ABSTRACT
Altered immune cell phenotype and chronic inflammation are key features shared by various chronic diseases. Evidence from nutritional interventions aimed at alleviating inflammation could be a promising approach for the prevention of adverse health outcomes. We therefore aimed to conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) to summarize the recent evidence on the effects of dietary patterns on inflammatory and immune-related biomarkers in humans. PubMed, Medline, and Web of Science databases were searched for publications up to October 2020. In total, 22 RCTs were included in the meta-analysis conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The Mediterranean diet appeared as the dietary pattern that showed the most prominent reductions of inflammatory biomarkers such as IL-6 [mean difference (MD): –1.07 pg/mL (95% CI: –1.94, –0.20); I2: 96%], IL-1β [MD: –0.46 pg/mL (95% CI: –0.66, –0.25); I2: 0%], and C-reactive protein [MD: –1.00 mg/L (95% CI: –2.02, 0.01); I2: 100%]. No substantial effects were observed for the additional dietary patterns studied in intervention research, including the Dietary Adherence to Stop Hypertension diet, and the vegetarian or vegan diets. Future large-scale multifactorial intervention studies are warranted to allow direct comparison of various dietary patterns in relation to a range of biomarkers reflecting multiple inflammatory and immune-related pathways.
Keywords: dietary patterns, inflammation, biomarkers, randomized controlled trials, meta-analysis, systematic review
Statement of Significance: The overall evidence highlights the beneficial anti-inflammatory effects of the Mediterranean diet in adults.
Introduction
Altered immune cell phenotype and chronic inflammation are key features shared by various chronic diseases, including cardiovascular disease, diabetes, and cancer (1). Compromised immune system function has proven to be one of the main determinants of disease severity and fatality (2), in particular during the COVID-19 pandemic. The pandemic has infected >133 million people worldwide (as of April 2021) and accounted for 2.8 million deaths (3), with unprecedented consequences on the global economy and public health (4, 5).
As opposed to acute inflammation, chronic inflammation is not resolved over time and is characterized by the sustained production of proinflammatory molecules with detrimental actions on various organ and system levels (1). Phenotypes associated with chronic inflammation can include aging and immune senescence, dysfunctional mitochondria and sustained oxidative stress, autoimmune reactions, obesity-induced immune cell dysregulation and inflammasome activation, and infections (e.g., SARS-CoV-2), amongst others (1, 6–8). As an example, both older and obese individuals are particularly prone to increased production of proinflammatory molecules and reduced production of anti-inflammatory molecules, i.e., increased macrophages that are responsible for cytokine production including TNF-α and IL-6 (9). Common to all phenotypes, inflammation and disturbed immune balance are associated with more severe clinical outcomes (1, 10). Thus, in COVID-19 patients, excess cytokine production was shown to lead to a deficiency in control of viral replication and a prolonged proinflammatory response (11). Elevated concentrations of IL-6 and C-reactive protein (CRP) are present in mild and severe COVID-19 patients, regardless of their comorbidities (12). Interventions targeted at modifying circulating biomarkers of immune response and inflammation may therefore provide valid means for preventing disease development and progression.
In this context, a number of dietary factors with anti-inflammatory potential have been explored in mechanistic and/or epidemiologic studies, including specific nutrients, e.g., ω-3 fatty acids (13), polyphenols (14), or individual food groups such as whole grains, fruits, and vegetables (15). However, in those studies the synergistic or interactive effects of food components could not be taken into account (16, 17). Evaluation of dietary patterns represents a real-life approach towards examining diet–health associations (16) and the development of contemporary dietary guidelines (18). In recent years, nutrition research has shifted towards examining the effects of composite dietary patterns instead of focusing on single nutrients. Consequently, the number of studies that have explored various dietary patterns in relation to inflammatory biomarkers has constantly grown (17, 19–21). Several meta-analyses have assessed the relation between individual food components or specific dietary patterns (15, 21–25); however, to our knowledge, to date, there has not been a comprehensive study that reviews the effects of various dietary patterns on biomarkers of immune response and inflammation. Therefore, we conducted a systematic review and a meta-analysis of randomized controlled trials (RCTs) to summarize the recent evidence on the effects of dietary patterns on inflammatory and immune-related biomarkers in humans.
Methods
This systematic review and meta-analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (26). The protocol for the current review was registered at PROSPERO (registration number: CRD42021218829).
Search strategy
Relevant studies were identified by systematically searching for publications between January 2015 to October 2020 in the following electronic databases: PubMed, Medline, and Web of Science. The following combination of Medical Subject Heading (MeSH) terms and text words were used: (“Eat-Lancet diet”[All Fields] OR “planetary health diet”[All Fields] OR “portfolio diet”[All Fields] OR “DASH”[All Fields] OR “Dietary Approaches to Stop Hypertension”[All Fields] OR “Dietary Inflammatory Index”[All Fields] OR “Nordic diet”[All Fields] OR “paleolithic diet”[All Fields] OR “plant-based diet”[All Fields] OR “vegetarian diet”[All Fields] OR “vegan diet”[All Fields] OR “Mediterranean diet”[All Fields] OR “dietary pattern*”[All Fields] OR “eating pattern*”[All Fields] OR “food pattern*”[All Fields] OR “diet index”[All Fields] OR “dietary index”[All Fields] OR “diet score”[All Fields] OR “dietary score”[All Fields]) AND (“immun*”[All Fields] OR “inflammat*”[All Fields] OR “CRP”[All Fields] OR “C-reactive protein”[All Fields] OR “IL”[All Fields] OR “interleukin*”[All Fields] OR “TNF”[All Fields] OR “tumor necrosis factor”[All Fields] OR “acute-phase protein*”[All Fields] OR “adipokin*”[All Fields] OR “cytokine*”[All Fields]).
Eligibility criteria
Studies were included if they reported on the effect (intervention studies) of dietary patterns (as exposure) with biomarkers of inflammation (as outcome). The inclusion criteria were as follows: 1) assessment of dietary patterns (based on whole foods) as the main exposure; 2) plasma/serum measurements of biomarkers as main outcome measures; 3) enrolled humans at adult and old age (≥18 y); 4) RCTs with parallel or crossover design; 5) studies written in English and published in peer-reviewed journals, and 6) reported data to allow calculation of the mean difference (MD) in biomarker concentrations in intervention and control groups.
The exclusion criteria were: 1) no original research (e.g., reviews, editorials, nonresearch letters); 2) observational studies (cross-sectional, case-control, or prospective cohort studies) and nonrandomized intervention studies; 3) case reports or case series; 4) ecological studies; 5) lack of data on dietary patterns (e.g., examined only individual nutrients or did not examine all dietary components); 6) no repeated measurements of biomarkers; 7) short duration of intervention (<4 wk); 8) studies not conducted in humans; 9) studies not conducted in an adult population (<18 y); 10) studies conducted in pregnant women; and 11) studies without reported data to allow the calculation of effect estimates. Additionally, intervention studies were excluded if they used lifestyle interventions in conjunction with diet intervention (e.g., exercise or behavioral management). The exclusion of short-duration interventions was applied to allow characterizing the potential effect of habitual diet on changes of biomarker concentrations over a longer period of time rather than acute effects of initial drastic dietary change.
Study selection and data extraction
Identified records were imported into EndNote referencing software (version X7,2013; Thomson Reuters) and their titles and abstracts were screened by 2 independent reviewers (LK and TH). Full-text articles were retrieved if the article was considered eligible and subjected to a second evaluation by the 2 independent reviewers (LK and TH). Any discrepancies and disagreements were discussed and resolved by 2 other independent reviewers (CER and KA). After retrieval of full-text articles, the reference lists of the articles and other reviews on dietary patterns and inflammation were checked to identify additional potentially relevant articles that could be included.
Data extraction was performed by 2 independent reviewers (LK and TH) using a predefined data extraction form. The following information was extracted: first author, publication year, country, study design (parallel or crossover RCT) and duration of the intervention, participant characteristics including sample size, mean age, proportion male, mean BMI, health status of participants, dietary patterns in intervention and control groups, biomarkers measured, and main findings (pre-/postintervention values and/or net change values wherever possible).
Risk-of-bias judgment and quality assessment
The assessment of study bias was done using the Cochrane Risk-of-Bias Tool for RCTs (27). Risk of bias was judged as low, high, or unclear for individual elements: 1) random sequence generation (selection bias); 2) allocation concealment (selection bias); 3) blinding of outcome assessment (detection bias) and self-reported outcomes; 4) incomplete outcome data addressed (attrition bias); 5) selective reporting (reporting bias); 6) other bias (other sources of bias that have been detected by the reviewer). Two figures were generated to demonstrate the risk-of-bias assessment of individual and overall studies that have been included in the systematic review and meta-analysis.
Meta-analyses
Meta-analyses were performed with the “meta” package in R (version 3.4.3, R Foundation for Statistical Computing) after assessing comparability and risk of bias of the included RCTs. MDs in changes of biomarkers, comparing diet intervention and control groups, were used to calculate the overall effect size. A standardized data-extraction tool suggested by Cochrane Collaboration (Review Manager) was used to calculate pre–post changes in the means, and where necessary, we converted reported SEs, 95% CIs, and IQRs to SDs using relevant formulas. The units of the biomarkers were standardized to make the results of the studies comparable. Log-transformed values were converted back to crude/normal values.
In the case of intervention studies with multiple intervention arms, results on 1 intervention were reported, and if the interventions differed substantially from each other, the control group was pooled with the other intervention arm. In the case of publications reporting biomarker data from the same trial, the study with the larger sample size was included in the analysis. In the case of studies reporting stratified analyses within the intervention and control groups, the results were merged within the group. Crossover studies were dealt with as though the interventions were paired.
The overall effect sizes were calculated using random-effects models to determine the association between dietary patterns and concentrations of inflammatory biomarkers, taking individual study variations into account. Forest plots were generated to illustrate the study-specific effect sizes with 95% CI. A P value of <0.05 was considered as statistically significant.
Subgroup analyses were further conducted to detect probable sources of heterogeneity. Subgroup analyses were based on stratification of the following categories: age (<60 y compared with ≥60 y), sex (male compared with female), geographic region (Americas, Asia, Europe, or Oceania), participants’ health conditions (healthy, with prevalent metabolic syndrome, cardiovascular disease, type 2 diabetes, or other), BMI (<30 compared with ≥30 kg/m2), duration of intervention (<6 mo compared with ≥6 mo; <1 y compared with ≥1 y), study design (parallel compared with crossover trial), and weight loss (in both intervention and control groups, in the intervention group only, in the control group only, or in neither intervention nor control groups).
In sensitivity analyses, the extent to which inferences may depend on study effect size (i.e., with smaller sample size) were explored. In studies with multiple follow-up measurements, sensitivity analyses were done including earlier follow-up values. Potential publication bias was examined by visual inspection of funnel plots for meta-analysis with n ≥10 studies included, and asymmetry was tested using Egger's test. Duval and Tweedie's trim-and-fill procedure was performed to estimate what the actual effect size would be had the small studies been published. This procedure assumes that the initial results were overestimated due to publication bias and calculates the true effect after controlling for selective publication.
Results
Search results
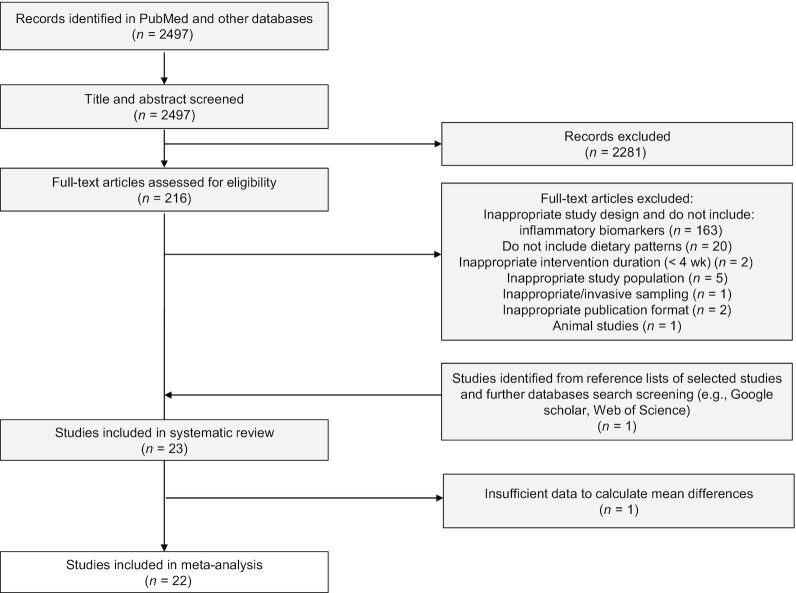
In Figure 1, the PRISMA flow diagram including the identification, screening, eligibility, and number of studies included is shown. The initial search yielded a total of 2497 studies. After title and abstract screening, 216 publications were included in full-text evaluation. Following the screening of the full text, articles were excluded because of the following reasons:
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection and number of excluded studies.
1) inappropriate study design (no RCT); 2) no data on inflammatory biomarkers as outcomes (n = 163); 3) no reports on dietary patterns as intervention (n = 20); 4) inappropriate intervention duration (<4 wk) (n = 2); 5) inappropriate study population (aged <18 y or pregnant women) (n = 5); 6) inappropriate or invasive sampling (n = 1); 7) inappropriate publication format (n = 2); 8) animal studies (n = 1). Among these, 22 articles were identified as meeting the eligibility criteria. In addition, 1 study was found through the manual searching of reference lists of selected studies. A total of 23 articles have been included in the systematic review. For the meta-analysis, 1 additional study was excluded due to insufficient data to calculate mean biomarker differences. In total, 22 studies were eligible for performing a meta-analysis.
Characteristics of included studies
Table 1 summarizes the main characteristics of the studies from the systematic literature search. Overall, there were 23 RCTs on dietary pattern interventions and inflammatory biomarkers that measured between-group effect (28–50). Of these, 20 studies had a parallel design (28–30, 32–35, 37–42, 44–50) and 3 studies used a crossover design (31, 36, 43). The studies were conducted in 11 different countries, including Australia (n = 5), Spain (n = 4), Poland (n = 3), USA (n = 3), Iran (n = 2), Greece (n = 1), United Kingdom (n = 1), Italy (n = 1), Sweden (n = 1), Denmark (n = 1), and Brazil (n = 1) (28–50). The study size among included studies ranged from 23 to 1139 participants (28–50). Nineteen studies included both sexes, with prevailing proportions of participating women than men (28, 29, 31, 33–36, 38–46, 49, 50), whereas 4 studies included only women (32, 37, 48) or men (30), respectively. With regards to prevalent diseases, 3 studies included adults with cardiovascular disease (28, 33, 34), whereas 11 studies included adults characterized by existing cardiovascular disease risk factors (including obesity and type 2 diabetes) (31, 32, 36, 37, 39, 43–45, 50). Within all studies included in the meta-analysis, 4 included older participants (aged >55 y) (38, 40, 42, 46) among which 2 studies included only healthy participants (38, 40) and the other 2 included older adults with a high risk of cardiovascular disease (42, 46). Duration of interventions ranged from 4 wk to 5 y (28–50). Among the dietary patterns assessed, the Mediterranean diet was the most investigated diet (n = 16) (30–33, 35–44, 46, 47), followed by the Dietary Adherence to Stop Hypertension (DASH) diet (n = 4) (28, 29, 45, 48). Other studies assessed a vegan diet (n = 1) (34), a vegetarian diet (n = 1) (29), the New Nordic Diet (n = 1) (49), and the Nordic prudent diet (n = 1) (50) collectively categorized as “vegetarian/vegan diet” in the current meta-analysis.
TABLE 1.
Summary of study characteristics and outcomes of included randomized controlled trials investigating the effects of dietary patterns and biomarkers of immune inflammation
| Study, year (reference) | Participants, n | Sex (% female) | Age, 1 y | BMI, 1 kg/m2 | Study design, duration | Study population, country | Diet intervention | Control | Biomarkers measured with results2 | Application of intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Baguley, 2020 (30) | 23 | 0% | 65.9 ± 7.8 | 28.9 ± 3.4 | Parallel, 12 wk | Adults with prostate cancer, Australia | Mediterranean diet | Habitual diet | IL-6—, IL-8— | Personalized nutrition consultations every 2 wk |
| Jaacks, 2018 (35) | 30 | 73.3% | 51.6 ± 6.6 | 34 | Parallel, 8 wk | Adults consuming >10% of total daily calories in saturated and trans fats and total cholesterol intake of >300 mg/d, USA | Mediterranean diet | Habitual diet | CRP—, IL-6—, IL-8—, adiponectin— | 3 meals with beverages and 2 snacks per day provided; verbal and written dietary instructions throughout the study |
| Rallidis, 2017 (39) | 82 | 47.6% | 50.4 ± 7.3 | 32.2 ± 4.3 | Parallel, 2 mo | Adults with abdominal obesity without CVD or diabetes, Greece | Mediterranean diet | Habitual diet | CRP—, IL-6—, sICAM-1—, sVCAM-1—, sE-selectin— | Weekly phone calls and appointments with a dietitian |
| Davis, 2017 (40) | 152 | 55.9% | 70.9 ± 4.8 | 27.0 ± 3.9 | Parallel, 24 wk | Healthy older adults, Australia | Mediterranean diet | Habitual diet | hs-CRP—, F2-Isoprostanes ↓ | Participants attended the clinic every 2 wk to meet with a dietitian to ensure high adherence to the dietary protocol |
| Dyer, 2017 (41) | 99 | 88.8% | 63.0 ± 11.8 | 71.0 ± 15.4 | Parallel, 16 wk | Older adults with osteoarthritis, United Kingdom | Mediterranean diet | Habitual diet | IL-1α ↓, sCOMP—, IL-1β—, IL-2—, IL-4—, IL-6—, IL-8—, IL-10—, IFN-γ—, TNF-α—, VEGF—, EGF—, IL-6sR—, IL-2sR—, TNF-sR1—, TNF-sR2—, MCP-1—, MMP-9— | Nutritional information and dietary advice were provided. Dietitian was available for support via phone calls |
| Wade, 2019 (31) | 33 | 69.7% | 61 ± 7.1 | 30.6 ± 5.1 | Crossover, 8 wk | Adults with CVD risk factors, Australia | Mediterranean diet with extra pork | Low-fat diet | CRP— | Dietary resources and dietary counseling sessions every 2 wk |
| Mayr, 2018 (33) | 56 | 16.1% | 62.3 ± 8.8 | 29.9 ± 5.2 | Parallel, 6 mo | Adults with CHD, Australia | Mediterranean diet | Low-fat diet | hs-CRP—, hs-IL-6— | 2-wk model meal plan and resource kits provided. Counseling with dietitian at baseline, and after 3 and 6 mo. Additional short phone interviews at weeks 3, 6, and 9 and months 4 and 5 |
| Wade, 2018 (36) | 41 | 68.3% | 60.2 ± 6.9 | 30.8 ± 3.8 | Crossover, 8 wk | Adults with CVD risk factors, Australia | Mediterranean diet with extra dairy | Low-fat diet | CRP— | Dietary resources and dietary counseling sessions every 2 wk |
| Dus-Zuchowska, 2018 (37) | 144 | 100% | 60.6 ± 4.7 | 33.7 ± 4.9 | Parallel, 16 wk | Postmenopausal women with central obesity, Poland | Mediterranean diet | Low-fat diet | hs-CRP— | Main meals provided; other meals prepared by participants |
| Medina-Remon, 2017 (38) | 1139 | 55.1% | 67.6 ± 5.9 | 29.3 ± 3.4 | Parallel, 12 mo | Community-dwelling adults, Spain | Mediterranean diet with extra virgin olive oil, Mediterranean diet with extra nuts | Low-fat diet | In both diets: IL‐6 ↓, TNF‐α ↓, MCP‐1 ↓, VCAM‐1 ↓, ICAM‐1 ↓ | Personalized nutrition consultation at the baseline, advises every 3 mo, and group educational sessions every 3 mo |
| Casas, 2017 (42) | 44 | 56.8% | 66.9 ± 6.1 | 29.1 ± 3.7 | Parallel, 5 y | Older adults at high risk for CVD, Spain | Mediterranean diet with extra-virgin olive oil | Low-fat diet | IL-1β ↓, IL-5 ↓, IL-6—, IL-7 ↓, IL-8 ↓, IL-10—, IL-12p70—, IL-13 ↑, IL-18 ↓, TNF-α ↓, MCP-1 ↓, RANTES/CCL5 ↓, MIP-1β/CCL4 ↓, IP-10/CXCL10—, IFN-γ ↓, GCSF ↓, GMCSF—, E-selectin—, P-selectin—, sVCAM-1— | Individual sessions annually with dietitian, individual sessions every 3 mo, and group educational sessions every 3 mo |
| Casas, 2016 (46) | 106 | 54.3% | 66.5 ± 6.1 | 29.3 ± 3.9 | Parallel, 5 y | Older adults at high risk for CVD, Spain | Mediterranean diet with extra-virgin olive oil | Low-fat diet | hs-CRP ↓, IL-6 ↓, TNF-α ↓, MCP-1 ↓ | Individual sessions annually with dietitian, individual sessions every 3 mo, and group educational sessions every 3 mo |
| Maiorino, 2016 (44) | 215 | 50.7% | 52.2 ± 10.9 | 29.6 ± 3.5 | Parallel, 1 y | Adults with type 2 diabetes, Italy | Mediterranean diet | Low-fat diet | CRP ↓, adiponectin ↑, HMW adiponectin ↑, non-HMW adiponectin ↑ | Participants had dietary advice sessions every mo |
| Gomez-Delgado, 2015 (47) | 897 | NA | 59.5 ± 8.9 | 31.2 ± 4.5 | Parallel, 1 y | Adults with CHD, Spain | Mediterranean diet | Low-fat diet | CRP3 | Individual sessions with dietitian at baseline and every 6 mo |
| Chmurzynska, 2019 (32) | 95 | 100% | 60 ± 0.5 | 33.0 ± 4.4 | Parallel, 16 mo | Postmenopausal women with central obesity, Poland | Mediterranean diet | Central European diet | TNF-α—, IL-6— | Main meals were provided, other meals prepared by themselves |
| Monfort-Pires, 2017 (43) | 80 | 66.3% | 51.7 ± 9.3 | 30.5 ± 4.2 | Crossover, 4 wk | Adults with CVD risk factor, Brazil | Mediterranean modified type breakfast | Brazilian breakfast | CRP ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, IL-8 ↓, IFN-γ ↓, E-selectin ↓ | Participants received weekly calls and were instructed to maintain their normal diet, with exception to breakfast foods that were provided for consumption |
| Makarewicz-Wujec, 2020 (28) | 81 | 38.3% | 59.6 ± 7.9 | 29.2 ± 3.6 | Parallel, 6 mo | Patients with coronary artery disease, Poland | DASH diet | Habitual diet | hs-CRP—, CXCL4 ↓ | 6 dietary counseling sessions within 6 mo |
| Razavi-Zade, 2016 (45) | 60 | 50% | 41.3 ± 9.2 | 28.4 ± 3.2 | Parallel, 8 wk | Adults with overweight or obesity and NAFLD, Iran | DASH diet with calorie restriction | Traditional Iranian diet | hs-CRP ↓ | Participants received 7‐d cycle menus, dietary instructions, and a 45-min session with a dietitian |
| Asemi, 2015 (48) | 48 | 100% | 30.1 ± 6.4 | 30.3 ± 4.7 | Parallel, 8 wk | Overweight women with polycystic overage syndrome, Iran | DASH diet | Traditional Iranian diet | hs-CRP ↓ | Personalized dietary advice, and 7-d menu cycle |
| Juraschek, 2020 (29) | 217 | 47.9% | 45.2 ± 0.6 | 28.1 ± 0.2 | Parallel, 8 wk | Adults without CVD, diabetes, morbid obesity, or binge drinkers, USA | DASH diet, vegetarian diet | Typical American diet | DASH diet:hs-CRP—, hs-cTnl ↓vegetarian diet:hs-CRP—, hs-cTnl ↓ | 21 meals provided per week |
| Shah, 2018 (34) | 100 | 15.0% | 60 ± 10.0 | 30.3 ± 5.7 | Parallel, 8 wk | Adults with history of CAD, USA | Vegan diet | American Heart Association diet | hs-CRP ↓ | Personalized nutrition consultations every 4 wk and contact with dietitian per telephone and e‐mail. Weekly groceries and a cookbook were provided |
| Fritzen, 2015 (49) | 64 | 67.2% | 44.7 ± 4.4 | 30.9 ± 1.3 | Parallel, 26 wk | Healthy adults, Denmark | New Nordic Diet | Average Danish diet | CRP—, TNF-α—, adiponectin— | All foods and beverages were provided |
| Adamsson, 2015 (50) | 79 | 61.5% | 54.64 ± 8.4 | 28.3 ± 2.5 | Parallel, 12 wk | Healthy overweight adults, Sweden | Nordic prudent breakfast | Usual breakfast | CRP ↓, TNF-R2 ↓ | All breakfast foods were provided |
Values presented as mean ± SD
Differences of changes in biomarkers between intervention compared with control: ↑, significant increase of biomarker in intervention compared with control (P <0.05); ↓, significant decrease of biomarker in intervention compared with control (P <0.05); —, nonsignificant difference of biomarker between intervention compared with control (P ≥0.05).
Between group differences not available among all participants.
CAD, coronary artery disease; CHD, coronary heart disease; CRP, C-reactive protein; CXCL4, platelet factor-4; DASH, Dietary Approaches to Stop Hypertension; EGF, epidermal growth factor; GMCSF, granulocyte-macrophage colony-stimulating factor; GCSF, granulocyte colony-stimulating factor; HMW, high-molecular weight; hs, high sensitivity; hs-cTnl, high-sensitivity troponin l; ICAM‐1, intercellular adhesion molecule 1; IP-10/CXCL10, interferon γ-induced protein 10/C-X-C motif chemokine ligand 10; MCP-1, monocyte chemoattractant protein 1; MMP-9, matrix metallopeptidase 9; MIP-1β/CCL4, macrophage inflammatory protein-1 β/C-C motif chemokine ligand 4; NA, not available; NAFLD, non-alcoholic fatty liver disease; RANTES/CCL5, regulated upon activation, normal T cell expressed and secreted/chemokine ligand 5; sCOMP, serum cartilage oligomeric matrix protein; sVCAM-1, soluble vascular cell adhesion molecule-1; TNF-R2, TNF receptor-2; VEGF, vascular endothelial growth factor.
Table 2 provides a summary of the results for all evaluated biomarkers of inflammation, including information on significant between-group differences in intervention compared with control groups. Overall, CRP was the most commonly evaluated inflammatory biomarker (n = 18) (28, 29, 31, 33–37, 39, 40, 43–50), followed by IL-6 (n = 9), (30, 32, 35, 38, 39, 41–43, 46), TNF-α (n = 7) (32, 38, 41–43, 46, 49), and IL-8 (n = 5) (30, 35, 41–43). Remaining inflammatory biomarkers that were evaluated in 2 or more studies include adiponectin (n = 3) (35, 44, 49), E-selectin (n = 3) (39, 42, 43), and IFN-γ (n = 3) (41, 43).
TABLE 2.
Summary of pooled estimates for mean differences in biomarker concentrations after the Mediterranean diet, DASH diet, and vegetarian/vegan diet
| Diet | Biomarker | Number of studies | Total number of participants | Mean difference (95% CI) | I 2 |
|---|---|---|---|---|---|
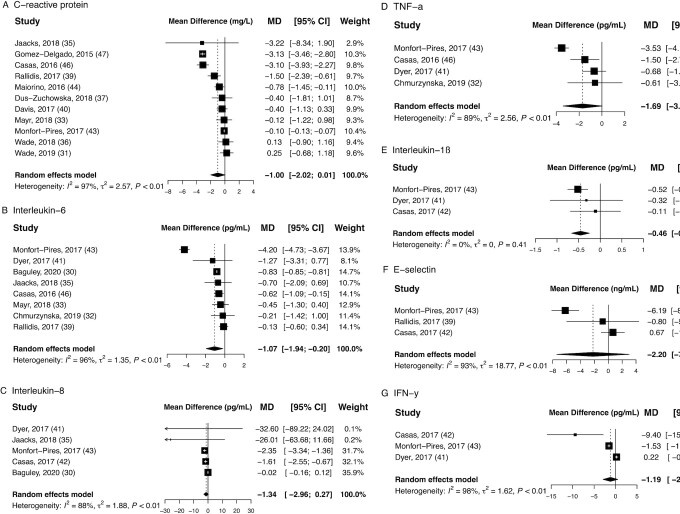
| Mediterranean | CRP, mg/L | 11 | 1805 | –1.00 (–2.02, 0.01) | 97% |
| IL-6, pg/mL | 8 | 524 | –1.07 (–1.94, –0.20) | 96% | |
| IL-8, pg/mL | 5 | 229 | –1.34 (–2.96, 0.14) | 88% | |
| TNF-α, pg/mL | 4 | 335 | –1.69 (–3.40, 0.02) | 89% | |
| IL-1β, pg/mL | 3 | 178 | –0.46 (–0.66, –0.25) | 0% | |
| E-selectin, ng/mL | 3 | 206 | –2.20 (–7.43, 3.02) | 93% | |
| IFN-γ, pg/mL | 3 | 178 | –1.19 (–2.91, 0.53) | 98% | |
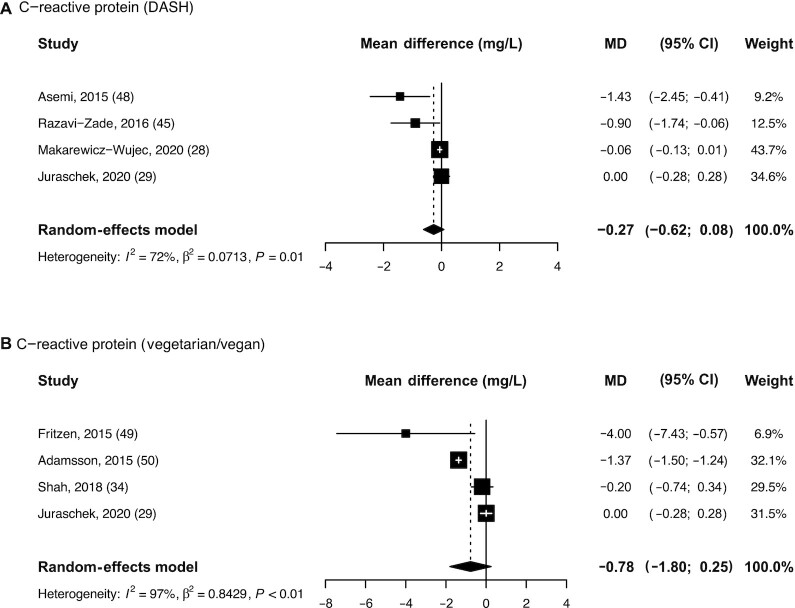
| DASH | CRP, mg/L | 4 | 348 | –0.27 (–0.62, 0.08) | 72% |
| Vegetarian/vegan | CRP, mg/L | 4 | 413 | –0.78 (–1.80, 0.25) | 97% |
CRP, C-reactive protein; DASH; Dietary Approaches to Stop Hypertension.
Pooled effects of Mediterranean diets on inflammatory biomarkers
The Mediterranean dietary pattern was significantly associated with a reduction of IL-6 [MD: –1.07 pg/mL (95% CI: –1.94, –0.20); I2: 96%] and IL-1β [MD: –0.46 pg/mL (95% CI: –0.66, –0.25); I2: 0%] (Table 2). Biomarkers CRP, IL-8, and TNF-α also showed a tendency to decrease after a Mediterranean diet, albeit pooled estimates did not reach statistical significance. No effects were revealed for the remaining biomarkers E-selectin and IFN-γ after following a Mediterranean diet (Figure 2).
FIGURE 2.
Pooled estimates for mean differences in concentrations of (A) C-reactive protein, (B) IL-6, (C) IL-8, (D) TNF-α, (E) IL-1β, (F) E-selectin, and (G) IFN-γ after interventions following a Mediterranean diet. Subcaption: single study effects are depicted as squares with error bars indicating 95% CIs. Diamonds represent pooled estimates from the random-effects model for each biomarker. CRP, C-reactive protein.
Pooled effects of DASH diets and vegetarian/vegan diets on CRP concentrations
Figure 3 presents the pooled effects of the DASH diet (A) and the vegetarian/vegan diets (B) on CRP concentrations. Both dietary patterns led to a decrease in CRP concentrations, yet pooled estimates did not reach statistical significance. Mean differences in CRP concentrations between intervention and control groups were –0.27 mg/L (95% CI: –0.62, 0.08; I2: 72%) for the DASH diet and –0.78 mg/L (95% CI: –1.80, 0.25; I2: 97%) for the vegetarian/vegan diet.
FIGURE 3.
Pooled estimates for mean differences in concentrations of C-reactive protein after interventions following a (A) DASH diet and (B) vegetarian/vegan diet. Subcaption: single study effects are depicted as squares with error bars indicating 95% CIs. Diamonds represent pooled estimates from the random-effects model for each biomarker. DASH, Dietary Approaches to Stop Hypertension.
Subgroup analysis and metaregression
All pooled studies showed considerable heterogeneity, except for the effect of Mediterranean diet on IL-1β (Table 2). In stratified analyses, studies conducted in the Americas, Europe, and Oceania showed stronger effects in reducing TNF-α (43), CRP (37, 39, 44, 46, 47), and IL-6 (30, 33), respectively. Studies with a length of intervention over 1 y showed stronger effects for CRP (44, 46, 47) and IFN-γ (42) (Supplemental Table 1). Studies with a parallel design showed stronger effects in reducing CRP [MD: –1.45 mg/L (95% CI: –2.54, –0.37)] (33, 35, 37, 39, 40, 44, 46, 47) compared with crossover studies [MD: –0.10 mg/L (95% CI: –0.13, –0.07); P-group difference: 0.01] (31, 36, 43). Studies with predominantly female participants showed stronger effects of IL-8 and CRP after following the Mediterranean diet (35, 41–43), and DASH (45, 48) or vegetarian/vegan diet (49, 50), respectively. In stratified analysis by weight loss, a study reporting no weight loss following a Mediterranean diet showed stronger effects for IL-6, IL-8, TNF-α, IL-1β, E-selectin, and IFN-γ (43). Studies reporting weight loss in intervention and control groups found significantly stronger reductions of CRP (37, 39, 44). In the vegetarian/vegan diet, stronger effects were observed in studies reporting weight loss in the intervention group (35, 46). However, these potential sources of heterogeneity should be interpreted with caution due to the small size of the subgroups (Supplemental Table 2).
In sensitivity analysis with shorter follow-up measurements, the pooled effect estimate of the Mediterranean diet and TNF-α was stronger and significant [MD: –1.79 pg/mL (95% CI: –3.52, –0.07)], whereas the effect estimate of the Mediterranean diet and CRP attenuated towards null [MD: –0.91 mg/L (95% CI: 1.99, 0.16)]. Results for IL-6 and IL-1β remained stable (Supplemental Table 3). In sensitivity analysis excluding a study on gene–diet interactions (47), the effect estimate for CRP became stronger [MD: –0.71 mg/L (95% CI: –1.34, –0.09)].
Multivariable metaregression yielded significant results for the Mediterranean diet and CRP with regard to duration of the study [β-coefficient (SE): –0.01 (0.002); P ≤0.0001] and study size [β-coefficient (SE): –0.003 (0.001); P ≤0.0001] (Supplemental Table 4). No significant results were seen for study size, sex, BMI, or age. For the remaining biomarkers and dietary patterns, the number of studies was too low to conduct metaregression analysis (n <10).
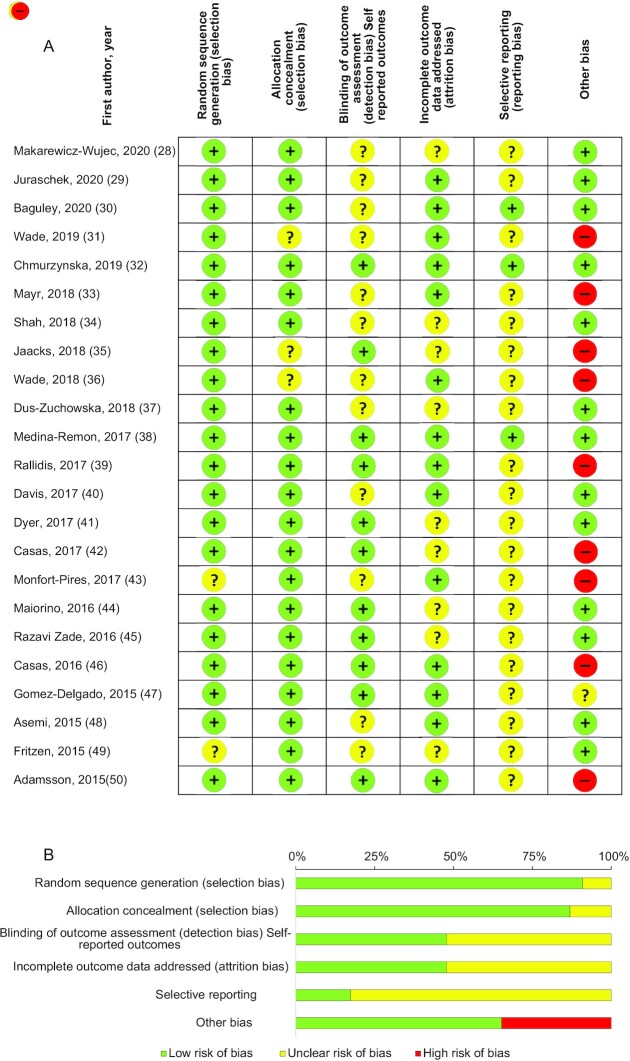
Risk of bias and quality of included studies
Figure 4 shows the quality assessment results of the selected studies and the estimated risk of bias. Above 90% and 85% of the studies had adequate random sequence generation and low risk of bias for allocation concealment, respectively, rendering an overall low risk of selection bias for included studies. Above 50% of the studies displayed unclear risk for blinding of outcome assessment (detection bias), whereas <40% of the studies presented an unclear risk of bias for incomplete outcome data addressed (attrition bias). Above 80% of the studies had an unclear risk of bias for selective reporting, rendering overall unclear risk of reporting bias in included studies. Other identified potential sources of bias were the only source of high risk of bias, around 35% of the studies presented a high risk of bias and ∼65% a low risk of bias.
FIGURE 4.
Risk of bias assessment using the Cochrane Risk of Bias Tool. Subcaption: assessment of risk of bias for (A) each individual study (n = 23) and (B) bias summarized from all studies.
Visual inspection of the generated funnel plot for Mediterranean diets and CRP suggested a possible publication bias (see Supplemental Figure 1). However, the Egger's regression test did not confirm it (P = 0.14). The trim-and-fill procedure added 4 theoretically missing studies and produced a pooled estimate of –0.15 mg/L (95% CI: –1.13, 0.83; I2: 98%).
Discussion
This systematic review and meta-analysis of RCTs published in the last 5 y provides the most recent evaluation of the effects of various dietary patterns on biomarkers of immune response and inflammation. Overall, the findings highlight that following a Mediterranean-type diet leads to a pronounced reduction in the concentrations of the majority of the evaluated inflammatory biomarkers, including IL-6, IL-1β, CRP, IL-8, and TNF-α. No such pronounced effects could be observed for the DASH, vegetarian, or vegan diets.
This review is distinguished from previous reviews in that it evaluates various dietary patterns and a wide range of inflammatory and immune biomarkers studied in recent RCTs conducted in real-world settings. Notably, the results pointed to the Mediterranean diet as the dietary pattern with the most pronounced anti-inflammatory potential. This type of diet is well known both in research and among the general public as a health-promoting diet with various beneficial properties and is implicated in the prevention of chronic diseases. The Mediterranean diet is characterized by the high consumption of plant-derived foods including vegetables, fruits, whole grains, nuts and seeds, and olive oil as the main culinary fat (51). The dietary pattern further consists of a moderate intake of seafood and dairy products—especially yogurt and cheese, and poultry and eggs, whereas low amounts of red and processed meats and sweet desserts are consumed (51). In comparison to the DASH diet, the consumption of fruit and fish is higher and dairy is lower, whilst in comparison to a vegetarian or vegan diet, animal-based foods are limited but not eliminated. Our evidence comes in support of previous research that demonstrated significant reductions in inflammatory biomarkers following the Mediterranean diet (19, 25, 52–55).
Despite increasing evidence on the protective inflammatory effects of vegan and vegetarian diets (20, 22, 56) and DASH diet (24), CRP was not significantly reduced following either. This may be explained by the small number of studies included. The vegetarian and vegan diet groups consisted of a combination of vegan, vegetarian, and Nordic diets that encompass different combinations of food groups, which may have further contributed to the attenuated effect estimate.
The precise mechanisms by which diet could modulate and mitigate inflammatory processes and, accordingly, decrease circulating concentrations of inflammatory biomarkers remain unclear. There are a number of plausible pathways through which a diet may favorably influence immune function and modulate inflammation, including inhibition of proinflammatory mediators, promotion of anti-inflammatory functions, modulation of cell-mediated immunity, alteration of antigen-presenting cell functions, and communication between the innate and adaptive immune systems (57). In particular, the Mediterranean diet and its components have been suggested to modulate pro- and anti-inflammatory processes as well as oxidative stress and antioxidant defenses through several complex and interrelated mechanisms (58). Inflammation, on the one hand, may lead to a significant increase in the secretion of reactive oxygen species (ROS) (59), whereas ROS, on the other hand, may activate signaling pathways that increase proinflammatory gene expression (60). We have previously reported that the Mediterranean diet is associated with reduced concentrations of several biomarkers of oxidative stress and with increased concentrations of antioxidant defenses (52). The high antioxidant potential of this dietary pattern due to its components may represent a lead hypothesis to explain this effect (52). Another plausible explanation for the anti-inflammatory effect observed for the Mediterranean diet as compared with other evaluated plant-based dietary patterns may be sought in the relatively high proportion of MUFAs. Approximately one-third of total calories in the Mediterranean diet is consumed in the form of fats, with ≤3 times higher amounts of MUFAs compared to SFAs (61). Although the high intake of SFAs is considered to be a proinflammatory factor, displaying induction of the expression of cytokines such as IL-1β, IL-6, and TNF-α and activation of the NF-κB proinflammatory signaling pathways, MUFAs have been shown to be able to decrease proinflammatory cytokine concentrations such as CRP, TNF-α, monocyte chemoattractant protein 1 (MCP-1), IFN-γ, IL-18, and IL-6 and to inhibit NF-κB proinflammatory signaling pathways (62). Moreover, the vast variety of phytochemicals and bioactive compounds such as carotenoids, flavonoids, and polyphenols may contribute directly or indirectly to pro- and anti-inflammatory effects of the Mediterranean diet (63, 64). Although the specific mechanisms have not yet been fully elucidated, the synergy and the balanced combination of all dietary components of the Mediterranean diet undoubtedly possess strong anti-inflammatory and immune-balancing properties that justify recommending its application for sustaining strong immune health (21).
In contrast, evidence from observational studies suggests that dietary patterns characterized by high amounts of refined starches, sugar, processed meats, alcohol, salt, and saturated and trans fatty acids and by low intakes of fruits, vegetables, and whole grains may lead to innate immune system activation and increased production of proinflammatory cytokines (65, 66). Western-type dietary patterns were particularly shown to contribute to metaflammation, a state of disturbed immune-metabolic homeostasis, predisposing chronic disease development and progression (67). Moreover, accumulating evidence suggests that dietary patterns high in processed and ultraprocessed foods affect the composition of the gut microbiota, leading to changes in low-grade systemic inflammation (68). Further epidemiological studies are still needed to better understand the interaction of dietary patterns, the gut microbiome and systemic metabolic immune-inflammatory mechanisms.
Our approach focused on the evaluation of whole-food dietary patterns as a suitable strategy for sustaining long-term dietary modification. Other dietary approaches proposed to exert effects in modulating inflammation take into account the distribution of macronutrients, i.e., carbohydrates, proteins, and lipids. In our literature search, we identified 2 RCTs that focused on low-carbohydrate (69) and ketogenic (low-carb, moderate protein, higher-fat) (70) diets reporting beneficial effects on inflammatory biomarkers after short and long intervention periods, respectively. Further studies are warranted to specifically evaluate the inflammatory response associated with the intake of diets characterized by various macronutrient composition.
Since weight loss may partly account for the reduction in inflammatory levels and may thereby mask the effect of dietary patterns alone (71), we explored to what extent the studies accounted for change in weight of study participants. The majority of the studies included in the current review did not report on substantial influence by weight loss. These results were supported by the metaregression analysis that did not suggest BMI change to have influenced the results. These results are in contrast with some previous reports, suggesting that dietary patterns per se may not modify circulating markers of inflammation in weight-stable individuals (72). However, those previous studies addressed diets with higher calorie restriction that could have led to stronger weight loss compared with the dietary patterns, i.e., the Mediterranean Diet, evaluated in the current review (72).
It should be noted that many of the evaluated studies focused on CRP as a proxy biomarker denoting inflammation, which may limit the interpretation of the observed effects. CRP is a nonspecific acute phase and proinflammatory response biomarker that may not be fully capturing the spectrum of inflammatory and immune responses important for chronic disease pathophysiology (73). Indeed, there is an ongoing debate whether CRP may be causally linked to chronic disease etiology since the majority of Mendelian Randomization studies did not support its causal role (74–76). Also in healthy individuals, the intraindividual variation in CRP measurements can be substantial and prone to influence by various modifying factors such as individual age, BMI, physical activity, and even season (77, 78). Only a few studies in the current review evaluated a wider range of inflammatory biomarkers, including cytokines that reflect different pathways and are able to capture various aspects of inflammation and immune response. To date, there is a lack of established biomarkers that indicate a specific inflammatory pathway and can differentiate between acute and chronic inflammation and that can be used as target endpoints in nutrition interventions (73). The research is further challenged by a lack of reliability studies to ensure that biomarkers are quantifiable and stable over time (79). The studies included in the current review applied various assessment methods to measure the inflammatory biomarkers with different kits with varying inter- and intra-assay coefficients of variability affecting the overall accuracy of the reported effect estimates. Furthermore, multiple biomarkers representing both pro- and anti-inflammatory pathways may provide more useful insights when investigating anti-inflammatory potentials of a diet. This calls for the need of the development of a harmonized, standardized, and accepted assay to measure a panel of biomarkers representing a spectrum of immune-inflammatory pathways. Another challenge to providing a concise evaluation of the dietary patterns is the large variation of interventions amongst studies and lack of standardized instruments for the quality assessment of nutritional studies employing biomarkers. A promising approach to overcome methodological issues between studies, and to directly assess different diets in relation to a specific outcome, is to conduct a single large-scale intervention study with a strong multifactorial design.
The current study has a number of strengths. The results go beyond previous meta-analyses and provide updated evidence on the effect of dietary patterns on a wider range of inflammatory biomarkers, including specific cytokines and adhesion molecules, i.e., IL-1β and E-selectin. Wholefood diets were evaluated instead of a focus on adjusting the macronutrient ratio of diet, specific nutrients, or food groups. We included RCTs only, so the study design and quality of evidence is relatively high, and we provide a comprehensive overview of the most recent studies within this field.
This study is also prone to limitations. Participants included in the studies were mostly of older age, i.e., above 50 y, limiting the generalizability of our results. The studies differed by design, characteristics of included participants, and analyses, causing heterogeneity between studies and limiting comparability. Differences in compliance of diets or weight loss were not taken into account as a source of heterogeneity. Weight loss is directly related to a decline in CRP (80) so may be an important confounder. The reduced concentrations of CRP may have resulted from shifting to a “healthy” diet with less calories rather than the components of the diet per se (80). However, most studies designed the interventions and control groups to be isocaloric and reported changes in biomarkers independent of weight loss. Crossover studies were incorporated into the meta-analysis as though the design was parallel, thereby not taking time in the different intervention periods into account, and the studies may have been underweighted. In addition, some studies had multiple assessments of biomarkers over the duration of the trial. We consistently included only the last measurement to standardize the approach across studies and remove selection bias related to the intermediate measurements. Sensitivity analyses were performed with the intermediate measurements to see whether different follow-up times had an influence on the pooled effect estimate. Finally, although the current review aimed to capture a variety of dietary patterns, its results showed that the research has been dominated by studies on the most popular types of diets, i.e., the Mediterranean diet. This outlines an important niche in research that requires building upon novel hypotheses on specific anti-inflammatory diet plans generated in observational epidemiologic research.
In conclusion, the current systematic review and meta-analysis of RCTs published in the last 5 y highlighted the beneficial anti-inflammatory effects of the Mediterranean diet and suggested that these can be maintained in the long term. Large-scale intervention studies with a strong multifactorial design are warranted in future research to enable the direct comparison of various dietary patterns approached in relation to a range of biomarkers reflecting multiple inflammatory and immune-related pathways. This research would pave the way towards the development of dietary strategies to alleviate inflammation and sustain adequate immune balance in the battle against inflammation-related chronic diseases.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tom Heinze (Institute of Nutritional Sciences, University of Potsdam) for his assistance with literature search and data extraction. We thank the following authors for providing clarification on their published articles and/or additional unpublished data: Stephen Juraschek (Beth Israel Deaconess Medical Center, USA), Lindsay Jaacks (University of Edinburgh, United Kingdom), Glen Davison (University of Kent, United Kingdom), Joanna Bajerska (Poznan University of Life Sciences, Poland), and Brenton Baguley (Deakin University, Australia).
The authors’ responsibilities were as follows—KA: contributed to the conception and design of the systematic review and meta-analysis; LK and CER: reviewed the publications; LK: extracted the data; CER: verified the accuracy of the extraction; CER: conducted the risk of bias assessment; LK: analyzed the data; KA, LK, and CER: interpreted the data, and prepared the manuscript; all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–4 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CRP, C-reactive protein; DASH, Dietary Adherence to Stop Hypertension; MCP-1, monocyte chemoattractant protein 1; MD, mean difference; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; ROS, reactive oxygen species.
Contributor Information
Liselot Koelman, Department of Molecular Epidemiology, German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Nuthetal, Germany; Institute of Nutritional Science, University of Potsdam, Potsdam, Germany.
Caue Egea Rodrigues, Department of Pharmacology and Toxicology, Institute of Pharmacy, Free University of Berlin, Berlin, Germany.
Krasimira Aleksandrova, Department Epidemiological Methods and Etiological Research, Leibniz Institute for Prevention Research and Epidemiology, Bremen, Germany; Faculty of Human and Health Sciences, University of Bremen, Bremen, Germany.
References
- 1. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27(1):28–33. [DOI] [PubMed] [Google Scholar]
- 3. Dong E, Du H, Gardner L. [Internet] (accessed 8 April, 2021). Available from: (accessed 8 April, 2021) https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. [Google Scholar]
- 4. Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet North Am Ed. 2020;395(10227):912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, Alsukait RF, Alluhidan M, Alazemi N, Shekar M. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Liu S, Leng SX. Chronic low-grade inflammatory phenotype (CLIP) and senescent immune dysregulation. Clin Ther. 2019;41(3):400–9. [DOI] [PubMed] [Google Scholar]
- 7. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–5S. [DOI] [PubMed] [Google Scholar]
- 10. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet North Am Ed. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwamura APD, Tavares da Silva MR, Hümmelgen AL, Soeiro Pereira PV, Falcai A, Grumach AS, Goudouris E, Neto AC, Prando C. Immunity and inflammatory biomarkers in COVID-19: a systematic review. Rev Med Virol. 2021;31:e2199. [DOI] [PubMed] [Google Scholar]
- 13. Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6(6):461–7. [DOI] [PubMed] [Google Scholar]
- 14. Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwingshackl L, Hoffmann G, Iqbal K, Schwedhelm C, Boeing H. Food groups and intermediate disease markers: a systematic review and network meta-analysis of randomized trials. Am J Clin Nutr. 2018;108(3):576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39(2):99–110. [DOI] [PubMed] [Google Scholar]
- 17. Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(S3):S5–S78. [DOI] [PubMed] [Google Scholar]
- 18. Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7(3):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. [DOI] [PubMed] [Google Scholar]
- 20. Craddock JC, Neale EP, Peoples GE, Probst YC. Vegetarian-based dietary patterns and their relation with inflammatory and immune biomarkers: a systematic review and meta-analysis. Adv Nutr. 2019;10(3):433–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev. 2016;17(11):1067–79. [DOI] [PubMed] [Google Scholar]
- 22. Menzel J, Jabakhanji A, Biemann R, Mai K, Abraham K, Weikert C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci Rep. 2020;10(1):21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwingshackl L, Christoph M, Hoffmann G. Effects of olive oil on markers of inflammation and endothelial function – a systematic review and meta-analysis. Nutrients. 2015;7(9):7651–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37(2):542–50. [DOI] [PubMed] [Google Scholar]
- 25. Wu P-Y, Chen K-M, Tsai W-C. The Mediterranean dietary pattern and inflammation in older adults: a systematic review and meta-analysis. Adv Nutr. 2021;12(2):363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–30. [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons; 2019, p.205–28. [Google Scholar]
- 28. Makarewicz-Wujec M, Henzel J, Kruk M, Kępka C, Wardziak Ł, Trochimiuk P, Parzonko A, Demkow M, Kozłowska-Wojciechowska M. DASH diet decreases CXCL4 plasma concentration in patients diagnosed with coronary atherosclerotic lesions. Nutrition, Metabolism and Cardiovascular Diseases. 2020;30(1):56–9. [DOI] [PubMed] [Google Scholar]
- 29. Juraschek SP, Kovell LC, Appel LJ, Miller ER 3rd, Sacks FM, Christenson RH, Rebuck H, Chang AR, Mukamal KJ. Associations between dietary patterns and subclinical cardiac injury: an observational analysis from the DASH trial. Ann Intern Med. 2020;172(12):786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baguley BJ, Skinner TL, Jenkins DG, Wright ORL. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: a pilot randomised control trial. Clin Nutr. 2021;40(1):245–54. [DOI] [PubMed] [Google Scholar]
- 31. Wade AT, Davis CR, Dyer KA, Hodgson JM, Woodman RJ, Murphy KJ. Effects of Mediterranean diet supplemented with lean pork on blood pressure and markers of cardiovascular risk: findings from the MedPork trial. Br J Nutr. 2019;122(8):873–83. [DOI] [PubMed] [Google Scholar]
- 32. Chmurzynska A, Muzsik A, Krzyżanowska-Jankowska P, Walkowiak J, Bajerska J. The effect of habitual fat intake, IL6 polymorphism, and different diet strategies on inflammation in postmenopausal women with central obesity. Nutrients. 2019;11(7):1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayr HL, Thomas CJ, Tierney AC, Kucianski T, George ES, Ruiz-Canela M, Hebert JR, Shivappa N, Itsiopoulos C. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvement in Dietary Inflammatory Index scores in patients with coronary heart disease: the AUSMED Heart Trial. Nutr Res. 2018;55:94–107. [DOI] [PubMed] [Google Scholar]
- 34. Shah B, Newman JD, Woolf K, Ganguzza L, Guo Y, Allen N, Zhong J, Fisher EA, Slater J. Anti-inflammatory effects of a vegan diet versus the American Heart Association-recommended diet in Coronary Artery Disease Trial. J Am Heart Assoc. 2018;7(23):e011367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaacks LM, Sher S, Staercke C, Porkert M, Alexander WR, Jones DP, Vaccarino V, Ziegler TR, Quyyumi AA. Pilot randomized controlled trial of a Mediterranean diet or diet supplemented with fish oil, walnuts, and grape juice in overweight or obese US adults. BMC Nutr. 2018;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wade AT, Davis CR, Dyer KA, Hodgson JM, Woodman RJ, Murphy KJ. A Mediterranean diet supplemented with dairy foods improves markers of cardiovascular risk: results from the MedDairy randomized controlled trial. Am J Clin Nutr. 2018;108(6):1166–82. [DOI] [PubMed] [Google Scholar]
- 37. Duś-Żuchowska M, Bajerska J, Krzyżanowska P, Chmurzyńska A, Miśkiewicz-Chotnicka A, Muzsik A, Walkowiak J. The Central European diet as an alternative to the Mediterranean diet in atherosclerosis prevention in postmenopausal obese women with a high risk of metabolic syndrome – a randomized nutritional trial. Acta Sci Pol Technol Aliment. 2018;17(4):399–407. [DOI] [PubMed] [Google Scholar]
- 38. Medina-Remón A, Casas R, Tressserra-Rimbau A, Ros E, Martínez-González MA, Fitó M, Corella D, Salas-Salvadó J, Lamuela-Raventos RM, Estruch R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol. 2017;83(1):114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rallidis LS, Kolomvotsou A, Lekakis J, Farajian P, Vamvakou G, Dagres N, Zolindaki M, Efstathiou S, Anastasiou-Nana M, Zampelas A. Short-term effects of Mediterranean-type diet intervention on soluble cellular adhesion molecules in subjects with abdominal obesity. Clin Nutr ESPEN. 2017;17:38–43. [DOI] [PubMed] [Google Scholar]
- 40. Davis CR, Bryan J, Hodgson JM, Woodman R, Murphy KJ. A Mediterranean diet reduces F(2)-isoprostanes and triglycerides among older Australian men and women after 6 months. J Nutr. 2017;147(7):1348–55. [DOI] [PubMed] [Google Scholar]
- 41. Dyer J, Davison G, Marcora SM, Mauger AR. Effect of a Mediterranean type diet on inflammatory and cartilage degradation biomarkers in patients with osteoarthritis. J Nutr Health Aging. 2017;21(5):562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Casas R, Urpi-Sardà M, Sacanella E, Arranz S, Corella D, Castañer O, Lamuela-Raventós RM, Salas-Salvadó J, Lapetra J, Portillo MP et al. Anti-inflammatory effects of the Mediterranean diet in the early and late stages of atheroma plaque development. Mediators Inflamm. 2017;2017:3674390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monfort-Pires M, Ferreira SRG. Inflammatory and metabolic responses to dietary intervention differ among individuals at distinct cardiometabolic risk levels. Nutrition. 2017;33:331–7. [DOI] [PubMed] [Google Scholar]
- 44. Maiorino MI, Bellastella G, Petrizzo M, Scappaticcio L, Giugliano D, Esposito K. Mediterranean diet cools down the inflammatory milieu in type 2 diabetes: the MÉDITA randomized controlled trial. Endocrine. 2016;54(3):634–41. [DOI] [PubMed] [Google Scholar]
- 45. Razavi Zade M, Telkabadi MH, Bahmani F, Salehi B, Farshbaf S, Asemi Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int. 2016;36(4):563–71. [DOI] [PubMed] [Google Scholar]
- 46. Casas R, Sacanella E, Urpí-Sardà M, Corella D, Castañer O, Lamuela-Raventos RM, Salas-Salvadó J, Martínez-González MA, Ros E, Estruch R. Long-term immunomodulatory effects of a Mediterranean diet in adults at high risk of cardiovascular disease in the PREvención con DIeta MEDiterránea (PREDIMED) randomized controlled trial. J Nutr. 2016;146(9):1684–93. [DOI] [PubMed] [Google Scholar]
- 47. Gomez-Delgado F, Garcia-Rios A, Alcala-Diaz JF, Rangel-Zuñiga O, Delgado-Lista J, Yubero-Serrano EM, Lopez-Moreno J, Tinahones FJ, Ordovas JM, Garaulet M et al. Chronic consumption of a low-fat diet improves cardiometabolic risk factors according to the CLOCK gene in patients with coronary heart disease. Mol Nutr Food Res. 2015;59(12):2556–64. [DOI] [PubMed] [Google Scholar]
- 48. Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm Metab Res. 2015;47(3):232–8. [DOI] [PubMed] [Google Scholar]
- 49. Fritzen AM, Lundsgaard AM, Jordy AB, Poulsen SK, Stender S, Pilegaard H, Astrup A, Larsen TM, Wojtaszewski JF, Richter EA et al. New Nordic Diet-induced weight loss is accompanied by changes in metabolism and AMPK signaling in adipose tissue. J Clin Endocrinol Metab. 2015;100(9):3509–19. [DOI] [PubMed] [Google Scholar]
- 50. Adamsson V, Reumark A, Marklund M, Larsson A, Risérus U. Role of a prudent breakfast in improving cardiometabolic risk factors in subjects with hypercholesterolemia: a randomized controlled trial. Clin Nutr. 2015;34(1):20–6. [DOI] [PubMed] [Google Scholar]
- 51. Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M, Ros E. Benefits of the Mediterranean diet: insights from the PREDIMED Study. Prog Cardiovasc Dis. 2015;58(1):50–60. [DOI] [PubMed] [Google Scholar]
- 52. Aleksandrova K, Koelman L, Rodrigues CE. Dietary patterns and biomarkers of oxidative stress and inflammation: a systematic review of observational and intervention studies. Redox Biol. 2021;42:101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hart MJ, Torres SJ, McNaughton SA, Milte CM. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutrition Journal. 2021;20(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Papadaki A, Nolen-Doerr E, Mantzoros CS. The effect of the Mediterranean diet on metabolic health: a systematic review and meta-analysis of controlled trials in adults. Nutrients. 2020;12(11):3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–39. [DOI] [PubMed] [Google Scholar]
- 56. Haghighatdoost F, Bellissimo N, Totosy de Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2017;20(15):2713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2018;9:3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martucci M, Ostan R, Biondi F, Bellavista E, Fabbri C, Bertarelli C, Salvioli S, Capri M, Franceschi C, Santoro A. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr Rev. 2017;75(6):442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mittal M, Rizwan Siddiqui M, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients. 2015;7(11):9139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ravaut G, Légiot A, Bergeron K-F, Mounier C. Monounsaturated fatty acids in obesity-related inflammation. Int J Mol Sci. 2020;22(1):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Watzl B. Anti-inflammatory effects of plant-based foods and of their constituents. Int J Vitam Nutr Res. 2008;78(6):293–8. [DOI] [PubMed] [Google Scholar]
- 64. Teodoro AJ. Bioactive compounds of food: their role in the prevention and treatment of diseases. Oxidative Medicine and Cellular Longevity. 2019;2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48(4):677–85. [DOI] [PubMed] [Google Scholar]
- 66. Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017;8:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51(5):794–811. [DOI] [PubMed] [Google Scholar]
- 68. Telle-Hansen VH, Holven KB, Ulven SM. Impact of a healthy dietary pattern on gut microbiota and systemic inflammation in humans. Nutrients. 2018;10(11):1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gyorkos A, Baker MH, Miutz LN, Lown DA, Jones MA, Houghton-Rahrig LD. Carbohydrate-restricted diet and high-intensity interval training exercise improve cardio-metabolic and inflammatory profiles in metabolic syndrome: a randomized crossover trial. Cureus. 2019;11(9):e5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Perticone M, Maio R, Sciacqua A, Suraci E, Pinto A, Pujia R, Zito R, Gigliotti S, Sesti G, Perticone F. Ketogenic diet-induced weight loss is associated with an increase in vitamin D levels in obese adults. Molecules. 2019;24(13):2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bianchi VE. Weight loss is a critical factor to reduce inflammation. Clinical Nutrition ESPEN. 2018;28:21–35. [DOI] [PubMed] [Google Scholar]
- 72. Cowan SF, Leeming ER, Sinclair A, Dordevic AL, Truby H, Gibson SJ. Effect of whole foods and dietary patterns on markers of subclinical inflammation in weight-stable overweight and obese adults: a systematic review. Nutr Rev. 2020;78(1):19–38. [DOI] [PubMed] [Google Scholar]
- 73. Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(7):999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang X, Dai JY, Albanes D, Arndt V, Berndt SI, Bézieau S, Brenner H, Buchanan DD, Butterbach K, Caan B et al. Mendelian randomization analysis of C-reactive protein on colorectal cancer risk. Int J Epidemiol. 2019;48(3):767–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, Engert JC, Clarke R, Davey-Smith G, Nordestgaard BG et al. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brunner EJ, Kivimäki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP et al. Inflammation, insulin resistance, and diabetes – Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5(8):e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chiriboga DE, Ma Y, Li W, Stanek EJ, Hébert JR, Merriam PA, Rawson ES, Ockene IS. Seasonal and sex variation of high-sensitivity C-reactive protein in healthy adults: a longitudinal study. Clin Chem. 2009;55(2):313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bower JK, Lazo M, Juraschek SP, Selvin E. Within-person variability in high-sensitivity C-reactive protein. Arch Intern Med. 2012;172(19):1519–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koelman L, Pivovarova-Ramich O, Pfeiffer AFH, Grune T, Aleksandrova K. Cytokines for evaluation of chronic inflammatory status in ageing research: reliability and phenotypic characterisation. Immunity & Ageing. 2019;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167(1):31–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.