Abstract
Primary infection with Epstein-Barr virus (EBV) is associated with post-transplant lymphoproliferative disease and severe disease in patients with X-linked lymphoproliferative disease; no therapies are approved to prevent EBV infection in these patients. Hyperimmune globulin has been used to prevent some virus infections in immunocompromised persons. Here, we identified plasma donors with high titers of EBV gp350 and EBV B cell neutralizing antibodies. Pooled IgG isolated from these donors was compared to intravenous immunoglobulin (IVIG) for its ability to reduce viral load in the blood in humanized mice challenged with EBV. Mice that received EBV hyperimmune globulin had significantly reduced EBV DNA copy numbers compared to animals that received saline control; however, while animals that received EBV hyperimmune globulin had lower EBV DNA copies than those that received IVIG, the difference was not significant. Thus, while EBV hyperimmune globulin reduced viral load compared to IVIG, the effect was modest.
Keywords: Epstein-Barr virus, glycoprotein 350, hyperimmune globulin, herpesvirus
1. Introduction
Epstein-Barr virus (EBV) is one of the most prevalent human viruses with more than 90% of the world’s adult population infected with EBV (Cohen et al., 2011). EBV is the primary cause of infectious mononucleosis and is also associated with certain B cell lymphomas and epithelial cell malignancies. In immunocompromised individuals who undergo solid organ or hematopoietic stem cell transplant, EBV is responsible for > 70% of cases of post-transplant lymphoproliferative disease (PTLD) (San-Juan et al., 2014). PTLD usually develops within first year after transplant in hematopoietic stem cell transplant recipients and in the first 2 years in solid organ transplant recipients (Fujimoto and Suzuki, 2020; Wistinghausen, Gross, and Bollard, 2013). The frequency of PTLD is 24- to 33-fold higher in persons with primary EBV infection after transplant than in EBV seropositive persons (Preiksaitis and Cockfield, 1998). EBV infection can also result in fatal disease in boys with X-linked lymphoproliferative disease (XLPD) and in certain other primary immunodeficiencies (Cohen, 2015).
Intravenous immunoglobulin (IVIG) or antiviral therapy has been used to try to prevent infection in some patients at high risk of PTLD or XLPD; however, fatal breakthroughs have occurred. Neither IVIG nor antivirals affect latent EBV infection; thus, they have only been used to try to reduce infection or in the case of antivirals to inhibit EBV lytic replication. IVIG contains polyclonal antibodies prepared from pooled plasma of >15,000 donors (Orange et al., 2006). While plasma donors are not screened for antibodies to EBV, since 90% of adults are infected with the virus, IVIG contains EBV antibodies, although antibody levels to different EBV proteins can vary from batch to batch.
Hyperimmune globulin is available to prevent or attenuate virus infections (Slifka and Amanna, 2018). FDA licensed products include varicella, rabies, and hepatitis B hyperimmune globulins that are used to prevent disease after exposure (post-exposure prophylaxis) to the corresponding virus infections. Cytomegalovirus and hepatitis B hyperimmune globulins are used to prevent disease before exposure, while vaccinia hyperimmune globulin is used to treat persons with progressive vaccinia. The FDA recently issued an emergency use authorization for convalescent plasma from persons with high titers of antibody to SARS-CoV-2 to treat hospitalized persons with COVID-19.
Currently there is no approved hyperimmune globulin for EBV. In view of the limited effectiveness associated with IVIG for prevention of EBV PTLD (Humar et al., 2006) and prevention of primary infection in patients with XLPD (Seemayer et al. 1993), EBV hyperimmune globulin may be more effective than IVIG due to the higher titer of neutralizing antibody. Here, we isolated EBV hyperimmune globulin from plasma of donors with high titers of EBV gp350 and EBV B cell neutralizing antibodies and evaluated its effect on protection against EBV infection using a humanized mouse model.
2. Materials and Methods
2.1. Cells and viruses
293/2089 cells containing the B95-8/F EBV genome, which expresses green fluorescent protein (GFP), were cultured in DMEM with 10% fetal bovine serum, penicillin (100U/mL), streptomycin (100U/mL) and hygromycin (100 μg/mL) (Delecluse et al., 1998). Raji cells, an EBV-positive Burkitt lymphoma cell line, were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS), penicillin, and streptomycin.
EBV was prepared by transfecting 293/2089 cells with plasmids expressing EBV BZLF1 and BALF4 (Neuhierl et al., 2002) using PEI Max (Polysciences, Warrington, PA). After 7 days, medium was collected and filtered through a 0.80 μm MCE membrane filter (Millipore, Burlington, MA). Filtered supernatant was concentrated by ultracentrifugation at 21,000 rpm for 1 hr at 4°C. The virus pellet was resuspended in DMEM with 10% FBS and EBV B95-8/F was stored at −70°C.
2.2. Commercial IVIG, plasma collection and EBV IgG purification
Plasma donors were consented using FDA approved informed consents for plasmapheresis. Plasma was separated from red blood cells during plasmapheresis and the red cells were returned to the donors. Prior to donation, donors were screened in accordance with FDA regulations to protect the donor and ensure the safety, purity, and potency of the collected plasma. All plasma units were tested for presence of antigens and antibodies to human immunodeficiency viruses I and II, hepatitis A, B, and C, and parvovirus.
IgG was purified from plasma using Protein G Sepharose Fast Flow (Cytiva Life Sciences) using the manufacturer’s protocol. Briefly, plasma was diluted with the binding buffer, centrifuged for 15 min to clarify, the supernatant was loaded onto the Protein G column, and the IgG was eluted with low pH buffer. The pH of the purified IgG was neutralized, extensively dialyzed with PBS, and sterile filtered. The concentration was determined by OD280 using 1 mg/ml = 1.4 AU.
2.3. Virus titration, EBV B cell neutralization, and gp350 antibody assay
EBV B95-8/F was serially diluted in 2-fold steps in a 96 well plate and incubated with 75 μl of 1 x 105 Raji cells. After 72 hr, cells were fixed with 2% paraformaldehyde in PBS. GFP-positive Raji cells were counted using a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) and analyzed by FlowJo software (Tree Star Inc., Ashland, OR). The EBV titer was measured in Green Raji units (GRU) as previously described (Sashihara et al., 2009).
100 μg/mL of IVIG (Bivigam, ADMA Biologics) or EBV hyperimmune globulin was serially diluted 23 times in 2-fold dilutions. 25 μL of diluted IVIG or EBV hyperimmune globulin was added to each well of a 96-well plate. 25 μL of B95-8/F virus was added to each of the wells and incubated for 2 hr at 37°C. After incubation, 50 μL of 1 x 105 Raji cells were added and incubated for 3 days at 37°C. Cells were fixed in 2% paraformaldehyde in PBS.
GFP-positive cells were quantified using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) and BD FlowJo software (BD Biosciences, Ashland, OR). The dilution of IVIG or EBV hyperimmune globulin that inhibited infectivity by 50% (IC50) based on reduction of the number of GFP-positive cells was calculated by non-linear regression analysis using GraphPad PRISM software (GraphPad Software, San Diego, CA). Neutralizing activity was considered absent when the software program failed to fit the results to an appropriate regression curve.
EBV gp350 antibody titers were determined by a luciferase immunoprecipitation system (LIPS) assay (Sashihara et al., 2009). Briefly, 293T cells were transfected with pREN3s-gp350 which expresses EBV p350 fused with Renilla luciferase. Cell lysates were incubated with either human plasma or IVIG, and immunoprecipitated with protein A/G beads for 1 hr. Coelenterazine substrate was added and luciferase activity was measured in light units (LU) by a luminometer. Each sample was tested in triplicate.
2.4. Passive transfer in humanized mice
All animal experiments were performed in accordance with federal regulations and NIH guidelines and approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases. Female HSCCB-NOG-F (NOD.Cg-Prkdcscid Il2rgtm1Sug/ JicTac) mice that were 19 to 20 weeks post-engraftment was purchased from Taconic. IVIG (0.5 mg), EBV hyperimmune globulin (0.5 mg), or 300 μl of PBS was injected to each mouse by intraperitoneal injection on days −1, 0, 1, 4, 7, and 10 of challenge. On day 0, at least 4 hours after receiving immune globulin or PBS, all animals were challenged with 1 x 106 GRU of EBV B95-8/F virus given intravenously. Mouse weights and blood were collected weekly for 15 weeks after EBV infection. Mice were euthanized when they lost 30% of their original weight, were unable to obtain food or water, or were non-responsive to stimuli.
2.5. Quantifying the number of EBV DNA copies in the blood by qPCR
EBV DNA was quantified in blood as previously reported (Strowig et al., 2009) with minor modifications. DNA was isolated from mouse blood using a DNeasy Blood and Tissue kit (Qiagen, Germantown, MD). Forward primer 5’-GGACCACTGCCCCTGGTATAA-3’and reverse primer 5’-TTTGTGTGGACTCCTGGCG-3’were used to amply the EBV BamHI W repeat fragment and probe 5’-FAM-TCCTGCAGCTATTTCTGGTCGCATCA-3’ was used to detect the PCR product. Human GAPDH was amplified using primers 5’-AGGGTGGTGGACCTCAT-3’ and 5’-TGAGTGTGGCAGGGACT-3’ and the PCR product was detected with probe 5’-HEX-CAGCAAGAGCACAAGAGGAAGAGAGA-3’. EBV viral DNA was quantified using a TaqMan Universal PCR Master Mix kit and CFX96 Touch Real-Time PCR Detection System (BioRad, Hercules, CA). p-values were calculated using the Welch two sample t-test.
2.6. Total human IgG quantification by ELISA
Plasma was separated from mouse blood by centrifuging at 800 x g for 10 min. Total human IgG concentration in plasma was measured using an IgG (Total) Human Uncoated ELISA kit (Invitrogen, Carlsbad, CA). Plasma was diluted to fall within the standard range of the kit. Briefly, 96 well plates were coated with purified anti-human IgG monoclonal antibody and incubated overnight at 4°C. Plates were washed with wash buffer and blocked with blocking buffer for 2 hr at room temperature. Plates were washed again, and plasma samples and standards were added to wells and incubated for 2 hr at room temperature. After incubation, plates were washed, and HRP-conjugated anti-human IgG monoclonal antibody was added. After incubation for 1 hr at room temperature, tetramethylbenzidine substrate solution was added and incubated for 15 min. 1M H3PO4 was added as a stop solution and wells were read at 450 nm.
2.7. Pathology of tissues in mice
Mice were euthanized at the end of the study and portions of the liver, spleen, lung, and kidney were fixed with 10% neutral-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Organs from 6 animals in each treatment group were examined for evidence of lymphoproliferative disease or lymphoma by a pathologist who was blinded to the treatment arms.
3. Results
3.1. Screening of plasma from EBV seropositive donors for EBV gp350 antibody and B cell neutralizing titers
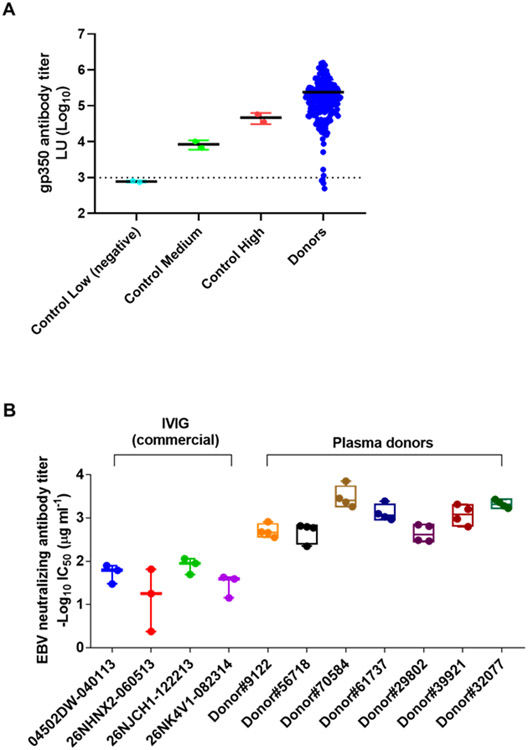
Plasma from 200 EBV seropositive donors were evaluated for EBV gp350 antibody titers by LIPS assay. As a control, plasma previously obtained from NIH blood bank donors that had been categorized as having high, medium, or low levels of gp350 antibody were used as a control (Fig. 1A). Many of the plasma donors had similar or higher levels of gp350 antibody titers as compared to the high titer gp350 antibody control group. Based on these results, a subset of plasma donors was evaluated for EBV B cell neutralizing titers to select donors for preparation of EBV hyperimmune globulin. Seven donors were selected that had high titers of both gp350 and EBV B cell neutralizing antibodies (Fig. 1B). All 7 donors had higher EBV neutralizing titers (1-2 log lower IC50 values) than 4 different preparations of IVIG.
Fig. 1. EBV gp350 antibody levels in plasma from donors and EBV B cell neutralizing titers in lots of IVIG and selected plasma donors with high EBV gp350 antibody titers.
(A) gp350 antibody levels in human plasma donors were quantified using a gp350 LIPS assay. Plasma previously obtained from blood bank donors that had been categorized with high, medium, and low gp350 antibody titers were used as controls. Antibody titers are expressed in light units (LUs) based on the luciferase immunoprecipitation system (LIPS) assay. Each dot indicates the mean titer of triplicate results for each donor and the solid black line indicates the mean. The dotted line represents the cutoff value defined as the mean plus 2 standard deviations of the antibody titer of EBV negative sera. (B) EBV B cell neutralizing antibody titers from separate lots of IVIG and 7 plasma donors with the highest EBV gp350 antibody titers based on LIPS assay. Each dot represents the IC50 value of individual neutralization assay result.
3.2. Comparison of IVIG and EBV hyperimmune globulin by passive transfer in humanized mice and EBV challenge
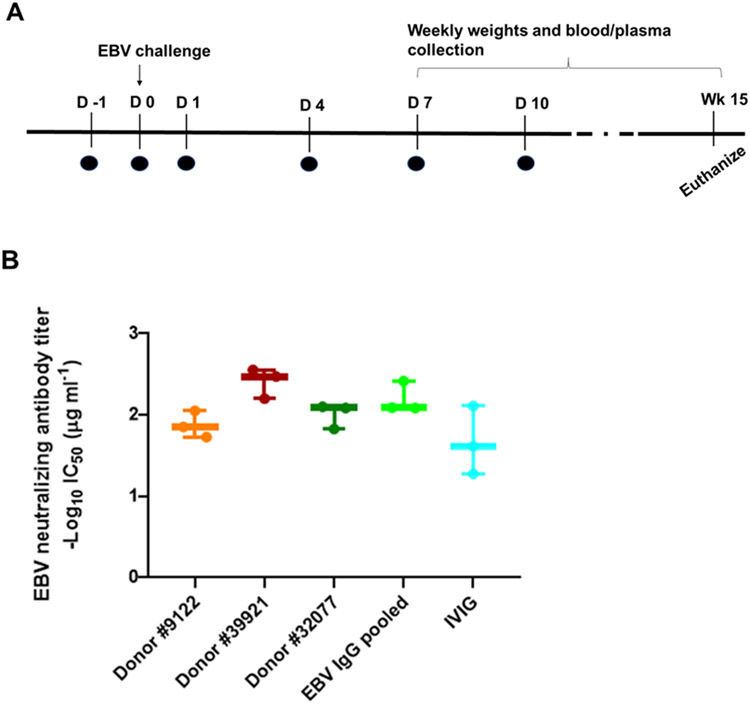
Three plasma donors (donors 1, 6, 7), available for further plasma donation after their initial screening, were chosen from the 7 donors with the highest gp350 and EBV B cell neutralizing titers, and IgG was purified from their plasma and pooled. This pooled IgG was termed EBV hyperimmune globulin and compared with IVIG and PBS for its ability to reduce the number of EBV DNA copies in the blood in humanized mice challenged with EBV. Groups of 6 mice received 0.5 mg of either IVIG or EBV hyperimmune globulin on days −1, 0, 1, 4, 7, and 10 of challenge (Fig. 2A). Another group of mice was given PBS at the same time points as a control. On the day of challenge, 1 x 106 GRU of EBV B95-8/F was given by intravenous injection. Mouse weights were recorded and peripheral blood was collected weekly from week 1 to 15 after infection. The concentration of total human IgG in plasma and the level of EBV DNA in whole blood was measured. EBV hyperimmune globulin had comparable levels of EBV B cell neutralizing titers as IgG obtained from individual plasma donors (Fig. 2B). Differences in the individual donors’ neutralizing titers in Fig 1B and 2B were due to the use of plasma from donors in the former and purified IgG from donors in the latter.
Fig. 2. Passive transfer protocol for humanized mice and EBV B cell neutralizing titers of IVIG or EBV hyperimmune globulin.
(A) Six doses of either IVIG or EBV hyperimmune globulin, indicated by the black circles, were given by intraperitoneal injection and 1x106 GRU of EBV was used to challenge mice by intravenous injection. (B) EBV B cell neutralization titers for IgG isolated from individual donors, hyperimmune globulin from pooled donors, and IVIG were plotted.
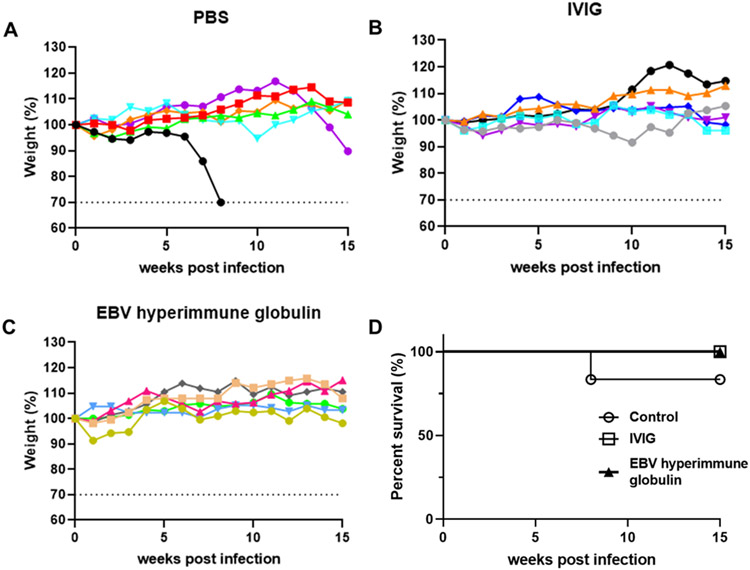
Mice in all three groups were monitored for weight changes. In the PBS group, one mouse reached the endpoint for 30% weight loss and was euthanized, while none of the mice in the other groups had significant weight loss (Fig. 3A-C). One mouse in the PBS control group was euthanized, while all mice in the IVIG and hyperimmune EBV globulin groups survived (Fig. 3D).
Fig. 3. Changes of weight and survival rate for mice receiving IVIG, EBV hyperimmune globulin, or PBS and challenged with EBV.
Percentage weights relative to initial weights (set at 100%) are plotted for mice that received PBS control (A), IVIG (B), or EBV hyperimmune globulin (C). Each line represents one animal and dotted lines indicate study end point 30% weight loss. Survival rates were plotted for each group of mice up to week 15 after infection (D).
3.3. Mice receiving hyperimmune EBV globulin had the lowest EBV viral load in the blood after virus challenge
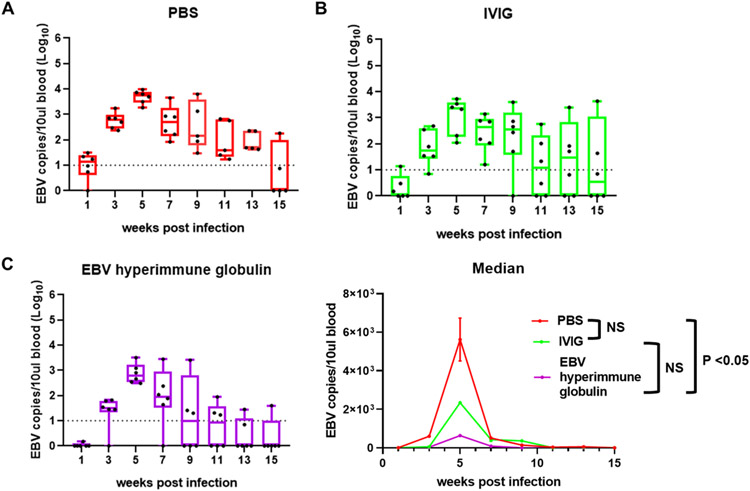
DNA was extracted from the blood of mice after challenge with EBV and the level of viral DNA was measured by qPCR using a probe for the EBV BamHI W sequence. There are 11 copies of BamHI W, a repeat sequence, in the EBV B95-8 genome (Quynh-Thu et al., 2005); therefore, amplifying this repeat sequence increases the sensitivity of the assay. All three groups of mice had peak levels of EBV DNA in the blood 5 weeks after infection and thereafter the levels decreased (Fig. 4A-C). When the number of EBV DNA copies in the blood was compared over the full 15-week study period, an area under the curve analysis showed that mice that received EBV hyperimmune globulin had a significantly lower viral load in the blood compared to PBS group (p = 0.004, Welch two sample t test); however, animals that received IVIG did not have significantly lower viral load than those that received PBS (p = 0.19) (Fig. 4D). While mice that received EBV hyperimmune globulin had a lower viral load in the blood than those that received IVIG over the 15-week study period, the difference was not significant (p = 0.13).
Fig. 4. Quantification of EBV viral load from weeks 1 to 15 after challenge.
EBV viral load expressed as EBV copy numbers per 10 μl of blood was plotted for each mouse over time. Each dot represents one mouse. Whiskers are the minimum and maximum data points and box represents upper and lower quartiles with the horizontal line at the median. EBV DNA was quantified in mice in the PBS control group (A), IVIG group (B), and EBV hyperimmune globulin group (C). Median EBV DNA copies for each group at separate time points were calculated (D). NS indicates not significant.
Mice were euthanized at the end of the study, and sections of liver, spleen, lung, and kidney were examined for evidence of lymphoproliferative disease or lymphoma. Tissues from 33.3% of mice in the PBS group, 16.7% of mice in the IVIG group, and none of the mice in the EBV hyperimmune globulin group showed lymphoproliferative disease or lymphoma (Table 1).
Table 1.
Number of animals with lymphoproliferative disease (LPD) or lymphoma after challenge with EBV
| Treatment group | No LPD or lymphoma (%) | LPD or lymphoma (%) |
|---|---|---|
| PBS | 4/6 (66.7%) | 2/6 (33.3%) |
| IVIG | 5/6 (83.3%) | 1/6 (16.7%) |
| EBV hyperimmune globulin | 6/6 (100%) | 0/6 (0%) |
3.4. Mice that received IVIG or EBV hyperimmune globulin had similar levels of total human IgG in the plasma
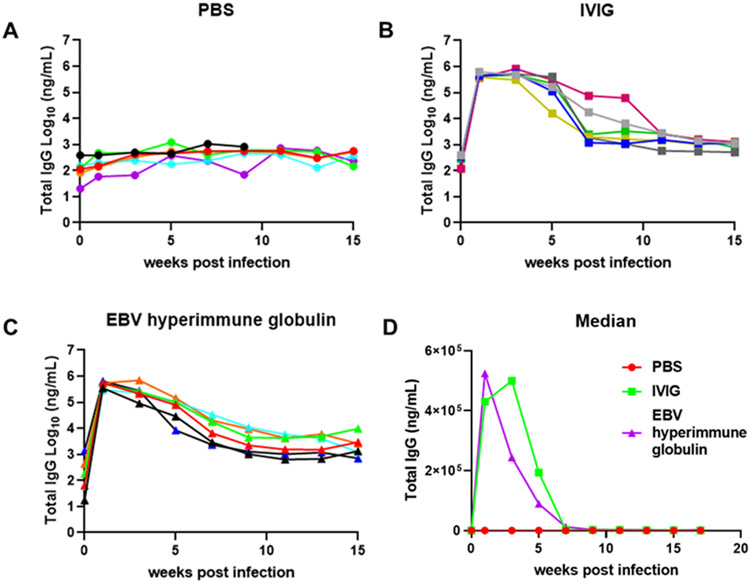
To be certain that differences in EBV DNA copy number in the blood between mice receiving EBV hyperimmune globulin and IVIG was not due to differences in plasma levels of the two immune globulin products, plasma from mice was tested for total human IgG concentration by ELISA. Mice that received IVIG or EBV hyperimmune globulin showed an increase in the level of human IgG that peaked at 1 to 3 weeks after infection (Fig. 5A-C), while animals that received PBS showed no increase in IgG levels. Mice that received IVIG or EBV hyperimmune globulin had a decline in human IgG from weeks 3 to 11 after infection and then remained stable. Similar levels of IgG were present in plasma of mice receiving IVIG or EBV hyperimmune globulin each week with the exception of week 3 when IgG was significantly higher in animals receiving IVIG than those receiving EBV hyperimmune globulin (p = 0.04) (Fig. 5D). Therefore, the lower number of EBV DNA copies in the blood of mice that received EBV hyperimmune globulin compared to those that received IVIG was not due to a difference in the plasma level of total human IgG.
Fig. 5. Quantification of total human IgG in mouse plasma.
The total human IgG concentration was measured by ELISA in the plasma of mice that received PBS (A), IVIG (B), or EBV hyperimmune globulin (C). Each line represents one mouse and plasma samples collected bi-weekly were used in the assay. Median total IgG level of each group at different time points was calculated (D).
4. Discussion
We have shown that humanized mice that received EBV hyperimmune globulin isolated from the plasma of donors with high levels of gp350 and B cell neutralizing antibodies had significantly reduced EBV DNA copies in the blood compared with mice that received PBS after challenge with EBV. However, EBV hyperimmune globulin was not significantly better than IVIG to reduce EBV DNA copies in the blood of mice after challenge.
We screened plasma donors for those with high gp350 titers since EBV gp350 is the major target of B cell neutralizing antibodies in human plasma (Bu et al., 2019) and gp350 antibody titers correlate strongly with EBV neutralizing antibodies in human plasma (Sashihara et al, 2009). While EBV gp350 has been the major target for an EBV vaccine (Sokal et al., 2007) and EBV monoclonal antibodies have been developed as potential therapeutics (Herrman et al., 2016; Tanner, Hu, and Alfieri, 2018; Mutsvunguma et al., 2019), recent studies have focused on other EBV glycoproteins including gH/gL as vaccine candidates or therapeutics. gp350 is important for virus attachment to B cells through CD21 (Nemerow et al., 1987) or CD35 (Ogembo et al., 2013), but is not essential for infection in vitro (Janz et al., 2000). In contrast, EBV gH/gL is essential for virus fusion to B cells and epithelial cells (Wu and Hutt-Fletcher, 2007). In addition, a vaccine containing gH/gL nanoparticles induced higher titers of antibody to neutralize virus infection of B cells and epithelial cells than a similar gp350 nanoparticle vaccine (Bu et al., 2019). Monoclonals to EBV gH/gL have been isolated that block fusion of the virus to both B cells and epithelial cells and potently neutralize infection of both cell types (Bu et al., 2019; Snijder et al. 2018). Thus, isolation of EBV hyperimmune globulin from donors with high titers of antibodies to EBV gH/gL may have provided more effective reduction in the number of EBV DNA copies in the blood than EBV hyperimmune globulin selected for antibody to gp350.
We found that EBV hyperimmune globulin did not completely eliminate detection of EBV DNA in the blood of humanized mice challenged with EBV intravenously. EBV does not naturally infect small animals. Lymphocryptoviruses that are closely related to EBV can infect rhesus monkeys (Cho et al., 1999), but the sequence of EBV and rhesus lymphocryptovirus gp350 share only 49% amino acid similarity and EBV cannot infect rhesus macaques (Herrman et al., 2016). Humanized mice engrafted with CD34 hematopoietic stem cells are currently the best small animal model to study EBV infection. Infection of these mice with high doses of EBV results in viremia and B cell lymphoproliferative disease or lymphoma with similar histopathology and latent EBV gene expression as EBV infected immunocompromised patients; infection with low doses of EBV cause persistent infection without lymphoproliferative disease (Yajima et al., 2008). Humanized mice reach their peak viral load 4-to-6 weeks after primary infection which is similar to the course in symptomatic infections in humans (Munz, 2017). Despite the similarities between EBV infection in humans and humanized mice, there are several limitations in the mice model. Humanized mice have engraftment of human lymphocytes, but not human epithelial cells. Since mouse epithelial cells cannot be infected with EBV in the absence of expression of human CD21 (Ahearn et al., 1988), only B cells in humanized mice can be infected. Thus, these animals require intravenous inoculation with EBV for efficient infection and therefore the natural route of EBV infection, the oropharynx, cannot be studied in humanized mice. This difference in the route of infection may affect the evaluation of the efficacy of EBV hyperimmune globulin. Thus, administration of EBV hyperimmune globulin to humans, may or may not reach sufficient levels in the mucosa of the oropharynx to protect them from the natural route of infection, despite protection observed in the humanized mice model.
While hyperimmune globulin may be more effective than IVIG due to its higher EBV antibody and neutralizing titers, monoclonal antibody (mAb) to EBV glycoproteins may be even more effective. In fact, a gp350 monoclonal antibody (72A1) prevented EBV tumor development in severe combined immunodeficient mice injected with EBV seronegative donor peripheral blood mononuclear cells and challenged intravenously with EBV (Haque et al., 2006). More recent studies with a gH/gL mAb showed protection of humanized mice from challenge with EBV (Singh et al, 2020). Future studies might use a combination of EBV mAbs to reduce the chance of immune escape by the virus.
5. Conclusion
In conclusion, we have isolated EBV hyperimmune globulin from donors with high gp350 titers and EBV B cell neutralizing titers and shown that the EBV hyperimmune globulin significantly reduced EBV DNA copies in the blood when compared to PBS control group. However, this reduction was suboptimal and EBV hyperimmune globulin did not show significantly better protection than IVIG. Future studies using hyperimmune globulin derived from donors with high titers of antibody to other glycoproteins or mAbs might provide better protection.
Acknowledgements
We thank Jing Qin for help with statistics.
Funding
JungHyun Kim was supported by NIH T32 grant AI125186. This work was funded by the intramural research program of the National Institute of Allergy and Infectious Diseases (NIAID) and in part through a cooperative research and development agreement between NIAID and ADMA. This project has been funded in part with federal funds from the NIH, National Cancer Institute, under contract number HHSN261200800001E.
Footnotes
CRediT authorship contribution statement
JungHyun Kim: methodology, formal analysis, investigation, resources, data curation, writing-original draft, visualization. Wei Bu: conceptualization, methodology, investigation, formal analysis, data curation, resources, writing-review & editing, supervision, project administration. Sohtaro Mine, Zeshan Tariq, Yanmei Wang, Cynthia Tolman: investigation. James Mond: conceptualization, writing- review and editing, funding acquisition. Jeffrey Cohen: conceptualization, writing-review, and editing, supervision, project administration, funding acquisition.
Declaration of competing interest
James Mond and Cynthia Tolman are employed by ADMA Biologics; James Mond is the chief medical and scientific officer of the company.
References
- Ahearn JM, Hayward SD, Hickey JC, Fearon DT, 1988. Epstein-Barr virus (EBV) infection of murine L cells expressing recombinant human EBV/C3d receptor. Proc. Natl. Acad. Sci. U.S.A 85(23), 9307–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu W, Joyce GM, Nguyen H, Banh DV, Aguilar F, Tariq Z, Yap ML, Tsujimura Y, Gillespie RA, Tsybovsky Y, Andrews SF, Narpala SR, McDermott AB, Rossmann MG, Yasutomi Y, Nabel GJ, Kanekiyo M, Cohen JI, 2019. Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells. Immunity. 50(5), 1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Gordadze AV, Ling PD, Wang F, 1999. Evolution of Two Types of Rhesus Lymphocryptovirus Similar to Type 1 and Type 2 Epstein-Barr Virus. J Virol. 73(11), 9206–9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Fauci AS, Varmus H, Nabel GJ, 2011. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci. Transl. Med 3, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, 2015. Primary Immunodeficiencies Associated with EBV Disease. Curr. Top. Microbiol. Immunol 390, 241–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W, 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. U.S.A 95, 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Suzuki R, 2020. Epstein-Barr Virus-Associated Post-Transplant Lymphoproliferative Disorders after Hematopoietic Stem Cell Transplantation: Pathogenesis, Risk Factors and Clinical Outcomes. Cancers (Basel). 12, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque T, Johannessen I, Dombagoda D, Sengupta C, Burns DM, Bird P, Hale G, Mieli-Vergani G, Crawford DH, 2006. A mouse monoclonal antibody against Epstein-Barr virus envelope glycoprotein 350 prevents infection both in vitro and in vivo. J Infect Dis. 194(5), 584–587. [DOI] [PubMed] [Google Scholar]
- Herrman M, Mühe J, Quink C, Wang F, 2016. Epstein-Barr virus gp350 can functionally replace the rhesus lymphocryptovirus major membrane glycoprotein and does not restrict infection of rhesus macaques. J Virol. 90, 1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humar A, Hébert D, Davies HD, Humar A, Stephens D, O'Doherty B, Allen U, 2006. A randomized trial of ganciclovir versus ganciclovir plus immune globulin for prophylaxis against Epstein-Barr virus related posttransplant lymphoproliferative disorder. Transplantation. 81(6), 856–861. [DOI] [PubMed] [Google Scholar]
- Janz A, Oezel M, Kurzeder C, Mautner J, Pich D, Kost M, Hammerschmidt W, Delecluse HJ, 2000. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J Virol, 74, 10142–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz C, 2017. Humanized mouse models for Epstein Barr virus infection. Curr Opin Virol. 25, 113–118. [DOI] [PubMed] [Google Scholar]
- Mutsvunguma LZ, Rodriguez E, Escalante GM, Muniraju M, Williams JC, Warden C, Qin H, Wang J, Wu X, Barasa A, Mulama DH, Mwangi W, Ogembo JG, 2019. Identification of multiple potent neutralizing and non-neutralizing antibodies against Epstein-Barr virus gp350 protein with potential for clinical application and as reagents for mapping immunodominant epitopes. Virology. 536, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow GR, Mold C, Schwend VK, Tollefson V, Cooper NR, 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J Virol. 61(5), 1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhierl B, Feederle R, Hammerschmidt W, Delecluse HJ, 2002. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. U.S.A 99, 15036–15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogembo JG, Kannan L, Ghiran I, Nicolson-Weller A, Finberg R, Tsokos GC, Fingeroth JD, 2013. Human complement receptor type 1/CD35 is an Epstein-Barr virus receptor. Cell Rep. 3(2), 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson RP, Patel DD, Secord E, Sorensen RU, Wasserman RL, Cunningham-Rundles C, 2006. Use of intravenous immunoglobulin in human disease a review of evidence by members of the primary immunodeficiency committee of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol. 117, S525–553. [DOI] [PubMed] [Google Scholar]
- Preiksaitis JK Cockfield SM, 1998. Epstein-Barr virus and lymphoproliferative disease after transplantation. In: Bowden RA, Ljungman P, Pava CV (Eds.) Transplant infections. Lippincott-Raven, Philadelphia, pp. 245–263. [Google Scholar]
- San-Juan R, Comoli P, Caillard S, Moulin B, Hirsch HH, Meylan P, 2014. Epstein-Barr virus related post-transplant proliferative disorder in solid organ transplant recipients. Clin Microbiol Infect. 20, 109–118. [DOI] [PubMed] [Google Scholar]
- Sashihara J, Burbelo PD, Savoldo B, Pierson TC, and Cohen JI, 2009. Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology. 391(2), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemayer TA, Gross TG, Egeler RM, Pirruccello SJ, Davis JR, Kelly CM, Okano M, Lanyi A, Sumegi J, 1995. X-linked lymphoproliferative disease: twenty-five years after the discovery. Pediatr Res. 38, 471–478. [DOI] [PubMed] [Google Scholar]
- Singh S, Homad LJ, Akins NR, Stoffers CM, Lackhar S, Malhi H, Wan YH, Rawlings DJ, McGuire AT, 2020. Neutralizing antibodies protect against oral transmission of lymphocryptovirus. Cell Rep Med. 1, 100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka J Amanna IJ 2017. Passive Immunization. In: Plotkin S, Orenstein W, Offit P Edwards KM (Eds.) Vaccines. 7th. Elsevier, Philadelphia, pp. 84–95. [Google Scholar]
- Snijder J, Ortego MS, Weidle C, Stuart AB, Gray MD, McElrath MJ, Pancera M, Veesler D, McGuire AT, 2018. An Antibody Targeting the Fusion Machinery Neutralizes Dual-Tropic Infection and Defines a Site of Vulnerability on Epstein-Barr Virus. Immunity. 48(4):799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, Haumont M, Bollen A, Smets F, Denis M, 2007. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis. 196(12), 1749–1753. [DOI] [PubMed] [Google Scholar]
- Strowig T, Gurer C, Ploss A, Liu Y, Arrey F, Sashihara J, Koo G, Rice CM, Young JW, Chadburn A, Cohen JI, Munz C, 2009. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J. Exp. Med 206, 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JE, Hu J, Alfieri C, 2018. Construction and characterization of a humanized anti-Epstein- Barr virus gp350 antibody with neutralizing activity in cell culture. Cancers. 10(4), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistinghausen B, Gross TG, Bollard C, 2013. Post-transplant lymphoproliferative disease in pediatric solid organ transplant recipients. Pediatr. Hematol. Oncol 6, 520–531. [DOI] [PubMed] [Google Scholar]
- Wu L, Hutt-Fletcher LM, 2007. Point mutations in EBV gH that abrogate or differentially affect B cell and epithelial cell fusion. Virology. 363(1), 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, Nakamura H, Ito M, Shimizu N, Honda M, Yamamoto N, Fujiwara S, 2008. A New Humanized Mouse Model of Epstein-Barr Virus Infection That Reproduces Persistent Infection, Lymphoproliferative Disorder, and Cell-Mediated and Humoral Immune Responses. J Infect Dis. 198(5), 673–682. [DOI] [PubMed] [Google Scholar]