Abstract
Background
Concomitant seasonal influenza vaccination with a COVID-19 vaccine booster could help to minimise potential disruption to the seasonal influenza vaccination campaign and maximise protection against both diseases among individuals at risk of severe disease and hospitalisation. This study aimed to assess the safety and immunogenicity of concomitant administration of high-dose quadrivalent influenza vaccine (QIV-HD) and a mRNA-1273 vaccine booster dose in older adults.
Methods
This study is an ongoing, phase 2, multicentre, open-label, descriptive trial at six clinical research sites in the USA. We describe the interim results up to 21 days after vaccination (July–August, 2021). Community-dwelling adults aged 65 years and older, who were previously vaccinated with a two-dose primary schedule of the mRNA-1273 SARS-CoV-2 vaccine, were eligible for inclusion. The second dose of the primary mRNA-1273 vaccination series was required to have been received at least 5 months before enrolment in the study. Participants were randomly assigned (1:1:1) using a permuted block method stratified by site and by age group (<75 years vs ≥75 years), to receive concomitant administration of QIV-HD and mRNA-1273 vaccine, QIV-HD alone, or mRNA-1273 vaccine alone. Randomisation lists, generated by Sanofi Pasteur biostatistics platform, were provided to study investigators for study group allocation. Unsolicited adverse events occurring immediately, solicited local and systemic reactions up to day 8, and unsolicited adverse events, serious adverse events, adverse events of special interest, and medically attended adverse events up to day 22 were reported. Haemagglutination inhibition antibody responses to influenza A/H1N1, A/H3N2, B/Yamagata, and B/Victoria strains and SARS CoV-2 binding antibody responses (SARS-CoV-2 pre-spike IgG ELISA) were assessed at day 1 and day 22. All analyses were descriptive. The study is registered with ClinicalTrials.gov, NCT04969276.
Findings
Between July 16 and Aug 31, 2021, 306 participants were enrolled and randomly assigned, of whom 296 received at least one vaccine dose (100 in the coadministration group, 92 in the QIV-HD, and 104 in the mRNA-1273 group). Reactogenicity profiles were similar between the coadministration and mRNA-1273 groups, with lower reactogenicity rates in the QIV-HD group (frequency of solicited injection site reactions 86·0% [95% CI 77·6–92·1], 91·3% [84·2–96·0], and 61·8% [50·9–71·9]; frequency of solicited systemic reactions 80·0%, [70·8–87·3], 83·7% [75·1–90·2], and 49·4% [38·7–60·2], respectively). Up to day 22, unsolicited adverse events were reported for 17·0% (95% CI 10·2–25·8) of participants in the coadministration group and 14·4% (8·3–22·7) of participants in the mRNA-1273 group, and tended to be reported at a slightly lower rate (10·9% [5·3–19·1]) in participants in the QIV-HD group. Seven participants each reported one medically attended adverse event (three in the coadministration group, one in the QIV-HD group, and three in the mRNA-1273 group). There were no serious adverse events, adverse events of special interest, or deaths. Haemagglutination inhibition antibody geometric mean titres increased from day 1 to day 22 to similar levels in the coadministration and QIV-HD groups, for each influenza strain (A/H1N1: 363 [95% CI 276–476] vs 366 [272–491]; A/H3N2: 286 [233–352] vs 315 [257–386]; B/Yamagata: 429 [350–525] vs 471 [378–588]; B/Victoria: 377 [325–438] vs 390 [327–465] for the coadministration and QIV-HD groups, respectively). SARS-CoV-2 binding antibody geometric mean concentrations also increased to similar levels in the coadministration and mRNA-1273 groups at day 22 (7634 [95% CI 6445–9042] and 7904 [6883–9077], respectively).
Interpretation
No safety concerns or immune interference were observed for concomitant administration of QIV-HD with mRNA-1273 booster in adults aged 65 years and older, supporting co-administration recommendations.
Funding
Sanofi Pasteur.
Research in context.
Evidence before this study
To support optimal uptake of seasonal COVID-19 vaccines, numerous governments currently provide guidance on concomitant administration of seasonal influenza vaccine with a dose of COVID-19 vaccine in individuals at risk of severe disease and hospitalisation. We searched the following terms in PubMed on Oct 20, 2021: (“covid-19 vaccine” OR “sars-cov-2 vaccine”), (“influenza vaccine” OR “flu vaccine”), (“concomitant” OR “coadminist*”), (“flu” OR “influenza”), and (“COVID-19” OR “SARS-CoV-2”), limited to clinical trials (no limit on date or language), and searched the preprint servers medRxiv and bioRxiv using combinations of the same terms. Although no peer-reviewed publications reporting clinical evaluation of concomitant administration of SARS-CoV-2 and influenza vaccines were retrieved from the PubMed search, two studies of interest were retrieved from the preprint servers (now published in peer-reviewed journals). Toback and colleagues describe the results of a small substudy of a phase 3 randomised trial of two doses of the SARS-CoV-2 vaccine NVX-CoV2373 (primary series), wherein around 400 participants with a median age of 39 years received NVX-CoV2373 (first dose) or placebo concomitantly with an age-appropriate influenza vaccine (cellular quadrivalent influenza vaccine for those aged 18–64 years of age or adjuvanted trivalent influenza vaccine for those ≥65 years). No early safety concerns were identified in those who received NVX-CoV2373 with influenza vaccine, and immunogenicity of the influenza vaccination was preserved in the co-administered group compared with those who received influenza vaccine alone, although a modest decrease in the immunogenicity of NVX-CoV2373 was observed. Lazarus and colleagues provide data supporting the concomitant administration of age-appropriate seasonal influenza vaccine (adjuvanted trivalent vaccine or a cellular or recombinant quadrivalent vaccine) with a second dose of a COVID-19 vaccine (ChAdOx1 or BNT162b2; primary series) in adults, including older adults (≥65 years), in the UK (ComFluCOV study). The study found acceptable safety and tolerability after concomitant administration of the two vaccines, without negative interference on the immunogenicity of either vaccine given alone.
Added value of this study
To our knowledge, we show for the first time in this study the acceptable safety and tolerability of concomitant administration of a high-dose quadrivalent influenza vaccine (Fluzone High-Dose Quadrivalent; Sanofi Pasteur; Lyon, France) with a booster (third) dose of the mRNA-1273 vaccine (Moderna), with no evidence of immune interference compared with administration of each vaccine dose alone in older adults (≥65 years) who had received a second dose of mRNA-1273 around 5 months before this study.
Implications of all the available evidence
This descriptive analysis adds to the available evidence supporting the concomitant administration of vaccine doses against seasonal influenza and COVID-19. Furthermore, this is the first study to our knowledge to suggest acceptable safety and immunogenicity of concomitant administration of a booster dose of COVID-19 vaccine with high-dose quadrivalent influenza vaccine in older adults, who are a priority target group.
Introduction
COVID-19, the disease caused by SARS-CoV-2, first emerged in humans in December, 2019, and spread rapidly to global pandemic status within a few months. The COVID-19 pandemic continues to have a devastating impact on public health systems, economies, and societies worldwide. Risk factors for severe illness and mortality include age older than 75 years, male sex, severe obesity (BMI >40 kg/m2), and active cancer.1 The accelerated development and deployment of vaccines against SARS-CoV-2 have helped to limit COVID-19-related severe disease, hospitalisations, and deaths in parts of the world where vaccines have been effectively rolled out, with mRNA COVID-19 vaccines among the first to be made available.2, 3, 4, 5, 6 The mRNA-1273 vaccine (100 μg dose)7 has been registered in multiple countries for active two-dose immunisation in individuals aged 12 years and older, with some countries also recommending a third 100 μg dose for individuals aged 18 years and older with severe immunosuppression.8, 9 On October 21 2021, the US Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee and the US Centers for Disease Control and Prevention Advisory Committee on Immunization Practices' (ACIP) expanded the eligibility for a 50 μg booster dose to adults with frequent institutional or occupational exposure to SARS-CoV-2 or at high risk of severe COVID-19, including those aged 65 years and older.10, 11
Reductions in vaccine effectiveness against SARS-CoV-2 infections over time have been observed, which might reflect waning immunity from primary vaccination or reduced efficacy against emerging variants.4, 5, 6, 12 Available data on the safety and immunogenicity of third doses of SARS-CoV-2 vaccines mRNA-1273, BNT162b2, and ChAdOx1, support the administration of a booster vaccine dose to enhance protection against COVID-19.13, 14, 15, 16, 17, 18 Several countries in the northern hemisphere have thus launched a COVID-19 booster vaccine campaign, mostly targeting at-risk individuals, including those with autoimmune disease and older adults, since these groups were among the first to be vaccinated and therefore are more likely to have waning immunity.19
Influenza is an acute viral respiratory disease that affects all age groups, but has a disproportionate disease burden in older adults. Global influenza-associated respiratory excess mortality rates have been estimated to be 0·1–6·4 per 100 000 individuals for people younger than 65 years, 2·9–44·0 per 100 000 individuals for people aged between 65 and 74 years, and 17·9–223·5 per 100 000 for people older than 75 years.20 Broader, non-respiratory consequences of influenza (including exacerbation of chronic underlying conditions, increased susceptibility to secondary microbial infections, cardiovascular events, and functional decline) add substantial disease burden.21
Seasonal influenza vaccination reduces influenza-associated morbidity and mortality in groups at increased risk for complications, including older adults.22, 23 A high-dose influenza vaccine, which contains four times as much haemagglutinin as standard-dose influenza vaccines, was developed to improve protection against influenza in adults aged 65 years and older.24, 25 In a meta-analysis of multiple randomised and observational studies over the past 10 years of licensure in the USA, high-dose trivalent influenza vaccine was consistently more effective than was standard dose influenza vaccine at reducing influenza disease and associated clinical complications.26 A quadrivalent formulation of the high-dose vaccine, containing an additional B strain, was licensed by the FDA in November, 2019 (QIV-HD; Fluzone High-Dose Quadrivalent; Sanofi Pasteur; Lyon, France).
The public health measures put in place to curb SARS-CoV-2 transmission in 2020 led to substantially reduced influenza activity during the 2020–21 northern hemisphere influenza season;27, 28 WHO reported that less than 0·2% of tested specimens were positive for influenza virus during that period, compared with 17% for the period 2017–20.27 As countries lift restrictions on everyday life, including travel, and with potentially increased susceptibility of individuals to influenza virus due to reduced circulation the previous season, a consequent resurgence of influenza during the 2021–22 influenza season is predicted.29, 30
There is a risk of disruption to, or delay of, the seasonal influenza vaccination campaign through prioritised efforts to vaccinate individuals against COVID-19, particularly in older adults, during the same period. To help maintain influenza vaccine uptake and ease pressure on health systems during the northern hemisphere winter season, WHO and several countries have provided guidance on the concomitant administration of influenza and COVID-19 vaccines, with the aim of shortening the vaccination period, reducing the number of visits to health-care providers, and minimising missed opportunities to immunise against influenza.31, 32, 33, 34, 35, 36
The current study aimed to assess safety and immunogenicity after concomitant administration of QIV-HD and a third (booster) dose of mRNA-1273 vaccine in adults aged ≥65 years in the USA.
Methods
Study design and participants
This study is an ongoing, phase 2, multicentre, open-label, descriptive trial at six clinical research sites in the USA. The study was initiated on July 16, 2021, and has a planned completion date of Feb 15, 2022. The study included an active phase, from day 1 (enrolment, pre-vaccination blood draw, and vaccination) through to day 22 (post-vaccination blood draw), and a 6-month safety follow-up (day 181). Completion of the active phase of the study, reported here, was on Aug 31, 2021.
Community-dwelling adults aged 65 years and older, who were previously vaccinated with a two-dose primary schedule of the mRNA-1273 SARS-CoV-2 vaccine, were eligible for inclusion. The second dose of the primary mRNA-1273 vaccination series was required to have been received at least 5 months before enrolment in the study. Individuals with immunodeficiency, or who had received immunosuppressive therapy (in the preceding 6 months) or long-term systemic corticosteroid therapy, or who had thrombocytopenia or received anticoagulants in the 3 weeks before study inclusion, were excluded. Other exclusion criteria included any vaccination in the 4 weeks before the study, or planned receipt of any vaccine between study days 1 and 28 (with the exception of participants receiving QIV-HD at the end of the study); known, or a history of, systemic hypersensitivity or life-threatening reaction to any of the study intervention components; previous dermal filler injection (lips or face); chronic illness that might interfere with study conduct; moderate or severe acute illness or infection, or temperature of 100·4°F (38·0°C) or greater, on the day of study vaccination; history of serious adverse reaction to any influenza or COVID-19 vaccines; history of Guillain-Barré syndrome, or of clinically significant developmental delay, neurological disorder, or seizure disorder; known seropositivity for HIV, hepatitis B, or hepatitis C; any condition that might pose a health risk if enrolled or could interfere with the evaluation of the vaccine; receipt of blood-derived IgGs, blood, or blood-derived products in the past 3 months; participation at the time of study enrolment (or in the 30 days preceding the first study vaccination) or planned participation during the study period in another clinical study investigating a vaccine, drug, medical device, or medical procedure; deprived of freedom by an administrative or court order, or in an emergency setting, or hospitalised involuntarily; or identified as an investigator, employee, or an immediate family member of the investigator or study centre, with direct involvement in the proposed study.
Individuals were enrolled after public notice of ongoing research at dedicated clinical research sites in US regions with high proportions of primary series mRNA-1273 deployment, at a time of public interest in boosters amid waning immunity concerns.
The study conduct is in compliance with the International Council for Harmonisation Good Clinical Practice and ethical principles derived from international guidelines, including the Declaration of Helsinki. All participants provided written informed consent.
Ethics approval was obtained through the independent Institutional Review Board Advarra.
Randomisation and masking
Participants were randomly assigned (1:1:1) using a permuted block method stratified by site and by age group (<75 years vs ≥75 years), to receive concomitant administration of QIV-HD and mRNA-1273 vaccine, QIV-HD alone, or mRNA-1273 vaccine alone. Investigators were provided with scratchable randomisation lists by Sanofi Pasteur biostatistics platform. Investigators scratched the list to reveal the randomisation order and the corresponding study group for each participant.
Procedures
The QIV-HD vaccine (Fluzone High-Dose Quadrivalent; Sanofi Pasteur; Lyon, France) used was the 2021–22 formulation for the northern hemisphere influenza season. One dose (0·7 mL) contained 60 μg of haemagglutinin of each of the two A strains (A/H1N1, A/H3N2) and two B strains (from the Yamagata and Victoria lineages). The mRNA-1273 vaccine (Moderna; Cambridge, MA, USA) was supplied by the Biomedical Advanced Research and Development Authority (BARDA), part of the office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services, and was given as a 0·5 mL dose, containing 100 μg of mRNA.
Participants received one or both vaccines by intramuscular injection in the upper arm—one injection on each side for the concomitant administration group. Participants who were randomly assigned to the mRNA-1273 group were offered the QIV-HD vaccine after completion of the day 22 study visit, on a voluntary basis as part of routine medical care and aligned with recommendations of ACIP; no additional data related to this additional vaccination were collected and vaccine receipt was not considered exclusionary for continuing in the trial.
Haemagglutination inhibition antibody responses to influenza A/H1N1, A/H3N2, B/Yamagata, and B/Victoria strains were evaluated using a validated haemagglutinin inhibition assay by Global Clinical Immunology (Sanofi Pasteur laboratory; Swiftwater, PA, USA), as described previously.37 The endpoint of the assay is the highest serum dilution in which complete inhibition of haemagglutination occurs. The lower limit of quantification was 1:10 dilution (dil). Titres below this level were reported as less than 10 (1/dil).
The SARS-CoV-2 Pre-Spike IgG enzyme-linked immunosorbent assay was a validated assay, done by Nexelis (Laval, QC, Canada), as previously described.38 A reference standard was used to quantify antibodies against SARS-CoV-2 pre-spike (ELISA laboratory units [ELU] per mL). The concentration units were converted from ELU/mL to binding antibody units (BAU)/mL as follows: result in BAU/mL = result in ELU/mL÷7·9815.
Immunogenicity was evaluated for the three study groups using samples taken before vaccination (day 1) and 21 days (+3 days) after vaccination.
Outcomes
Safety endpoints were the number and frequency of: immediate unsolicited systemic adverse events and adverse reactions occurring within 30 min after vaccine injection; solicited injection site and systemic reactions occurring up to 7 days after injection; unsolicited adverse events and adverse reactions up to 21 days after injection; and serious adverse events, adverse events of special interest, and medically attended adverse events up to 6 months after injection (see appendix p 2 for definitions). In this study, we describe safety endpoints up to day 22. Adverse events were assessed for intensity and relatedness to study vaccine (see appendix p 3 for intensity grading definitions). The adverse events of special interest that were monitored for each vaccine are listed in the appendix (p 4).
To describe the haemagglutination inhibition antibody response, the following immunogenicity endpoints were reported for each treatment group: haemagglutination inhibition geometric mean titres (GMTs; 1/dil) on day 1 and day 22, day 22:day 1 titre ratios, detectable haemagglutination inhibition titres (≥10), and haemagglutination inhibition titres of 40 or greater on day 1 and day 22, and seroconversion rates (titre <10 on day 1 and post-vaccination titre ≥40 on day 22; or titre ≥10 on day 1 and a ≥4-times rise in titre on day 22). To describe the SARS-CoV-2 binding antibody response, the following endpoints were reported for each treatment group: geometric mean concentration (GMC) of anti-spike binding IgG on day 1 and day 22, and day 22:day 1 ratios; and 2-times or greater rise and 4-times or greater rise in anti-spike binding IgG on day 22. Additional analyses were done to assess the ratios of post-vaccination GMTs or GMCs, between the coadministration and single administration groups, for each influenza strain, and for the SARS-CoV-2 antigen.
Statistical analysis
No study power calculation was done for this study, since all analyses were descriptive and no hypothesis testing was done. 300 participants (around 100 per treatment group) were planned for enrolment, and we assumed a drop-out rate of 5%, meaning a total of 95 evaluable patients per study group. Safety endpoints were described for each study intervention group in the safety analysis set (all participants who received at least one dose of the study vaccine). Immunogenicity endpoints were described for each study intervention group in the immunogenicity analysis set (subset of randomised participants who received at least one dose of study vaccine). All analyses were done according to the vaccine(s) received. For immunogenicity results, 95% CIs for the point estimates were calculated using the normal approximation after log transformation for quantitative data (GMC and geometric mean of individual ratios) and exact binomial distribution (Clopper-Pearson method, quoted by Newcombe39) for single proportions. For the ratios of post-vaccination GMTs or GMCs between the coadministration group and single administration groups, a baseline adjustment was applied using an ANCOVA model of the day 22 log10 titre or concentration with vaccine group and day 1 log10 titre as factor and covariate, respectively. For the derivation of immunogenicity endpoints, all values under the lower limit of quantification were treated as lower limit of quantification divided by 2.
All statistical analyses were done using SAS version 9.4 or above. The study is registered with ClinicalTrials.gov, NCT04969276.
Role of the funding source
The funder of the study was involved in the study design, data collection, data analysis, data interpretation, writing of the report, and the decision to submit the paper for publication.
Results
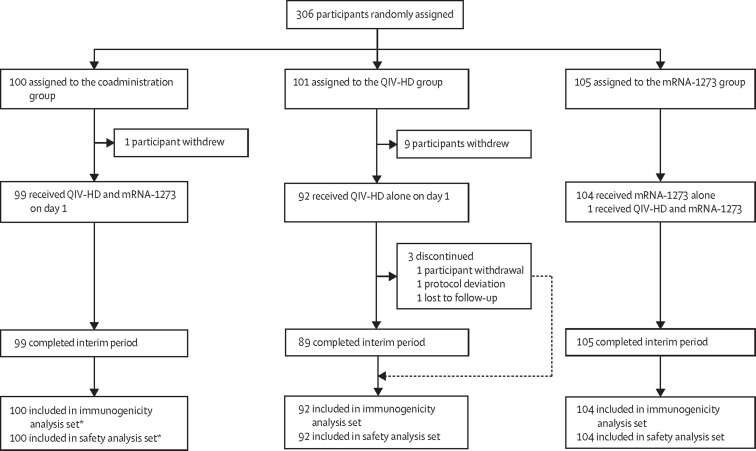
Between July 16 and Aug 31, 2021, 306 participants were enrolled and randomly assigned, of whom 296 received at least one vaccine dose (100 in the coadministration group, 92 in the QIV-HD, and 104 in the mRNA-1273 group). 293 participants completed the active phase (through to day 22); 11 participants withdrew (one in the coadministration group and ten in the QIV-HD group) and one participant in the QIV-HD group discontinued because of a protocol deviation (figure 1 ). The higher number of withdrawals in the QIV-HD group was due to participants who had wanted to receive a COVID-19 vaccine discontinuing from the study once they were not randomly assigned to receive mRNA-1273. One participant randomly assigned to the mRNA-1273 group was mistakenly administered both the QIV-HD and mRNA-1273 vaccine and was therefore included in the coadministration group for safety and immunogenicity analyses. Overall, in the safety analysis set, there were more female participants (166 [56%] of 296) than male participants (130 [44%]), particularly in the mRNA-1273 group (table 1 ). The median age of participants was 71 years (IQR 68–74); 71 (24%) of 296 participants were aged 75 years or older. Most participants (282 [95%] of 296 participants) were white or non-Hispanic or Latino and most (287 [94%] of 306 participants) had received seasonal influenza vaccination in the 2020–21 season (table 1). The characteristics of participants in the immunogenicity analysis set were similar to those in the safety analysis set (data not shown).
Figure 1.
Trial profile (active phase)
QIV-HD=high-dose quadrivalent influenza vaccine. *Including the patient from the mRNA-1273 group who was coadminsitered the vaccines in error.
Table 1.
Population characteristics at baseline (safety analysis set)
| Coadministration group (n=100) | QIV-HD alone group (n=92) | mRNA-1273 vaccine alone group (n=104) | ||
|---|---|---|---|---|
| Sex | ||||
| Male | 46 (46%) | 43 (47%) | 41 (39%) | |
| Female | 54 (54%) | 49 (53%) | 63 (61%) | |
| Age, years | 71·0 (67·5–74·0) | 71·0 (68·0–74·5) | 72·0 (69·0–74·0) | |
| Age group, years | ||||
| 65–74 | 77 (77%) | 69 (75%) | 79 (76%) | |
| 75–84 | 22 (22%) | 20 (22%) | 23 (22%) | |
| ≥85 | 1 (1%) | 3 (3%) | 2 (2%) | |
| Ethnicity | ||||
| Hispanic or Latino | 4 (4%) | 2 (2%) | 2 (2%) | |
| Not Hispanic or Latino | 93 (93%) | 89 (97%) | 100 (96%) | |
| Not reported or unknown | 3 (3%) | 1 (1%) | 2 (2%) | |
| Race | ||||
| White | 94 (94%) | 86 (93%) | 102 (98%) | |
| Black or African American | 1 (1%) | 4 (4%) | 0 | |
| Native Hawaiian or other Pacific Islander | 0 | 1 (1%) | 2 (2%) | |
| Asian | 2 (2%) | 0 | 0 | |
| Multiple | 1 (1%) | 1 (1%) | 0 | |
| Not reported | 2 (2%) | 0 | 0 | |
| Received the 2020–21 seasonal influenza vaccine* | 94/100 (94%) | 95/101 (94%) | 98/105 (93%) | |
| Duration between mRNA-1273 vaccine dose 2 and dose 3, days | 159 (153–167) | .. | 159 (154–167) | |
Data shown are n (%) or median (IQR). QIV-HD=high-dose quadrivalent influenza vaccine.
Denominators for these percentages are the randomly assigned group totals without patient withdrawals.
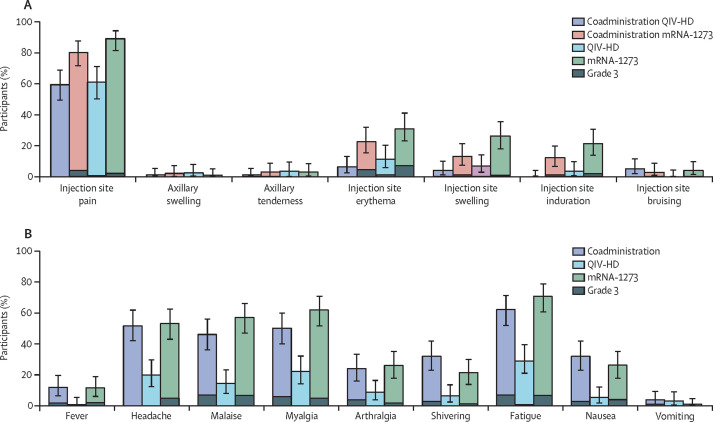
Four immediate unsolicited adverse events were reported—three by one participant in the coadministration group (anxiety and dizziness, both grade 1 and resolved spontaneously in one day, and grade 3 hypertension, which resolved by day 10) and one in a participant in the QIV-HD group (dizziness, grade 1, and assessed by the investigator as related to study vaccination, which resolved spontaneously after one day; appendix p 5). Solicited injection site reactions occurring up to 7 days after mRNA-1273 vaccine injection were reported at similar rates between the coadministration group (86·0% [95% CI 77·6–92·1]; 86 of 100 participants) and the mRNA-1273 group (91·3% [84·2–96·0]; 95 of 104 participants). Solicited injection site reactions occurring after QIV-HD injection in the coadministration group were reported less frequently than those after mRNA-1273 vaccine injection in the coadministration group (61·0% [95% CI 50·7–70·6] vs 82·0% [73·1–89·0] in the QIV-HD and mRNA-1273 injected limbs, respectively), and at a similar rate to the QIV-HD group (61·8% [50·9–71·9]; 55 of 89 participants; appendix p 5). Injection site pain was the most frequently reported solicited injection site reaction in each treatment group; the most frequently reported grade 3 injection site reactions were pain (ranging between 0·0 [95% CI 0·0–3·6] and 4·0% [1·1–9·0] per group) and erythema (ranging between 0·0 [0·0–3·6] and 6·7% [2·7–13·4]; figure 2A ). Solicited systemic reactions were also reported at similar rates between the coadministration and mRNA-1273 groups (80·0% [95% CI 70·8–87·3]; 80 of 100 participants and 83·7% [75·1–90·2]; 87 of 104 participants), and at a lower rate in the QIV-HD group (49·4% [38·7–60·2]; 44 of 89 participants; appendix p 5). The most frequently reported solicited systemic reaction in each group was fatigue (figure 2B). The most frequently reported grade 3 solicited systemic reactions were malaise, fatigue, and myalgia in the coadministration group and malaise, fatigue, headache, and myalgia in the mRNA-1273 group (ranging from 4·8% [95% CI 1·6–10·9] to 7·0% [2·9–13·9]); only one participant reported a grade 3 reaction (fatigue) in the QIV-HD group.
Figure 2.
Solicited injection site reactions (A) and solicited systemic reactions (B) occurring up to 7 days after injection (immunogenicity analysis set)
Error bars show 95% CIs. Coadministration QIV-HD shows the solicited reactions observed in the QIV-HD-injected limb of participants in the coadministration group. Coadministration mRNA-1273 shows the solicited reactions observed in the mRNA-1273-injected limb of participants in the coadministration group. QIV-HD=high-dose quadrivalent influenza vaccine.
Unsolicited adverse events through to day 22 were reported at similar frequencies between the coadministration and mRNA-1273 groups (17·0% [95% CI 10·2–25·8]; 17 of 100 participants and 14·4% [8·3–22·7]; 15 of 104 participants, respectively), and tended to be reported slightly less frequently in the QIV-HD group (10·9% [5·3–19·1]; 10 of 92 participants). Two grade 3 unsolicited adverse events were reported, one each in the coadministration and QIV-HD groups (appendix p 5): hypertension (immediate adverse event) and chemical burns to the eye, respectively. Four participants reported five unsolicited injection site adverse reactions—two participants in the coadministration group experienced injection site pruritis (for one participant, in both limbs with onset on day 6 and resolving by day 8; for the other participant, in the mRNA-1273 injected limb with onset on day 2 and resolving by day 4); one participant reported injection site warmth after injection of QIV-HD alone, with onset on the day of injection and resolving on day 5; and one participant reported injection site pruritis on day 3 after injection of mRNA-1273 alone, which resolved on day 5. Unsolicited systemic adverse reactions were reported for four participants in the coadministration group (four adverse reactions), three participants in the QIV-HD group (four adverse reactions), and six participants in the mRNA-1273 group (nine adverse reactions). These adverse reactions included a medically attended adverse event of muscle spasms occurring in the left calf of a participant in the mRNA-1273 group, 6 days after injection; the spasms were grade 1 and resolved after 2 days with health-care contact. No grade 3 unsolicited adverse reactions were reported up to day 22. There were no serious adverse events, adverse events of special interest, or deaths reported up to day 22. Seven participants each reported one medically attended adverse events (three in the coadministration group, one in the QIV-HD group, and three in the mRNA-1273 group).
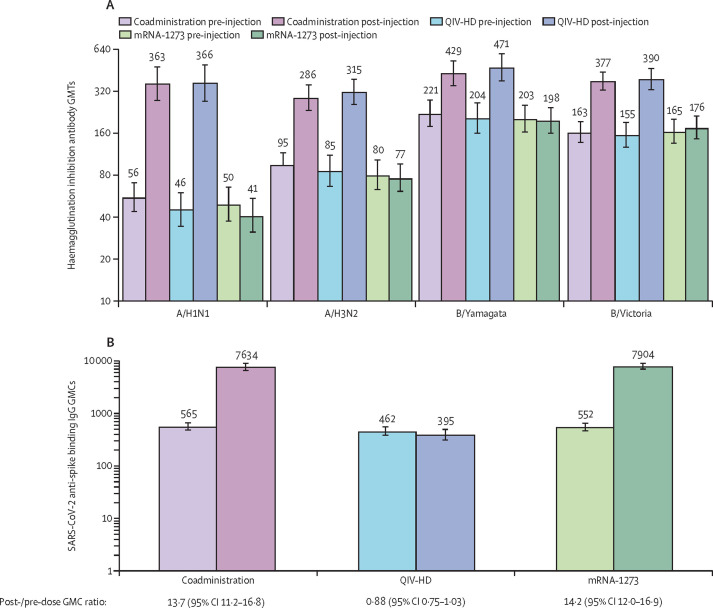
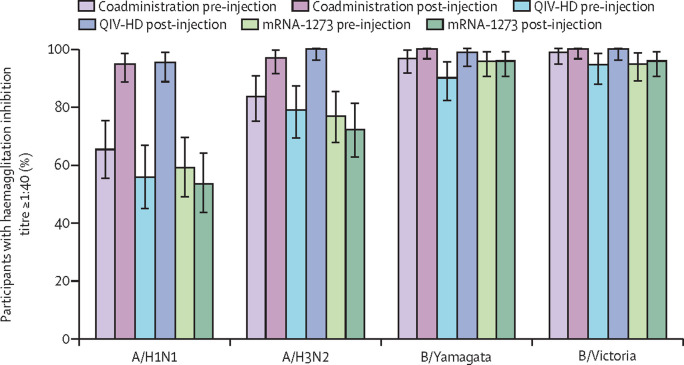
For each influenza strain, haemagglutination inhibition GMTs were similar across all groups at baseline (day 1), and increased after vaccination at day 22 to similar levels in the coadministration and QIV-HD groups (A/H1N1 GMT 363 [95% CI 276–476] vs 366 [272–491]; A/H3N2 286 [233–352] vs 315 [257–386]; B/Yamagata 429 [350–525] vs 471 [378–588]; B/Victoria 377 [325–438] vs 390 [327–465], for the coadministration and QIV-HD groups, respectively). Post-vaccination haemagglutination inhibition GMTs remained close to baseline levels in the mRNA-1273 group (figure 3A ). Day 22 GMT ratios relative to baseline are presented in table 2 . From day 1 to day 22 the proportion of participants with haemagglutination inhibition titres of 40 or greater for each strain increased in the coadministration and QIV-HD groups, to similar levels between the two groups (figure 4 ). Seroconversion rates were similar between the coadministration and QIV-HD groups for each strain (table 2).
Figure 3.
Influenza haemagglutinin inhibition (A) and SARS-CoV-2 anti-spike binding IgG (B) antibody responses on day 1 and day 22 for each treatment group (immunogenicity analysis set)
Error bars show 95% CIs. Annotations above each bar indicate GMTs (A) or GMCs (B) for each group or timepoint. GMCs are expressed in binding antibody units per mL. GMC=geometric mean concentration. GMT=geometric mean titre. QIV-HD=high-dose quadrivalent influenza vaccine.
Table 2.
Haemagglutination inhibition antibody responses for each influenza strain and SARS-CoV-2 anti-spike binding antibody responses after vaccination (day 22; immunogenicity analysis set)
|
Coadministration group (n=100) |
QIV-HD alone group (n=92) |
mRNA-1273 vaccine alone group (n=104) |
|||||
|---|---|---|---|---|---|---|---|
| M or n | Ratio or % (95% CI) | M or n | Ratio or % (95% CI) | M or n | Ratio or % (95% CI) | ||
| Haemagglutination inhibition antibody responses | |||||||
| A/H1N1 | |||||||
| Post-vaccination to pre-vaccination GMT ratio | 96 | 6·4 (4·9–8·3) | 86 | 8·1 (6·1–10·8) | 100 | 0·9 (0·8–0·9) | |
| Seroconversion rate | 66 | 68·8 (58·5–77·8) | 62 | 72·1 (61·4–81·2) | 0 | 0 (0–3·6) | |
| A/H3N2 | |||||||
| Post-vaccination to pre-vaccination GMT ratio | 96 | 3·1 (2·5–3·7) | 86 | 3·5 (2·8–4·4) | 100 | 0·9 (0·9–1·0) | |
| Seroconversion rate | 42 | 43·8 (33·6–54·3) | 41 | 47·7 (36·8–58·7) | 0 | 0 (0–3·6) | |
| B/Yamagata | |||||||
| Post-vaccination to pre-vaccination GMT ratio | 96 | 1·9 (1·7–2·2) | 86 | 2·3 (1·8–2·8) | 100 | 1·0 (0·9–1·1) | |
| Seroconversion rate | 17 | 17·7 (10·7–26·8) | 18 | 20·9 (12·9–31·0) | 1 | 1·0 (0–5·4) | |
| B/Victoria | |||||||
| Post-vaccination to pre-vaccination GMT ratio | 96 | 2·3 (2·0–2·6) | 86 | 2·4 (2·0–3·0) | 100 | 1·1 (1·0–1·1) | |
| Seroconversion rate | 29 | 30·2 (21·3–40·4) | 21 | 24·4 (15·8–34·9) | 1 | 1·0 (0–5·4) | |
| SARS-CoV-2 binding antibody responses | |||||||
| Post-vaccination to pre-vaccination GMT ratio | 96 | 13·7 (11·2–16·8) | 86 | 0·9 (0·7–1·0) | 102 | 14·2 (12·0–16·9) | |
| ≥2-times rise | 94 | 97·9 (92·7–99·7) | 3 | 3·5 (0·7–9·9) | 100 | 98·0 (93·1–99·8) | |
| ≥4-times rise | 89 | 92·7 (85·6–97·0) | 2 | 2·3 (0·3–8·1) | 97 | 95·1 (88·9–98·4) | |
GMC=geometric mean concentration. Seroconversion rate, titre <10 (1/dil) study day 1 and post-vaccination titre ≥40 (1/dil) at day 22, or titre ≥10 (1/dil) at day 1 and a ≥4-times rise in titre (1/dil) at day 22. GMT=geometric mean titre. M=number of participants with GMC or GMT data at the specified time point. n=number of participants seroconverted or with a ≥2-times or ≥4-times rise in concentration. QIV-HD=high-dose quadrivalent influenza vaccine.
Figure 4.
Proportion of participants in each vaccine group with influenza haemagglutinin inhibition antibody titres of 1:40 or greater for each influenza strain pre-vaccination (day 1) and post-vaccination (day 22; immunogenicity analysis set)
Error bars show 95% CIs. QIV-HD=high-dose quadrivalent influenza vaccine.
Haemagglutination inhibition GMT ratios between the coadministration and QIV-HD groups at day 22 were 0·87 (95% CI 0·61–1·23) for H1N1, 0·89 (0·70–1·14) for H3N2, 0·88 (0·71–1·09) for B/Yamagata, and 0·96 (0·79–1·16) for B/Victoria.
SARS-CoV-2 binding antibody GMCs were similar across all groups at baseline and increased to similar levels in the coadministration and mRNA-1273 groups at day 22 (7634 [95% CI 6445–9042] and 7904 [6883–9077], respectively; figure 3B). The GMC for participants in the QIV-HD group remained close to baseline levels. At day 22, the proportions of participants in the coadministration and mRNA-1273 groups with 2-times or greater and 4-times or greater rises in antibody concentration from baseline were high and similar between groups (table 2). The SARS-CoV-2 anti-spike binding IgG GMC ratio between the coadministration and mRNA-1273 groups at day 22 was 0·97 (95% CI 0·79–1·19).
Discussion
In this descriptive interim analysis up to 21 days after vaccination, we did not identify any safety concerns or any evidence of immune interference on influenza haemagglutination inhibition or SARS-CoV-2 binding antibody responses after concomitant administration of QIV-HD with a third dose of the mRNA-1273 vaccine (100 μg) in older adults (≥65 years) who were immunised with two mRNA-1273 doses around 5 months previously.
We observed similar rates of local reactogenicity in the QIV-HD-injected limb of participants in the coadministration group compared with participants who received QIV-HD alone (any solicited injection site reaction) and in the mRNA-1273 injected limb of participants in the coadministration group compared with the mRNA-1273 vaccine alone group; solicited systemic reactions were reported at similar frequencies in the coadministration and mRNA-1273 groups, with lower frequencies observed in participants who received QIV-HD alone. Grade 3 solicited reactions and unsolicited adverse reactions were infrequently reported for all groups and no serious adverse events, adverse events of special interests, or deaths were reported. In terms of immunogenicity, similar haemagglutination inhibition antibody responses were observed between the coadministration and QIV-HD groups and similar SARS-CoV-2 binding antibody responses were observed between the coadministration and mRNA-1273 groups. The safety and immunogenicity profiles described here for QIV-HD administered concomitantly with the mRNA-1273 vaccine or administered alone are in line with previous descriptions of the reactogenicity, safety, and immunogenicity of high-dose influenza vaccine in adults aged 65 years and older40, 41 or after two 100 μg doses of mRNA-1273 in adults aged 55 years and older,42, 43 and are in line with safety descriptions in the QIV-HD and mRNA-1273 vaccine product information, respectively.44, 45 Our findings of the reactogenicity and immunogenicity of a booster dose of mRNA-1273 vaccine (100 μg) administered concomitantly with QIV-HD or administered alone are also in line with the results of an open-label phase 2a study showing acceptable safety and immunogenicity of a single booster 50 μg dose of mRNA-1273 in participants who received a two-dose primary series of the COVID-19 vaccine mRNA-1273 around 6 months earlier.14
A 2021 study in the UK, involving 679 participants aged 18 years and older (randomly assigned to 12 study groups), showed that the coadministration of a second dose of the SARS-CoV-2 mRNA vaccine BNT162b2 (Pfizer-BioNTech) with one of three seasonal inactivated influenza vaccines had acceptable reactogenicity and tolerability, with no evidence of negative immune interference compared with administration of each vaccine alone.46 Based on those preliminary data, the UK Government updated their guidance to support concomitant administration of the BNT162b2 vaccine with influenza vaccines (in separate arms)47 and encouraged concomitant administration of the influenza vaccine with COVID-19 booster vaccination, where practical to do so.34 An exploratory substudy of a phase 3 trial also reported no overall effect of coadministration of a first dose of COVID-19 protein-based vaccine NVX-CoV2373 coadministered with licensed seasonal quadrivalent (18–64 years old) or trivalent (≥65 years old) influenza vaccine on the safety and efficacy of either vaccine alone.48
To our knowledge, this study provides the first data supporting concomitant administration of QIV-HD with a COVID-19 booster vaccine dose in older adults. Our data thus provide additional insights to support current public health recommendations to implement seasonal influenza vaccination concomitantly with COVID-19 booster vaccination. Concomitant implementation of these vaccination campaigns could help avoid potential delays in influenza vaccination during the northern hemisphere influenza season due to prioritisation of COVID-19 booster vaccination, which is particularly important among individuals at increased risk of severe illness and hospitalisation from both COVID-19 and influenza infection. Therefore, concomitant vaccination might be needed to reduce morbidity and mortality due to these infectious diseases as much as possible. This strategy could also have broader public health implications by easing growing pressure on health systems across affected countries.
This study used previously validated assays to measure haemagglutination inhibition antibody responses37 and SARS-CoV-2 pre-spike IgG antibody responses.38 High influenza haemagglutination inhibition antibody titres were observed before vaccination, especially for the influenza B strains, which could be due to the fact that this study population was vaccinated very early in the influenza season (July–August) and that the same B strains were used in the 2020–21 and 2021–22 vaccine compositions. Moreover, more than 90% of the study population had received influenza vaccine in the previous season. Although we included around 100 participants in each intervention group, this study was not powered for statistical comparisons between study groups. Furthermore, the dose of mRNA-1273 (100 μg) was greater than the dose (50 μg) that has been authorised for a booster. We used the 100 μg dose in the current study since this was the authorised dose for primary vaccination with mRNA-1273 at the time of study design, and ACIP had not yet provided guidance for a booster (third) dose of the mRNA-1273 vaccine. In the absence of an increase in incidence or severity of adverse events and adverse reactions and without evidence of interference in the immune response, the results of this study should enable extrapolation to coadministration of lower doses with longer intervals between the second and third dose. Notably, the study population did not sufficiently represent the ethnic and racial diversity of the US population, which was in part due to the very short time window for enrolment of study participants (around 2 weeks), which should be prioritised and improved in future studies.
In conclusion, our findings suggest that QIV-HD and the mRNA-1273 vaccine can be administered together without any safety concerns or interference in the immune response, thus supporting existing guidance on the implementation of concomitant vaccination campaigns against both influenza disease and SARS-CoV-2.
Data sharing
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Declaration of interests
RI, J-SB, MF, TMM, AP, LP, NS, AS, SW, and SIS are Sanofi Pasteur employees and might hold stock. PB is an employee of Hospices Civils de Lyon, a service provider for Sanofi. DB declares no competing interests.
Acknowledgments
Acknowledgments
We thank all participants, investigators, and study site personnel who took part in this study. In particular, we would like to thank Mark H Hutchens, Peter Levins, Isabel Pereira, Thomas Starkey, and Patrick Yassini; from Sanofi Pasteur, Iris Depaz, Stephanie Pepin, Kevin Yin, Camille Salamand, and Cynthia Tabar; from Moderna, Hamilton Bennett, Julie Vanas, Andrea Sutherland, Deborah Manzo, Amparo Figueroa, and Brett Leav; and the US Department of Health & Human Services-Department of Defense COVID-19 Countermeasures Acceleration Group and Biomedical Advanced Research and Development Authority (BARDA). We also acknowledge Juliette Gray of inScience Communications (Springer Healthcare; London, UK) for editorial assistance with the preparation of this manuscript, funded by Sanofi Pasteur, and Isabel Grégoire for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur. This work was funded by Sanofi Pasteur and done in collaboration with BARDA and Moderna. Doses of the mRNA-1273 vaccine (Moderna) were provided by BARDA, which is part of the office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Contributors
RI, DB, MF, and SIS contributed to the concept or design of the study. DB contributed to data acquisition and all authors were involved in the analysis or interpretation of the data. All authors were involved in drafting or critically revising the manuscript, approved the final version, and are accountable for the accuracy and integrity of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. RI and PB have accessed and verified the data.
Supplementary Material
References
- 1.Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status—New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1306–1311. doi: 10.15585/mmwr.mm7037a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1156–1162. doi: 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency Comirnaty and Spikevax: EMA recommendations on extra doses and boosters. Oct 4, 2021. https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters
- 9.Regulatory Affairs Professionals Society COVID-19 vaccine tracker. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
- 10.US Food and Drug Administration Coronavirus (COVID-19) update: FDA takes additional actions on the use of a booster dose for COVID-19 vaccines. Oct 20, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines
- 11.US Centers for Disease Control and Prevention CDC expands eligibility for COVID-19 booster shots. Oct 21, 2021. https://www.cdc.gov/media/releases/2021/p1021-covid-booster.html
- 12.Baden LR, El Sahly HM, Essink B, et al. COVID-19 in the phase 3 trial of mRNA-1273 during the delta-variant surge. medRxiv. 2021 doi: 10.1101/2021.09.17.21263624. published online Sept 22. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atmar RL, Lyke KE, Deming ME, et al. Heterologous SARS-CoV-2 booster vaccinations —preliminary report. medRxiv. 2021 doi: 10.1101/2021.10.10.21264827. published online Oct 15. (preprint). [DOI] [Google Scholar]
- 14.Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27:2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falsey AR, Frenck RW, Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baden LR, El Sahy HM, Essink B, et al. Phase 3 trial of mRNA-1273 during the delta-variant surge. N Engl J Med. 2021;385:2485–2487. doi: 10.1056/NEJMc2115597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuters Factbox: countries weigh need for booster COVID-19 shots. https://www.reuters.com/business/healthcare-pharmaceuticals/countries-weigh-need-booster-covid-19-shots-2021-09-24/
- 20.Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macias AE, McElhaney JE, Chaves SS, et al. The disease burden of influenza beyond respiratory illness. Vaccine. 2021;39(suppl 1):A6–14. doi: 10.1016/j.vaccine.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017–18 influenza season. MMWR Recomm Rep. 2017;66:1–20. doi: 10.15585/mmwr.rr6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 24.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 25.Diaco M, Chang LJ, Seet B, et al. Introductory paper: high-dose influenza vaccine. Vaccine. 2021;39(suppl 1):A1–A5. doi: 10.1016/j.vaccine.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee JKH, Lam GKL, Shin T, Samson SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine. 2021;39(suppl 1):A24–A35. doi: 10.1016/j.vaccine.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization Recommended composition of influenza virus vaccines for use in the 2021–2022 northern hemisphere influenza season. Wkly Epidemiol Rec. 2021;96:77–88. [Google Scholar]
- 28.Tang JW, Bialasiewicz S, Dwyer DE, et al. Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID-19 pandemic. J Med Virol. 2021;93:4099–4101. doi: 10.1002/jmv.26964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCauley J, Barr IG, Nolan T, Tsai T, Rockman S, Taylor B. The importance of influenza vaccination during the COVID-19 pandemic. Influenza Other Respir Viruses. 2022;16:3–6. doi: 10.1111/irv.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K, Jalal H, Raviotta JM, et al. Predicting the impact of low influenza activity in 2020 on population immunity and future influenza season in the United States. medRxiv. 2021 doi: 10.1101/2021.08.29.21262803. published online Aug 30. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sante publique, sécurité de la chaine alimentair et environmente Co-administration des vaccins contre la COVID-19 avec d'autres vaccins (vaccination simultanée) Oct 7, 2021. https://www.health.belgium.be/fr/avis-9675-vaccination-simultanee-covid-19
- 32.Haute Autorité de Santé Avis n° 2021.0069/AC/SESPEV du 23 septembre 2021 du collège de la Haute Autorité de santé venant compléter l'avis du 23 août 2021 relatif à la définition des populations à cibler par la campagne de rappel vaccinal chez les personnes ayant eu une primovaccination complète contre la Covid-19. September, 2021. https://www.has-sante.fr/jcms/p_3288556/fr/avis-n-2021-0069/ac/sespev-du-23-septembre-2021-du-college-de-la-haute-autorite-de-sante-venant-completer-l-avis-du-23-aout-2021-relatif-a-la-definition-des-populations-a-cibler-par-la-campagne-de-rappel-vaccinal-chez-les-personnes-ayant-eu-une-primovaccination-complete-contre-la-covid-19
- 33.Government of Canada Canada's National Advisory Committee on Immunization. Summary of National Advisory Committee on Immunization (NACI) statement: recommendations on the use of COVID-19 vaccines. Sept 28, 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/summary-updates-september-28-2021.html
- 34.UK Department of Health and Social Care Joint Committee on Vaccination and Immunisation. JCVI statement regarding a COVID-19 booster vaccine programme for winter 2021 to 2022. Sept 14, 2021. https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-booster-vaccine-programme-for-winter-2021-to-2022/jcvi-statement-regarding-a-covid-19-booster-vaccine-programme-for-winter-2021-to-2022#:~:text=In%20JCVI%20's%20view%2C%20the,and%20to%20protect%20the%20NHS
- 35.US Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States (updated Sept 27, 2021) https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html#Coadministration
- 36.WHO Coadministration of seasonal inactivated influenza and COVID-19 vaccines: Interim guidance. https://apps.who.int/iris/bitstream/handle/10665/346897/WHO-2019-nCoV-SAGE-Vaccines-coadministration-Influenza-2021.1-eng.pdf?sequence=1&isAllowed=y
- 37.Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31:770–776. doi: 10.1016/j.vaccine.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 38.Goepfert PA, Fu B, Chabanon AL, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infect Dis. 2021;21:1257–1270. doi: 10.1016/S1473-3099(21)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:878–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 40.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 41.Chang LJ, Meng Y, Janosczyk H, Landolfi V, Talbot HK. Safety and immunogenicity of high-dose quadrivalent influenza vaccine in adults ≥65 years of age: a phase 3 randomized clinical trial. Vaccine. 2019;37:5825–5834. doi: 10.1016/j.vaccine.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39:2791–2799. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanofi Pasteur Fluzone high-dose prescribing information. Mar 29, 2019. https://www.fda.gov/media/119870/download
- 45.US Food and Drug Administration Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) 2021. https://www.fda.gov/media/144637/download
- 46.Lazarus R, Baos S, Cappel-Porter H, et al. The safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. Lancet. 2021;398:2277–2287. doi: 10.1016/S0140-6736(21)02329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.GOV.UK; UK Medicines & Healthcare products Regulatory Agency Information for healthcare orofessionals on COVID-19 vaccine Pfizer/BioNTech (regulation 174) 2021. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/information-for-healthcare-professionals-on-pfizerbiontech-covid-19-vaccine
- 48.Toback S, Galiza E, Cosgrove C, et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00409-4. published online Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.