This editorial relates to ‘Longitudinal strain is an independent predictor of survival and response to therapy in patients with systemic AL amyloidosis’, by O.C. Cohen et al., https://doi.org/10.1093/eurheartj/ehab507.
Over the last years, research on light-chain (AL) amyloidosis has focused on the search for variables able to detect and quantify cardiac and renal involvement, predict survival, and monitor disease progression and response to treatment, in terms of the plasma cell disorder and organ damage.1 Finding reliable indicators of disease status becomes increasingly important given the growing number of therapeutic options.
Graphical Abstract.
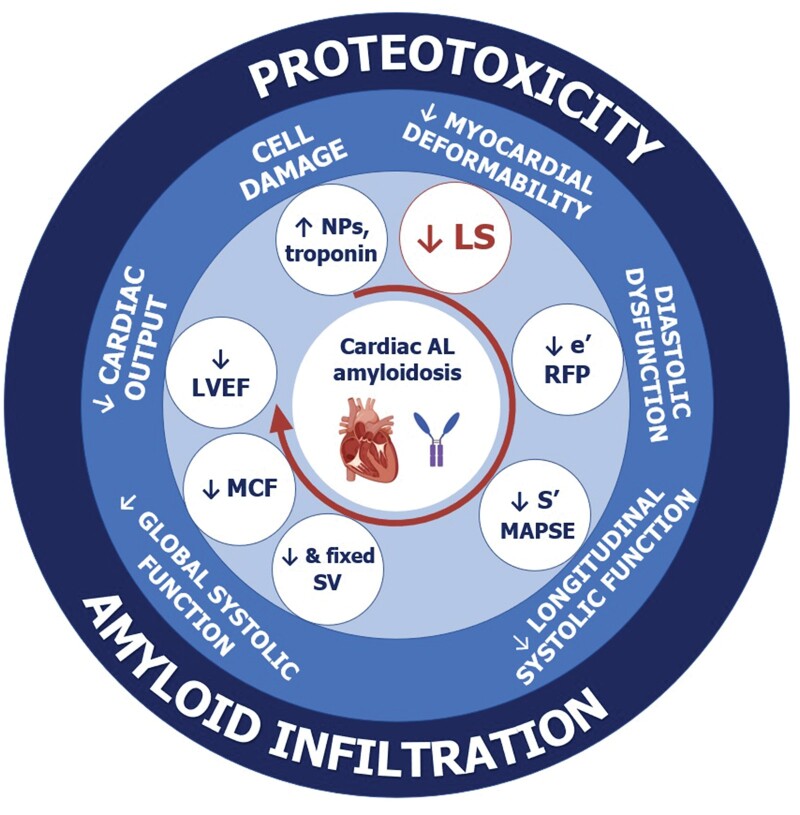
The place of longitudinal strain (LS) alterations in the natural history of amyloid light-chain (AL) amyloidosis. The three concentric circles report, from outside to inside, the mechanisms of cardiac damage, the main pathophysiological abnormalities, and the corresponding echocardiographic findings. The order of the pathophysiological abnormalities and echocardiographic findings broadly reflect the timing of their development. LS, longitudinal strain; LVEF, left ventricular ejection fraction; MAPSE, mitral anular plane systolic excursion; MCF, myocardial contraction fraction; NPs, natriuretic peptides; RFP, restrictive filling pattern; SV, stroke volume.
In this issue of the European Heart Journal, Cohen et al. report the results of a detailed characterization of echocardiographic and haematology findings in 915 patients with AL amyloidosis [69% of whom had cardiac amyloidosis (CA)].2 The authors report that longitudinal strain (LS) of the left ventricle provides important information about the whole natural history of AL amyloidosis. Indeed, LS values at baseline testify to the presence of cardiac involvement, which is an established predictor of outcome in patients with AL amyloidosis.3 Accordingly, LS also predicted survival independently of several variables from standard echocardiographic examination.2 Interestingly, patients with the most severe impairment in LS had a poor prognosis even regardless of the Mayo staging system (which includes biomarkers of cardiac damage) and other variables including left ventricular ejection fraction (LVEF).2 Changes in LS over time also reflect variations in cardiac function and, in treated patients, the response to therapy, with a possible added value over indicators reflecting the plasma cell disorder alone, such as the difference between involved and non-involved free light chains (dFLCs). Using this, the authors identify patients with an absolute improvement of LS ≥2% and a final dFLC <10 mg/L after 12 months of treatment as the subgroup with the best prognosis.2
Despite some methodological limitations acknowledged by the authors,2 the study is very stimulating and raises a number of questions.
Why has LS become so important to characterize cardiac involvement in patients with amyloidosis? What is the added value of LS beyond other echocardiographic indicators of cardiac function?
An increased myocardial stiffness is a hallmark of CA, leading to an impairment in diastolic and then systolic function and a restrictive phenotype.4 Several echocardiographic parameters allow exploration of LV kinesis, not exclusively along the longitudinal axis (as in the case of LS). These indicators include LVEF, myocardial tissue velocities by Doppler imaging, mitral annular plane systolic excursion, and even tricuspid annular plane systolic excursion (which investigates right ventricular function, which may also be also impaired).4 , 5 Three indicators particularly well suited to investigate LV function are the mitral flow patterns (which investigate diastolic function and in advanced cases demonstrate a restrictive LV filling), LV strain (which quantifies myocardial deformation and therefore systolic function), and LV stroke volume (which is a less sensitive measure of systolic dysfunction than LV strain).4 , 5 LV strain, assessed through 2D speckle-tracking echocardiography (STE), is particularly sensitive for detecting increased myocardial stiffness, which is a direct consequence of amyloid infiltration, and (particularly in AL-CA) also the toxic myocardial damage from circulating amyloid precursors.4 – 6 LV strain is able to accurately detect cardiac damage in patients with systemic AL amyloidosis, as demonstrated by its inclusion in a multiparametric diagnostic score for AL-CA.7 The close link with myocardial stiffness and high sensitivity fdor the detection of cardiac involvement represent the main strengths of STE. This technique also allows detection of apical sparing, i.e. the relative preservation of apical contractility of the left ventricle compared with basal segments, which is an almost pathognomonic finding and helps distinguish CA from other forms of cardiac hypertrophy.4–6 An indirect confirmation of the value of STE derives from the study by Cohen et al., where baseline LV strain yielded greater prognostic significance than other echocardiographic findings.2 The same observation had already been reported in at least two other studies.8 , 9 Conversely, LVEF was reported to be a poor parameter to diagnose AL-CA, being reduced only in late disease stages.10 LVEF was also a worse predictor of outcome than the myocardial contraction fraction (MCF), defined as the ratio between stroke volume and myocardial volume, reflecting the characteristic discrepancy between LV mass and contractility in CA.11 LV stroke volume has a similar ability to MCF and LS in predicting outcome in AL amyloidosis, also independently of cardiac biomarkers.11
When comparing indicators of LV function, it is important to remember that the performance of diagnostic and prognostic markers probably changes according to the disease stage. This has been well documented in patients with amyloid transthyretin (ATTR) CA.12 The same is likely to apply to AL-CA, although it is not necessarily the case since in AL-CA there is also a direct cardiotoxic effect of amyloid precursors. In one study on patients in advanced disease stages, stroke volume was superior even to strain.9 This is not surprising if we consider that a fixed stroke volume indicates an advanced restrictive cardiomyopathy, with cardiac output relying mostly on heart rate.4 , 5 Overall, the characterization of LV strain in AL-CA cannot ignore the assessment of its diagnostic and prognostic value during the different stages of the natural history of this condition.
Do we really need an echocardiographic predictor of outcome in a disease where circulating biomarkers are able to effectively stratify patient outcome and monitor the response to chemotherapy?
The goals of treatment in AL amyloidosis are to block the production of light chains and to block (and then reverse) organ damage. There are several moments when the need for an accurate patient profiling is particularly important to guide treatment decisions: the search for cardiac involvement and risk prediction at baseline; the decision as to whether a patient can undergo autologous bone marrow transplantation; the quantification of cardiac damage and its evolution over time; and the assessment of cardiac and haematological response to treatment. Biomarkers of cardiac damage, most commonly N-terminal probrain natriuretic peptide (NT-proBNP) and high-sensitivity (hs) troponin T, together with free light chains may help clinicians to navigate through these steps, and are regarded with interest in clinical trials as early indicators of response to treatment and as surrogate endpoints.
The results of Cohen et al. do not allow the proposal of a change to established decision-making criteria, but they do show the perspective to optimize these criteria. The proposed combined staging system may represent the basis for future larger international collaborative studies. Notably, LS yielded added prognostic value on top of the Mayo staging system, and it is the first time that an indicator of cardiac function adds prognostic significance over a very high baseline NT-proBNP (>8500 ng/L).2 A very interesting finding is the possibility to combine LS with the complete haematological response, identifying a subgroup with a better survival at both 12 and 24 months.2
Can LV strain already be implemented in the clinical practice of patients with AL-CA?
This study identifies a promising potential application of LV strain to the management of patients with AL-CA. Nonetheless, these findings require robust confirmation before being universally adopted in clinical practice. LV strain analysis has several drawbacks that are correctly acknowledged by the authors:2 differences in LV strain calculation between different vendors, time-consuming image optimization and post-processing, and different metrics (e.g. global LS vs. LS from four-chamber views) and methods of calculation (such as a 16- or 6-segment analysis, as in the study of Cohen et al.2 We may add the still limited application of STE in current clinical practice. All these factors may limit the large-scale applicability of a staging system incorporating LV strain. Furthermore, the absolute change in LS associated with better survival and the optimal timing for assessment should be reappraised in large cohorts. An early indicator of treatment response and then outcome would be important in a serious condition such as AL-CA, which also has several possible lines of treatment. On the other hand, LS improvement in responders is expected to be much slower than cardiac biomarkers (NT-proBNP but also possibly hs-troponin T), reflecting the long time needed for the reabsorption of amyloid deposits, as compared with the rapid relief from cardiac damage. The different kinetics of the two processes might justify the combined assessment of NT-proBNP or hs-troponin T as indicators of early response, and LS as a late indicator.
In conclusion, the growing body of evidence about LS in CA is transforming a simple echocardiographic functional parameter into a multipurpose biomarker, useful for diagnosis, risk stratification, and decision-making in patients with AL-CA (Table 1; Graphical Abstract). The study by Cohen et al. represents a relevant step during this journey.
Table 1.
Longitudinal strain (LS) and N-terminal probrain natriuretic peptide (NT-proBNP) for the management of amyloid light-chain (AL) amyloidosis
| LS | NT-proBNP | Potential for combination | |
|---|---|---|---|
| Intra-/interobserver variability | Low (but need for local expertise) | High | NA |
| Recognition of cardiac involvement | ++ | ++ | +++ |
| Differential diagnosis with other cardiomyopathies | + | ± | TBA |
| Risk stratification at baseline | ++ | +++ | +++ |
| Monitoring disease progression | + | ++ | +++ |
| Monitoring initial response to treatment (3 and 6 months) | + | +++ | + |
| Monitoring final response to treatment (12 months) | ++ | ++ | +++ |
| Monitoring chemotherapy for cardiotoxicity | ++ | + | +++ |
| Endpoint for clinical trials | TBA | ++ | +++ |
The importance LS and NT-proBNP was graded by the authors of this Editorial from ‘TBA’ (to be adjudicated) to +++ (very important). High-level evidence is reported in bold.
Conflict of interest: none declared.
Contributor Information
Claudio Rapezzi, Cardiologic Centre, University of Ferrara, Italy; Maria Cecilia Hospital, GVM Care & Research, Cotignola (Ravenna), Italy.
Alberto Aimo, Institute of Life Sciences, Scuola Superiore Sant’Anna, Pisa, Italy; Cardiology Division, Fondazione Toscana Gabriele Monasterio, Pisa, Italy.
Rita Pavasini, Cardiologic Centre, University of Ferrara, Italy.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Law S, Fontana M, Gillmore JD. Advances in diagnosis and treatment of cardiac and renal amyloidosis. Cardiol Clin 2021;39:389–402. [DOI] [PubMed] [Google Scholar]
- 2. Cohen OC, Ismael A, Pawarova B, Manwani R, Ravichandran S, Law S, Foard D, Petrie A, Ward S, Douglas B, Martinez-Naharro A, Chacko L, Quarta CC, Mahmood S, Sachchithanantham S, Lachmann HJ, Hawkins PN, Gillmore JD, Fontana M, Falk RH, Whelan CJ, Wechalekar AD. Longitudinal strain is an independent predictor of survival and response in patients with systemic AL amyloidosis. Eur Heart J 2021;43:333–341. [DOI] [PubMed] [Google Scholar]
- 3. Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM, Kurland LT. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992;79:1817–1822. [PubMed] [Google Scholar]
- 4. Falk RH, Quarta CC. Echocardiography in cardiac amyloidosis. Heart Fail Rev 2015;20:125–131. [DOI] [PubMed] [Google Scholar]
- 5. Siddiqi OK, Sanchorawala V, Ruberg FL. Echocardiography and survival in light chain cardiac amyloidosis: back to basics. Circ Cardiovasc Imaging 2018;11:e007826. [DOI] [PubMed] [Google Scholar]
- 6. Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 2016;68:1323–1341. [DOI] [PubMed] [Google Scholar]
- 7. Boldrini M, Cappelli F, Chacko L, Restrepo-Cordoba MA, Lopez-Sainz A, Giannoni A, Aimo A, Baggiano A, Martinez-Naharro A, Whelan C, Quarta C, Passino C, Castiglione V, Chubuchnyi V, Spini V, Taddei C, Vergaro G, Petrie A, Ruiz-Guerrero L, Moñivas V, Mingo-Santos S, Mirelis JG, Dominguez F, Gonzalez-Lopez E, Perlini S, Pontone G, Gillmore J, Hawkins PN, Garcia-Pavia P, Emdin M, Fontana M. Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 2020;13:909–920. [DOI] [PubMed] [Google Scholar]
- 8. Milani P, Dispenzieri A, Scott CG, Gertz MA, Perlini S, Mussinelli R, Lacy MQ, Buadi FK, Kumar S, Maurer MS, Merlini G, Hayman SR, Leung N, Dingli D, Klarich KW, Lust JA, Lin Y, Kapoor P, Go RS, Pellikka PA, Hwa YL, Zeldenrust SR, Kyle RA, Rajkumar SV, Grogan M. Independent prognostic value of stroke volume index in patients with immunoglobulin light chain amyloidosis. Circ Cardiovasc Imaging 2018;11:e006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee Chuy K, Drill E, Yang JC, Landau H, Hassoun H, Nahhas O, Chen CL, Yu AF, Steingart RM, Liu JE. Incremental value of global longitudinal strain for predicting survival in patients with advanced AL amyloidosis. JACC CardioOncol 2020;2:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kristen AV, Perz JB, Schonland SO, Hegenbart U, Schnabel PA, Kristen JH, Goldschmidt H, Katus HA, Dengler TJ. Non-invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail 2007;9:617–624. [DOI] [PubMed] [Google Scholar]
- 11. Tendler A, Helmke S, Teruya S, Alvarez J, Maurer MS. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid 2015;22:61–66. [DOI] [PubMed] [Google Scholar]
- 12. Knight DS, Zumbo G, Barcella W, Steeden JA, Muthurangu V, Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Kotecha T, Francis R, Rezk T, Quarta CC, Whelan CJ, Lachmann HJ, Wechalekar AD, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Cardiac structural and functional consequences of amyloid deposition by cardiac magnetic resonance and echocardiography and their prognostic roles. JACC Cardiovasc Imaging 2019;12:823–833. [DOI] [PubMed] [Google Scholar]