ABSTRACT
Prebiotics, synbiotics, and SCFAs have been shown to decrease systemic inflammation and play a protective role in chronic respiratory conditions. However, their effects on infection and immune function are unclear. The objective of this systematic review was to summarize the current evidence for prebiotic, synbiotic, and SCFA supplementation on respiratory tract infections (RTIs) and immune function. The protocol for this systematic review was registered with PROSPERO (National Institute for Health Research, University of York, UK), accessed online at https://www.crd.york.ac.uk/prospero (CRD42019118786). Relevant English-language articles up to May 2021 were identified via online databases: MEDLINE, EMBASE, CINAHL, and Cochrane Library. Included studies (n = 58) examined the effect of prebiotics, synbiotics, or SCFA, delivered orally, on the incidence, severity, or duration of RTIs and/or markers of immune function (e.g., peripheral blood immunophenotyping, NK cell activity). The majority of studies were randomized controlled trials reporting on RTIs in infants and children. The meta-analysis indicated that the numbers of subjects with ≥1 RTI were reduced with prebiotic (OR, 0.73; 95% CI: 0.62–0.86; P = 0.0002; n = 17) and synbiotic (OR, 0.75; 95% CI: 0.65–0.87; P = 0.0001; n = 9) supplementation compared to placebo. Further, NK cell activity was increased with synbiotic (standardized mean difference, 0.74; 95% CI: 0.42–1.06; P < 0.0001, n = 3) supplementation. This review provides evidence that prebiotic, specifically oligosaccharide, supplementation may play a protective role in RTIs in infants and children. There is less evidence for this effect in adults. Supplementation with prebiotic and synbiotic treatment may alter immune function by increasing NK cell activity, though effects on immunophenotype were less clear.
Keywords: fructooligosaccharide, galactooligosaccharide, xylooligosaccharide, inulin, inulin-type fructans, respiratory tract infection, NK cell activity
Statement of Significance: This is the first systematic review to provide a comprehensive summary of available evidence for the effects of prebiotic and synbiotic supplementation in the context of immunity and respiratory tract infections.
Introduction
A growing body of evidence suggests the importance of the gut-lung axis in respiratory health, as evidenced by a number of studies linking the consumption of pre-, pro-, and synbiotics with beneficial effects in respiratory conditions, such as asthma (1, 2) and chronic obstructive pulmonary disease (3). However, these effects may not be limited to chronic disease and may play a protective role in infectious disease episodes, such as respiratory tract infections (RTIs). Previous systematic reviews have reported evidence for a protective effect of probiotic therapy on the prevention of ventilator-associated pneumonia (4) and upper respiratory tract infections (URTIs) (5). Emerging evidence suggests protective effects of both pre- and synbiotic interventions on RTI prevalence, duration, and symptom severity in human subjects of various ages (6–13).
A prebiotic is defined as a “selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health” (14). Food ingredients are classed as prebiotics if they satisfy specific criteria, including resistance to digestion and the ability to undergo fermentation by colonic bacteria, leading to the growth of “health-promoting” bacteria. Prebiotics primarily consist of soluble fibers such as resistant starch, pectin, gums and oligosaccharide carbohydrates, including fructooligosaccharides (FOS), galactooligosaccharides (GOS), xylooligosaccharides (XOS), and glucooligosaccharides. Synbiotics are products which consist of a combination of prebiotic carbohydrates with probiotic organisms, the latter being defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host” (15).
Prebiotics and synbiotics are hypothesized to exert a number of immunomodulatory effects through modification of the microflora within the colon, as well as direct and indirect effects on the intestinal epithelium and innate immune cells [e.g., dendritic cells (DCs), monocytes/macrophages, neutrophils, NK cells] (16). Indirect effects are carried out through the elimination of pathogenic bacteria and via postbiotic products, such as SCFAs. These SCFAs exert their effects primarily through the activation of free fatty acid receptors (FFARs) and the inhibition of histone deacetylase (HDAC) enzymes. In humans, pre- and synbiotic supplementation has been reported to increase NK cell cytotoxicity (17–20). Further, evidence from animal models suggests pre- and synbiotic supplementation modulates DC maturation (21), macrophage phenotype (22, 23), regulatory T cell (Treg) expansion (24), and a number of neutrophil functions (25, 26).
Prebiotic- and synbiotic-induced changes in immune function, particularly the activity of specific immune cell subsets, could improve responses to pathogens during infection. Oral supplementation of humans with pre- and synbiotics have reported increased NK cell activity (17, 18, 20), reduced CD16/56 expression on natural killer T (NKT) cells (27), and increased toll-like receptor (TLR) 2 and 4 on myeloid dendritic cells (mDCs) (28). Human NK cells are identified using CD markers present on the cell surface, and are defined as CD56+ lymphocytes negative for other markers specific to different classes of lymphocytes (e.g., CD3 on T cells, CD14 on monocytes, CD19 on B cells) (29). These cells are an important component of the innate immune system, responsible for nonspecific antiviral and antitumor activity. NKT cells are a class of T cells which express a highly conserved αβ T-cell receptor and play a regulatory role on lymphocytes via releasing large amounts of cytokines (30). The nature of the antigen-presenting cell (APC) determines the function of NKT cells. For example, IL-12 release from DCs during antigen presentation induces NKT cells to produce IFN-γ, which leads to a type 1 T helper (TH1) response via the activation and expansion of NK cells, neutrophils, and CD4+ TH1 or CD8+ T cells. TLR2 and TLR4 are activated by a number of different microbial factors, and are important in recognition of a wide array of pathogens. Changes to TLR expression on DCs could be associated with an increased capacity to recognize pathogen-associated molecular patterns.
To our knowledge, no systematic review has summarized the evidence for the effects of prebiotic, synbiotic, or SCFA intervention for the prevention of RTIs and on markers of immune function. Therefore, the objective of this systematic review was to synthesize the available evidence to determine the effects of prebiotics, synbiotics, and SCFAs on RTIs and immune function in humans. Hence, this systematic review aimed to answer the following question: does oral supplementation with prebiotics or synbiotics affect the incidence, duration, and severity of RTIs or markers of immune function (e.g., NK cell activity, peripheral immune cell populations)? Secondary aims were to establish what intervention dose and duration are required for protective effects against RTIs and modification of immune cell functions.
Methods
Protocol registration
The protocol for this systematic review was registered with PROSPERO (National Institute for Health Research, University of York, UK), accessed online at https://www.crd.york.ac.uk/prospero (CRD42019118786). The review was performed according to a prospective protocol using Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Search strategy
The electronic databases Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane Library, and Medline were searched for English-language articles from 1947 to 4 December 2018 with the use of predetermined keywords and Medical Subject Heading (MeSH) terms of the National Library of Medicine (Supplemental Table 1). Additional hand searching of recently published review articles was conducted. The search strategy was repeated in December 2019 and May 2021 to identify additional relevant articles published after the initial search was conducted.
Inclusion and exclusion criteria
Included articles were primary research studies that delivered a prebiotic or synbiotic supplement with or without other interventions to human participants of any age, or studies which treated human cells ex vivo with SCFA. Research studies with the following designs were included: randomized controlled trials (RCTs), non-randomized controlled trials (NRCTs), before-and-after studies, and prospective cohort studies. Included studies were categorized by the type of intervention to assess the evidence regarding prebiotics and synbiotics. The exclusion criteria were studies using prebiotics as an adjuvant for influenza vaccination, probiotic-only interventions, interventions administering a prebiotic or synbiotic to mothers without supplementing the infant, participants in critical care settings, and studies which focused on immune changes within the gastrointestinal tract.
Data collection
Following the search strategy, studies were evaluated for relevancy using Covidence systematic review software (Veritas Health Innovation; www.covidence.org). The number of records acquired from each database were noted, with duplicate studies noted and removed. Two reviewers used the inclusion and exclusion criteria to assess the retrieved studies using titles only (LMW and ILS). Studies considered irrelevant were noted, with the reason, then removed. The remaining studies were assessed on the abstract, keywords, and MeSH terms using the inclusion and exclusion criteria outlined above. Irrelevant articles were noted, with the reason, then removed. The full texts of remaining articles, including articles with unclear relevancy, were retrieved and assessed for relevancy according to the inclusion and exclusion criteria by 2 reviewers (LMW and ILS). If there was any disagreement between the 2 reviewers regarding the relevancy of an article, a third independent reviewer decided the outcome (LGW).
The remaining studies were assessed for quality by 2 reviewers (LMW and ILS) using a standardized critical appraisal checklist designed by the American Dietetic Association (ADA) (31). This tool assessed the methodological quality and risk of bias of each study by evaluating their reliability, validity, and generalizability, giving studies a rating of positive, neutral, or negative quality. The tool evaluates each study based on the appropriateness of the study design and the quality of how the study was conducted using 10 validity questions that address scientific soundness. Studies received a negative quality rating if 6 or more of the validity criteria questions were not satisfied. These negative-quality studies were excluded from the review. The evidence level for each article was defined according to the National Health and Medical Research Council (NHMRC) of Australia levels of evidence hierarchy (32).
After the elimination of duplicate, irrelevant, and negative-quality articles, the following data were extracted: author, publication year, country, baseline characteristics (age, gender, and sample size), type of intervention (prebiotic or synbiotic composition, placebo composition, and intervention duration), and outcomes of interest, which were the incidence of RTIs, duration of RTIs, NK cell activity, and peripheral blood immune cell populations. Studies were reported qualitatively in a table format, with columns for study design/level of evidence; quality, as determined by the ADA checklist; participant characteristics, including age and sample size; details of the intervention; details of the control; and the effects of the intervention on outcomes of interest. WebPlotDigitizer (version 4.4; https://apps.automeris.io/wpd/) was used to extract data from published figures. For further analysis, all the feasible data were entered into Review Manager (RevMan, Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The primary manuscripts included are available in PubMed. Data extraction tables and analyses performed can be obtained by sending an email to the corresponding author.
Meta-analysis
The meta-analysis was performed using Review Manager (RevMan, version 5.3; Nordic Cochrane Centre). Heterogeneity was investigated using the χ2 test (P values < 0.1 were considered to indicate significant heterogeneity) and I2 parameter [with 30%–60% indicating moderate heterogeneity, 50%–90% indicating substantial heterogeneity, and 75%–100% indicating considerable heterogeneity (33)]. When considerable heterogeneity was identified, subgroup analyses were conducted to investigate potential contributing factors. When studies were considered heterogeneous regarding the type and dosage of the prebiotic or synbiotic, the treatment duration, and/or the study population, random-effects meta-analysis models were applied. Otherwise, fixed-effects meta-analysis models were used. The inverse-variance statistical method was used, with OR (effect size) or standardized mean difference (SMD; effect size) and corresponding 95% CIs calculated. The Cochrane Handbook for Systematic Reviews of Interventions was used to determine whether it was appropriate to include data from crossover studies in the meta-analyses, based on the ability to rule out significant carry-over effects (33). If there were insufficient data in a publication or data were in the incorrect format, the corresponding authors were contacted for further information. Following this, if data were still unavailable, articles were excluded from the meta-analysis. In the event the SD was not reported in a publication, it was calculated using the SE or 95% CI values. If the OR was not reported, this was calculated from the number of participants with ≥1 event in each group and the total number of subjects per group. If a study reported on incidences for URTIs and lower respiratory tract infections (LRTIs) separately, we combined outcomes as described in Borenstein's “Introduction to Meta-Analysis: Complex Data Structures” (34), using a correlation coefficient of r = 1. This correlation coefficient was used because it is the most conservative approach, given it underestimates precision and overestimates variance. Publication bias was examined using a visual assessment of asymmetry in the funnel plot. For meta-analyses with ≥10 studies included, Egger's Test (35) for publication bias was also performed using STATA15 (StataCorp).
Results
Study selection
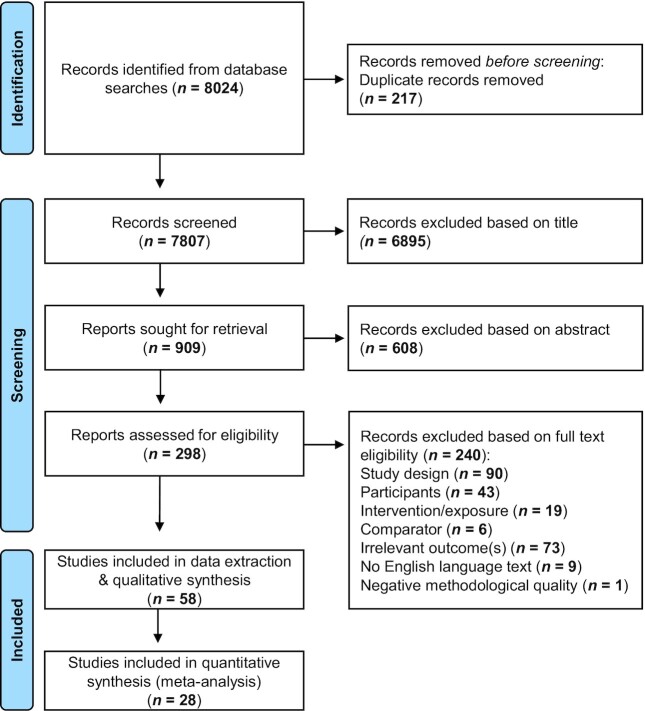
The search identified 8024 articles, and 217 duplicate articles were excluded (Figure 1). The remaining 7807 articles were reviewed for relevancy via the title, of which 909 (∼10%) were retrieved for abstract evaluation (Figure 1). Abstracts from 298 articles met the inclusion criteria, of which full texts were retrieved for further review. Full texts from 59 articles met the inclusion criteria and were assessed for methodological quality. One article received a negative quality rating according to the ADA checklist, which was excluded (36). Data extraction was completed on 58 articles.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of articles for inclusion in a systematic review of the effect of prebiotic and synbiotic supplementation on respiratory tract infections and immune function.
Study characteristics
Of the 58 studies included, 37 evaluated the effects of prebiotic or synbiotic interventions on RTIs, 7 reported the effects on NK cell activity, and 21 examined the peripheral blood immunophenotype (Figure 1). The publication year ranged from 2003 to 2021. There were 33 studies (57%) from Europe (6, 11–13, 20, 27, 37–64); 10 (17%) from Asia (9, 10, 65–72); 8 (14%) from North America (7, 8, 17, 18, 28, 50, 73, 74); 3 (5%) from South America (19, 75, 76); 1 (2%) from Australia (77); 1 (2%) from Africa (78); and 2 (3%) that included participants from multiple continents (79, 80). There were 19 studies (33%) conducted in infants, 13 (22%) in children, 19 (33%) in adults, and 7 (12%) in older adults (aged ≥ 60 y). There were 34 (59%) studies that used a prebiotic only (6–9, 17, 18, 28, 37, 38, 43, 46, 47, 50, 51, 54–58, 61, 63, 65, 66, 69–72, 74–80), 22 (38%) that used a synbiotic only (10–13, 19, 20, 39–42, 44, 45, 48, 49, 52, 53, 59, 60, 64, 67, 68, 73), 1 (2%) that had both pre- and synbiotic arms (27), and 1 (2%) that used an SCFA (62). The intervention period ranged from 5 d to 12 mo (median, 9 wk). There were 54 (93%) RCTs, 2 (3%) NRCTs, 1 (2%) retrospective cohort study, and 1 (2%) case-control trial.
Effects of prebiotic supplementation on RTIs
There were 23 studies that examined the effects of prebiotic supplementation on RTIs (6–9, 27, 37, 38, 46, 50, 51, 54, 56, 58, 61, 65, 69–72, 75, 76, 78, 80). The characteristics of these studies are presented in Table 1, of which 8 (∼35%) reported a reduction in the number of subjects experiencing ≥1 episode of at least 1 type of RTI or number of RTI episodes (6, 9, 37, 69, 72, 76, 78, 80). No studies reported an increase in the number of RTI episodes or number of subjects experiencing ≥1 episode of RTI. Four studies investigated the effect of prebiotic supplementation on the duration of RTIs (7–9, 71), of which 3 (75%) reported a reduction in the duration of RTIs following prebiotic supplementation compared to controls. One study reported an increase in the RTI duration in children receiving formula supplemented with 300 mg 2′fucosyl lactose per 100 mL (71). Of the 23 studies, 18 (78%) supplemented with an oligosaccharide, while interventions in the remaining 5 studies included rice bran exo-polymer (65), inulin (46), resistant maltodextrin (70), partially hydrolyzed guar gum (72), or an unspecified soluble fiber (76). The majority (n = 11; 48%) of studies were conducted in infants (6, 37, 38, 50, 51, 54, 56, 58, 61, 69, 78), 6 (26%) were performed in children (9, 46, 71, 75, 76, 80), and 4 (26%) were in adults (7, 8, 27, 65, 70, 72). The intervention period ranged from 3 wk to 12 mo (median, 6 mo). Out of 19 studies, 11 (48%) reported RTI cases with a doctor diagnosis, 9 (39%) studies used self- or parent-reported symptoms, and 3 (13%) studies used an investigator diagnosis.
TABLE 1.
Effect of prebiotics on respiratory tract infections in participants of all ages1
| Author, Year (Country) | Design/Level of evidence | Quality2 | Participants, age, n | Intervention, daily dose | Control, daily dose | Duration | Effect of intervention on respiratory tract infections |
|---|---|---|---|---|---|---|---|
| Infants | |||||||
| Arslanoglu et al., 2007(Germany) (37) | RCT/II | + | Healthy term infants, gestational age 37–42 wk, n = 206 | Infant formula supplemented with scGOS/lcFOS, 8 g/L | Infant formula supplemented with MDX, 8 g/L | 6 mo | ↓ number of subjects with ≥1 URTI3 episode |
| Arslanoglu et al., 2008(Germany) (6) | RCT/II | + | Healthy term infants with parental history of atopy,4 gestational age 37–42 wk, n = 134 | Infant formula supplemented with scGOS/lcFOS, 8 g/L | Infant formula supplemented with MDX, 8 g/L | 6 mo | ↓ number of URTI3 episodes |
| ↔ number LRTI3 episodes | |||||||
| Bruzzese et al., 2009(Italy) (38) | RCT/II | + | Infants with birthweight >2500 g, gestational age 37–42 wk, n = 342 | Infant formula supplemented with GOS/FOS, 4 g/L | Standard infant formula | 12 mo | ↔ number of subjects with ≥1 URTI5 episode |
| ↔ number of subjects with ≥1 LRTI3 episode | |||||||
| Moore et al., 2003(USA) (50) | RCT/II | + | Healthy term infants, 4–12 mo, n = 56 | Infant cereal supplemented with FOS, 0.75 g/25 g serve (mean ± SD, 35 ± 22.5 g/d cereal intake) | Infant cereal supplemented with MDX, 0.75 g/25 g serve (mean ± SD, 35 ± 27.5g/d cereal intake) | 4 wk | ↔ number of subjects with ≥1 URTI6 episode |
| Niele et al., 2013(The Netherlands) (51) | RCT/II | + | Infants with gestational age <32 wk and/or birthweight <1500 g admitted to NICU, n = 94 | Enteral supplementation with scGOS/lcFOS/pAOS, 1.5 g/kg (mean ± SD, 1.4 ± 0.4 kg birthweight) | Enteral supplementation with MDX, 1.5 g/kg (mean ± SD, 1.3 ± 0.3 kg birthweight) | 6 mo | ↔ number of subjects with ≥1 URTI7 episode |
| ↔ number of subjects with ≥1 LRTI8 episode | |||||||
| Paganini et al., 2017(Kenya) (78) | RCT/II | + | Infants, 6.5–9.5 mo, n = 96 | GOS powder, 7.5 g | MDX powder, 7.5 g | 4 mo | ↓ number of subjects with ≥1 RTI3 episode |
| Puccio et al., 2017(Italy) (54) | RCT/II | + | Healthy infants with gestational age 37–42 wk and birthweight 2500–4500 g, ≤2 wk, n = 175 | Infant formula containing 2′FL, 1 g/Llacto-N-neotetratose, 0.5 g/L (908 mL, mean formula intake) | Standard infant formula (929 mL, mean formula intake) | 6 mo | ↔ number of subjects with ≥1 LRTI3 episode |
| Ranucci et al., 2018(Italy) (56) | RCT/II | + | Infants with gestational age 37–42 wk and birthweight >2500 g, n = 222 | Formula containing GOS/PDX, 4 g/L | Standard formula | 48 wk | ↔ number of subjects with ≥1 RTI3 episode |
| ↔ number of subjects with ≥1 LRTI3 episode | |||||||
| Sierra et al., 2015(Spain) (58) | RCT/II | + | Healthy term infants, <2 y, n = 264 | Formula supplemented with GOS, 5 g/L | Standard formula | 12 mo | ↔ number of subjects with ≥3 URTI3 episode |
| ↔ total URTI3 episodes | |||||||
| Shahramian et al., 2018(Iran) (69) | RCT/II | Ø | Infants with gestational age 38–42 wk with birthweight >2500 g, n = 120 | Formula supplemented with scGOS/lcFOS, n/a | Standard formula | 12 mo | ↓ total URTI9 episodes |
| Westerbeek et al., 2010(The Netherlands) (61) | RCT/II | + | Infants with gestational age <32 wk and birthweight <1500 g admitted to NICU, n = 113 | Enteral preterm formula with scGOS/lcFOS/pAOS, max 1.5 g/kg (mean ± SD, 0.48 ± 0.31g/kg) | Enteral preterm formula with MDX, 1.5g/kg | 27 d | ↔ number of subjects with ≥1 LRTI10 episode |
| Children | |||||||
| Anaya-Loyola et al., 2020(Mexico) (76) | RCT/II | + | Children, 6–8 y, n = 65 | Mango juice extract containing soluble dietary fiber, 285 mg | Flavored water | 2 mo | ↓ Patient reported URTI symptoms11 |
| Chatchatee et al. 2014(Multiple) (80) | RCT/II | Ø | Children attending daycare ≥2 d/wk, 11–29 mo, n = 767 | GUM supplemented with scGOS/lcFOS, 12 g/L; Omega-3 lcPUFAs, 192 mg/L | GUM | 12 mo | ↓ number of subjects with ≥1 URTI12 episode |
| Leung et al., 2020(Hong Kong) (71) | RCT/II | + | Healthy children of Chinese ethnicity, 1–2.5 y, n = 456 | Control formula with added 100 mg Ig, 170 mg lactoferrin, 1.5 g TGF-β, 1.7 g milk fat, 281 mg linoleic acid, 300 mg 2′FL per 100 mL, 400 mL | Formula containing 2.7 g protein, 2.5 g fat (337 mg linoleic acid), 10 g carbohydrates (400 mg GOS) per 100 mL, 400 mL | 6 mo | ↔ URTI13 episodes |
| ↔ URTI13 duration | |||||||
| ↔ number of subjects with ≥1 URTI13 episode | |||||||
| Control formula with added 300 mg 2′FL per 100 mL, 400 mL | ↔ URTI13 episodes | ||||||
| ↑ URTI13 duration | |||||||
| ↔ number of subjects with ≥1 URTI13 episode | |||||||
| Li et al., 2014 (China) (9) | RCT/II | + | Children attending childcare for up to 3 mo, 3–4 y, n = 310 | Follow-up formula PDX/GOS, 3.6 gB-glucan, 26.1 mg | Standard follow-up formula | 28 wk | ↓ number of subjects with ≥1 URTI14 episode |
| ↓ URTI14 duration | |||||||
| Lohner et al., 2018(Hungary) (46) | RCT/II | + | Children attending kindergarten, 3–7 y, n = 219 | Inulin-type fructan, 6 g | MDX, 6 g | 24 wk | ↔ total URTI15 episodes |
| ↔ total LRTI16 episodes | |||||||
| Pontes et al., 2016(Brazil) (75) | RCT/II | Ø | Children attending childcare, 1–4 y,n = 97 | Follow-up formula GOS, 1.8gβ-glucan, 26.1 mg | Standard follow-up formula | 28 wk | ↔ number of subjects with ≥1 RTI17 episode |
| Adults | |||||||
| Childs et al., 2014 (UK) (27) | RCT/II, x-over | + | Healthy adults, 25–65 y, n = 42 | XOS powder, 8 g | MDX powder, 8 g | 3 wk/arm 4 wk w/o | ↔ number of subjects with ≥1 URTI7 episode |
| Choi et al., 2014 (Japan) (65) | RCT/II | + | Healthy individuals with WBC 4000–8000 cells/μL, 25–70 y, n = 77 | RBEP capsule, 18 g | Corn starch capsule, 18 g | 8 wk | ↔ number of subjects with ≥1 URTI18 episode |
| Hughes et al., 2011(USA) (7) | RCT/II | + | Healthy full-time university students, ≥18 y, n = 419 | GOS powder, 2.5 g or 5.0 g | Baker's sugar, 5 g | 8 wk | ↓ URTI17 duration19 |
| Kitagawa et al., 2020(Japan) (70) | RCT/II | + | Volunteers with BMI between 23–30 kg/m2, 20–65 y, n = 138 | Tea beverage containing resistant MDX, 5 g | Control tea beverage | 12 wk | ↔ number of subjects with ≥1 episode of cold symptoms20 |
| Langkamp-Henken et al.,2004 (USA) (8) | RCT/II | + | Individuals in assisted- and independent-living facilities, ≥65 y, n = 34 | Formula containing FOS, 4.41 g | Control formula | 6 mo | ↔ number of subjects with ≥1 URTI21 episode |
| ↓ URTI21 duration | |||||||
| Takahashi and Kozawa,2021 (Japan) (72) | RCS/III-2 | + | Patients consuming food orally who were hospitalized in the convalescent rehabilitation ward, n = 492 | PHGG, 5.2 g | Nonsupplemented | 2 mo | ↓ number of subjects with ≥1 influenza22 episode |
| Patients consuming food orally who were hospitalized in the long-term care ward, n = 30 | ↓ number of subjects with ≥1 influenza22 episode | ||||||
Abbreviations: 2′FL, 2′fucosyl lactose; FOS, fructooligosaccharide; GOS, galactooligosaccharide; GUM, growing up milk formulated milk drink; lc, long chain; LRTI, lower respiratory tract infection; MDX, maltodextrin; n/a, not available; NICU, neonatal intensive care unit; pAOS, pectin-derived acidic oligosaccharide; PDX, polydextrose; PHGG, partially hydrolyzed guar gum; RBEP, rice bran exo-polymer; RCS, retrospective cohort study; RCT, randomized controlled trial; RTI, respiratory tract infection; sc, short chain; TGF, transforming growth factor; URTI, upper respiratory tract infection; WBC, white blood cell count; w/o, washout; XOS, xylooligosaccharide; x-over, crossover study; Ø, neutral study quality; +, positive study quality; ↓ indicates decrease; ↑ indicates increase; ↔ indicates no change.
Methodological study quality was determined using the American Dietetic Association critical appraisal checklist.
Doctor diagnosis.
Parental history of atopy includes: atopic eczema, allergic rhinitis, or asthma.
Doctor-diagnosed otitis, pharyngitis, laryngitis, tracheitis, and bronchitis were considered as URTIs.
Parent-reported adverse event.
URTIs included at least 1 doctor-diagnosed episode of severe rhinitis, pharyngitis, or otitis media.
LRTIs included at least 1 physician-diagnosed episode of bronchitis, bronchiolitis, or pneumonia.
Doctor-diagnosed in the presence of symptoms of common cold, wheezing, watery nose, cough, rhinitis, and pharyngitis.
Doctor-diagnosed pneumonia with a positive bacterial culture.
Reduction in URTI symptoms of runny nose, crystalline mucus, itchy nose, itchy throat, sore nose, and sneezing. No change in congested nose, yellow mucus, bloody mucus, hoarseness, dry cough, and cough with phlegm.
Parent-reported diary of respiratory symptoms, including cough, fever, blocked or runny nose, sore throat, wheeze, and/or ear discharge.
Parent-reported episodes verified by investigators and nurses from study diary.
ARI was defined as upper respiratory infections, including common cold, pharyngitis, tonsillitis, otitis media, infectious sinusitis and rhinitis, and lower respiratory infections, including pneumonia, bronchiolitis, and bronchitis.
Acute URTIs included doctor-diagnosed common cold (rhinitis acuta, rhinosinusitis acuta), sinusitis maxillaris (acute bacterial sinusitis), pharyngitis acuta/tonsillitis acuta/tonsillopharyngitis acuta, croup (laryngotracheobronchitis/laryngotracheitis), and acute infectious laryngitis.
Acute LRTIs included doctor-diagnosed acute bronchitis/acute tracheobronchitis and pneumonia.
Acute respiratory infections comprised doctor-diagnosed URTIs, including common cold, pharyngitis, tonsillitis, otitis media, infectious sinusitis and rhinitis; and LRTIs, including pneumonia, bronchiolitis, and bronchitis.
Self-reported episodes of cold or flu-like symptoms.
Healthy weight (BMI, 18.5–24.9 kg/m2) group only.
Cold symptoms included.
Self-reported symptoms of cough, running or congested nose, sore throat, stiffness or chills, fever, achiness, and headache. Combinations of 2 or more symptoms, including 1 or more of the first 3 listed symptoms, were defined as a day of URTI symptoms. A URTI episode was defined as symptoms lasting 2 or more consecutive days, while a new URTI was defined as an initial infection separated from a new URTI episode by 5 or more symptom-free days.
Positive influenza test result using a rapid diagnostic kit.
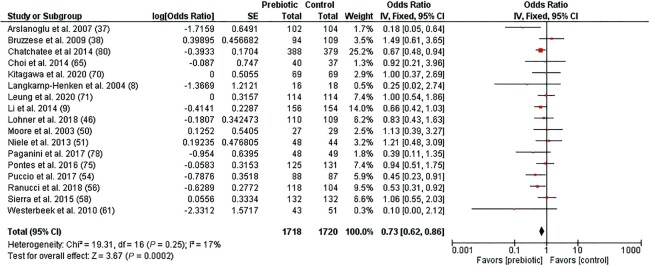
The meta-analysis indicates that prebiotic supplementation significantly decreased the odds of a subject experiencing ≥1 RTI episode compared to the placebo/control group (OR, 0.73; 95% CI: 0.62–0.86; P = 0.0002; I2 = 17%; n = 17; Figure 2). A subgroup analysis by RTI type indicated no difference between studies reporting on URTI and LRTI outcomes separately (χ2 = 0.16; P = 0.69; I2 = 0%; n = 14; Supplemental Figure 1). Egger's test was not significant (P = 0.632) for publication bias, suggesting no small study effects. In this meta-analysis, 6 studies were not included due to incidence data being reported in a different format (i.e., total RTI episodes, episodes per infant) (37, 69, 72), incidence data not being reported (i.e., study reported RTI duration only) (7), or being inappropriate for a meta-analysis (27, 72). Out of 6 of these studies, 4 (67%) reported a protective effect of prebiotic intervention on RTI episodes. The other 2 studies either reported no effect (27) or did not report on the incidence at all (7).
FIGURE 2.
Forest plot of clinical studies examining the effect of prebiotic intervention on the incidence of respiratory tract infections in participants of all ages. Individual study effect estimates (red boxes) and the pooled effect estimate (diamond) are shown. Values are ORs with 95% CIs determined using generic inverse-variance fixed-effects models. Heterogeneity was quantified by I2 at a significance of P < 0.10. Abbreviation: IV, inverse variance.
Effects of synbiotic supplementation on RTIs
Characteristics of the 15 synbiotic studies assessing RTIs are presented in Table 2 (10–12, 27, 39–41, 44, 45, 48). Five studies reported on the total number of RTI episodes during the intervention period (10, 13, 39–41), of which 2 studies reported a reduction in the total number of episodes following synbiotic supplementation compared to controls (10, 13). Ten studies reported on the number of subjects experiencing ≥1 episode of at least 1 type of RTI (11, 12, 27, 40, 44, 45, 48, 53, 67, 68), of which 3 studies reported a reduction in RTIs following synbiotic supplementation compared to controls (48, 67, 68). Three studies reported on the duration of RTIs (11, 13, 73), of which 2 (67%) reported a reduction in the RTI duration (11, 13). One study, which included 3 individual trials, found synbiotic supplementation reduced symptom severity during URTIs (13).
TABLE 2.
Effect of synbiotics on respiratory tract infections in participants of all ages1
| Author, Year (Country) | Design/Level of evidence | Quality2 | Participants, age, n | Intervention, daily dose | Control, daily dose | Duration | Effect of synbiotic on respiratory tract infections |
|---|---|---|---|---|---|---|---|
| Infants | |||||||
| Cohen et al., 2013(France) (40) | RCT/II | + | Healthy infants, 7–13 mo, n = 166 | NAN 3 follow-up formula with:Probiotic—Streptococcus thermophiles, 1 × 107 CFU/g; S. salivarius, 2.5 × 107 CFU/g; Lactobacillus rhamnosus, 1 × 107 CFU/gPrebiotic - Inulin-like fructans, n/a | NAN 3 follow-up formula | 12 mo | ↔ number of subjects with ≥1 URTI3 episode |
| ↔ total LRTI4 episodes | |||||||
| Kukkonen et al., 2008(Finland) (12) | RCT/II | + | Infants at high risk of atopy, gestational age ≥37 wk, n = 939 | Synbiotic capsule containing:Probiotic—L. rhamnosus, 9 × 109 CFU; Bifidobacterium breve, 9 × 109 CFU; Propionibacterium freudenreichii subsp. shermanii, 9 × 109 CFUPrebiotic—GOS, 0.8 g | Placebo capsule | 6 mo | ↔ number of subject with ≥1 RTI5 episode |
| Luoto et al., 2014(Finland) (48) | RCT/II | + | Infants with birthweight >1500 g, gestational age 32–36 wk, n = 47 | Probiotic—L. rhamnosus, 1 × 109 CFU (d 1–30) then 2 × 109 CFU (d 31–60)Prebiotic—PDX/GOS, 0.6 g (d 1–30) then 1.2 g (d 31–60) | Cellulose/dextrose anhydrate | 60 d | ↓ number of children with >3 viral RTI6 episodes |
| Panigrahi et al., 2017(India) (67) | RCT/II | + | Infants with suspected sepsis or possible severe bacterial infection, gestational age ≥35 wk, n = 4556 | Probiotic—Lactobacillus plantarum ATCC strain 202195, 1 × 109 CFUPrebiotic—FOS, 150 mg | MDX, 250 mg | 60 d | ↓ number of subjects with ≥1 LRTI7 requiring abx treatment |
| Picaud et al., 2010(France) (53) | RCT/II | + | Infants, 4–6 mo, n = 771 | Synbiotic formula enriched with:Probiotic— Bifidobacterium longum 1 × 107 CFU/g; S. thermophilus 1 × 106 CFU/gPrebiotic—FOS, 28 mg/g(mean ± SD, 748 ± 180 mL prescribed formula) | Standard formula (mean ± SD, 752 ± 176 mL prescribed formula) | 3 mo | ↔ number of subjects with ≥1 URTI8 episode |
| ↔ number of subjects with ≥1 LRTI9 episode | |||||||
| Children | |||||||
| Ahanchian et al., 2016(Iran) (10) | RCT/II | Ø | Children with mild persistent asthma, 6–12 y, n = 72 | Probiotic—Lactobacillus casei, L. rhamnosus, S. thermophiles, B. breve, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus bulgaricus, 1×109 CFU/capsulePrebiotic—FOS, n/a | Placebo capsule, n/a | 8 wk | ↓ total viral RTI10 episodes |
| Cazzola et al., 2010(Italy) (39) | RCT/II | + | Children, 3–7 y, n = 135 | Probiotic—Lactobacillus helveticus, B. longum subsp. infantis, Bifidobacterium bifidum, 5 × 109 CFU/capsulePrebiotic—FOS, 750 mg | Placebo capsule, n/a | 3 mo | ↔ total RTI9 episodes |
| Fox et al., 2019 (Multiple) (44) | RCT/II | Ø | Children with a clinical history or suspicion of non-IgE-mediated CMA, >13 mo, n = 35 | Formula containing:Probiotic—B. breve, 1.47 × 108 CFU/LPrebiotic - FOS/inulin, 6.3 g/L (0–6 mo, 500 mL; 6–8 mo, 450 mL; and >9 mo, 350 mL) | Control formula | 8 wk | ↔ number of subjects with ≥1 URTI11 episode |
| Gerasimov et al., 2016(Ukraine) (11) | RCT/II | + | Healthy children with household expressed symptoms of ARI, 3–12 y, n = 225 | Probiotic—L. acidophilus, 5 × 109 CFU; Bifidobacterium lactis, 5 × 109 CFUPrebiotic—FOS powder, 50 mg | MDX powder, 1 g | 2 wk | ↔ number of subjects with ≥1 RTI12 episode |
| ↓ RTI12 duration | |||||||
| Gerasimov et al., 2010(Ukraine) (45) | RCT/II | + | Children with atopic dermatitis, 12–36 mo, n = 90 | Probiotic—L. acidophilus 1 × 1010 CFU; B. lactis, 1 × 1010 CFUPrebiotic—FOS powder, 50 mg | MDX powder, 1g | 8 wk | ↔ number of subjects with ≥1 URTI9 episode |
| ↔ number of subjects with ≥1 LRTI9 episode | |||||||
| Ringel-Kulka et al., 2015(USA) (73) | RCT/II | + | Healthy children attending childcare ≥5 d/wk for >4 h, 12–48 mo, n = 149 | Synbiotic yogurt drink containing:Probiotic - S. thermophiles, 1 × 108 CFU/g; L. bulgaricus, 1 × 108 CFU/g; B. lactis, 5 × 109 CFU/g (97 g/serve)Prebiotic — Inulin, 1g | Acidified, flavored milk drink | 16 wk | ↔ URTI13 duration |
| Sazawal et al., 2010(India) (68) | RCT/II | + | Children from permanent resident families, 1–3 y, n = 624 | Milk powder fortified with:Probiotic—B. lactis, 1.9 × 109 CFUPrebiotic—Oligosaccharide, 2.4g | Unfortified milk powder | 12 mo | ↓ number of subjects with ≥1 severe LRTI14 episode |
| ↓ number of subjects with ≥1 pneumonia episode | |||||||
| Adults | |||||||
| Childs et al., 2014 (UK) (27) | RCT/II, x-over | + | Healthy adults, 25–70 y, n = 41 | Probiotic—B. lactis, 1 × 109 CFUPrebiotic—XOS powder, 8 g | MDX powder, n/a | 3 wk/arm4 wk w/o | ↔ number of subjects with ≥1 URTI15 episode |
| Coman et al., 2017 (Italy) (41) | RCT/II | Ø | Healthy adults in an intense gym-training program, 20–45 y, n = 10 | Fermented milk enriched with:Probiotic—L. rhamnosus, 1 × 109 CFU; L. paracasei, 1 × 109 CFUPrebiotic—Oat bran fiber, n/a | Fermented milk | 28 d | ↔ total URTI16 symptoms |
| Pregliasco et al., 2008(Italy) (13)Stage 1 | RCT/II | + | Healthy adults with a lifestyle favoring the development of respiratory infection, ≥18 y, n = 219 | Synbiotic powder containing:Probiotic—L. plantarum, 1 × 1010 CFU; L. rhamnosus, 1 × 1010 CFU; B. lactis, 1 × 1010 CFUPrebiotic—FOS, 3 g | MDX powder, 5 g | 90 d | ↓ URTI17 episodes |
| ↓ URTI17 severity | |||||||
| ↓ URTI17 duration | |||||||
| Pregliasco et al., 2008(Italy) (13)Stage 2 | RCT/II | + | Healthy adults with a lifestyle favoring the development of respiratory infection, ≥18 y, n = 212 | Synbiotic powder containing:Probiotic—L. plantarum, 1 × 1010 CFU; L. rhamnosus, 1 × 1010 CFU; B. lactis, 1 × 1010 CFUPrebiotic—FOS, 3 g; Lactoferrin, 0.3 g | MDX powder, 5 g | 90 d | ↓ URTI17 episodes |
| ↓ URTI17 severity | |||||||
| ↓ URTI17 duration | |||||||
| Pregliasco et al., 2008(Italy) (13)Stage 3 | RCT/II | + | Healthy adults with a lifestyle favoring the development of respiratory infection, ≥18 y, n = 250 | Synbiotic powder containing:Probiotic—L. plantarum, 5 × 109 CFU; L. rhamnosus, 5 × 109 CFU; B. lactis, 5 × 109 CFUPrebiotic—GOS, 2.5 g | MDX powder, 3.5 g | 90 d | ↓ URTI17 episodes |
| ↓ URTI17 severity | |||||||
| ↓ URTI17 duration | |||||||
Abbreviations: abx, antibiotics; ARI, acute respiratory infection; ATCC, American Type Culture Collection; CMA, cow's milk allergy; FOS, fructooligosaccharide; GOS, galactooligosaccharide; LRTI, lower respiratory tract infection; MDX, maltodextrin; n/a, not available; PDX, polydextrose; RCT, randomized controlled trial; RTI, respiratory tract infection; URTI, upper respiratory tract infection; w/o, washout; XOS, xylooligosaccharide; x-over, crossover study; Ø, neutral study quality; +, positive study quality; ↓ indicates decrease; ↔ indicates no change.
Methodological study quality was determined using the American Dietetic Association critical appraisal checklist.
Doctor diagnosis of acute otitis media, common cold, pharyngitis, or sinusitis.
Doctor diagnosis of bronchiolitis, bronchitis, pneumonia, or laryngitis.
RTI requiring hospitalization.
One or more parent-reported symptoms of fever, rhinitis, or cough along with nasal swab positive for viral RNA.
Supported diagnosis by chest X-ray or strong auscultatory findings.
Doctor diagnosis of URTIs including pharyngitis, laryngitis, and tracheitis.
Diagnosis method not described.
Doctor-diagnosed viral URTI, including acute nasopharyngitis or common cold.
Parent-reported symptoms of blocked nose, cough, and wheeze.
Parent-reported Canadian Acute Respiratory Illness and Flu Scale symptoms.
Doctor diagnosis of URTI.
Defined as respiratory rate ≥50/min.
Self-reported cold or flu-like symptoms.
Wisconsin Upper Respiratory Symptom Survey (self-reported), where 0 indicates no symptoms, 3 indicates mild symptoms, 5 indicates moderate symptoms, and 7 indicates severe symptoms, including throat soreness, sneeze, blocked or runny nose, and cough.
Self-reported symptoms of influenza-like illness, bronchitis, laryngitis, tracheitis, or common cold with a severity score where 1 indicates no symptoms; 2 indicates mild, non-persistent symptoms; 3 indicates mild, persistent symptoms; 4 indicates moderate symptoms; and 5 indicates severe symptoms.
Twelve (80%) studies used an oligosaccharide prebiotic component (10–13, 27, 39, 44, 45, 48, 53, 67, 68), while the remaining 3 studies used a polysaccharide prebiotic component (40, 41, 73). Out of 15 studies, 11 (73%) used Bifidobacterium strains in the probiotic component, with Bifidobacterium lactis being the most commonly used probiotic strain (n = 6; 55%). Out of 15 studies, 10 (67%) used Lactobacillus probiotic strains, with Lactobacillus rhamnosus the most commonly used probiotic strain (n = 7; 70%). The majority of studies were conducted in infants (12, 40, 48, 53, 67) (n = 5; 33%) and children (10, 11, 39, 44, 45, 68, 73) (n = 7; 47%), with only 3 (20%) studies conducted in adults (13, 27, 41). The intervention period ranged from 2 wk to 12 mo (median, 8 wk).
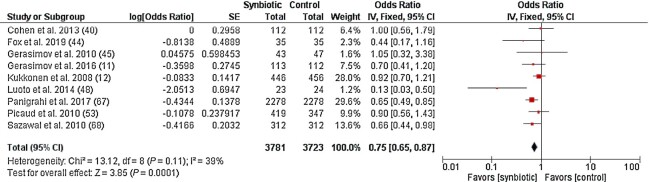
The meta-analysis showed synbiotic supplementation significantly decreased the odds of a subject experiencing ≥1 RTI episode compared to the placebo/control group (OR, 0.75; 95% CI: 0.65–0.87; P = 0.0001; I2 = 39%; n = 9; Figure 3). A subgroup analysis by RTI type indicated no difference between studies reporting on URTI and LRTI outcomes separately (χ2 = 0.04; P = 0.84; I2 = 0%; n = 6; Supplemental Figure 2). Egger's test was not significant (P = 0.328) for publication bias, suggesting no small study effects. In this meta-analysis, 6 studies were not included due to incidence data being reported in a different format (10, 13, 39, 41), incidence data not being reported (73), or being inappropriate for a meta-analysis (27). Two (∼33%) out of these studies reported a protective effect of synbiotic supplementation on RTIs (10, 13).
FIGURE 3.
Forest plot of clinical studies examining the effect of synbiotic intervention on the incidence of respiratory tract infections in participants of all ages. Individual study effect estimates (red boxes) and the pooled effect estimate (diamond) are shown. Values are ORs with 95% CIs determined using generic inverse-variance fixed-effects models. Heterogeneity was quantified by I2 at a significance of P < 0.10. Abbreviation: IV, inverse variance.
Effects of prebiotic supplementation on NK cell activity
Characteristics of the 4 prebiotic studies which examined NK cell activity are presented in Table 3 (17, 18, 47, 65). Two (50%) studies reported an increase in NK cell activity at ≥1 effector-to-target cell [E/T; ratio of effector (NK cells) to target (K562) cells] ratio (17, 18). The remaining 2 studies reported no change in NK cell activity at any E/T ratio in the intervention group (Table 3) (47, 65). Out of 4 studies, 2 used an oligosaccharide supplement (17, 18), both of which reported an increase in NK cell activity. The remaining 2 studies used a polysaccharide prebiotic intervention (47, 65). All studies were conducted in adults, with 50% of studies being conducted in older adults (aged ≥60 y) (17, 18). The intervention period ranged from 4 to 10 wk (median, 9 wk). Due to the availability of data, we could not perform a meta-analysis for this outcome.
TABLE 3.
Effect of prebiotic supplementation on NK cell activity in participants of all ages1
| Author, Year (Country) | Design/Level of evidence | Quality2 | Participants, age, n | Intervention, daily dose | Control, daily dose | Duration | Effect of prebiotic supplementation on NK cell activity |
|---|---|---|---|---|---|---|---|
| Choi et al., 2014 (Japan) (65) | RCT/II | + | Healthy individuals with WBC 4000–8000 cells/μL, 25–70 y, n = 77 | RBEP capsule, 18 g | Corn starch capsule, 18 g | 8 wk | ↔ NK cell activity3 |
| Lomax et al., 2012 (UK) (47) | RCT/II | + | Adults in good general health, 45–65 y, n = 49 | Inulin powder, 8 g | MDX powder, 8 g | 4 wk4 | ↔ NK cell activity5 |
| Vulevic et al., 2008 (USA) (17) | RCT/II, x-over | Ø | Elderly free-living subjects, 64–79 y, n = 40 | B-GOS powder, 5.5 g | MDX powder, 5.5 g | 10 wk/arm4 wk w/o | ↑ NK cell activity3,6,7,8 |
| Vulevic et al., 2015 (USA) (18) | RCT/II, x-over | Ø | Elderly volunteers, 65–80 y, n = 41 | B-GOS powder, 5.5 g | MDX powder, 5.5 g | 10 wk/arm4 wk w/o | ↑ NK cell activity3,8 |
Abbreviations: B-GOS, bimuno-galactooligosaccharide; LDH, lactate dehydrogenase; MDX, maltodextrin; PBMC, peripheral blood mononuclear cells; RBEP, rice bran exo-polymer; RCT, randomized controlled trial; WBC, white blood cell count; w/o, washout; x-over, crossover; Ø, neutral study quality; +, positive study quality; ↑ indicates increase; ↔ indicates no change.
Methodological study quality was determined using the American Dietetic Association critical appraisal checklist.
Percentage lysis was determined by LDH release following PBMC-mediated lysis of K562 target cells.
The intervention duration was 8 wk but only data from the first 4 wk were reported.
Percentage lysis was determined via flow cytometry following PBMC-mediated lysis of K562 target cells.
For 100:1 and 50:1 effector-to-target cell ratios only.
Change within group compared to baseline values.
A difference in post-intervention measurements between groups.
Effects of synbiotic supplementation on NK cell activity
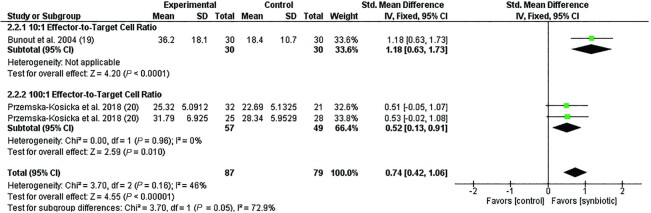
Characteristics of the 3 synbiotic studies that examined NK cell activity are presented in Table 4 (19, 20, 42). Out of 3 studies, 2 (67%) reported an increase in NK cell activity at ≥1 E/T ratio (19, 20). One study reported no change in NK cell activity at any E/T in the intervention group (42), while 1 study reported no change in NK cell activity in the younger cohort (20). Out of 3 studies, 2 used an oligosaccharide supplement (19, 20), both of which reported an increase in NK cell activity following synbiotic supplementation compared to controls. The remaining study used a polysaccharide prebiotic (42). Out of 3 studies, 2 (67%) used a Lactobacillus strain as a probiotic component of the intervention (19, 42), while the remaining study used a Bifidobacterium probiotic strain (20). All studies were conducted in adults, with intervention periods ranging from 3 to 16 wk (median, 4 wk). The meta-analysis showed synbiotic supplementation significantly increased NK cell activity compared to the control/placebo group (SMD, 0.74; 95% CI: 0.42–1.06; P < 0.0001; I2 = 46%; n = 3; Figure 4). A subgroup analysis suggests differences between studies measuring NK cell activity at different E/T ratios (Figure 4; P = 0.05).
TABLE 4.
Effect of synbiotic supplementation on NK cell activity in participants of all ages1
| Author, Year (Country) | Design/Level of evidence | Quality2 | Participants, age, n | Intervention, daily dose | Control, daily dose | Duration | Effect of synbiotic supplementation on NK cell activity |
|---|---|---|---|---|---|---|---|
| Bunout et al., 2004 (Chile) (19) | Comparative study/IIIa | + | Elderly subjects in a geriatric preventative program, >70 y, n = 60 | Nutritional supplement powder containing:Probiotic—L. paracasei, 109 CFUPrebiotic—FOS, 6 g | Nil | 16 wk | ↑ NK cell activity3,4 |
| Costabile et al., 2017 (UK) (42) | RCT/II, x-over | Ø | Healthy individuals, 60–80 y, n = 37 | Dry powder containing:Probiotic— Lactobacillus rhamnosus, 1010 CFUPrebiotic—SCF, 6 g | MDX powder, 6 g | 3 wk/arm 2 wk w/o | ↔ NK cell activity5 |
| Przemska-Kosicka et al., 2018 (UK) (20) | RCT/II | + | Healthy elderly subjects, 60–85 y, n = 53 | Probiotic— Bifidobacterium longum, 109 CFUPrebiotic—Gl-OS, 8 g | MDX, 9 g | 4 wk | ↑NK cell activity5– 7 |
| Healthy subjects, 18–35 y, n = 53 | ↔NK cell activity5 |
Abbreviations: FOS, fructooligosaccharide; Gl-OS, glucooligosaccharide; MDX, maltodextrin; PBMC, peripheral blood mononuclear cells; RCT, randomized controlled trial; SCF, soluble corn fiber; w/o, washout; x-over, crossover; Ø represents neutral study quality; + represents positive study quality; ↑ indicates increase; ↔ indicates no change.
Methodological study quality was determined using the American Dietetic Association critical appraisal checklist.
Percentage lysis was determined by 51Cr release following PBMC-mediated lysis of K562 target cells.
Difference in change compared to baseline between groups.
Percentage lysis was determined via flow cytometry following PBMC-mediated lysis of K562 target cells.
For 100:1 effector-to-target cell ratios only.
Change within group compared to baseline values.
FIGURE 4.
Forest plot of clinical studies examining the effect of synbiotic supplementation on NK cell activity in participants of all ages. Individual study effect estimates (red boxes) and the pooled effect estimate (diamond) are shown. Values are standardized mean differences with 95% CIs determined using generic inverse-variance fixed-effects models. Heterogeneity was quantified by I2 at a significance of P < 0.10. The first entry in this meta-analysis for Przemska-Kosicka et al. (20) is the younger cohort (18–35 y), with the second entry including subjects of the older cohort (60–85 y). Abbreviation: IV, inverse variance.
Effects of prebiotic supplementation on peripheral immune cell populations
Characteristics of the 13 studies examining the effects of a prebiotic intervention on immune cell populations in whole blood are described in Table 5 (9, 27, 28, 43, 47, 55, 57, 63, 66, 74, 75, 77, 79), of which 10 studies (77%) reported a change in ≥1 immune cell population. Out of the 13 studies, 6 (46%) used an oligosaccharide intervention (9, 27, 43, 55, 75, 79), 6 used a polysaccharide supplement (28, 47, 57, 63, 66, 74), and 1 (8%) included multiple soluble fiber types in a dietary strategy (77). Of the 13 studies, 8 (62%) were conducted in adults (27, 28, 43, 47, 57, 63, 66, 77), 3 (23%) in children (9, 74, 75), and 2 (15%) in infants (55, 79). The intervention period ranged from 5 d to 28 wk (median, 6 mo).
TABLE 5.
Effect of prebiotic supplementation on immune cell populations in peripheral blood in participants of all ages1
| Author, Year (Country) | Design/Level of evidence | Quality2 | Participants, age, n | Intervention, daily dose | Control, daily dose | Duration | Effect of prebiotic intervention on peripheral immune cell populations |
|---|---|---|---|---|---|---|---|
| Infants | |||||||
| Boyle et al., 2016 (Multiple) (79) | RCT/II | + | Infants with parental atopy, ≥36 wk gestational age, n = 863 | Infant formula containing: scGOS/lcFOS, 6.8 g/LpAOS, 1.2 g/L | Standard infant formula | 6 mo | ↑ Regulatory T cells (%)3 |
| ↑Plasmacytoid DC (%)3 | |||||||
| Raes et al., 2010 (Belgium) (55) | RCT/II | + | Healthy infants, 37–42 wk gestational age, n = 156 | Infant formula supplemented with scGOS/lcFOS, 6 g/L | Standard infant formula | 26 wk | ↔ CD2+ cells (%) |
| ↔ CD3+ cells (%) | |||||||
| ↔ CD4+ cells (%) | |||||||
| ↔ CD4+ CD25+ cells (%) | |||||||
| ↔ CD8+ cells (%) | |||||||
| ↔ CD8+ CD38+ cells (%) | |||||||
| ↔ CD8+ CD25+ cells (%) | |||||||
| ↔ CD19+ (B) cells (%) | |||||||
| ↔ CD22+ cells (%) | |||||||
| ↔ CD38+ cells (%) | |||||||
| ↔ NK cells (%) | |||||||
| Children | |||||||
| Li et al., 2014 (China) (9) | RCT/II | + | Children attending day care, 3–4 y, n = 264 | Follow-up formula containing: PDX/GOS, 3.6 g; β-glucan, 26.1 mg | Nonenriched cow's milk–based beverage | 28 wk | ↑ Leukocyte count3,4 |
| ↔ Neutrophils (%) | |||||||
| ↔ Lymphocytes (%) | |||||||
| Pontes et al., 20165 (Brazil) (75) | RCT/II | Ø | Children attending childcare, 1–4 y, n = 97 | Follow-up formula containing: GOS, 1.8 g; β-glucan, 26.1 mg | Isocaloric, nonsupplemented cow's milk–based beverage | 28 wk | ↔ Leukocyte count |
| ↔ Neutrophil (%) | |||||||
| ↔ Lymphocyte (%) | |||||||
| Adults | |||||||
| Bloomer et al., 2020 (USA) (74) | RCT/II | Ø | Volunteers that partake in ≥2 times per week for ≥30 min, 20–65 y, n = 75 | Advanced Ambrotose (aloe vera inner leaf gel, arabinogalactan, ghatti gum, glucosamine HCl, gum tragacanth, vitamin A, β-carotenem wakame algae extract, and rice starch), 2 g or 4 g | MDX | 8 wk | ↔ Leukocyte count |
| ↓ Lymphocytes (%)6,7 | |||||||
| ↔ Granulocytes (%) | |||||||
| ↓ Monocytes (%)6,7 | |||||||
| ↓ Monocyte count6,7 | |||||||
| Ambrotose LIFE (aloe vera inner leaf gel, arabinogalactan, ghatti gum, glucosamine HCL, gum tragacanth, vitamin A, β-carotenem wakame algae extract, and rice starch, rice bran, citrus pectin with sodium alginate), 2 g or 4 g | ↔ Leukocyte count | ||||||
| ↔ Lymphocytes (%) | |||||||
| ↔ Granulocytes (%) | |||||||
| ↓ Monocytes (%)7,8 | |||||||
| ↓ Monocyte count7,8 | |||||||
| Childs et al., 2014 (UK) (27) | RCT/II, x-over | + | Healthy volunteers with BMI 25–30 kg/m2, 25–65 y, n = 79 | XOS powder, 8 g | MDX powder, 8 g | 21 d/arm 28 d w/o | ↓ CD16/56 on NKT cells7 |
| ↔ T cells (%) | |||||||
| ↔ Helper T cells (%) | |||||||
| ↔ Cytotoxic T cells (%) | |||||||
| ↔ NKT cells (%) | |||||||
| ↔ NK cells (%) | |||||||
| ↔ B cells (%) | |||||||
| Clarke et al., 2016 (Canada) (28) | RCT/II, x-over | + | Healthy volunteers, 18–50 y, n = 30 | Inulin powder, 15 g | MDX powder, 15 g | 28 d/arm 2 wk w/o | ↑ TLR2+ mDC3 |
| ↑ TLR4+ mDC3 | |||||||
| ↔ T cells (%) | |||||||
| ↔ Helper T cells (%) | |||||||
| ↔ Cytotoxic T cells (%) | |||||||
| ↔ B cells | |||||||
| ↔ NK cells (%) | |||||||
| ↔ γδ T cells (%) | |||||||
| ↔ Monocytes (%) | |||||||
| ↔ Granulocytes (%) | |||||||
| Elison et al., 20165 (Denmark) (43) | RCT/II | Ø | Healthy volunteers, 18–60 y, n = 40 | HMOs (2’FL, LNnT) powder, 5, 10, or 20 g | Glucose powder, 5, 10, or 20 g | 14 d | ↓ Monocyte count7,9 |
| ↔ Lymphocyte count | |||||||
| ↔ Granulocyte count | |||||||
| Farhangi et al., 2018 (Iran) (66) | RCT/II | + | Female type 2 diabetics with BMI 25–35 kg/m2, 30–50 y, n = 55 | Nutriose 06 (maize supplement powder), 10 g | MDX powder, 10 g | 8 wk | ↑ Monocytes (%)7 |
| ↑ Cytotoxic T cells (%)7 | |||||||
| ↔ Neutrophils (%) | |||||||
| ↔ Lymphocytes (%) | |||||||
| ↔ Helper T cells (%) | |||||||
| ↔ CD4/CD8 ratio | |||||||
| Gill et al., 2020 (Australia) (77) | RCT/II, x-over | + | Healthy volunteers, 18–65 y, n = 10 | High-fiber diet containing resistant starch, 6.7 g; oligosaccharides, 6.8 g (fructans, 3.9 g, GOS, 2.8 g) | Low-fiber diet containing resistant starch, 2.1 g; oligosaccharides, 1.2 g (fructans, 0.9 g; GOS, 0.2 g) | 5 d | ↔ CD4+ T cells (%) |
| ↔ Regulatory T cells (% CD4+ cells) | |||||||
| Kiewiet et al., 2020(The Netherlands) (63) | RCT/II | + | Healthy Caucasian subjects, 55–80 y, n = 26 | Inulin, 8 g | Glucose, 5 g | 7 d | ↔ Helper T cells (%) |
| ↔ Cytotoxic T cells (%) | |||||||
| ↔ Regulatory T cells (% CD4+ cells) | |||||||
| ↔ TH1 cells (% CD4+ cells) | |||||||
| ↔ Memory T cells (%) | |||||||
| ↔ Memory TH1 cells (%) | |||||||
| Lomax et al., 2012 (UK) (47) | RCT/II | + | Adults in good general health, 45–65 y, n = 49 | Inulin powder, 8 g | MDX powder, 8 g | 4 wk10 | ↑ CD8+ cells3 |
| ↑ CD3+/CD8+ cells3 | |||||||
| ↓ CD4/CD83 | |||||||
| ↔ CD3+/CD4+ cells (%) | |||||||
| ↔ CD4+ cells (%) | |||||||
| ↔ CD3–CD16+ (%) | |||||||
| ↔ CD3–CD19+ (%) | |||||||
| ↔ CD14+ cells (%) | |||||||
| ↔ CD4+/CD25+/CD127low/– (%) | |||||||
| Seidel et al., 2007 (Germany) (57) | RCT/II, x-over | + | Males, ≥18 y, n = 19 | Prebiotic bread containing: inulin, 4 g; soy fiber, 6 g | Control bread | 5 wk/arm no w/o | ↑ Lymphocyte count 7 |
| ↑ Granulocyte count 7 | |||||||
| ↑ CD3+ HLA-DR+ cells 7 | |||||||
| ↓ NK cell count 7 | |||||||
| ↔ Leukocyte count | |||||||
Studies were determined peripheral blood cell populations using flow cytometry unless otherwise specified. Abbreviations: 2′FL, 2′fucosyl lactose; DC, dendritic cell; FOS, fructooligosaccharide; GOS, galactooligosaccharide; HLA-DR+ human leukocyte antigen-DR positive; HMO, human milk oligosaccharide; lc, long chain; LNnT, lacto-N-neotetraose; mDC, myeloid dendritic cell; MDX, maltodextrin; NKT, natural killer T; pAOS, pectin-derived acidic oligosaccharide; PDX, polydextrose; RCT, randomized controlled trial; TH1, type 1 T helper; TLR, toll-like receptor; sc, short chain; w/o, washout period; XOS, xylooligosaccharide; x-over, crossover; Ø represents neutral study quality; + represents positive study quality; ↓ indicates decrease; ↑ indicates increase; ↔ indicates no change.
Methodological study quality was determined using the American Dietetic Association critical appraisal checklist.
Difference in postintervention measurements between groups.
Difference in change compared to baseline between groups.
Outcome determined by differential cell count.
Advanced Ambrotose at 2 g.
Change within group compared to baseline values.
Advanced LIFE at 4 g/d.
Change in 5-g and 10-g intervention groups only.
The intervention period was 8 wk, but only data from the first 4 wk were reported.
Adaptive immune cell subsets
Four studies (31%) reported on the total white blood cell count, of which 1 study showed an increase in the leukocyte count following 28 wk of polydextrose and GOS supplementation compared to controls (9). Seven studies reported on the effects of prebiotics on the proportion of helper T cells, determined using CD3+ and CD4+ markers (27, 28, 47, 55, 63, 66, 77). None of these studies reported a change in the helper T cell proportion following prebiotic supplementation. Six studies reported on changes to the cytotoxic T cell population, determined using CD3+ and CD8+ markers (27, 28, 47, 55, 63, 66). Out of 6 studies, 2 reported an increase in the population of cytotoxic T cells following prebiotic supplementation (47, 66), both of which were conducted in adults with a polysaccharide prebiotic intervention. The remaining 3 studies reported no change in the proportion of cytotoxic T cells. Five studies reported on the population of Tregs following prebiotic supplementation, determined using CD4+, CD25+, and Foxp3+ or CD127low/– markers (47, 55, 63, 77, 79). Out of 5 studies, 1 (20%) showed an increase in the proportion of Tregs in term infants with a parental history of atopy after 6 mo of consuming an oligosaccharide supplement (79). The other 4 studies reported no change in the proportion of Tregs following prebiotic supplementation compared to controls. One study reported an increase in the proportion of activated T cells, identified using CD3+ and human leukocyte antigen-DR positive (HLA-DR+) markers, following 5 wk of supplementation with prebiotic bread in adult males (57). Another study reported a decrease in the expression of CD16/56 on NKT cells compared to baseline following 8 g/d of XOS supplementation for 3 wk, despite no change in the proportion of NKT cells (27). Four studies reported on the effects of prebiotic supplementation on the B cell population, determined using CD3– and CD19+ markers (27, 28, 47, 55), none of which reported a change in the proportion of B cells following a prebiotic intervention.
Innate immune cell subsets
Five studies examined the effects of prebiotic supplementation on changes in the NK cell population, determined using CD3–, CD16+, and CD56+ markers (27, 28, 47, 55, 57). One study reported a decrease in the NK cell count following 5 wk of inulin and soy fiber supplementation compared to baseline (57), but not compared to controls. However, the other 4 studies that reported on the proportion of NK cells found no change following prebiotic supplementation. Five studies reported on the effects of prebiotic supplementation on the peripheral monocyte population, determined using CD14+ markers (28, 43, 47, 66, 74). Out of 5 studies, 3 (60%) reported a decrease in either the monocyte count or monocyte percentage following prebiotic intervention compared to controls (43, 66, 74). The remaining 2 studies found no change in the monocyte population following prebiotic supplementation. One study reported increases in TLR2+ and TLR4+ on mDCs following 4 wk of supplementation with 15 g/d inulin (28). Three studies examined the effects of prebiotic supplementation on the peripheral neutrophil population (9, 66, 75), all of which reported no change following prebiotic supplementation.
The effect of prebiotic supplementation compared to a control/placebo on peripheral blood immune cell subsets was investigated via meta-analyses. Results from these meta-analyses indicate prebiotic supplementation did not significantly change the proportion of any cell population (Supplemental Figures 3–5).
Effects of synbiotic supplementation on peripheral immune cell populations
Characteristics of the 8 studies examining the effects of prebiotic interventions on immune cell populations in whole blood are described in Table 6 (19, 27, 45, 49, 52, 59, 60, 64), and 4 (50%) of the studies reported a change in ≥1 immune cell population (19, 45, 52, 64). Out of 8 studies, 7 (88%) used an oligosaccharide as the prebiotic component of the synbiotic. However, the dosage varied considerably, from 50 mg to 16 g (median, 7 g). The remaining study used arabinogalactan as the prebiotic component. Studies primarily used Bifidobacteria (n = 4; 50%) and Lactobacillus (n = 6; 75%) strains as the probiotic components of supplements. Of the 8 studies, 6 (75%) were conducted in adults (19, 27, 49, 52, 59, 64) and the remaining 2 were performed in infants and children (45, 60). The intervention period ranged from 3 to 16 wk (median, 5 wk).
TABLE 6.
Effect of synbiotic supplementation on immune cell populations in peripheral blood in participants of all ages1
| Author, Year (Country) | Design/Level of evidence | Quality2 | Participants, age, n | Intervention, daily dose | Control, daily dose | Duration | Effect of synbiotic intervention on peripheral immune cell populations |
|---|---|---|---|---|---|---|---|
| Infants | |||||||
| van der Aa et al., 2012(The Netherlands) (60) | RCT/II | + | Full-term infants aged 0–7 mo with atopic dermatitis, n = 65 | Whey-based formula containing:Probiotic—B. breve, 1.3 × 1010 CFU/LPrebiotic—scGOS/lcFOS, 8g/L(mean ± SD, 778 ± 135 mL/d formula intake) | Nonsupplemented whey-based infant formula (mean ± SD, 760 ± 148ml/d formula intake) | 12 wk | ↔ CD3+ cells (%) |
| ↔ CD4+ CD8- cells (%) | |||||||
| ↔ FOXP3+ CD25+ cells (%) | |||||||
| ↔ Regulatory T cells (%) | |||||||
| Children | |||||||
| Gerasimov et al., 2010 (Ukraine) (45) | RCT/II | + | Healthy children with household expressed symptoms of ARI, 3–12 y, n = 225 | Probiotic— Lactobacillus acidophilus, 5 × 109 CFU; Bifidobacterium lactis, 5 × 109 CFUPrebiotic—FOS powder, 50 mg | MDX powder, 1 g | 8 wk | ↑ Cytotoxic T cells (%)3 |
| ↓ CD4+ cells (%)3 | |||||||
| ↓ CD25+ cells (%)3 | |||||||
| ↔ CD16+ cells (%) | |||||||
| ↔ CD22+ cells (%) | |||||||
| Adults | |||||||
| Bunout et al., 2004 (Chile) (19) | RCT/II | + | Elderly subjects in a geriatric preventative program, >70 y, n = 60 | Nutritional supplement powder containing:Probiotic—L. paracasei, 109 CFUPrebiotic—FOS, 6 g | Nil | 16 wk | ↑ T cells with NK activity (%)3 |
| ↔ T cells (%) | |||||||
| ↔ B cells (%) | |||||||
| ↔ Helper T cells (%) | |||||||
| ↔ Cytotoxic T cells (%) | |||||||
| ↔ Monocytes (%) | |||||||
| ↔ CD4/CD8 ratio | |||||||
| Childs et al., 2014 (UK) (27) | RCT/II, x-over | + | Healthy volunteers with BMI 25–30 kg/m2, 25–65 y n = 77 | Probiotic—B. lactis, 1 × 109 CFUPrebiotic—XOS powder, 8 g | MDX powder, 8 g | 21 d/arm 28 d w/o | ↔ T cells (%) |
| ↔ Helper T cells (%) | |||||||
| ↔ Cytotoxic T cells (%) | |||||||
| ↔ NKT cells (%) | |||||||
| ↔ NK cells (%) | |||||||
| ↔ B cells (%) | |||||||
| Macfarlane et al., 20134(Scotland) (49) | RCT/II, x-over | Ø | Healthy volunteers with BMI 18.5–30 kg/m2, 65–90 y, n = 42 | Synbiotic capsule containing:Probiotic— Bifidobacterium longum, 2 × 1011 CFUPrebiotic—inulin/FOS, 12 g | Capsule containing: Potato starch; MDX, 12 g | 4 wk/arm 4 wk w/o | ↔ Leukocyte count |
| ↔ Neutrophil count | |||||||
| Nova et al., 2011 (Spain) (52) | RCT/II | + | Healthy individuals aged 25–45 y, n = 36 | Synbiotic tablet containing:Probiotic—L. acidophilus, Bifidobacterium animalis, L. bulgaricus, S. thermophiles, and L. paracasei, 2.4 × 109 CFUPrebiotic—FOS, 1.4 g | Placebo tablet | 6 wk | ↑ Helper T cells (%)3 |
| ↔ Cytotoxic T cells (%) | |||||||
| ↔ NK cells (%) | |||||||
| ↔ CD4/CD8 ratio | |||||||
| van de Pol et al., 2011(The Netherlands) (59) | RCT/II | + | Adult allergic patients with intermittent to mild asthma, n = 29 | Food supplement containing:Probiotic—B. breve, 2 × 1010 CFUPrebiotic—scGOS/lcFOS, 16 g | Food supplement containing MDX, 18 g | 4 wk | ↔ Regulatory T cells |
| Velikova et al., 20205 (Bulgaria) (64) | CC/III-2 | Ø | Healthy subjects, n = 20 | 4 × 400 mg capsule containing:Probiotic (67.5%): L. acidophilus LLA-10, L. helveticus LLH-108, Lactobacillus rhamnosus LLR-L1, Lactobacillus fermentum LLF-01, and L. bulgaricus LLB-06.Prebiotic (37.5%): arabinogalactan | n/a | 21 d | ↔ Total NK cells |
| ↑ Activated (HLA-DR+) NK cells5 | |||||||
| Patients with IBS according to Rome III criteria, n = 20 | ↔ Total NK cells | ||||||
| ↔ Activated (HLA-DR+) NK cells | |||||||
Studies determined peripheral blood cell populations using flow cytometry unless otherwise specified.
Abbreviations: ARI, acute respiratory infection; CC, case-control study; FOS, fructooligosaccharide; FOXP3, forkhead box P3; GOS, galactooligosaccharide; HLA-DR+; human leukocyte antigen-DR positive; IBS, irritable bowel syndrome; lc, long chain; MDX, maltodextrin; n/a, not available; NKT, natural killer T; RCT, randomized controlled trial; sc, short chain; x-over, crossover; w/o, washout; XOS, xylooligosaccharide; Ø represents neutral study quality; + represents positive study quality; ↓ indicates decrease; ↑ indicates increase; ↔ indicates no change.
Methodological study quality was determined using the American Dietetic Association critical appraisal checklist.
Difference in postintervention measurements between groups.
Outcome determined by differential cell count.
Change within group compared to baseline values.
Adaptive immune cell subsets
Five studies reported on changes in the helper T cell population, determined using CD4+ and CD8+ markers (19, 27, 45, 52, 60). One study reported an increase in the proportion of helper T cells following 6 wk of synbiotic supplementation in healthy adults (52), while another showed a decrease in the percentage of helper T cells following 2 wk of synbiotic supplementation in children (45). Four studies reported on changes in the cytotoxic T cell population, determined using CD3+ and CD8+ markers (19, 27, 45, 52). One study reported an increase in the proportion of cytotoxic T cells following 2 wk of synbiotic supplementation compared to placebo (45). The remaining 3 studies reported no change in the population of cytotoxic T cells. Two studies reported on changes to the population of Tregs following synbiotic supplementation (59, 60), neither of which reported a significant change in the proportion of Tregs following intervention. One study reported an increase in the proportion of T cells with NK activity compared to controls following 16 wk of supplementation with 109 CFU/d of Lactobacillus paracasei and 6 g/d of FOS in older adults (19). Results from the meta-analyses indicate synbiotic supplementation did not significantly change the proportions of the helper T cell populations (Supplementary Figure 6). However, a meta-analysis indicated the proportion of cytotoxic T cells increased following synbiotic supplementation compared to control/placebo (mean difference, 4.85; 95% CI: 2.27–7.43; P = 0.0002; I2 = 57%; n = 3; Supplementary Figure 7).
Innate immune cell subsets
Three studies reported on changes in the NK cell population, determined using CD3–, CD16+, and CD56+ markers (27, 52, 64). None of the studies reported a change in the percentage of NK cells following synbiotic supplementation. However, 1 study reported an increase in the proportion of activated (HLA-DR+) NK cells compared to baseline following 3 wk of supplementation in healthy subjects (64).
Effects of SCFA supplementation on peripheral immune cell populations
Characteristics of the 1 study using an oral SCFA intervention are reported in Table 7 (62). This study reported no changes in immune cell populations following a 4-wk intervention with 4 g/d of oral sodium butyrate capsules.
TABLE 7.
Effect of SCFA supplementation on immune cell populations in peripheral blood in participants of all ages1
| Author, Year (Country) | Design/Level of evidence | Quality2 | Participants, age, n | Intervention, daily dose | Control, daily dose | Duration | Effect of SCFA intervention on peripheral immune cell populations |
|---|---|---|---|---|---|---|---|
| de Groot et al., 2020 (The Netherlands) (62) | RCT/II, x-over | Ø | Subjects with longstanding type 1 diabetes with BMI 19–25 kg/m2, 18–45 y, n = 30 | Sodium butyrate capsules, 4 g | Placebo | 4 wk/arm4 wk w/o | ↔ CD8+ T cells (×1000 PBMC) |
| ↔ β7/CD49d CD8+ T cells (×1000 PBMC) | |||||||
| ↔ CXCR3/CCR5 CD8+ T cells (×1000 PBMC) | |||||||
| ↔ CD4+ T cells (×1000 PBMC) | |||||||
| ↔ β7/CD49d CD4+ T cells (×1000 PBMC) | |||||||
| ↔ CXCR3/CCR5 CD4+ T cells (×1000 PBMC) | |||||||
| ↔ B cells (×1000 PBMC) | |||||||
| ↔ NK cells (×1000 PBMC) | |||||||
| ↔ Regulatory T cells (×1000 PBMC) |
Outcomes determined peripheral blood cell populations using flow cytometry. Abbreviations: CCR5, C-C chemokine receptor type 5; CXCR3, C-X-C motif chemokine receptor 3; PBMC, peripheral blood mononuclear cells; RCT, randomized controlled trial; x-over, crossover; Ø represents neutral study quality; + represents positive study quality. ↓ indicates decrease; ↑ indicates increase; ↔ indicates no change.
Methodological study quality was determined using the American Dietetic Association critical appraisal checklist.
Discussion
This systematic review and meta-analysis aimed to investigate the effects of prebiotic, synbiotic, and SCFA supplementation on the incidences of RTIs and markers of immune function (including innate immune cell activity and peripheral immune cell populations). We found that ∼45% of studies (n = 4 in infants, n = 3 in children, n = 3 in adults) reported a protective effect of prebiotic supplementation on the incidence and/or duration of RTIs. The remaining studies (n = 7 in infants, n = 3 in children, n = 3 in adults) did not show any effect of prebiotic supplementation on the incidence and/or duration of RTIs. Out of 23 studies, 17 (∼75%) were included in meta-analysis examining the effects of prebiotic supplementation on RTIs, indicating prebiotic supplementation reduced the odds of an RTI. Given the majority of studies excluded from the meta-analyses showed a protective effect of the prebiotic on RTIs, it is unlikely the difference between qualitative and quantitative analyses is due to the direction of outcomes between excluded and included studies. We found 40% (n = 6) of synbiotic studies reported a protective effect on the incidence, severity, and/or duration of RTIs. The remaining studies (n = 9) did not show any effect of synbiotic supplementation on RTIs. Out of 15 studies, 9 (60%) were included in a meta-analysis that indicated synbiotic supplementation reduced the odds of an RTI. The proportions of studies included and excluded from the meta-analysis reporting a protective effect were similar (∼33%; included, n = 3/9; excluded, n = 2/6). The effect of oral SCFA supplementation on the prevention of RTIs was unable to be determined, as no studies were identified to evaluate this aim of the review.
The protective effects of pre- and synbiotic interventions on RTIs observed in the meta-analysis, particularly in infants and children, are consistent with evidence in the literature. A recent systematic review examining the effect of probiotic supplementation on RTIs in individuals attending childcare reported a reduced risk in experiencing ≥1 RTI (5). Furthermore, evidence suggests that the consumption of pre-, pro-, and synbiotics alters the microflora of formula-fed infants to be similar to that of breastfed infants (81, 82), which has been associated with a reduced risk of RTIs (83, 84). While there appears to be a benefit for supplementation in infants and children, there is insufficient evidence to establish a clear effect in the adult population. This lack of evidence may be due to the limited number of studies that have investigated the effects of pre- and synbiotic interventions on RTIs in this population. Furthermore, the current evidence was not sufficient to suggest whether prebiotic or synbiotic supplementation was more effective at protecting against RTIs. It is hypothesized that the combination of a probiotic with a prebiotic is more effective than use of a prebiotic or probiotic alone, given synbiotics provide both the “health-promoting” bacteria and the substrate for fermentation (85). However, there is limited evidence from clinical trials to support this theoretical view. Future research should investigate whether prebiotic or synbiotic supplementation is more effective at conferring protection against RTIs.
The protective doses and durations of prebiotics and synbiotics associated with prevention of RTIs are unclear, and variable across age categories. Interestingly, both studies by Arslanoglu et al. (6, 37) reported protective effects of prebiotic supplementation on RTIs, using oligosaccharide supplementation of infant formula at a dose of 8 g/L for 6 mo. However, the reporting of dose, particularly in infant studies, is unclear. Out of 11 studies in infants, 10 (∼91%) used a prebiotic-supplemented formula or cereal as the intervention, with only 4 (40%) of those 10 studies providing adequate information to calculate the total daily prebiotic intake. Hence, variability in formula intake across different studies, in conjunction with different prebiotic concentrations, potentially leads to differences in total daily intakes of prebiotics, which cannot be accounted for using published data. In the adult population, the intervention durations were short in comparison to studies conducted in infants and children. This may contribute to a lack of evidence in our narrative synthesis supporting pre- and synbiotic supplementation for the prevention of RTIs in this population. Further, prebiotic and synbiotic studies in adults used intervention periods of 3 wk to 6 mo and 3 wk to 90 d, respectively. It is unlikely these study periods are long enough to see true differences in RTI incidences between groups.
A number of mechanisms have been hypothesized that may explain the protective effects of prebiotics and synbiotics, and the products of their microbial fermentation, against RTIs. These include increased NK cell activity, effector T cell differentiation, and Treg expansion (86). The mechanisms by which prebiotics and synbiotics exert these effects include postbiotic mechanisms, including HDAC inhibition and FFAR activation by SCFAs, as well as a direct interaction between prebiotics and carbohydrate receptors (e.g., galectins, C-type lectin receptors, and mannose receptors) that impacts immune cells (87). Meta-analyses from this systematic review indicate that the cytotoxicity of ex vivo NK cells was increased following both pre- and synbiotic supplementation compared to controls. Interestingly, oral probiotic supplementation in mice has been shown to be protective against an influenza challenge, an effect associated with increased NK cell activity in the lungs and spleen (88, 89). However, whether oral pre- or synbiotic supplementation in humans alters NK cell activity in the lungs has not yet been investigated.
There was limited evidence to suggest that pre- or synbiotic supplementation altered peripheral blood immunophenotypes. This is similar to the outcome of a recent systematic review that found no effect of probiotic supplementation on circulating immune cells and inflammatory markers in healthy adults (90). However, qualitative analysis of individual studies in this review suggests that pre- and synbiotic supplementation may have effects on the maturation and function of immune cells. Velikova et al. (64) reported the proportion of activated NK cells increased following 3 wk of synbiotic supplementation, despite no change in the total number of NK cells. Childs et al. (27) showed that 3 wk of supplementation with XOS reduced the expression of CD16 and CD56 on NKT cells compared to baseline (27). Given this study reported a decrease in type 2 inflammation, it suggests a drive towards a TH1 immune response, which may be relevant in the context of the elderly or excessive type 2 immunity, such as allergic asthma. Similarly, Clarke et al. (28) reported that the expression of TLR2/4 was increased on mDC following 4 wk of inulin supplementation compared to placebo (28). Interestingly, evidence from animal models has shown a protective effect of increased TLR2 and TLR4 expression following probiotic supplementation in the context of intestinal immunity (91). However, there is a lack of evidence available to determine whether changes in TLR expression on circulating APCs are protective in human subjects or in the context of an infection that originates outside of the gastrointestinal tract.
The strengths of this review include a wide-ranging literature search, well-defined inclusion and exclusion criteria, prospective protocol registration, and comprehensive data extraction methods and analyses. All studies were assessed for methodological quality using a validated checklist (31), with all negative-quality articles removed from analysis. Furthermore, the majority of studies are categorized as level II evidence according to the NHMRC evidence hierarchy (32). Also, the majority of studies defined an RTI on the basis of a doctor diagnosis. Despite a number of strengths, this systematic review had some limitations. The scope of this systematic review was wide, where the effects of interventions were examined for multiple outcomes. However, the meta-analyses of multiple outcomes could have contributed to a type I error. The diet composition is an important confounding element of these interventions, as many foods contain both prebiotic (e.g., fruits, vegetables, cereals) and probiotic (e.g., cheese, live yogurt, fermented food products) components. Despite all studies excluding subjects who were currently taking a prebiotic, probiotic, or synbiotic supplement, only 22 out of 34 (65%) prebiotic studies and 9 out of 22 (41%) synbiotic studies reported, accounted for, or controlled the background diet. Future research should perform appropriate dietary assessments (e.g., FFQ, 24-h food recalls, 7-d food records, etc.) and account for this during analyses. Included studies were variable in terms of the dose, sample size, duration, and participant age. Limited evidence is available for prebiotic and synbiotic supplementation in both adults and children for the prevention of RTIs. Thus, future research should investigate whether a protective effect exists for these populations. Meta-analyses included fewer studies compared to the qualitative data synthesis, due to a lack of usable data. Hence, these analyses are subject to some outcome reporting bias. For example, some studies reported the number of RTI episodes without taking into consideration subjects experiencing more than 1 episode during the study period, while others only reported the RTI duration and/or severity. Further, whether outcomes were reported differently depending on the direction or significance of an outcome (i.e., selective reporting bias) is unclear.
Future studies are required, including adequately powered clinical trials with longer supplementation periods for studies reporting on RTIs, particularly in the adult population. Dietary fiber intake should either be controlled for or assessed and accounted for during analyses. Further, little research to date has examined the effects of dose frequency on RTI or immune-related outcomes. The immunomodulatory effects of prebiotics and synbiotics are partially attributed to the generation of postprandial SCFA. However, these are rapidly metabolized, with peak circulating SCFA occurring at 4–5 h following soluble fiber consumption (92, 93). Therefore, future studies should examine whether maintaining elevated circulating SCFAs via multiple doses per day is of greater benefit compared to a single daily dose.
In conclusion, the findings of this systematic review suggest that pre- and synbiotic supplementation may have beneficial effects for the prevention of RTIs. However, there was considerable variation in individual study results, particularly across different age categories. Furthermore, evidence indicates pre- and synbiotic supplementation increases NK cell cytotoxicity, but does not induce changes in immune cell populations. More research is needed to confirm the effects of prebiotics and synbiotics on RTIs, particularly in the adult population. There should also be a focus on whether synbiotics are more effective than prebiotics. Future research concerning the effects of prebiotic and synbiotic supplementation should focus on changes in activation markers of immune cells rather than just changes in peripheral cell populations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LMW, BSB, LGW: designed the research and had primary responsibility for the final content; LMW, ILS: conducted the research; LMW: analyzed the data and wrote the paper; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: LMW and ILS are supported by Australian Government Research Training Program (RTP) scholarships. All other authors report no conflicts of interest.
Supplemental Figures 1–7 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ADA, American Dietetic Association; APC, antigen-presenting cell; DC, dendritic cell; E/T, effector-to-target cell; FFAR, free fatty acid receptor; FOS, fructooligosaccharide; GOS, galactooligosaccharide; HDAC, histone deacetylase; HLA-DR+, human leukocyte antigen-DR positive; LRTI, lower respiratory tract infection; mDC, myeloid dendritic cell; MeSH, medical subject heading; NHMRC, National Health and Medical Research Council; NKT, natural killer T; NRCT, nonrandomized controlled trial; OR, odds ratio; RCT, randomized controlled trial; RTI, respiratory tract infection; SMD, standardized mean difference; TH1, type 1 T helper; TLR, toll-like receptor; Treg, regulatory t cell; URTI, upper respiratory tract infection; XOS, xylooligosaccharide.
Contributor Information
Lily M Williams, School of Biomedical Sciences & Pharmacy, University of Newcastle, Callaghan, Australia; Priority Research Centre for Healthy Lungs, Hunter Medical Research Institute, New Lambton Heights, Australia.
Isobel L Stoodley, School of Biomedical Sciences & Pharmacy, University of Newcastle, Callaghan, Australia.
Bronwyn S Berthon, School of Biomedical Sciences & Pharmacy, University of Newcastle, Callaghan, Australia; Priority Research Centre for Healthy Lungs, Hunter Medical Research Institute, New Lambton Heights, Australia.
Lisa G Wood, School of Biomedical Sciences & Pharmacy, University of Newcastle, Callaghan, Australia; Priority Research Centre for Healthy Lungs, Hunter Medical Research Institute, New Lambton Heights, Australia.
References
- 1. Williams NC, Johnson MA, Shaw DE, Spendlove I, Vulevic J, Sharpe GR, Hunter KA. A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstriction and markers of airway inflammation. Br J Nutr. 2016;116(5):798–804. [DOI] [PubMed] [Google Scholar]
- 2. McLoughlin R, Berthon BS, Rogers GB, Baines KJ, Leong LEX, Gibson PG, Williams EJ, Wood LG. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine. 2019;46:473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim T, Choi H, Kim J. Association between dietary nutrient intake and chronic obstructive pulmonary disease severity: A nationwide population-based representative sample. COPD. 2020;17(1):49–58. [DOI] [PubMed] [Google Scholar]
- 4. Batra P, Soni KD, Mathur P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: An updated systematic review and meta-analysis of randomized control trials. J Intensive Care. 2020;8(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laursen RP, Hojsak I. Probiotics for respiratory tract infections in children attending day care centers–A systematic review. Eur J Pediatr. 2018;177(7):979–94. [DOI] [PubMed] [Google Scholar]
- 6. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G, Arslanoglu S, Moro GE, Schmitt J, Tandoi L et al. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138(6):1091–5. [DOI] [PubMed] [Google Scholar]
- 7. Hughes C, Davoodi-Semiromi Y, Colee JC, Culpepper T, Dahl WJ, Mai V, Christman MC, Langkamp-Henken B. Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: A randomized, double-blind, controlled trial in healthy university students. Am J Clin Nutr. 2011;93(6):1305–11. [DOI] [PubMed] [Google Scholar]
- 8. Langkamp-Henken B, Bender BS, Gardner EM, Herrlinger-Garcia KA, Kelley MJ, Murasko DM, Schaller JP, Stechmiller JK, Thomas DJ, Wood SM. Nutritional formula enhanced immune function and reduced days of symptoms of upper respiratory tract infection in seniors. J Am Geriatr Soc. 2004;52(1):3–12. [DOI] [PubMed] [Google Scholar]
- 9. Li F, Jin X, Liu B, Zhuang W, Scalabrin D. Follow-up formula consumption in 3- to 4-year-olds and respiratory infections: An RCT. Pediatrics. 2014;133(6):e1533–40. [DOI] [PubMed] [Google Scholar]
- 10. Ahanchian H, Jafari SA, Ansari E, Ganji T, Kiani MA, Khalesi M, Momen T, Kianifar H. A multi-strain synbiotic may reduce viral respiratory infections in asthmatic children: a randomized controlled trial. Electron Physician. 2016;8:2833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerasimov SV, Ivantsiv VA, Bobryk LM, Tsitsura OO, Dedyshin LP, Guta NV, Yandyo BV. Role of short-term use of L. acidophilus DDS-1 and B . lactis UABLA-12 in acute respiratory infections in children: A randomized controlled trial. Eur J Clin Nutr. 2016;70(4):463–9. [DOI] [PubMed] [Google Scholar]
- 12. Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: Randomized, double-blind, placebo-controlled trial. Pediatrics. 2008;122(1):8–12. [DOI] [PubMed] [Google Scholar]
- 13. Pregliasco F, Anselmi G, Fonte L, Giussani F, Schieppati S, Soletti L. A new chance of preventing winter diseases by the administration of synbiotic formulations. J Clin Gastroenterol. 2008;42(Suppl 3):S224–33. [DOI] [PubMed] [Google Scholar]
- 14. Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–75. [DOI] [PubMed] [Google Scholar]
- 15. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 16. Pujari R, Banerjee G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol Cell Biol. 2021;99(3):255–73. [DOI] [PubMed] [Google Scholar]
- 17. Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008;88(5):1438–46. [DOI] [PubMed] [Google Scholar]
- 18. Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, Gibson GR. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr. 2015;114(4):586–95. [DOI] [PubMed] [Google Scholar]
- 19. Bunout D, Barrera G, Hirsch S, Gattas V, De La Maza MP, Haschke F, Steenhout P, Klassen P, Hager C, Avendano M et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. JPEN J Parenter Enteral Nutr. 2004;28(5):348–54. [DOI] [PubMed] [Google Scholar]
- 20. Przemska-Kosicka A, Childs CE, Maidens C, Dong H, Todd S, Gosney MA, Tuohy KM, Yaqoob P. Age-related changes in the natural killer cell response to seasonal influenza vaccination are not influenced by a synbiotic: A randomised controlled trial. Front Immunol. 2018;9:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72(6):3299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernando MR, Saxena A, Reyes JL, McKay DM. Butyrate enhances antibacterial effects while suppressing other features of alternative activation in IL-4-induced macrophages. Am J Physiol Gastrointest Liver Physiol. 2016;310(10):G822–31. [DOI] [PubMed] [Google Scholar]
- 23. Ji J, Shu D, Zheng M, Wang J, Luo C, Wang Y, Guo F, Zou X, Lv X, Li Y et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep. 2016;6(1):24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandoval A, Triviños F, Sanhueza A, Carretta D, Hidalgo MA, Hancke JL, Burgos RA. Propionate induces pH(i) changes through calcium flux, ERK1/2, p38, and PKC in bovine neutrophils. Vet Immunol Immunopathol. 2007;115(3–4):286–98. [DOI] [PubMed] [Google Scholar]
- 26. Vinolo MA, Hatanaka E, Lambertucci RH, Newsholme P, Curi R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct. 2009;27(1):48–55. [DOI] [PubMed] [Google Scholar]
- 27. Childs CE, Roytio H, Alhoniemi E, Fekete AA, Forssten SD, Hudjec N, Lim YN, Steger CJ, Yaqoob P, Tuohy KM et al. Xylo-oligosaccharides alone or in synbiotic combination with B ifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: A double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr. 2014;111(11):1945–56. [DOI] [PubMed] [Google Scholar]
- 28. Clarke ST, Green-Johnson JM, Brooks SPJ, Ramdath DD, Bercik P, Avila C, Inglis GD, Green J, Yanke LJ, Selinger LB et al. Beta2-1 fructan supplementation alters host immune responses in a manner consistent with increased exposure to microbial components: Results from a double-blinded, randomised, cross-over study in healthy adults. Br J Nutr. 2016;115(10):1748–59. [DOI] [PubMed] [Google Scholar]
- 29. Freud AG, Yu J, Caligiuri MA. Human natural killer cell development in secondary lymphoid tissues. Semin Immunol. 2014;26(2):132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25(1):297–336. [DOI] [PubMed] [Google Scholar]
- 31. American Dietetic Association. ADA evidence analysis manual. 4th ed. Chicago, IL: American Dietetic Association; 2003. [Google Scholar]
- 32. NHMRC. Additional levels of evidence and grades for recommendations for developers of guidelines. Canberra, Australia: National Health and Medical Research Council; 2009. [Google Scholar]
- 33. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. Cochrane handbook for systematic review of interventions 6.1. London, UK: Cochrane Library; 2020. [Google Scholar]
- 34. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, Higgins D, Proquest EC. Introduction to meta-analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons, Incorporated; 2011. [Google Scholar]
- 35. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maeda H, Ichihashi K, Fujii T, Omura K, Zhu X, Anazawa M, Tazawa K. Oral administration of hydrolyzed rice bran prevents the common cold syndrome in the elderly based on its immunomodulatory action. Biofactors. 2004;21(1–4):185–7. [DOI] [PubMed] [Google Scholar]
- 37. Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137(11):2420–4. [DOI] [PubMed] [Google Scholar]
- 38. Bruzzese E, Volpicelli M, Squeglia V, Bruzzese D, Salvini F, Bisceglia M, Lionetti P, Cinquetti M, Iacono G, Amarri S et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin Nutr. 2009;28(2):156–61. [DOI] [PubMed] [Google Scholar]
- 39. Cazzola M, Pham-Thi N, Kerihuel JC, Durand H, Bohbot S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: A randomized, double-blind, placebo-controlled pilot study. Ther Adv Respir Dis. 2010;4(5):271–8. [DOI] [PubMed] [Google Scholar]
- 40. Cohen R, Martin E, de La Rocque F, Thollot F, Pecquet S, Werner A, Boucherat M, Varon E, Bingen E, Levy C. Probiotics and prebiotics in preventing episodes of acute otitis media in high-risk children: A randomized, double-blind, placebo-controlled study. Pediatr Infect Dis J. 2013;32(8):810–14. [DOI] [PubMed] [Google Scholar]
- 41. Coman MM, Verdenelli MC, Silvi S, Cecchini C, Gabbianelli R, Amadio E, Orpianesi C, Cresci A. Knowledge and acceptance of functional foods: A preliminary study on influence of a synbiotic fermented milk on athlete health. Int J Probiotics Prebiotics. 2017;12(1):33–41. [Google Scholar]
- 42. Costabile A, Bergillos-Meca T, Rasinkangas P, Korpela K, de Vos WM, Gibson GR. Effects of soluble corn fiber alone or in synbiotic combination with L actobacillus rhamnosus GG and the pilus-deficient derivative GG-PB12 on fecal microbiota, metabolism, and markers of immune function: A randomized, double-blind, placebo-controlled, crossover study in healthy elderly (Saimes study). Front Immunol. 2017;8:1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elison E, Vigsnaes LK, Rindom Krogsgaard L, Rasmussen J, Sorensen N, McConnell B, Hennet T, Sommer MOA, Bytzer P. Oral supplementation of healthy adults with 2'-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br J Nutr. 2016;116(8):1356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fox AT, Wopereis H, Van Ampting MTJ, Oude Nijhuis MM, Butt AM, Peroni DG, Vandenplas Y, Candy DCA, Shah N, West CE et al. A specific synbiotic-containing amino acid-based formula in dietary management of cow's milk allergy: a randomized controlled trial. Clin Transl Allergy. 2019;9(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: A randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol. 2010;11(5):351–61. [DOI] [PubMed] [Google Scholar]
- 46. Lohner S, Jakobik V, Mihalyi K, Soldi S, Vasileiadis S, Theis S, Sailer M, Sieland C, Berenyi K, Boehm G et al. Inulin-type fructan supplementation of 3- to 6-year-old children is associated with higher fecal B ifidobacterium concentrations and fewer febrile episodes requiring medical attention. J Nutr. 2018;148(8):1300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lomax AR, Cheung LV, Tuohy KM, Noakes PS, Miles EA, Calder PC. β2-1 fructans have a bifidogenic effect in healthy middle-aged human subjects but do not alter immune responses examined in the absence of an in vivo immune challenge: Results from a randomised controlled trial. Br J Nutr. 2012;108(10):1818–28. [DOI] [PubMed] [Google Scholar]
- 48. Luoto R, Ruuskanen O, Waris M, Kalliomaki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133(2):405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther. 2013;38(7):804–16. [DOI] [PubMed] [Google Scholar]
- 50. Moore N, Chao C, Yang LP, Storm H, Oliva-Hemker M, Saavedra JM. Effects of fructo-oligosaccharide-supplemented infant cereal: A double-blind, randomized trial. Br J Nutr. 2003;90(3):581–7. [DOI] [PubMed] [Google Scholar]
- 51. Niele N, van Zwol A, Westerbeek EA, Lafeber HN, van Elburg RM. Effect of non-human neutral and acidic oligosaccharides on allergic and infectious diseases in preterm infants. Eur J Pediatr. 2013;172(3):317–23. [DOI] [PubMed] [Google Scholar]
- 52. Nova E, Viadel B, Warnberg J, Carreres JE, Marcos A. Beneficial effects of a synbiotic supplement on self-perceived gastrointestinal well-being and immunoinflammatory status of healthy adults. J Med Food. 2011;14(1–2):79–85. [DOI] [PubMed] [Google Scholar]
- 53. Picaud JC, Chapalain V, Paineau D, Zourabichvili O, Bornet FR, Duhamel JF. Incidence of infectious diseases in infants fed follow-on formula containing synbiotics: An observational study. Acta Paediatr. 2010;99(11):1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Puccio G, Alliet P, Cajozzo C, Janssens E, Corsello G, Sprenger N, Wernimont S, Egli D, Gosoniu L, Steenhout P. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. J Pediatr Gastroenterol Nutr. 2017;64(4):624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Raes M, Scholtens PA, Alliet P, Hensen K, Jongen H, Boehm G, Vandenplas Y, Rummens JL. Exploration of basal immune parameters in healthy infants receiving an infant milk formula supplemented with prebiotics. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e377–85. [DOI] [PubMed] [Google Scholar]
- 56. Ranucci G, Buccigrossi V, Borgia E, Piacentini D, Visentin F, Cantarutti L, Baiardi P, Felisi M, Spagnuolo MI, Zanconato S et al. Galacto-oligosaccharide/polidextrose enriched formula protects against respiratory infections in infants at high risk of atopy: A randomized clinical trial. Nutrients. 2018;10(3):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seidel C, Boehm V, Vogelsang H, Wagner A, Persin C, Glei M, Pool-Zobel BL, Jahreis G. Influence of prebiotics and antioxidants in bread on the immune system, antioxidative status and antioxidative capacity in male smokers and non-smokers. Br J Nutr. 2007;97(2):349–56. [DOI] [PubMed] [Google Scholar]
- 58. Sierra C, Bernal M-J, Blasco J, Martínez R, Dalmau J, Ortuño I, Espín B, Vasallo M-I, Gil D, Vidal M-L et al. Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: A multicentre, randomised, double-blind and placebo-controlled trial. Eur J Nutr. 2015;54(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van de Pol MA, Lutter R, Smids BS, Weersink EJ, van der Zee JS. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy. 2011;66(1):39–47. [DOI] [PubMed] [Google Scholar]
- 60. van der Aa LB, Lutter R, Heymans HS, Smids BS, Dekker T, van Aalderen WM, Sillevis Smitt JH, Knippels LM, Garssen J, Nauta AJ et al. No detectable beneficial systemic immunomodulatory effects of a specific synbiotic mixture in infants with atopic dermatitis. Clin Exp Allergy. 2012;42(4):531–9. [DOI] [PubMed] [Google Scholar]
- 61. Westerbeek EA, van den Berg JP, Lafeber HN, Fetter WP, Boehm G, Twisk JW, van Elburg RM. Neutral and acidic oligosaccharides in preterm infants: A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91(3):679–86. [DOI] [PubMed] [Google Scholar]
- 62. de Groot PF, Nikolic T, Imangaliyev S, Bekkering S, Duinkerken G, Keij FM, Herrema H, Winkelmeijer M, Kroon J, Levin E et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: A randomised controlled trial. Diabetologia. 2020;63(3):597–610. [DOI] [PubMed] [Google Scholar]
- 63. Kiewiet MBG, Elderman ME, Aidy SE, Burgerhof JGM, Visser H, Vaughan EE, Faas MM, Vos PD. Flexibility of gut microbiota in ageing individuals during dietary fiber long-chain inulin intake. Mol Nutr Food Res. 2020;65(4):2000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Velikova T, Tumangelova-Yuzeir K, Georgieva R, Ivanova-Todorova E, Karaivanova E, Nakov V, Nakov R, Kyurkchiev D. Lactobacilli supplemented with larch arabinogalactan and colostrum stimulates an immune response towards peripheral NK activation and gut tolerance. Nutrients. 2020;12(6):1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Choi JY, Paik DJ, Kwon DY, Park Y. Dietary supplementation with rice bran fermented with Lentinus edodes increases interferon-gamma activity without causing adverse effects: A randomized, double-blind, placebo-controlled, parallel-group study. Nutr J. 2014;13(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Farhangi MA, Javid AZ, Sarmadi B, Karimi P, Dehghan P. A randomized controlled trial on the efficacy of resistant dextrin, as functional food, in women with type 2 diabetes: Targeting the hypothalamic-pituitary-adrenal axis and immune system. Clin Nutr. 2018;37(4):1216–23. [DOI] [PubMed] [Google Scholar]
- 67. Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, Baccaglini L, Mohapatra A, Mohapatra SS, Misra PR et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548(7668):407–12. [DOI] [PubMed] [Google Scholar]
- 68. Sazawal S, Dhingra U, Hiremath G, Sarkar A, Dhingra P, Dutta A, Verma P, Menon VP, Black RE. Prebiotic and probiotic fortified milk in prevention of morbidities among children: Community-based, randomized, double-blind, controlled trial. PLoS One. 2010;5(8):e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shahramian I, Kalvandi G, Javaherizadeh H, Khalili M, Noori NM, Delaramnasab M, Bazi A. The effects of prebiotic supplementation on weight gain, diarrhoea, constipation, fever and respiratory tract infections in the first year of life. J Paediatr Child Health. 2018;54(8):875–80. [DOI] [PubMed] [Google Scholar]
- 70. Kitagawa M, Nakagawa S, Suzuki T, Kishimoto Y, Kanahori S, Hatakeyama Y, Tomita S, Fukuhara I. Visceral fat-reducing effect and safety of continuous consumption of beverage containing resistant maltodextrin: A randomized, double-blind, placebo-controlled, parallel-group clinical trial. J Nutr Sci Vitaminol. 2020;66(5):417–26. [DOI] [PubMed] [Google Scholar]
- 71. Leung TF, Ulfman LH, Chong MKC, Hon KL, Khouw I, Chan PKS, Delsing DJ, Kortman GAM, Bovee-Oudenhoven IMJ. A randomized controlled trial of different young child formulas on upper respiratory and gastrointestinal tract infections in Chinese toddlers. Pediatr Allergy Immunol. 2020;31(7):745–54. [DOI] [PubMed] [Google Scholar]
- 72. Takahashi C, Kozawa M. The effect of partially hydrolyzed guar gum on preventing influenza infection. Clin Nutr ESPEN. 2021;42:148–52. [DOI] [PubMed] [Google Scholar]
- 73. Ringel-Kulka T, Kotch JB, Jensen ET, Savage E, Weber DJ. Randomized, double-blind, placebo-controlled study of synbiotic yogurt effect on the health of children. J Pediatr. 2015;166(6):1475–81.e3. [DOI] [PubMed] [Google Scholar]
- 74. Bloomer RJ, Butawan M, van der Merwe M, Keating FH. An assessment of the glyconutrient ambrotose TM on immunity, gut health, and safety in men and women: A placebo-controlled, double-blind, randomized clinical trial. Nutrients. 2020;12(6):1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pontes MV, Ribeiro TC, Ribeiro H, de Mattos AP, Almeida IR, Leal VM, Cabral GN, Stolz S, Zhuang W, Scalabrin DM. Cow's milk-based beverage consumption in 1- to 4-year-olds and allergic manifestations: An RCT. Nutr J. 2016;15(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Anaya-Loyola MA, Garcia-Marin G, Garcia-Gutierrez DG, Castano-Tostado E, Reynoso-Camacho R, Lopez-Ramos JE, Enciso-Moreno JA, Perez-Ramirez IF. A mango (Mangifera indica L.) juice by-product reduces gastrointestinal and upper respiratory tract infection symptoms in children. Food Res Int. 2020;136:109492. [DOI] [PubMed] [Google Scholar]
- 77. Gill PA, van Zelm MC, Ffrench RA, Muir JG, Gibson PR. Successful elevation of circulating acetate and propionate by dietary modulation does not alter T-regulatory cell or cytokine profiles in healthy humans: A pilot study. Eur J Nutr. 2020;59(6):2651–61. [DOI] [PubMed] [Google Scholar]
- 78. Paganini D, Uyoga MA, Kortman GAM, Cercamondi CI, Moretti D, Barth-Jaeggi T, Schwab C, Boekhorst J, Timmerman HM, Lacroix C et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut. 2017;66(11):1956–67. [DOI] [PubMed] [Google Scholar]
- 79. Boyle RJ, Tang ML, Chiang WC, Chua MC, Ismail I, Nauta A, Hourihane JOB, Smith P, Gold M, Ziegler J et al. Prebiotic-supplemented partially hydrolysed cow's milk formula for the prevention of eczema in high-risk infants: A randomized controlled trial. Allergy. 2016;71(5):701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chatchatee P, Lee WS, Carrilho E, Kosuwon P, Simakachorn N, Yavuz Y, Schouten B, Graaff PL, Szajewska H. Effects of growing-up milk supplemented with prebiotics and LCPUFAs on infections in young children. J Pediatr Gastroenterol Nutr. 2014;58(4):428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, Klarczyk M, Schöpfer H, Böckler HM, Wells J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: More like breast-fed infants. J Pediatr Gastroenterol Nutr. 2005;40(1):36–42. [DOI] [PubMed] [Google Scholar]
- 82. Bakker-Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJ, Bindels JG. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable B ifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr. 2005;94(5):783–90. [DOI] [PubMed] [Google Scholar]
- 83. Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1):e18–25. [DOI] [PubMed] [Google Scholar]
- 84. Tromp I, Kiefte-de Jong J, Raat H, Jaddoe V, Franco O, Hofman A, de Jongste J, Moll H. Breastfeeding and the risk of respiratory tract infections after infancy: The Generation R study. PLoS One. 2017;12(2):e0172763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. [DOI] [PubMed] [Google Scholar]
- 86. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Batista VL, da Silva TF, de Jesus LCL, Coelho-Rocha ND, Barroso FAL, Tavares LM, Azevedo V, Mancha-Agresti P, Drumond MM. Probiotics, prebiotics, synbiotics, and paraprobiotics as a therapeutic alternative for intestinal mucositis. Front Microbiol. 2020;11:544490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kawahara T, Takahashi T, Oishi K, Tanaka H, Masuda M, Takahashi S, Takano M, Kawakami T, Fukushima K, Kanazawa H et al. Consecutive oral administration of B ifidobacterium longum MM-2 improves the defense system against influenza virus infection by enhancing natural killer cell activity in a murine model. Microbiol Immunol. 2015;59(1):1–12. [DOI] [PubMed] [Google Scholar]
- 89. Goto H, Sagitani A, Ashida N, Kato S, Hirota T, Shinoda T, Yamamoto N. Br J Nutr. 2013;110(10):1810–18. [DOI] [PubMed] [Google Scholar]
- 90. Mohr AE, Basile AJ, Crawford MS, Sweazea KL, Carpenter KC. Probiotic supplementation has a limited effect on circulating immune and inflammatory markers in healthy adults: A systematic review of randomized controlled trials. J Acad Nutr Diet. 2020;120(4):548–64. [DOI] [PubMed] [Google Scholar]
- 91. Castillo NA, Perdigón G, de Moreno de Leblanc A. Oral administration of a probiotic L actobacillus modulates cytokine production and TLR expression improving the immune response against S almonella enterica serovar typhimurium infection in mice. BMC Microbiol. 2011;11(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rahat-Rozenbloom S, Fernandes J, Cheng J, Gloor GB, Wolever TM. The acute effects of inulin and resistant starch on postprandial serum short-chain fatty acids and second-meal glycemic response in lean and overweight humans. Eur J Clin Nutr. 2017;71(2):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35(1):9–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.