | |
| 3 | Singapore | NUS | Healthy controls | 0.500 | 52 | --- | 52 | --- | --- | 3 | 52 | 0 | 52 | 0 | 0 | 52 | 52 | 0 | 52 | 52 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 | 0 | |
| Singapore | NUS | Sleep deprivation in healthy participants | 0.480 | 35 | --- | --- | 35 | --- | 3 | 35 | 0 | 35 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | Greifswald, Germany | DPPUMG | Insomnia disorders | 0.850 | 1,217 | --- | --- | 676 | 541 | 1.5 | 841 | 0 | 0 | 0 | 0 | 0 | 1,062 | 1,150 | 1,007 | 0 | 1,062 | 1,217 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1,175 | 0 | 0 | 0 | ESS, CTQ, PHQ–9, BDI–2, SF–12, TAS–20 |
| Greifswald, Germany | DPPUMG | OSA | 0.850 | 1,109 | --- | --- | 592 | 517 | 1.5 | 763 | 0 | 0 | 0 | 0 | 0 | 1,109 | 1,046 | 913 | 0 | 1,109 | 1,062 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1,067 | 0 | 0 | 0 | ESS, CTQ, PHQ–9, BDI–2, SF–12, TAS–20 | |
| 5 | Stockholm, Sweden | Karolinska Institutet | Sleep restriction in healthy participants | 1.047 | 84 | --- | --- | 47 | 39 | 3 | 86 | 0 | 68 | 0 | 86 | 0 | 86 | 0 | 84 | 86 | 0 | 86 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 86 | 86 | 86 | HADS, frozen plasma, frozen DNA |
| Stockholm, Sweden | Karolinska Institutet | Sleep deprivation in healthy participants | 0.650 | 47 | --- | --- | 47 | --- | 3 | 47 | 47 | 38 | 0 | 38 | 26 | 0 | 0 | 38 | 38 | 0 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 47 | 0 | 0 | HADS, frozen plasma, frozen DNA | |
| 6 | Rotterdam, Netherlands | Erasmus MC | Population-based sample | 1.446 | 14,926 | --- | --- | 1606 | 13,320 | 1.5 | 5,286 | 5,286 | 2,668 | 0 | 0 | 2,430 | 912 | 11,502 | 9,950 | 2,430 | 796 | 0 | 926 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | IRLS |
| 7 | Freiburg, Germany | UFMC | Insomnia | 1.770 | 50 | --- | --- | --- | --- | 3 | 50 | 24 | 20 | 20 | 28 | 0 | 42 | 0 | 50 | 50 | 42 | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Freiburg, Germany | UFMC | Healthy controls | 1.300 | 47 | --- | --- | --- | --- | 3 | 47 | 35 | 20 | 20 | 38 | 0 | 39 | 0 | 47 | 47 | 39 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 8 | Amsterdam, Netherlands | NINS | Insomnia | 2.984 | 251 | --- | --- | 164 | 87 | 1.5,3 | 251 | 251 | 251 | 0 | 251 | 251 | 251 | 0 | 251 | 251 | 0 | 251 | 251 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Amsterdam, Netherlands | NINS | Healthy controls | 2.406 | 109 | --- | --- | 76 | 33 | 1.5, 3 | 109 | 109 | 109 | 0 | 109 | 109 | 109 | 0 | 109 | 109 | 0 | 109 | 109 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 9 | France | NeuroSpin | Bipolar | 0.643 | 28 | --- | --- | 28 | --- | 3 | 28 | 28 | 28 | 0 | 0 | 28 | 0 | 28 | 28 | 28 | 0 | 0 | 0 | 0 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| France | NeuroSpin | Healthy controls | 0.867 | 28 | --- | --- | 28 | --- | 3 | 28 | 28 | 28 | 0 | 0 | 28 | 0 | 28 | 28 | 28 | 0 | 0 | 0 | 0 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 10 | Melbourne, Victoria, Australia | Deakin University | ADHD | 0.377 | 84 | --- | 84 | --- | --- | 3 | 84 | 84 | 84 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Melbourne, Victoria, Australia | Deakin University | Healthy controls | 0.900 | 76 | --- | 76 | --- | --- | 3 | 76 | 76 | 76 | 0 | 0 | 0 | 0 | 76 | 0 | 0 | 0 | 0 | 0 | 76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 11 | Amsterdam, Netherlands | AMC | ADHD | 0.000 | 48 | 21 | 27 | --- | --- | 3 | 48 | 48 | 48 | 0 | 48 | 48 | 0 | 0 | 0 | 48 | 0 | 0 | 0 | 0 | 0 | 48 | 0 | 48 | 0 | 48 | 0 | 0 | 0 | 0 | |
| 12 | Houston, Texas, Texas | UTHSCH | BD, MDD | 1.829 | 99 | --- | --- | 62 | 37 | 3 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Houston, Texas, Texas | UTHSCH | Healthy controls | 1.579 | 49 | --- | --- | 28 | 21 | 3 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 13 | California, United States | UCSF | MDD | 1.895 | 55 | --- | 55 | --- | --- | 3 | 53 | 50 | 49 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PHQ–9, RADS–2, CDRS-R |
| California, United States | UCSF | Healthy controls | 1.400 | 24 | --- | 24 | --- | --- | 3 | 22 | 19 | 21 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PHQ–9, RADS–2, CDRS-R | |
| 14 | Groningen, Netherlands | UG | MDD | 2.833 | 23 | --- | --- | 18 | 5 | 3 | 23 | 18 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | BDI-II, FAS–10, TAS–20 |
| Groningen, Netherlands | UG | Healthy controls | 2.429 | 24 | --- | --- | 18 | 6 | 3 | 24 | 20 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | BDI-II, FAS–10, TAS–20 | |
| 15 | Brisbane, Australia | UQL | Healthy controls | 0.936 | 422 | 174 | 248 | --- | --- | 3 | 406 | 395 | 310 | 0 | 0 | 188 | 0 | 0 | 0 | 247 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PDSS |
| 16 | Maryland, United States | UMSOM | Family-based recruitment sample focused on cross-diagnostic illnesses research | 1.363 | 560 | --- | --- | 420 | 140 | 3 | 560 | 560 | 560 | 560 | 560 | 0 | 0 | 560 | 560 | 560 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PSS, LIS |
| 17 | Melbourne, Victoria, Australia | ACU | Sleep deprivation in healthy participants | 1.000 | 20 | --- | --- | 20 | --- | 3 | 20 | 0 | 0 | 0 | 20 | 20 | 0 | 0 | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Melbourne, Victoria, Australia | ACU | Healthy Controls | 1.030 | 67 | --- | --- | --- | 67 | 3 | 67 | 67 | 67 | 0 | 0 | 0 | 0 | 0 | 67 | 67 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 18 | Oslo, Norway | OUH | Sleep deprivation in healthy participants | 0.600 | 25 | --- | --- | 25 | --- | 3 | 25 | 25 | 25 | 0 | 0 | 25 | 0 | 25 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Oslo, Norway | OUH | Healthy Controls | 0.600 | 25 | --- | --- | 25 | --- | 3 | 25 | 25 | 25 | 0 | 0 | 25 | 0 | 25 | 25 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PVT, HMEQ, BIS | |
| 19 | Trondheim, Norway | NTNU | Habitual sleep patterns + deprivation in healthy participants | 0.494 | 92 | --- | --- | 92 | --- | 3 | 88 | 88 | 88 | 0 | 88 | 88 | 0 | 0 | 92 | 88 | 0 | 92 | 0 | 0 | 0 | 0 | 0 | 0 | 92 | 0 | 0 | 0 | 92 | 0 | ESS, FSS, CFS, PSS, MCQ–30, CAS–1, BRIEF-A, HSCL-25, NEO-PI-3, neuropsychological testing |
| 20 | Liège, Belgium | GIGA-CRC | Healthy Controls | 2.000 | 90 | --- | --- | --- | 90 | 3 | 90 | 90 | 90 | 0 | 90 | 90 | 90 | 0 | 90 | 90 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 0 | 0 | 90 | 0 | Educational level, ESS, BDI, BAI, STAI Y-A, MILL HILL, SPAQ, PSS, (BMI, waist to hip, blood pressure), leasure, sport, profession, NEO-FFI, SF36, neuropsychological testing |
| Liège, Belgium | GIGA-CRC | Healthy Controls | 2.194 | 101 | --- | --- | --- | 101 | 3 | 101 | 101 | 0 | 0 | 0 | 101 | 101 | 101 | 101 | 101 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 101 | 0 | 0 | 0 | 101 | 0 | As above | |
| Liège, Belgium | GIGA-CRC | sleep deprivation in healthy participants | 0.000 | 364 | --- | --- | 364 | --- | 3 | 360 | 360 | 0 | 0 | 0 | 364 | 364 | 364 | 364 | 364 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 364 | 0 | 0 | 0 | 364 | 0 | As above | |
ACU, Australian Catholic University; AHI, apnea-hypopnea index; AMC, Academic Medical Center; ASWS, Adolescent Sleep-Wake Scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BIS, Bergen Insomnia Scale; BRIEF-A, Behaviour Rating Inventory of Executive Function for Adults; CAS, Confirmation of Acceptance for Studies; CDRS-R, Children's Depression Rating Scale, Revised; CFS, Chalder Fatigue Scale; CSM, Composite Scale of Morningness; CTQ, Childhood Trauma Questionnaire; DPPUMG, Department of Psychiatry and Psychotherapy, University Medicine Greifswald; DSM-V, Diagnostic and Statistical Manual of Mental Disorders; DTS, Diurnal Type Scale; ELIT, Evaluation List Insomnia Therapy; Erasmus MC, Erasmus University Medical Center; ESS, Epworth Sleepiness Scale; FAS, Fatigue Assessment Scale; FSS, Fatigue Severity Scale; GIGA-CRC, GIGA-Cyclotron Reaserch Center in Vivo Imaging; GSAQ, Global Sleep Assessment Questionnaire; GWAS, Genome-Wide Association Study; HADS, Hospital Anxiety and Depression Scale; HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression; HMEQ, Horne-Østberg Morningness-Eveningness Questionnaire; HSCL-25, Hopkins Symptom Checklist; HSDQ, Holland Sleep Disorders Questionnaire; IRCCS, Scientific Institute San Raffaele Hospital; IRLS, International Restless Legs Scale; ISI, Insomnia Severity Index; JHRLSS, Johns Hopkins Restless Legs Severity Scale; KSNS, Karolinska Sleepiness Scale; KSQ, Karolinska Sleep Questionnaire; KUMS, Sleep Disorders Research Center, Kermanshah University of Medical Sciences; LIS, Laboratory information system; LSI, Lifetime Stressor Inventory; MCQ-30, Metacognition Questionnaire; MEQ, Morningness-Eveningness Questionnaire; NEO-PI, Neo Personality Inventory-Revised; NINS, Netherlands Institute for Neuroscience; NTNU, Norges Teknisk-Naturvitenskapelige Universitet; NUS, National University of Singapore; OUH, Oslo University Hospital; PDSS, Paediatric Daytime Sleepiness Scale; PHQ-9, Patient Health Questionnaire 9; PSQI, Pittsburgh Sleep Quality Index; PSS, Perceived Stress Scale; PVT, Psychomotor vigilance test; RADS, Reynolds Adolescent Depression Scale; RLS-DI, Restless Legs Syndrome-Diagnostic Index; SF12, Short Form (12); SPAQ, Seasonal Pattern Assessment Questionnaire; STAI, State-Trait Anxiety Inventory; STAI, State-Trait Anxiety Inventory; TAS-20, Toronto Alexithymia Scale; UCSF, University of California San Francisco; UFMC, University of Freiburg Medical Center; UG, University of Groningen; UMSOM, University of Maryland School of Medicine; UQL, University of Queensland; UTHSCH, The University of Texas Health Science Center at Houston.
5 ∣. CURRENT PROJECTS
The projects proposed within the ENIGMA-Sleep framework will focus on functional, microstructural and structural changes associated with sleep disorders and individual differences in sleep health. Multimodal neuroimaging/genetics data available as a part of the consortium allow us to probe biologically specific biomarkers associated with sleep physiology.
5.1 ∣. Neural correlates of insomnia disorder
Several structural and functional neuroimaging studies, using resting-state or cognitive and emotional tasks, have been conducted on patients with insomnia disorder. However, so far, the results of these studies appear to be largely inconsistent (Tahmasian, Noori, et al., 2018). Nonetheless, some reviews have highlighted the involvement of the salience and the default mode networks in altered emotion processing, hyperarousal and sleep-to-wake transitions in these patients (Bagherzadeh-Azbari et al., 2019; Khazaie et al., 2017; Schiel et al., 2020; Spiegelhalder et al., 2015). Thus, the ENIGMA-Sleep group aims to perform large-scale between-group comparisons of structural and functional imaging findings in insomnia patients compared with healthy controls, to gain more detailed insights into the neurobiology of insomnia disorder.
5.2 ∣. Association between circadian rhythms and aging
Circadian rhythm is essential for mental health and cognitive ability, and individuals with a disrupted circadian rhythm are at a greater risk for mental disorders, particularly MDD and bipolar disorder (McClung, 2007). For instance, a lower difference in activity profiles between daytime activity and night-time activity increases the risk of mood disorder and leads to poorer subjective well-being (Lyall et al., 2018). In this project, structural and functional data are assessed in parallel to actigraphy and in-lab measurements of night-time (PSG) and daytime (circadian sleep–wake propensity PSG) measures, as well as assessment of drowsiness using Karolinska Drowsiness Test (KDT; Åkerstedt & Gillberg, 1990), cortical excitability and vigilance, in addition to cognitive phenotyping of healthy older adults. Thus, we aim to investigate the relationship between circadian rhythm, brain structure/function, gene and mental health, and then use mediation analysis and Bayesian network to access the underlying mechanisms that link circadian rhythm to the cognitive aging process.
5.3 ∣. Neural correlates of sleep duration and quality in healthy subjects across the lifespan
The prevalence of short sleep duration and poor sleep quality in the general population are noticeable, but little is known about the potential impact of such chronic sleep disturbances on the brain. Sleeping too little or too much can be detrimental to cognitive functioning, which begs the question of whether and how sleep duration affects brain function. It is also well known that people are generally poor at assessing their actual sleep length, and there is often a large discrepancy between self-reported and objective measures of sleep – to such a degree that subjective and objective sleep parameters are believed to measure different constructs and present distinct biological underpinnings (Rezaie et al., 2018;Tahmasian et al., 2017). Leveraging the datasets of ENIGMA-sleep enables us to associate objective measures of naturalistic sleep (e.g. measured using actigraphy) and perceived sleep quality with brain activity and structure.
5.4 ∣. Neural basis of selective vulnerability to lapses following sleep deprivation
Inter-individual differences in vigilance and visual attention have been observed in previous studies of human performance following acute sleep deprivation (Chee et al., 2008; Chuah & Chee, 2008). Neuroimaging studies have shown that such differences between individuals may be explained by differences in task-related activation (Chee & Chuah, 2008), functional connectivity (Wang et al., 2016; Yeo et al., 2015), dynamics and structural organization of the brain (Hawes et al., 2020; Poudel et al., 2018; Toppi et al., 2016). The ENIGMA-Sleep group aims to conduct neuroimaging mega- and meta-analyses on individuals undergoing sleep deprivation, by investigating the association between cortical morphological features (such as thickness and volume) or functional dynamics (from resting-state fMRI studies) with the frequency of performance lapses after sleep deprivation.
5.5 ∣. Structural dysconnectivity in individuals with poor sleep quality or sleep disorders
Understanding the structural connectivity alterations of healthy subjects with sleep impairment or patients with sleep disorders are important features in sleep medicine (Raikes et al., 2018; Rostampour et al., 2020). DTI is a method to assess white matter brain integrity and identify microstructural abnormalities. Here, we aim to perform tract-based spatial statistics (TBSS; Smith et al., 2006) on the pooled multi-centre neuroimaging data, based on the ENIGMA protocols, which uses a skeleton of white matter tracts for assessing statistical differences in regional fractional anisotropy (FA) values between healthy subjects with good or poor sleep quality/quantity and between healthy subjects and patients with sleep disorders. Next, the association between the subjective or objective sleep measurements and regional diffusion alterations will be assessed.
5.6 ∣. Transdiagnostic neural correlates of sleep across mental illnesses
Sleep disturbances are prevalent symptoms in many neuropsychiatric disorders including, but not limited to, Alzheimer’s disease and dementia (Emamian et al., 2016; Peter-Derex et al., 2015), Parkinson’s disease (Mantovani et al., 2018), PTSD (Ahmadi et al., 2020; Germain, 2013), MDD (Bagherzadeh-Azbari et al., 2019; Emamian et al., 2019; Murphy & Peterson, 2015), ADHD (Cortese et al., 2009), autism spectrum disorder (Kotagal & Broomall, 2012; Souders et al., 2017) or anxiety disorders (Baglioni et al., 2016; Cox & Olatunji, 2020). Such studies have a great impact on society and health policy makers to decrease the risk of neuropsychiatric disorders by improving the sleep quality and alerting clinicians to carefully screen and treat sleep disturbances. However, precise neural mechanisms of the link between these neuropsychiatric disorders and sleep problems are still poorly understood. In dementia, for example, it has been suggested that sleep apnea can increase the risk of Alzheimer’s disease through inducing hypoxia and inflammation, which in turn increases the concentration of amyloid-beta and tau proteins (Bucks et al., 2017; Ju et al., 2014; Rosenzweig et al., 2015). Moreover, monoamine system impairment and alterations within and between the salience and default mode networks have been suggested as the neurobiological link between depression and insomnia (Bagherzadeh-Azbari et al., 2019). In general, there is a clear need for collaboration between researchers in the ENIGMA-Sleep and other ENIGMA working groups to evaluate the neural basis of sleep disturbances in various neurological and mental illnesses using a transdiagnostic approach.
6 ∣. CHALLENGES AND OPPORTUNITIES FOR THE ENIGMA-SLEEP WORKING GROUP
Studying the neural correlates of sleep quality/quantity comes with its own unique challenges compared with other neuropsychiatric disorders. In particular, the number of eligible included studies (< 20) in the recent neuroimaging meta-analyses on insomnia (Jiang et al., 2020; Tahmasian, Noori, et al., 2018; Wu et al., 2020) or OSA (Huang et al., 2019; Shi et al., 2017; Tahmasian et al., 2016; Weng et al., 2014) were considerably lower than the ones on other psychiatric disorders such as depression (N = 97; Gray et al., 2020), ADHD (N = 96; Samea et al., 2019) or obsessive-compulsive disorder (N = 54; Rasgon et al., 2017). In addition, a PubMed search for “functional magnetic resonance imaging” and (insomnia or OSA) in December 2020 retrieved 112 studies, while this figure is much higher for, for example, depression (n = 2,132), anxiety (n = 1,532), schizophrenia (n = 1,756), and dementia or Alzheimer’s disease (n = 1,227). The lower appeal of neuroimaging research in sleep disorders can be attributed to several reasons, including: (a) ignoring the importance of sleep disturbance in clinical practice and the resulting underdiagnosis of such disorders; (b) lower priority for research funders and policy makers to support sleep research (Roach et al., 2020); and (c) the costs and difficulties of objective sleep measurement (see below). The worldwide collaboration in the ENIGMA-Sleep may help individual centres to share their data and expertise to include more subjects, obtain more funding, apply sophisticated methodology, work together to answer upcoming questions, and finally alert our societies to the importance of sleep disturbance on human health and well-being.
The diversity of sleep measurement methods used in different sleep laboratories is an additional challenge for the ENIGMA-Sleep group. Self-report questionnaires, such as PSQI (Buysse et al., 1989), ISI (Bastien et al., 2001), STOP-Bang (Chung et al., 2016), ESS (Johns, 1991) and Berlin questionnaires (Netzer et al., 1999) are commonly used due to their wider availability and their ability to capture subjective sleep symptoms, which is important for different sleep disorders such as insomnia disorder. These tools have medium to high diagnostic performance (Buysse et al., 1989; Ng et al., 2019), but could be susceptible to recall bias. Prospective sleep diaries are an inexpensive alternative to the questionnaires that could rectify the recall bias problem and also allow investigation of day to day variability of sleep but, similar to the questionnaires, they are also prone to misestimating objective aspects of sleep (e.g. sleep duration; Van Den Berg et al., 2008). Although valid objective measurements in many psychiatric disorders are not available for diagnosis and phenomenology, objective sleep measurement does exist. PSG is the gold-standard tool for the measurement of objective sleep parameters such as sleep duration and sleep efficiency, as well as diagnosis of sleep disorders (Douglas et al., 1992; Kushida et al., 2005). This method is typically performed in the sleep laboratories by trained experts, and detects sleep stages by simultaneous recording of brain activity, eye movements, jaw and leg movements, nasal airflow, respiratory effort, oxygen saturation, heart rhythms and body position throughout the sleep–wake cycle (Douglas et al., 1992). However, despite some progress in making it more convenient and even ambulatory, it is still rather difficult, costly, time-consuming, often inaccessible worldwide and inconvenient for many patients (Scott et al., 2020). A conventional ambulatory sleep measurement device, i.e. actigraphy, is a more available/convenient alternative that has become increasingly popular in sleep and neuropsychiatric research (Sadeh, 2011; Tahmasian et al., 2013). An actigraph is a wearable gyro sensor, commonly worn as a wristwatch, that is suitable for longitudinal monitoring of sleep-wake patterns and circadian rhythms over days or weeks (Park & Choi, 2019), as well as measuring rest–activity fragmentation or locomotor activity dynamics over the night (Lim et al., 2011; Winnebeck et al., 2018). Newer devices integrate sleep, heart rate and physical activity information to show how major life events like the recent COVID-19 pandemic objectively affect all three (Ong et al., 2020). Furthermore, some large-scale databases have actigraphy data, which allow to link brain level parameters with objective sleep patterns in a large number of people. Actigraphy has been widely used to study rest–activity patterns and sleep, with a growing interest in the analysis of large population-based samples. Within this perspective, efforts have been made to develop open-source toolboxes that allow users to preprocess, visualize and quantify various properties of the rest–activity rhythms using actigraphy data from various brands of devices, as well as to perform more advanced signal processing analyses (Hammad et al., 2020). However, actigraphy has low specificity and tends to overestimate sleep time in comparison to PSG and, unlike PSG, cannot discriminate between different stages of sleep (Park & Choi, 2019), for which an alternative is multimodal wearable sleep sensors (Boe et al., 2019). Taken together, an important challenge in the field of sleep research is the lack of widely accessible, convenient and inexpensive, yet highly accurate, sleep measurement methods. The ENIGMA-Sleep can provide a chance to investigate novel research questions based on similar subjective or objective sleep data across sites that are not possible when only analysing data from a single site.
In addition, although recently there has been remarkable advances in facilitating open data sharing (White et al., 2020), traditionally not many researchers are willing to share their raw data (Krawczyk & Reuben, 2012; Wicherts et al., 2006) due to legal/ethical prohibitions or other reasons. The reluctance of researchers to share data might be due to lack of time/resources for curating and documenting the data such that it can be properly used by others, being unable to receive proper credit/recognition for the shared data, or worries about being scooped by other teams, who may be able to publish prior to them (White et al., 2020). As a result, and to mitigate these problems, within the ENIGMA consortium, we are developing the guidelines and protocols for data sharing to facilitate the curation and documentation of the data. In addition, to avoid legal/personal problems with raw data sharing, using a meta-analytic approach, we ask each site to share the first- or second-level statistics obtained using pre-defined analysis protocols; and in the mega-analytic approach, when we do need raw data for a specific question, we will acquire it following careful ethical considerations and based on the ENIGMA guidelines and institution/regional laws of data sharing (e.g. General Data Protection Regulation [GDPR] in the European Union) ensuring anonymity of the subjects. In addition, the PIs have complete control over their own data, and thus actively agree to participate in the individual projects they wish to. Members are credited for their contribution by having authorship in the resulting publications (Thompson et al., 2020).
The heterogeneity of imaging data is another challenge for the ENIGMA-Sleep group. There are inevitable variations in data acquisition in terms of different field strengths (1.5, 3, or 7T), scanning sequences, type of acquired tasks and preprocessing (Bas-Hoogendam et al., 2020; Dennis et al., 2020; Schmaal et al., 2020; van den Heuvel et al., 2020). However, applying unified preprocessing and first- and second-level analysis pipelines using the available ENIGMA protocols can help decrease further inference divergence and replicability/reproducibility issues, which is an important issue in neuroimaging currently (Botvinik-Nezer et al., 2020; Kharabian Masouleh et al., 2019; Poldrack, 2008, 2019). Moreover, the clinical phenotypes of study samples and their in-/exclusion criteria are heterogeneous in terms of diagnosis criteria, age and gender of subjects, severity and stage of the disorders, comorbidities, and pharmacological or non-pharmacological treatments. Our approach for addressing this problem in the ENIGMA-Sleep is to use unified selection criteria for the samples and to control for the particular covariates/comorbidities properly.
Our current datasets mostly include cross-sectional data, and we will need more longitudinal data to identify neural trajectories of sleep disorders and how they are affected by specific treatments. Moreover, on the one side, the ENIGMA projects can generate hypotheses that can be further tested in the single studies. On the other hand, these projects can also be used for validating findings from single studies in a larger setting.
Last but not least, there are few imaging-genetic studies investigating the link between genotypes and brain structures in sleep disorders (Jansen et al., 2019; Tahmasian et al., 2020; Takeuchi et al., 2018). However, finding individual imaging samples on sleep disorders with genotyping data (e.g. GWAS studies) is confined and mostly limited to a particular country and, therefore, cannot assess the variety of genotypes across different populations. In addition, these studies have often identified sleep problems using subjective sleep questionnaires or even a single question, rather than objective measurements or diagnosing sleep disorders according to standard criteria. This highlights a huge gap between the numbers of genetic data on sleep studies in comparison with other neuropsychiatric disorders.
7 ∣. CONCLUSION
In order to truly elucidate the neural correlates of sleep quality/quantity in the general population and their changes across the lifespan, brain alterations after sleep deprivation, as well as structural and functional abnormalities in patients with sleep disorders, or the neural correlates of sleep disturbances in various neuropsychiatric disorders, large-scale meta- and mega-analysis studies are needed. In this article, we have discussed some of the challenges, opportunities, as well as the road map of the newly-established ENIGMA-Sleep working group. The core purpose of this initiative is to unite sleep clinicians/scientists across different countries to work together and join us in this exciting initiative (http://enigma.ini.usc.edu/ongoing/enigma-sleep/). Moreover, as many neuropsychiatric disorders present with sleep disturbance symptoms, this group could work with other ENIGMA working groups and other neuroimaging cohorts on various mental illnesses. This review highlights a clear need for the global sharing of cross-sectional and longitudinal imaging-genetic data, and the development of harmonized data collection and analysis approaches for studying sleep and its disorders. We hope that ENIGMA-Sleep group will advance the field of sleep medicine by obtaining robust and replicable results using large-scale datasets.
ACKNOWLEDGEMENTS
GN was supported by Riksbankens Jubileumsfond (grant no. P15-0310:1). SLV was supported by the Otto Hahn award of the Max Planck Society. AO was supported by the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU). GV and CS are funded by National Funds FNRS Scientific Research (FRS-FNRS Belgium). The research included here was funded by Wallonia-Brussels Federation (ARC - 09/14-03), WELBIO/Walloon Excellence in Life Sciences and Biotechnology Grant (WELBIO-CR-2010-06E), FNRS-Belgium (FRS-FNRS, F.4513.17 & T.0242.19 & 3.4516.11), University of Liège (ULiège), Fondation Simone et Pierre Clerdent, European Regional Development Fund (Radiomed project), European Research Council (ERC-Starting Grant - GA 757763). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. TCH is supported by the National Institute of Mental Health (K01MH117442) and the Ray and Dagmar Dolby Family Fund. AW was supported by the Deutsche Forschungsgemeinschaft (DFG, grant number: GR 1912/13-1). The Study of Health in Pomerania (SHIP) is supported by the German Federal State of Mecklenburg-West Pomerania. MRI scans have been supported by a joint grant from Siemens Healthineers, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania, and PSG assessment was in part supported by the Deutsche RLS e.V. (German Restless Legs Syndrome Society). HJG has received travel grants and speakers honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag as well as research funding from Fresenius Medical Care.
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Ahmadi R, Rahimi S, Javaheripour N, Emamian F, Ghadami M, Khazaie H, & Zarei M (2020). Insomnia and post-traumatic stress disorder: A meta-analysis on prevalence and interrelated association. PsyArXiv, November 3. 10.31234/osf.io/7n84u [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, & Gillberg M (1990). Subjective and objective sleepiness in the active individual. International Journal of Neuroscience, 52(1–2), 29–37. 10.3109/00207459008994241 [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, & Kecklund G (2002). Sleep disturbances, work stress and work hours: A cross-sectional study. Journal of Psychosomatic Research, 53(3), 741–748. 10.1016/s0022-3999(02)00333-1 [DOI] [PubMed] [Google Scholar]
- Allen RP, & Earley CJ (2001). Validation of the Johns Hopkins restless legs severity scale. Sleep Medicine, 2(3), 239–242. 10.1016/s1389-9457(00)00080-0 [DOI] [PubMed] [Google Scholar]
- Bagherzadeh-Azbari S, Khazaie H, Zarei M, Spiegelhalder K, Walter M, Leerssen J, Van Someren EJW, Sepehry AA, & Tahmasian M (2019). Neuroimaging insights into the link between depression and Insomnia: A systematic review. Journal of Affective Disorders, 258, 133–143. 10.1016/j.jad.2019.07.089 [DOI] [PubMed] [Google Scholar]
- Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, Reynolds CF, & Riemann D (2016). Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychological Bulletin, 142(9), 969–990. 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas-Hoogendam JM, Groenewold NA, Aghajani M, Freitag GF, Harrewijn A, Hilbert K, & Stein DJ (2020). ENIGMA-anxiety working group: Rationale for and organization of large-scale neuroimaging studies of anxiety disorders. Human Brain Mapping, 10.1002/hbm.25100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallières A, & Morin CM (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Benes H, & Kohnen R (2009). Validation of an algorithm for the diagnosis of Restless Legs Syndrome: The Restless Legs Syndrome-Diagnostic Index (RLS-DI). Sleep Medicine, 10(5), 515–523. 10.1016/j.sleep.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Boe AJ, McGee Koch LL, O’Brien MK, Shawen N, Rogers JA, Lieber RL, Reid KJ, Zee PC, & Jayaraman A (2019). Automating sleep stage classification using wireless, wearable sensors. NPJ Digit Med, 2, 131. 10.1038/s41746-019-0210-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinik-Nezer R, Holzmeister F, Camerer CE, Dreber A, Huber J, Johannesson M, Kirchler M, Iwanir R, Mumford JA, Adcock RA, Avesani P, Baczkowski BM, Bajracharya A, Bakst L, Ball S, Barilari M, Bault N, Beaton D, Beitner J, … Schonberg T (2020). Variability in the analysis of a single neuroimaging dataset by many teams. Nature, 582(7810), 84–88. 10.1038/s41586-020-2314-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks RS,Olaithe M, Rosenzweig I, & Morrell MJ(2017). Reviewing the relationship between OSA and cognition: Where do we go from here? Respirology, 22(7), 1253–1261. 10.1111/resp.13140 [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, & Munafò MR (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, & Miller MA (2017). Sleep and cardio-metabolic disease. Current Cardiology Reports, 19(11), 110. 10.1007/s11886-017-0916-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, & Chuah LY (2008). Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Current Opinion in Neurology, 21(4), 417–423. 10.1097/WCO.0b013e3283052cf7 [DOI] [PubMed] [Google Scholar]
- Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, & Dinges DF (2008). Lapsing during sleep deprivation is associated with distributed changes in brain activation. Journal of Neuroscience, 28(21), 5519–5528. 10.1523/jneurosci.0733-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Ruan H, & Feng J (2018). Functional Connectivities in the Brain That Mediate the Association Between Depressive Problems and Sleep Quality. JAMA Psychiatry, 75(10), 1052. 10.1001/jamapsychiatry.2018.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah LY, & Chee MW (2008). Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. Journal of Neuroscience, 28(44), 11369–11377. 10.1523/jneurosci.4045-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Abdullah HR, & Liao P (2016). STOP-Bang Questionnaire: A practical approach to screen for obstructive sleep Apnea. Chest, 149(3), 631–638. 10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- Cortese S, Faraone SV, Konofal E, & Lecendreux M (2009). Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. Journal of the American Academy of Child and Adolescent Psychiatry, 48(9), 894–908. 10.1097/CHI.0b013e3181ac09c9 [DOI] [PubMed] [Google Scholar]
- Cox RC, & Olatunji BO (2020). Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Medicine Reviews, 51, 101282. 10.1016/j.smrv.2020.101282 [DOI] [PubMed] [Google Scholar]
- Dennis EL, Baron D, Bartnik-Olson B, Caeyenberghs K, Esopenko C, Hillary FG, & Wilde EA (2020). ENIGMA brain injury: Framework, challenges, and opportunities. Human Brain Mapping. 10.1002/hbm.25046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas NJ, Thomas S, & Jan MA (1992). Clinical value of polysomnography. Lancet, 339(8789), 347–350. 10.1016/0140-6736(92)91660-z [DOI] [PubMed] [Google Scholar]
- Durmer JS, & Dinges DF (2005). Neurocognitive consequences of sleep deprivation. Seminars in Neurology, 25(1), 117–129. 10.1055/s-2005-867080 [DOI] [PubMed] [Google Scholar]
- Dyken ME, Afifi AK, & Lin-Dyken DC (2012). Sleep-related problems in neurologic diseases. Chest, 141(2), 528–544. 10.1378/chest.11-0773 [DOI] [PubMed] [Google Scholar]
- Emamian F, Khazaie H, Okun ML, Tahmasian M, & Sepehry AA (2019). Link between insomnia and perinatal depressive symptoms: A meta-analysis. Journal of Sleep Research, 28(6), e12858. 10.1111/jsr.12858 [DOI] [PubMed] [Google Scholar]
- Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung G-Y, Rosenzweig I, & Sepehry AA (2016). The association between obstructive sleep apnea and Alzheimer's disease: A meta-analysis perspective. Frontiers in Aging Neuroscience, 8, 78. 10.3389/fnagi.2016.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Sørensen Ø, Amlien IK, Bartrés-Faz D, Bros DM, Buchmann N, Demuth I, Drevon CA, Düzel S, Ebmeier KP, Idland A-V, Kietzmann TC, Kievit R, Kühn S, Lindenberger U, Mowinckel AM, Nyberg L, Price D, Sexton CE, … Walhovd KB (2020). Self-reported sleep relates to hippocampal atrophy across the adult lifespan: Results from the Lifebrain consortium. Sleep, 43(5). 10.1093/sleep/zsz280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Malhotra N, & Simonovits G (2014). Social science. Publication bias in the social sciences: Unlocking the file drawer. Science, 345(6203), 1502–1505. 10.1126/science.1255484 [DOI] [PubMed] [Google Scholar]
- Freeman D, Sheaves B, Waite E, Harvey AG, & Harrison PJ (2020). Sleep disturbance and psychiatric disorders. Lancet Psychiatry, 7(7), 628–637. 10.1016/s2215-0366(20)30136-x [DOI] [PubMed] [Google Scholar]
- Germain A (2013). Sleep disturbances as the hallmark of PTSD: Where are we now? American Journal of Psychiatry, 170(4), 372–382. 10.1176/appi.ajp.2012.12040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA (2017). Sleep, health, and society. Sleep Medicine Clinics, 12(1), 1–22. 10.1016/j.jsmc.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JP, Müller VI, Eickhoff SB, & Fox PT (2020). Multimodal abnormalities of brain structure and function in major depressive disorder: A meta-analysis of neuroimaging studies. American Journal of Psychiatry, 177(5), 422–434. 10.1176/appi.ajp.2019.19050560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad G, Reyt M, Beliy N, Baillet M, Deantoni M, Lesoinne A, Muto V, & Schmidt C (2020). pyActigraphy: open-source python package for actigraphy data visualisation and analysis. bioRxiv, 2020.2012.2003.400226. 10.1101/2020.12.03.400226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, & Soehner A (2011). Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clinical Psychology Review, 31(2), 225–235. 10.1016/j.cpr.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes S, Innes CRH, Parsons N, Drummond SPA, Caeyensberghs K, Jones RD, & Poudel GR (2020). Sleeping while awake: The intrusion of neural activity associated with sleep onset in the awake human brain. bioRxiv, 2020.2006.2004.133603. 10.1101/2020.06.04.133603 [DOI] [Google Scholar]
- Heuvel OA, Boedhoe PSW, Bertolin S, Bruin WB, Francks C, Ivanov I, Jahanshad N, Kong X-Z, Kwon JS, O'Neill J, Paus T, Patel Y, Piras F, Schmaal L, Soriano-Mas C, Spalletta G, Wingen GA, Yun J-Y, Vriend C, … Stein DJ (2020). An overview of the first 5 years of the ENIGMA obsessive-compulsive disorder working group: The power of worldwide collaboration. Human Brain Mapping. 10.1002/hbm.24972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, & Ostberg O (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110. [PubMed] [Google Scholar]
- Huang X, Tang S, Lyu X, Yang C, & Chen X (2019). Structural and functional brain alterations in obstructive sleep apnea: A multimodal meta-analysis. Sleep Medicine, 54, 195–204. 10.1016/j.sleep.2018.09.025 [DOI] [PubMed] [Google Scholar]
- Ioannidis JP (2005). Why most published research findings are false. PLoS Med, 2(8), e124. 10.1371/journal.pmed.0020124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, de Leeuw CA, Benjamins JS, Muñoz-Manchado AB, Nagel M, Savage JE, Tiemeier H, White T, Tung JY, Hinds DA, Vacic V, Wang X, Sullivan PF, van der Sluis S, … Posthuma D (2019). Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nature Genetics, 51(3), 394–403. 10.1038/S41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- Javaheripour N, Shahdipour N, Noori K, Zarei M, Camilleri JA, Laird AR, Fox PT, Eickhoff SB, Eickhoff CR, Rosenzweig I, Khazaie H, & Tahmasian M (2019). Functional brain alterations in acute sleep deprivation: An activation likelihood estimation meta-analysis. Sleep Medicine Reviews, 46, 64–73. 10.1016/j.smrv.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, He D, Guo Z, & Gao Z (2020). Effect-size seed-based d mapping of resting-state fMRI for persistent insomnia disorder. Sleep Breath, 24(2), 653–659. 10.1007/s11325-019-02001-3 [DOI] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14(6), 540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- Ju YE, Lucey BP, & Holtzman DM (2014). Sleep and Alzheimer disease pathology–a bidirectional relationship. Nature Reviews. Neurology, 10(2), 115–119. 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof G (1999). Evaluatie Lijst Insomnie Therapie. Den Haag, The Netherlands. [Google Scholar]
- Kerkhof GA, Geuke ME, Brouwer A, Rijsman RM, Schimsheimer RJ , & Van Kasteel V (2013). Holland Sleep Disorders Questionnaire: A new sleep disorders questionnaire based on the International Classification of Sleep Disorders-2. Journal of Sleep Research, 22(1), 104–107. 10.1111/j.1365-2869.2012.01041.x [DOI] [PubMed] [Google Scholar]
- Kharabian Masouleh S, Eickhoff SB, Hoffstaedter F, & Genon S (2019). Empirical examination of the replicability of associations between brain structure and psychological variables. Elife, 8. 10.7554/eLife.43464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaie H, Veronese M, Noori K, Emamian F, Zarei M, Ashkan K, Leschziner GD, Eickhoff CR, Eickhoff SB, Morrell MJ, Osorio RS, Spiegelhalder K, Tahmasian M, & Rosenzweig I (2017). Functional reorganization in obstructive sleep apnoea and insomnia: A systematic review of the resting-state fMRI. Neuroscience and Biobehavioral Reviews, 77, 219–231. 10.1016/j.neubiorev.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagal S, & Broomall E (2012). Sleep in children with autism spectrum disorder. Pediatric Neurology, 47(4), 242–251. 10.1016/j.pediatrneurol.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, & Walker MP (2017). The sleep-deprived human brain. Nature Reviews Neuroscience, 18(7), 404–418. 10.1038/nrn.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, & Reuben E (2012). (Un)available upon request: Field experiment on researchers' willingness to share supplementary materials. Accountability in Research, 19(3), 175–186. 10.1080/08989621.2012.678688 [DOI] [PubMed] [Google Scholar]
- Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Loube DL, Owens J, Pancer JP, & Wise M (2005). Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep, 28(4), 499–521. 10.1093/sleep/28.4.499 [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, & Fox PT (2009). ALE meta-analysis workflows via the brainmap database: Progress towards a probabilistic functional brain atlas. Front Neuroinform, 3, 23. 10.3389/neuro.11.023.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere S, Paquola C, Park BY, Royer J, Wang Y, Benkarim O, de Wael RV, Valk SL, Thomopoulos SI, Kirschner M, & Sisodiya S (2020). The ENIGMA Toolbox: Cross-disorder integration and multiscale neural contextualization of multisite neuroimaging datasets. bioRxiv, 2020.2012.2021.423838. 10.1101/2020.12.21.423838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBourgeois MK, Giannotti F, Cortesi F, Wolfson AR, & Harsh J (2005). The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics, 115(1 Suppl), 257–265. 10.1542/peds.2004-0815H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, & Van Cauter E (2010). Role of sleep and sleep loss in hormonal release and metabolism. Endocrine Development, 17, 11–21. 10.1159/000262524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Costa MD, Buchman AS, Bennett DA, Leurgans SE, & Saper CB (2011). Quantification of the fragmentation of rest-activity patterns in elderly individuals using a state transition analysis. Sleep, 34(11), 1569–1581. 10.5665/sleep.1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall LM, Wyse CA, Graham N, Ferguson A, Lyall DM, Cullen B, Celis Morales CA, Biello SM, Mackay D, Ward J, Strawbridge RJ, Gill JMR, Bailey MES, Pell JP, & Smith DJ (2018). Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: A cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry, 5(6), 507–514. 10.1016/s2215-0366(18)30139-1 [DOI] [PubMed] [Google Scholar]
- Ma N, Dinges DF, Basner M, & Rao H (2015). How acute total sleep loss affects the attending brain: A meta-analysis of neuroimaging studies. Sleep, 38(2), 233–240. 10.5665/sleep.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo AC, Balouch S, & Tabet N (2017). Is sleep disruption a risk factor for Alzheimer's disease? Journal of Alzheimer's Disease, 58(4), 993–1002. 10.3233/jad-161287 [DOI] [PubMed] [Google Scholar]
- Mantovani S, Smith SS, Gordon R, & O'Sullivan JD (2018). An overview of sleep and circadian dysfunction in Parkinson's disease. Journal of Sleep Research, 27(3), e12673. 10.1111/jsr.12673 [DOI] [PubMed] [Google Scholar]
- McClung CA (2007). Circadian genes, rhythms and the biology of mood disorders. Pharmacology & Therapeutics, 114(2), 222–232. 10.1016/j.pharmthera.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medic G, Wille M, & Hemels ME (2017). Short- and long-term health consequences of sleep disruption. Nature and Science of Sleep, 9, 151–161. 10.2147/nss.s134864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, & Spiegelhalder K (2015). Insomnia disorder. Nature Reviews Disease Primers, 1, 15026. 10.1038/nrdp.2015.26 [DOI] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, Wager TD, & Eickhoff SB (2018). Ten simple rules for neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 84, 151–161. 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MJ, & Peterson MJ (2015). Sleep disturbances in depression. Sleep Medicine Clinics, 10(1), 17–23. 10.1016/j.jsmc.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, & Goldman SA (2020). Glymphatic failure as a final common pathway to dementia. Science, 370(6512), 50–56. 10.1126/science.abb8739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP (1999). Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine, 131(7), 485–491. 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- Ng SS, Tam W, Chan T-O, To K-W, Ngai J, Chan KKP, Yip W-H, Lo RL, Yiu K, Ko FW, & Hui DS (2019). Use of Berlin questionnaire in comparison to polysomnography and home sleep study in patients with obstructive sleep apnea. Respiratory Research, 20(1), 40. 10.1186/s12931-019-1009-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn CL, Samson DR, & Krystal AD (2016). Shining evolutionary light on human sleep and sleep disorders. Evol Med Public Health, 2016(1), 227–243. 10.1093/emph/eow018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Lemoine P, Arnaud-Briant V, & Dreyfus M (2002). Prevalence and consequences of sleep disorders in a shift worker population. Journal of Psychosomatic Research, 53(1), 577–583. 10.1016/s0022-3999(02)00438-5 [DOI] [PubMed] [Google Scholar]
- Ong JL, Lau TY, Massar SAA, Chong ZT, Ng BKL, Koek D, Zhao W, Yeo BTT, Cheong K, & Chee MWL (2020). COVID-19 related mobility reduction: heterogenous effects on sleep and physical activity rhythms. Sleep, 44. 10.1093/sleep/zsaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, & Choi SH (2019). Smart technologies toward sleep monitoring at home. Biomedical Engineering Letters, 9(1), 73–85. 10.1007/s13534-018-0091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Derex L, Yammine P, Bastuji H, & Croisile B (2015). Sleep and Alzheimer's disease. Sleep Medicine Reviews, 19, 29–38. 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2008). The role of fMRI in cognitive neuroscience: Where do we stand? Current Opinion in Neurobiology, 18(2), 223–227. 10.1016/j.conb.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2019). The Costs of Reproducibility. Neuron, 101(1), 11–14. 10.1016/j.neuron.2018.11.030 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline J-B, Vul E, & Yarkoni T (2017). Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience, 18(2), 115–126. 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel GR, Innes CR, & Jones RD (2013). Distinct neural correlates of time-on-task and transient errors during a visuomotor tracking task after sleep restriction. NeuroImage, 77, 105–113. 10.1016/j.neuroimage.2013.03.054 [DOI] [PubMed] [Google Scholar]
- Poudel GR, Innes CRH, & Jones RD (2018). Temporal evolution of neural activity and connectivity during microsleeps when rested and following sleep restriction. NeuroImage, 174, 263–273. 10.1016/j.neuroimage.2018.03.031 [DOI] [PubMed] [Google Scholar]
- Raikes AC, Bajaj S, Dailey NS, Smith RS, Alkozei A, Satterfield BC, & Killgore WDS (2018). Diffusion tensor imaging (DTI) correlates of self-reported sleep quality and depression following mild traumatic brain injury. Frontiers in Neurology, 9, 468. 10.3389/fneur.2018.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon A, Lee WH, Leibu E, Laird A, Glahn D, Goodman W, & Frangou S (2017). Neural correlates of affective and non-affective cognition in obsessive compulsive disorder: A meta-analysis of functional imaging studies. European Psychiatry, 46, 25–32. 10.1016/j.eurpsy.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Rezaie L, Fobian AD, McCall WV, & Khazaie H (2018). Paradoxical insomnia and subjective-objective sleep discrepancy: A review. Sleep Medicine Reviews, 40, 196–202. 10.1016/j.smrv.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Roach M, Juday T, Tuly R, Chou JW, Jena AB, & Doghramji PP (2020). Challenges and opportunities in insomnia disorder. International Journal of Neuroscience, 1–8. 10.1080/00207454.2020.1773460 [DOI] [PubMed] [Google Scholar]
- Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, & Morrell MJ (2015). Sleep apnoea and the brain: A complex relationship. The Lancet Respiratory Medicine, 3(5), 404–414. 10.1016/s2213-2600(15)00090-9 [DOI] [PubMed] [Google Scholar]
- Rostampour M, Noori K, Heidari M, Fadaei R, Tahmasian M, Khazaie H, & Zarei M (2020). White matter alterations in patients with obstructive sleep apnea: A systematic review of diffusion MRI studies. Sleep Medicine, 75, 236–245. 10.1016/j.sleep.2020.06.024 [DOI] [PubMed] [Google Scholar]
- Sadeh A (2011). The role and validity of actigraphy in sleep medicine: An update. Sleep Medicine Reviews, 15(4), 259–267. 10.1016/j.smrv.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Saletin JM, Jackvony S, Rodriguez KA, & Dickstein DP (2019). A coordinate-based meta-analysis comparing brain activation between attention deficit hyperactivity disorder and total sleep deprivation. Sleep, 42(3), 10.1093/sleep/zsy251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, & Nichols TE (2009). Meta-analysis of neuroimaging data: A comparison of image-based and coordinate-based pooling of studies. Neuroimage, 45(3), 810–823. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Samea F, Soluki S, Nejati V, Zarei M, Cortese S, Eickhoff SB, Tahmasian M, & Eickhoff CR (2019). Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neuroscience & Biobehavioral Reviews, 100, 1–8. 10.1016/j.neubiorev.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Pozzi E, Ho CT, van Velzen LS, Veer IM, Opel N, Van Someren EJW, Han LKM, Aftanas L, Aleman A, Baune BT, Berger K, Blanken TF, Capitão L, Couvy-Duchesne B, Cullen RK, Dannlowski U, Davey C, Erwin-Grabner T, … Veltman DJ (2020). ENIGMA MDD: Seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry, 10(1), 172. 10.1038/s41398-020-0842-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel JE, Holub F, Petri R, Leerssen J, Tamm S, Tahmasian M, Riemann D, & Spiegelhalder K (2020). Affect and Arousal in Insomnia: Through a Lens of Neuroimaging Studies. Current Psychiatry Reports, 22(9). 10.1007/s11920-020-01173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H, Lack L,& Lovato N (2020). A systematic review of the accuracy of sleep wearable devices for estimating sleep onset. Sleep Medicine Reviews, 49, 101227. 10.1016/j.smrv.2019.101227 [DOI] [PubMed] [Google Scholar]
- Shattuck NL, Matsangas P, Mysliwiec V, & Creamer JL (2019). The role of sleep in human performance and well-being. In Human performance optimization. Oxford University Press. [Google Scholar]
- Sheng LQ, Zhao PW, Ma HR, Qi L, Yi ZQ, Shi YY, Zhong JG, Shi HC, Dai ZY, & Pan PL (2020). Gray matter alterations in restless legs syndrome: A coordinate-based meta-analysis. Medicine (Baltimore), 99(29), e21374. 10.1097/md.0000000000021374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen L, Chen T, Li L, Dai J, Lui SU, Huang X, Sweeney JA, & Gong Q (2017). A meta-analysis of voxel-based brain morphometry studies in obstructive sleep apnea. Scientific Reports, 7(1), 10095. 10.1038/s41598-017-09319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Reilly C, & Midkiff K (1989). Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology, 74(5), 728–738. 10.1037/0021-9010.74.5.728 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, & Behrens TEJ (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Souders MC, Zavodny S, Eriksen W, Sinko R, Connell J, Kerns C, Schaaf R, & Pinto-Martin J (2017). Sleep in children with autism spectrum disorder. Current Psychiatry Reports, 19(6), 34. 10.1007/s11920-017-0782-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K, Regen W, Baglioni C, Nissen C, Riemann D, & Kyle SD (2015). Neuroimaging insights into insomnia. Current Neurology and Neuroscience Reports, 15(3), 9. 10.1007/s11910-015-0527-3 [DOI] [PubMed] [Google Scholar]
- Stickgold R (2005). Sleep-dependent memory consolidation. Nature, 437(7063), 1272–1278. 10.1038/nature04286 [DOI] [PubMed] [Google Scholar]
- Tahmasian M, Jamalabadi H, Abedini M, Ghadami MR, Sepehry AA, Knight DC, & Khazaie H (2017). Differentiation chronic post traumatic stress disorder patients from healthy subjects using objective and subjective sleep-related parameters. Neuroscience Letters, 650, 174–179. 10.1016/j.neulet.2017.04.042 [DOI] [PubMed] [Google Scholar]
- Tahmasian M, Khazaie H, Golshani S, & Avis KT (2013). Clinical application of actigraphy in psychotic disorders: A systematic review. Current Psychiatry Reports, 15(6), 359. 10.1007/s11920-013-0359-2 [DOI] [PubMed] [Google Scholar]
- Tahmasian M, Noori K, Samea F, Zarei M, Spiegelhalder K, Eickhoff SB, Van Someren E, Khazaie H, & Eickhoff CR (2018). A lack of consistent brain alterations in insomnia disorder: An activation likelihood estimation meta-analysis. Sleep Medicine Reviews, 42, 111–118. 10.1016/j.smrv.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Rosenzweig I, Eickhoff SB, Sepehry AA, Laird AR, Fox PT, Morrell MJ, Khazaie H, & Eickhoff CR (2016).Structural and functional neural adaptations in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Neuroscience and Biobehavioral Reviews, 65, 142–156. 10.1016/j.neubiorev.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Samea F, Khazaie H, Zarei M, Kharabian Masouleh S, Hoffstaedter F, Camilleri J, Kochunov P, Yeo BTT, Eickhoff SB, & Valk SL (2020). The interrelation of sleep and mental and physical health is anchored in grey-matter neuroanatomy and under genetic control. Communications Biology, 3(1), 171. 10.1038/s42003-020-0892-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Sepehry AA, Samea F, Khodadadifar T, Soltaninejad Z, Javaheripour N, Khazaie H, Zarei M, Eickhoff SB, & Eickhoff CR (2019). Practical recommendations to conduct a neuroimaging meta-analysis for neuropsychiatric disorders. Human Brain Mapping, 40(17), 5142–5154. 10.1002/hbm.24746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Zarei M, Noori K, Khazaie H, Samea F, Spiegelhalder K, Eickhoff SB, Van Someren E, & Eickhoff CR (2018). Reply to Hua Liu, HaiCun Shi and PingLei Pan: Coordinate based meta-analyses in a medium sized literature: Considerations, limitations and road ahead. Sleep Medicine Reviews, 42, 236–238. 10.1016/j.smrv.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Yokoyama R, Kotozaki Y, Nakagawa S, Sekiguchi A, lizuka K, Yamamoto Y, Hanawa S, Araki T, Miyauchi CM, Shinada T, Sakaki K, Nozawa T, Ikeda S, Yokota S, Daniele M, Sassa Y, & Kawashima R (2018). Shorter sleep duration and better sleep quality are associated with greater tissue density in the brain. Scientific Reports, 8(1), 5833. 10.1038/s41598-018-24226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Jahanshad N, Ching CRK, Salminen LE, Thomopoulos SI, Bright J, Baune BT, Bertolín S, Bralten J, Bruin WB, Bülow R, Chen J, Chye Y, Dannlowski U, de Kovel CGF, Donohoe G, Eyler LT, Faraone SV, Favre P, … Zelman V (2020). ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry, 10(1), 100. 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppi J, Astolfi L, Poudel GR, Innes CRH, Babiloni F, & Jones RD (2016). Time-varying effective connectivity of the cortical neuroelectric activity associated with behavioural microsleeps. NeuroImage, 124(Pt A), 421–432. 10.1016/j.neuroimage.2015.08.059 [DOI] [PubMed] [Google Scholar]
- Torsvall L, & Akerstedt T (1980). A diurnal type scale. Construction, consistency and validation in shift work. Scandinavian Journal of Work, Environment & Health, 6(4), 283–290. 10.5271/sjweh.2608 [DOI] [PubMed] [Google Scholar]
- Toschi N, Passamonti L, & Bellesi M (2020). Sleep quality relates to emotional reactivity via intra-cortical myelination. Sleep, 44(1). 10.1093/sleep/zsaa146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den berg JF, Van rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, Neven AK, Tiemeier H (2008). Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. Journal of Sleep Research, 17(3), 295–302. 10.1111/j.1365-2869.2008.00638.x [DOI] [PubMed] [Google Scholar]
- Walker MP (2009). The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences, 1156, 168–197. 10.1111/j.1749-6632.2009.04416.x [DOI] [PubMed] [Google Scholar]
- Wang C,Ong JL, Patanaik A,Zhou J, & Chee MW (2016). Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states. Proceedings of the National Academy of Sciences, 113(34), 9653–9658. 10.1073/pnas.1523980113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H-H, Chen C-F,Tsai Y-H, Wu C-Y, Lee M, Lin Y-C, Yang C-T, Tsai Y-H, & Yang C-Y (2015). Gray matter atrophy in narcolepsy: An activation likelihood estimation meta-analysis. Neuroscience and Biobehavioral Reviews, 59, 53–63. 10.1016/j.neubiorev.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Weng HH, Tsai YH, Chen CF, Lin YC, Yang CT, Tsai YH, & Yang CY (2014). Mapping gray matter reductions in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Sleep, 37(1), 167–175. 10.5665/sleep.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Blok E, & Calhoun VD (2020). Data sharing and privacy issues in neuroimaging research: Opportunities, obstacles, challenges, and monsters under the bed. Human Brain Mapping. 10.1002/hbm.25120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherts JM, Borsboom D, Kats J, & Molenaar D (2006). The poor availability of psychological research data for re-analysis. American Psychologist, 61(7), 726–728. 10.1037/0003-066x.61.7.726 [DOI] [PubMed] [Google Scholar]
- Winnebeck EC, Fischer D, Leise T, & Roenneberg T (2018). Dynamics and Ultradian Structure of Human Sleep in Real Life. Current Biology, 28(1), 49–59.e45. 10.1016/j.cub.2017.ll.063 [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhuang Y, & Qi J (2020). Explore structural and functional brain changes in insomnia disorder: A PRISMA-compliant whole brain ALE meta-analysis for multimodal MRI. Medicine, 99(14), e19151. 10.1097/md.0000000000019151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, & Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Tandi J, & Chee MW (2015). Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. NeuroImage, 111, 147–158. 10.1016/j.neuroimage.2015.02.018 [DOI] [PubMed] [Google Scholar]
- Zugman A, Harrewijn A, Cardinale EM, Zwiebel H, Freitag GF, Werwath KE, & Winkler AM (2020). Mega-analysis methods in ENIGMA: The experience of the generalized anxiety disorder working group. Human Brain Mapping, 10.1002/hbm.25096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.