Abstract
In preeclamptic pregnancies, a variety of intrauterine alterations lead to abnormal placentation, release of inflammatory and/or antiangiogenic factors, and subsequent fetal growth restriction with significant potential to cause a primary insult to the developing fetal lung. Thus, modulation of the maternal intrauterine environment may be a key therapeutic avenue to prevent preeclampsia-associated developmental lung injury. A biologic therapy of interest is mesenchymal stromal cell–derived extracellular vesicles (MEx), which we have previously shown to ameliorate preeclamptic physiology through intrauterine immunomodulation. To evaluate the therapeutic potential of MEx to improve developmental lung injury in experimental preeclampsia, using the heme oxygenase-1–null mouse (Hmox1−/−) model, preeclamptic pregnant dams were administered intravenous antenatal MEx treatment during each week of pregnancy followed by analysis of fetal and postnatal lung tissues, amniotic fluid protein profiles, and lung explant and amniotic fluid cocultures in comparison with control and untreated preeclamptic pregnancies. We first identified that a preeclamptic intrauterine environment had a significant adverse impact on fetal lung development, including alterations in fetal lung developmental gene profiles in addition to postnatal alveolar and bronchial changes. Amniotic fluid proteomic analysis and fetal lung explant and amniotic fluid cocultures further demonstrated that maternally administered MEx altered the expression of multiple inflammatory mediators in the preeclamptic intrauterine compartment, resulting in the normalization of fetal lung branching morphogenesis and developmental gene expression. Our evaluation of fetal and postnatal parameters overall suggests that antenatal MEx treatment may provide a highly valuable preventative therapeutic modality for amelioration of lung development in preeclamptic disease.
Keywords: lung development, preeclampsia, exosomes, bronchopulmonary dysplasia
Preeclampsia is a gestational-specific disease that impacts ∼3–5% of pregnancies worldwide (1). Well recognized as a maternal systemic vasculopathy, its primary manifestations include systemic hypertension, proteinuria, and, if untreated, progressive liver dysfunction and cerebral edema and seizures. Although the maternal effects of preeclampsia have been evaluated for decades, the fetal sequelae of this disorder have only been more recently understood. Most notably, preeclampsia is the leading cause of intrauterine growth restriction in nonanomalous infants (1). Each year, an estimated 23 million growth-restricted infants are born in developing countries (∼20% of live births), and growth restriction puts both full-term and preterm infants at an increased risk for mortality (2). Growth restriction has multisystem effects with long-term impacts on fetal health, particularly in the developing lung. Infants with intrauterine growth restriction have an overall higher incidence of bronchopulmonary dysplasia (BPD), with the combination of extreme prematurity and growth restriction putting infants at the highest risk for BPD (3, 4). Preeclampsia itself has also been significantly implicated in BPD risk (5, 6).
Within the spectrum of preeclamptic disease, a subset of early-onset severe preeclamptic pregnancies is driven by immunological alterations of the intrauterine milieu, with resultant abnormal placentation and fetal growth restriction (7). An immunologically driven preeclamptic phenotype has been explored in many preclinical models, including the Hmox1 (heme oxygenase-1)-null mouse, which has significant alterations in intrauterine leukocytes in conjunction with maternal hypertension, altered placentation, and fetal growth restriction (8). Although dangerous for maternal health during pregnancy, an early-onset severe preeclamptic intrauterine environment also contains an adverse mix of antiangiogenic as well as proinflammatory factors that have the potential to cause significant harm to the developing fetus (9). Thus, preeclampsia and its significant association with intrauterine growth restriction and neonatal lung disease have the potential for long-term impacts on newborn pulmonary health.
Preeclampsia remains difficult to prevent despite a variety of attempted interventions, likely because of its multifactorial pathogenesis (10). Although existing pharmacologic agents can alleviate maternal preeclamptic symptoms, no available clinical therapies to date are able to prevent and mitigate the fetal consequences of this disease. Of particular interest for preeclampsia therapeutics are mesenchymal stromal cells (MSCs), which are a well-characterized treatment modality for a variety of disease processes, particularly those with immunologic underpinnings (11, 12). The therapeutic capacity of MSCs is most potently contained among their secreted extracellular vesicles, or MEx, which range from 30 to 150 nm in size and contain a mix of mRNAs, microRNAs, cytokines, and immunomodulatory proteins (13–15). Using a model of experimental preeclampsia involving the Hmox1−/− (heme oxygenase–null) mouse (8), we have recently shown that systemic antenatal MEx therapy can prevent core features of maternal preeclamptic physiology and fetal growth restriction (16).
As severe immune-mediated preeclamptic disease is the most likely to impact the developing fetus, we next hypothesized that preeclampsia causes significant lung developmental alteration, which can be prevented through antenatal systemic MEx therapy. We thus employed the Hmox1−/− model of experimental preeclampsia in this new study, extending our analysis beyond pregnancy to examine the influence of preeclamptic physiology on the neonatal lung and test the therapeutic capacity of MEx to ameliorate potential alterations in lung development secondary to preeclampsia.
Methods
Timed Pregnancies and MEx Treatment and Lung Tissue and Amniotic Fluid Collection and Analysis
An extended description of the Hmox1−/− pregnancy model of experimental preeclampsia and intravenous MEx treatment used for the current study are described in full detail in our previous publication (16). Full details of lung and amniotic fluid collection and analysis are included in the data supplement. All murine studies were approved by the Boston Children’s Hospital Animal Care and Use Committee.
MEx Isolation and Characterization
Human umbilical cord–derived MSCs and MEx were isolated using previously established protocols from our group (14). Briefly, MEx (i.e., extracellular vesicles sizing 30–150 nm in diameter) were isolated from cell culture supernatants (conditioned media) after a 36-hour serum-free incubation period. After serial centrifugation (which clarified cellular and apoptotic debris), conditioned media was concentrated through Tangential Flow Filtration, and MEx was isolated from the retentate via density flotation on a cushion of OptiPrep (iodixanol; Sigma). Intravenous MEx doses used for the current study were obtained from the exact same MSC-MEx preparations that were fully characterized in our recently published study on MEx therapy in the Hmox1−/− model of preeclampsia (16). Intravenous bolus doses (5 × 106 cell equivalents) were given via tail vein injection at three time points throughout pregnancy (Figure 1). The MEx dose of 5 × 106 cell equivalents has been previously established in our laboratory as capable of preventing preeclamptic physiology in the Hmox1−/− model system as well as having therapeutic effects in adult models of pulmonary fibrosis and pulmonary hypertension (13, 17). Additional details on MSC and MEx isolation and characterization can also be found in the data supplement.
Figure 1.
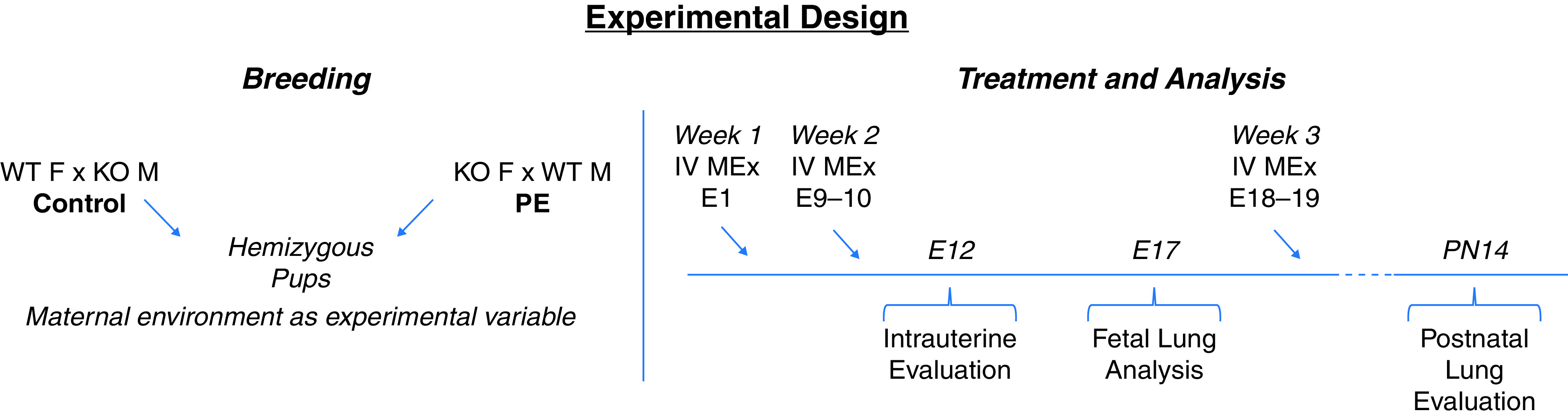
Experimental design. A schematic of hemizygous breeding strategies and serial MEx treatment in the Hmox1−/− model system of experimental preeclampsia is shown. E = Embryonic Day; F = female; IV = intravenous; KO = Knockout; Hmox1−/− = heme oxygenase-1–null mouse; M = male; MEx = mesenchymal stromal cell derived extracellular vesicles; PE = preeclampsia; PN = Postnatal Day; WT = wild-type.
Statistical Analysis
Statistical analysis of pup weights, histological and morphological quantifications of lung tissues and lung explants, and RT-PCR relative expression values were performed using GraphPad Prism software (GraphPad version 8.0; GraphPad Software Inc.). One-way ANOVA followed by a Tukey’s post hoc test for multiple comparisons was used in the statistical evaluation between all experimental groups. Significance was considered at P < 0.05. Investigators were blinded to experimental groups for all histological analyses. Statistical analysis of amniotic fluid proteomic data is described in full detail in the data supplement.
Results
Preeclampsia-associated Alterations in Lung Development Are Ameliorated by Antenatal MEx Treatment
Using a combination of wild-type (WT) and Hmox1−/− matings, hemizygous, genotypically equivalent pups were generated, for which the main experimental difference was the maternal environment (Figure 1). This allowed us to first evaluate the influence of a preeclamptic maternal environment on fetal lung development and also whether the alterations we observed with antenatal MEx treatment in preeclampsia had any downstream effects on the fetal lung. In this series of experiments, a total of three doses of MEx were administered, one during each week of gestation (Figure 1). As a control treatment, human dermal fibroblast-derived extracellular vesicles were also delivered at the same time points throughout gestation.
Consistent with our previous study (16), significant preeclamptic features, including placental spiral artery modification and fetal growth restriction, were noted at Embryonic Day (E) 12 among all pregnancies in which the mother was Hmox1−/−, all of which were ameliorated by antenatal MEx therapy (Figure E1 in the data supplement). Biodistribution studies also demonstrated that intravenously injected, labeled MSC-derived extracellular vesicles trafficked to uterine cells of Hmox1−/− females at multiple time points in pregnancy (Figure E2). These combined data established the intrauterine presence and pregnancy-specific effects of MEx in this model system.
We next evaluated fetal lungs with a targeted panel of established lung developmental genes at E17 (Figure 2). At this stage of gestation, the fetal lungs are in the canicular stage, which is a highly active phase of concomitant alveolar formation, airway branching, and vascularization (18). Following harvest, fetal lung tissue was processed for quantitative PCR analysis and evaluated for expression changes in Nkx 2–1 (NK2 homeobox 1), Fgf-10 (fibroblast growth factor–10), and eNOS (endothelial nitric oxide synthase) (Nos3) transcripts, which are involved in canonical pathways of alveolarization, branching morphogenesis, and pulmonary vascular development, respectively (18, 19). All three genes showed significant changes in lungs resulting from preeclamptic pregnancies, which were subsequently normalized when dams were treated with antenatal MEx. Importantly, MEx administered to control pregnancies did not alter gene expression of these targets in comparison to untreated control pregnancies, and human dermal fibroblast-derived extracellular vesicle administration had similar expression to untreated preeclamptic pregnancies (Figure 2).
Figure 2.
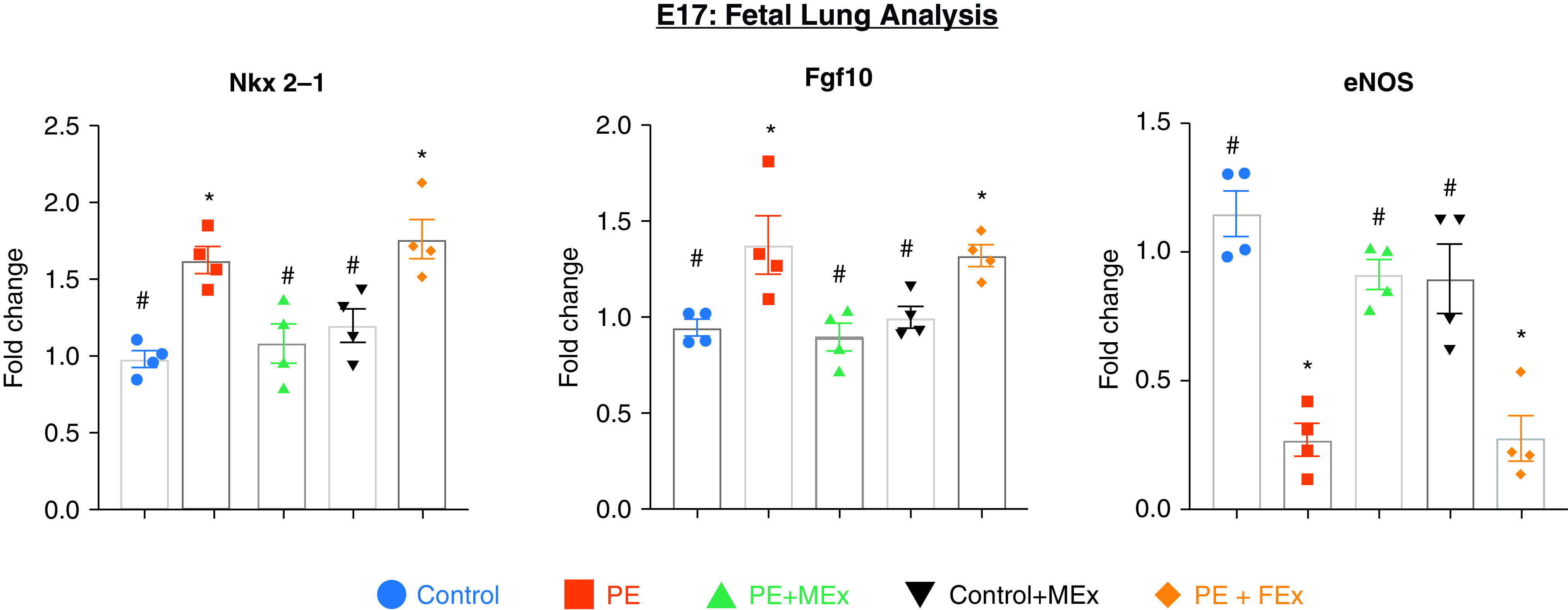
Antenatal MEx treatment normalizes preeclampsia-mediated alterations in lung developmental genes at E17. A quantitative PCR analysis of Nkx 2–1, Fgf10, and eNOS from E17 fetal lung tissue is shown. Data are representative of four independent experiments (n = 3–4 pregnant dams per experiment). *#Statistically significant (P < 0.05) changes between experimental groups as determined by one-way ANOVA are denoted by different symbols. Control + MEx = control pregnancies receiving MEx treatment; eNOS = endothelial nitric oxide synthase; PE + FEx = preeclamptic pregnancies receiving fibroblast derived extracellular vesicle treatment; PE + MEx = preeclamptic pregnancies receiving MEx treatment.
We next evaluated whether these molecular changes observed during fetal development resulted in postnatal changes in lung morphology. After birth, pups were maintained under routine (normoxic) conditions, and equal litter sizes were maintained between all experimental groups, averaging 7–8 pups per litter. Pup weight and lung tissue were evaluated at Postnatal Day 14 (PN14), a standard postnatal time point at which lung alveolarization and airway epithelium can be histologically quantified (Figures 3A–3D) (15). Comparisons of pup weight at PN14 reflected our findings of fetal growth at mid-pregnancy, with smaller pups resulting from the preeclamptic maternal environment, which was reversed after maternal MEx treatment (Figure 3B). Upon evaluating lung morphology, we further observed that pups resulting from the preeclamptic maternal environment had disrupted alveolar formation (Figure 3A). Quantification of alveolarization using mean linear intercept (MLI) analysis showed significant increases in MLI in pups from knockout mothers compared with pups from WT mothers (Figure 3C), indicating alveolar simplification and disrupted lung development under normoxic conditions. Interestingly, pups born from MEx-treated preeclamptic mothers had a restoration of alveolarization and MLI values similar to that of pups from control pregnancies (Figures 3A and 3C). Additionally, bronchial epithelium, as assessed by CCSP (clara cell secretory protein) staining, showed significant changes in morphology in preeclamptic pregnancies, which was normalized after MEx treatment (Figures 3A and 3D). Taken together, these molecular and histological data at multiple time points demonstrate that the maternal preeclamptic environment is associated with altered fetal lung development, which can be ameliorated by antenatal MEx therapy.
Figure 3.
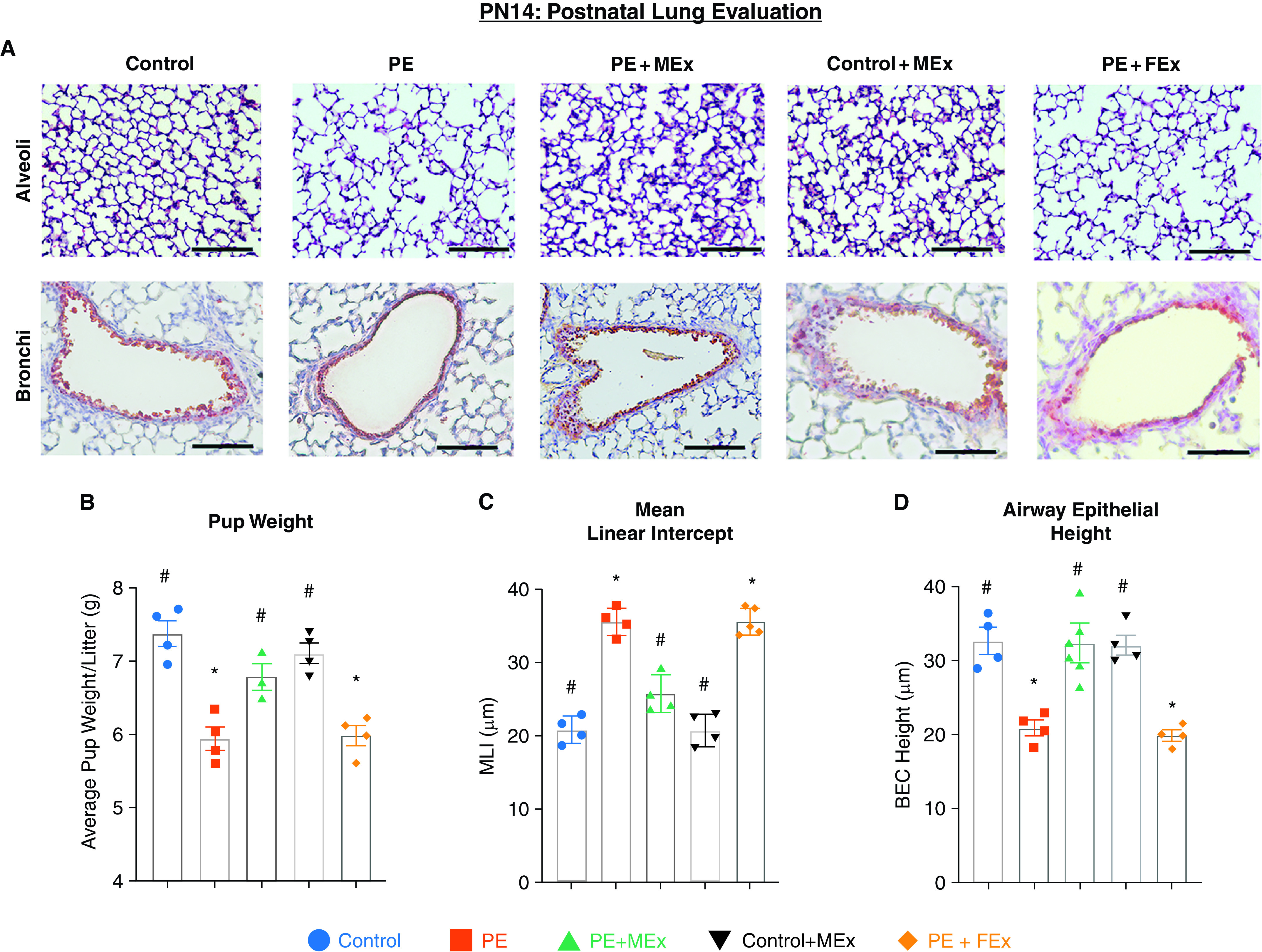
Preeclampsia-associated disruptions in postnatal alveolar and bronchial epithelial morphology are ameliorated by antenatal MEx therapy. (A) Representative hematoxylin and eosin images of alveoli and bronchi from PN14 lung histology showing disrupted alveolarization and abnormal airway epithelium. Scale bars, 60 µm. (B) Pup weight analysis was determined at PN14. (C) Mean linear intercept analysis quantifying lung alveolarization. (D) Measurement of airway epithelial height was assayed by clara cell secretory protein (CCSP) staining in lung tissue sections. *#Statistically significant (P < 0.05) changes between experimental groups as determined by one-way ANOVA are denoted by different symbols. Data are representative of four independent experiments (n = 3–4 litters per experiment). BEC = bronchial epithelial cell; MLI = mean linear intercept.
Lung-Adverse Secretome of Preeclamptic Amniotic Fluid Is Ameliorated by Antenatal MEx Treatment
Based on the changes we observed in lung development related to MEx modulation of preeclamptic physiology, we next sought to identify a particular aspect of the intrauterine environment conferring these effects. Among the varied biological components within the maternal–fetal interface, amniotic fluid has the most consistent, direct contact with fetal lungs throughout development. In early pregnancy, amniotic fluid is produced as a filtrate of maternal plasma, passing through the fetal membranes by osmotic and hydrostatic forces. Amniotic fluid also contains the secreted products (primarily cytokines) from the surrounding uteroplacental leukocyte populations throughout gestation. Later in pregnancy, amniotic fluid contains increasing amounts of fetal components, namely, fetal urine and fetal lung secretions with more minor contributions from fetal oral–nasal secretions (20). Increased inflammatory and antiangiogenic factors have been identified within human preeclamptic amniotic fluid (21, 22), and preclinical studies have also demonstrated that intraamniotic exposure of LPS or antiangiogenic soluble sFLT-1 (fms-like tyrosine kinase–1) within the amniotic compartment are associated with significant changes within the developing lung (23).
For analysis of amniotic fluid within our preeclamptic model system, amniotic fluid was collected from E12 and subsequently subjected to comparative analysis among experimental groups using both a Luminex multiplex assay as well as broad-spectrum proteomics (Figure 4). Within our Luminex assay, we evaluated a targeted panel of cytokines that have been associated with both increased risk of BPD and preeclampsia, including IL-10, IFN-γ, IL-6, and TNF-α (24–29). Of these analytes, IL-6 showed a significant increase in preeclamptic amniotic fluid, which was significantly decreased in those pregnancies receiving antenatal MEx treatment (Figure 4A). IL-10 did not show a significant difference between experimental groups, and IFN-γ as well as TNF-α were below the detectable limits of the assay (data not shown).
Figure 4.
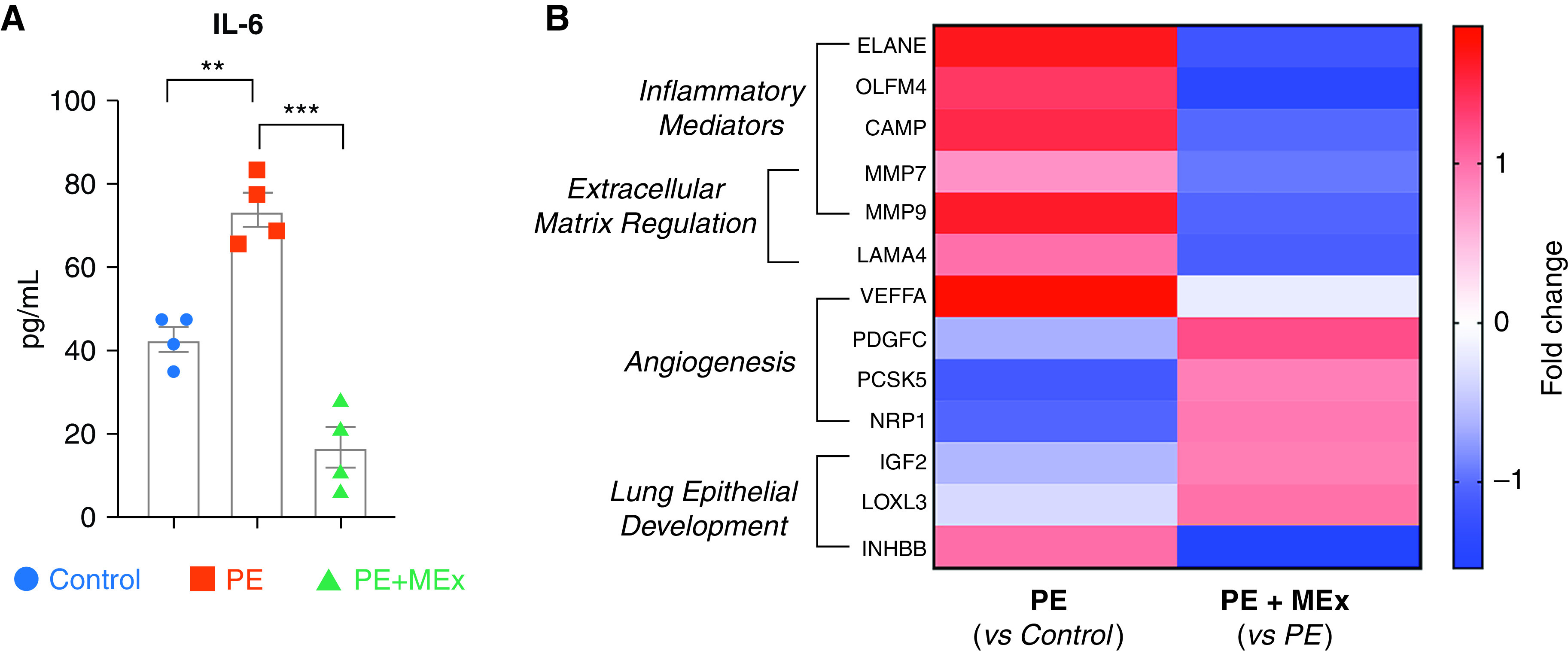
Antenatal maternal MEx therapy significantly alters preeclamptic amniotic fluid (AF) cytokine and proteomic profiles. An analysis of AF collected from control, PE, or PE + MEx pregnancies at E12 is shown. Data are representative of 3–4 independent experiments with AF pooled from 3–4 pregnant dams per experiment. (A) Quantification of IL-6 protein levels by luminex cytokine analysis. **P < 0.01 and ***P < 0.001. (B) Heatmap of fold changes from pairwise comparisons of Control versus PE or PE versus PE + MEx amniotic proteomic profiles. Targets represent protein changes with a false discovery rate–adjusted P value of <0.05 in addition to Ingenuity Pathway Analysis z-scores of >2 for targets relevant to intrauterine physiology and lung development.
We next conducted proteomic analysis of amniotic fluid samples, which identified a total of 3,512 proteins present in control, preeclamptic, and preeclamptic + MEx conditions (Table E1). Of these, 130 proteins were found to have significant fold changes among all experimental groups based on a false discovery rate–adjusted P value of <0.05 and an absolute fold change value >2. These were then evaluated using Ingenuity Pathway Analysis to explore the enrichment of pathways related to intrauterine biology and lung development. Of these, a final panel of 13 targets were identified that had significant fold changes among pairwise comparisons of control versus preeclampsia and preeclampsia versus preeclampsia + MEx pregnancies (Figure 4B, Table E2). Ingenuity Pathway Analysis first identified common targets among inflammatory mediators and extracellular matrix regulation that were significantly upregulated in preeclamptic pregnancies. Targets related to angiogenesis and lung epithelial development overall appeared to be downregulated in preeclampsia, with the exception of vascular endothelial growth factor A and inhibin BB, which were both upregulated in preeclamptic conditions (Figure 4B). Interestingly, antenatal MEx treatment significantly shifted the preeclamptic amniotic fluid proteomic profile within multiple pathways, demonstrating an overall downregulation of proinflammatory and extracellular matrix regulators and restoration of angiogenic and lung development pathways.
Antenatal MEx Treatment Abolishes the Detrimental Impact of Preeclamptic Amniotic Fluid on Fetal Lung Development
Noting the multifaceted amniotic fluid protein alterations that were altered in preeclampsia and subsequently with MEx treatment, we next sought to explore the collective effects of this fluid on the developing lung by employing an in vitro model system of fetal lung explants exposed to various types of amniotic fluid. Fetal lung explants are a previously established model system to evaluate the influence of exogenous exposures on the developing lung, particularly inflammatory mediators (30). In our system, WT lung explants (E15) were exposed to amniotic fluid from control, preeclamptic, or MEx-treated preeclamptic pregnancies (Figure 5A) for a period of 48 hours. Amniotic fluid for these experiments was collected at E12, a targeted time point in which the fluid still has a significant component of maternal and secreted uteroplacental contents and a timeframe in which we observed a peak of preeclamptic symptoms in our previous analysis of the Hmox1−/− model system (16, 20). Media only and media + MEx control conditions were also included in the analysis. At the end of the 48-hour incubation period with amniotic fluid, explants were imaged via brightfield microscopy (Figure 5) followed by harvest for quantitative PCR analysis of lung developmental genes evaluated in E17 lungs as well as those identified related to lung epithelial development from amniotic fluid proteomic analysis (Figure 6).
Figure 5.
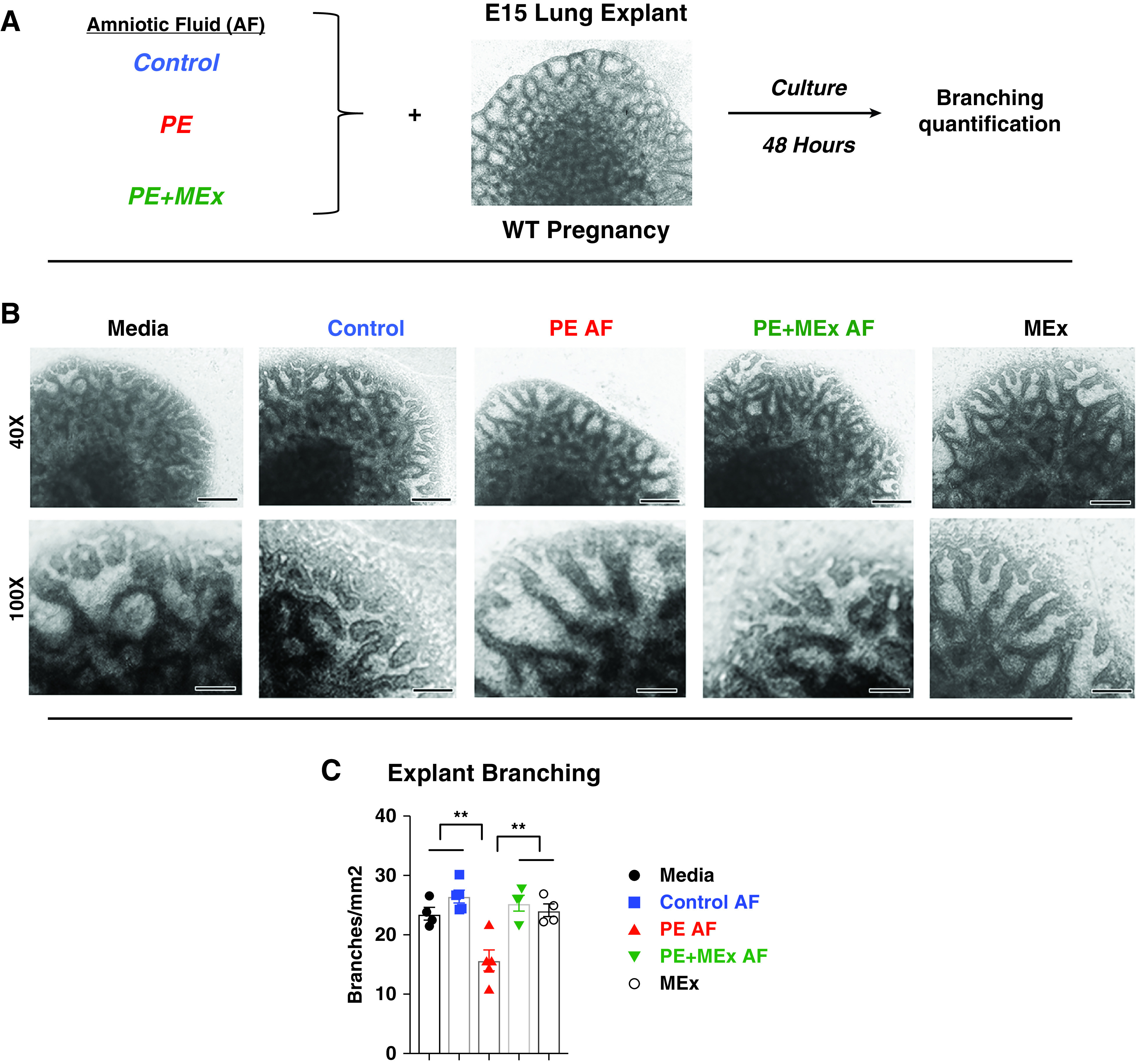
AF confers therapeutic effects of antenatal MEx treatment to improve fetal lung branching morphology in preeclamptic pregnancies. (A) Experimental design. (B) Representative images of lung explants. Scale bars, 150 µm and 60 µm. (C) Quantification of average new branches per squared millimeter at the end of a 48-hour culture period. Data are representative of four independent experiments, with lung explants harvested from 3–4 pregnant dams per experiment plated into 4–5 explants per condition in each experiment. **P < 0.01. Control amniotic fluid (AF) = explants cultured with AF from control pregnancies; Media = explants cultured with media only; MEx = explants cultured with MEx alone (no AF); PE AF = explants cultured with PE AF; PE + MEx AF = explants cultured with AF from preeclamptic pregnancies treated with MEx therapy.
Figure 6.
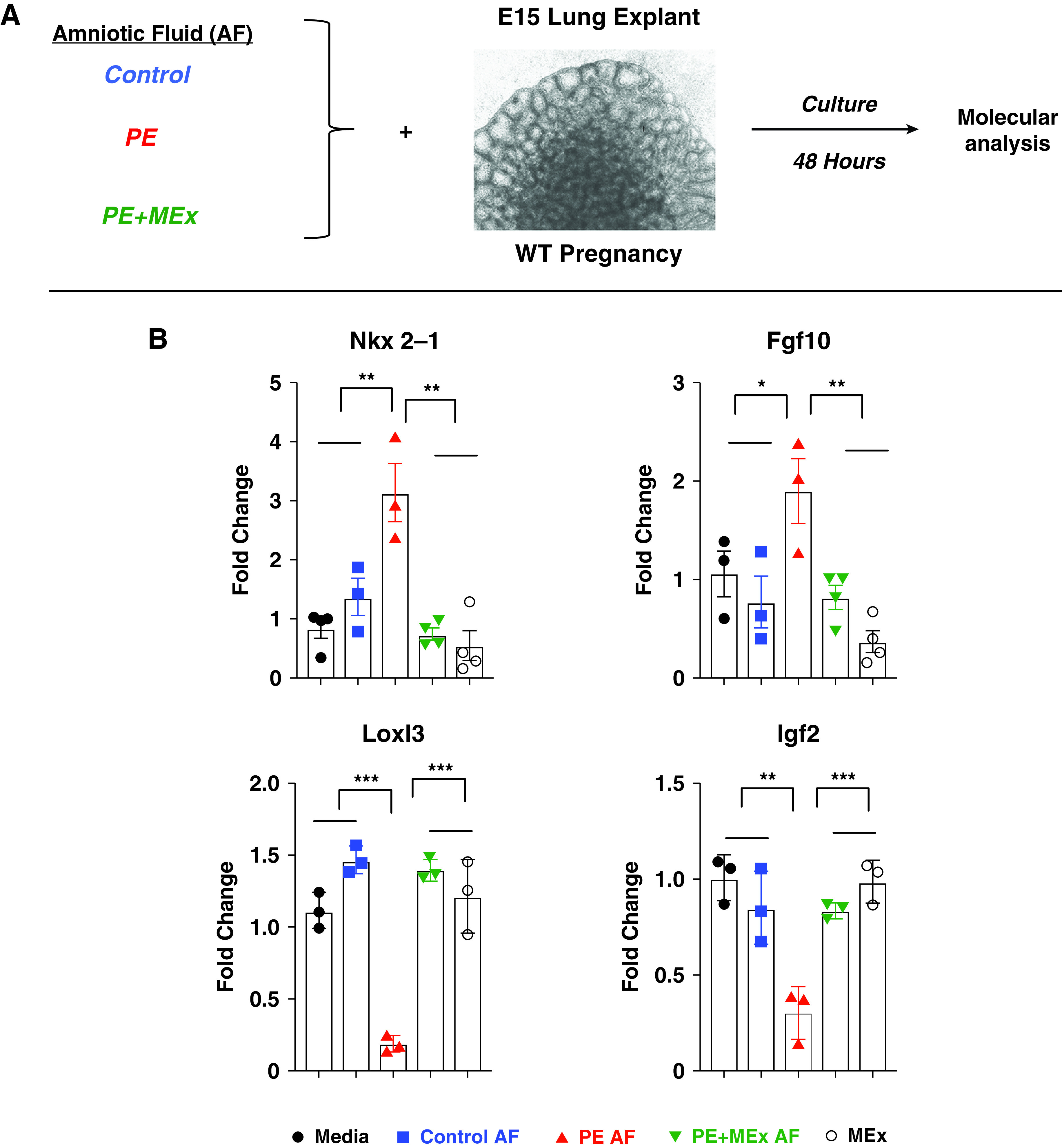
AF from MEx-treated preeclamptic pregnancies normalizes fetal lung explant developmental gene profiles. (A) Experimental design. (B) Quantitative PCR analysis showing the expression of lung developmental genes. Data are representative of four independent experiments, with lung explants harvested from 3–4 pregnant dams per experiment plated into 4–5 explants per condition in each experiment. *P < 0.05, **P < 0.01, and ***P < 0.001. Labels are as described for Figure 5.
Explants exposed to media and control amniotic fluid conditions had similar branching values, which were comparable to those previously reported for explants cultured under baseline conditions (Figures 5B and 5C) (31). Exposure to preeclamptic amniotic fluid was associated with a significant decrease in the number of new branches, suggesting diminished airway morphogenesis during the culture period (Figures 5B and 5C). Proximal regions of the developing airways also appeared more dilated, consistent with decreased branching. In contrast, exposure to amniotic fluid from MEx-treated preeclamptic pregnancies resulted in branching morphogenesis levels similar to those of controls. Finally, direct MEx exposure on the WT explants did not cause any significant changes in branching as compared with media conditions. Thus, in this ex vivo model system, we could not detect a direct effect of MEx on WT explant morphology, buttressing the hypothesis that the antenatal MEx effect is a systemic process.
Molecular (quantitative PCR) analysis of explant mRNA after 48 hours of culture revealed significant upregulation in both Nkx2.1 and Fgf-10 after exposure to preeclamptic amniotic fluid, which was reversed in explants exposed to amniotic fluid from MEx-treated preeclamptic pregnancies (Figure 6B). An analysis of lung epithelial development targets from amniotic proteomic analysis showed that Igf2 and Loxl3 were significantly downregulated in explants after exposure to preeclamptic amniotic fluid (Figure 6B). Inhbb transcripts were not detected within explants (data not shown). Culture with preeclamptic MEx-treated amniotic fluid normalized expression of these genes to those of control amniotic fluid conditions.
In a final series of experiments, we evaluated Hmox1−/− (knockout) explants at E15 using the same model system and noted that, unlike WT explants, these lungs were abnormal at baseline and manifested decreased and dilated branching. Interestingly, there was no change in branching or developmental gene expression after amniotic fluid or MEx treatment in this ex vivo environment (Figure E3). Taken together, these in vitro experiments suggest that lung development is directly impacted by amniotic fluid contents, and modulation of the intrauterine milieu is a key interface through which antenatal MEx therapy in preeclampsia ameliorates fetal lung development.
Discussion
The results of this study add to the growing body of literature associating maternal preeclampsia, fetal growth restriction and poor neonatal respiratory outcomes, namely, increased risk of BPD. Through the study of the Hmox1−/− model of pregnancy, we have identified a link between an early-onset, immune-mediated preeclamptic intrauterine environment and alterations in fetal lung development that persist into postnatal life. Given the sum considerations of our data, we propose that MEx treatment has significant therapeutic potential as a preventative modality for both preeclampsia and preeclamptic developmental lung injury.
At multiple stages in fetal and postnatal life, we identified alterations in lung development tied to the preeclamptic maternal environment. Evaluation of E15 lung explant cultures showed that branching morphogenesis was blunted by exposure to preeclamptic amniotic fluid. As this culture period encompassed the formation of terminal saccules that will subsequently develop into alveolar ducts (18, 19), these explant changes likely reflect early signs of altered alveolarization that were subsequently noted in our PN14 postnatal lung histology. Histological changes in alveolarization at PN14 were further accompanied by significant molecular changes in Nkx 2–1, Fgf-10, and eNOS. Downregulation of eNOS in preeclampsia likely reflects the antiangiogenic contributions within this preeclamptic model system (32). This finding, in addition to significant alterations in angiogenesis signals within the amniotic fluid proteomic profile, strongly indicates that further evaluation of blood vessel growth and vasculogenic pathways will be a key area of future analysis for this model.
Nkx 2–1 and Fgf-10 upregulation in preeclamptic fetal lungs may be caused by a more complex physiology. As both genes are significantly involved in the development of lung epithelium (18), Nkx 2–1 and Fgf-10 expression could be a compensatory mechanism in response to altered fetal lung development. Alternatively, Nkx 2–1 and Fgf-10 upregulation could be a reflection of arrested lung development or altered epithelial–mesenchymal interactions that are key for lung morphogenesis (33, 34). Although these mechanisms require ongoing investigation, our findings do suggest overall that preeclamptic fetal growth restriction is a global developmental delay and not just a smaller size fetus.
The combined data from our amniotic fluid analysis and ex vivo explant experiments suggest that alterations in intraamniotic secretome may be a key interface through which preeclampsia-associated developmental lung injury is ameliorated by antenatal MEx therapy. This is supported by our findings that direct MEx treatment alone did not impact the WT fetal lung explants and the aberrant Hmox1−/− explants were not rescued by amniotic fluid or direct MEx exposure ex vivo. Thus, alteration of the uteroplacental interface is the main therapeutic benefit of MEx rather than a direct impact on the developing lung itself.
The preeclamptic amniotic fluid contents from our model system contained a significant inflammatory component that was abrogated after antenatal MEx therapy. This is likely a downstream result of MEx-mediated alterations in preeclamptic intrauterine immune cell activation, which we previously demonstrated in the Hmox1−/− preeclampsia model (16). This prior work identified a MEx-mediated shift toward an antiinflammatory phenotype, particularly among intrauterine macrophages, consistent with macrophage immunomodulation conferred by MEx treatment in multiple studies from our group (13, 15). As inflammatory mediators are known to cause similar disruptions in fetal lung branching morphogenesis (30), MEx-associated alterations of the intrauterine immune environment and, subsequently, the amniotic fluid inflammatory profile could thus be a key influence governing lung developmental changes in preeclamptic pregnancies. Inflammatory mediators could also be coming from the developing fetal immune system, particularly as endogenous fetal lung macrophage signaling has also been shown to alter lung development (35). As these cells could potentially be affected by antenatal MEx therapy, additional studies will be required to evaluate the relative impact of this treatment on the maternal and fetal contributions to the inflammatory components of preeclamptic amniotic fluid.
However, as our proteomic data also showed shifts in multiple physiologic pathways after MEx treatment, attenuation of inflammation could be one key alteration among many working in combination to ultimately improve the uteroplacental environment and prevent preeclamptic developmental lung injury. The additional amniotic fluid signals related to extracellular matrix regulation or angiogenesis may reflect MEx-induced placental structural changes and fetal organ contributions to the intraamniotic secretome. This is suggested by our findings of altered Loxl3 and Igf2 expression in fetal lung explants, which directly reflected changes noted from the preeclamptic and MEx-treated amniotic fluid proteomic analysis. As both of these genes participate in lung developmental pathways (36, 37), their expression changes in combination with those of Nkx 2–1 and Fgf-10 reflect the complex interplay of pathways governing the alterations of fetal lung development in the preeclamptic intrauterine environment (18).
The current study has several limitations. First, the preeclamptic physiology found in our Hmox1−/− model encompasses the early-onset, immune-mediated subtype of this disease. The pathogenesis of preeclampsia is multifactorial, and the severity and timing of onset is highly heterogenous. Thus, an additional investigation using other preclinical models of preeclampsia (38) is warranted to more fully evaluate how the full spectrum of preeclamptic disease impacts fetal lung development. In addition, lung explant models have several limitations. First, ex vivo explant cultures can only evaluate a snapshot of the complex cascade of mechanisms orchestrating fetal lung development and the role of a preeclamptic environment in this process. Also, explants cultured under 21% oxygen are exposed to relative hyperoxia compared with that of the intrauterine environment. Finally, explants are cultured in the absence of physiologic blood flow, which likely contains many additional key factors for the developing lung. Future investigations using additional lung developmental models to evaluate the hierarchy and interrelated mechanisms of these pathways will be highly informative to further elucidate the key drivers of the alveolar and airway changes associated with preeclampsia in our model system.
Finally, the specific mechanisms through which MEx ultimately ameliorate the amniotic fluid secretome in preeclampsia are likely multifaceted. Our biodistribution analysis found that intravenously administered MEx traffic to the uterus, and our previous studies identified MEx treatment to be associated with significant shifts in the abundance and phenotype of intrauterine myeloid populations (16). As MEx contain a variety of nucleic acid, lipid, and protein moieties, ongoing multiparameter analyses will be required to identify pregnancy-specific synergistic pathways through which MEx shift the secreted contents of uteroplacental leukocytes and resultant amniotic fluid contents toward prohomeostatic pathways beneficial for lung development (14, 16). In conclusion, this study provides key foundational evidence for the intrauterine origins of preeclamptic neonatal lung disease and highlights the highly innovative potential of antenatal MEx therapy to ameliorate the preeclamptic intrauterine environment for improvement of fetal lung health and development.
Acknowledgments
Acknowledgment
The authors thank Stephanie Byrum and Sam Mackintosh at the University of Arkansas Proteomics Core facility for their efforts in establishing novel acquisition and analysis protocols for amniotic fluid proteomics.
Footnotes
Supported by National Heart, Lung, and Blood Institute grant R01 HL146128 (S.K.), National Institute of Allergy and Infectious Diseases grant R21 AI134025 (S.K.), Charles H. Hood Foundation Major Grants Initiative to Advance Child Health (S.K.), United Therapeutics Research Grant (S.A.M. and S.K.), AAP Marshall Klaus Award (E.S.T.), National Institutes of Health T32HL007633 Integrated Training in Respiratory Research (E.S.T.), and National Institute of Child Health and Human Development T32HD098061 Neonatal Research Training Program (E.S.T.).
Author Contributions: E.S.T., A.F.-G., and G.R.W. participated in study design and execution, data collection, analysis, and manuscript writing. M.R. participated in study design and execution, analysis, and manuscript writing. V.Y. participated in mesenchymal stromal cell–derived extracellular vesicle preparation and characterization. X.L. participated in technical support. L.S.P. participated in supervision of lung explant study design and execution and final manuscript editing and approval. S.A.M. and S.K. contributed to study design, supervision of study execution, analysis, manuscript writing, and the editing and approval of the final article.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2021-0307OC on October 6, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet . 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 2. Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, et al. CHERG Small-for-Gestational-Age-Preterm Birth Working Group. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: analysis of CHERG datasets. BMJ . 2017;358:j3677. doi: 10.1136/bmj.j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lal MK, Manktelow BN, Draper ES, Field DJ. Population-based study. Chronic lung disease of prematurity and intrauterine growth retardation: a population-based study. Pediatrics . 2003;111:483–487. doi: 10.1542/peds.111.3.483. [DOI] [PubMed] [Google Scholar]
- 4. Soudée S, Vuillemin L, Alberti C, Mohamed D, Becquet O, Farnoux C, et al. Fetal growth restriction is worse than extreme prematurity for the developing lung. Neonatology . 2014;106:304–310. doi: 10.1159/000360842. [DOI] [PubMed] [Google Scholar]
- 5. Hansen AR, Barnés CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr . 2010;156:532–536. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 6. Tagliaferro T, Jain D, Vanbuskirk S, Bancalari E, Claure N. Maternal preeclampsia and respiratory outcomes in extremely premature infants. Pediatr Res . 2019;85:693–696. doi: 10.1038/s41390-019-0336-5. [DOI] [PubMed] [Google Scholar]
- 7. Laresgoiti-Servitje E, Gómez-López N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update . 2010;16:510–524. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 8. Ozen M, Zhao H, Lewis DB, Wong RJ, Stevenson DK. Heme oxygenase and the immune system in normal and pathological pregnancies. Front Pharmacol . 2015;6:84. doi: 10.3389/fphar.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stojanovska V, Scherjon SA, Plösch T. Preeclampsia as modulator of offspring health. Biol Reprod . 2016;94:53. doi: 10.1095/biolreprod.115.135780. [DOI] [PubMed] [Google Scholar]
- 10. Grimes S, Bombay K, Lanes A, Walker M, Corsi DJ. Potential biological therapies for severe preeclampsia: a systematic review and meta-analysis. BMC Pregnancy Childbirth . 2019;19:163. doi: 10.1186/s12884-019-2268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suvakov S, Richards C, Nikolic V, Simic T, McGrath K, Krasnodembskaya A, et al. Emerging therapeutic potential of mesenchymal stem/stromal cells in preeclampsia. Curr Hypertens Rep . 2020;22:37. doi: 10.1007/s11906-020-1034-8. [DOI] [PubMed] [Google Scholar]
- 12. Phinney DG, Pittenger MF. Concise review: Msc-derived exosomes for cell-free therapy. Stem Cells . 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 13. Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight . 2019;4:e128060. doi: 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willis GR, Kourembanas S, Mitsialis SA. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med . 2017;4:63. doi: 10.3389/fcvm.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med . 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taglauer ES, Fernandez-Gonzalez A, Willis GR, Reis M, Yeung V, Liu X, et al. Mesenchymal stromal cell-derived extracellular vesicle therapy prevents preeclamptic physiology through intrauterine immunomodulation. Biol Reprod . 2021;104:457–467. doi: 10.1093/biolre/ioaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation . 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development . 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol Rev . 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brace RA, Cheung CY. Regulation of amniotic fluid volume: evolving concepts. Adv Exp Med Biol . 2014;814:49–68. doi: 10.1007/978-1-4939-1031-1_5. [DOI] [PubMed] [Google Scholar]
- 21. Kupferminc MJ, Peaceman AM, Aderka D, Wallach D, Socol ML. Soluble tumor necrosis factor receptors and interleukin-6 levels in patients with severe preeclampsia. Obstet Gynecol . 1996;88:420–427. doi: 10.1016/0029-7844(96)00179-2. [DOI] [PubMed] [Google Scholar]
- 22. Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmäki E. Amniotic fluid—soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol . 2000;95:353–357. doi: 10.1016/s0029-7844(99)00565-7. [DOI] [PubMed] [Google Scholar]
- 23. Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol . 2012;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA. Interferon gamma in successful pregnancies. Biol Reprod . 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol . 2013;70:412–427. doi: 10.1111/aji.12138. [DOI] [PubMed] [Google Scholar]
- 26. LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep . 2007;9:480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 27. Köksal N, Kayik B, Çetinkaya M, Özkan H, Budak F, Kiliç Ş, et al. Value of serum and bronchoalveolar fluid lavage pro- and anti-inflammatory cytokine levels for predicting bronchopulmonary dysplasia in premature infants. Eur Cytokine Netw . 2012;23:29–35. doi: 10.1684/ecn.2012.0304. [DOI] [PubMed] [Google Scholar]
- 28. Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics . 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aghai ZH, Saslow JG, Mody K, Eydelman R, Bhat V, Stahl G, et al. IFN-γ and IP-10 in tracheal aspirates from premature infants: relationship with bronchopulmonary dysplasia. Pediatr Pulmonol . 2013;48:8–13. doi: 10.1002/ppul.22540. [DOI] [PubMed] [Google Scholar]
- 30. Stouch AN, McCoy AM, Greer RM, Lakhdari O, Yull FE, Blackwell TS, et al. Il-1beta and inflammasome activity link inflammation to abnormal fetal airway development. J Immunol . 2016;196:3411–3420. doi: 10.4049/jimmunol.1500906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prince LS. Fgf10 and human lung disease across the life spectrum. Front Genet . 2018;9:517. doi: 10.3389/fgene.2018.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res . 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax . 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 34. Itoh N. FGF10: A multifunctional mesenchymal-epithelial signaling growth factor in development, health, and disease. Cytokine Growth Factor Rev . 2016;28:63–69. doi: 10.1016/j.cytogfr.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 35. Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, et al. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol . 2011;187:2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Gao B, Xiao X, Li S, Jia X, Sun W, et al. Exome sequencing identified null mutations in LOXL3 associated with early-onset high myopia. Mol Vis . 2016;22:161–167. [PMC free article] [PubMed] [Google Scholar]
- 37. Silva D, Venihaki M, Guo WH, Lopez MF. Igf2 deficiency results in delayed lung development at the end of gestation. Endocrinology . 2006;147:5584–5591. doi: 10.1210/en.2006-0498. [DOI] [PubMed] [Google Scholar]
- 38. Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Nephrol . 2004;24:596–606. doi: 10.1016/s0270-9295(04)00131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


