Abstract
Highly portable, cloud-enabled neuroimaging technologies will fundamentally change neuroimaging research. Instead of participants traveling to the scanner, the scanner will now come to them. Field-based brain imaging research, including populations underrepresented in neuroscience research to date, will enlarge and diversify databases and pave the way for clinical and direct-to-consumer (DTC) applications. Yet these technological developments urgently require analysis of their ethical, legal, and social implications (ELSI). No consensus ethical frameworks for mobile neuroimaging exist, and existing policies for traditional MRI research are inadequate. Based on literature review and ethics analysis of neurotechnology development efforts, Shen et al. identify seven foundational, yet unresolved, ELSI issues posed by portable neuroimaging: (1) informed consent; (2) privacy; (3) capacity to accurately communicate neuroimaging results to remote participants; (4) extensive reliance on cloud-based artificial intelligence (AI) for data analysis; (5) potential bias of interpretive algorithms in diverse populations; (6) return of research results and incidental (or secondary) findings to research participants; and (7) responding to participant requests for access to their data. The article proposes a path forward to address these urgent issues.
The Challenge of Research with Highly Portable Neuroimaging
The emergence of new, highly portable neuroimaging technologies will fundamentally change neuroimaging research. Instead of participants traveling to the scanner, the scanner will now come to them. Here, we identify pressing ethical issues posed by this shift to field-based brain imaging research.
Research teams have already deployed functional near-infrared spectroscopy (fNIRS), high-density diffuse optical tomography, mobile electroencephalography (EEG), and ultra-low field magnetic resonance imaging (MRI) in the field. Researchers are now developing highly portable, high-field MRI; mobile positron emission tomography (PET); and mobile magnetoencephalography (MEG). A core feature of highly portable neuroimaging is likely to be its reliance on cloud-based data processing and interpretation. This permits functions traditionally performed by large MRI machines and local computer arrays to instead be carried out by much smaller and cheaper image acquisition devices, transmitting the data for remote cloud-based processing.
These emerging technologies will allow researchers to conduct field-based research with underserved and economically marginalized populations that have thus far been underrepresented in neuroimaging research. Highly portable neuroimaging has great potential to revolutionize field-based neuroscience research.
Achieving that potential, however, requires careful analysis of the ethical, legal, and social implications (ELSI) of this new technology. In research conducted as part of a National Institutes of Health (NIH) Neuroethics Administrative Supplement to a parent grant developing highly portable MRI (3U01EB025153–02S2), we conductedliteraturereviewsplusbioethics analysis embedded in the multi-institutionalparent-grantteam.Wealsoconducted semi-structured interviews of key experts participating in this technological revolution. Our neuroethics research identified seven unresolved ELSI challenges: (1) informed consent; (2) data security and privacy; (3) capacity to accurately communicate neuroimaging results to remote participants; (4) extensive reliance on cloud-based artificial intelligence (AI) for data analysis; (5) potential bias of interpretive algorithms in diverse populations; (6) return of research results and incidental (or secondary) findings to research participants; and (7) responding to participant requests for access to their data.
MRI research is regulated by a mix of federal, state, and local institutional policy (Kulynych 2007). The U.S. Food and Drug Administration (FDA) publishes guidance on MRI machine safety and efficacy, and the American College of Radiology (ACR) sets standards and facilitates an accreditation process that MRI machines must meet for the facility to bill Medicare for scans on the machine. But guidelines focused on safety and efficacy do not reach the new ELSI issues posed by neuroimaging research in the field.
This article examines these emerging ELSI issues. To concretize the analysis, we focus on one of the most powerful of these technologies, highly portable MRI. We show how field deployment in new and underserved settings will pose acute ELSI challenges, and we suggest the analyses needed to meet these challenges.
How Highly Portable MRI Works
MRI traditionally requires a large, heavy scanner, a powerful magnet, a supply of liquid helium for cooling, and a dedicated room with radiofrequency (RF) shielding, sound proofing, and a large power supply. Because even a 1.5 T magnet produces a magnetic field 30,000 times stronger than the Earth’s own magnetic field, access to the scanner room must be restricted.
With advances in engineering, physics, and AI, clinical-grade magnetic resonance (MR) images (images with high spatial and contrast resolution, equivalent to those generated by MRI with 1.5 T magnetic field strength) can be produced from machines that are much smaller; can sit in a room without RF shielding; can run on batteries, a power generator, or a standard 120 v outlet; and do not require an elaborate helium cooling system. As one company developing such technology describes it on their website, highly portable MRI systems will be “able to generate images in places never thought possible…[operating] wherever there is a power outlet…” (Hyperfine, 2019, https://www.hyperfine.io/).
How can high-quality MR images be produced without the need for the bulky magnet? Two strategies are promising. The first, called “ultra-low field” MRI, uses a smaller, less powerful magnet to acquire imaging data and then relies upon advanced techniques to extract signals from noisy data (Sarracanie et al., 2015). The second approach, “high field” MRI, retains the high signal-to-noise ratio of 1.5 T, even while reducing the size of the magnet. The challenge with high-field MRI using a small magnet is that the resulting magnetic field is very non-uniform. To address this, new RF pulse and image reconstruction strategies are being pursued (Garwood et al., 2020, Intl. Soc. Magn. Reson. Med., abstract).
Cloud-Enabled and Field-Based Neuroimaging Research
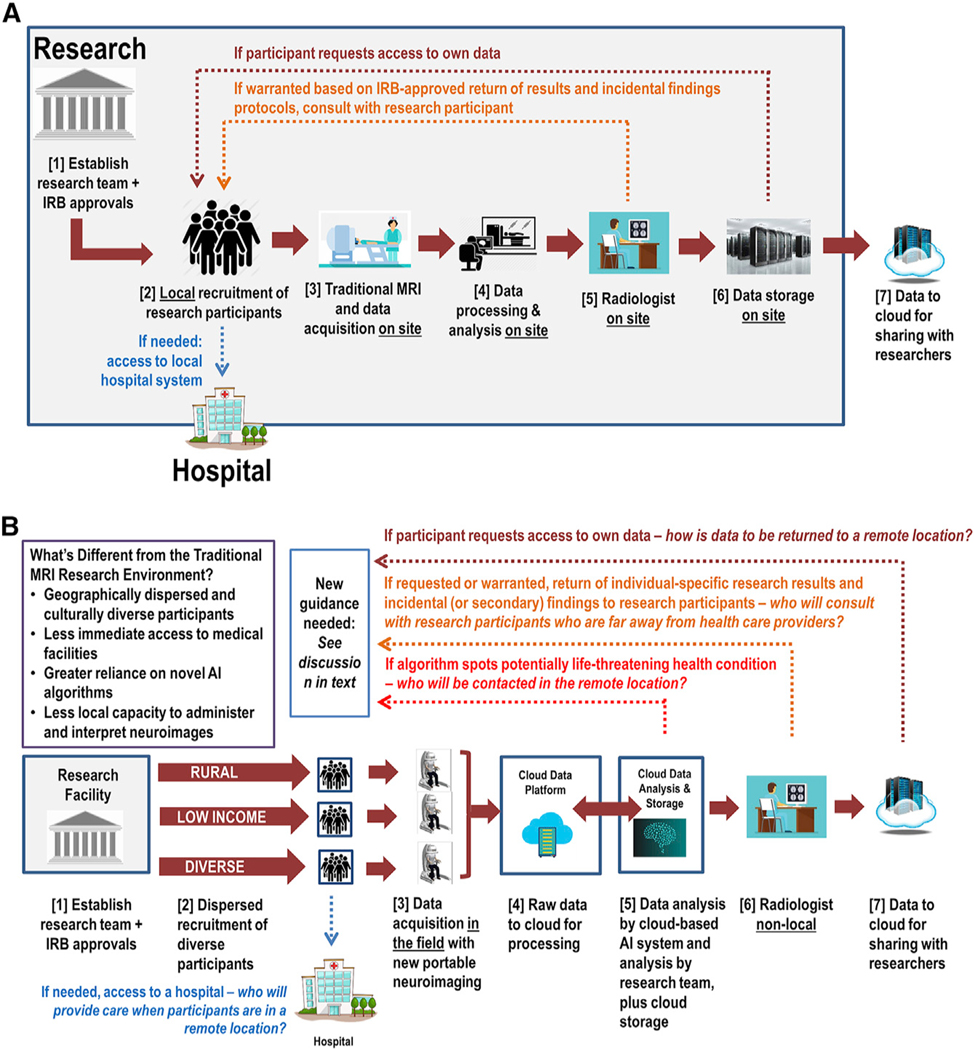
Highly portable, low-cost, cloud-enabled MRI will introduce significant differences in the way that brain data will be acquired, processed, stored, and shared in research (see Figure 1).
Figure 1. Comparing the Data Flow in Current MRI Research (A) with the Anticipated Data Flow in Emerging, Field-Based MRI Research.
(A) Current data flow for MRI research with local analysis and local data storage.
(B) New data flow for MRI research that is highly portable, cloud-enabled, and field-based.
Figure 1A depicts data flow for traditional MRI research in which the investigators, relevant clinicians, and facilities are contained within a single institution or geographic area. With conventional MRI, the researchers (1) obtain institutional review board (IRB) approval and then (2) recruit local participants and obtain informed consent. Participants who consent (3) undergo an MRI scan locally. The data are (4) processed and analyzed on site. During the data analysis, the research team (5) may send all or some subset of the acquired images to a radiologist on site for clinical review. If the radiologist identifies concerns, the investigator and/ or radiologist consult with the research participant. A hospital visit may be required for follow-up evaluation. Finally, the data (6) are stored locally (typically in de-identified form) and (7) are shared with other researchers per NIH and other relevant guidelines. As noted by the top dotted lines in Figure 1A, participants may also request access to their brain data, exercising their right of access to final laboratory results under the Health Insurance Portability and Accountability Act (HIPAA) (42 C.F.R. 493; 45 C.F.R. 164).
Current approaches to neuroimaging research thus largely assume that researchers are utilizing an MRI machine located within a medical or research facility, that participants can readily travel to that location, and that data analysis will be conducted locally. In highly portable neuroimaging, however, these assumptions are disrupted (Figure 1B).
Figure 1B illustrates how data may be collected and analyzed using highly portable and cloud-enabled MRI after (1) IRB approval of the research. The differences from conventional research become evident in step 2, as highly portable MRI will allow researchers access to previously underrepresented and unavailable participant populations in new locations across the country and world. This dispersed recruitment will require a new process for eliciting informed consent, as local interaction with participants for informed consent and image acquisition may involve local and non-expert personnel. For field-based neuroimaging, there may be no medical expertise or medical facility nearby.
The brain scan data will (3) be acquired remotely and will (4) be sent to the cloud for processing. The cloud data will (5) then be analyzed by an AI-driven system and the research team. The cloud will also store both the raw and processed data. Should the AI system identify an abnormality on the scan that requires immediate intervention, it is conceivable that the system might send the scan directly to a radiologist. The research team may also (6) send all or some subset of the images to a radiologist for review for incidental findings. If the radiologist identifies concerns, the radiologist or research team may reach out to the research participant, though it is unclear how that communication will occur if the participant is in a remote location. If clinical follow-up and evaluation are warranted, it is also unclear where a research participant in a remote and underserved location will go for a clinical work-up and how it will be funded. As in traditional MRI research, (7) data will be shared with other researchers (typically in de-identified form). If a participant requests their data, it is unclear how that request will be accommodated.
Seven Pressing ELSI Questions
These stark differences in data acquisition and information flow raise seven key ELSI issues that urgently need analysis and solutions.
1. What Is Meaningful Informed Consent for Field-Based and Cloud-Enabled Neuroimaging?
Although guidance exists for eliciting informed consent for MRI research, there is no agreed-upon consensus for uniform best practices (Racine and Illes, 2007). One of the problems with existing informed consent approaches in MRI research is understating and inaccurately presenting the risks, for instance, by making no mention of re-identification and related brain privacy risks. Moreover, there is inadequate guidance about how to meaningfully inform potential research participants about the Figure 1B steps related to the flow of data to the cloud, the distance between researcher and participant, and the use of AI for analysis. Our review of publicly available IRB guidance on informed consent for MRI research found no consent templates that considered the unique risks associated with core features of highly portable MRI: the geographic distance between researcher and participant, the possibility that mobile MRI research in remote settings will be performed by technicians rather than investigators, the use of machine learning and AI in analyzing brain scans, and the storage and sharing of brain data on cloud-based platforms. The federal Common Rule governs the requirements for informed consent in a wide swath of research, including research that is federally funded, federally conducted, and conducted by an institution that renders a broad Federalwide Assurance (45 C.F.R. Part 46). To comply with the federal Common Rule and, where applicable, the FDA’s informed consent requirements (21 C.F.R. 50), improved guidance is required for researchers to ensure meaningful informed consent in the context of highly portable MRI.
2. How Will Researchers Ensure the Privacy and Security of Brain Data?
As noted in the 2019 NIH Neuroethics Roadmap, “[b]rain privacy is at the forefront of concerns” about the development of new brain technologies (National Institutes of Health, 2019). In the context of highly portable and cloud-enabled brain imaging, privacy concerns include (1) effectively de-identifying brain data; (2) securing data held and processed in the cloud, including protection from data breach; (3) ensuring that all business associates that store and analyze brain data provide adequate data security; and (4) ensuring accountability to participants regarding privacy and security, including respecting their rights of access to their data. There is no consensus yet in the neuroimaging community on how to optimally de-identify neuroimaging data. Although methods exist for at least partially de-identifying data, such as skull stripping and defacing, there is significant variability in the implementation of these and other methods. Moreover, these may be insufficient because every individual’s brain anatomy is unique, and researchers can now identify individuals based on brain anatomy alone (Valizadeh et al., 2018).
Concerns about privacy are further heightened in highly mobile neuroimaging because of its expected reliance on teleradiology and cloud-based computing. It is unclear which platforms will be most utilized in portable MRI research, but many of these entities may not be covered by HIPAA regulations. If HIPAA applies to the researchers, they must ensure that HIPAA business associate agreements are in place and that cooperating entities comply with state-specific privacy laws as well. Data security additionally requires that the systems by which data will be processed and interpreted are not excessively vulnerable to attack.
3. Is There Sufficient Capacity to Accurately Communicate Neuroimaging Results to Remote Participants in Culturally Appropriate Ways?
Brain data—without culturally appropriate and scientifically accurate communication—can easily be misunderstood. Current ethics frameworks do not adequately address how to provide expertise and communication mechanisms to explain the research and results at the site of the participant, especially when the research is conducted far from the home site of the research team. In portable MRI research, the research team may never visit the site in person, relying instead on a local technician to acquire the images. One solution may be teleradiology—outsourcing the reading of scans to experts in another locale, which is now common in many hospitals. But teleradiology introduces ethical concerns, such as reliability, safety, and security, which are not typically addressed in traditional MRI research.
4. How Should Ethical Frameworks Incorporate Cloud-Based AI Analyzing Brain Scan Data?
Highly portable neuroimaging is likely to be significantly cloud-enabled in order to remove pressure from dispersed and remote field sites to process, analyze, and store the data generated. As one mobile MRI company announces on its web page,“...MRI data sets can be uploaded to the cloud, ready for the next available teleradiology consultation. Built-in Artificial Intelligence, based on the latest in deep learning, helps with image interpretation.” (Hyperfine, 2019). Neuroscience researchers are increasingly using the cloud for data storage and AI-enabled data analysis, and computer-assisted imaging is also becoming part of clinical practice. Yet there is no relevant guidance specific to AI identification of incidental findings in brain MRI research, even though the use of AI in radiology is being explored in other clinical domains. New guidance is needed to address the ethical issues emerging from the use of cloud-enabled AI systems to analyze brain data.
5. Will an AI-Driven Mobile MRI Analytic Platform Trained on a Limited Dataset Be Biased When Assessing Brains from More Diverse Populations?
A further problem posed by reliance on AI is the potential for bias in analyzing data from participant populations that are more diverse than the AI’s training data. The potential for biased outcomes in AI-based algorithms is being explored and debated across multiple fields. The NIH has formed an Artificial Intelligence Working Group to address these AI challenges, and the Institute of Electrical and Electronics Engineers Standards Association has created a new standard-setting effort on algorithmic bias considerations. But because AI technology is only beginning to be introduced into neuroimaging, consensus is lacking on best practices, and guidance from professional associations and regulatory agencies remains in development.
6. How Will the Research Team Return Incidental (or Secondary) Findings to Research Participants?
Although estimates of incidental finding rates in brain MRI vary by the participant population and imaging approach, incidental findings are not uncommon (Shoemaker et al., 2016). Disclosure of incidental brain findings can have a significant impact on the lives of participants, and there is a robust literature on the ethics of managing incidental findings in neuroimaging research (Wolf et al., 2008). But current ethical guidelines were developed for MRI in research facilities, and presently, there is no guidance for return of results in field-based MRI research. To develop this guidance, neuroimagers can learn from recent developments in genetics and genomics research about how to handle return of results and incidental (or secondary) findings (Wolf, 2013).
7. What Level of Access Will Research Participants Have to Their Brain Data?
Evidence suggests that neuroimaging research participants overwhelmingly desire to receive their brain scan findings, with one study finding that “87% of research participants expressed a preference to receive all scan findings” (Shoemaker et al., 2016). In both research and clinical care, HIPAA gives individuals a federal right of access to the contents of their “designated record set” (DRS) held by a HIPAA-covered entity (Wolf and Evans, 2018). The DRS includes any information used by the HIPAA-covered entity to make decisions about any individual, not just the person in question. Thus, individuals may have access to raw data and interpreted results. But as previous legal analysis has revealed, “[c]orporate device manufacturers that sponsor or conduct research, as well as investigators in nonclinical academic departments.are unlikely to be covered by HIPAA” (Kulynych, 2007). Given the introduction of multiple institutions and firms handling mobile MRI data, clearer guidance is needed on data access.
Further guidance is also required to determine what should be returned to a research participant asserting a right of access to their raw data. Participants may wish to take this brain data to a clinician for interpretation. At present, there is not consensus on how to respond to such requests. For instance, in neuroimaging studies of Alzheimer’s disease, research participants who request their brain data will have their requests denied because conventional practice has been to withhold such information (Shulman et al., 2013). Meanwhile, a survey of Alzheimer’s Disease Neuroimaging Initiative (ADNI) researchers found that the field is “experiencing tremendous flux” and that a majority of researchers would support return of some results if evidence-based guidelines could be developed (Shulman et al., 2013). Looking ahead, highly portable neuroimaging needs to anticipate and address requests for data and interpreted results. Moreover, communicating data and results requires consensus terminology and standards.
Next Steps
No published guidance squarely addresses these ethical issues in highly portable and cloud-enabled neuroimaging research. In the coming years, these ELSI issues will multiply in complexity as highly portable neuroimaging modalities are deployed not only in research but also clinically and in direct-to-consumer (DTC) contexts, both domestically and internationally.
Now is the moment in the development of mobile neuroimaging to critically examine informed consent practices, data privacy policies, end-user license agreements, and return of results protocols. Empirical research is needed on the characteristics of emerging field-based neuroimaging, the participant populations involved, the precise challenges presented, and stakeholder concerns.
Professional associations and government agencies can contribute by convening meetings to develop guidance on these issues. Stakeholder organizations include the NIH, FDA, and Federal Trade Commission, along with the Radiological Society of North America, ACR, and International Society for Magnetic Resonance in Medicine, in addition to those firms building the technology and the accompanying software and hardware. Moreover, international perspectives can be included through dialogue with the Human Brain Project in Europe, the NIH BRAIN Neuroethics Working Group, and the Neuroethics Workgroup of the International Brain Initiative.
Guidance for highly portable and cloud-enabled neuroimaging research is urgently needed, as portable neuroimaging technologies are developed and move into field settings. We have suggested the most pressing challenges, the importance of empirical research, and the need for normative consensus building to ensure ethical deployment of this powerful emerging technology.
ACKNOWLEDGMENTS
This work was supported by a National Institutes of Health Neuroethics Administrative Supplement (3U01EB025153-02S2). For research assistance, we thank Travis Panneck, Sydney Diekmann, Warren Cormack, Julie Griep, Andrew Park, and Jacob Hauschild. For grants management support, we thank Audrey Boyle, Deb Morgan, and Carolyn Wielde. All views are those of the authors and not the funders.
REFERENCES
- Kulynych JJ (2007). The regulation of MR neuroimaging research: disentangling the Gordian knot. Am. J. Law Med. 33, 295–317. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (2019). The BRAIN Initiative and neuroethics: enabling and enhancing neuroscience advances for Society. https://braininitiative.nih.gov/strategic-planning/acdworking-groups/brain-initiative-and-neuroethicsenabling-and-enhancing. [DOI] [PubMed]
- Racine E, and Illes J (2007). Emerging ethical challenges in advanced neuroimaging research: review, recommendations and research agenda. J. Empir. Res. Hum. Res. Ethics 2, 1–10. [DOI] [PubMed] [Google Scholar]
- Sarracanie M, LaPierre CD, Salameh N, Waddington DEJ, Witzel T, and Rosen MS (2015). Low-cost high-performance MRI. Sci. Rep. 5, 15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JM, Cole C, Petree LE, Helitzer DL, Holdsworth MT, Gluck JP, and Phillips JP (2016). Evolution of universal review and disclosure of MRI reports to research participants. Brain Behav. 6, e00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman MB, Harkins K, Green RC, and Karlawish J (2013). Using AD biomarker research results for clinical care: a survey of ADNI investigators. Neurology 81, 1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valizadeh SA, Liem F, Mérillat S, Hänggi J, and Jäncke L (2018). Identification of individual subjects on the basis of their brain anatomical features. Sci. Rep. 8, 5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM (2013). Return of individual research results and incidental findings: facing the challenges of translational science. Annu. Rev. Genomics Hum. Genet. 14, 557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, and Evans BJ (2018). Defending the return of results and data. Science 362, 1255–1256. [DOI] [PubMed] [Google Scholar]
- Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, Fletcher JG, Georgieff MK, Hammerschmidt D, Hudson K, et al. (2008). Managing incidental findings in human subjects research: analysis and recommendations. J. Law Med. Ethics 36, 219–248, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]