Abstract
The discipline of Cardio-Oncology has seen tremendous growth over the past decade. It is devoted to the cardiovascular (CV) care of the cancer patient, especially to the mitigation and management of CV complications or toxicities of cancer therapies, which can have profound implications on prognosis. To that effect, many studies have assessed CV toxicities in patients undergoing various types of cancer therapies; however, direct comparisons have proven difficult due to lack of uniformity in CV toxicity endpoints. Similarly, in clinical practice, there can be substantial differences in the understanding of what constitutes CV toxicity, which can lead to significant variation in patient management and outcomes. This document addresses these issues and provides consensus definitions for the most commonly reported CV toxicities, including cardiomyopathy/heart failure and myocarditis, vascular toxicity, and hypertension, as well as arrhythmias and QTc prolongation. The current document reflects a harmonizing review of the current landscape in CV toxicities and the definitions used to define these. This consensus effort aims to provide a structure for definitions of CV toxicity in the clinic and for future research. It will be important to link the definitions outlined herein to outcomes in clinical practice and CV endpoints in clinical trials. It should facilitate communication across various disciplines to improve clinical outcomes for cancer patients with CV diseases.
Keywords: Cardiomyopathy, Cardio-oncology, Cardiotoxicity, Hypertension, Myocarditis, Vascular disease, QTc prolongation
Graphical Abstract
Introduction
As advancements in cancer therapy have led to improvement in survival, there has been increasing recognition of the short- and long-term complications of cancer therapies that affect morbidity and mortality, including cardiovascular (CV) toxicities.1 , 2 The discipline of Cardio-Oncology (CO) has emerged, in particular, to prevent, mitigate, and manage CV diseases and complications in cancer patients in addition to providing assistance in balancing the risks and benefits of cancer therapy.3 , 4 A critical element of such efforts, important for both clinical practice and research endeavours, is a uniform understanding and agreement regarding what constitutes a CV toxicity.
Cardiovascular toxicities of cancer therapies encompass a broad spectrum of entities; however, this document will focus on the categories most commonly reported in the literature and illustrated in Figure 1.3 Furthermore, it is outside the scope of this document to provide specific management recommendations for CV toxicities. The intent of this document is to provide clinically meaningful definitions of commonly encountered CV adverse events during contemporary cancer therapy (Graphical Abstract). It is to facilitate cross-disciplinary communication to allow effective clinical description of CV events and enhance the clinical research that is ongoing in CO (thereby universal). By incorporation of these standards into routine clinical practice and research, direct comparisons of clinically relevant events in various subpopulations of patients will be strengthened to allow advances in evidence-based CO practice.
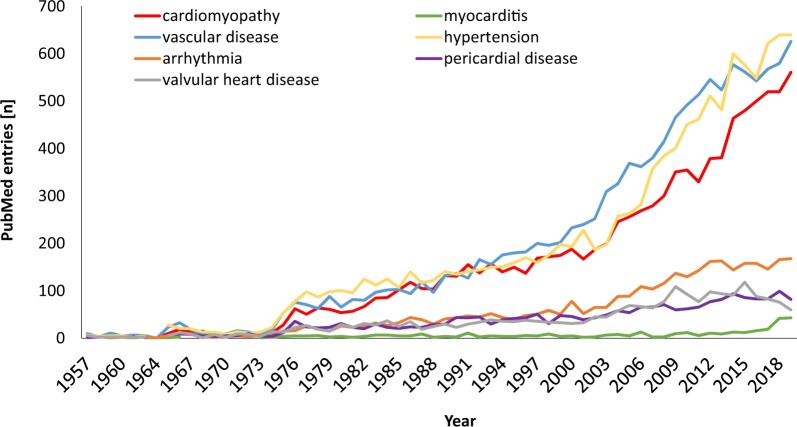
Figure 1.
PubMed entries over time for case reports, clinical, observational, or multicentre studies, and randomized controlled clinical trials based on the following search terms: cardiotoxicity OR cardiac dysfunction OR cardiomyopathy OR heart failure AND cancer, myocarditis AND cancer, vascular toxicity OR atherosclerosis OR thrombosis OR vasospasm AND cancer, hypertension AND cancer, pericarditis OR pericardial disease AND cancer, valvular heart disease AND cancer.
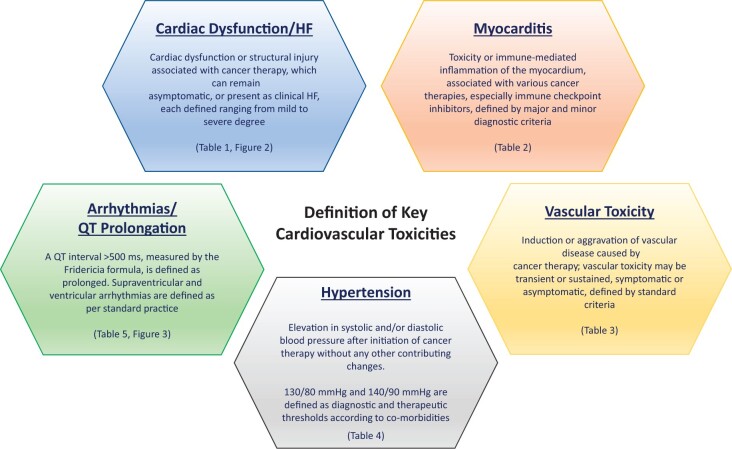
Graphical Abstract.
Outline of the five focus areas of cardiovascular toxicities covered in this definitions document. HF, heart failure.
Methodology
The consensus definitions of CV toxicities encountered during cancer therapy were developed by a writing group consisting of multidisciplinary experts in the fields of cardiology, haematology, and oncology convened by the Scientific Council of the International Cardio-Oncology Society (IC-OS). Bimonthly webinars/teleconferences were held from July 2020 until January 2021, during which subgroups discussed individual topics with an accompanying extensive literature review. Consensus definitions were developed applicable to clinical practice as well as clinical trials following accepted guidelines and representing the agreement of the writing group members.5 The definitions pertain to the most common adverse CV events during contemporary cancer therapy, which can be categorized into five main categories: (i) cardiac dysfunction: cardiomyopathy/heart failure (HF), (ii) myocarditis, (iii) vascular toxicity, (iv) hypertension, and (v) arrhythmias and QTc prolongation (Graphical abstract). It is recognized that societal consensus documents and guidelines [e.g. by the American College of Cardiology (ACC), the American Heart Association (AHA) and the European Society of Cardiology (ESC)] have already defined cardiac adverse events encountered in the general population; this document specifically focuses on those adverse CV events uniquely encountered during cancer therapy.
Cardiac dysfunction/heart failure
What constitutes cardiac (or myocardial) dysfunction as a cardiovascular toxicity?
Cancer therapy can adversely impact cardiac structure and/or function, emerging as asymptomatic cardiac dysfunction or symptomatic HF, collectively termed cancer therapy-related cardiac dysfunction (CTRCD).
Which cancer therapeutics are associated with cardiomyopathy and heart failure?
CTRCD has been described in association with many cancer therapies including conventional chemotherapeutics (anthracyclines) and different classes of targeted therapies such as HER2-targeted agents, certain small molecule kinase inhibitors, and specific proteasome inhibitors. The incidence and details of CTRCD associated with specific cancer therapeutics have been described extensively elsewhere.6–9 For the purposes of this document, a summary of agents, for which a direct causative association with CTRCD has been described in clinical trials, is presented in the Supplementary material online, Table S1.
It is important to note that routine baseline left ventricular ejection fraction (LVEF) assessment and/or monitoring of cardiac function is recommended by the package insert/drug label for only a few subgroups of therapies/agents, while for all others only symptom-based surveillance is recommended. In clinical practice, the lack of a baseline LVEF can pose a challenge when evaluating the likelihood of true CTRCD. In addition, the multitargeted nature of many cancer therapeutics means that other CV toxicities may be present, especially ischaemia and thromboembolism, which may complicate and contribute to the development of HF.
Which cardiac dysfunction definitions have been used in cancer patients?
The definition of cardiac dysfunction associated with chemotherapy and other cancer treatments has evolved over the years from recognition of clinical HF to declines in cardiac function, elevation of cardiac biomarkers, and (previously) histological evidence of cardiac injury on endomyocardial biopsies, especially with anthracycline use.10–14 The first step towards a set of established criteria for asymptomatic and symptomatic cardiac dysfunction was taken after the emergence of an unexpected incidence of HF events associated with trastuzumab (a monoclonal antibody to HER2 receptor), confirmed by a post hoc investigation by the independent Cardiac Review and Evaluation Committee (CREC).15 , 16 These criteria were incorporated in subsequent clinical trials and, ultimately, into regulatory package inserts and professional society guidelines for monitoring of cardiac function during trastuzumab-based therapy.16 Subsequently, many professional groups developed modifications of the CREC definitions to define CTRCD, albeit with some notable differences (Table 1). In the most recent version (5th) of Common Terminology Criteria for Adverse Events (CTCAE), CTRCD can be reported as LVEF change, systolic dysfunction and/or HF events with unique severity grading within each category. These categories overlap with each other and are not aligned with standard terminology used in HF and cardiology guidelines, thus making them difficult to apply in a practical, multidisciplinary care model. Apart from these developments, investigators have used their own, independent definitions of CTRCD in research reports, impeding efforts to compare study findings directly and to generate an evidence base for clinical practice. There henceforth is a need to harmonize the multiple classification systems in the discipline of CO.
Table 1.
Definitions for Cancer Treatment Related Cardiac Dysfunction
| Cardiac Review and Evaluation Committee, Definition of Chemotherapy-induced Cardiotoxicity16 | Any one of the following:
|
||||
| NYHA Classification |
|
|
|
|
|
| ACCF/AHA Stages of HF |
|
|
|
|
|
|
|
|
|
||
|
|
|
|||
|
|
|
|
|
|
| Package Insert Guidelines to Hold Cancer Therapy Due to LV Dysfunction |
|
|
|||
| 2014 Echo Guidelines for Subclinical LV Dysfunction27 |
|
|
|||
| 2016 ESC Position Statement |
|
|
|||
| 2017 ASCO Guideline | Cardiotoxicity not specifically defined | ||||
|
2020 ESMO Guideline |
|
All Cancer Therapy |
|
|
|
| Anthracycline or Trastuzumab Related |
|
||||
|
| |||||
| IC-OS 2021 Consensus | |||||
| Asymptomatic CTRCD (with or without additional biomarkers, LVEF values are based on 2D echocardiography) |
|
|
|
||
| Symptomatic CTRCD (with LVEF and supportive diagnostic biomarkers) |
|
|
|
|
|
ACCF=American College of Cardiology Foundation. AHA = American Heart Association. ASE = American Society of Echocardiography. CTCAE = Common Terminology Criteria for Adverse Events. CTRCD = Cancer-therapeutics Related Cardiac Dysfunction. HF = Heart Failure. GLS = Global Longitudinal Strain. LVEF = Left ventricular ejection fraction. NYHA: New York Heart Association.
Oncology trial investigators can choose to classify a given event under “ejection fraction decreased,” “LV systolic dysfunction,” or “Heart Failure” with associated grades if they decide the adverse effect is related to the intervention. This contributes to difficulty in comparing results of trials and effects of cancer therapies. Grade 1 – Grade 4 (mild to severe). Death = Grade 5. No Grade 5 for “ejection fraction decreased.”
Cardiac troponin I/T>99th percentile, BNP ≥ 35 pg/ml, NT-proBNP≥125 pg/mL (or new significant rise from baseline beyond the biological and analytical variation of the assay used)
How is this definition of cancer therapy-related cardiac dysfunction different or improved?
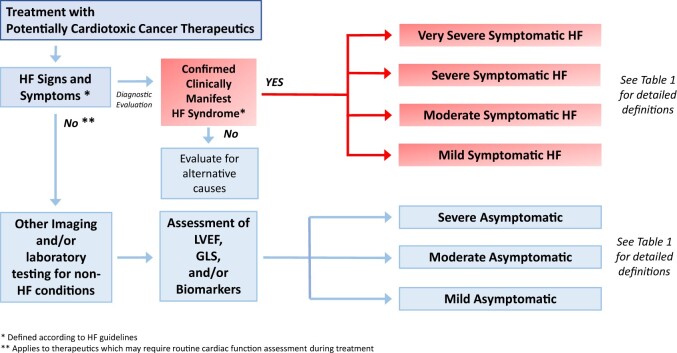
The challenges of reconciling multiple classification systems are not unique to CO, where differences reflect growth and evolution of science (including preferences for terms such as HF over congestive HF, or the need for a universal definition of myocardial infarction), sophistication of cardiac imaging techniques, and numerous new targeted cancer therapeutics. In this document, we aim to harmonize previous and currently used definitions of cardiac dysfunction in CO practice and research with a contemporary approach to HF put forward by professional CV societies. Under the umbrella of CTRCD, we make the critical distinction between symptomatic HF and asymptomatic CTRCD and define the criteria for severity assessment in both categories, analogous to the CTCAE system (Figure 2). By utilizing this approach, the proposed definitions are applicable to CO clinical practice as well as clinical research in oncology treatment, registries, and clinical trials. The diagnosis of CTRCD includes a comprehensive evaluation of clinical symptoms, signs, cardiac imaging and cardiac biomarkers, in the context of exposure to potentially cardiotoxic agents.
Figure 2.
Diagnostic algorithm for cancer therapy-related cardiac dysfunction. GLS, global longitudinal strain; HF, heart failure; LVEF, left ventricular ejection fraction.
What defines symptomatic cancer therapy-related cardiac dysfunction?
Symptomatic CTRCD is characterized by a HF syndrome including typical symptoms with signs of volume overload and/or inadequate perfusion, that are caused by structural and/or functional abnormalities of the heart consistent with AHA/ACC Stage C/D HF (Supplementary material online, Table S2). However, these symptoms can be non-specific and, therefore, in a patient presenting with symptoms of HF, a careful history and physical examination, accompanied by appropriate diagnostic tests, should be performed to differentiate between cardiac and non-cardiac disorders. As such, the history should focus on potential cardiotoxic exposures and pre-existing CV risk factors or conditions placing the patient at risk for HF. Symptoms and signs should be assessed with particular attention to volume overload. However, rarely patients can present with signs of hypoperfusion in the absence of congestion. Symptoms of HF correlate with survival and even patients with mild symptoms are at increased risk of hospitalization and death.18 These principles are especially pertinent in patients with cancer, in whom many of these symptoms could result from cancer therapy. A combination of signs, symptoms, and objective findings has been utilized in the PROTECT (Prospective Observation of Cardiac Safety With Proteasome Inhibition) study to diagnose HF in a cancer population undergoing cancer therapy and was noted to correlate with worse overall outcomes.19
Measurement of natriuretic peptides (NPs) [B-type natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP)] can help establish or exclude the diagnosis of symptomatic HF20 , 21 and cut-off values (BNP < 100 pg/mL or NT-proBNP < 300 pg/mL) have been proposed to exclude HF in the acute setting.22 In the subacute setting, lower values may be more appropriate with BNP <35 pg/mL or NT-proBNP <125 pg/mL having a negative predictive value of 93–97% for symptomatic HF.23 Natriuretic peptide levels, especially NT-proBNP, increase with age and declining renal function and decrease with obesity (body mass index > 30 kg/m2). All values should ideally be compared to a pre-treatment baseline in order to confirm new findings. Troponin elevation above the 99th percentile cut-off for the specific assay used can serve a supportive role as a biomarker indicating cardiac injury.24–27 Isolated elevations of these biomarkers without imaging parameters may be considered as biochemical evidence of cardiotoxicity. Decisions regarding cancer treatment continuation vs. discontinuation should not be based on biomarker abnormalities alone. The same applies to imaging studies other than substantial LVEF changes.
Cardiac imaging, typically with an echocardiogram (Echo), should be performed to define LVEF as well as chamber sizes, diastolic filling parameters and, preferably, global longitudinal strain (GLS).17 We used the intensity of therapy needed to resolve symptoms as a method for classifying the severity of symptomatic HF (Table 1). This combines and builds on the CTCAE v5.0 categories of left ventricular systolic dysfunction and HF.28
What defines asymptomatic cancer therapy-related cardiac dysfunction?
Asymptomatic CTRCD is much more common during cancer therapy than symptomatic HF. Its identification is often based on threshold changes of LVEF on screening Echo during cancer treatment or as an incidental finding during survivorship surveillance. There have been multiple cut-offs of LVEF changes attempting to describe CTRCD, and there is uncertainty regarding which of these criteria is most prognostically relevant. A fall in LVEF to <50% appears to be prognostically important and can affect continuation of cancer therapy as well as cancer prognosis.29–32 More importantly, a reduction in LVEF to <50% followed by persistent LVEF decline, or lack of recovery, despite optimal HF treatment, is associated with subsequent risk of major adverse CV events.29 , 30 This phenomenon is more common with anthracycline therapy, but has been observed with other cancer therapies as well.33 Therefore, identification and treatment of asymptomatic CTRCD remains important. In addition to accurate LVEF assessment, the cardiac imaging technique needs to reliably detect a significant change in LVEF from baseline, as LVEF reduction is part of the CTRCD definitions in both asymptomatic and symptomatic populations (Table 1). The LVEF decline by >10 percentage points has been the most commonly accepted threshold value; however, it is important to emphasize that test–retest validity of the chosen imaging technique should be established and confirmed for each laboratory prior to being able to reliably diagnose CTRCD.34 , 35 Given these recognized challenges with serial LVEF measurements, more sensitive methods to detect and confirm cardiac dysfunction should be considered, including GLS and cardiac biomarkers (e.g. troponins and NPs).
Global longitudinal strain is a measure of myocardial deformation and thereby of myocardial function; a reduction of GLS (less negative) henceforth indicates myocardial dysfunction. This tool can detect changes in myocardial function prior to a significant threshold change in LVEF.36 As the calculation of GLS varies between vendors of Echo machines and analytical equipment and software, it is recommended to use the same system to be able to accurately compare values over time.17 Much of the literature on the use of GLS applies classically to patients with breast cancer receiving anthracyclines and/or trastuzumab therapy, thought data in patients on immune checkpoint inhibitor (ICI) therapy are emerging.37 Two studies have demonstrated a significant concurrent association between temporal changes in GLS and LVEF.38 , 39 Therefore, a change in GLS can be used as an arbiter of whether a true change in LVEF has occurred (Table 1). Conceptually, however, the value of GLS is greatest in the absence of a significant change in LVEF. In this scenario, a change in GLS >15% relative to baseline has been suggested as the threshold to identify subclinical cardiomyopathy.17 Other thresholds have been considered, and the recently published SUCCOUR trial used a 12% relative change in GLS as a cut-off for the initiation of cardioprotective therapy.40 Similar to GLS, an increase in troponin and NP levels has also been considered to have utility for the early detection of cardiotoxicity, and in some cases a prognostic value especially in the context of exposure to anthracyclines and HER2-targeted therapy or proteasome inhibitors.19 , 41 , 42
Considering these findings, asymptomatic CTRCD is graded on the basis of LVEF change and includes measures of GLS and/or biomarkers to help further determine severity (Table 1). A reduction in LVEF to <40% indicates severe asymptomatic CTRCD, with recent data suggesting an association with poor prognosis in multiple cancers and treatment regimens.43 Moderate asymptomatic CTRCD requires (i) a fall in LVEF into a clearly abnormal range (40–49%) with a change in LVEF beyond the described variability of the most commonly used Echo-based 2D LVEF measurements (i.e. by >10 percentage points), or (ii) a smaller change in LVEF but with a concomitant significant fall in GLS and/or new rise in cardiac biomarkers. Mild asymptomatic CTRCD is defined as preserved LVEF (i.e. LVEF ≥ 50%) with >15% reduction in GLS relative to baseline and/or new rise in troponin or NPs.
Myocarditis
What constitutes myocarditis as a cardiovascular toxicity?
Myocarditis is an inflammatory disease of heart muscle cells. In cancer patients, most commonly myocarditis can be seen as a result of direct toxicity or as an immune-mediated event.44
Which cancer therapies have been associated with myocarditis?
Traditional cytotoxic cancer therapies (e.g. doxorubicin, fluorouracil, and cyclophosphamide), radiation therapy, and ICIs have been associated with the development of myocarditis.6 , 45
Which myocarditis definitions have been used in cancer patients?
Historically, CTCAE has served as a reference for adverse events coding in cancer patients (Table 2). More recently, a specific set of criteria for adjudicating myocarditis in clinical trials with cancer therapeutics was forwarded by Bonaca et al.46 in 2019 (Table 2).
Table 2.
Definitions for Immune Checkpoint Inhibitor Associated Myocarditis
|
CTCAE Version 5*
| ||
|
A disorder characterized by inflammation of the muscle tissue of the heart.
| ||
|
|
|
|
| ||
| Bonaca et al. | ||
| Definitive |
|
|
| Probable |
|
|
| Possible |
|
|
|
| ||
| IC-OS 2021 Consensus | ||
| ||
| ||
| ||
| ||
| ||
| Modifiers | ||
| Severity of Myocarditis | ||
| Severe | Hemodynamic instability, heart failure requiring non-invasive or invasive ventilation, complete or high-grade heart block, and/or significant ventricular arrhythmia | |
| Non-Severe (clinically significant) | Symptomatic but hemodynamically and electrically stable, may have reduced LVEF, no features of severe disease | |
| Smoldering (sub-clinical) | Incidentally diagnosed myocarditis without any clinical signs or symptoms | |
| Steroid Refractory | Non-resolving or worsening myocarditis (clinical worsening or persistent troponin elevation after exclusion of other etiologies) despite high dose methylprednisolone | |
| Recovery from Myocarditis | ||
| Complete Recovery | Patients with complete resolution of acute symptoms, normalization of biomarkers and recovery of LVEF after discontinuation of immunosuppression are considered to have achieved complete recovery. CMR may still show LGE or elevated T1 due to fibrosis but any suggestion of acute edema should be absent. | |
| Recovering | Ongoing improvement in patient clinical symptoms, signs, biomarkers and imaging parameters, but not yet normalized, while on tapering doses of immunosuppression. | |
CMR, cardiac magnetic resonance; CTCAE, Common Terminology Criteria for Adverse Events; cTn, cardiac troponin; ECG, electrocardiogram; Echo, echocardiogram; EMB, endomyocardial biopsy; IC-OS, International Cardio-Oncology Society; IV, intravenous; LBBB, left bundle branch block; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; PET, positron emission tomography; RBBB, right bundle branch block; WMA, wall motion abnormalities.
*Grade 5 = death.
How is this definition of myocarditis different or improved?
The CTCAE definition and grading system is rather generic and lacks specific criteria for diagnosis. While specific criteria and a grading system of possible, proable, and definite myocarditis were provided by Bonaca et al.,46 the goal of their definition was to facilitate identification and ascertainment of cases of myocarditis in clinical trials. As specifically stated, their definition was not intended for clinical use. This is, however, very much the goal of the definition outlined herein, which may also be used in clinical trials to align clinical practice and research. The current definition focuses on immune checkpoint inhibitor myocarditis and takes into consideration additional data on ICI myocarditis that have become available since the publication of the document by Bonaca et al. [including the utility and limitations of the electrocardiogram (ECG), various imaging modalities and treatment implications].47–49
In distinction from prior definitions, the current definition is first of all binary: a diagnosis of myocarditis is either made or not made, based on meeting major and/or minor criteria. In keeping with the concept of grading schemes, these were provided for severity, steroid refractory myocarditis, and the degree of recovery from myocarditis. These are crucial aspects for the management of myocarditis, including decisions on further antineoplastic therapies, especially if re-challenge with ICI therapy is being considered.
What defines immune checkpoint inhibitor-mediated myocarditis?
Consistent data have shown that myocarditis caused by an ICI is a T-cell-mediated inflammatory disease of myocardium leading to cell death. The mechanisms involved in the development of T-cell-mediated cardiac myocyte cell death with ICI therapy are incompletely understood. Possible aetiologies include the development of auto-antigens, allo-antigens, or allergens.50 , 51 Lack of specificity in the clinical presentation, potential overlap with other CV and general medical conditions, and limited sensitivity and specificity of routine CV testing, make the diagnosis of ICI-associated myocarditis challenging.37 , 52 , 53 Similar to prior criteria, we propose using a combination of clinical, electrocardiographic, cardiac biomarker, CV imaging (echo and cardiac magnetic resonance imaging), and tissue pathology findings with some modifications based on recent data to increase the accuracy of the diagnosis (Table 2 and Supplementary material online, Table S3).46 We recognize that many of these tests can be abnormal in a variety of other conditions, emphasizing the importance of maintaining a broad differential in patients being evaluated for myocarditis.
Timely diagnosis of ICI myocarditis is critical since prompt initiation of immunosuppression can substantially improve CV outcomes.53 Conversely, an incorrect diagnosis of myocarditis can lead to the discontinuation of a potentially effective cancer therapy and worsen cancer-related outcomes. We have therefore further classified myocarditis based on the severity of the clinical presentation (Table 2), as well as refractoriness to treatment with corticosteroids. We also define recovery from myocarditis with the intention that severity of the index presentation, the response to treatment, and the degree of recovery may help guide further cancer therapy, especially if re-challenge with ICI therapy is being considered (Table 2).
While ICIs are currently the primary immune therapy associated with myocarditis, it is possible that other novel immunomodulatory agents may also cause myocarditis. Application of uniform diagnostic criteria to identify myocarditis in clinical trials of novel immunotherapies might enable us to better understand the incidence, severity, and implications of myocarditis associated with a particular cancer therapy.54–60
Vascular toxicities
What constitutes vascular toxicity in the cancer patient?
Vascular toxicity is the induction or aggravation of vascular disease in the setting of cancer therapy.
Which cancer therapies have been associated with vascular toxicity?
This topic emerged with the introduction of 5-fluorouracil into cancer therapy regimens but has been noted with several other cancer drugs including: platinum drugs, cyclophosphamide, gemcitabine, bleomycin, vinca alkaloids, the immunomodulatory drugs interferon alpha 2B and lenalidomide, the proteasome inhibitor carfilzomib, and the mTOR inhibitor everolimus (Supplementary material online, Table S4).61 Vascular toxicities gained further interest with the introduction of targeted therapies, namely vascular endothelial growth factor (VEGF) signalling pathway inhibitors (VSPI), breakpoint cluster region-abelson (BCR-Abl) tyrosine kinase inhibitors such as nilotinib and ponatinib, and the epidermal growth factor receptor inhibitor erlotinib.62 , 63 Last but not least, vascular toxicity can also be seen with radiation injury but does not emerge until sometime after completion of therapy.
Which vascular toxicity definitions have been used in cancer patients?
‘Vascular toxicity’ has been used as an umbrella term rather than a designated event or endpoint in clinical studies despite the common use of composite endpoints. The most commonly used composite endpoint in research studies in this area is arterial thromboembolism (ATE), variably defined, for instance, as (i) any inpatient or outpatient diagnosis of myocardial infarction or ischaemic stroke, or (ii) arterial thrombosis, cerebral infarct, cerebral ischaemia, cerebrovascular accident, myocardial infarction, and myocardial ischaemia, or (iii) angina pectoris, arterial thrombosis, cerebral infarct, cerebral ischaemia, cerebrovascular accident, myocardial infarction, and myocardial ischaemia.64–66 The other terminology that has been used is arterial occlusive event, categorized based on a broad collection of >400 Medical Dictionary for Regulatory Activities preferred terms related to vascular ischaemia or thrombosis.67 Peripheral arterial occlusive disease (PAOD) is another term in the CO literature, and an alternate term for peripheral arterial disease, also known as peripheral vascular disease or lower extremity arterial disease.68 The only standardized approach to the various aspects of vascular toxicity is found in the CTCAE catalogue, which has been used in clinical studies, especially trials in cancer patients (Table 3).
Table 3.
Definitions for Vascular Toxicities with Cancer Therapies
| CTCAE Version 5*
| ||
|---|---|---|
| Event | Definition | Grades |
| Arterial injury | A finding of damage to an artery. |
|
| Arterial thromboembolism | A disorder characterized by occlusion of an arterial vessel by a blood clot that develops in an artery. |
|
| Chest pain (cardiac) | A disorder characterized by substernal discomfort due to insufficient myocardial oxygenation e.g., angina pectoris. |
|
| Cerebrovascular ischemia | A disorder characterized by a decrease or absence of blood supply to the brain caused by obstruction (thrombosis or embolism) of an artery resulting in neurological damage. |
|
| Myocardial infarction | A disorder characterized by gross necrosis of the myocardium; this is due to an interruption of blood supply to the area. |
|
| Peripheral ischemia | A disorder characterized by impaired circulation to an extremity. |
|
| Stroke | A disorder characterized by a decrease or absence of blood supply to the brain caused by obstruction (thrombosis or embolism) of an artery resulting in neurological damage. |
|
| Thromboembolic event | A disorder characterized by occlusion of a vessel by a thrombus that has migrated from a distal site via the blood stream. |
|
| Transient ischemic attack | A disorder characterized by a brief attack (less than 24 hours) of cerebral dysfunction of vascular origin, with no persistent neurological deficit. |
|
| Vasculitis | A disorder characterized by inflammation involving the wall of a vessel. |
|
| Vascular disorder |
|
|
| Venous injury | A finding of damage to a vein |
|
|
| ||
| IC-OS 2021 Consensus | ||
| Asymptomatic vascular toxicity | ||
| Atherosclerosis |
|
|
|
||
|
||
| Thrombosis |
|
|
|
||
| Abnormal vasoreactivity |
|
|
|
||
|
||
| Symptomatic vascular toxicity | ||
| Stroke | ||
| Transient ischemic attack | ||
| Myocardial infarction | 4th Universal Definition of MI86 | |
| Acute coronary syndromes |
|
|
| Chronic coronary syndromes | 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC)81 | |
| Peripheral arterial disease | 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS)80 | |
| Vasospastic angina | 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC)81 | |
| International standardization of diagnostic criteria for vasospastic angina89 | ||
| Microvascular angina | 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC)81 | |
| International standardization of diagnostic criteria for microvascular angina90 | ||
| Raynaud’s phenomenon | Meeting the diagnostic criteria of an international consensus panel of recurrent episodes bilateral blanching or tricolor change of the fingers.78 , 79 | |
*Grade 5 = death.
How is this definition of vascular toxicity different or improved?
As outlined, there is no standardized definition of vascular toxicity other than the approach provided by CTCAE. Even so, the CTCAE definitions do not necessarily match events taken into account otherwise. For instance, the CTCAE Version 5 definition of ATE is that of ‘a disorder characterized by an occlusion of an arterial vessel by a blood clot that develops in an artery’ and grading of severity starts with Grade 3 (urgent intervention indicated). Arterial thromboembolism definitions used in clinical studies deviate from this by including presentations of ischaemia not requiring urgent interventions or being life-threatening and may focus only on one or two arterial territories while the scope could be broader. Also, the origin of the thrombus may not always be the vasculature but would still qualify as an ATE, e.g. if a thrombus embolized from cardiac chambers into an arterial territory. The definition proposed herein encourages the definition of the vascular disease entity and its mode of presentation using established societal criteria and guidelines (Table 3).
Which pathophysiological types of vascular toxicity have been noted?
As outlined, vascular toxicity has a broad spectrum of presentations, varying in type and by vascular bed involved. From a pathophysiological perspective, three main scenarios can be encountered that lead to luminal obstruction and reduction in blood flow with related sequelae: (i) altered vascular reactivity, (ii) vascular thrombosis, and (iii) atherosclerosis.61 A fourth one that can be seen is vasculitis, which may lead to all of the above (altered vasoreactivity, thrombosis, and/or structural obstruction).
What is the clinical presentation of vascular toxicity?
Vascular toxicity can be clinically silent (asymptomatic) or apparent (symptomatic). Asymptomatic vascular toxicity is detected by testing modalities and, while of interest for research studies, it is also important clinically, especially for the early recognition and prevention of symptomatic disease and complications. For instance, recognition of progressive narrowing of the peripheral arteries by a decline in ankle-brachial indices over time in a patient with chronic myelogenous leukaemia on nilotinib may prevent progression to the point of critical limb ischaemia, which can result in gangrene and amputation.63 Conversely, presentation with claudication or critical limb ischaemia may lead to the detection of peripheral arterial disease which was not present before the start of cancer therapy, and thus might have been provoked by it.
In cases of suspected vascular toxicity, in addition to documenting a change from baseline, it is important to establish the likelihood of an association with cancer therapy based on current knowledge (definite, probable, possible, unlikely), akin to the adverse event adjudication process in clinical trials. At times, and especially with new drugs, the appropriate association may not have been previously noted; recognition and reporting of potential toxicities is therefore extremely important.
Asymptomatic vascular changes
These reflect disease processes recognized by changes in diagnostic testing parameters beyond what can be expected based on analytical and biological variability. In addition to recognizing significant changes, taking common thresholds for abnormality into account is important for aligning with common practice standards and guidelines. The margin or reserve from the threshold of abnormality for vascular structure or function is reduced in patients with underlying CV disease and/or risk factors (Table 3).69–72 , 74–77
Symptomatic presentations
These are defined by societal guidelines as it is common clinical practice (Table 3).78–86 , 88 , 91 Conventional terms such as peripheral arterial disease should be used in lieu of non-conventional terms such as PAOD. Furthermore, it is recommended avoiding the use of combination and overlap terms such as ATEs. Instead, the specific component should be reported in keeping with standard definitions.
Hypertension
What constitutes hypertension as a cardiovascular toxicity?
An increase in systolic and/or diastolic blood pressure (BP) after initiation of cancer therapy, without any other contributing changes, constitutes an adverse effect which can be of various grading. Distinct from chronic hypertension, which can be present in the cancer patient and has been generally associated with an increased risk of CV events, less is known about the effects of short-term increases in BP in patients with cancer.92–96
Which cancer therapies are associated with hypertension?
Several cancer therapies have been associated with hypertension and in particular newer targeted agents such as VSPIs. Other agents include the proteasome inhibitor carfilzomib, mTOR inhibitors, and tyrosine kinase inhibitors of b-raf (rapidly accelerated fibrosarcoma) (BRAF), mitogen-activated protein/Extracellular signal-regulated kinase (MEK), and bruton tyrosine kinase (BTK) (Supplementary material online, Table S5). Patients receiving VSPIs can develop hypertension within days of starting therapy and there is potential for life-threatening complications.97–101 Of note, different agents may have variable hypertensive effects and there is remarkable inter-individual variation. Uncontrolled hypertension is associated with diverse cardiac and non-cardiac complications.102–104 Hypertension is a potent risk factor for cardiotoxicity and CV events in patients with cancer, both during cancer therapy and after its completion. Therefore, defining diagnostic and therapeutic thresholds is particularly important.
Which hypertension definitions have been used in cancer patients?
Multiple definitions and grading schemes exist for hypertension that have come out by groups such as ACC/AHA, European Society of Cardiology, and International Society of Hypertension (Table 4). However, none of them specifically address hypertension in the cancer patient.
Table 4.
Definition of Hypertension in Cancer Patients
| CTCAE Version 5* 28 |
|
|
|
|
|
|
| ACC/AHA 201793 |
|
|
|
|
|
|
| ESC 201892 |
|
|
|
|
|
|
| ISH 2020105 |
|
|
|
|
|
|
|
| ||||||
| IC-OS 2021 Consensus* |
|
|
|
|
|
|
ACC, American College of Cardiology; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; BP, Blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CTCAE, Common Terminology Criteria for Adverse Events; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; ESC, European Society of Cardiology; HMOD, Hypertension-Mediated Organ Damage; HTN, hypertension; ICOS, International Cardio-Oncology Society; ISH, International Society of Hypertension; SBP, systolic blood pressure; WNL, within normal limits.
Definition of hypertension aspect in the cancer patient
These values are based on office blood pressure measurement; home blood pressure measurement cutoffs are 5 mmHg points lower.
ESC Council on hypertension position document on the management of hypertensive emergencies.104
Grade 5 = death.
How is this definition of hypertension different or improved?
Hypertension in the cancer patient presents a unique situation in which hypertension may be temporary and due to treatment, but with more abrupt onset that can lead to end-organ damage and other complications. Uncontrolled hypertension may also lead to the holding of cancer treatment, which can have significant implications on the oncologic aspect of a patient’s care. Our definition and perspective of hypertension, as outlined in Table 4, was created with these considerations in mind.
What defines hypertension in the cancer patient?
The inaccuracy of BP measurements in the office setting has led to the recommendation for out-of-office BP measurements (ambulatory and home BP monitoring) to confirm a diagnosis of hypertension (Table 4).107 Home BP monitoring should be adopted by all patients with cancer receiving therapy known to cause or worsen hypertension.94 In those with elevated BP, it remains important to rule out reversible causes such as obstructive sleep apnoea, pain, and emotional stressors.
The diagnostic threshold for hypertension in patients with malignancy before or after cancer therapy is >130/80 mmHg (Table 4).93 This is also the BP treatment threshold for patients during cancer treatment with pre-existing CV disease, proteinuric renal disease, or diabetes.93 In other patients during cancer treatment, the threshold for the initiation of antihypertensive therapy can be extended to 140/90 mmHg. If BP is >180 mmHg systolic or 110 mmHg diastolic, the competing cancer and CV risks should be evaluated, and any cancer therapy associated with hypertension should be deferred or temporarily withheld until BP is controlled to values below 160 mmHg systolic and 100 mmHg diastolic. The same holds true for an emergency hypertensive response, defined as very high BP elevations associated with acute hypertension-mediated organ damage. This may include hypertensive encephalopathy, posterior reversible encephalopathy syndrome, papilledema, stroke, myocardial infarction, acutely decompensated HF, aortic dissection, and acute kidney injury. Any such condition needs to be managed, along with BP control, before cancer therapy can resume after proper risk/benefit discussion. Patients with greater BP variability and/or an exaggerated response such as an absolute increase in systolic BP >20 mmHg and/or mean arterial BP >15 mmHg from baseline need particular attention as high BP levels may be reached precipitously and with clinical consequences.
Arrhythmias and QTc prolongation
What constitutes QTc prolongation as a cardiovascular toxicity?
The full scope of abnormalities in cardiac electrophysiology can be seen in patients with cancer.6 , 108 These may be related to cancer therapy, underlying predisposition/risk, or both. While atrial fibrillation occurs commonly in this population, its definition as well as the definition of other supraventricular and ventricular arrhythmias does not differ from those applied to the general population. As such, they will not be discussed in this document. QTc prolongation, which is a lengthening of the cardiac repolarization interval is of particular importance due to the risk of sudden cardiac death and its direct relation to cancer therapy and related treatments (anti-emetics, etc.). There is substantial variability in the literature regarding significant QT interval changes. No standardized definitions and recommendations exist, and cancer care providers are referred to the individual drug labels.109 The goal of this section is to provide a harmonized definition of QT prolongation in the cancer patient population.110
Which cancer therapies are associated with QTc prolongation and the risk of sudden cardiac death?
Several cancer therapies have been recognized to cause QTc prolongation including arsenic trioxide, HDAC inhibitors, tyrosine kinase inhibitors (esp. vandetanib, vemurafenib, ceritinib, gilteritinib, trametinib, and those targeting BCR-Abl and the VEGF signalling pathway) and Cyclin-dependent kinase (CDK) 4–6 inhibitors (ribociclib) (Supplementary material online, Table S6).6 , 111 , 112
Which arrhythmia definitions have been used in cancer patients?
The CTCAE criteria have been used to define degrees of QT prolongation in clinical trials (Table 5). Grade 1 prolongation is an average QTc 450–480 ms; Grade 2 is an average QTc 481–500 ms; and Grade 3 is an average QTc ≥501 or 60 ms change from baseline; however, these are not uniformly incorporated into routine clinical practice decision-making.
Table 5.
Definition of QTc prologation and Arrhythmias with Cancer Therapies
| CTCAE Version 5*
| ||
|---|---|---|
| Event | Definition | Grades |
| QTc prolongation | A finding of a cardiac dysrhythmia characterized by an abnormally long corrected QT interval. |
|
| Arrhythmias | ||
| Ventricular arrhythmia | A disorder characterized by a dysrhythmia that originates in the ventricles. |
|
| Ventricular fibrillation | A disorder characterized by a dysrhythmia without discernible QRS complexes due to rapid repetitive excitation of myocardial fibers without coordinated contraction of the ventricles | Grade 4: Life-threatening consequences; hemodynamic compromise |
| Ventricular tachycardia | A disorder characterized by a dysrhythmia with a heart rate greater than 100 beats per minute that originates distal to the bundle of His. |
|
| Atrial fibrillation | A disorder characterized by a dysrhythmia without discernible P waves and an irregular ventricular response due to multiple reentry circuits. The rhythm disturbance originates above the ventricles. |
|
| Atrial flutter | A disorder characterized by a dysrhythmia with organized rhythmic atrial contractions with a rate of 200-300 beats per minute. The rhythm disturbance originates in the atria. |
|
| (Paroxysmal) atrial tachycardia | A disorder characterized by a dysrhythmia with abrupt onset and sudden termination of atrial contractions with a rate of 150-250 beats per minute. The rhythm disturbance originates in the atria. |
|
| Supraventricular tachycardia | A disorder characterized by a dysrhythmia with a heart rate greater than 100 beats per minute that originates above the ventricles. |
|
| Sinus tachycardia | A disorder characterized by a dysrhythmia with a heart rate greater than 100 beats per minute that originates in the sinus node. |
|
| Atrioventricular block, first degree | A disorder characterized by a dysrhythmia with a delay in the time required for the conduction of an electrical impulse through the atrioventricular (AV) node beyond 0.2 seconds; prolongation of the PR interval greater than 200 milliseconds. |
|
| Atrioventricular block, second degree, Mobitz (type) II | A disorder characterized by a dysrhythmia with relatively constant PR interval prior to the block of an atrial impulse. This is the result of intermittent failure of atrial electrical impulse conduction through the atrioventricular (AV) node to the ventricles. |
|
| Atrioventricular block, second degree, Mobitz type I | A disorder characterized by a dysrhythmia with a progressively lengthening PR interval prior to the blocking of an atrial impulse. This is the result of intermittent failure of atrial electrical impulse conduction through the atrioventricular (AV) node to the ventricles. |
|
| Atrioventricular block, complete (third degree) | A disorder characterized by a dysrhythmia with complete failure of atrial electrical impulse conduction through the AV node to the ventricles. |
|
| Conduction disorder | A disorder characterized by pathological irregularities in the cardiac conduction system. |
|
| Sick sinus syndrome | A disorder characterized by a dysrhythmia with alternating periods of bradycardia and atrial tachycardia accompanied by syncope, fatigue and dizziness. |
|
| Sinus bardycardia | A disorder characterized by a dysrhythmia with a heart rate less than 60 beats per minute that originates in the sinus node. |
|
| IC-OS 2021 consensus | ||
|---|---|---|
| QTc prolongation | QTcF < 480ms | Acceptable: continue current treatment |
| QTcF 480-500ms | Prolonging: proceed with caution; minimize other QT prolonging medications, replete electrolytes | |
| QTcF >500ms | Prolonged: stop treatment and evaluate. May require dose reduction or alternative therapy | |
| Arrhythmias | ||
| Ventricular arrhythmia | ||
| Ventricular tachycardia (VT), including polymorphic VT (torsades de pointes) | ||
| Ventricular fibrillation | ||
| Atrial fibrillation | ||
| Atrial flutter | ||
| Atrial tachycardia |
|
|
| Supraventricular tachycardia |
|
|
| Sinus tachycardia |
|
|
| Sinus bradycardia | 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay122 | |
| Sick sinus syndrome | 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay122 | |
| Atrioventricular block first, second and third degree | 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay122 | |
| Conduction disorder (disease) | 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay122 | |
Grade 5 = death.
How is this definition of arrhythmia different or improved?
While the CTCAE criteria provide grades of QT interval prolongation, they do not provide any guidance regarding management, specifically as it relates to withholding or dose reduction of cancer therapies. As such, each pharmaceutical manufacturer provides different recommendations and guidance. Our definition of significant QT interval prolongation is based on epidemiologic data demonstrating increased risk of arrhythmias and can be applied universally to all cancer therapies which will significantly improve and simplify care delivery (Table 5).
What defines QTc prolongation in the cancer patient?
QT interval assessment can be challenging, especially in the setting of arrhythmia, conduction delays due to bundle branch block or pacing, and abnormal T wave morphologies. Due to variations in the absolute QT interval with heart rate fluctuations, several correction formulae have been developed to standardize these measurements.109 , 112 , 113 In the oncology setting, we recommend using the Fridericia formula QTc = QT × RR− 1/3 as this is relatively easy to calculate and has demonstrated less error than other correction methods such as Bazett at both tachy- and bradycardic heart rates.109 , 112–114 Electrocardiogram machines provide an automated QT measurement; however, these systems are generally defaulted to the Bazett algorithm. We recommend re-programming machines being used for cancer patients to provide corrected QT measurements using the Fridericia formula. While it is acceptable to use the automated QT values reported on the ECG tracing in most circumstances, any value that is abnormal or concerning should be manually evaluated by a cardiologist and/or electrophysiologist with CO expertise. This is particularly true for patients with ventricular pacing or bundle branch blocks as the associated QRS prolongation must be accounted for when assessing the QT interval.
In the general population, the upper 99% limit of normal for QT interval is 470 ms for males and 480 ms for females.114 Other cut-offs for a normal QTc interval, however, have been used, i.e. 450 ms for males and 470 ms for females. In general, the risk of malignant arrhythmias is considered to increase with QTc intervals in excess of 500 ms or an increase by >60 ms from baseline, although this may not always apply to cancer patients.110 The exact frequency of malignant arrhythmias in clinical practice is not precisely defined, ranging from well under 1% to nearly 5% with tyrosine kinase inhibitors.110 , 111 The differences may be explained by the number of factors that can affect the QT interval and arrhythmogenic risk including cancer drugs, concomitant medications (i.e. antibiotics, psychiatric medications), and electrolyte abnormalities, co-morbidities, and underlying/concomitant CV disease.
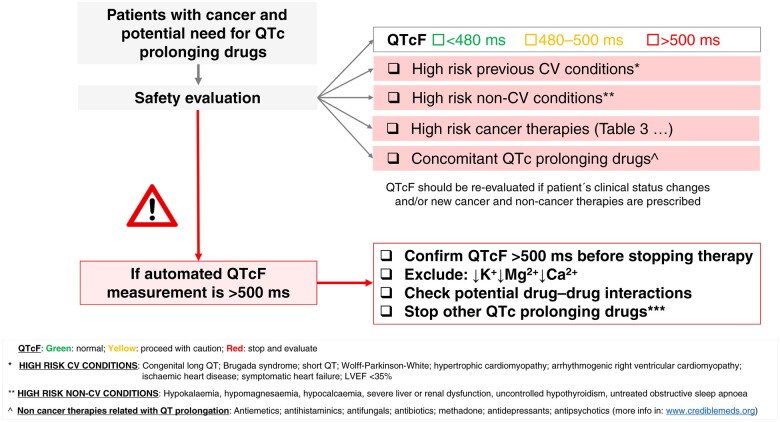
In general, if the corrected QT interval is <500 ms, the risk of torsade de pointes is exceedingly low.115 As such, we recommend considering a change in cancer therapy only when the corrected QT interval is >500 ms. Moreover, changes in the QT interval of >60 ms from baseline are clinically insignificant if the QT remains <500 ms and should not routinely affect treatment decisions. It is important to remember that the QT interval is not stagnant and should be re-assessed if the clinical status (e.g. electrolyte disturbances) of the patient changes or dose changes have been applied (Figure 3).
Figure 3.
Overview of the approach to QTc prolongation in cancer patients. CV, cardiovascular; LVEF, left ventricular ejection fraction; QTcF, QT interval corrected by the Fridericia formula.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The consensus document was reviewed and endorsed by the international executive committee of IC-OS. Those members of IC-OS are listed here: Darryl Leong, Canada; Sebastian Szmit, Poland; Hasan Farhan Ali, Iraq; Zaza Iakobishvili, Israel; Aaron Sverdlov, Australia; Cafer Zorkun, Turkey; Sergey Kozhukhov, Ukraine; Li Ling Tan, Singapore; Daniel Cehic, Australia; and Christine Brezden-Masley, Canada.
Funding
J.H. and K.J.R. are supported by the National Institutes of Health/National Cancer Institute (R01CA233601). J.H. is supported by the Miami Heart Foundation/Research Institute. N.N.L. is supported by the British Heart Foundation (PG/19/64/34434). A.R.L. is supported by the Foundation Leducq Network of Excellence in Cardio-Oncology. J.E.L. is supported by the National Institutes of Health/National Cancer Institute (NIH/NCI) Cancer Center Support Grant (P30 CA008748). J.D.M. has received research funding from the Longer Life Foundation and Children’s Discovery Institute. T.G.N. is supported by the National Institutes of Health/National Heart, Lung, Blood Institute (R01HL137562, R01HL130539, and K24HL150238). T.G.N. was also supported, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg. A.N. is supported by the Gelb Master Clinician Award and Catherine Fitch Geoff Fund at Brigham and Women’s Hospital. P.T. is supported by a Canada Research Chair in Cardiooncology and the Canadian Institutes of Health Research (137132 and 142456). P.V. is supported by Austrian Science Fund (FWF) Herzfelder'sche Familienstiftung P30627-B25.
Conflict of interest: J.H. has been a consultant to Amgen, BMS, and Ariad Pharmaceuticals. D.L. has been a consultant to Lilly, Acorda, BMS, Roche and has received research funding from Myocardial Solutions. A.B. has received consultancy fees from Takeda Inc. S.D. has grant funding from Novartis US and received consultant fees from Novartis Canada. T.L.-F. received speakers fees and conference support outside the current document from Janssen, Amgen, Daiichi-Sankyo, Bayer, Pfizer, MSD, Incyte, TEVA, Philips, iQuone. M.G.F. received research support from Medtronic and consultant fees from Takeda and Abbott. N.N.L. has grant funding from Roche Diagnostics, has been a consultant to Vifor Pharma and Pharmacosmos outside the current work, and has received speaker’s fees from Pfizer and Novartis. J.E.L. has received consultant fees from Pfizer and Caption Health and speakers fees from Philips Medical. J.D.M. has received research support and modest consulting fees from Pfizer. T.G.N. has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, BMS, H3-Biomedicine, and Abbvie Pharmaceuticals, outside of the current work. A.N. receives research support from Amgen and is a consultant for Takeda Oncology and AstraZeneca. R.O. has received consultant fees for educational endeavours from AstraZeneca and Bracco. P.T. has received speaker’s fee from Amgen, Takeda, Janssen, and BI and consultation fees from Bay Labs. All other authors declared no conflict of interest.
Contributor Information
Joerg Herrmann, (Chair), Department of Cardiovascular Diseases, Mayo Clinic, 200 First Street SW, Rochester, MN 55902, USA.
Daniel Lenihan, (Co-chair), International Cardio-Oncology Society, 465 Lucerne Ave., Tampa, FL 33606, USA.
Saro Armenian, City of Hope Comprehensive Cancer Center, Department of Population Sciences, 500 E Duarte Rd, Duarte, CA 91010, USA.
Ana Barac, MedStar Heart and Vascular Institute, Georgetown University, 10 Irving Street Northwest Suite NW, Washington, DC 20010, USA.
Anne Blaes, University of Minnesota, Division of Hematology/Oncology, 420 Delaware Street SE, Minneapolis, MN 55455, USA.
Daniela Cardinale, Cardioncology Unit, European Institute of Oncology, IRCCS, Via Adamello 16, 20139 Milan, Italy.
Joseph Carver, Abraham Cancer Center, University of Pennsylvania, Philadelphia, 3400 Civic Center Boulevard, Pavilion 2nd Floor, Philadelphia, PA 19104, USA.
Susan Dent, Duke Cancer Institute, Department of Medicine, Duke University, 20 Duke Medicine Circle, Durham, NA 27704, USA.
Bonnie Ky, Division of Cardiology, University of Pennsylvania, 3400 Civic Center Blvd, Philadelphia, PA 19104, USA.
Alexander R Lyon, Cardio-Oncology Service, Royal Brompton Hospital, Imperial College, Sydney St, London SW3 6NP, United Kingdom.
Teresa López-Fernández, Division of Cardiology; Cardiac Imaging and Cardio-Oncology Unit; La Paz University Hospital, IdiPAZ Research Institute, CIBER CV, C. de Pedro Rico, 6, 28029 Madrid, Spain.
Michael G Fradley, Division of Cardiology, University of Pennsylvania, 3400 Civic Center Blvd, Philadelphia, PA 19104, USA.
Sarju Ganatra, Cardio-Oncology Program, Department of Cardiovascular Medicine, Lahey Hospital and Medical Center, 41 Burlington Mall Road, Burlington, MA 01805, USA.
Giuseppe Curigliano, Department of Oncology and Hemato-Oncology, University of Milano, Via Festa del Perdono 7. 20122 Milano, Italy; European Institute of Oncology, IRCCS, Via Adamello 16, 20139 Milan, Italy.
Joshua D Mitchell, Cardio-Oncology Center of Excellence, Washington University, 4921 Parkview Pl, St. Louis, MO 63110, USA.
Giorgio Minotti, Department of Medicine, University Campus Bio-Medico, Via Álvaro del Portillo, 21, 00128 Roma, Italy.
Ninian N Lang, British Heart Foundation Centre for Cardiovascular Sciences, University of Glasgow, 126 University Place, Glasgow, G12 8TA Scotland, United Kingdom.
Jennifer E Liu, Memorial Sloan Kettering Cancer Center, Department of Medicine/Cardiology Service, 1275 York Ave, New York, NY 10065, USA.
Tomas G Neilan, Cardio-oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St, Boston, MA 02114, USA.
Anju Nohria, Cardio-Oncology Program, Brigham and Women’s Hospital and Dana Farber Cancer Institute, Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA.
Rupal O'Quinn, Division of Cardiology, University of Pennsylvania, 3400 Civic Center Blvd, Philadelphia, PA 19104, USA.
Iskra Pusic, Washington University School of Medicine, Division of Oncology, 4921 Parkview Place, St. Louis, MO 63110, USA.
Charles Porter, Cardiovascular Medicine, Cardio-Oncology Unit, University of Kansas Medical Center, 4000 Cambridge Street, Kansas City, KS 66160, USA.
Kerry L Reynolds, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit St, Boston, MA 02114, USA.
Kathryn J Ruddy, Department of Oncology, Mayo Clinic, 200 First Street SW, Rochester, MN, 55902, USA.
Paaladinesh Thavendiranathan, Department of Medicine, Division of Cardiology, Ted Rogers Program in Cardiotoxicity Prevention, Peter Munk Cardiac Centre, University Health Network, University of Toronto, 585 University Ave, Toronto, ON M5G 2N2, Canada.
Peter Valent, Department of Internal Medicine I, Division of Hematology and Hemostaseology and Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria.
References
- 1. Jemal A, Ward EM, Johnson CJ et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst 2017;109:djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strongman H, Gadd S, Matthews A et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019;394:1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curigliano G, Lenihan D, Fradley M et al. ; ESMO Guidelines Committee. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alvarez-Cardona JA, Ray J, Carver J et al. ; Cardio-Oncology Leadership Council. Cardio-oncology education and training: JACC council perspectives. J Am Coll Cardiol 2020;76:2267–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham R, Mancher M, Miller Wolman D et al. , eds. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 6. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 2020;17:474–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kenigsberg B, Jain V, Barac A. Cardio-oncology related to heart failure: epidermal growth factor receptor target-based therapy. Heart Fail Clin 2017;13:297–309. [DOI] [PubMed] [Google Scholar]
- 8. Agunbiade TA, Zaghlol RY, Barac A. Heart failure in relation to tumor-targeted therapies and immunotherapies. Methodist Debakey Cardiovasc J 2019;15:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agunbiade TA, Zaghlol RY, Barac A. Heart failure in relation to anthracyclines and other chemotherapies. Methodist Debakey Cardiovasc J 2019;15:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Billingham ME, Mason JW, Bristow MR et al. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep 1978;62:865–872. [PubMed] [Google Scholar]
- 11. Goorin AM, Borow KM, Goldman A et al. Congestive heart failure due to adriamycin cardiotoxicity: its natural history in children. Cancer 1981;47:2810–2816. [DOI] [PubMed] [Google Scholar]
- 12. Friedman MA, Bozdech MJ, Billingham ME et al. Doxorubicin cardiotoxicity. Serial endomyocardial biopsies and systolic time intervals. JAMA 1978;240:1603–1606. [DOI] [PubMed] [Google Scholar]
- 13. Alexander J, Berger HJ, Zaret BL. Testing for doxorubicin cardiotoxicity. N Engl J Med 1979;300:1393. [PubMed] [Google Scholar]
- 14. Schwartz RG, McKenzie WB, Alexander J et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med 1987;82:1109–1118. [DOI] [PubMed] [Google Scholar]
- 15. Slamon DJ, Leyland-Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 16. Seidman A, Hudis C, Pierri MK et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002;20:1215–1221. [DOI] [PubMed] [Google Scholar]
- 17. Plana JC, Galderisi M, Barac A et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911–939. [DOI] [PubMed] [Google Scholar]
- 18. Chen MH, Colan SD, Diller L. Cardiovascular disease: cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res 2011;108:619–628. [DOI] [PubMed] [Google Scholar]
- 19. Cornell RF, Ky B, Weiss BM et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol 2019;37:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 21. Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 22. Mueller C, McDonald K, de Boer RA et al. ; on behalf of the Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715–731. [DOI] [PubMed] [Google Scholar]
- 23. Hn K, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011;123:2015–2019. [DOI] [PubMed] [Google Scholar]
- 24. Ky B, Putt M, Sawaya H et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sawaya H, Sebag IA, Plana JC et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawaya H, Sebag IA, Plana JC et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pudil R, Mueller C, Čelutkienė J et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail 2020;22:1966–1983. [DOI] [PubMed] [Google Scholar]
- 28. Freites-Martinez A, Santana N, Arias-Santiago S et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr 2021;112:90–92. [DOI] [PubMed] [Google Scholar]
- 29. Cardinale D, Colombo A, Lamantia G et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
- 30. Cardinale D, Colombo A, Bacchiani G et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 31. Rushton M, Lima I, Tuna M et al. Impact of stopping trastuzumab in early breast cancer: a population-based study in Ontario, Canada. J Natl Cancer Inst 2020;112:1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Copeland-Halperin RS, Al-Sadawi M, Patil S et al. Early trastuzumab interruption and recurrence-free survival in ERBB2-positive breast cancer. JAMA Oncol 2020;6:1971–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Telli ML, Witteles RM, Fisher GA et al. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 2008;19:1613–1618. [DOI] [PubMed] [Google Scholar]
- 34. Lambert J, Lamacie M, Thampinathan B et al. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart 2020;106:817–823. [DOI] [PubMed] [Google Scholar]
- 35. Thavendiranathan P, Grant AD, Negishi T et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013;61:77–84. [DOI] [PubMed] [Google Scholar]
- 36. Oikonomou EK, Kokkinidis DG, Kampaktsis PN et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol 2019;4:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Awadalla M, Mahmood SS, Groarke JD et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol 2020;75:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Houbois CP, Nolan M, Somerset E et al. Serial cardiovascular magnetic resonance strain measurements to identify cardiotoxicity in breast cancer: comparison with echocardiography. JACC Cardiovasc Imaging 2021;14:962–974. [DOI] [PubMed] [Google Scholar]
- 39. Finkelman BS, Putt M, Wang T et al. Arginine-nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. J Am Coll Cardiol 2017;70:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thavendiranathan P, Negishi T, Somerset E et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol 2021;77:392–401. [DOI] [PubMed] [Google Scholar]
- 41. Demissei BG, Hubbard RA, Zhang L et al. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc 2020;9:e014708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Michel L, Mincu RI, Mahabadi AA et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail 2020;22:350–361. [DOI] [PubMed] [Google Scholar]
- 43. Lopez-Sendon J, Alvarez-Ortega C, Zamora Aunon P et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020;41:1720–1729. [DOI] [PubMed] [Google Scholar]
- 44. Caforio AL, Pankuweit S, Arbustini E et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 45. Wang DY, Salem JE, Cohen JV et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bonaca MP, Olenchock BA, Salem JE et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zlotoff DA, Hassan MZO, Zafar A et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer 2021;9:e002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Higgins AY, Arbune A, Soufer A et al. Left ventricular myocardial strain and tissue characterization by cardiac magnetic resonance imaging in immune checkpoint inhibitor associated cardiotoxicity. PLoS One 2021;16:e0246764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schiffer WB, Deych E, Lenihan DJ et al. Coronary and aortic calcification are associated with cardiovascular events on immune checkpoint inhibitor therapy. Int J Cardiol 2021;322:177–182. [DOI] [PubMed] [Google Scholar]
- 50. Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist 2018;23:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang L, Awadalla M, Mahmood SS et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang L, Zlotoff DA, Awadalla M et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 2020;141:2031–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Escudier M, Cautela J, Malissen N et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017;136:2085–2087. [DOI] [PubMed] [Google Scholar]
- 55. Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ganatra S, Parikh R, Neilan TG. Cardiotoxicity of immune therapy. Cardiol Clin 2019;37:385–397. [DOI] [PubMed] [Google Scholar]
- 57. Friedrich MG, Sechtem U, Schulz-Menger J et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferreira VM, Schulz-Menger J, Holmvang G et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 59. Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987;18:619–624. [DOI] [PubMed] [Google Scholar]
- 60. Aretz HT, Billingham ME, Edwards WD et al. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987;1:3–14. [PubMed] [Google Scholar]
- 61. Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol 2020;17:503–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Herrmann J, Yang EH, Iliescu CA et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation 2016;133:1272–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Valent P, Hadzijusufovic E, Schernthaner GH et al. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015;125:901–906. [DOI] [PubMed] [Google Scholar]
- 64. Scappaticci FA, Skillings JR, Holden SN et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007;99:1232–1239. [DOI] [PubMed] [Google Scholar]
- 65. Matsumura C, Chisaki Y, Sakimoto S et al. Evaluation of thromboembolic events in cancer patients receiving bevacizumab according to the Japanese Adverse Drug Event Report database. J Oncol Pharm Pract 2018;24:22–27. [DOI] [PubMed] [Google Scholar]
- 66. Choueiri TK, Schutz FA, Je Y et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol 2010;28:2280–2285. [DOI] [PubMed] [Google Scholar]
- 67. Cortes JE, Kim DW, Pinilla-Ibarz J et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood 2018;132:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–1526. [DOI] [PubMed] [Google Scholar]
- 69. Hijmering ML, Stroes ES, Pasterkamp G et al. Variability of flow mediated dilation: consequences for clinical application. Atherosclerosis 2001;157:369–373. [DOI] [PubMed] [Google Scholar]
- 70. De Roos NM, Bots ML, Schouten EG et al. Within-subject variability of flow-mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound Med Biol 2003;29:401–406. [DOI] [PubMed] [Google Scholar]
- 71. Bots ML, Westerink J, Rabelink TJ et al. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J 2005;26:363–368. [DOI] [PubMed] [Google Scholar]
- 72. Moerland M, Kales AJ, Schrier L et al. Evaluation of the EndoPAT as a tool to assess endothelial function. Int J Vasc Med 2012;2012:904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herrmann J, Lerman A. The endothelium: dysfunction and beyond. J Nucl Cardiol 2001;8:197–206. [DOI] [PubMed] [Google Scholar]
- 74. Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J 2012;33:2771–2782b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aboyans V, Criqui MH, Abraham P et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 76. Willeit P, Tschiderer L, Allara E et al. ; PROG-IMT and the Proof-ATHERO Study Groups. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation 2020;142:621–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reeh J, Therming CB, Heitmann M et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J 2019;40:1426–1435. [DOI] [PubMed] [Google Scholar]
- 78. Maverakis E, Patel F, Kronenberg DG et al. International consensus criteria for the diagnosis of Raynaud's phenomenon. J Autoimmun 2014;48–49:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Belch J, Carlizza A, Carpentier PH et al. ESVM guidelines—the diagnosis and management of Raynaud's phenomenon. Vasa 2017;46:413–423. [DOI] [PubMed] [Google Scholar]
- 80. Aboyans V, Ricco JB, Bartelink MEL et al. ; ESC Scientific Document Group. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO). The Task Force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 81. Knuuti J, Wijns W, Saraste A et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 82. O'Gara PT, Kushner FG, Ascheim DD et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 83. Amsterdam EA, Wenger NK, Brindis RG et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 84. Roffi M, Patrono C, Collet JP et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 85. Ibanez B, James S, Agewall S et al. ; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 86. Thygesen K, Alpert JS, Jaffe AS et al. ; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 87. Powers WJ, Rabinstein AA, Ackerson T et al. ; American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 88. Sacco RL, Kasner SE, Broderick JP et al. ; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century. Stroke 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beltrame JF, Crea F, Kaski JC et al. ; on behalf of the Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 90. Ong P, Camici PG, Beltrame JF et al. ; on behalf of the Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 91. Gerhard-Herman MD, Gornik HL, Barrett C et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2017;69:e71–e126. [DOI] [PubMed] [Google Scholar]
- 92. Williams B, Mancia G, Spiering W et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 93. Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 94. Plummer C, Michael A, Shaikh G et al. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br J Cancer 2019;121:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang YX, Song L, Xing AJ et al. Predictive value of cumulative blood pressure for all-cause mortality and cardiovascular events. Sci Rep 2017;7:41969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Armstrong GT, Oeffinger KC, Chen Y et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Robinson ES, Matulonis UA, Ivy P et al. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol 2010;5:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Maitland ML, Kasza KE, Karrison T et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res 2009;15:6250–6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Khakoo AY, Kassiotis CM, Tannir N et al. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer 2008;112:2500–2508. [DOI] [PubMed] [Google Scholar]
- 100. Abdel-Qadir H, Ethier JL, Lee DS et al. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev 2017;53:120–127. [DOI] [PubMed] [Google Scholar]
- 101. Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med 2008;358:95–97. [DOI] [PubMed] [Google Scholar]
- 102. Totzeck M, Mincu RI, Mrotzek S et al. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: a meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol 2018;25:482–494. [DOI] [PubMed] [Google Scholar]
- 103. Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med 2006;354:980–982. [DOI] [PubMed] [Google Scholar]
- 104. Hamnvik OP, Choueiri TK, Turchin A et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015;121:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Unger T, Borghi C, Charchar F et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension 2020;75:1334–1357. [DOI] [PubMed] [Google Scholar]
- 106. van den Born BJH, Lip GYH, Brguljan-Hitij J et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur J Cardiovasc Pharmocother 2019;5:37–46. [DOI] [PubMed] [Google Scholar]
- 107. Pickering TG, Hall JE, Appel LJ et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 108. Alomar M, Fradley MG. Electrophysiology translational considerations in cardio-oncology: QT and beyond. J Cardiovasc Transl Res 2020;13:390–401. [DOI] [PubMed] [Google Scholar]
- 109. Fradley MG, Moslehi J. QT prolongation and oncology drug development. Card Electrophysiol Clin 2015;7:341–355. [DOI] [PubMed] [Google Scholar]
- 110. Abu Rmilah AA, Lin G, Begna KH et al. Risk of QTc prolongation among cancer patients treated with tyrosine kinase inhibitors. Int J Cancer 2020;147:3160–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Porta-Sanchez A, Gilbert C, Spears D et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J Am Heart Assoc 2017;6:e007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chandrasekhar S, Fradley MG. QT interval prolongation associated with cytotoxic and targeted cancer therapeutics. Curr Treat Options Oncol 2019;20:55. [DOI] [PubMed] [Google Scholar]
- 113. Rautaharju PM, Surawicz B, Gettes LS et al. ; American Heart Association Electrocardiography and Arrhythmias Committee Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009;119:e241–e250. [DOI] [PubMed] [Google Scholar]
- 114. Vandenberk B, Vandael E, Robyns T et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 2016;5:e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Drew BJ, Ackerman MJ, Funk M et al. ; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular Nursing; American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol 2010;55:934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Priori SG, Blomström-Lundqvist C, Mazzanti A et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 117. Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. [DOI] [PubMed] [Google Scholar]
- 118. Hindricks G, Potpara T, Dagres N et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 119. January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 120. Brugada J, Katritsis DG, Arbelo E et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 121. Page RL, Joglar JA, Caldwell MA et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2016;67:e27–e115. [DOI] [PubMed] [Google Scholar]
- 122. Kusumoto FM, Schoenfeld MH, Barrett C et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018; doi: 10.1016/j.jacc.2018.10.044. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.