To the Editor:
Older age is a major risk factor for severe coronavirus disease (COVID-19) (1). Understanding the biological mechanisms linking age to the pathogenesis of COVID-19 is essential for developing preventive and therapeutic strategies. We hypothesized that cell senescence, a basic aging process that plays a pivotal role in health deterioration and diseases, particularly those targeting the lung (2), is involved in the pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–induced lung disease, including the development of long-lasting lung alterations. Senescent cells exhibit a stable proliferation arrest and acquire a specific senescence-associated secretory phenotype characterized by the release of inflammatory cytokines, immune modulators, proteases, profibrotic factors, and various effectors that can alter tissue organization and function (3). Senescence is triggered by a myriad of stressors that promote a DNA damage response leading to p53-dependent upregulation of the CDK inhibitor p21 and/or expression of p16, which is used as a reliable marker of senescent cells. Cell senescence is pivotal in age-associated lung diseases, notably lung emphysema, fibrosis, and chronic obstructive pulmonary disease (2, 4–6). In recent studies, SARS-CoV-2 Spike protein-1 was shown to exacerbate the senescence-associated secretory phenotype of human senescent cells, thereby contributing to the exuberant inflammatory response seen in severe COVID-19. Targeting senescent cells using senolytic drugs reduced mortality in old mice infected with a mouse β-coronavirus (7). To further evaluate potential links between SARS-CoV-2 infection and cell senescence, we analyzed publicly available single-cell RNA sequencing data sets obtained using BAL fluid (BALF) cells from patients with moderate or severe-to-critical COVID-19 (8). We also monitored lung cell senescence in SARS-CoV-2–infected macaques, which constitute a relevant model for studying human COVID-19 (9).
First, we extracted data from publicly available, BALF cell, single-cell RNA sequencing data sets from patients with moderate or severe-to-critical COVID-19 versus healthy control subjects to analyze senescence-related genes (8). In BALFs collected 10–16 days after symptom onset, mRNA of the senescence marker CDKN2A encoding p16 was mainly detected in epithelial cells, macrophages, and T cells, with higher levels in epithelial cells from patients with severe-to-critical disease compared with control subjects (Figure 1A). Expression of the senescence markers CDKN2A, CDKN1A (encoding p21), uPAR (urokinase plasminogen activator surface receptor), CXCL8, IGFBP3, and GDF15 was significantly increased in epithelial ciliated and club cells from patients with severe COVID-19 compared with those with moderate disease and with healthy control subjects, suggesting that lung cell senescence induction coincided with virus detection (Figure 1B). Of note, patients with severe COVID-19 were older than those with moderate disease, whereas age was comparable between patients with moderate disease and healthy control subjects (Figure 1). In single-cell data sets from another study (10), which compared same-age patients with mild versus critical disease (see Fig. E1 in the data supplement), variations were similar, although CDKN1A and CDKN2A were less affected than in the first data set. To further assess the extent of SARS-CoV-2–induced lung cell senescence and the fate of senescent lung cells over time, we investigated macaques at 4 and 30 dpi, or in other words, at the viral load peak and at the first negative airway sample qRT-PCR, respectively (9). Immunohistochemical studies of lung sections at 4 dpi revealed SARS-CoV-2 antigen–stained cells, including lung endothelial cells (ECs) and parenchymal cells, as well as numerous p16- and p21-immunofluorescence–stained cells predominating at sites of alveolar damage (Figure 2A). Cells positive for p16 were also positive for SARS-CoV-2 Spike protein-1 at 4 dpi, indicating that senescent lung cells were infected with the virus. SARS-CoV-2 antigen–stained cells were rarer at 30 dpi, whereas massive accumulation of p16- and p21-positive cells throughout the lung indicated persistence of senescent lung cells after virus clearance (Figure 2A). Cells stained for p16 were also stained for the DNA damage markers γ-H2AX protein and p53-binding protein 1 at both 4 and 30 dpi (Figure 2A).
Figure 1.
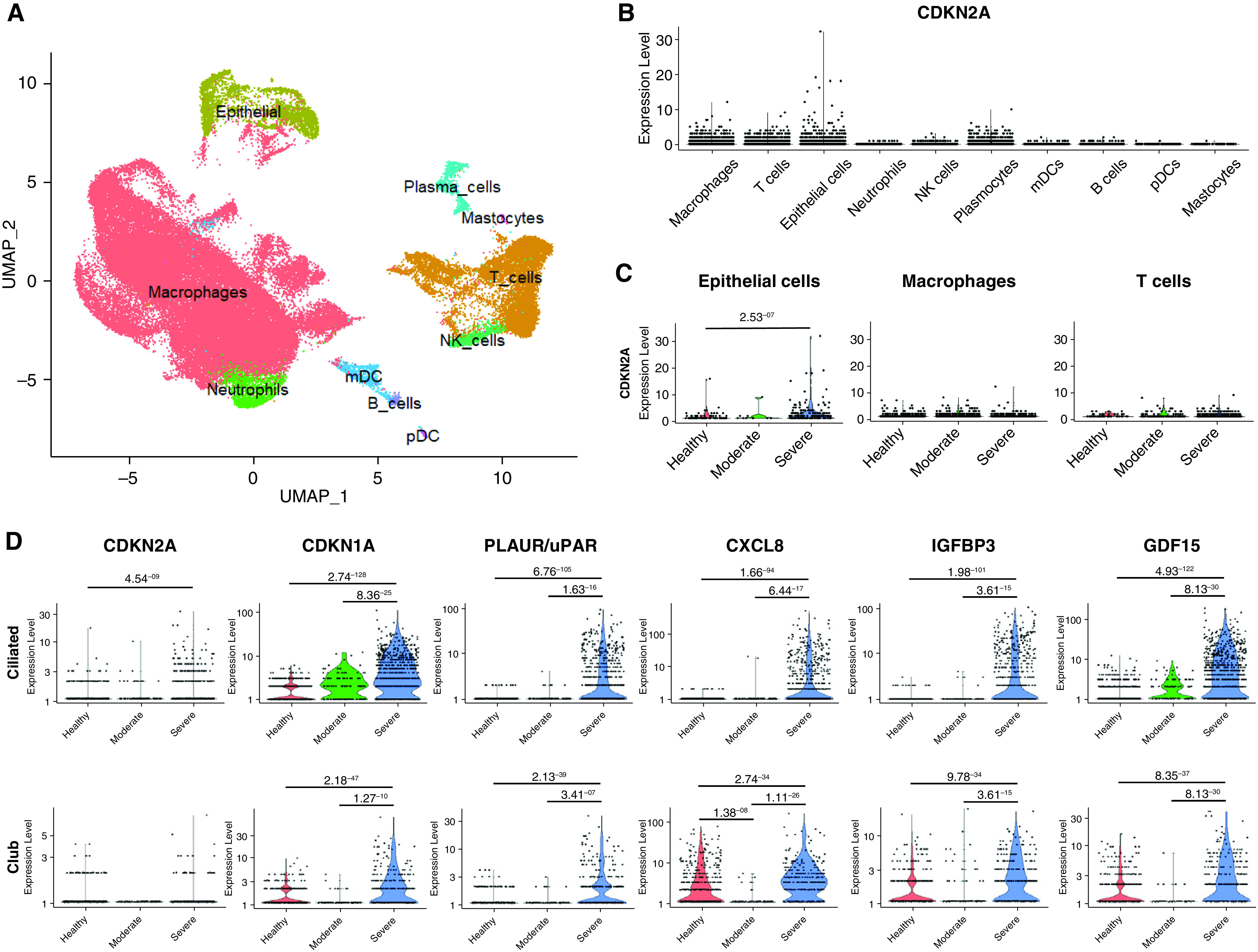
Single-cell RNAseq of cells from patients with COVID-19 revealed increased expression of senescence markers in epithelial cells. (A) UMAP plot of cell types identified in BALFs (n = 13) from the GSE145926 data set (7). (B) CDKN2A mRNA was predominantly detected in epithelial cells, macrophages, and T cells. (C) CDKN2A expression was significantly upregulated in epithelial cells from patients with severe COVID-19. (D) The expression of several senescence markers (i.e., CDKN2A, CDKN1A, uPAR, CXCL8, IGFBP3, and GDF15) was significantly increased in ciliated and club cells in BALFs from patients with severe COVID-19 pneumonia compared with patients with moderate disease and with healthy control subjects. The statistical tests were performed using the model-based analysis of single-cell transcriptomics (MAST) package (Finak G and colleagues Genome Biology 2015), and adjusted P values are reported. BALF = BAL fluid; COVID-19 = coronavirus disease; mDC = myeloid dendritic cells; pDC = plasmacytoid dendritic cells.
Figure 2.
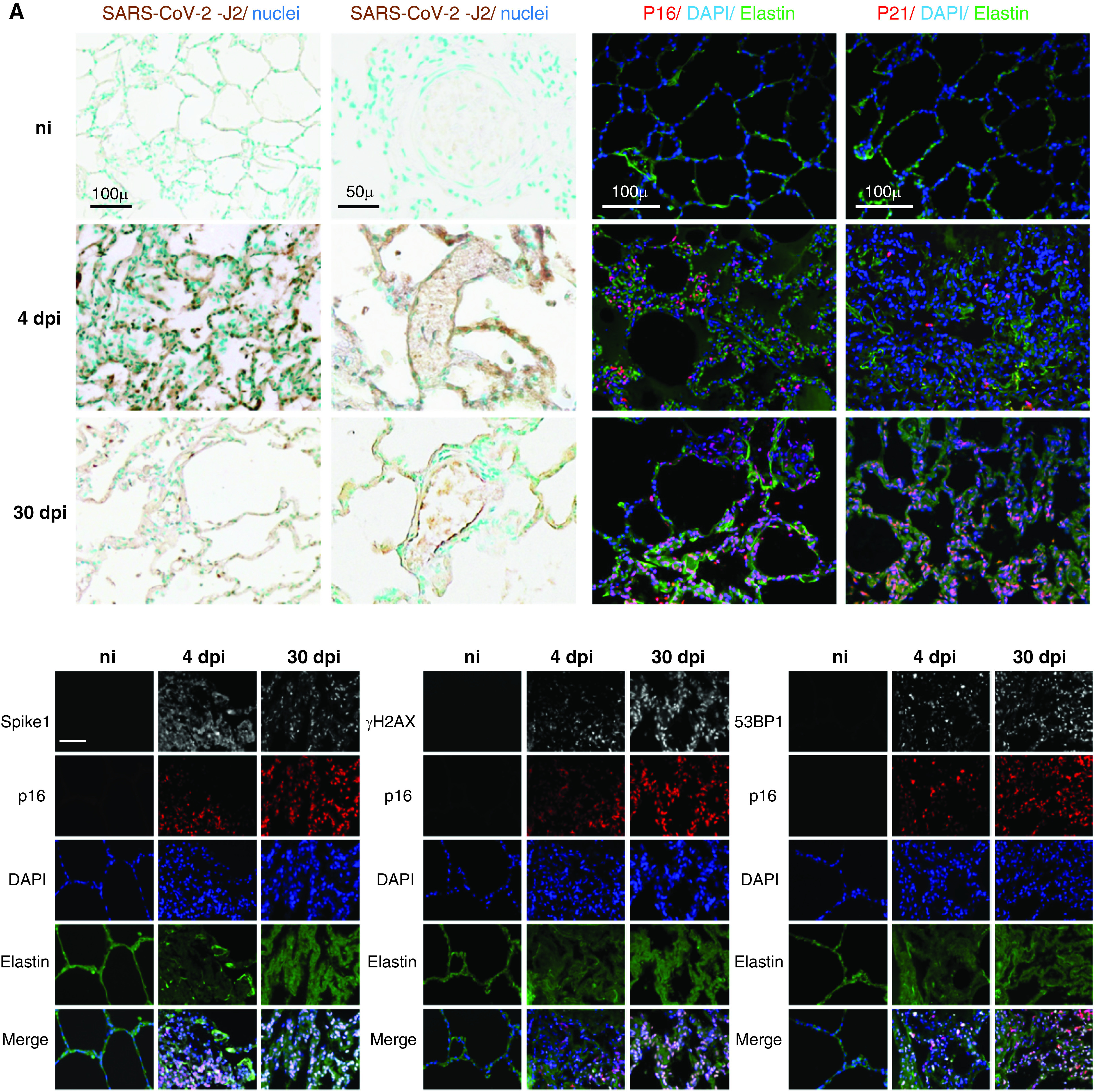
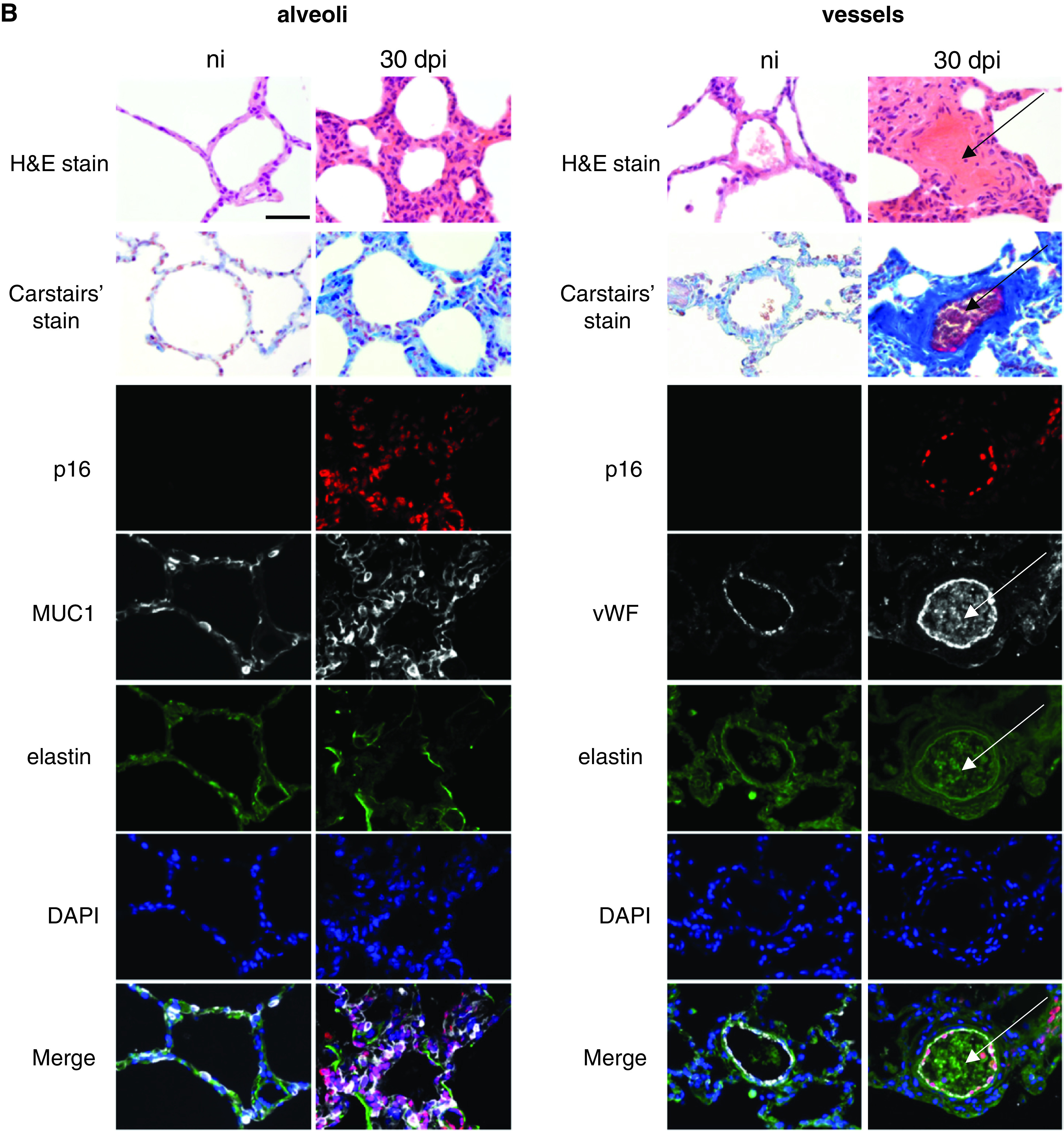
(A) SARS-CoV-2 infection induced lung cell senescence in cynomolgus macaques. Top-left and middle-left panels: representative micrographs of lung tissue from noninfected animals and from animals at 4 and 30 dpi showing viral double-stranded RNA immunostaining (SARS-CoV-2-J2, brown) in the parenchyma (left panel) and vessels (middle-left panel). The mAb SCICONS J2 recognizes dsRNA provided that the length of the helix is greater than or equal to 40 bp (viral dsRNA). Nuclei were stained with methyl green (blue). Middle-right and right panels: representative micrographs showing immunofluorescence of the senescence markers p16 (red) and p21 (red) in lung tissues. Green elastin autofluorescence. Nuclei were stained with DAPI (blue). Bottom: Double immunolabeling showing colocalization (pink in the merged images) of p16 (red) with SARS-CoV-2 capsid protein Spike-1 (white, left panel), as well as with the DNA damage markers γH2AX (white, middle panel) and 53BP1 (white, right panel). Green elastin autofluorescence. Nuclei were stained with DAPI (blue). Scale bars, 100 μ and 50 µm. (B) Lung lesions associated with cell senescence. Representative micrographs of lung tissue from noninfected animals and from animals at 30 dpi showing lung lesions associated with cell senescence in the alveoli (left panel) and vessels (right panel). Top: The lung lesions identified by hematoxylin & eosin (H&E) staining (alveolar thickening and vascular thrombosis) were confirmed by the Carstairs’ staining showing increased collagen deposition (bright blue) and luminal fibrin (bright red) at 30 dpi. Bottom: Double immunofluorescence showing colocalization of p16-positive alveolar cells (red) with mucin 1 (Muc1, white), a marker of type II pneumocytes (left panel), and with von Willebrand factor (vWF, white), a marker of endothelial cells (right panel). Note the intraluminal vWF staining indicating thrombosis. Green elastin autofluorescence. The nuclei were labeled with DAPI (blue). The arrows indicate thrombosis. Scale bar, 50 µm. H&E = hematoxylin and eosin; ni = noninfected animals; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Interestingly, the lungs at 30 dpi no longer exhibited the consolidated parenchymal areas seen at 4 dpi but showed extensive lung parenchyma remodeling, with thickening of the alveolar and pulmonary vessel walls and abundant extracellular matrix deposits as assessed by collagen staining (Figure 2B and Figure E2). These advanced lesions were accompanied by massive accumulation of p16- and p21-positive cells, most of which were alveolar type II cells and ECs, as shown by double immunofluorescence staining for p16 and mucin 1 and for von Willebrand factor, respectively (Figure 2B). Of note, most ECs stained for p16 in many lung vessels, notably those occluded by thrombi and showing intraluminal von Willebrand factor and fibrin staining. Collectively, our data constitute the first evidence of temporal and topographic relations between senescent cell accumulation and pulmonary lesions induced by SARS-CoV-2.
Cell senescence is usually viewed as a response to chronic stressors that severely impedes healthy aging and promotes age-related noncommunicable diseases (11). Here, BALF cells from patients with severe COVID-19 expressed high levels of senescent cell markers. This original observation was confirmed in a macaque COVID-19 model: early massive senescent lung cell accumulation occurred in areas of severe COVID-19-related lung damage. Moreover, senescent cells persisted in the lungs over time, and many of them appeared concomitantly with the development of long-term lung alterations, including remodeling of the alveolar septa and pulmonary vessels. Given the deleterious effect of cell senescence on tissue repair and inflammation, these results suggest that senescent cell accumulation may contribute to the early lung alterations caused by SARS-CoV-2 infection and, potentially, to the postviral lung pathology seen in a substantial proportion of patients (12). Most ECs in thrombosed vessels were senescent, suggesting a causal relationship between EC senescence and vascular thrombosis. Thus, counteracting the cell senescence process or eliminating senescent lung cells might lessen lung damage severity. This may be of therapeutic importance as strategies are now proposed to control senescence in various lung diseases, as well as in acute respiratory distress syndrome due to other causes (13, 14). A recent study in mice showed that lung inflammation caused by a mouse β-coronavirus was markedly reduced by senolytic treatment, which also decreased mortality in old mice (7). These findings also support senescence as a major mechanism in the pathogenesis of COVID-19 and of other viral infections (15) and suggest that senescent lung cell persistence after virus clearance may contribute to postviral lung disease, namely emphysema or fibrosis.
Footnotes
Supported by Ligue Contre le Cancer (RS21/75-24), the Institut National Du Cancer, the Fondation pour la Recherche Médicale (AM-CoV-Path), the Agence Nationale de la Recherche (REACTing/ANRS-MIE SENOCOVID - ANR-20-COV3-0006, Lustra, INFLUENZAGING-ANR-20-CE14-0023-02), and Electricité de France (CT9818).
Author Contributions: L.L., V.S., E.B., F.T., and S.A. designed the study, examined the lung tissues, interpreted the data, and wrote the manuscript. P.M., Q.P., and R.L.G. designed and conducted the animal experiments, processed the samples, and acquired and interpreted the data. C.F., J.-M.F., A.L.-V., and D.B. analyzed and interpreted the human pathology data. All authors reviewed the manuscript for important intellectual content, approved the final version and its submission for publication, and take responsibility for the integrity of the study data.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2021-0205LE on October 14, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China medical treatment expert group for c. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;58:711–712. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature . 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov . 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adnot S, Amsellem V, Boyer L, Marcos E, Saker M, Houssaini A, et al. Telomere dysfunction and cell senescence in chronic lung diseases: therapeutic potential. Pharmacol Ther . 2015;153:125–134. doi: 10.1016/j.pharmthera.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 5. Amsellem V, Gary-Bobo G, Marcos E, Maitre B, Chaar V, Validire P, et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2011;184:1358–1366. doi: 10.1164/rccm.201105-0802OC. [DOI] [PubMed] [Google Scholar]
- 6. Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol . 2014;51:323–333. doi: 10.1165/rcmb.2013-0382PS. [DOI] [PubMed] [Google Scholar]
- 7. Camell CD, Yousefzadeh MJ, Zhu Y, Prata LGPL, Huggins MA, Pierson M, et al. Senolytics reduce coronavirus-related mortality in old mice. Science . 2021;373:eabe4832. doi: 10.1126/science.abe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med . 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 9. Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R, et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature . 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 10.Wauters E, Van Mol P, Garg AD, Jansen S, Van Herck Y, Vanderbeke L, et al. CONTAGIOUS collaborators. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res. 2021;31:272–290. doi: 10.1038/s41422-020-00455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature . 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J . 2021;57:2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med . 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown R, McKelvey MC, Ryan S, Creane S, Linden D, Kidney JC, et al. The impact of aging in acute respiratory distress syndrome: a clinical and mechanistic overview. Front Med (Lausanne) . 2020;7:589553. doi: 10.3389/fmed.2020.589553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez I, García-Carpizo V, Guijarro T, García-Gomez A, Navarro D, Aranda A, et al. Induction of DNA double-strand breaks and cellular senescence by human respiratory syncytial virus. Virulence . 2016;7:427–442. doi: 10.1080/21505594.2016.1144001. [DOI] [PMC free article] [PubMed] [Google Scholar]


