Abstract
Current evidence suggests that severity and mortality of COVID-19 is higher in men than in women, whereas women might be at increased risk of COVID-19 reinfection and development of long COVID. Differences between sexes have been observed in other infectious diseases and in the response to vaccines. Sex-specific expression patterns of proteins mediating virus binding and entry, and divergent reactions of the immune and endocrine system, in particular the hypothalamic–pituitary–adrenal axis, in response to acute stress might explain the higher severity of COVID-19 in men. In this Personal View, we discuss how sex hormones, comorbidities, and the sex chromosome complement influence these mechanisms in the context of COVID-19. Due to its role in the severity and progression of SARS-CoV-2 infections, we argue that sexual dimorphism has potential implications for disease treatment, public health measures, and follow-up of patients predisposed to the development of long COVID. We suggest that sex differences could be considered in future pandemic surveillance and treatment of patients with COVID-19 to help to achieve better disease stratification and improved outcomes.
Introduction
Evolution has led to a substantial divergence in endocrine, metabolic, and immune functions between males and females that is reflected in sex-specific differences in disease susceptibility and outcomes.1, 2 Factors responsible for sex-specific differences include sex hormones, gender-dependent lifestyle, and environmental aspects, such as smoking, diet, and alcohol consumption. Additionally, the sex chromosome complement, which leads to sex-specific, age-specific, and tissue-specific variations in gene transcription, contributes to these differences.3
A striking example of sexual dimorphism is the currently observed difference in severity and survival between men and women infected with SARS-CoV-2.4 Early reports of COVID-19 already suggested that men are at higher risk of developing severe disease, which is associated with increased case fatality compared with women.5, 6 Sex-disaggregated data from several governments compiled by the Global Health 50/50 research initiative confirmed an increased mortality in men despite similar numbers of COVID-19 cases in men and women.7 Biological sex is associated with life span differences in humans, with a substantially higher life expectancy in women. However, mechanisms responsible for shorter life expectancy in men have already been ruled out as major drivers of excess mortality in men with COVID-19.8
Patients with comorbidities such as diabetes, hypertension, and cancer are at higher risk of a severe course of SARS-CoV-2 infection, with men being significantly more likely to have these comorbidities than women.9 Even before the onset of clinical symptoms or diagnosis of diabetes and metabolic disease, there are striking intrinsic sex hormone-dependent distinctions in metabolic regulation, including insulin sensitivity. Genetic differences are associated with disparate regulation of glycaemic control, insulin sensitivity, lipid metabolism, and adipose tissue homoeostasis; these metabolic processes are primarily, but not exclusively, controlled by sex hormones.10, 11 In an observational study of people with diabetes admitted to hospital for COVID-19, female sex was associated with a lower incidence of severe outcomes but similar mortality compared with men (NCT04324736). The authors of this study concluded that diabetes might reduce the protection that women have over men in terms of susceptibility to severe COVID-19.12 In another cohort study of people with diabetes, male sex, among other factors, such as living in residential care, was identified as a risk factor for developing fatal or critical care unit-treated COVID-19.13 Cardiovascular disease is the leading cause of death among men and women, although epidemiological observations indicate that women have a lower risk of major adverse cardiovascular events than age-matched men and that the manifestation of cardiovascular disease in women is delayed, as cardiovascular risk increases in women predominantly after the menopause. Thus, oestrogens are generally assumed to have protective effects on the cardiovascular system.14
Sex-specific differences in the regulation of the hormonal stress response and inflammatory processes might also contribute to sexual dimorphism in COVID-19.15 This notion is supported by the fact that some diseases with an autoimmune background, such as Grave's disease, systemic lupus erythematosus, and rheumatoid arthritis, show a clear predominance in women.16 Although the cellular and molecular basis for the sex-specific increased incidence of some diseases remains largely unknown, genetic mechanisms have been discussed in the literature. For example, expression of specific innate immune-associated genes (eg, TLR4, TLR7, TLR8) is X-chromosome-linked and the pattern of cytokine expression varies between sexes.17, 18, 19, 20 Moreover, the gene encoding angiotensin-converting enzyme 2 (ACE2), the host receptor that binds coronaviruses such as SARS-CoV-221 is located at specific sites of the X chromosome that commonly escape the inactivation of one X chromosome in mammalian XX cells. This silencing mechanism avoids redundant gene expression in female cells by up to 90%; consequently, XX cells overexpress genes such as ACE2. 22 Males are usually more affected by X-linked pathogenic variants, which might also contribute to the increased severity of COVID-19 in males.23
Moreover, susceptibility to virus infections differs between males and females. Thus, even in previous endemic infections, including SARS-CoV (2002) and MERS-CoV, men were more severely affected than women.4 In general, men with severe acute respiratory syndrome have a significantly higher mortality rate than women.24 Given the close link between the pathophysiological mechanism of COVID-19 and endocrine, metabolic, and immune regulation,25, 26, 27 we argue that various aspects of sexual dimorphism should be more thoroughly considered in disease surveillance and treatment of patients with COVID-19. In this Personal View, we present the current knowledge of sexual dimorphism in COVID-19 and speculate about its clinical and public health implications.
Sex differences in COVID-19
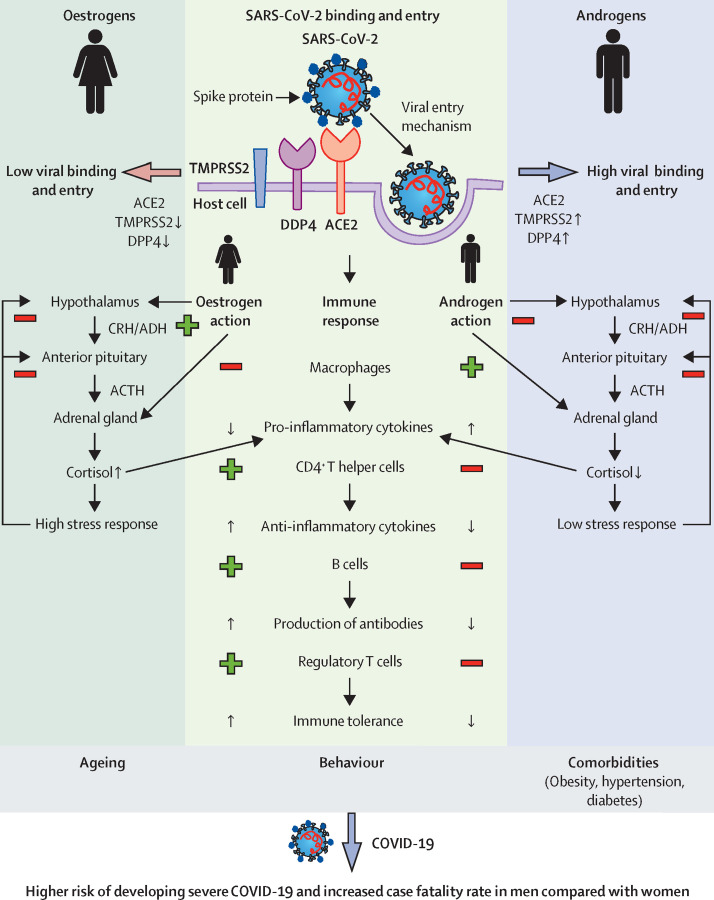
Women are less affected by insulin resistance, have fewer cardiovascular risk factors, and have more favourable protein, microbiome, lipidome, and microRNA expression profiles than men.28, 29, 30, 31, 32 Given that insulin resistance and impaired glucose metabolism are key risk factors for developing severe COVID-19, females might have a more advantageous metabolism, thereby preventing disease progression (figure 1 ).
Figure 1.
Systemic and molecular basis for sexual dimorphism in COVID-19
SARS-CoV-2 uses its spike glycoprotein (S) to attach to the host cell, triggering fusion between virus and lipid membrane (exocytosis). ACE2, TMPRSS2, and DPP4 are critical mediators of this process and show tissue-specific and sex-specific expression patterns. Higher expression of TMPRSS2 and DDP4 in men is associated with increased viral binding and entry, leading to higher viral load compared with women. Oestrogens and androgens regulate the subsequent immune response. Women exhibit a stronger immune response, which is characterised by, among other factors, the release of anti-inflammatory cytokines (eg, IL-6, IL-1β, TNFα). In men, on the other hand, pro-inflammatory cytokines (eg, IL-4, IL-10) dominate. Sex-related differences in the response to stress further contribute to the predisposition of men to a pro-inflammatory state. An overall increased basal activity of the hypothalamic–pituitary–adrenal axis in women, mediated by oestrogens, results in increased cortisol concentrations compared with men. Decreased concentrations of anti-inflammatory cortisol further contribute to the pro-inflammatory status in men, possibly causing more severe COVID-19 courses in men compared with women. Other factors, such as age, smoking, alcohol consumption, and presence of comorbidities further contribute to the increased risk of severe disease and higher case fatality rates in men. Plus symbols mark an activation and minus symbols mark an inhibition. ACE2=angiotensin-converting enzyme 2. ACTH=adrenocorticotropic hormone. ADH=antidiuretic hormone. CRH=corticotropin-releasing hormone. DPP4=dipeptidyl peptidase-4. TMPRSS2=transmembrane protease serine subtype 2.
Higher predisposition to inflammation in men
Infections with a range of pathogens are associated with different immune responses and disease outcomes depending on sex.33 Men are more likely to have a less potent immune response and thus a higher susceptibility or vulnerability to infections.33 Obesity has previously been described as a predictor of severe COVID-19 course.34 However, beyond BMI, the distribution of fat deposits also seems to be important; it has been shown that visceral fat, which accumulates more in men than women, is associated with more severe COVID-19.35, 36 Furthermore, adipose tissue in males contains more macrophages and immune cells with higher and longer-raised cytokine concentrations than in females.37, 38, 39 This might become the source of more rapid and intense systemic inflammation in men contributing to the detrimental rise of cytokines (cytokine storm) observed in critical SARS-CoV-2 infections. It is conceivable that this rapid cytokine response, mediated by adipose tissue among other factors, could even be beneficial initially by providing an immediate immune response. In patients with moderate COVID-19 on no immunomodulatory medications, men had higher plasma concentrations of innate immune cytokines (eg, IL-8, IL-18) together with a more robust induction of non-classical monocytes.40 However, women showed stronger activation of T cells than men during SARS-CoV-2 infections.40 Furthermore, age-dependent effects on the immune system contribute to vulnerability and a more severe course of COVID-19.41 Interestingly, immunosenescence also shows sex-specific effects. Prominent examples in this context are the observation that the number of naive T cells generally decreases with age in both men and women, with a more pronounced drop in men, while a profound decline of B cells is observed only in men.42 In females, high oestradiol and progesterone concentrations suppress pro-inflammatory cytokine production by macrophages and stimulate anti-inflammatory cytokines in CD4+ T helper cells.43, 44 Moreover, oestradiol stimulates antibody production by B cells.45 The stronger immune response mediated by oestradiol and progesterone in females might contribute to less severe COVID-19 infections and lower mortality rates in women compared with men (figure 1).
Sex-dependent DPP4 activity and COVID-19
In addition to ACE2, SARS-CoV-2 uses dipeptidyl peptidase-4 (DPP4) as a co-receptor when entering cells.46 DPP4 is involved in the regulation of the immune response and autoimmune processes and has been identified as a druggable target.47 Therefore, continued administration of DPP4 inhibitors, commonly used in people with diabetes, has been discussed in patients infected with SARS-CoV-2.48, 49 Although a retrospective analysis showed that DPP4 inhibitors can improve mortality in patients with COVID-19 and type 2 diabetes,50 an open-label, prospective, multicenter, randomised clinical trial in three Israeli hospitals found no differences in the time to clinical improvement between hospitalised patients with diabetes and COVID-19 who received linagliptin and a control group receiving standard of care.51
Interestingly, in mice, notable changes in DPP4 activity occur during the oestrous cycle, with a low activity at oestrus and a high activity at dioestrus.52 Exposure to oestrogens diminishes DPP4 activity in ovariectomised mice52 and application of phytoestrogens leads to inhibition of DPP4 activity.53 Conversely, DPP4 inhibitors decrease free androgens in patients with polycystic ovary syndrome54 and might have the potential to reduce the risk of autoimmune disease and inflammation.55 Recent evidence suggests that DPP4 inhibitors alter specific aspects of the innate immune response.56 DPP4 inhibition could potentially also modulate the higher plasma concentrations of innate immune cytokines in males that have also been described in the context of COVID-19.57 Sex hormone-dependent regulation of DPP4 activity could be another important factor in determining different outcomes in COVID-19 severity and mortality between men and women.
TMPRSS2 mediates sex differences in COVID-19 severity
Once SARS-CoV-2 binds to host ACE2, the transmembrane protease serine subtype 2 (TMPRSS2) mediates cleavage of SARS-CoV-2 spike proteins, allowing fusion with the cell membrane.58, 59 Age-dependent regulation of TMPRSS2 in lung epithelium, characterised by increased expression with age, might explain the relative protection of infants and children from severe respiratory illness.60 Single-cell RNA sequencing of lung tissue from 13 healthy men and 13 healthy women revealed no sex-related differences in expression of ACE2, but TMPRSS2 expression was significantly increased in men.61 Moreover, ACE2 + TMPRSS2 + double-positive cells were more than threefold higher in men than in women.61 The tissue-specific higher expression of TMPRSS2 in men might be because TMPRSS2, like ACE2,62 is a known target of the androgen receptor.61, 63 In line with this hypothesis, the anti-androgen enzalutamide lowers TMPRSS2 expression in human lung cells and mouse lungs; moreover, it significantly reduces SARS-CoV-2 entry and infection in lung cells.64 Unfortunately, treatment with the TMPRSS2 inhibitor camostat mesilate did not shorten the time to clinical recovery and failed to reduce mortality in hospitalised patients with COVID-19 in a double-blind, randomised, placebo-controlled multicentre trial (NCT04321096).65 This study highlights that addressing virus uptake alone might not be sufficient to improve the outcome of patients with COVID-19. Therefore, combination therapies that address viral uptake and the pro-inflammatory state, particularly in men, should be considered.
Sexual dimorphism in adrenal stress response and COVID-19
The hypothalamic–pituitary–adrenal (HPA) axis, responsible for integrating and managing internal and external stress stimuli of the organism, demonstrates a clear sex-biased activity, with striking sex differences in the neuroendocrine response particularly to acute stress.66 Females generally present with increased glucocorticoid secretion in response to various acute stressors.67 Adult sex differ—ences in the neuroendocrine response to acute stress are partly the result of interactions between the HPA axis and the endocrine system, which controls reproduction. Therefore, by increasing the production of dihydrotestosterone or oestradiol, the hypothalamic–pituitary–gonadal axis modulates the function of the HPA axis in adults in a sex-dependent manner. Oestradiol treatment enhances the activity of the HPA axis, but endogenous oestrogens have also been reported to have inhibitory effects.66 The importance of the HPA axis, and particularly of the adrenal glands, in the context of COVID-19 is supported by our recent findings demonstrating that the adrenal glands are a potential target for SARS-CoV-2 infection; the resulting cellular damage could potentially predispose patients with COVID-19 to adrenal dysfunction.68
Evolution has resulted in profound differences between sexes that extend to non-reproductive organs. As an example, adrenal gland tissue renewal is highly active and sexually dimorphic, with female mice demonstrating a threefold higher turnover than males.1 Interestingly, females employ an additional stem-cell compartment throughout life, located in the adrenal capsule. In males, these stem cells become inactive by adulthood. Sex-specific stem-cell activity in adrenal development is driven by androgens that repress recruitment of stem cells from the capsule and cell proliferation.1
A more robust and enhanced release of stress hormones by the adrenal glands, including glucocorticoids, in response to acute stressors might contribute to greater protection against severe COVID-19 and mortality in women (figure 1). In this context, it is not surprising that potent glucocorticoids such as dexamethasone have been shown to be the most effective therapy currently available to limit the progression of severe COVID-19 and inflammation.69, 70 In a controlled, open-label trial of 6425 hospitalised patients with COVID-19, treatment with dexamethasone resulted in lower 28-day mortality in those receiving either invasive mechanical ventilation or oxygen only at randomisation.69 A small prospective, triple-blind, randomised controlled trial (84 patients) demonstrated superiority of methylprednisolone compared with dexamethasone in terms of clinical status and length of hospitalisation in patients with COVID-19.71
Besides glucocorticoid concentrations, differential action of cortisol between sexes might contribute to a more favourable response of women to severe COVID-19 (figure 1). Recently, it has been shown that cytokine secretion and responsiveness of lymphomonocytes following cortisol exposure occurs in a sex-dependent manner.72 Thus, following cortisol exposure, the concentrations of the pro-inflammatory cytokines IL-6 and IL-8 were increased in cells derived from males, whereas in female cells, IL-6 release was unchanged and IL-8 concentrations decreased. Furthermore, anti-inflammatory cytokines such as IL-4 and IL-10 did not change in male cells but increased in female cells. Therefore, these results suggest that cortisol can differentially affect lymphomonocytes in males and females, changing the cytokine release from a pro-inflammatory pattern in male cells to a more anti-inflammatory secretion profile in females.72 Sex differences in cytokine storm, as well as in concentrations and effects of endogenous glucocorticoids, might provide a rational pathophysiological basis for explaining the potential advantage of women in managing severe COVID-19.
Clinical and public health implications of sex-based differences in COVID-19
Since men have a higher risk of developing severe COVID-19, the question arises whether older men (≥50 years) with severe comorbidities might require special consideration concerning prevention, screening, surveillance, and vaccination strategies. Conversely, women appear to be at increased risk of some vaccine-related adverse effects, vaccine breakthroughs, and long COVID (discussed in detail later). Therefore, a sex-specific approach could be desirable in making optimal recommendations of prevention and treatment strategies in the context of the COVID-19 pandemic. However, we are only just beginning to define sex-specific preventive and therapeutic approaches for COVID-19. Considering that we are still far away from sex-specific treatment in other areas of clinical practice, it becomes obvious that there is still a long way to go until we reach the goal of a sex-specific or even individualised treatment of our patients.
Oestrogens versus androgens: novel therapeutic approaches in COVID-19
Regarding the development of novel therapeutic approaches in COVID-19, it has been hypothesised that increased oestrogen or progesterone signalling or decreased androgen signalling can be beneficial for improving COVID-19 outcomes in men. Therefore, pharmacological intervention modulating the biological effects of oestrogens and androgens appears to be a plausible approach. Since many drugs targeting these hormonal pathways are approved and have been in clinical use for years and even decades, there are a number of registered clinical trials (eg, NCT04728802, NCT04865029, NCT05172050) addressing this question, some of which have been completed and are awaiting publication. In a small randomised controlled pilot study of 42 men hospitalised with moderate-to-severe COVID-19, subcutaneous administration of progesterone in addition to standard treatment was associated with shorter hospitalisation and reduced requirement for oxygen supplementation compared with standard treatment alone.73 The authors of a community-based study from the Veneto region of northern Italy with 4532 patients concluded that androgen deprivation therapy—used for the treatment of prostate cancer—might reduce the risk of infection with SARS-CoV-2.74 Data from a small prospective cohort of 77 hospitalised men suggested that anti-androgenic treatment might have beneficial effects for the clinical outcome in patients with severe COVID-19.75
A retrospective cohort study of 5451 women with COVID-19 found that women who received hormone replacement therapy (HRT) had a lower mortality rate than those who did not receive HRT.76 This was somewhat expected, as HRT is associated with therapeutic benefits, including reduced incidence of cardiovascular disease.77 In addition, postmenopausal women present with increased concentrations of pro-inflammatory cytokines (IL-6, IL-1) and the use of oestrogen-associated HRT has shown potential to decrease the cytokine concentrations to the premenopausal levels.78 In men with COVID-19, low circulating testosterone concentrations during hospitalisation were associated with increased disease severity, inflammation, and mortality in two observational studies.79, 80 Testosterone administration might also be beneficial in men hospitalised with COVID-19, because testosterone has been reported to effectively reduce inflammation by increasing anti-inflammatory cytokines (IL-10) and decreasing pro-inflammatory cytokines (IL-1β, IL-6, and TNFα).81
Dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) are precursors for sex hormones that decrease with age and are higher in males. DHEA is a powerful inhibitor of glucose-6-phosphate dehydrogenase (G6PD), which has relevance in the context of the COVID-19 pandemic since the reduction of normal G6PD activity has been shown to sensitise human cells to coronavirus 229E infections.82 Elevated DHEA concentrations exhibit toxic effects on endothelial cells, which might enhance SARS-CoV-2-induced vascular endothelialitis.83 These effects are of particular relevance for people with diabetes, as they already have reduced G6PD activity in blood.84 DHEA is an over-the-counter drug in the USA and is commonly used by men to compensate for age-related decline in circulating androgens. In view of the ongoing COVID-19 pandemic, such unrestricted distribution of DHEA might contribute to a more severe course of the disease in individual men, although there are no data currently available to support this theory. Placebo-controlled studies are required to investigate the use of DHEA in elderly men in the context of COVID-19.85
Due to sustained high concentrations of oestrogens during gestation, maternal outcomes in pregnant women with COVID-19 were analysed in a systematic review and meta-analysis based on data from 24 articles including 1100 pregnancies.86 The authors concluded that pregnancy itself does not substantially affect maternal and neonatal outcomes; however, patient numbers were low, especially considering that pregnant women are usually of younger age without specific comorbidities. Conversely, a large US cohort study of women of reproductive age (15–44 years; 91 412 women) revealed that pregnancy (8207 women) was associated with increased risk of hospitalisation, intensive care unit admission, and requirement for mechanical ventilation, but no difference in mortality was found.87 In another cohort study from the UK (427 pregnant women), comorbidities such as hypertension, asthma, and diabetes were identified as major risk factors for hospitalisation of pregnant women.88 Although physiological changes during pregnancy, including hormonal status, have implications for immune response, cardiovascular function, and the respiratory system, current knowledge regarding the course and risks of COVID-19 in pregnancy is still limited.89
Overall, these data suggest that hormone status should be given greater consideration in COVID-19 disease stratification and might offer potential therapeutic approaches for selected patients with COVID-19, but no defined recommendations can be made at this stage. For this purpose, it should first be clarified whether treatment with sex hormones is helpful during acute COVID-19 infection, or whether preventive use is beneficial only for certain populations with increased risk. Potential side effects of sex hormones, such as the occurrence of thrombosis, should also be taken into account.
Sex-dependent effects of COVID-19 vaccines and reinfections
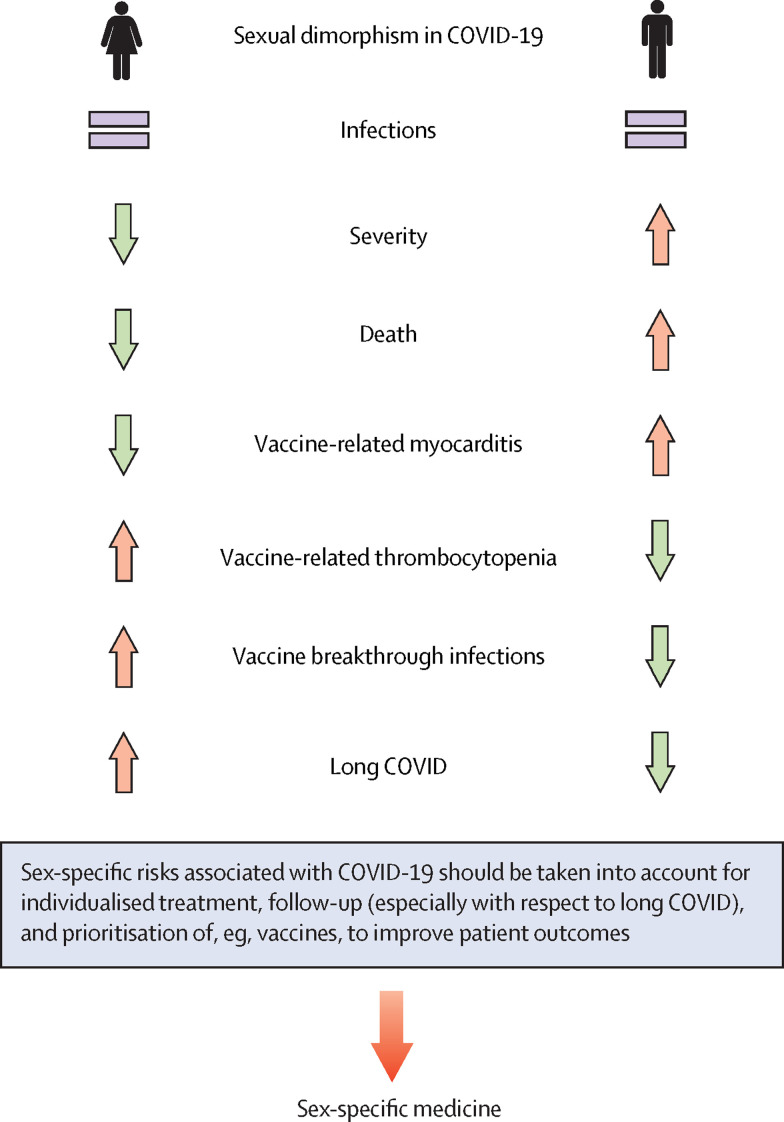
Data from the past 2 years suggest that sex differences might also have implications for responses to SARS-CoV-2 vaccination (figure 2 ) and reinfection. Smaller studies propose that COVID-19 reinfections might be associated with increased severity compared with initial infection in both sexes; moreover, there is evidence that women are more commonly affected by COVID-19 reinfections than men.90, 91 The higher rate of reinfection in women is unexpected, since women show a stronger immune response. The reasons for this apparent paradox are unclear but might be related to increased antibody responses found in male convalescent plasma donors.57, 92 Described differences in social behaviour during the COVID-19 pandemic would also suggest increased susceptibility to reinfection in men compared with women. Panel evidence suggests that women are more likely to perceive COVID-19 as a very serious health issue and therefore more likely to agree with and comply with restrictive policies.93 A study using mobile phone data from 1·2 million devices in Austria found gender differences in social behaviour during different phases of the COVID-19 pandemic; for example, women avoided larger shopping malls during lockdown, and after lockdown, men returned to their normal social behaviour faster than women.94
Figure 2.
Sexual dimorphism in severity and mortality of COVID-19
Men experience more severe disease courses and more deaths connected to COVID-19, but women appear to be at increased risk of long COVID. Therefore, we argue that sex should be taken in consideration regarding COVID-19 treatment, follow-up, and establishment of public health measures.
An asymptomatic or mild course of a first COVID-19 infection seems to increase the likelihood of reinfection compared with patients with symptomatic disease, which could be explained by a weaker immune response after a first infection with a mild course.95, 96 However, the COVID-19 reinfection rate associated with the beta and delta SARS-CoV-2 variants is low. Population-based data now indicate that the new omicron variant, first described in South Africa in November, 2021, is associated with a substantial ability to bypass immunity to previous infection,97 potentially resulting in an increase in the number of reinfections.98 COVID-19 vaccines are an effective tool to reduce the risk of reinfection.99
Women exhibit a more robust immune response to vaccines, associated with higher and longer-lasting protective antibody responses,33 but report more frequently adverse side effects, including fever, pain, and inflammation, compared with vaccinated men.100 This could be because women are more likely than men to report adverse side effects.100 As discussed earlier, oestrogens and androgens differentially modulate immune responses (figure 1), including responses to vaccines; however, other factors, such as epigenetic and genetic differences and an additional X chromosome, are likely to play a role.
Another point of discussion is the general safety of currently available COVID-19 vaccines and, specifically, how extremely rare cases of unusual thrombocytopenia should be handled. These events, first reported after immunisation with ChAdOx1 nCoV-19 (AstraZeneca), have also been observed after administration of Ad26.COV2.S (Johnson & Johnson/Janssen)101 and appear to occur preferentially in women below the age of 50 years.102 The pathophysiology of these venous thromboembolic events affecting the cerebral sinus and splanchnic and pulmonary veins, and the reason why predominantly women are affected, remains largely unclear. This form of thrombocytopenia is mediated by platelet-activating autoantibodies against platelet factor 4 (PF4) and carries some resemblance to autoimmune heparin-induced thrombocytopenia.102, 103 While the European Medicines Agency continues to classify ChAdOx1 nCoV-19 (AstraZeneca) as safe and effective and recommends its use without restriction, several national and regional authorities in Europe, as well as in Australia and the UK, have restricted the use of this vaccine. In most countries, its use is recommended for people older than 60 years. Recent case reports describe rare cases of myocarditis (24 cases per million) after a second dose of mRNA-based COVID-19 vaccine, occurring predominantly in young men (18–29 years).104, 105 Moreover, rare cases of Guillain-Barré syndrome have been described after vaccination with Ad26.COV2.S (Johnson & Johnson/Janssen), particularly in men aged 50–64 years.105 Despite these extremely rare potential adverse events, benefits of these vaccines clearly outweigh their risks.
Although all approved vaccines are highly effective, even fully vaccinated individuals can develop symptomatic or asymptomatic infection with SARS-CoV-2. Breakthrough infections in two women were reported in a fully vaccinated cohort (417 individuals), which received the second dose of BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna) vaccine at least 2 weeks earlier.106 In the USA, 10 262 SARS-CoV-2 vaccine breakthrough infections had been reported as of April 13, 2021, of which 6446 (63%) occurred in women.107 These preliminary data indicate that females appear to be more at risk of vaccine breakthrough infections than males. With the emergence of the new omicron variant, which might bypass immunity, an adjustment of existing vaccines might be necessary to prevent increasing numbers of breakthrough infections.98 The outlined differences between sexes with respect to susceptibility to reinfection and response to SARS-CoV-2 vaccines are rooted most likely in their specific immune responses; hence, we suggest that future recommendations for the allocation and prioritisation of certain vaccines should take biological sex into consideration.
Sex-related predisposition to long COVID
Another phenomenon with potential sex-related predisposition is long COVID (also named post-COVID syndrome), which is defined as a complex of non-specific persisting symptoms, such as chronic fatigue, muscle weakness, sleep difficulties, anxiety, and depression, that are observed in individuals after acute COVID-19 and are not explained by other diagnoses.108 Post-virus syndromes, including chronic fatigue syndrome, are not uncommon after a variety of viral infections with extended course—eg, caused by cytomegalovirus or Epstein-Barr virus.109, 110 An increasing number of long COVID cases have been reported during the past months, and a female predominance is emerging, similar to chronic fatigue syndrome.111, 112 In a cohort study of 5838 individuals in Switzerland, women reported more frequently at least one persistent symptom, with reduced resilience being the most common symptom in both men and women.113 In women, cardiovascular risk factors, pre-existing mental illness, and self-reported domestic stress increased the risk of long COVID.113 Besides female sex, number of symptoms in the first week, BMI, and increasing age were found to be predictors for long COVID.114 For long COVID in particular, as well as for any other symptoms reported to the physician, it is important to consider that there might also be sex-related differences in how symptoms are perceived and reported, which might affect outcomes of studies. For example, women with West Nile virus infection reported significantly more symptoms compared with men despite a similar viral load in men and women.115
Increasing evidence suggests that autoantibodies, whose concentrations exhibit also sex-specific differences,116, 117 play a crucial role in the extended multi-organ illness in patients with long COVID.118, 119 Pre-existing asthma, which is more prevalent in women than in men, has been reported to further increase the risk of developing long COVID.114, 120 Further characterisation of predictors for long COVID, such as sex and comorbidities, might help to identify patients at high risk of developing long COVID and allow early intervention to address their individual needs and improve outcomes.
Search strategy and selection criteria.
We searched PubMed and Google Scholar to identify relevant articles published up to Dec 15, 2021, using combinations of the search items “sexual dimorphism”, “gender differences”, “gender”, “females”, “males”, “coronavirus”, “COVID-19”, “SARS-CoV-2”, “long COVID”, “post-COVID”, “reinfections”, “vaccine”, “breakthrough infections”, “DPP4”, “TMPRSS2”, “hormones”, “hormone replacement”, “estrogens”, “androgens”, “glucocorticoids”, “cardiovascular disease”, “diabetes”, “obesity”, “risk factors”, and “co-morbidities”. The reference list of original articles, position statements, narrative and systematic reviews, and meta-analyses was screened for relevant publications. We also partially reviewed relevant references cited in the articles. Only manuscripts published in English were considered. The final reference list was prepared based on relevance to the topic of this Personal View, with preference given to the most recent relevant publications.
Conclusion
Taken together, there is evidence that sexual dimorphism in COVID-19 has potential implications that should be considered in treatment of COVID-19 and follow-up of patients predisposed to the development of long COVID, as well as for vaccine prioritisation. While COVID-19 infections are more frequently associated with a severe course and higher mortality in men, women appear to be predisposed to long COVID (figure 2). Although general molecular mechanisms do not differ between males and females, differences in the expression patterns of several cell surface proteins responsible for virus binding and entry, as well as sex-specific differences in the stress and immune response, likely contribute to the observed sexual dimorphism in COVID-19. Reanalysis of our own data121 regarding sexual dimorphism suggests that male patients have a higher expression of ACE2 and inflammatory markers in the coronary tree than female patients with similar cardiovascular diseases. This might further underline a specific predisposition of men to having a higher susceptibility to severe and fatal COVID-19. Lifestyle and behavioural factors, differences in the presence of comorbidities, and sex-specific risk factors also contribute to sexual dimorphism in COVID-19 and should always be considered. Although there are no known clear mechanisms yet to explain sexual dimorphism in COVID-19, as reviewed here, there are many possible leads, some of which are worthy of further exploration. Therefore, larger prospective and mechanistic studies are required to provide scientifically robust evidence to draw improved conclusions that would allow for clear recommendations for the prevention and management of patients with COVID-19 based on sex differences.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Susan Richter (Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Carl Gustav Carus, Technische Universität Dresden) for the scientific discussion and editorial review. The authors are supported by the Deutsche Forschungsgemeinschaft within the CRC/Transregio 205, project number 314061271 - TRR205 “The Adrenal: Central Relay in Health and Disease” (NB, SH, CLA, and SRB) and project HA 8297/1-1 (CH).
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
NB, AB, and SRB conceptualised this Personal View. NB created the figures. NB, AB, and SRB wrote the original draft. NB, AB, AS, SH, ZV, MM, CH, FB, CW, LP, CLA, RS, RRG, AD, YS, GM, and SRB reviewed and edited the manuscript.
References
- 1.Grabek A, Dolfi B, Klein B, Jian-Motamedi F, Chaboissier MC, Schedl A. The adult adrenal cortex undergoes rapid tissue renewal in a sex-specific manner. Cell Stem Cell. 2019;25:290–296.e2. doi: 10.1016/j.stem.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Manuel RSJ, Liang Y. Sexual dimorphism in immunometabolism and autoimmunity: impact on personalized medicine. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2021.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185–201. doi: 10.1038/nrneph.2017.189. [DOI] [PubMed] [Google Scholar]
- 4.Alwani M, Yassin A, Al-Zoubi RM, et al. Sex-based differences in severity and mortality in COVID-19. Rev Med Virol. 2021;31 doi: 10.1002/rmv.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao S, Cao P, Chong MKC, et al. COVID-19 and gender-specific difference: analysis of public surveillance data in Hong Kong and Shenzhen, China, from January 10 to February 15, 2020. Infect Control Hosp Epidemiol. 2020;41:750–751. doi: 10.1017/ice.2020.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Health 5050 The sex, gender and COVID-19 project. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/dataset/
- 8.Geldsetzer P, Mukama T, Jawad N, Riffe T, Rogers A, Sudharsanan N. Sex differences in the mortality rate for coronavirus disease 2019 compared to other causes of death. medRxiv. 2021 doi: 10.1101/2021.02.23.21252314. published online Feb 26. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarty D, Nair SS, Hammouda N, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol. 2020;3:374. doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris A. Sex differences for fasting levels of glucose and insulin: expanding our understanding. Nat Rev Endocrinol. 2021;17:131. doi: 10.1038/s41574-021-00472-7. [DOI] [PubMed] [Google Scholar]
- 11.Lagou V, Mägi R, Hottenga J-J, et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat Commun. 2021;12:1–18. doi: 10.1038/s41467-020-19366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tramunt B, Smati S, Coudol S, et al. Sex disparities in COVID-19 outcomes of inpatients with diabetes: insights from the CORONADO study. Eur J Endocrinol. 2021;185:299–311. doi: 10.1530/EJE-21-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGurnaghan SJ, Weir A, Bishop J, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8:33. doi: 10.1186/s13293-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldhuis JD, Sharma A, Roelfsema F. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab Clin North Am. 2013;42:201–225. doi: 10.1016/j.ecl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amur S, Parekh A, Mummaneni P. Sex differences and genomics in autoimmune diseases. J Autoimmun. 2012;38:J254–J265. doi: 10.1016/j.jaut.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Umiker BR, Andersson S, Fernandez L, et al. Dosage of X-linked Toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur J Immunol. 2014;44:1503–1516. doi: 10.1002/eji.201344283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Driscoll DN, De Santi C, McKiernan PJ, McEneaney V, Molloy EJ, Greene CM. Expression of X-linked Toll-like receptor 4 signaling genes in female vs. male neonates. Pediatr Res. 2017;81:831–837. doi: 10.1038/pr.2017.2. [DOI] [PubMed] [Google Scholar]
- 19.Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13:2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 21.Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 22.Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. doi: 10.1038/s41420-020-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Migeon BR. X-linked diseases: susceptible females. Genet Med. 2020;22:1156–1174. doi: 10.1038/s41436-020-0779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barthel A, Mohanraj K, Biener AM, Bornstein SR. Metabolic syndrome and COVID-19: endocrine-immune vascular interactions shape the clinical course. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steenblock C, Schwarz PEH, Ludwig B, et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elzinga SE, Savelieff MG, O'Brien PD, Mendelson FE, Hayes JM, Feldman EL. Sex differences in insulin resistance, but not peripheral neuropathy, in a diet-induced prediabetes mouse model. Dis Model Mech. 2021;14 doi: 10.1242/dmm.048909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broussard JL, Perreault L, Macias E, et al. Sex differences in insulin sensitivity are related to muscle tissue acylcarnitine but not subcellular lipid distribution. Obesity (Silver Spring) 2021;29:550–561. doi: 10.1002/oby.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng L, Jena PK, Hu Y, Wan YY. Age-specific microbiota in altering host inflammatory and metabolic signaling as well as metabolome based on the sex. Hepatobiliary Surg Nutr. 2021;10:31–48. doi: 10.21037/hbsn-20-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17:47–66. doi: 10.1038/s41574-020-00431-8. [DOI] [PubMed] [Google Scholar]
- 32.Karere GM, Cox LA, Bishop AC, et al. Sex differences in microRNA expression and cardiometabolic risk factors in hispanic adolescents with obesity. J Pediatr. 2021;235:138–143.e5. doi: 10.1016/j.jpeds.2021.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 34.Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14:655–659. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen A, Bressem K, Albrecht J, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110 doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Földi M, Farkas N, Kiss S, et al. Visceral adiposity elevates the risk of critical condition in COVID-19: a systematic review and meta-analysis. Obesity (Silver Spring) 2021;29:521–528. doi: 10.1002/oby.23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa A, Wada T, Nishimura S, et al. Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camporez JP, Lyu K, Goldberg EL, et al. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol. 2019;597:3885–3903. doi: 10.1113/JP277270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varghese M, Griffin C, McKernan K, et al. Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Endocrinology. 2019;160:293–312. doi: 10.1210/en.2018-00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65 doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Márquez EJ, Chung CH, Marches R, et al. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wray S, Arrowsmith S. The physiological mechanisms of the sex-based difference in outcomes of COVID19 infection. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.627260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers MJ, Petersen BH. Estradiol induced alterations of the immune system--I. Enhancement of IgM production. Int J Immunopharmacol. 1985;7:207–213. doi: 10.1016/0192-0561(85)90028-1. [DOI] [PubMed] [Google Scholar]
- 46.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subbarayan K, Ulagappan K, Wickenhauser C, Bachmann M, Seliger B. Immune interaction map of human SARS-CoV-2 target genes: implications for therapeutic avenues. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.597399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hariyanto TI, Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;20:1–8. doi: 10.1007/s40200-021-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khunti K, Knighton P, Zaccardi F, et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol. 2021;9:293–303. doi: 10.1016/S2213-8587(21)00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Cai Z, Zhang J. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: a meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abuhasira R, Ayalon-Dangur I, Zaslavsky N, et al. A randomized clinical trial of linagliptin vs. standard of care in patients hospitalized with diabetes and COVID-19. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.794382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohta N, Takahashi T, Mori T, et al. Hormonal modulation of prolyl endopeptidase and dipeptidyl peptidase IV activities in the mouse uterus and ovary. Acta Endocrinol (Copenh) 1992;127:262–266. doi: 10.1530/acta.0.1270262. [DOI] [PubMed] [Google Scholar]
- 53.Rajput MS, Sarkar PD, Nirmal NP. Inhibition of DPP-4 activity and neuronal atrophy with genistein attenuates neurological deficits induced by transient global cerebral ischemia and reperfusion in streptozotocin-induced diabetic mice. Inflammation. 2017;40:623–635. doi: 10.1007/s10753-017-0509-5. [DOI] [PubMed] [Google Scholar]
- 54.Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther Adv Endocrinol Metab. 2021;12 doi: 10.1177/2042018821989238. 2042018821989238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis. 2015;74:1968–1975. doi: 10.1136/annrheumdis-2014-205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yazbeck R, Jaenisch SE, Abbott CA. Dipeptidyl peptidase 4 inhibitors: applications in innate immunity? Biochem Pharmacol. 2021;188 doi: 10.1016/j.bcp.2021.114517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi T, Iwasaki A. Sex differences in immune responses. Science. 2021;371:347–348. doi: 10.1126/science.abe7199. [DOI] [PubMed] [Google Scholar]
- 58.Ou T, Mou H, Zhang L, Ojha A, Choe H, Farzan M. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuler BA, Habermann AC, Plosa EJ, et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J Clin Invest. 2021;131 doi: 10.1172/JCI140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okwan-Duodu D, Lim E-C, You S, Engman DM. TMPRSS2 activity may mediate sex differences in COVID-19 severity. Signal Transduct Target Ther. 2021;6:100. doi: 10.1038/s41392-021-00513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuel RM, Majd H, Richter MN, Ghazizadeh Z, Zekavat SM, Navickas A, et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27:876–889. doi: 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020;12:10087–10098. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leach DA, Mohr A, Giotis ES, et al. The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells. Nat Commun. 2021;12 doi: 10.1038/s41467-021-24342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunst JD, Staerke NB, Pahus MH, et al. Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19 —a double-blind randomized controlled trial. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis' response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44:45–58. doi: 10.1038/s41386-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanczkowski W, Evert K, Stadtmüller M, et al. COVID-19 targets human adrenal glands. Lancet Diabetes Endocrinol. 2021 doi: 10.1016/S2213-8587(21)00291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Águas R, Mahdi A, Shretta R, Horby P, Landray M, White L. Potential health and economic impacts of dexamethasone treatment for patients with COVID-19. Nat Commun. 2021;12:1–8. doi: 10.1038/s41467-021-21134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21:1–8. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Da Pozzo E, Giacomelli C, Cavallini C, Martini C. Cytokine secretion responsiveness of lymphomonocytes following cortisol cell exposure: sex differences. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghandehari S, Matusov Y, Pepkowitz S, et al. Progesterone in addition to standard of care vs standard of care alone in the treatment of men hospitalized with moderate to severe COVID-19: a randomized, controlled pilot trial. Chest. 2021;160:74–84. doi: 10.1016/j.chest.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goren A, Wambier CG, Herrera S, et al. Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men. J Eur Acad Dermatol Venereol. 2021;35:e13–e15. doi: 10.1111/jdv.16953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dambha-Miller H, Hinton W, Joy M, Feher M, de Lusignan S. Mortality in COVID-19 amongst women on hormone replacement therapy or combined oral contraception: a cohort study. medRxiv. 2021 doi: 10.1101/2021.02.16.21251853. published online Feb 19. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nappi RE, Simoncini T. Menopause transition: a golden age to prevent cardiovascular disease. Lancet Diabetes Endocrinol. 2021;9:135–137. doi: 10.1016/S2213-8587(21)00018-8. [DOI] [PubMed] [Google Scholar]
- 78.Okpechi SC, Fong JT, Gill SS, et al. Global sex disparity of COVID-19: a descriptive review of sex hormones and consideration for the potential therapeutic use of hormone replacement therapy in older adults. Aging Dis. 2021;12:671–683. doi: 10.14336/AD.2020.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhindsa S, Zhang N, McPhaul MJ, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohamad N-V, Wong SK, Hasan WNW, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2018 doi: 10.1080/13685538.2018.1482487. [DOI] [PubMed] [Google Scholar]
- 82.Wu Y-H, Tseng C-P, Cheng M-L, Ho H-Y, Shih S-R, Chiu DT-Y. Glucose-6-phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. J Infect Dis. 2008;197:812–816. doi: 10.1086/528377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heymann AD, Cohen Y, Chodick G. Glucose-6-phosphate dehydrogenase deficiency and type 2 diabetes. Diabetes care. 2012;35:e58. doi: 10.2337/dc11-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nyce J. Alert to US physicians: DHEA, widely used as an OTC androgen supplement, may exacerbate COVID-19. Endocr Relat Cancer. 2021;28:R47–R53. doi: 10.1530/ERC-20-0439. [DOI] [PubMed] [Google Scholar]
- 86.Di Toro F, Gjoka M, Di Lorenzo G, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:36–46. doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wastnedge EAN, Reynolds RM, van Boeckel SR, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101:303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adrielle Dos Santos L, Filho PGG, Silva AMF, et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J Infect. 2021;82:399–406. doi: 10.1016/j.jinf.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdelrahman MM, Abd-Elrahman NM, Bakheet TM. Persistence of symptoms after improvement of acute COVID19 infection, a longitudinal study. J Med Virol. 2021;93:5942–5946. doi: 10.1002/jmv.27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klein SL, Pekosz A, Park H-S, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130:6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galasso V, Pons V, Profeta P, Becher M, Brouard S, Foucault M. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. Proc Natl Acad Sci USA. 2020;117:27285–27291. doi: 10.1073/pnas.2012520117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reisch T, Heiler G, Hurt J, Klimek P, Hanbury A, Thurner S. Behavioral gender differences are reinforced during the COVID-19 crisis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-97394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2021;21:3–5. doi: 10.1016/S1473-3099(20)30783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Elslande J, Vermeersch P, Vandervoort K, et al. Symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection by a phylogenetically distinct strain. Clin Infect Dis. 2021;73:354–356. doi: 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pulliam JR, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv. 2021 doi: 10.1101/2021.11.11.21266068. published online Dec 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Callaway E, Ledford H. How bad is Omicron? What scientists know so far. Nature. 2021;600:197–199. doi: 10.1038/d41586-021-03614-z. [DOI] [PubMed] [Google Scholar]
- 99.Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination—Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muir K-L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26. COV2. S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cines DB, Bussel JB. SARS-CoV-2 Vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021;6:1115–1117. doi: 10.1001/jamacardio.2021.2821. [DOI] [PubMed] [Google Scholar]
- 105.Rosenblum HG, Hadler SC, Moulia D, et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the Advisory Committee on Immunization Practices—United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1094–1099. doi: 10.15585/mmwr.mm7032e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.CDC COVID-19 Vaccine Breakthrough Case Investigations Team COVID-19 vaccine breakthrough infections reported to CDC—United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:792–793. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ortega-Hernandez OD, Shoenfeld Y. Infection, vaccination, and autoantibodies in chronic fatigue syndrome, cause or coincidence? Ann N Y Acad Sci. 2009;1173:600–609. doi: 10.1111/j.1749-6632.2009.04799.x. [DOI] [PubMed] [Google Scholar]
- 110.Appel S, Chapman J, Shoenfeld Y. Infection and vaccination in chronic fatigue syndrome: myth or reality? Autoimmunity. 2007;40:48–53. doi: 10.1080/08916930701197273. [DOI] [PubMed] [Google Scholar]
- 111.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 112.Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gebhard CE, Suetsch C, Bengs S, et al. Sex-and gender-specific risk factors of post-COVID-19 syndrome: a population-based cohort study in Switzerland. medRxiv. 2021 doi: 10.1101/2021.06.30.21259757. published online July 6. (preprint). [DOI] [Google Scholar]
- 114.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoffman KW, Lee JJ, Foster GA, Krysztof D, Stramer SL, Lim JK. Sex differences in cytokine production following West Nile virus infection: Implications for symptom manifestation. Pathog Dis. 2019;77 doi: 10.1093/femspd/ftz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pandey JP, Fudenberg HH, Ainsworth SK, Loadholt CB. Autoantibodies in healthy subjects of different age groups. Mech Ageing Dev. 1979;10:399–404. doi: 10.1016/0047-6374(79)90021-6. [DOI] [PubMed] [Google Scholar]
- 117.Williams AJ, Norcross AJ, Dix RJ, Gillespie KM, Gale EA, Bingley PJ. The prevalence of insulin autoantibodies at the onset of type 1 diabetes is higher in males than females during adolescence. Diabetologia. 2003;46:1354–1356. doi: 10.1007/s00125-003-1197-2. [DOI] [PubMed] [Google Scholar]
- 118.Khamsi R. Rogue antibodies could be driving severe COVID-19. Nature. 2021;590:29–31. doi: 10.1038/d41586-021-00149-1. [DOI] [PubMed] [Google Scholar]
- 119.Bornstein SR, Voit-Bak K, Donate T, et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01148-4. published online June 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax. 2012;67:625–631. doi: 10.1136/thoraxjnl-2011-201249. [DOI] [PubMed] [Google Scholar]
- 121.Maccio U, Zinkernagel AS, Shambat SM, et al. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103182. [DOI] [PMC free article] [PubMed] [Google Scholar]