Abstract
Hypertension is characterized by systemic microvascular endothelial dysfunction, in part due to a functional absence of hydrogen sulfide (H2S)-mediated endothelium-dependent dilation. Treatment with a sulfhydryl-donating ACE inhibitor (SH-ACE inhibitor) improves endothelial function in preclinical models of hypertension. To date, no studies have directly assessed the effects of SH-ACE-inhibitor treatment on H2S-dependent vasodilation in humans with hypertension. We hypothesized that SH-ACE-inhibitor treatment would improve H2S-mediated endothelium-dependent vasodilation. Ten adults with hypertension [1 woman and 9 men; 56 ± 9 yr; systolic blood pressure (SBP): 141 ± 8.5 mmHg; diastolic blood pressure (DBP): 90.3 ± 6 mmHg] were treated (16 wk) with the SH-ACE-inhibitor captopril. Red blood cell flux (laser-Doppler flowmetry) was measured continuously during graded intradermal microdialysis perfusion of the endothelium-dependent agonist acetylcholine (ACh; 10−10 to 10−1 M) alone (control) and in combination with an inhibitor of enzymatic H2S production [10−3 M aminooxyacetate (AOAA)] preintervention and postintervention. Cutaneous vascular conductance (CVC; flux/mmHg) was calculated and normalized to the site-specific maximal CVC (0.028 M sodium nitroprusside and local heat to 43°C). Area under the curve was calculated using the trapezoid method. The 16-wk SH-ACE-inhibitor treatment resulted in a reduction of blood pressure (systolic BP: 129 ± 10 mmHg; diastolic BP: 81 ± 9 mmHg, both P < 0.05). Preintervention, inhibition of H2S production had no effect on ACh-induced vasodilation (316 ± 40 control vs. 322 ± 35 AU AOAA; P = 0.82). Captopril treatment improved ACh-induced vasodilation (316 ± 40 pre vs. 399 ± 55 AU post; P = 0.04) and increased the H2S-dependent component of ACh-induced vasodilation (pre: −6.6 ± 65.1 vs. post: 90.2 ± 148.3 AU, P = 0.04). These data suggest that SH-ACE-inhibitor antihypertensive treatment improves cutaneous microvascular endothelium-dependent vasodilation in adults with hypertension, in part via H2S-dependent mechanisms.
NEW & NOTEWORTHY This is the first study to prospectively assess the effects of sulfhydryl antihypertensive treatment on microvascular endothelial function in adults with hypertension. Our data suggest that 16 wk of SH-ACE-inhibitor antihypertensive treatment improves cutaneous microvascular endothelium-dependent vasodilation in middle-aged adults with hypertension, in part via H2S-dependent mechanisms.
Keywords: blood pressure, endothelium-dependent dilation, hydrogen sulfide, intradermal microdialysis, nitric oxide-dependent dilation
INTRODUCTION
Cardiovascular disease remains the leading cause of morbidity and mortality worldwide. Hypertension is a primary risk factor for fatal cardiovascular events and affects nearly half (46%) of all American adults (1). A hallmark characteristic of hypertension is systemic microvascular dysfunction (2–8), an early manifestation of the atherosclerotic disease process that precedes larger vessel dysfunction and clinically apparent target organ damage (9–11).
Hypertension-associated microvascular dysfunction is characterized by a loss of endothelium-dependent signaling pathways, including nitric oxide (NO) and hydrogen sulfide (H2S) (3, 5, 7, 8, 12–15). NO and H2S vasodilatory pathways are synergistically interdependent, making them essential for the maintenance of vascular homeostasis (5, 16–18). For example, inhibiting H2S production attenuates NO-dependent vasodilation (16–18), and conversely, inhibition of NO synthase blunts the vasodilatory response to H2S (16). Studies from our laboratory (3, 5, 15) and others (7, 8, 14) demonstrate significant impairments in NO-mediated endothelium-dependent vasodilation in naïve-to-treatment adults with hypertension. Hypertension-associated microvascular endothelial dysfunction is also mediated by a diminished bioavailability of the vasodilatory signaling molecule H2S. We recently found that H2S-mediated vasodilation is functionally absent in adults with hypertension due to reductions in endogenous enzymatic production of H2S through cystathionine γ-lyase (CSE) and 3-mercaptopyruvate (5). Given that these critical vasoprotective pathways are diminished in hypertension, interventions that can improve both NO and H2S bioavailability are needed to mitigate hypertensive-associated peripheral vascular dysfunction.
Using a cross-sectional approach, we reported lower blood pressure, as well as improvements in NO-mediated endothelium-dependent vasodilation, in adults with hypertension treated with antihypertensive pharmacotherapy compared with a naïve to therapy group (3). In that study, medicated participants were treated with a variety of medications, including angiotensin receptor blockers, ACE inhibitors, diuretics, and calcium channel blockers; the specific medications and dosages were not specified. However, to date, no studies have prospectively assessed the effects of chronic treatment with a sulfhydryl-donating (SH) ACE inhibitor on either the NO- or H2S-dependent components of endothelium-dependent dilation in humans with hypertension. Based on this, the purpose of this small, open-label, proof-of-concept study was to examine the effect of chronic SH-ACE-inhibitor treatment (16 wk) on microvascular endothelium-dependent dilation, with a specific focus on the relative contribution of H2S- and NO-dependent signaling. We hypothesized that chronic SH-ACE-inhibitor treatment would improve ACh-induced endothelium-dependent dilation via increases in both H2S- and NO-dependent dilation.
METHODS
The Institutional Review Board at The Pennsylvania State University approved all experimental procedures and protocols (NCT03179163). A Food and Drug Administration Investigational Drug Number was obtained for all protocols (IND 120,058). Verbal and written informed consent were voluntarily obtained from all participants before participation and in accordance with the guidelines set forth by the Declaration of Helsinki.
Participants
All participants were screened by clinical staff including a medical screening, including a medical health history questionnaire, a physical examination, and a blood chemistry analysis (Quest Diagnostics, Pittsburgh, PA). All participants were free from any signs or symptoms of overt cardiovascular disease, aside from elevated blood pressure (BP), or any evidence or diagnosis of cardiovascular disease comorbidities, including renal, pulmonary, neurological, or dermatological disease. Participants did not use tobacco products and were not taking over-the-counter or prescription medications with primary or secondary cardiovascular effects (e.g., antihypertensives, statins, anticoagulants, antidepressants, etc.) at the time of testing. Two participants that were taking antihypertensive medications (lisinopril, 10 mg/day) performed a 2-wk washout period before being tested and enrolled in the captopril intervention (19). BP after the 2-wk washout period was used to determine study eligibility. Consistent with the 2017 AHA/ACC guidelines, adults with stage I or stage II hypertension were eligible for participation [systolic BP (SBP) >130 mmHg or diastolic BP (DBP) ≥80 mmHg] (20). Because of evidence suggesting that hormone therapy can influence the vasculature, specifically endothelial function (21), women taking such therapies were excluded. All women were postmenopausal (>1 yr since last menses) during the time of testing (self-report).
At the laboratory screening visit, resting BP measurements were obtained by a trained research nurse using brachial auscultation, in accordance with standard AHA guidelines (20, 22). Twenty-four-hour ambulatory BP monitoring (Ambulo 2400; Mortara Instrument, Inc., Milwaukee, WI) was used to confirm hypertension. Ambulatory BP measures were obtained every 30 min when awake and every 60 min when asleep and averaged to obtain 24-h values. Due to nocturnal dipping, average BP values obtained using the 24-h ambulatory BP monitor are generally lower than those obtained in a clinic; thus, the consensus values for defining hypertension are also lower (24-h systolic BP ≥125 mmHg or diastolic BP ≥75 mmHg) (23). Eligible participants were classified as hypertensive by both seated and 24-h ambulatory BP monitoring.
Antihypertensive Intervention
Participants were treated with the SH-ACE-inhibitor captopril for 16 wk (starting dose: 50 mg twice daily). Participants returned to the laboratory every 4 wk throughout the intervention to assess medication compliance and for resting seated brachial BP. BP monitoring (24 h) was conducted at 8 and 16 wk. Pharmacokinetic and pharmacodynamic data indicate that the effects of BP-lowering treatments on the peripheral vasculature are evident after 12 wk of treatment and maintained thereafter (24–28). Thus, to ensure the detection of any potential changes in vascular function, 16 wk was selected as the protocol time frame. The dosage of captopril was titrated in accordance with the Food and Drug Administration-approved labeling to achieve a reduction in BP. Final doses ranged from 50 to 100 mg/day. Any increases in dosage were complete by week 12 of the intervention. One participant was excluded from data analysis because BP was not reduced by week 12 of the intervention.
Intradermal Microdialysis
Experimental visits occurred before and immediately after the 16-wk intervention. Before each experimental session, participants were instructed to abstain from caffeine and alcohol for 12 h and strenuous physical activity for 24 h. Five intradermal microdialysis fibers (10 mm, 55 kDa, CMA Linear 31 probe, Harvard Apparatus, Holliston, MA) were inserted into the ventral forearm skin for the local delivery of either lactated Ringer’s solution (control; 3 sites), 0.015 M NG-nitro-l-arginine methyl ester (l-NAME; Calbiochem, EMD Millipore, Billerica, MA; 1 site) to nonselectively inhibit NO synthase, or 0.001 M aminooxyacetic acid (AOAA; Sigma-Aldrich Corp., St. Louis, MO; 1 site) to inhibit H2S biosynthesis (5, 15, 29).
After microdialysis fiber insertion, 60–90 min were allowed for hyperemia resolution during which site-specific pharmacological agents were perfused (2 μmol/L/min; Hive controller and microinfusion pumps; BASi, West Lafayette, IN). Cutaneous red blood cell flux was continuously measured directly over each microdialysis site with an integrated laser-Doppler flowmeter probe placed in a local heating unit (VP12 and VHP2; Moor Instruments, Wilmington, DE) set to a thermoneutral 33°C. After baseline measurements, in three sites, progressively increasing concentrations of the endothelium-dependent agonist acetylcholine (ACh; 10−10 to 10−1 M; USP, Rockville, MD) were coperfused with Ringer’s solution, l-NAME, or AOAA sequentially for 5 min each. In the remaining two sites, progressively increasing concentrations of either 1) sodium sulfide (Na2S; 10−6 to 10−1 M; USP) for the assessment of exogenous H2S-induced vasodilation or 2) norepinephrine (NE; 10−12 to 10−2 M; USP) for exogenous NE-induced vasoconstriction, were coperfused with Ringer’s solution sequentially for 5 min each. At the conclusion of the dose-response protocols, 0.028 M sodium nitroprusside (USP) was perfused and the local temperature was increased to 43°C to elicit maximal cutaneous vasodilation (5, 15, 29). Automated brachial BP (Connex Spot Monitor, Welch Allyn, Skaneateles Falls, NY) was measured every 5 min throughout the protocol.
Data and Statistical Analysis
Intradermal microdialysis data collection procedures and the pharmacological efficacy of the site-specific inhibitors have been previously reported (5, 15, 29). Data were recorded at 40 Hz and stored for offline analysis (Powerlab and LabChart, ADInstruments, Bella Vista, NSW, Australia). Average values for red cell flux (perfusion units) were obtained during 5 min of baseline, during the last minute of each dose, and at maximum. Cutaneous vascular conductance (CVC) was calculated as red cell flux divided by mean arterial pressure. Due to the heterogeneity of capillary density at each microdialysis site, both flux and CVC were normalized as a percentage of either the site-specific maximum (%max; ACh and Na2S) or baseline (%base; NE), which is a standard normalization procedure for data derived from laser-Doppler flowmetry (5, 15, 29–31). Area under the dose-response curve (AUC) was calculated using the trapezoid rule (Prism v8.1; GraphPad Software, La Jolla, CA). The H2S- and NO-dependent contributions were calculated as the difference between the AUC of the control site and either the AOAA or l-NAME sites, respectively (32–34).
A priori power analysis (power = 0.80, α = 0.05) confirmed a sample size of n = 10 was needed to determine a meaningful difference of 10% in endothelium-dependent vasodilation among sites. All data were analyzed with either a one- or two-way repeated-measures ANOVA (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, v. 26.0. Armonk, NY: IBM Corp; SAS v. 9.4; Cary, NC). When appropriate, post hoc Tukey–Kramer corrections were applied to correct for multiple comparisons. Significance was set a priori at α < 0.05. Text and table results are presented as means ± SD. Figure results are presented as means ± SE for visual clarity of main effects.
RESULTS
Ten middle-aged adults (56 ± 9 yr; 1 woman) participated. All participants had hypertension based on resting seated brachial BP (Fig. 1) (20). These results were confirmed with 24-h ambulatory monitoring (Table 1). Other than hypertension, all participants were clinically healthy [body mass index (BMI): 27.7 ± 3.7 kg/m2; HbA1c: 5.3 ± 0.3%; HDL-C: 55 ± 10 mg/dL; LDL-C: 125 ± 25 mg/dL; heart rate (HR): 68 ± 8 beats/min].
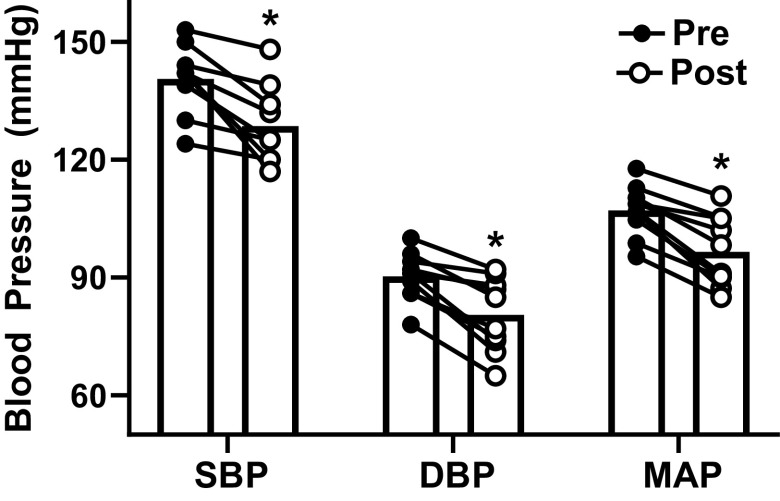
Figure 1.
Seated systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) in participants (n = 10, 9 men and 1 woman) before and after 16-wk captopril intervention. In all measures, blood pressure was significantly lower postintervention. *P < 0.05 vs. preintervention (RMANOVA).
Table 1.
Ambulatory blood pressures (24 h)
| Pretreatment | 8 Wk | Posttreatment (16 Wk) | |
|---|---|---|---|
| 24 h, mmHg | |||
| SBP | 132 ± 9 (121–151) | 128 ± 11 (106–142) | 121 ± 13 (102–145)*‡ |
| DBP | 83 ± 6 (75–94) | 80 ± 6 (69–88) | 77 ± 6 (66–87)* |
| MAP | 100 ± 6 (93–113) | 96 ± 8 (81–106) | 92 ± 8 (78–107)*‡ |
| Day, mmHg | |||
| SBP | 135 ± 7 (124–148) | 131 ± 13 (106–150) | 123 ± 13 (103–148)*‡ |
| DBP | 86 ± 5 (75–92) | 83 ± 8 (69–93) | 78 ± 7 (66–89)*‡ |
| MAP | 102 ± 5 (93–110) | 99 ± 9 (81–112) | 93 ± 9 (94–132)*‡ |
| Night, mmHg | |||
| SBP | 113 ± 13 (91–130) | 107 ± 11 (90–123) | 105 ± 12 (94–132) |
| DBP | 72 ± 7 (62–78) | 68 ± 6 (56–78) | 68 ± 6 (59–80) |
| MAP | 86 ± 8 (71–94) | 81 ± 8 (67–91) | 80 ± 8 (71–98) |
Values are means ± SD (minimum-maximum); n = 9 men and 1 woman. DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
*P < 0.05 vs. pretreatment; ‡P < 0.05 vs. 8 wk.
Seated BP was significantly reduced by week 12 of treatment [SBP: 126 ± 9 mmHg, DBP: 77 ± 7 mmHg, mean arterial pressure (MAP): 93 ± 7 mmHg, all P < 0.05 vs. preintervention] and remained reduced at week 16 following the intervention (Fig. 1). Twenty-four-hour BP ambulatory monitoring confirmed these results (Table 1).
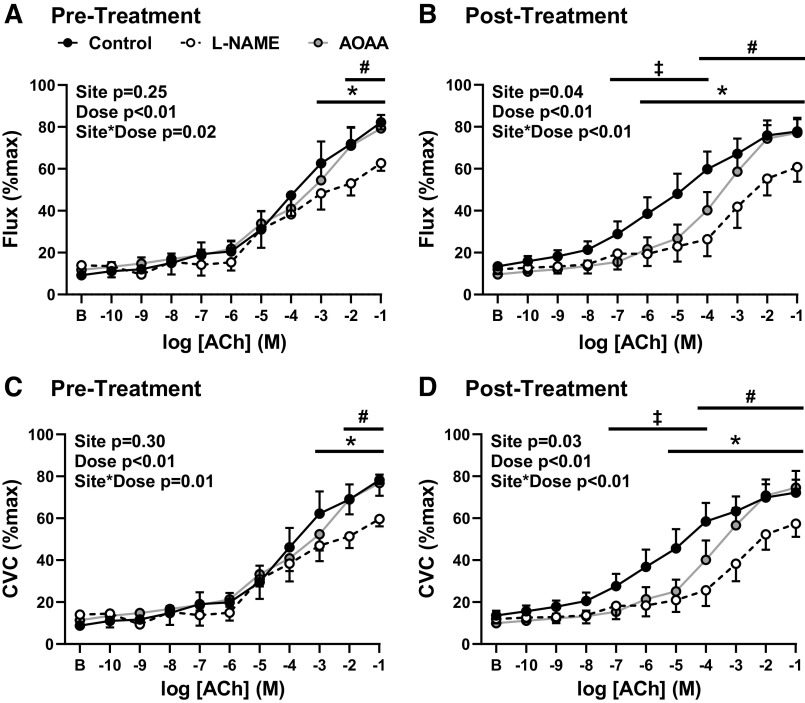
Baseline and maximal flux and CVC were not different pre- to postintervention, or between sites (Table 2). Although the present study did include two subjects who underwent a 2-wk washout of antihypertensive medication when they were enrolled, their baseline vasodilatory responses (control CVC AUC: 121 and 167 AU) were not different from naïve to medication participants (control CVC AUC: 379 ± 85, range = 224–490) following the 2-wk washout. Before treatment, NO synthase inhibition blunted ACh-induced vasodilation (Fig. 2, A and C); however, the inhibition of enzymatic H2S production had no effect (Fig. 2, A and C). ACh-induced vasodilation was modestly improved posttreatment (CVC AUC: pre 316 ± 40 vs. post 399 ± 55 AU, P = 0.04; ANOVA main treatment effect, both P < 0.01; Fig. 2). Postintervention, both NO and H2S inhibition blunted ACh-induced vasodilation (both P < 0.01; Fig. 2, B and D), such that both the NO-dependent (flux: pre 59.1 ± 131.2 vs. post 119.0 ± 167.7 AU, P = 0.05; CVC: pre 57.4 ± 127.8 vs. post 150.7 ± 159.6 AU, P = 0.03) and H2S-dependent (flux: pre 5.3 ± 57.2 vs. post 80.5 ± 152.2 AU, P = 0.11; CVC: pre −6.6 ± 65.1 vs. post 90.2 ± 148.3 AU, P = 0.04) contributions to ACh-induced dilation were increased following captopril treatment.
Table 2.
Microdialysis baseline and maximum values
| ACh Control | ACh + l-NAME | ACh + AOAA | Na2S | NE | |
|---|---|---|---|---|---|
| Baseline | |||||
| Flux, PU | |||||
| Pre | 15 ± 9 (9–36) | 28 ± 38 (9–121) | 22 ± 8 (11–38) | 23 ± 15 (3–36) | 29 ± 31 (10–107) |
| Post | 17 ± 9 (6–34) | 12 ± 5 (7–20) | 15 ± 9 (8–35) | 13 ± 4 (8.4–20.4) | 25 ± 16 (13–55) |
| CVC, flux/mmHg | |||||
| Pre | 0.2 ± 0.1 (0.03–0.5) | 0.3 ± 0.4 (0.1–1.2) | 0.2 ± 0.1 (0.1–0.3) | 0.2 ± 0.1 (0.03–0.5) | 0.2 ± 0.2 (0.1–0.6) |
| Post | 0.3 ± 0.3 (0.1–0.9) | 0.2 ± 0.1 (0.1–0.3) | 0.2 ± 0.1 (0.1–0.4) | 0.1 ± 0.03 (0.1–0.2) | 0.3 ± 0.2 (0.1–0.6) |
| Maximum | |||||
| Flux, PU | |||||
| Pre | 207 ± 129 (57–423) | 192 ± 68 (68–287) | 197 ± 195 (83–290) | 196 ± 106 (42.4–341) | |
| Post | 139 ± 79 (45–260) | 128 ± 82 (53–328) | 195 ± 119 (56–433) | 158 ± 55 (86–254) | |
| CVC, flux/mmHg | |||||
| Pre | 2.0 ± 1.2 (0.6–4.3) | 1.8 ± 0.7 (0.7–2.8) | 1.9 ± 0.6 (0.9–2.8) | 2.1 ± 1.1 (0.4–3.7) | |
| Post | 1.4 ± 0.9 (0.4–3.0) | 1.3 ± 0.7 (0.6–3.0) | 2.0 ± 1.4 (0.5–5.0) | 1.7 ± 0.6 (0.9–2.8) |
Values are means ± SD (minimum-maximum). ACh, acetylcholine; AOAA, aminooxyacetic acid; CVC, cutaneous vascular conductance; l-NAME, NG-nitro-l-arginine methyl ester; Na2S, sodium sulfide; NE, norepinephrine.
All P > 0.05.
Figure 2.
Flux (A and B) and cutaneous vascular conductance (CVC, C and D) in response to increasing concentrations of acetylcholine (ACh) with Ringer’s solution (control, closed circles) or during concurrent nitric oxide synthase inhibition [NG-nitro-l-arginine methyl ester (l-NAME), open circles] or hydrogen sulfide biosynthesis inhibition [aminooxyacetic acid (AOAA), gray circles] prepharmacological (A and C) and postpharmacological (B and D) intervention (16 wk captopril; n = 10, 9 men and 1 women). Preintervention, l-NAME blunted ACh-induced vasodilation (P = 0.02; A and C); however, AOAA did not alter the response to ACh (P = 0.99, A and C). Postintervention, the control site increased (both flux and CVC, P < 0.01). Posttreatment, both l-NAME and AOAA blunted ACh-induced vasodilation (both P < 0.01, B and D). Data are means ± SE. *P < 0.05 control vs. l-NAME, ‡P < 0.05 control vs. AOAA, #P < 0.05 AOAA vs. l-NAME (RMANOVA).
Na2S-induced vasodilation was not different postintervention (flux: intervention P = 0.62, dose P < 0.01, intervention × dose P = 0.64; CVC: intervention P = 0.15, dose P < 0.01, intervention × dose P = 0.52; AUC flux: pre 374.5 ± 129.9 vs. post 316.9 ± 87.4 AU, P = 0.12; AUC CVC: pre 345.0 ± 128.0 vs. post 298.6 ± 82.6 AU, P = 0.15). Similarly, NE-induced vasoconstriction was not different following captopril treatment (flux: intervention P = 0.39, dose P < 0.01, intervention × dose P = 0.48; CVC: intervention P = 0.21, dose P < 0.01, intervention × dose P = 0.38; AUC flux: pre 993.2 ± 294.7 vs. post 928.4 ± 397.8, P = 0.73; AUC CVC: pre 989.5 ± 272.9 vs. post 897.0 ± 355.6, P = 0.57).
DISCUSSION
The results of this open-label, proof-of-concept trial demonstrate that 16 wk of SH-ACE-inhibitor treatment significantly lowered BP (by design) and improved microvascular endothelium-dependent vasodilation through increases in both H2S- and NO-dependent mechanisms. SH-ACE-inhibitor treatment did not affect vasodilatory sensitivity to exogenous H2S. In addition, there was no effect on exogenous NE-induced vasoconstriction, suggesting that chronic SH-ACE-inhibitor treatment may not influence vascular smooth muscle sensitivity to sympathetic stimulation. Collectively, these data provide compelling support for future randomized clinical trials investigating H2S-targeted interventions for the treatment of hypertension-associated microvascular dysfunction.
Pharmacological treatment of hypertension is a critical component of therapy to mitigate coronary heart disease and stroke risk (20). Using a cross-sectional approach, our laboratory has previously reported that adults with hypertension treated with antihypertensive pharmacotherapy (and thus with lower resting BP) have greater microvascular endothelial function compared with unmedicated adults with hypertension (3). In the current proof-of-concept study, 16 wk of SH-ACE-inhibitor treatment resulted in reductions in BP. We also demonstrate a modest but significant increase in the vasodilatory response to the endothelium-dependent agonist acetylcholine. Whether SH-ACE-inhibitor treatment improves microvascular endothelium-dependent dilation independent of, or synergistically with, reductions in BP cannot be determined from the present data. Although methodologically challenging, future randomized placebo-controlled clinical trials are necessary to determine the degree to which antihypertensive pharmacotherapy-induced improvements in the mechanisms regulating peripheral microvascular function are separate from the concomitant BP-lowering effects. We also acknowledge the lack of inclusion of a group of normotensive adults, as well as an imbalance of sexes, in the present study. However, H2S-dependent vasodilation is intact in normotensive adults and the purpose here was to determine if SH-ACE-inhibitor treatment improves the H2S-mediated endothelium-dependent vasodilation in adults in whom it is functionally absent (i.e., unmedicated adults with hypertension) (5). Additionally, the incidence of hypertension differs between sexes throughout the lifetime (35). Another limitation of the current pilot study is that it is underpowered and imbalanced for the determination of sex differences in the current protocol. Although mechanisms underlying these sex differences have been purported, to our knowledge, no studies have investigated the impact of H2S-dependent vascular dysfunction in humans.
In rodent models of hypertension, increasing H2S bioavailability via exogenous H2S donation via sodium hydrosulfide (NaHS) administration improved endothelial vascular function (36–38), in part via an upregulation of enzymatic H2S synthesis (37). Translating this to humans, in the present study, we tested the effectiveness of the sulfhydryl moiety-containing ACE-inhibitor captopril to improve the H2S-dependent contribution to endothelium-dependent vasodilation in adults with hypertension. Consistent with previous findings from our laboratory in unmedicated adults with hypertension (5), the present data confirm a functional absence of H2S-mediated dilation before beginning captopril treatment. After 16 wk of treatment, there was a significant increase in the H2S-dependent contribution to ACh-induced vasodilation. Importantly, we did not detect any alterations in the vasodilatory response to exogenous H2S following captopril treatment, suggesting that the improvements in H2S-dependent vasodilation were secondary to increased enzymatic production and vascular bioavailability of H2S and not a result of enhanced vascular sensitivity to H2S. Interestingly, we previously demonstrated that microvascular sensitivity to exogenous H2S was not different between normotensive and adults with hypertension, despite a profound hypertension-associated reduction in the expression and activity of H2S-producing enzymes (5). This provides additional support for our interpretation that SH-ACE-inhibitor treatment improved H2S-mediated endothelium-dependent dilation due to increased bioavailability of H2S (37). Speculatively, the increase in bioavailability of vascular H2S may be a result of a release of the H2S moiety, as well as an increase in internal enzymatic production of H2S (37). Indeed, the exogenous administration of H2S or SH-ACE-inhibitor has been shown to increase the activity of H2S-producing enzymes CSE and cystathionine-β-synthase (37, 39). Additionally, the increase in H2S bioavailability may be the result of an increase in endogenous production of H2S via initiation of a positive feedback loop (37, 39).
Along with an increase in H2S-mediated vasodilation, we also observed improvements in the NO-dependent component of endothelium-dependent vasodilation following captopril treatment. NO and H2S synergistically interact at multiple points throughout their respective signaling pathways (16–18). For example, H2S directly increases phosphorylation of endothelial NO synthase, augmenting NO biosynthesis (16–18), and, reciprocally, inhibition of NO synthase blunts NaHS-induced dilation (16). In addition to having direct vasodilatory effects, NO and H2S also have secondary effects that could also conceivably contribute to improvements in microvascular endothelial function. That is, H2S can enhance anti-inflammatory cytokines and inhibit inflammatory cytokines and leukocyte adhesion molecules, thus potentially improving vascular function via antioxidant properties (17, 40, 41). Further, in rodent and human models, H2S-donation blunted inward eutrophic microvessel remodeling, thereby beneficially impacting microvessel structure (42, 43). These mechanistic possibilities warrant future targeted investigation.
In conclusion, the results of this initial proof-of-concept study suggest that 16 wk of treatment with the SH-ACE-inhibitor captopril improved microvascular endothelium-dependent vasodilation in adults with hypertension, via an augmentation of both the H2S- and NO-dependent components of ACh-induced vasodilation. As such, intervention strategies specifically targeting the H2S vasodilatory signaling pathway should be considered for mitigating vascular damage in human hypertension.
GRANTS
This work was supported by National Institutes of Health Grants T-32-5T32AG049676 (to G.A.D. and C.S.) and HL093238 (to L.M.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E.S., J.L.G., and L.M.A. conceived and designed research; G.A.D., A.E.S., C.S., and J.L.G. performed experiments; G.A.D. and J.L.G. analyzed data; G.A.D., A.E.S., C.S., J.L.G., and L.M.A. interpreted results of experiments; G.A.D. prepared figures; G.A.D. drafted manuscript; G.A.D., A.E.S., C.S., J.L.G., and L.M.A. edited and revised manuscript; G.A.D., A.E.S., C.S., J.L.G., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the time and effort given by study volunteers and Sue Slimak, Jane Pierzga, and Sean Shank for assistance.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018]. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Bruning RS, Kenney WL, Alexander LM. Altered skin flowmotion in hypertensive humans. Microvasc Res 97: 81–87, 2015. doi: 10.1016/j.mvr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craighead DH, Smith CJ, Alexander LM. Blood pressure normalization via pharmacotherapy improves cutaneous microvascular function through NO-dependent and NO-independent mechanisms. Microcirculation 24: 2017. doi: 10.1111/micc.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farkas K, Kolossváry E, Járai Z, Nemcsik J, Farsang C. Non-invasive assessment of microvascular endothelial function by laser Doppler flowmetry in patients with essential hypertension. Atherosclerosis 173: 97–102, 2004. doi: 10.1016/j.atherosclerosis.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, Alexander LM. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension 69: 902–909, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung F, Pindur G, Ohlmann P, Spitzer G, Sternitzky R, Franke RP, Leithäuser B, Wolf S, Park JW. Microcirculation in hypertensive patients. Biorheology 50: 241–255, 2013. doi: 10.3233/BIR-130645. [DOI] [PubMed] [Google Scholar]
- 7.Lindstedt IH, Edvinsson ML, Edvinsson L. Reduced responsiveness of cutaneous microcirculation in essential hypertension—a pilot study. Blood Press 15: 275–280, 2006. doi: 10.1080/08037050600996586. [DOI] [PubMed] [Google Scholar]
- 8.Rossi M, Bradbury A, Magagna A, Pesce M, Taddei S, Stefanovska A. Investigation of skin vasoreactivity and blood flow oscillations in hypertensive patients: effect of short-term antihypertensive treatment. J Hypertens 29: 1569–1576, 2011. doi: 10.1097/HJH.0b013e328348b653. [DOI] [PubMed] [Google Scholar]
- 9.Cohuet G, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmacol Ther 111: 81–98, 2006. doi: 10.1016/j.pharmthera.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep 12: 448–455, 2010. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 12.Cupisti A, Rossi M, Placidi S, Fabbri A, Morelli E, Vagheggini G, Meola M, Barsotti G. Responses of the skin microcirculation to acetylcholine in patients with essential hypertension and in normotensive patients with chronic renal failure. Nephron 85: 114–119, 2000. doi: 10.1159/000045643. [DOI] [PubMed] [Google Scholar]
- 13.Gryglewska B, Nęcki M, Cwynar M, Baron T, Grodzicki T. Local heat stress and skin blood flowmotion in subjects with familial predisposition or newly diagnosed hypertension. Blood Press 19: 366–372, 2010. doi: 10.3109/08037051.2010.488053. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa H, Raij L. The link among nitric oxide synthase activity, endothelial function, and aortic and ventricular hypertrophy in hypertension. Hypertension 29: 235–241, 1997. doi: 10.1161/01.HYP.29.1.235. [DOI] [PubMed] [Google Scholar]
- 15.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 109: 9161–9166, 2012. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114: 730–737, 2014. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beermann B. Pharmacokinetics of lisinopril. Am J Med 85: 25–30, 1988. doi: 10.1016/0002-9343(88)90346-4. [DOI] [PubMed] [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138: e426–e483, 2018. doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA, Keaney JF Jr.. Hormone replacement therapy and endothelial function: the exception that proves the rule? Arterioscler Thromb Vasc Biol 21: 1867–1869, 2001. doi: 10.1161/atvb.21.12.1867. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45: 142–161, 2005. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 23.Head GA, Mihailidou AS, Duggan KA, Beilin LJ, Berry N, Brown MA, Bune AJ, Cowley D, Chalmers JP, Howe PR, Hodgson J, Ludbrook J, Mangoni AA, McGrath BP, Nelson MR, Sharman JE, Stowasser M; Ambulatory Blood Pressure Working Group of the High Blood Pressure Research Council of Australia. Definition of ambulatory blood pressure targets for diagnosis and treatment of hypertension in relation to clinic blood pressure: prospective cohort study. BMJ 340: c1104, 2010. doi: 10.1136/bmj.c1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accord Healthcare Ltd. (Online). Captopril 25 mg Tablets SmPC. medicines.org.uk/emc/product/6119/smpc [2021 Aug 13]. [Google Scholar]
- 25.CAPOTEN® (Package insert). Captopril Tablets, USP. Spring Valley, NY: Par Pharmaceutical Companies, Inc., 2014. [Google Scholar]
- 26.Borghi C, Ambrosioni E, Omboni S, Cicero AF, Bacchelli S, Esposti DD, Vinereanu D, Ambrosio G, Zava D; SMILE-4 Working Party. Zofenopril and ramipril and acetylsalicylic acid in postmyocardial infarction patients with left ventricular systolic dysfunction: a retrospective analysis in hypertensive patients of the SMILE-4 study. J Hypertens 31: 1256–1264, 2013. doi: 10.1097/HJH.0b013e3283605cd8. [DOI] [PubMed] [Google Scholar]
- 27.Borghi C, Bacchelli S, Degli Esposti D. Long-term clinical experience with zofenopril. Expert Rev Cardiovasc Ther 10: 973–982, 2012. [Erratum in Expert Rev Cardiovasc Ther 11: 649–652, 2013]. doi: 10.1586/erc.12.81. [DOI] [PubMed] [Google Scholar]
- 28.Neutel JM, Frishman WH, Oparil S, Papademitriou V, Guthrie G. Comparison of telmisartan with lisinopril in patients with mild-to-moderate hypertension. Am J Ther 6: 161–166, 1999. doi: 10.1097/00045391-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Craighead DH, Wang H, Santhanam L, Alexander LM. Acute lysyl oxidase inhibition alters microvascular function in normotensive but not hypertensive men and women. Am J Physiol Heart Circ Physiol 314: H424–H433, 2018. doi: 10.1152/ajpheart.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts KA, van Gent T, Hopkins ND, Jones H, Dawson EA, Draijer R, Carter HH, Atkinson CL, Green DJ, Thijssen DHJ, Low DA. Reproducibility of four frequently used local heating protocols to assess cutaneous microvascular function. Microvasc Res 112: 65–71, 2017. doi: 10.1016/j.mvr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Roustit M, Blaise S, Millet C, Cracowski JL. Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc Res 79: 102–108, 2010. doi: 10.1016/j.mvr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Alba BK, Greaney JL, Ferguson SB, Alexander LM. Endothelial function is impaired in the cutaneous microcirculation of adults with psoriasis through reductions in nitric oxide-dependent vasodilation. Am J Physiol Heart Circ Physiol 314: H343–H349, 2018. doi: 10.1152/ajpheart.00446.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dillon GA, Greaney JL, Shank S, Leuenberger UA, Alexander LM. AHA/ACC-defined stage 1 hypertensive adults do not display cutaneous microvascular endothelial dysfunction. Am J Physiol Heart Circ Physiol 319: H539–H546, 2020. doi: 10.1152/ajpheart.00179.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greaney JL, Saunders EFH, Santhanam L, Alexander LM. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res 124: 564–574, 2019. doi: 10.1161/CIRCRESAHA.118.313764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. [Erratum in Circulation 133: e599, 2016]. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 36.Al-Magableh MR, Kemp-Harper BK, Hart JL. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens Res 38: 13–20, 2015. doi: 10.1038/hr.2014.125. [DOI] [PubMed] [Google Scholar]
- 37.Bucci M, Vellecco V, Cantalupo A, Brancaleone V, Zhou Z, Evangelista S, Calderone V, Papapetropoulos A, Cirino G. Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ACE inhibition. Cardiovas Res 102: 138–147, 2014. doi: 10.1093/cvr/cvu026. [DOI] [PubMed] [Google Scholar]
- 38.Xue H, Zhou S, Xiao L, Guo Q, Liu S, Wu Y. Hydrogen sulfide improves the endothelial dysfunction in renovascular hypertensive rats. Physiol Res 64: 663–672, 2015. doi: 10.33549/physiolres.932848. [DOI] [PubMed] [Google Scholar]
- 39.Wu W, Hou C-L, Mu X-P, Sun C, Zhu Y-C, Wang M-J, Lv Q-Z. H2S donor NaHS changes the production of endogenous H2S and NO in D-galactose-induced accelerated ageing. Oxid Med Cell Longev 2017: 5707830, 2017. doi: 10.1155/2017/5707830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agabiti-Rosei E, Manolis A, Zava D, Omboni S; ZODIAC Study Group. Zofenopril plus hydrochlorothiazide and irbesartan plus hydrochlorothiazide in previously treated and uncontrolled diabetic and non-diabetic essential hypertensive patients. Adv Ther 31: 217–233, 2014. doi: 10.1007/s12325-013-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace JL, Ferraz JGP, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal 17: 58–67, 2012. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Napoli C, Bruzzese G, Ignarro LJ, Crimi E, de Nigris F, Williams-Ignarro S, Libardi S, Sommese L, Fiorito C, Mancini FP, Cacciatore F, Liguori A. Long-term treatment with sulfhydryl angiotensin-converting enzyme inhibition reduces carotid intima-media thickening and improves the nitric oxide/oxidative stress pathways in newly diagnosed patients with mild to moderate primary hypertension. Am Heart J 156: 1154.e1151–1158, 2008. doi: 10.1016/j.ahj.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids 39: 1161–1169, 2010. doi: 10.1007/s00726-010-0550-2. [DOI] [PubMed] [Google Scholar]