Abstract
Tissue-resident macrophages are of vital importance as they preserve tissue homeostasis in all mammalian organs. Nevertheless, appropriate cell culture models are still limited. Here, we propose a novel culture model to study and expand murine primary alveolar macrophages (AMs), the tissue-resident macrophages of the lung, in vitro over several months. By providing a combination of granulocyte-macrophage colony-stimulating factor, TGFβ, and the PPARγ activator rosiglitazone, we maintain and expand mouse ex vivo cultured AMs (mexAMs) over several months. MexAMs maintain typical morphologic features and stably express primary AM surface markers throughout in vitro culture. They respond to microbial ligands and exhibit an AM-like transcriptional profile, including the expression of AM-specific transcription factors. Furthermore, when transferred into AM-deficient mice, mexAMs efficiently engraft in the lung and fulfill key macrophage functions, leading to a significantly reduced surfactant load in those mice. Altogether, mexAMs provide a novel, simple, and versatile tool to study AM behavior in homeostasis and disease settings.
Keywords: tissue-resident macrophage, pulmonary alveolar proteinosis, alveolar macrophage, in vitro model
Tissue-resident macrophages (TRMs) are capable of self-renewal under homeostatic conditions in many organs, including the lung (1, 2). By continuous sensing of the surrounding milieu, TRMs adapt to microenvironmental signals, resulting in distinct, tissue-specific macrophage identities (3–5).
Alveolar macrophages (AMs), the TRMs of the lung, reside in the alveoli, the air–liquid interface of the lung, where they perform organ-specific functions such as the clearance of surfactant proteins and cell debris. AMs arise from fetal liver-derived monocytes that differentiate via granulocyte-macrophage colony-stimulating factor (GM-CSF) (1) and transforming growth factor (TGFβ) (6) induced expression of the transcription factor, peroxisome proliferator-activated receptor gamma (PPARγ) around Postnatal Day 3 (7, 8). The absence of autocrine TGFβ signaling in AMs of young mice resulted in reduced AM numbers, together with increased protein content in the bronchoalveolar lavage (6). The accumulation of surfactant and subsequent development of pulmonary alveolar proteinosis (PAP) is also observed in humans who lack mature AMs owing to development of anti-GM-CSF autoantibodies or, more rarely, a loss of GM-CSF receptor subunits, as well as different mouse models lacking parts of the GM-CSF signaling pathway (9–11). In patients, PAP is a rare lung disease-associated increased susceptibility to infections and pulmonary fibrosis, which requires regular bronchoscopic removal of the protein-rich liquid (11, 12). To fully appreciate the functional versatility and therapeutic potential of AMs, appropriate cell culture models are required.
In vitro research on macrophages is essentially limited to the use of bone marrow-derived macrophages (BMDMs) that can be expanded and cultured in sufficient numbers. In contrast, TRMs such as AMs must be isolated from mice and can only be kept in culture for a few days. In this study, we established a protocol for the ex vivo expansion and culture of primary AMs, which we termed mexAMs, by providing AMs with culture conditions that mimic lung microenvironmental factors. These cultured mexAMs expand rapidly and can be maintained and stored for several months, while continuously exhibiting characteristic features of primary AMs, including typical cell surface markers such as CD11c and Siglec-F and an AM-like transcriptional profile. Adoptively transferred mexAMs efficiently engraft in the lung and fulfill AM functions. This includes the reduction of surfactant and protein accumulation upon transfer into AM-deficient mice. Taken together, mexAMs represent a valuable and versatile tool to study primary AM functions in health and disease.
The accession number for the raw and processed RNA-seq data reported in this paper is GEO: GSE179504.
The data was published on bioRxiv (https://doi.org/10.1101/2021.02.11.430791).
Methods
Mice
C57BL/6J, CD45.1 (13), and UBI-GFP (14) mice were originally obtained from Jackson Laboratory. CD169Cre/+STAT5abfl/fl mice (STAT5ΔCD169) or STAT5fl/fl littermate controls were obtained by crossing CD169-Cre (15) provided by the RIKEN BRC and floxed STAT5ab (16) mice provided by R. Morrigl (University of Veterinary Medicine). Csf2rb−/−Csf2rb2−/− mice (10, 17) were provided by M. Busslinger (Research Institute of Molecular Pathology). All mice were maintained on a C57BL/6 background and bred and housed under specific pathogen-free conditions. Mice of both sexes, aged 7–14 weeks, were used. All animal experiments were approved by the Austrian Federal Ministry of Sciences and Research (BMBWF-66.009/0340-V/3b/2019).
Culture of Murine Postnatal Liver Cells
Livers were taken between Postnatal Day 0.5 and 5 and squeezed through a sterile 70-μm cell strainer. Red blood cells were lysed using ammonium chloride lysis buffer. Single cell suspensions (0.2 × 106/ml) were cultured in RPMI 1640 containing 10% FCS and 1% penicillin-streptomycin [pen-strep] supplemented with indicated combinations of murine GM-CSF (30 ng/ml), human TGFβ (10 ng/ml) and rosiglitazone (1 μM). After 6 days, adherent cells were detached and replated at 4 × 104 cells/cm2. Subsequently, cells were passaged when 70–90% confluency was reached. Cells were gently washed with PBS before lidocaine (Gebro Pharma) diluted in PBS was added to detach the cells at 37°C. After a centrifugation step, cells were counted and seeded at the indicated concentration.
Maintenance of mexAMs
BAL from single or a pool of three to four mice was used. Cells were counted, and 0.2 × 106 cells/ml were cultured in RPMI 1640 containing 10% FCS and 1% pen-strep supplemented with murine GM-CSF (30 ng/ml), human TGFβ (10 ng/ml), and rosiglitazone (1 μM). After 2 hours, nonadherent cells were washed off and fresh medium was added. Medium was changed every 5–6 days, and cells were passaged when 70–90% confluency was reached. For this, cells were gently washed with PBS before lidocaine (Gebro Pharma) diluted in PBS was added to detach the cells at 37°C. After a centrifugation step, cells were counted and seeded at the indicated concentration. All experiments were performed between passage 5 and 28.
Isolation of Murine Alveolar Macrophages, BMDMs, and Peritoneal Macrophages
BAL was performed using sterile saline (at room temperature). Retrieved cells were stored on ice before they were plated in RPMI 1640 containing 10% FCS and 1% pen-strep for 3 hours and then washed twice to remove nonadherent cells. Femurs and tibias were flushed with PBS, and mouse bone marrow cells were differentiated for 5 days in RPMI 1640 containing 10% FCS and 1% pen-strep supplemented with 10% L929 conditioned medium. Peritoneal lavage was performed using sterile PBS, and cells were plated in RPMI 1640 containing 10% FCS and 1% pen-strep for 3 hours and then washed twice to remove nonadherent cells. For detachment, cells were gently washed with PBS before lidocaine (Gebro Pharma) diluted in PBS was added for 4–6 minutes at 37°C. After centrifugation and removal of lidocaine, cells were immediately used for downstream experiments.
Flow Cytometry
Single-cell suspensions were incubated with antimouse CD16/CD32 monoclonal antibody for 10 minutes at 4°C. A mix of fluorescently labeled monoclonal antibodies was added for 30 minutes at 4°C (resource table). Sample acquisition was performed on an LSR Fortessa equipped with FACSDiva software (BD Biosciences).
Phagocytosis Assay
Cells were plated for 3 hours, followed by an incubation with FITC-labeled heat-inactivated S. pneumoniae (MOI 100) for 45 minutes at 37°C or 4°C (negative control). Uptake of bacteria was assessed via FITC expression using flow cytometry. Phagocytosis index was calculated as (mean fluorescence intensity × % positive cells at 37°C) − (mean fluorescence intensity × % positive cells at 4°C).
Electron Microscopy
Cells were fixed in Karnovsky fixative (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1-M cacodylate buffer), washed with cacodylate buffer and stored overnight at 4°C. Next, they were embedded in 1% agarose type IV and treated with 1% osmium tetroxide for 1 hour and dehydrated through an ethanol series. After embedding in resin, ultrathin sections were placed on copper 150 mesh grids and stained with 2% uranyl acetate and lead citrate. Samples were examined with a JEM-1400 Plus transmission electron microscope (JEOL).
Cell Stimulations
All cell types were stimulated in 96-well plates (5 × 105 cells/ml) with heat-inactivated S. pneumoniae (MOI 100) or LPS E. coli O55:B5 (10 ng/ml) in RPMI 1640 containing 3% FCS and 1% pen-strep. The concentrations of secreted cytokines were determined in supernatants after 16 h. For polarization experiments cells were seeded and let adhere for 2.5 h in RPMI 1,640 containing 10% FCS and 1% pen-strep, washed, and afterwards treated with LPS (100 ng/ml) and IFN-γ (200 U/ml), IL-4 (10 ng/ml), and IL-13 (10 ng/ml) or IL-10 (10 ng/ml) in RPMI 1640 containing 3% FCS and 1% pen-strep for 1.5 hours (qPCR) or 16 hours (nitrite measurement).
Cytokine Analysis
Indicated mouse cytokines were measured using the LEGENDplex Mouse Macrophage/Microglia Panel (BioLegend). Samples were prepared according to manufacturer's instructions and analyzed by flow cytometry. Data analysis was performed using the LEGENDplex data analysis software.
Proliferation Assay
To assess cell proliferation, intracellular ATP concentrations were measured according to the CellTiter-Glo assay (Promega) instructions. Luminescence was expressed as fold change compared with time-point of seeding.
Immunocytochemistry
Lungs of untreated Csf2rb−/−Csf2rb2−/− mice or Csf2rb−/−Csf2rb2−/− mice that received GFP mexAMs intranasally 4 weeks earlier were fixed in 4% PFA for 48 hours, dehydrated in 15% and 30% sucrose for 24 hours each as described earlier (18). Sections were stained with antipro + mature surfactant protein B antibody and DAPI.
qPCR
Total mRNA was isolated using the NucleoSpin kit (Macherey-Nagel) according to manufacturers’ instructions. Real-time PCR was performed using the Perfecta SYBR Green Master Mix (Quant Bio). The following primers were used: mMrc1 (TCTGGGCCATGAGGCTTCTC, CACGCAGCGCTTGTGATC TT) and mYm1 (TCTGGGTACAAGATCCCTGAACTG, GCTGCTCCATGGTCC TTCCA). Gene expression was normalized to mHprt and expressed as fold change to indicated control.
Nitrite Measurement
Nitrite in cell culture supernatants was measured using the Griess Reagent System (Promega) according to manufacturers’ instructions.
Adoptive Cell Transfer
Mice were anesthetized with isoflurane, and 0.4–1 × 106 cells per mouse were intranasally administered. Mice were killed at indicated time-points. The BAL was centrifuged at 300 g, and the optical density at 600 nm was recorded using an Ultrospec 10 (Amersham Biosciences). Cells were manually counted using a Neubauer chamber or a Z2 cell counter (Beckman Coulter).
RNA Sequencing
Total RNA from indicated cell types was isolated using RNeasy Micro kit (Qiagen). Libraries were prepared from 150 ng total RNA input using the QuantSeq 3' mRNA-Seq Library Prep Kit and UMI Second Strand Synthesis Module (Lexogen), according to the manufacturer’s instructions. Pooled libraries were 65 bp single-end sequenced on the HiSeq4000 (Illumina). Sequencing was performed at the biomedical sequencing facility (Research Center for Molecular Medicine and Medical University of Vienna). Demultiplexing of raw data and mapping to the mouse genome GRCm38 (mm10) was done using the Bluebee software (version Quantseq 2.3.6 FWD UMI).
Statistical Analysis
Data are presented as mean ± SEM. Comparisons were performed using unpaired two-tailed Student’s t-test for two groups or one-way ANOVA for more than two groups. Statistical significance was defined as P < 0.05. Number of animals is indicated as n. Sizes of tested animal groups were dictated by availability of the transgenic strains and litter sizes, allowing littermate controls.
Results
Murine Alveolar Macrophage-like Cells Can Be Derived from Postnatal Liver Cells
To identify the optimal culture conditions for the expansion of AMs, we considered a previously published protocol, where murine postnatal liver cells were cultured in the presence of GM-CSF (19). Aiming for a setting that more closely resembles the lung microenvironment, we cultured postnatal murine liver cells in the presence of indicated combinations of GM-CSF, TGFβ, and rosiglitazone, an activator of the AM transcription factor, PPARγ (20) (Figure 1A). Already after 6 days, all cells treated with GM-CSF plus TGFβ or the triple combination had a round shape, closely resembling primary AMs (Figures 1B and E1A in the data supplement). In contrast, two microscopically distinct cell populations, round and elongated, were observed when cells were grown in the presence of GM-CSF alone or GM-CSF plus rosiglitazone (Figures 1B and E1A). In addition, we noticed that postnatal liver cells expanded very slowly in the presence of GM-CSF alone. This was reflected in significantly lower intracellular ATP concentrations, as well as longer time intervals between passages in cells treated with GM-CSF alone (Figures 1C and E1B).
Figure 1.
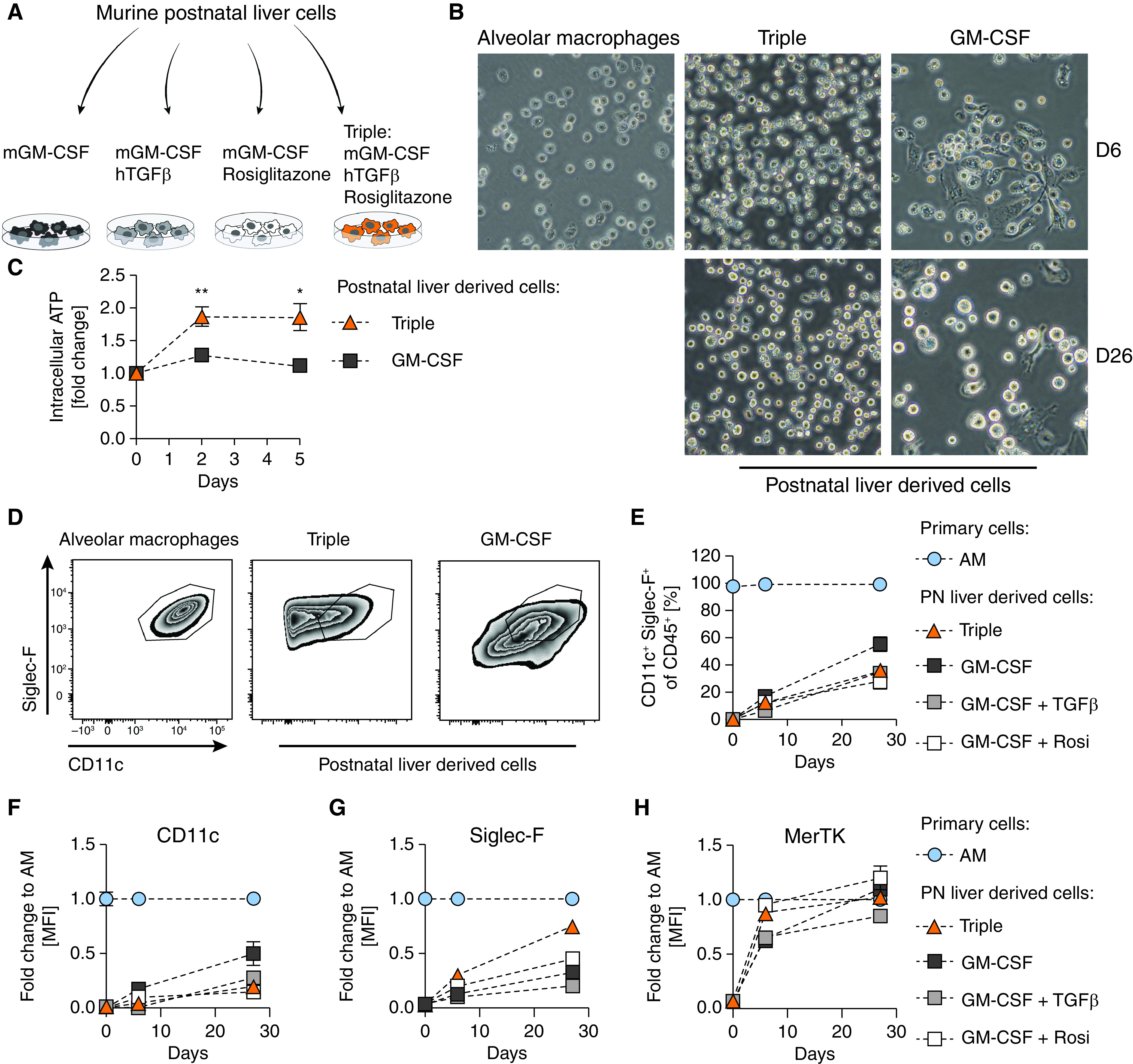
Murine AM-like cells can be derived from postnatal liver cells. (A) Experimental setup. (B) Primary AMs after 3 hours in culture and postnatal liver cells treated with murine GM-CSF (30 ng/ml) or murine GM-CSF (30 ng/ml) + human TGFβ (10 ng/ml) + rosiglitazone (1 µM) (Triple) after 6 days (D6) and 26 days (D26) in culture; 40 × magnification. (C) Cell proliferation of postnatal liver cells under indicated conditions over 5 days compared with time of seeding. (D) FACS analysis of Siglec-F and CD11c expression on primary AMs and postnatal liver cells grown under indicated conditions on D26. (E) Percentage of CD11c+Siglec-F+ cells in postnatal liver cell cultures at day of seeding, D6, and D26. (F) CD11c, (G) Siglec-F, and (H) MerTK mean fluorescence intensity degrees of postnatal liver cell cultures as fold change to primary AMs at indicated days. (D–H) Pregated on single, viable CD45+ cells. Graphs show means ± SEM of 3–4 biological replicates. Data are representative of at least two independent experiments. *P < 0.05 and **P < 0.01 (Student’s t test). AM = alveolar macrophages; GM-CSF = granulocyte-macrophage colony-stimulating factor; MFI = mean fluorescence intensity; PN = postnatal; Rosi = rosiglitazone; TGFβ = transforming growth factor β.
Next, we assessed the expression of AM-specific as well as panmacrophage surface markers to define the differentiation profile by flow cytometry. AMs typically express high amounts of Siglec-F and CD11c (21) (Figures 1D and E1C). From Day 6 onwards, CD11c+ Siglec-F+ cells emerged in the postnatal liver cell cultures, and the relative proportion of AM-like cells in culture gradually increased over time in all conditions (Figures 1E and E1C). Although CD11c was not expressed on freshly isolated postnatal liver cells, it increased over time, being highest on cells treated with GM-CSF only (Figure 1F). Siglec-F expression was upregulated within 26 days in all conditions (Figure 1G). Of note, cells treated with the combination of GM-CSF, TGFβ, and rosiglitazone reached 100% of primary AM Siglec-F expression degrees by Differentiation Day 77 (Figures E1D and E1E). Mer tyrosine kinase (MerTK), a receptor involved in the engulfment of apoptotic cells, is highly expressed on various macrophage populations, including AMs (22). Already on Differentiation Day 6, MerTK expression was comparable to primary AM concentrations when cells were treated with GM-CSF plus rosiglitazone or the triple combination (Figure 1H). As AMs develop from Ly-6C+CD11b+ fetal liver monocytes, we analyzed the expression of CD11b and Ly-6C over time and observed a gradual downregulation of Ly-6C and consistently very low concentrations of CD11b (Figures E1F and E1G).
These results show the ability of murine postnatal liver cells, cultured in the presence of GM-CSF, TGFβ and rosiglitazone, to progressively transition from a monocyte phenotype to an AM-like morphology and marker profile, while maintaining their proliferative capacity.
Murine Ex Vivo Cultured Alveolar Macrophages Are Functionally Similar to Primary Alveolar Macrophages
TRMs are terminally differentiated immune cell populations that retain self-renewing capacities (2, 23, 24). Being able to generate AM-like cells from murine postnatal liver cells, we continued to test our optimized protocol on mature, primary murine AMs (Figure 2A). Primary AMs expanded (Figure E2C) and maintained their CD11c+ Siglec-F+ cell expression profile in culture over 6 months when treated with the combination of GM-CSF, TGFβ and rosiglitazone (Figures 2B and E2A). These mexAMs appeared strikingly similar to primary AMs but distinct from BMDMs (Figures 2C and E2B).
Figure 2.
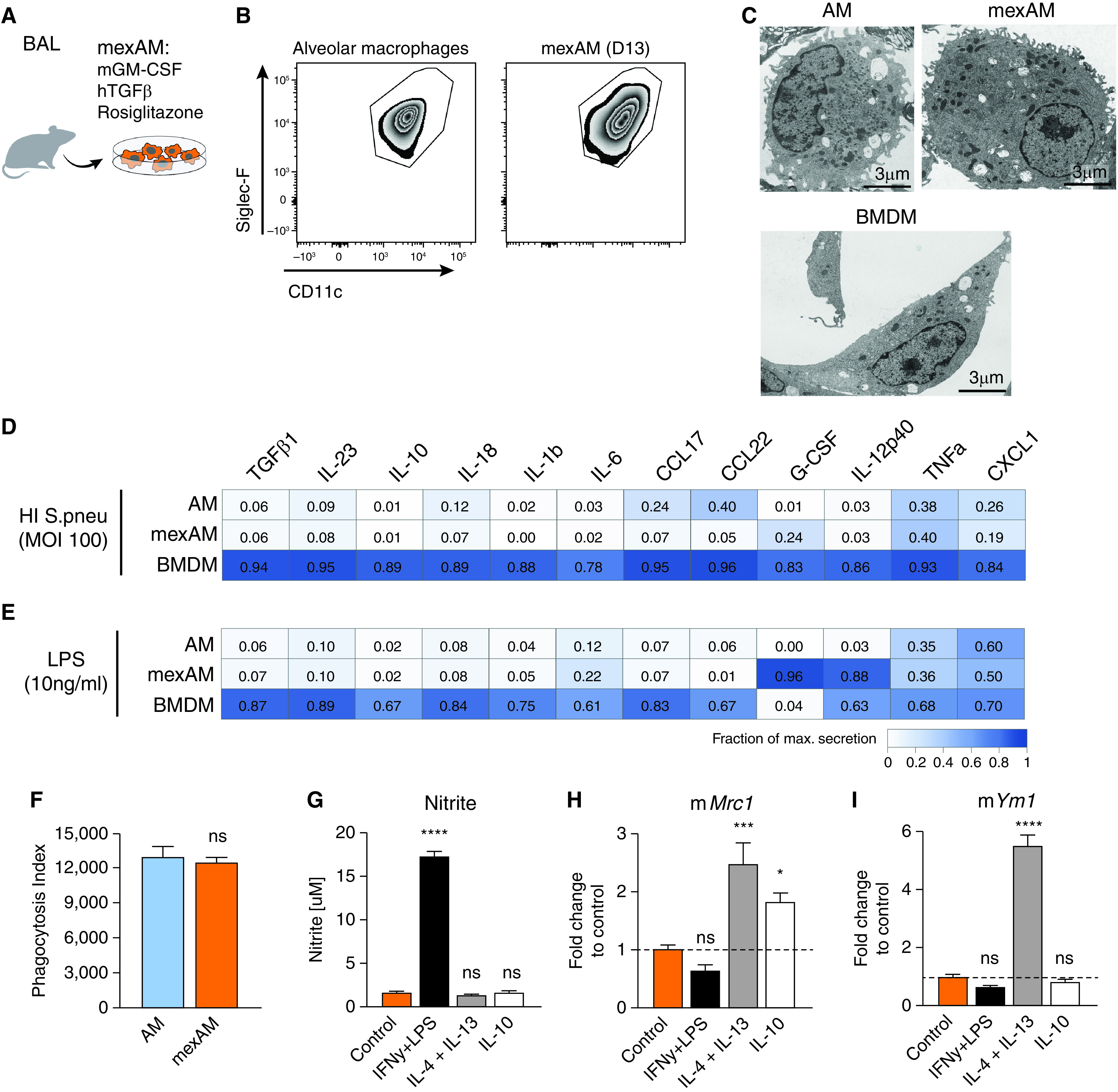
MexAMs are functionally similar to primary AMs. (A) Experimental setup. (B) FACS analysis of Siglec-F and CD11c expression on primary AMs and mexAMs on Day 13. Pregated on viable CD45+ cells. (C) Electron microscopy pictures of primary AMs, mexAMs, or BMDMs. Scale bars, 3 µm. (D and E) Measurement of indicated cytokines upon heat-inactivated S. pneumoniae (MOI 100) (D) or LPS (10 ng/ml) (E) stimulation of AMs, mexAMs, or BMDMs for 16 hours, expressed as fraction of maximal secretion. (F) Phagocytosis index of primary AMs and mexAMs. (G) Nitrite concentration in supernatants of polarized mexAMs after 16 hours. (H and I) M2 polarization markers mMrc1 (H) and mYm1 (I) assessed by qPCR in polarized mexAMs after 1.5 hours. (D–I) Graphs show means ± SEM of 3–4 biological replicates (D and E) or technical quadruplicates (F–I). Data are representative of at least two independent experiments. *P < 0.05, ***P < 0.001, and ****P < 0.0001 (one-way ANOVA followed by Dunnett’s multiple comparison test). BMDMs = bone marrow-derived macrophages; HI = heat-inactivated; mexAM = mouse ex vivo cultured alveolar macrophages; MOI = multiplicity of infection; ns = not significant; S.pneu = Streptococcus pneumoniae.
As innate immune cells, macrophages play a key role in the defense against pathogens by initiating a proinflammatory response. To test their functional properties, we exposed mexAMs, AMs, and BMDMs to heat-inactivated S. pneumoniae (HI S. pneu) (Figure 2D and Table E1) and LPS (Figure 2E and Table E1). With a few exceptions, mexAMs responded like primary AMs and showed a less vigorous release of cytokines and chemokines than BMDMs (Figures 2D and 2E). We also tested if a freeze–thaw cycle affects the responsiveness of mexAMs and discovered that IL-6 (Figure E2D) and CXCL1 (Figure E2E) concentrations did not differ between HI S. pneu stimulated thawed and continuously cultured mexAMs.
A main function of AMs in situ pertains to the phagocytosis of surfactant proteins and cellular debris in the alveoli. To test the phagocytic activity of mexAMs compared with primary AMs, we incubated them with FITC-labeled HI S. pneu and observed an efficient and comparable uptake of bacteria by both cell types (Figure 2F). Another key characteristic of macrophages is the plasticity in their response to stimuli they are exposed to while constantly surveying the surrounding tissue (25). To assess this, we polarized mexAMs with classically activating M1 (IFN-γ and LPS), alternatively activating M2 (IL-4 and IL-13), as well as deactivating (IL-10) stimuli. MexAMs maintained their plasticity, illustrated by the nitrite release upon M1-polarization (Figure 2G) and induction of mannose receptor, C type I (Mrc1) (Figure 2H) and chitinase-like 3 (Ym1) (Figure 2I) upon M2-polarization. This is in line with published results showing the plasticity of primary AMs in response to M1 and M2 stimuli (26). To investigate if mexAMs can be maintained without the combination of GM-CSF, TGFβ and rosiglitazone, we removed all trophic factors from the medium, which resulted in cell death of mexAMs within 1 week (Figure E2F).
Collectively, these data demonstrate that mexAMs, in contrast to BMDMs, exhibit phenotypic and functional properties of primary AMs.
MexAMs Are Phenotypically and Transcriptionally Closest to Primary Murine Alveolar Macrophages
Having established that mexAMs are functionally similar to primary AMs, we next compared their surface marker and transcriptional profile to different macrophage populations. These included BMDMs, as the macrophage type predominantly used for in vitro studies, arising from myeloid bone marrow progenitor cells, as well as peritoneal macrophages (PMs), a mature TRM population exposed to a different tissue microenvironment. Cell surface expression degrees of the panmacrophage marker F4/80 were comparable between all macrophage types, with slightly higher expression in mexAMs and PMs (Figure 3A). In contrast, the AM surface markers Siglec-F (Figure 3B) and CD11c (Figure 3C) were exclusively expressed in mexAMs and AMs, but not BMDMs or PMs.
Figure 3.
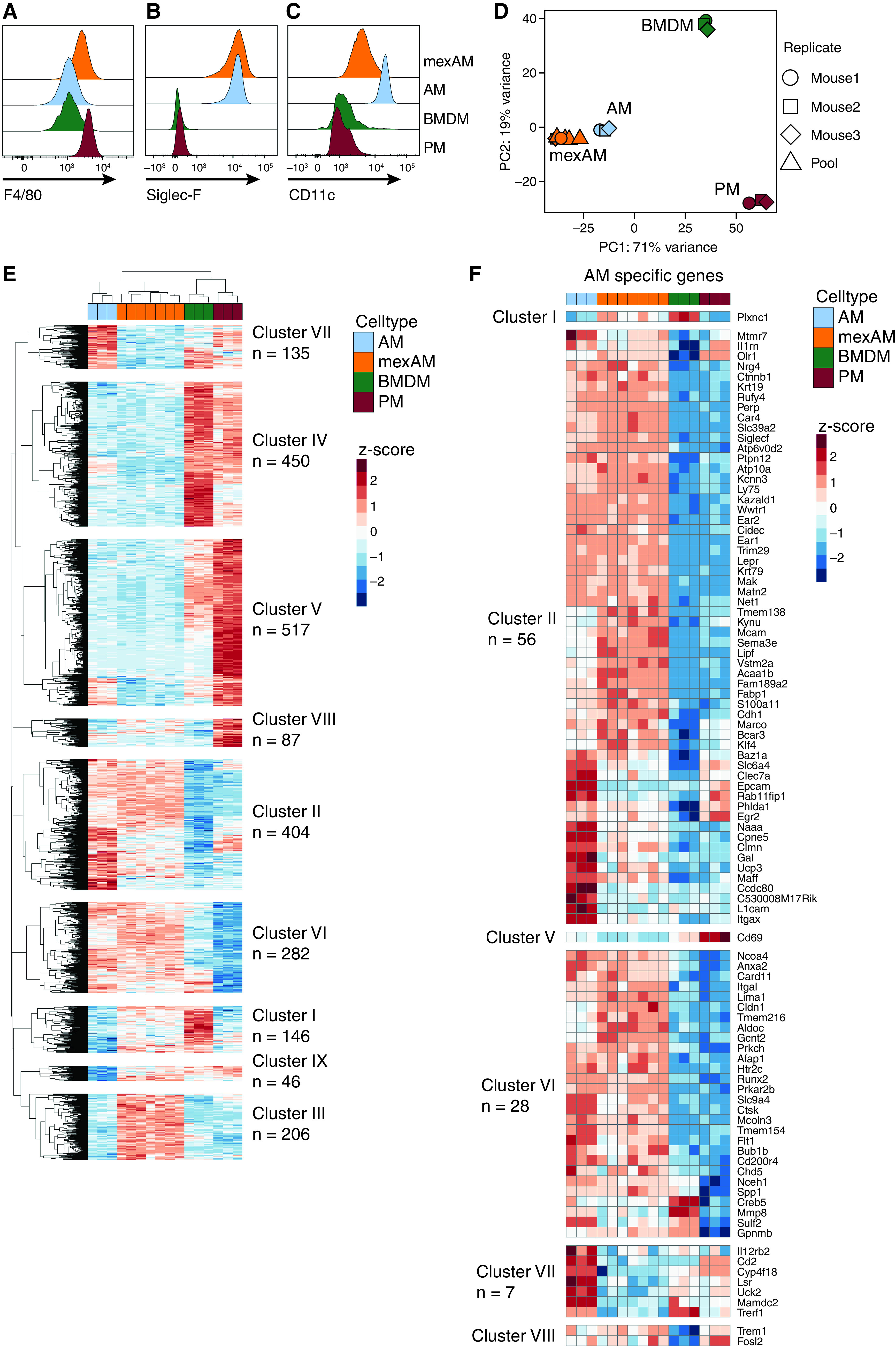
MexAMs are phenotypically and transcriptionally closest to primary murine alveolar macrophages. (A) F4/80, (B) Siglec-F and (C) CD11c cell surface expression on mexAMs, primary AMs, primary PMs, and BMDMs measured by FACS. Pregated on viable CD45+ cells. (D) PC analysis of indicated cell types of three biological replicates (mouse 1–3) plus mexAM samples derived from different passages of mexAM cultures (Pool). (E) Heatmap of genes differentially expressed (absolute log2fc value > 2, P-adj < 0.01) between primary AMs and any other cell type (PM, BMDM, or mexAM). n indicates total number of genes per cluster. Raw counts were rlog transformed, followed by z-score scaling. (F) Heatmap of AM-specific genes found in indicated clusters. fc = fold change; PC = principal component; PM = peritoneal macrophages.
By examining differences in the transcriptional profile of these four macrophage types, principal component analysis conclusively revealed that mexAMs and AMs clustered tightly together, whereas BMDMs and PMs showed distinct transcriptional profiles (Figure 3D). Consistently, hierarchical clustering of 2,273 differentially expressed genes revealed that mexAM samples clustered next to AMs, whereas BMDM samples were closest to PMs (Figure 3E). We identified nine different gene clusters (Figure 3E and Table E2) with cluster IV and V consisting of genes upregulated in BMDMs and PMs. Cluster VIII comprised PM-specific genes such as Klf2 and Naip1. The BMDM-specific cluster I contained genes previously shown to be highly expressed in BMDMs such as Trem2 (27). Most interesting were two prominent clusters of genes, clusters II and VI, because they were upregulated in AMs and mexAMs but downregulated in BMDMs and PMs. When we compiled a list of 133 am-specific transcription factors and genes (3, 28) (Table E3), we found 97 to be included in the differentially expressed gene list shown in Figure 3E. Most of these genes, including Ear2, Marco, and Fabp1, as well as the transcription factor Klf4, were part of the two clusters (II and VI) shared between mexAMs and AMs (Figure 3F). Similarly, the transcription factor Car4, which is uniquely expressed in AMs (4), was elevated in all mexAM and AM samples (cluster II). Itgax, the gene underlying CD11c, was highly upregulated in AMs but less expressed on a transcriptional level in mexAMs, whereas Siglecf was highly expressed in mexAM and AM samples, coinciding with the flow cytometry results shown before (Figures 3B and 3C). In the mexAM-specific cluster (cluster III), many metabolic genes such as Acly, Pdk1, or Fasn were upregulated (Figure E3A). This was also reflected in the enriched GO terms, which included different metabolic processes (Figure E3B). Seahorse experiments confirmed highly elevated basal oxygen consumption rate, ATP production, and basal extracellular acidification rate in actively expanding mexAMs when compared with primary AMs and terminally differentiated BMDMs (Figures E3C–E3G).
These data led us to conclude that mexAMs and primary AMs share a common transcriptional signature that differs from BMDMs and PMs.
MexAMs Engraft Efficiently in a Partially Depleted Alveolar Macrophage Niche In Vivo
To test whether mexAMs engraft in the physiological AM niche in vivo, we transferred CD45.1+ mexAMs by intranasal administration into CD45.2-expressing STAT5ΔCD169 mice or littermate controls (Figure 4A). STAT5 is required for the development of lung dendritic cells (DC) and AMs (29). The loss of STAT5 in CD169-expressing cells, which include AMs but not monocytes or DCs (30), led to a partially emptied AM niche, indicated by a significantly reduced number of AMs in the BAL fluid (BALF) (Figure E4A). Four weeks after transfer, a pronounced CD45.1+ mexAM population was found in the BALF of STAT5ΔCD169 and control mice (Figure 4B, upper and lower right panel). In STAT5ΔCD169 mice, we observed up to 50% of CD11c+Siglec-F+ cells to be of CD45.1+ mexAM origin, whereas in littermate controls approximately 9% of BALF (Figure 4C) or lung (Figure E4B) CD11c+Siglec-F+ cells expressed CD45.1. When expressed in absolute numbers, mexAM transfer sufficiently restored the AM niche in STAT5ΔCD169 animals after 4 weeks (Figure 4D).
Figure 4.
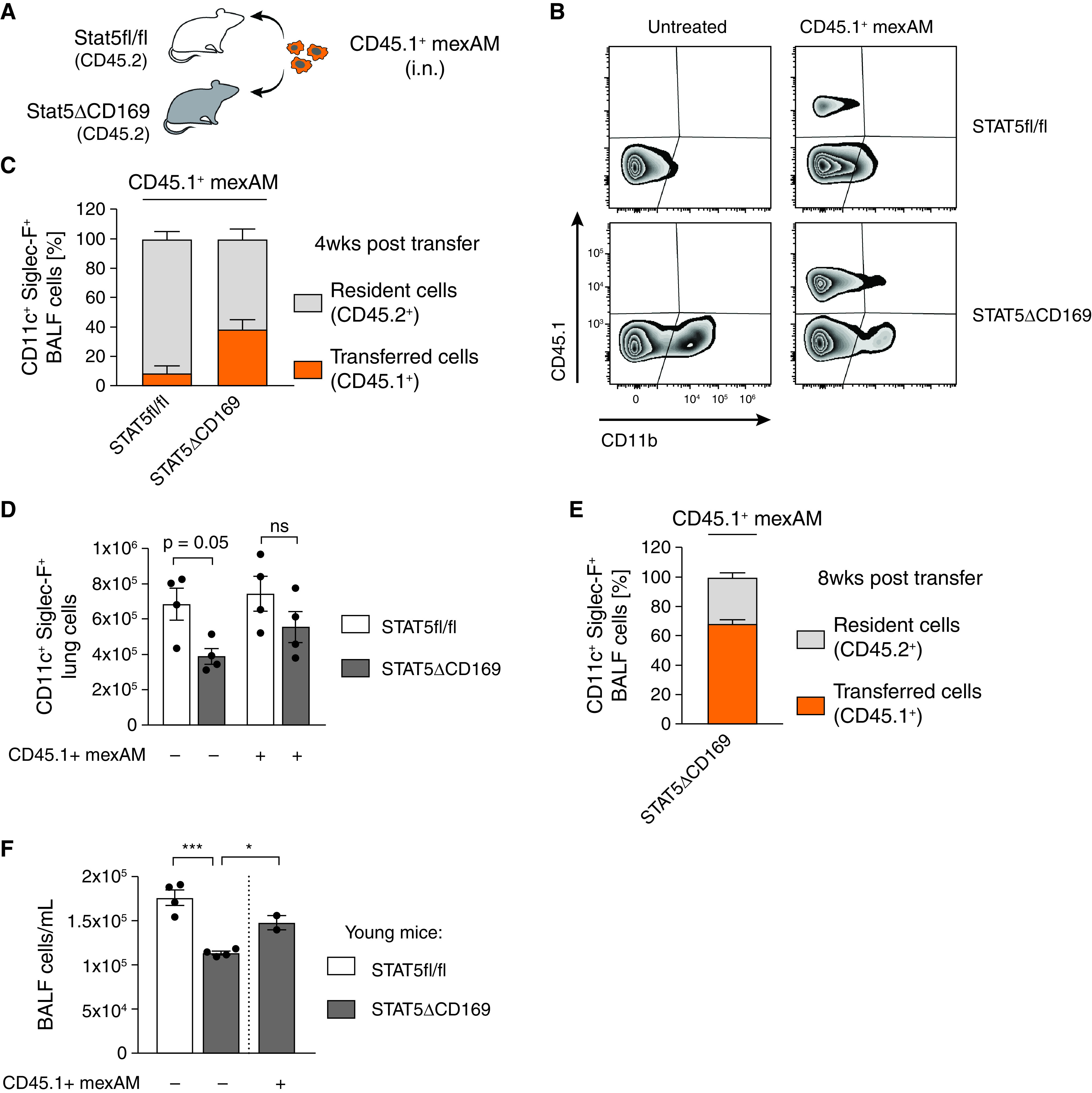
MexAMs engraft efficiently in a partially depleted AM niche in vivo. (A) Experimental setup. Intranasal transfer of CD45.1+ mexAMs into CD45.2-expressing control (STAT5fl/fl) or STAT5ΔCD169 mice. (B) FACS analysis of CD45.1 and CD11b expression of control (upper panel) and STAT5ΔCD169 (lower panel) BAL fluid (BALF) cells untreated, or 4 weeks after transfer of CD45.1+ mexAMs. Pregated on viable Siglec-F+/CD11c+ cells. (C) Percentage of resident (CD45.2+, gray) and transferred (CD45.1+, orange) cells in BALF of control (STAT5fl/fl, n = 4) and STAT5ΔCD169 mice (n = 4) 4 weeks after CD45.1+ mexAM transfer. (D) CD11c+Siglec-F+ lung cell number in STAT5fl/fl and STAT5ΔCD169 mice untreated and 4 weeks after transfer of CD45.1+ mexAMs. (E) Percentage of resident (CD45.2+, gray) and transferred (CD45.1+, orange) cells in BALF of STAT5ΔCD169 mice (n = 4–5) 8 weeks after CD45.1+ mexAM transfer. (F) BALF cell count per ml in STAT5fl/fl and STAT5ΔCD169 mice 14 weeks after transfer of CD45.1+ mexAMs into young (14 d) mice. Graphs show means ± SEM of 2–5 biological replicates. *P < 0.05, and ***P < 0.001 (one-way ANOVA followed by Sidak’s multiple comparison test). i.n. = intranasal.
To test if transferred mexAMs are capable of long-term repopulation of the alveolar niche (2), we transferred CD45.1+ mexAMs into the partially empty AM niche of STAT5ΔCD169 mice and analyzed the BALF 8 weeks later. We found a prominent CD45.1+ mexAM population, comprising up to 73% of the total AM population in STAT5ΔCD169 mice. This indicates that transferred mexAMs self-renew and repopulate the AM niche long-term (Figures 4E and E4C). Finally, we tested if mexAMs possess the ability to settle in and repopulate the lungs of newborn mice. Thus, we transferred CD45.1+ mexAMs into 2-week-old STAT5ΔCD169 animals. Consistent with the results in adult STAT5ΔCD169 mice, we found a significant increase in BALF cell numbers 14 weeks after mexAM transfer into young mice (Figure 4F). These findings demonstrate that mexAMs can efficiently engraft and replenish the AM niche in vivo.
MexAMs Restore Lung Function in a Murine Pulmonary Alveolar Proteinosis Model
To understand if mexAMs can home to the AM niche as efficiently as primary AMs, we transferred CD45.1+ mexAMs and GFP+ AMs in a 1:1 ratio into the partially depleted AM niche of STAT5ΔCD169 mice (Figure 5A). Using flow cytometry, we could clearly distinguish these two populations after the transfer (Figure 5B), and on average, 27% of BALF cells consisted of transferred cells after 4 weeks (Figure E5A). Analysis of the transferred CD11c- and Siglec-F-expressing population revealed that the ratio between CD45.1+ mexAMs and GFP+ primary AMs remained unchanged and that mexAMs and primary AMs contributed equally to the AM population (Figure 5C). Twelve weeks after transfer, BALF cells in STAT5ΔCD169 mice were mainly comprised of transferred cells (Figure E5C), consistent with data shown in Figure 4E. However, at this time, GFP+ primary AMs outnumbered CD45.1+ mexAMs (Figure E5B). To compare the proliferation and homing potential of two in vitro cultured macrophage types, we repeated the experimental setup described in Figure 5A and transferred GFP+ BMDMs and CD45.1+ mexAMs in a 1:1 ratio (Figure E5D). Already after 4 weeks, BMDMs made up a higher proportion of the transferred cells in the immune cell population of the BALF (Figures E5E and E5F).
Figure 5.
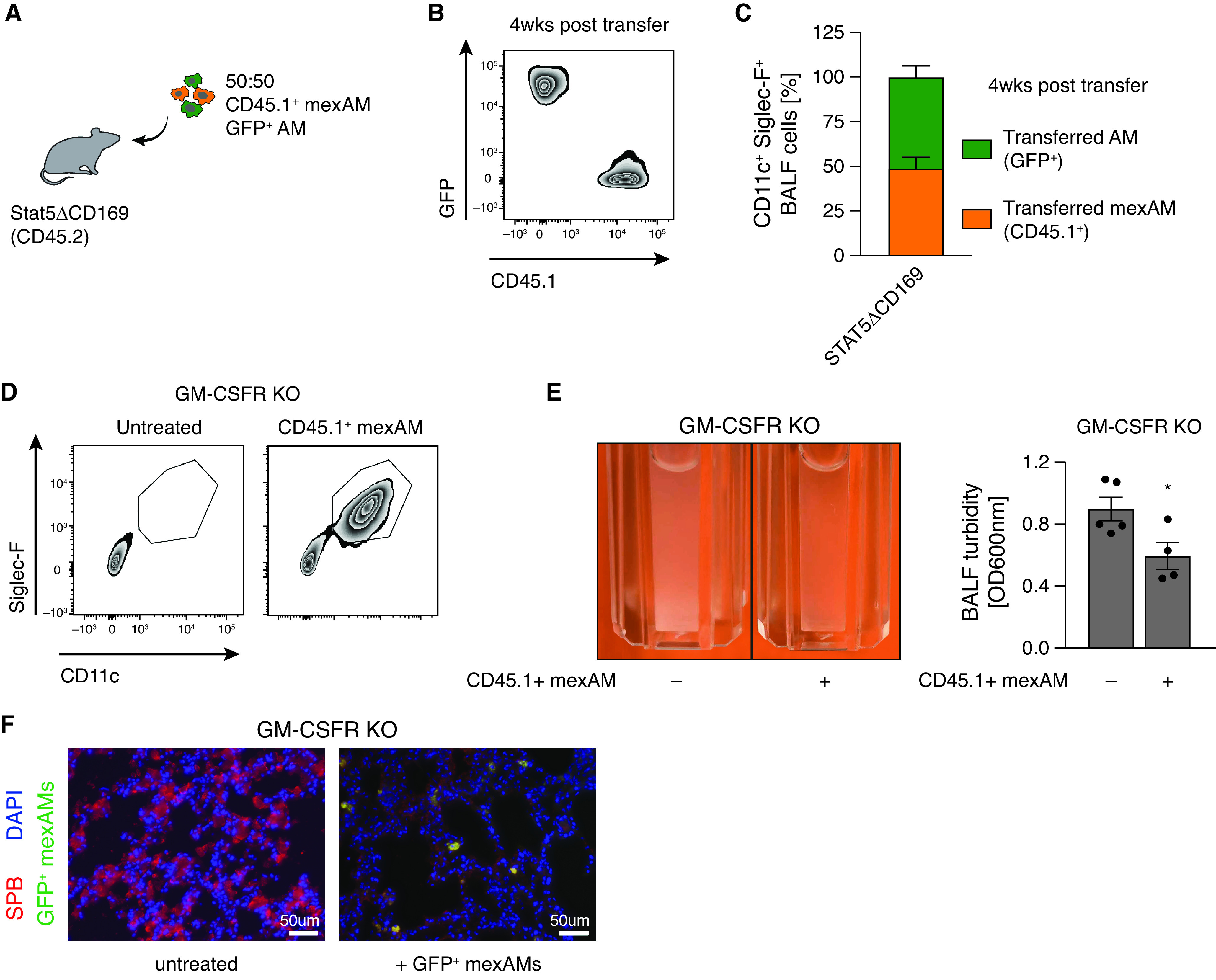
MexAMs restore lung function in a murine pulmonary alveolar proteinosis model. (A) Experimental setup. Intranasal transfer of CD45.1+ mexAMs and GFP+ AMs in a 1:1 ratio into CD45.2-expressing STAT5ΔCD169 mice. (B) FACS analysis of GFP and CD45.1 expression in BALF of STAT5ΔCD169 mice 4 weeks after transfer of 50% GFP+ AMs and 50% CD45.1+ mexAMs. Pregated on viable Siglec-F+/CD11c+ cells. (C) Percentage of AMs (GFP+, green) and mexAMs (CD45.1+, orange) Siglec-F+/CD11c+ cells in BALF of STAT5ΔCD169 (n = 4) mice 4 weeks after transfer. (D) FACS analysis of Siglec-F and CD11c expression on cells in BALF of GM-CSF receptor knockout mice with and without transferred CD45.1+ mexAMs. (E) Representative picture and quantification of BALF turbidity of GM-CSF receptor knockout (KO) mice control and after transfer of CD45.1+ mexAMs (4 wk). (F) Immunofluorescent picture of surfactant protein B accumulation (SPB, red) in GM-CSF receptor knockout mice without (left) and 4 weeks after transfer of GFP+ mexAMs (green). Scale bars, 50 µm. Graphs show means ± SEM of 4–5 biological replicates or representative pictures. *P < 0.05 (Student’s t test). OD = optical density; SPB = surfactant protein B.
One of the essential housekeeping functions of AMs is the clearance of lipids and proteins from the alveolar space (31). Impaired GM-CSF signaling leads to the absence of mature and functional AMs, increased accumulation of surfactant proteins, and subsequent development of PAP (11). To assess if mexAMs are capable of lipid and protein clearance, we transferred CD45.1+ mexAMs into lungs of GM-CSF receptor knockout mice (Csf2rb−/− Csf2rb2−/−) (GM-CSFR KO). Four weeks thereafter, we detected a CD11c+ Siglec-F+ AM population in the lavage (Figure 5D). Development of PAP and surfactant accumulation was significantly reduced upon mexAM transfer, as illustrated by a significantly reduced BALF turbidity (Figure 5E) and a decreased surfactant protein B content in the lungs of GM-CSFR KO mice (Figure 5F).
These data show that mexAMs engraft the alveolar niche and take over AM functions in vivo, thereby preventing PAP development in GM-CSFR KO mice. Collectively, these data support the utility of mexAMs to study AM behavior in homeostatic and disease settings.
Discussion
The tissue environment shapes the identity of macrophage subsets (3–5), making it almost mandatory to use specific, tissue-derived macrophages for in vitro studies. Furthermore, most TRM populations are of embryonic origin (2) and are thereby quite different in their development compared with the widely used adult hematopoietic stem cell-derived BMDMs.
By mimicking the lung microenvironment using GM-CSF, TGFβ and the PPARγ activator rosiglitazone, we first used postnatal liver cells to generate AM-like cells. Supplementing the medium with GM-CSF induced expression of the integrin molecule CD11c in accordance with established protocols that use GM-CSF to generate CD11c-expressing DCs from hematopoietic progenitors (32, 33). The addition of TGFβ to the culture medium induced constant expansion of postnatal liver-derived AM-like cells, confirming the importance of TGFβ in the early AM differentiation (6). Notably, only the use of the combination of GM-CSF, TGFβ, and rosiglitazone induced high Siglec-F expression degrees, a hallmark of primary AMs. We propose to use these postnatal liver-derived AM-like cells as a tool to study AM development in vitro.
In a next step, we cultured mature, fully differentiated primary AMs ex vivo. MexAMs expand rapidly while keeping their phenotypic and functional AM-like profile over several months in culture. In line with published results (1, 8), we found that expansion of mexAMs is dependent on GM-CSF and cannot be accomplished by supplementing medium with TGFβ or rosiglitazone alone (data not shown). As we never tested explicitly if rosiglitazone is as important for mexAM maintenance and cell surface marker expression as it is for postnatal liver cell-derived AM-like cells, we cannot rule out the possibility that it might be dispensable. Although limited GM-CSF concentrations regulate the population of the AM niche in vivo (20), excessive GM-CSF concentrations used under culture conditions allow constant mexAM proliferation and expansion.
A main advantage of any in vitro culture system is the unrestricted number of cells that can be used for high-throughput assays like whole-genome CRISPR screens. We used a common transfection reagent and could confirm that mexAMs can be efficiently transfected with established protocols (data not shown), supporting their usability for high-throughput screening experiments.
Analysis of the transcriptional profile of mexAMs revealed that these cells clustered most closely with AM samples and maintained an AM-specific gene expression profile including the transcription factors Klf4 (34) and Car4 (4). The fact that these mexAM samples derived from single biological replicates or pooled lavages as well as from different passaging numbers confirms the reproducibility and stability of the AM transcriptomic profile over time. Yet, it is important to note that mexAMs, as an actively proliferating population, exhibited features of expanding cells in their metabolic profile. We therefore recommend additional optimization strategies, when using mexAMs for metabolic assays. Interestingly, in contrast to published results (35), we did not observe a significant increase in extracellular acidification rate in BMDMs compared with primary AMs. This might result from differences in differentiation conditions of BMDM cultures where we used L929-conditioned medium in contrast to recombinant mouse M-CSF as well as a shorter time of differentiation. In addition, the method of detachment of cells might influence the metabolic state, highlighting the importance of detailed descriptions of experimental methods used.
In the next step, we demonstrated that transferred mexAMs replenished the partially depleted AM niche of STAT5ΔCD169 mice and could be detected up to 14 weeks later in the lavage. Remarkably, even when transferred to wild-type mice, mexAMs settled in the filled niche, albeit to a much lower extent than in STAT5ΔCD169 mice, supporting the idea that macrophage niches are self-regulating systems that contain a stable macrophage number (20). When performing competitive transfers with 1:1 ratio of mexAMs and AMs, we detected equal numbers of both populations after 4 weeks. Surprisingly, though, when we repeated the competitive transfer assay, mixing mexAMs and BMDMs, we saw a higher proliferative capacity of BMDMs as compared with mexAMs. The high proliferative capacity of transferred BMDMs is in line with data showing that adult bone marrow monocytes display a competitive advantage when refilling an emptied AM niche, as compared with the remaining donor-derived AM population (1, 2, 36).
We envision a wide range of potential applications for mexAMs, including coculture models with immune and structural cells, to better understand disease settings associated with macrophages, including lung fibrosis (36) and cancer (37). Here, we provide evidence that mexAMs can substitute for primary AMs as they restored impaired alveolar surfactant cleaning and prevented the development of PAP in mice lacking primary AMs in vivo.
Conclusions
In summary, our study highlights a previously underappreciated ability to culture and expand fully differentiated TRMs ex vivo over several months by maintaining their intrinsic, tissue-resident macrophage profile.
Acknowledgments
Acknowledgment
The authors thank the flow cytometry core facility and the animal facility of the Medical University of Vienna for their support. CD169cre mice were kindly provided by Miriam Merad (Icahn School of Medicine at Mount Sinai, New York, New York).
Footnotes
Supported by Austrian Science Fund (FWF) grant F 5410. S.K. is supported by the FWF within the Special Research Programs Chromatin Landscapes (L-Mac: F 6104) and Immunothrombosis (F 5410), as well as the Doctoral Program Cell Communication in Health and Disease (W1205). B.M. and V.S. are supported by the FWF Special Research Program (F 6107).
Author Contributions: A.-D.G., D.S., S.Z., K.L. and A.H. performed experiments and analyzed data. A.-D.G. analyzed bioinformatic data. B.L. and R.K. performed electron microscopy experiments. B.M. and V.S. provided valuable reagents and technical advice. A.-D.G. and S.K. wrote the manuscript with input from coauthors and conceptualized the study.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2021-0190OC on September 29, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med . 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity . 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Immunological Genome Consortium. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol . 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell . 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell . 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B, et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity . 2017;47:903–912.e4. doi: 10.1016/j.immuni.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 7. Saluzzo S, Gorki A-D, Rana BMJ, Martins R, Scanlon S, Starkl P, et al. First-breath-induced type 2 pathways shape the lung immune environment. Cell Rep . 2017;18:1893–1905. doi: 10.1016/j.celrep.2017.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol . 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 9. Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science . 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 10. Robb L, Drinkwater CC, Metcalf D, Li R, Köntgen F, Nicola NA, et al. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci USA . 1995;92:9565–9569. doi: 10.1073/pnas.92.21.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, Hamilton J, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers . 2019;5:16. doi: 10.1038/s41572-019-0066-3. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki T, Maranda B, Sakagami T, Catellier P, Couture CY, Carey BC, et al. Hereditary pulmonary alveolar proteinosis caused by recessive CSF2RB mutations. Eur Respir J . 2011;37:201–204. doi: 10.1183/09031936.00090610. [DOI] [PubMed] [Google Scholar]
- 13. Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood . 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 14. Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol . 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 15. Karasawa K, Asano K, Moriyama S, Ushiki M, Monya M, Iida M, et al. Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol . 2015;26:896–906. doi: 10.1681/ASN.2014020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, et al. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol . 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicola NA, Robb L, Metcalf D, Cary D, Drinkwater CC, Begley CG. Functional inactivation in mice of the gene for the interleukin-3 (IL-3)-specific receptor beta-chain: implications for IL-3 function and the mechanism of receptor transmodulation in hematopoietic cells. Blood . 1996;87:2665–2674. [PubMed] [Google Scholar]
- 18. Takata K, Kozaki T, Lee CZW, Thion MS, Otsuka M, Lim S, et al. Induced-pluripotent-stem-cell-derived primitive macrophages provide a platform for modeling tissue-resident macrophage differentiation and function. Immunity . 2017;47:183–198.e6. doi: 10.1016/j.immuni.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 19. Fejer G, Wegner MD, Györy I, Cohen I, Engelhard P, Voronov E, et al. Nontransformed, GM-CSF-dependent macrophage lines are a unique model to study tissue macrophage functions. Proc Natl Acad Sci USA . 2013;110:E2191–E2198. doi: 10.1073/pnas.1302877110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guilliams M, Thierry GR, Bonnardel J, Bajenoff M. Establishment and maintenance of the macrophage niche. Immunity . 2020;52:434–451. doi: 10.1016/j.immuni.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 21. Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol . 2013;49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee Y-J, Han J-Y, Byun J, Park HJ, Park EM, Chong YH, et al. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-κB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. J Leukoc Biol . 2012;91:921–932. doi: 10.1189/jlb.0611289. [DOI] [PubMed] [Google Scholar]
- 23. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature . 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity . 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity . 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, Lakovits K, et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest . 2013;123:3363–3372. doi: 10.1172/JCI67911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol . 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 28. Gibbings SL, Goyal R, Desch AN, Leach SM, Prabagar M, Atif SM, et al. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood . 2015;126:1357–1366. doi: 10.1182/blood-2015-01-624809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eddy WE, Gong K-Q, Bell B, Parks WC, Ziegler SF, Manicone AM. Stat5 is required for CD103+ dendritic cell and alveolar macrophage development and protection from lung injury. J Immunol . 2017;198:4813–4822. doi: 10.4049/jimmunol.1601777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med . 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lambrecht BN. Alveolar macrophage in the driver’s seat. Immunity . 2006;24:366–368. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 32. Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity . 2015;42:1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 33. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med . 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity . 2017;47:913–927.e6. doi: 10.1016/j.immuni.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woods PS, Kimmig LM, Meliton AY, Sun KA, Tian Y, O’Leary EM, et al. Tissue-resident alveolar macrophages do not rely on glycolysis for LPS-induced inflammation. Am J Respir Cell Mol Biol . 2020;62:243–255. doi: 10.1165/rcmb.2019-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med . 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mukaida N, Nosaka T, Nakamoto Y, Baba T. Lung macrophages: multifunctional regulator cells for metastatic cells. Int J Mol Sci . 2018;20:116. doi: 10.3390/ijms20010116. [DOI] [PMC free article] [PubMed] [Google Scholar]


