Abstract
The COVID-19 pandemic has led to a radical lifestyle change, which may unintendedly change physical activity levels. We aimed to perform a systematic review to investigate the physical activity changes in people with neurological diseases, and to examine the relationship between physical activity and disease symptoms, and psychosocial factors. The review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A systematic search of the literature across five databases (PubMed, CINAHL, Web of Science, SCOPUS, and Cochrane Library) was carried out using the keywords relating to COVID-19, physical activity, sedentary behaviour, exercise, and the name of the neurological diseases. The systematic search was updated on 4 February 2021 with the same keywords. Fourteen studies (n = 7662 persons with neurological diseases, n = 1663 healthy controls) were eligible for this review. The study populations were Parkinson disease (n = 7), dementia (n = 1), multiple sclerosis (n = 1), spinal cord injury (n = 1), hereditary spastic paraplegia (n = 1), neuromuscular diseases (n = 1), Charcot-Marie-Tooth neuropathy (n = 1), and epilepsy (n = 1). Thirteen studies reported a decreased physical activity level, one study reported a high interruption rate of physiotherapy/rehabilitation. Furthermore, the physical activity reduction was associated with worse disease symptoms, depression, perceived health, and mental and physical components of quality of life. The COVID-19 pandemic has a negative impact on the physical activity levels of people with neurological diseases, and this change was related to the worsening of disease symptoms and psychosocial factors. Registration number A protocol of the review was registered with the PROSPERO database (CRD42020207676).
Supplementary Information
The online version contains supplementary material available at 10.1007/s10882-022-09836-x.
Keywords: COVID-19, Coronavirus, Physical activity, Sedentary behaviour, Exercise, Neurological Disease
Introduction
Coronavirus Disease (COVID-19) has been declared as a global pandemic on 11 March 2020 by the World Health Organization (Jiménez-Pavón et al., 2020; Tison et al., 2020). To prevent or slow down the spread of COVID-19, the countries and territories have taken measures such as quarantine, closing schools and businesses, and banning cultural and sporting events to ensure social distance (Hammami et al., 2020; Jiménez-Pavón et al., 2020) These measures have led to a rapid change in lifestyle, and the physical activity level assessed by daily step counts have decreased compared to before the pandemic in the general population, which is an essential factor for physical and mental health (Tison et al., 2020; Tremblay et al., 2011) Neurological diseases are generally chronic and require using medications and rehabilitation services for a long time. Although there is no evidence that individuals with neurological diseases are more vulnerable to COVID-19, they may be more affected by healthcare disruptions, home confinement, and psychological stressors (Ferini-Strambi & Salsone, 2020) These factors can lead to more limitations of physical activity, either as routine physiotherapy or sports activities (Schirinzi et al., 2020; Woods et al., 2020).
Physical activity is any bodily movement produced by skeletal muscles that result in energy expenditure, and it improves the general health in both healthy population and people with neurological disabilities (Amekran & el Hangouche, 2021; Boysen & Krarup, 2009; Stennett et al., 2020) However, it has been well established that neurological diseases may negatively affect reaching a sufficient level of physical activity due to motor, sensory, and autonomic system involvement. Ultimately, a lack of physical activity causes deconditioning and worsening the disability (Ellis & Motl, 2013; Elsworth et al., 2009; Stroud & Minahan, 2009) However, it is known that physical activity improves the symptoms of neurological diseases and the quality of life of patients, so regular physical activity has been recommended as a first-line approach for many neurological diseases (Boysen & Krarup, 2009; Goodwin et al., 2008; Stennett et al., 2020).
There are more varied physical activity opportunities outdoor (Jiménez-Pavón et al., 2020) However, in the period of the COVID-19 pandemic, the outdoor activities have been limited to prevent or slow down the spread of infection. The time spent at home has increased, and normal routines have been interrupted (Hammami et al., 2020; Jiménez-Pavón et al., 2020) Even so, there are many possibilities for physical activity that can be performed at home, but indoor activities may need more autonomy and motivation during a stressful time like a pandemic (Hammami et al., 2020) Following the emergence of the COVID-19 pandemic, there are many efforts to encourage people to be physically active. The American College of Sports Medicine published strategies to stay active at home (ACSM, 2020) Research papers have been released to provide activity suggestions, video-based physical activity suggestions have been made, and the importance of the issue was emphasized in the newspapers, and telehealth approaches have been used (Hammami et al., 2020; Jiménez-Pavón et al., 2020; Quinn et al., 2020; Reynolds, 2020).
The evidence has indicated that physical activity is a substantial factor for the health of persons with neurologic diseases and the efforts targeting to improve physical activity during the pandemic are important. Therefore, the objectives of this systematic review were to examine the impact of quarantine and isolation during the COVID-19 pandemic on the physical activity level and sedentary behaviour in people with neurological diseases, and the differences of physical activity levels and sedentary behaviours between people with neurological diseases and healthy controls. Secondly, we aimed to investigate the relationship between physical activity level and psychosocial factors, and disease symptoms.
Methods
A protocol of the review was registered with the PROSPERO database (CRD42020207676). The review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statement (Moher et al., 2009).
Data Sources and Searches
A systematic search for relevant articles was conducted on 22 September 2020 using electronic databases, namely, PubMed, CINAHL (EBSCO host), Web of Science (WOS), SCOPUS, and the Cochrane Library, independently by the two authors (ZA, MK) until 22 September 2020. The search terms for PubMed were: (((((((COVID-19) OR (Novel Coronavirus)) OR (2019-nCoV)) OR (SARS-CoV-2)) OR (2019 novel coronavirus)) OR (SARS nCoV2)) AND (((((((((stroke) OR (multiple sclerosis)) OR (Parkinson)) OR (brain injury)) OR (spinal cord injury)) OR (Alzheimer’s disease)) OR (nervous system disease)) OR (neurological disorders)) OR (neurological diseases))) AND ((((((physical activity) OR (exercise)) OR (sedentary)) OR (sedentary behaviour)) OR (leisure activity)) OR (sitting)) and this strategy was adapted in each database. Since the review topic is very current, the systematic search was updated on 4 February 2021 using the same keywords.
Study Selection
We used the Population, Intervention, Comparison, Outcome (PICO) framework as the criterion for inclusion of articles in this review (Santos et al., 2007) Population: adults with neurological disease including stroke, Parkinson’s disease (PD), multiple sclerosis (MS), traumatic brain injury (TBI), spinal cord injury (SCI), Alzheimer’s disease, and others. Intervention: quarantine and isolation during the COVID-19 pandemic. Comparison: pre-pandemic physical activity level and sedentary behaviour or healthy controls. Outcomes: change in physical activity level or sedentary behaviour score from pre-pandemic to the last available follow-up after the pandemic, measured using the validated self-reported questionnaires or accelerometers/pedometers.
The observational studies, including cross-sectional, cohort, and case–control studies published in English, were included. Initially, two authors independently screened articles based on title and abstract, but if the abstract did not provide sufficient information, the full text was read. Two authors (ZA, MK) independently reviewed the full text of papers for eligibility. In case of any indecision or disagreement, a third author (TK) was consulted.
We had excluded studies that involved pediatric neurological disorders (e.g., cerebral palsy and epilepsy). The animal studies, conference papers, study protocols, opinion papers, commentaries, case reports, systematic reviews, and meta-analysis were also excluded.
Data Extraction and Quality Assessment
National Heart, Lung, and Blood Institute (NHLBI) study quality assessment tool for observational cohort and cross-sectional studies was used to assess the methodological quality of the included articles. The tool has 14 items and is scored as yes, no, or cannot determine/not applicable/not reported (NHLBI, 2014).
Data extraction and data synthesis were conducted according to a guideline for systematic reviews (van Tulder et al., 2003) Two independent reviewers (ZA and MK) manually extracted key data from the included articles in a Summary of Findings table. The two reviewers applied pilot testing for the data extraction form together to minimize misinterpretation. The following data were placed in the appropriate place in table: date and place of study, study design including methodological quality, participants characteristics (a number of participants, age, sex, neurological condition), inclusion and exclusion criteria, measurement of physical activity, and sedentary behaviour, change of physical activity, and sedentary behaviour between pre-pandemic and during the pandemic or differences between persons with a neurological condition and healthy controls.
Data Synthesis and Analysis
The impact of the COVID-19 pandemic on physical activity levels and sedentary behaviours was analysed by the narrative synthesis. Results were presented by grouping clinically similar studies. If convenient and sufficient data were available, it was performed narrative comparisons between the following groups: age, gender, disability group, and geographical area. A detailed analysis was conducted to examine the relationship between physical activity level and disease symptoms, and psychosocial factors.
Results
Search Results
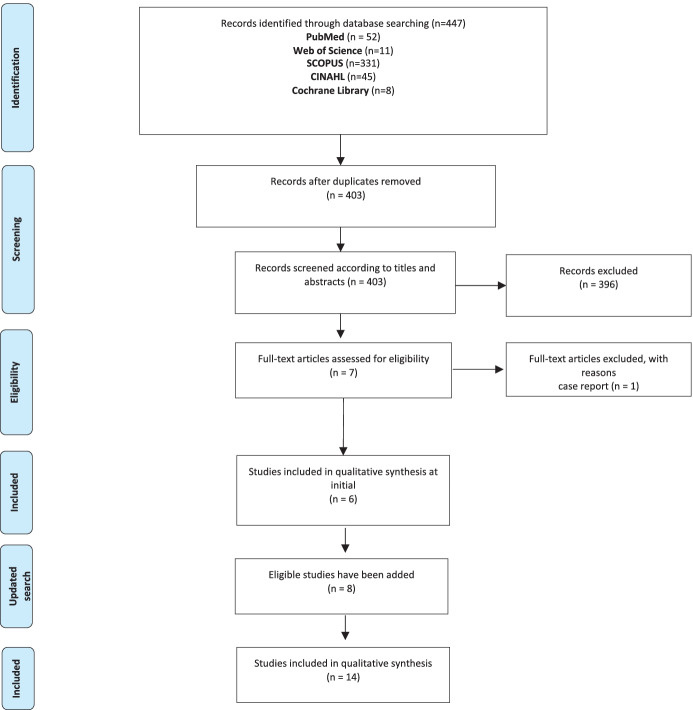
As shown in Fig. 1, 447 records initially were determined based on the search strategy. After deleting 44 duplicates, we removed 396 articles by reading their titles and abstracts. The full texts of the remaining seven articles were screened for eligibility. One article has been excluded because it was a case report. Six articles were included in this systematic review in the first systematic search on 22 September 2020. Eight more articles meeting the research criteria were added after the updated search on 4 February 2021.
Fig. 1.
PRISMA flow-chart
Study Characteristics
Table 1 indicates the descriptive characteristics of the 14 included articles. Seven of the articles included persons with PD. The other studies (n = 7) enrolled participants with different pathologies including dementia (n = 1), multiple sclerosis (n = 1), spinal cord injury (n = 1), hereditary spastic paraplegia (n = 1), neuromuscular diseases (n = 1), Charcot-Marie-Tooth neuropathy (n = 1), and epilepsy (n = 1). Twelve studies questioned whether their physical activity and/or sports Questionnaires (IPAQ) and Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) results assessed before and after the lockdown (Marco-Ahulló et al., 2021; Shalash et al., 2020) Three studies included healthy controls Brown et al., 2020; di Stefano et al., 2020; Shalash et al., 2020), but two of them compared physical activity levels of healthy controls and people with neurological diseases (di Stefano et al., 2020; Shalash et al., 2020). Two studies also included caregivers (Borges-Machado et al., 2020; Cavallieri et al., 2021) Answers were obtained from caregivers of dementia patients, whereas in the study that included persons with Parkinson’s disease, the caregivers were asked when patients could not respond (Borges-Machado et al., 2020; Cavallieri et al., 2021). Brown et al. included participants with and without COVID-19 and presented subgroup analysis for the baseline characteristics, but they provided data for information on physical activity or exercise of participants without COVID-19 (Brown et al., 2020).
Table 1.
Descriptive characteristics and quality assessment scores of the included studies
| Article | Country of origin | Period of observation | Neurological condition | Design | NHBLI Quality Rating |
|---|---|---|---|---|---|
| Shalash et al. (Shalash et al., 2020) | Egypt | Not reported | Parkinson Disease | Case–control | Fair |
| van der Heide et al. (van der Heide et al., 2020) | Netherlands | April 21 – May 25, 2020 | Parkinson Disease | Cross-sectional | Poor |
| Schirinzi et al. (Schirinzi et al., 2020) | Italy | April 20 – May 2,2020 | Parkinson Disease | Cross-sectional | Fair |
| Brown et al. (Brown et al., 2020) | USA | April 23 – May 23, 2020 | Parkinson Disease | Cross-sectional | Poor |
| Cavallieri et al. (Cavallieri et al., 2021) | Italy | May 25 – June 10, 2020 | Parkinson Disease | Cross-sectional | Poor |
| Kumar et al. (Kumar et al., 2021) | India | May 25 – July 20, 2020 | Parkinson Disease | Cross-sectional | Fair |
| Song et al. (Song et al., 2020) | Korea | May 1 – May 20, 2020 | Parkinson Disease | Cross-sectional | Fair |
| Borges-Machado et al. (Borges-Machado et al., 2020) | Portugal | During the June 2020 | Dementia | Cross-sectional | Poor |
| van de Venis et al. (van de Venis et al., 2020) | Netherlands | During the fifth week of the partial lockdown in the Netherlands | Hereditary Spastic Paraplegia | Cross-sectional | Poor |
| Di Stefano et al. (di Stefano et al., 2020) | Italy | April 20—May 4, 2020 | Neuromuscular Disease | Cross-sectional | Fair |
| Kalron et al. (Kalron et al., 2021) | Israel | May 15 – June 15, 2020 | Multiple Sclerosis | Cross-sectional | Poor |
| Marco-Ahulló et al. (Marco-Ahulló et al., 2021) | Spain | May 21- May 25, 2020 |
Complete Thoracic Spinal Cord Injury |
Cross-sectional | Fair |
| Prada et al. (Prada et al., 2020) | Italy | April 6 – May 11, 2020 | Charcot-Marie-Tooth Neuropathy | Cross-sectional | Poor |
| Sanchez-Larsen et al. (Sanchez-Larsen et al., 2020) | Spain | May 17—June 7, 2020 | Epilepsy | Cross-sectional | Fair |
The overall methodological quality is presented in the Supplementary Table and Table 1. All the included studies met criteria 1, 2, 3, and 7 because they well explained research questions and study populations, achieved high participation rates of eligible persons, and sufficient timeframes for assessments. However, none of the studies met criteria 5, 6, 10, and 12. There was no sample size calculation, power description, follow-up, and blindness to the exposure status. assessment in any papers. Also, since the COVID-19 pandemic unexpectedly affects all participants of the studies, none of the studies could assess the exposures of interest before the outcomes were measured.
The date was not reported in the study conducted in Egypt, (Shalash et al., 2020 but the data collection was carried out in other studies between April and June when the measures were strict.
Participants
Fourteen studies included a total of 7662 persons with neurological diseases and 1663 healthy participants. Most of the studies included persons with PD (90%, n = 6898). The mean age of the participants was 57.2 ranged from 42 to 74 years.
Effects of the Pandemic on Physical Activity
Three studies used the IPAQ to assess physical activity levels 6,22,25. In the 11 studies, a constructed survey was used to determine whether physical activity/sport activities/exercise has changed during the pandemic (Borges-Machado et al., 2020; Brown et al., 2020; Cavallieri et al., 2021; Kalron et al., 2021; Kumar et al., 2021; Prada et al., 2020; Sanchez-Larsen et al., 2020; Schirinzi et al., 2020; Song et al., 2020; van de Venis et al., 2020; van der Heide et al., 2020); (Schirinzi et al., 2020). used both IPAQ and structured surveys. Ahullo et al. applied PASIPD to manual wheelchair users with spinal cord injury (Marco-Ahulló et al., 2021).
Thirteen of the studies that evaluated physical activity changes reported a reduction in physical activity level following the COVID-19 pandemic. (Borges-Machado et al., 2020; Brown et al., 2020; Cavallieri et al., 2021; di Stefano et al., 2020; Kalron et al., 2021; Kumar et al., 2021; Marco-Ahulló et al., 2021; Prada et al., 2020; Sanchez-Larsen et al., 2020; Shalash et al., 2020; Song et al., 2020; van de Venis et al., 2020; van der Heide et al., 2020). Schrinzi et al. (Schirinzi et al., 2020) reported that the total number of patients playing sports was maintained but, during the pandemic, the total energy expenditure was 1994.7 ± 1971 metabolic equivalent (MET) min/week in persons with PD, and this score was the leading risk factor for perceived clinical worsening. Besides, the number of patients under physiotherapy/rehabilitation was decreased by 78% in their study (Schirinzi et al., 2020). In Cavallieri et al.’s study, 52.24% of the patients reported that the absence of physical activity is an unmet need during the pandemic (Cavallieri et al., 2021).
Across the studies that compared physical activity in persons with neurological diseases and healthy controls, both reported a greater physical activity reduction following the COVID-19 pandemic in persons with PD and neuromuscular disease (NMD) (di Stefano et al., 2020; Shalash et al., 2020). Brown et al. (Brown et al., 2020). compared only baseline characteristics, not the physical activity levels.
Relationship Between Physical Activity and Disease Symptoms, and Psychosocial Factors During the Pandemic
Of the studies involving persons with PD, Shalash et al. (Shalash et al., 2020). found that physical activity level is associated with depression, anxiety, pre-lockdown depression, and cognition, while van der Heide et al. (van der Heide et al., 2020). did not find a relationship between time of physical activity and perceived stress. The total MET score has been identified as the main risk factor for perceived deterioration of global health by Schirinzi et al. (Schirinzi et al., 2020). Heide et al. found that reduction of physical activity correlated with worsening of symptoms including rigidity, tremor, pain, and fatigue (van der Heide et al., 2020). Similarly, Brown et al. (Brown et al., 2020). reported that cancelled or postponed exercise activities increase the risk of the worsening of motor, cognitive, mood, autonomic, and sleep symptoms and Song et al. indicated that a decrease in the amount of exercise associated with worsening in motor and non-motor symptoms of Parkinson disease (Song et al., 2020). A decrease in physical activity and an increase in the number of patients with screen time of more than 3 h were also found to be associated with new-onset sleep disorders (Kumar et al., 2021).
The physical and mental components of the 12-Item Short Form Survey (SF-12) were correlated with a decrease in physical activity levels in persons with NMD (di Stefano et al., 2020). van de Venis et al. (van de Venis et al., 2020). showed that persons with hereditary spastic paraplegia (HSP) with a reduced level of physical activity experienced increased disease symptoms like increased muscle stiffness, pain, physical fatigue, and gait impairments. Also, it has been reported that a decrease in physical activity is associated with worsening of seizures in individuals with epilepsy (Sanchez-Larsen et al., 2020) (Table 2).
Table 2.
Main characteristics and summary of findings of the included studies
| Article | Participant demographics | Healthy control demographics | Outcome measures | Main Findings |
|---|---|---|---|---|
| Shalash et al. (Shalash et al., 2020) |
n = 38 Sex (F/M) = 9/29 Mean age = 55.58 ± 9.96 Neurological condition: Parkinson disease |
n = 20 Sex (F/M) = 6/14 Mean age = 55.55 ± 5.71 |
The short form of the International Physical Activity Questionnaire (IPAQ-SF), The 11 items survey for assessing perception of impact of COVID-19, The Depression, Anxiety, and Stress Scale–21 (DASS-21), Parkinson Disease Questionnaire (PDQ-39) (PDQ-39), Pre-lockdown Beck Depression Inventory (BDI) |
• Compared with controls, patients showed significantly worse stress (p = 0.028), depression(p = 0.015), anxiety (p = 0.001), total DASS (p = 0.006), moderate physical activity (p = 0.017), walking, total IPAQ-SF, total (p = 0.006) and most of the PDQ-39 dimensions • Persons with PD showed a significant decline in physical activity assessed by IPAQ-SF compared with pre-lockdown (p = 0.002) • Total IPAQ-SF scores were negatively correlated with total DASS (rs = − 0.354, p = 0.029), DASS depression (rs = − 0.441, p = 0.006), and BDI (rs = -0.333, p = 0.044) |
| van der Heide et al.(van der Heide et al., 2020) |
n = 358 Sex(F/M) = 138/220) Mean age = 62.8 ± 9.0 Mean disease duration: 3.9 Neurological condition: Parkinson disease |
None |
A 9-point (1 = much worse, 5 = no change, 9 = much improved) self-reported survey including: - the changes in physical activity, .duration of intensive exercise, - PD symptoms severity compared to the month preceding the start of the pandemic The Unified Parkinson Disease Rating Scale part Ib and II (MDS-UPDRS-self) Perceived Stress Scale (PSS) Parkinson Anxiety Scale (PAS) Beck’s Depression Inventory II (BDI-II) |
• Patients were significantly less active than before the pandemic (MD = –0.50, [95% CI –0.67, –0.33]) 46.6% of patients were less active compared to before the pandemic 33.0% of patients were equally active 20.4% of patients were more active • The reduction in physical activity correlated with worsening of PD symptoms severity (rs = 0.14 [95% CI 0.03, 0.25]) • There was no relationship between time of physical activity and the degree of perceived stress (PSS) (rs = –0.08 [95% CI, –0.18, 0.05]) |
| Schirinzi et al. (Schirinzi et al., 2020) |
n = 74 Mean age = 61.3 ± 9.3 Sex (F/M) = 37/37 Diseases duration: 6.5 ± 4.5 PD onset: 55.5 ± 10.8 Neurological condition: Parkinson disease |
None |
A structured survey including: - Motor activity habits before COVID-19 emergency: physiotherapy/rehabilitation practice, sports practice (type and weekly frequency) - Motor activity habits during lockdown: physiotherapy/rehabilitation practice, physical exercise practice (indoor/outdoor, type of activity) - The perception of own health during COVID-19 emergency - The use of technology-based tools International Physical Activity Questionnaires – Short Form (IPAQ–SF) Parkinson Well-Being Map (PWBM) Beck Depression Index (BDI) |
• The number of patients under physiotherapy/rehabilitation decreased from 32 (43%) to 7 (9.7%; 78% reduction) • The total number of patients playing sports remained stable, 59 (80%) before and 60 (81%) during the emergency (53 patients continued, 7 instead started during the lockdown) • The interruption of physiotherapy (n = 26) did not increase the number of sporting patients (reduced from 22 to 20, p > 0.05) • 60% of patients reported a significant worsening of their general conditions during the lockdown and the total MET (1994.7 ± 1971) min/week was inversely associated with worsening (OR = 0.2, p = 0.05) |
| Brown et al. (Brown et al., 2020) |
n = 5429 COVID-19 positive/negative = 51/5378 Age = 68 (range: 33–95) Sex (F/M) = 2625/2804 PD duration, years: 0–3: 1649 3–6: 1661 6–9: 990 > 9: 1123 Neurological condition: Parkinson disease |
n = 1452 COVID-19 positive/negative = 26/1426 Age = 61 (range: 19–94) Sex (F/M) = 1139/313 |
Constructed COVID-19 survey including: Disruptions of medical care: • Cancelled or postponed rehab therapy/ mental health care/ botox treatment/ DBS surgery/ DBS battery replacement/ DBS programming • Have lost or reduced in-home care services • Had to cancel healthcare appts • Problems obtaining meds for PD Activities: • Exercise • Seeing Family • Seeing Friends • Support Group Attendance • Volunteer Activities • Religious Activities • Community Activities PD related symptoms: • Motor • Cognitive • Autonomic • Sleep • Mood |
• 21% of patients reported cancelled exercise, 7.9% postponed, 41% conducted via alternative methods • 57% of patients reported cancelled social activities • The new PD symptoms emerged and existing symptoms worsened in all major domains (motor: 6.2% new, 41% worsened; mood: 6.5% new, 30% worsened; cognitive: 2.5% new, 16% worsened; sleep: 4.5% new, 32% worsened; autonomic: 2.6% new, 18% worsened • Respondents who experienced interruptions to exercise and social activities were also more likely to report worsening of PD symptoms including motor [OR = 1.31 (95%CI 1.16–1.49), p < 0.001], cognitive [OR = 1.28 (95% CI 1.10–1.50), p < 0.01], mood [OR = 1.21 (95% CI 1.07–1.38), p < 0.01], autonomic [OR = 1.23 (95% CI 1.06–1.42), p < 0.01], and sleep [OR = 1.34 (95% CI 1.18–1.52), p < 0.001] |
| Cavallieri et al. (Cavallieri et al., 2021) |
n = 67 Sex (F/M) = 26/41 Neurological condition: Parkinson disease |
n = 36 caregivers Sex (F/M) = 14/22 |
The online structured survey including: • Demographics and clinical condition • Neurological service provision and therapeutic relationship with the neurologist • Physical activities during the pandemic • Perceptions of healthcare related needs |
• 30.36% of the participants reported no PA, 39.29% home-only PA, 30.36 poor PA • 52.24% of the participants reported that the absence of physical activity is an unmet need |
| Kumar et al. (Kumar et al., 2021) |
n = 832 Age: < 50 years = 135 (16.2) ≥ 50 years = 697 (83.8) Sex (F/M) = 262/570 Neurological condition: Parkinson disease |
None |
The online validated questionnaire including: • change or new-onset of motor non-motor as well as sleep-related symptoms during home confinement • effect of home confinement on physical activity [change in screen time (< 3 h/day or > 3 h/day) and physical activity (< 1 h/day or > 1 h/day]. was compared by asking the subjects about time spent in these activities each day before and during home confinement |
• While the number of persons with PA duration < 1 h before the pandemic was 645 (77.5%), it was 660 (79.3%) during the pandemic • While the number of participants with screen time > 3 h was 139 (16.7%) before the pandemic, it was 240 (28.8%) during the pandemic • Decrease in physical activity (p < 0.003) and increase in screen time (p < 0.015) were associated with new-onset/worsening of sleep disturbances |
| Song et al. (Song et al., 2020) |
n = 100 Mean age = 70 (range:62.3–76) Sex (F/M) = 46/54 Neurological condition: Parkinson disease |
None |
The Unified Parkinson Disease Rating Scale part 3 Mini-Mental State Examination (MMSE) Schwab and England scale of activities of daily living (SE-ADL) The structured survey including subjective changes in PD symptoms and exercises (type, duration, frequency) The amount of exercise was evaluated using the Korean version of the Physical Activity Scale of the Elderly (PASE) questionnaire. Change in the amount of exercise during the pandemic were asked |
• Forty-five participants (45%) reported doing less amount of exercise after the onset of pandemic, while 55 participants (55%) continued to exercise as previous • The number of participants who do not exercise at all increased and less number of participants were exercising at sports facilities • There was a significant decrease in the amount of exercise [both duration (p = 0.003) and frequency (p = 0.011. checked by the PASE leisure part score (p < 0.001) • There was a significant association between reduced exercise amount and the subjective worsening of both motor and non-motor symptoms of parkinsonism (p < 0.001) |
| Borges-Machado et al. (Borges-Machado et al., 2020) |
n = 36 Mean age = 74.28 ± 6.76 Sex (F/M) = 24/12 Neurological condition: Dementia |
n = 36 caregivers Mean age = 64.94 ± 13.54 Sex (F/M) = 15/21 |
Questions were asked via telephone call to caregivers of patients - Current status of patients [independence in daily life via Barthel Index, neuropsychiatric symptoms and physical activity (comparisons between confinement period with pre-confinement period on the volume of physical activity and sitting time) and cognitive status]. and caregivers |
• PA decreased and sitting time increased in 80.6% of the patients • 66.7% of the patients showed physical decline during the pandemic • There was a significant decline in the independence in daily life (p = 0.003) .Neuropsychiatric symptoms worsened during the pandemic (p = 0.015) |
| van de Venis et al. (van de Venis et al., 2020) |
n = 58 Sex (F/M) = 31/27 Mean age = 57 (range:30–77) years Neurological condition: Hereditary spastic paraplegia |
None |
Web-based structured survey (5-point Likert scale) including changes in levels of: • physical activity • psychological stress • symptom severity including muscle stiffness, muscle cramps, restless legs, pain, physical fatigue, mental fatigue, balance problems, and gait problems |
• A reduction of physical activities was reported by 74% (33% strong decrease, 41% mild decrease), whereas 19% reported no change and 7% mild increase • Participants with reduced physical activity more often experienced increased muscle stiffness (p = 0.001), pain (p = 0.004), physical fatigue [χ2 (1) = 4.680, p = 0.031], and gait impairments [χ2 (1) = 5.129, p = 0.024]. compared to those with no change or an increase in physical activity • Decreased physical activity was independently associated with an increase in muscle stiffness. [R2 = 0.236, p < 0.001] and pain [R2 = 0.193, p = 0.003] |
| Di Stefano et al. (di Stefano et al., 2020) |
n = 149 Age = 57.3 ± 13.7 Sex (F/M) = 56/93 Ambulant/non-ambulant: 119/30 Type of disease: ○ Acquired or hereditary myopathy: n = 19 (13%) ○ Acquired or hereditary polyneuropathy: n = 69 (46%) ○ Disorder of the neuromuscular junction: n = 49 (33%) ○ Genetically confirmed degenerative disease: n = 12 (8%) Neurological condition: Neuromuscular disease |
n = 119 Age = 56 ± 6.8 Sex (F/M) = 45/74 |
International Physical Activity Questionnaire Short-Form (IPAQ-SF) was in before quarantine and during the quarantine to both groups Short-Form Health Survey (SF-12) was administered to persons with neuromuscular disease |
• In persons with neuromuscular disease, a significant reduction of physical activity was reported for walking activity (p < 0.0001), total physical activity level (p < 0.0001), and moderato-to-vigorous physical activity level (p = 0.04), while no difference was found for vigorous-intensity physical activity (p = 0.69) and moderate-intensity physical activity (p = 0.07) • There was significant difference in the distribution of each intensity physical activity between persons with neuromuscular disease and healthy controls in the before-quarantine and during quarantine • There were significant correlations between physical health component score of SF-12 and both ∆MET total (rs = − 0.276, p = 0.002) and moderate-to-vigorous physical activity (rs = − 0.229, p = 0.005) • The mental health component score of SF-12 was correlated with ∆MET total (rs = − 0.192, p = 0.036) |
| Kalron et al. (Kalron et al., 2021) |
n = 120 Sex (F/M) = 78/42 Mean age = 43 ± 12.9 Neurological condition: Multiple sclerosis |
None |
The online structured survey including: - Change in PA behaviour - Frequency per week they took part in PA - The duration of activity - The type of activity |
• 17.5% of the participants stopped performing PA • during the pandemic • 33.3% reduced PA during the pandemic • 20.0% continued PA as before • 18.3% increased PA during the pandemic |
| Marco-Ahulló et al. (Marco-Ahulló et al., 2021) |
n = 20 Mean age = 45.4 ± 9.48 Neurological condition: Spinal cord injury |
None | Physical Activity Scale for Individuals with Physical Disabilities |
• Total self-reported PA (p < 0.001), recreational PA (p < 0.001), occupational PA (p = 0.042), decreased during the pandemic. There was no change in housework PA (p = 0.69) • Total minutes spent (p < 0.001), minutes spent on recreational activities of moderate/vigorous intensity (p < 0.001), and minutes spent on occupational activities (p = 0.042) of moderate/vigorous intensity decreased during the pandemic |
| Prada et al. (Prada et al., 2020) |
n = 281 Sex (F/M) = 204/77 Neurological condition: Charcot-Marie-Tooth (CMT) Neuropathy Type of disease: ○ CMT1A: 49% (n = 137) ○ CMT2 forms: 16% (n = 45) ○ CMT1X: 10% (n = 28) ○ CMT1B: 5.38% (n = 15) ○ CMT4 C: 2% (n = 7) |
None |
The online structured survey including: • generic data (age, sex, CMT type) and the ‘‘home situation’’ (the presence of the lockdown in the country, the possibility of staying at home or not) • habits and general health situation before the outbreak (e.g., pain in the extremities, ability to perform exercises at home or outside) and how these habits changed after the outbreak • psychological situation and personal needs |
• There was a significant reduction in number of walks per week (p < 0.0001) • The perception reporting pain in arms and legs was significantly increased after the lockdown (p < 0.0001) • The fear of falling was also significantly increased (p = 0.0004) |
| Sanchez-Larsen et al. (Sanchez-Larsen et al., 2020) |
n = 100 Sex (F/M) = 52/48 Mean age = 42.4 Neurological condition: Epilepsy |
None |
Constructed survey including: • demographic and clinical data • seizure frequency during the period corresponding to the national state of emergency (from March 14, 2020, onward) • lifestyles and routines during the quarantine and social isolation (changes in mood, physical exercise, diet, rest and sleep, and adherence to treatment) |
• 31% of patients reported a reduction in physical activity during the pandemic • During the COVID-19 period, 27% of the patients presented an increase of > 50% of seizure frequency • A less physical activity (OR: 3.84, 95%CI:1.51–9.98; p = 0.004) was associated with worsening of seizure control |
Discussion
This paper is the first systematic review to reveal the change of physical activity level in people with neurological diseases during the COVID-19 pandemic period. The evidence presented in this review highlights the ongoing COVID-19 pandemic affects the physical activity levels of people with neurological disease. Studies recruiting 7662 persons with neurological disease and 1663 healthy controls indicate that physical activity level decreased in the period of the COVID-19 pandemic. Additionally, the reduction of physical activity was related to depression, perceived health, the mental and physical component of quality of life, and worse disease symptoms.
The sudden radical change in the lifestyle may lead to an increase in sedentary behaviour and a decrease in the physical activity level in the period of the COVID-19 pandemic. However, regular physical activity is required to counteract the negative effects of diseases in persons with neurological diseases (Block et al., 2016; Ellis & Motl, 2013). The unintended decrease in daily physical activity and increased sedentary time can culminate changes in skeletal muscles, including loss of muscle strength, muscle power, and muscle mass associated with disuse and deterioration in cardiovascular health in after a short period of inactivity (Pagano et al., 2018; Pecanha et al., 2020). These changes may prepare the ground for further motor deterioration caused by deconditioning in persons with neurological diseases who already have motor impairments. Additionally, the preventive role of exercise for COVID-19 and its helping manage recovery from the COVID-19 through the mechanism, including enhanced metabolic homeostasis, suppress inflammation, and improvements in cardio-respiratory fitness, have been discussed in the literature (M. Wang, Baker, et al., 2020; Wang, Chao, et al., 2020). Considering these factors, the decrease in physical activity in individuals with neurological diseases indicates a problem that requires an urgent solution. Although supervised physiotherapy interventions are generally prescribed for persons with neurological disease, the hospitals and external centres had to reduce/stop their outpatient activities. In the study of Schirinzi et al. (Schirinzi et al., 2020), 78% of physiotherapy sessions were interrupted in persons with PD. Although the effects of these interruptions are not known yet, they should be examined in future longitudinal studies.
There is evidence on that physical activity is not only related to physical health but also mental health through the brain and systemic effects in neurological diseases (Dauwan et al., 2019). It has been reported that neuropsychiatric sequel is caused by the immunological responses of the SARS-CoV-2 in the central nervous system and the psychological distress caused by a global pandemic (Troyer et al., 2020). Therefore, maintaining physical activity shows an important role in this period. There is growing evidence that physical activity and exercise positively affect anxiety and stress through their neurobiological effects (McDowell et al., 2019; Mochcovitch et al., 2016; Morgan et al., 2015). In line with this mechanism, three studies in this review demonstrated that physical activity is related to depression, anxiety, perceived global health, and mental and physical dimensions quality of life in persons with PD and NMD (di Stefano et al., 2020; Schirinzi et al., 2020; Shalash et al., 2020). However, in the study of van der Heide, (van der Heide et al., 2020). time of physical activity and perceived stress were not correlated in persons with PD. These conflicting results may result from using different measurement tools and statistical methods of the included papers.
It has been well-known that individuals with chronic neurological conditions need regular rehabilitation, and adequate physical activity is critical for their health status (Abbruzzese et al., 2016; Belagaje, 2017; Block et al., 2016; Charytoniuk et al., 2020; Ellis & Motl, 2013; Learmonth & Motl, 2016). In the literature, some reports state that neurorehabilitation should not be delayed or interrupted during the pandemic period, which will shape the lifestyle until the treatment becomes available (Leocani et al., 2020; Manto et al., 2020; Mantovani et al., 2020; C. C. Wang, Chao, et al., 2020; Wang, Baker, et al., 2020). However, the reorganization of the health system worldwide due to the COVID 19 pandemic deeply affected the patients' in-person rehabilitation session (Guidon & Amato, 2020; Mauri et al., 2020). When considering the close interaction between therapist and patient, it is not astounding that one of the most interrupted health services is rehabilitation sessions in the pandemic era (Boldrını et al., 2020). For this reason, the accessibility of technology-based approaches such as telehealth delivery can be promoted to monitor remotely and increase the level of physical activity during pandemic conditions. To continue the rehabilitation sessions remotely, methods such as sending videos and photographs, performing online home visits were used (Manto et al., 2020; Mantovani et al., 2020). However, it is not yet clear to what extent patients have access to these opportunities and how effective they find these methods.
Our study has some limitations. Firstly, in the vast majority of studies, physical activity change was questioned cross-sectionally with patient-reported outcomes, and confounders were not controlled except four studies (Brown et al., 2020; Kumar et al., 2021; Schirinzi et al., 2020; Song et al., 2020). Also, while a majority of the studies did not assess the sitting time, which could be important for sedentary behaviour. In some studies, the IPAQ was used, but the sitting sub-score has not been provided (di Stefano et al., 2020; Schirinzi et al., 2020; Shalash et al., 2020). Physical activity level was not objectively monitored using smart technologies in any study. Pre-pandemic physical activity level was known only in three studies (Shalash et al., 2020). Besides, in some studies, the lack of a control group may hinder understanding whether the results are disease specific. Lastly, although all studies present results in the same direction, the fact that the majority of the participants are persons with PD makes the generalizability of the results difficult.
An issue that attracts attention in the current systematic review was 7 of the 14 studies included in the research have poor quality (Borges-Machado et al., 2020; Brown et al., 2020; Cavallieri et al., 2021; Kalron et al., 2021; Prada et al., 2020; van de Venis et al., 2020; van der Heide et al., 2020). Although it can affect the generalizability of the results, it is understandable that the studies are planned and conducted quickly due to the pandemic's sudden nature. However, it is believed to be important that combining the results of existing research in line with a consociate conclusion until studies with high-quality study design offering more important evidence are published will make significant contributions to the literature.
There is a need for longitudinal studies that will examine the long-term effects of a reduction in physical activity during the quarantine period and examine the level of physical activity after progressive return to everyday life. However, these days some countries face the new mutation of the virus and an increase in the number of cases, which could lead to new challenges. Therefore, there is a need for urgent action to maintain the physical activity level. Since the end of the pandemic cannot be predicted, prevention strategies should be designed against these rapid changes in physical activity. Future studies should investigate the effects of interventions or alternative ways targeting physical activity during the pandemic.
Conclusions
This review highlights the negative indirect impact of the COVID-19 pandemic on physical activity levels in persons with neurological diseases. Moreover, the reduction of physical activity was associated with depression, perceived health, the mental and physical component of quality of life, and worse disease symptoms. Since the continuing spread of the pandemic and the associated global disruption, rapid modifications to increase physical activity should be sought within the measures' framework.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors' Contributions
Conceptualization: ZA, MK, TK; Methodology: ZA, MK, TK; Data curation: ZA, MK; Supervision: TK; Visualization: ZA, MK, TK; Writing-original draft: ZA, MK; Writing – review & editing: ZA, MK, TK.
Authors read and approved the final version of the manuscript; all authors indicated in the byline meet the criteria for authorship.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
The data of the study was not stored in a public database. However, in a necessary situation, data will be shared if data is requested from the author.
Code Availability
Not applicable.
Declarations
Conflicts of Interest
Authors have nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zuhal Abasıyanık, Email: zuhalabasiyanik@gmail.com.
Merve Kurt, Email: merveekurtt93@gmail.com.
Turhan Kahraman, Email: turhan.kahraman@yahoo.com.
References
- Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism and Related Disorders. 2016;22:S60–S64. doi: 10.1016/j.parkreldis.2015.09.005. [DOI] [PubMed] [Google Scholar]
- ACSM. (2020). Staying active during the Coronavirus pandemic. Exercise is Medicine.
- Amekran, Y., & el Hangouche, A. J. (2021). Coronavirus disease (COVID-19) and the need to maintain regular physical activity. The Journal of sports medicine and physical fitness. 10.23736/S0022-4707.20.11524-X [DOI] [PubMed]
- Belagaje SR. Stroke Rehabilitation. CONTINUUM Lifelong Learning in Neurology. 2017 doi: 10.1212/CON.0000000000000423. [DOI] [PubMed] [Google Scholar]
- Block VAJ, Pitsch E, Tahir P, Cree BAC, Allen DD, Gelfand JM. Remote physical activity monitoring in neurological disease: A systematic review. PLoS ONE. 2016 doi: 10.1371/journal.pone.0154335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini, P., Bernetti, A., & Fiore, P. (2020). Impact of COVID-19 outbreak on rehabilitation services and Physical and Rehabilitation Medicine physicians’ activities in Italy An official document of the Italian PRM Society (SIMFER). European Journal of Physical and Rehabilitation Medicine. 10.23736/S1973-9087.20.06256-5 [DOI] [PubMed]
- Borges-Machado, F., Barros, D., Ribeiro, Ó., & Carvalho, J. (2020). The Effects of COVID-19 Home Confinement in Dementia Care: Physical and Cognitive Decline, Severe Neuropsychiatric Symptoms and Increased Caregiving Burden. American journal of Alzheimer’s disease and other dementias, 35. 10.1177/1533317520976720 [DOI] [PMC free article] [PubMed]
- Boysen G, Krarup LH. Benefits of physical activity for stroke survivors. Expert Review of Neurotherapeutics. 2009 doi: 10.1586/14737175.9.2.147. [DOI] [PubMed] [Google Scholar]
- Brown EG, Chahine LM, Goldman SM, Korell M, Mann E, Kinel DR, et al. The Effect of the COVID-19 Pandemic on People with Parkinson’s Disease. Journal of Parkinson’s Disease. 2020;10(4):1365–1377. doi: 10.3233/JPD-202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallieri F, Sireci F, Fioravanti V, Toschi G, Rispoli V, Antonelli F, et al. Parkinson Patients’ needs during COVID-19 pandemic in a red zone: A Framework Analysis of Open-Ended Survey Questions. European Journal of Neurology. 2021 doi: 10.1111/ene.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charytoniuk T, Zywno H, Konstantynowicz-Nowicka K, Berk K, Bzdega W, Chabowski A. Can physical activity support the endocannabinoid system in the preventive and therapeutic approach to neurological disorders? International Journal of Molecular Sciences. 2020 doi: 10.3390/ijms21124221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwan, M., Begemann, M. J. H., Slot, M. I. E., Lee, E. H. M., Scheltens, P., & Sommer, I. E. C. (2019). Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: a transdiagnostic systematic review and meta-analysis of randomized controlled trials. Journal of Neurology, 1–25. 10.1007/s00415-019-09493-9 [DOI] [PMC free article] [PubMed]
- di Stefano, V., Battaglia, G., Giustino, V., Gagliardo, A., D’Aleo, M., Giannini, O., et al. (2020). Significant reduction of physical activity in patients with neuromuscular disease during COVID-19 pandemic: the long-term consequences of quarantine. Journal of Neurology, 1–7. 10.1007/s00415-020-10064-6 [DOI] [PMC free article] [PubMed]
- Ellis T, Motl RW. Physical activity behavior change in persons with neurologic disorders: Overview and examples from Parkinson disease and multiple sclerosis. Journal of Neurologic Physical Therapy. 2013 doi: 10.1097/NPT.0b013e31829157c0. [DOI] [PubMed] [Google Scholar]
- Elsworth, C., Dawes, H., Sackley, C., Soundy, A., Howells, K., Wade, D., et al. (2009). A study of perceived facilitators to physical activity in neurological conditions. International Journal of Therapy and Rehabilitation, 16(1), 17–23. 10.12968/ijtr.2009.16.1.37936
- Ferini-Strambi, L., & Salsone, M. (2020). COVID-19 and neurological disorders: are neurodegenerative or neuroimmunological diseases more vulnerable? Journal of Neurology, 1–11. 10.1007/s00415-020-10070-8 [DOI] [PMC free article] [PubMed]
- Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders. 2008 doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology. 2020 doi: 10.1212/WNL.0000000000009566. [DOI] [PubMed] [Google Scholar]
- Hammami A, Harrabi B, Mohr M, Krustrup P. Physical activity and coronavirus disease 2019 (COVID-19): Specific recommendations for home-based physical training. Managing Sport and Leisure. 2020 doi: 10.1080/23750472.2020.1757494. [DOI] [Google Scholar]
- Jiménez-Pavón D, Carbonell-Baeza A, Lavie CJ. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Progress in Cardiovascular Diseases. 2020 doi: 10.1016/j.pcad.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalron, A., Dolev, M., Greenberg-Abrahami, M., Menascu, S., Frid, L., Avrech-Shezifi, S., et al. (2021). Physical activity behavior in people with multiple sclerosis during the COVID-19 pandemic in Israel: Results of an online survey. Multiple Sclerosis and Related Disorders, 47. 10.1016/j.msard.2020.102603 [DOI] [PMC free article] [PubMed]
- Kumar N, Gupta R, Kumar H, Mehta S, Rajan R, Kumar D, et al. Impact of home confinement during COVID-19 pandemic on sleep parameters in Parkinson’s disease. Sleep Medicine. 2021;77:15–22. doi: 10.1016/j.sleep.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: A review and content analysis of qualitative research identifying perceived determinants and consequences. Disability and Rehabilitation. 2016 doi: 10.3109/09638288.2015.1077397. [DOI] [PubMed] [Google Scholar]
- Leocani L, Diserens K, Moccia M, Caltagirone C. Disability through COVID-19 pandemic: Neurorehabilitation cannot wait. European Journal of Neurology. 2020 doi: 10.1111/ene.14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M, Dupre N, Hadjivassiliou M, Louis ED, Mitoma H, Molinari M, et al. Management of Patients with Cerebellar Ataxia During the COVID-19 Pandemic: Current Concerns and Future Implications. Cerebellum. 2020;19(4):562–568. doi: 10.1007/s12311-020-01139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani E, Zucchella C, Bottiroli S, Federico A, Giugno R, Sandrini G, et al. Telemedicine and Virtual Reality for Cognitive Rehabilitation: A Roadmap for the COVID-19 Pandemic. Frontiers in Neurology. 2020;11:926. doi: 10.3389/fneur.2020.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Ahulló, A., Montesinos-Magraner, L., González, L.-M., Morales, J., Bernabéu-García, J. A., & García-Massó, X. (2021). Impact of COVID-19 on the self-reported physical activity of people with complete thoracic spinal cord injury full-time manual wheelchair users. The Journal of Spinal Cord Medicine, 1–5. 10.1080/10790268.2020.1857490 [DOI] [PMC free article] [PubMed]
- Mauri, E., Abati, E., Musumeci, O., Rodolico, C., D’Angelo, M. G., Mirabella, M., et al. (2020). Estimating the impact of COVID-19 pandemic on services provided by Italian Neuromuscular Centers: an Italian Association of Myology survey of the acute phase. Acta Myologica, 39(2), 57–66. 10.36185/2532-1900-008 [DOI] [PMC free article] [PubMed]
- McDowell CP, Dishman RK, Gordon BR, Herring MP. Physical Activity and Anxiety: A Systematic Review and Meta-analysis of Prospective Cohort Studies. American Journal of Preventive Medicine. 2019 doi: 10.1016/j.amepre.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Mochcovitch MD, Deslandes AC, Freire RC, Garcia RF, Nardi AE. The effects of regular physical activity on anxiety symptoms in healthy older adults: A systematic review. Revista Brasileira De Psiquiatria. 2016 doi: 10.1590/1516-4446-2015-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JA, Corrigan F, Baune BT. Effects of physical exercise on central nervous system functions: A review of brain region specific adaptations. Journal of Molecular Psychiatry. 2015;3(1):3. doi: 10.1186/s40303-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI. (2014). Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Bethesda, MD: National Institutes of Health, Department of Health and Human Services., 1–4. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort
- Pagano AF, Brioche T, Arc-Chagnaud C, Demangel R, Chopard A, Py G. Short-term disuse promotes fatty acid infiltration into skeletal muscle. Journal of Cachexia, Sarcopenia and Muscle. 2018;9(2):335–347. doi: 10.1002/jcsm.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecanha T, Goessler KF, Roschel H, Gualano B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. American Journal of Physiology - Heart and Circulatory Physiology. 2020 doi: 10.1152/ajpheart.00268.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada, V., Hamedani, M., Genovese, F., Zuppa, A., Benedetti, L., Bellone, E., et al. (2020). People with Charcot-Marie-Tooth disease and COVID-19: Impaired physical conditions due to the lockdown. An International cross-sectional survey. Annals of Physical and Rehabilitation Medicine. 10.1016/j.rehab.2020.10.001 [DOI] [PMC free article] [PubMed]
- Quinn L, MacPherson C, Long K, Shah H. Promoting physical activity via telehealth in people with parkinson disease: The path forward after the COVID-19 pandemic? Physical Therapy. 2020;100(10):1730–1736. doi: 10.1093/ptj/pzaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, G. (2020, January 4). Stuck Inside? Keep Walking. The New York Times.
- Sanchez-Larsen, A., Gonzalez-Villar, E., Díaz-Maroto, I., Layos-Romero, A., Martínez-Martín, Á., Alcahut-Rodriguez, C., et al. (2020). Influence of the COVID-19 outbreak in people with epilepsy: Analysis of a Spanish population (EPICOVID registry). Epilepsy and Behavior, 112. 10.1016/j.yebeh.2020.107396 [DOI] [PMC free article] [PubMed]
- Santos CMDC, Pimenta CADM, Nobre MRC. A estratégia PICO para a construção da pergunta de pesquisa e busca de evidências. Revista Latino-Americana De Enfermagem. 2007;15(3):508–511. doi: 10.1590/S0104-11692007000300023. [DOI] [PubMed] [Google Scholar]
- Schirinzi T, di Lazzaro G, Salimei C, Cerroni R, Liguori C, Scalise S, et al. Physical Activity Changes and Correlate Effects in Patients with Parkinson’s Disease during COVID-19 Lockdown. Movement Disorders Clinical Practice. 2020;7(7):797–802. doi: 10.1002/mdc3.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalash A, Roushdy T, Essam M, Fathy M, Dawood NL, Abushady EM, et al. Mental Health, Physical Activity, and Quality of Life in Parkinson’s Disease During COVID-19 Pandemic. Movement Disorders. 2020 doi: 10.1002/mds.28134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Ahn JH, Choi I, Mun JK, Cho JW, Youn J. The changes of exercise pattern and clinical symptoms in patients with Parkinson’s disease in the era of COVID-19 pandemic. Parkinsonism and Related Disorders. 2020;80:148–151. doi: 10.1016/j.parkreldis.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennett A, de Souza L, Norris M. The meaning of exercise and physical activity in community dwelling people with multiple sclerosis. Disability and Rehabilitation. 2020;42(3):317–323. doi: 10.1080/09638288.2018.1497715. [DOI] [PubMed] [Google Scholar]
- Stroud NM, Minahan CL. The impact of regular physical activity on fatigue, depression and quality of life in persons with multiple sclerosis. Health and Quality of Life Outcomes. 2009;7(1):68. doi: 10.1186/1477-7525-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison GH, Avram R, Kuhar P, Abreau S, Marcus GM, Pletcher MJ, Olgin JE. Worldwide Effect of COVID-19 on Physical Activity: A Descriptive Study. Annals of Internal Medicine. 2020 doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MS, Warburton DER, Janssen I, Paterson DH, Latimer AE, Rhodes RE, et al. New Canadian physical activity guidelines. Applied Physiology, Nutrition and Metabolism. 2011 doi: 10.1139/H11-009. [DOI] [PubMed] [Google Scholar]
- Troyer, E. A., Kohn, J. N., & Hong, S. (2020). Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 10.1016/j.bbi.2020.04.027 [DOI] [PMC free article] [PubMed]
- van de Venis L, van de Warrenburg BPC, Weerdesteyn V, van Lith BJH, Geurts ACH, Nonnekes J. COVID-19 reveals influence of physical activity on symptom severity in hereditary spastic paraplegia. Journal of Neurology. 2020 doi: 10.1007/s00415-020-10016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide A, Meinders MJ, Bloem BR, Helmich RC. The Impact of the COVID-19 Pandemic on Psychological Distress, Physical Activity, and Symptom Severity in Parkinson’s Disease. Journal of Parkinson’s Disease. 2020 doi: 10.3233/JPD-202251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine. 2003 doi: 10.1097/00007632-200306150-00014. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chao JK, Wang ML, Yang YP, Chien CS, Lai WY, et al. Care for Patients with Stroke During the COVID-19 Pandemic: Physical Therapy and Rehabilitation Suggestions for Preventing Secondary Stroke. Journal of Stroke and Cerebrovascular Diseases. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.105182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Baker JS, Quan W, Shen S, Fekete G, Gu Y. A Preventive Role of Exercise Across the Coronavirus 2 (SARS-CoV-2) Pandemic. Frontiers in Physiology. 2020 doi: 10.3389/fphys.2020.572718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Hutchinson NT, Powers SK, Roberts WO, Gomez-Cabrera MC, Radak Z, et al. The COVID-19 pandemic and physical activity. Sports Medicine and Health Science. 2020;2(2):55–64. doi: 10.1016/j.smhs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of the study was not stored in a public database. However, in a necessary situation, data will be shared if data is requested from the author.
Not applicable.