ABSTRACT
Micronutrient deficiencies are a major cause of morbidity and mortality in low- and middle-income countries worldwide. Climate change, characterized by increasing global surface temperatures and alterations in rainfall, has the capacity to affect the quality and accessibility of micronutrient-rich foods. The goals of this review are to summarize the potential effects of climate change and its consequences on agricultural yield and micronutrient quality, primarily zinc, iron, and vitamin A, of plant foods and upon the availability of animal foods, to discuss the implications for micronutrient deficiencies in the future, and to present possible mitigation and adaptive strategies. In general, the combination of increasing atmospheric carbon dioxide and rising temperature is predicted to reduce the overall yield of major staple crops, fruits, vegetables, and nuts, more than altering their micronutrient content. Crop yield is also reduced by elevated ground-level ozone and increased extreme weather events. Pollinator loss is expected to reduce the yield of many pollinator-dependent crops such as fruits, vegetables, and nuts. Sea-level rise resulting from melting of ice sheets and glaciers is predicted to result in coastal inundation, salt intrusion, and loss of coral reefs and mangrove forests, with an adverse impact upon coastal rice production and coastal fisheries. Global ocean fisheries catch is predicted to decline because of ocean warming and declining oxygen. Freshwater warming is also expected to alter ecosystems and reduce inland fisheries catch. In addition to limiting greenhouse gas production, adaptive strategies include postharvest fortification of foods; micronutrient supplementation; biofortification of staple crops with zinc and iron; plant breeding or genetic approaches to increase zinc, iron, and provitamin A carotenoid content of plant foods; and developing staple crops that are tolerant of abiotic stressors such as elevated carbon dioxide, elevated temperature, and increased soil salinity.
Keywords: climate change, food, iron deficiency, micronutrients, vitamin A deficiency, zinc deficiency
Statement of Significance: This comprehensive review shows that climate change and its consequences are likely to affect micronutrient malnutrition by limiting the availability of micronutrient-rich plant and animal foods rather than their micronutrient content. Various mitigating and adaptive strategies should be considered to reduce the risk of micronutrient malnutrition in vulnerable populations in the face of climate change.
Introduction
Micronutrient deficiencies are a leading cause of morbidity and mortality worldwide, especially among young children and pregnant women. Vitamin A, zinc, and iron deficiencies are highly prevalent in low- and middle-income countries (LMICs) and affect an estimated 2 billion people worldwide (1). The world population is expected to grow from 7.7 billion people in 2019 to 9.7 billion by 2050 and 10.9 billion by 2100, according to the United Nations (2). Five different shared socioeconomic pathways (SSPs), based upon integrated scenarios for future societal development, project a global population that ranges from 8.5 to 9.9 billion in 2050, depending upon scenario (3). The population projections diverge further after 2050, with the “middle of the road” scenario, or SPP2, predicting a world population of ∼9.0 billion by 2100 (3). Providing sufficient food and preventing micronutrient deficiencies for this growing population will be major challenges in the face of climate change. The ideal goal is to meet these needs by providing food in a sustainable manner within the boundaries of the planet (4). Food systems face the dual challenge of increasing demand, growing environmental pressures, and the prospect that climate change is influencing the quality and quantity of food that is produced (5).
Plant foods provide a large proportion of micronutrient intake in LMICs (6, 7). In predominantly plant-based diets, dark-green leafy vegetables and orange and yellow fruits are rich sources of pro-vitamin A carotenoids, and legumes, nuts, and cereals are important sources of zinc and iron (7). Small-scale inland capture and coastal fisheries are also major sources of micronutrients for many populations in LMICs (8–10). Animal-source foods, such as eggs, beef, pork, chicken, and dairy products, are rich sources of vitamin A, zinc, and iron, but are relatively expensive and not regularly consumed by poorer families. Any factors that limit the availability of staple crops such as cereals can lead to global increases in food prices and greater vulnerability of poor populations to micronutrient malnutrition (11, 12).
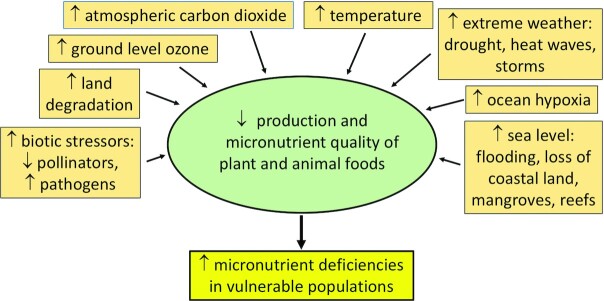
Climate change encompasses a broad set of environmental processes related to rising atmospheric carbon dioxide (CO2) and increasing global surface temperatures (13). These processes include more frequent occurrence of extreme weather events, degradation of the cryosphere, sea-level rise, increase in ocean hypoxia, land degradation, elevated ground-level ozone, and greater abiotic and biotic stress to plants. Both the yield and the micronutrient concentrations of foods can be affected by climate change and increase the risk of micronutrient deficiencies in the general population and especially in vulnerable populations. The goals of this scoping review are to summarize the potential effects of climate change on the yield and micronutrient quality of plant and animal foods and their implications for micronutrient deficiencies in the future and to discuss possible mitigation and adaptation strategies. A conceptual model for this review is shown in Figure 1. Climate change consists of abiotic and biotic factors that influence the quality and yield of plants and animals that provide micronutrients in the diet.
FIGURE 1.
Conceptual model of climate change and micronutrient deficiencies.
Rising Atmospheric CO2Concentrations and Increasing Temperature
The major greenhouse gas (GHG) arising from human activities is carbon dioxide (14). Prior to the Industrial Revolution, atmospheric carbon dioxide concentrations were relatively stable at ∼280 ppm for at least 10,000 y (13). Atmospheric carbon dioxide has increased steadily by 2–3 ppm annually (15) and is predicted to increase from 415 ppm in 2020 (16) to 550 ppm by 2050 (14). Without mitigating activities, atmospheric carbon dioxide is expected to double between now and the end of the century (14).
The accumulation of GHGs in the atmosphere alters the balance between incoming solar radiation, which heats the Earth's surface, and re-emitted infrared energy, which is absorbed by atmospheric carbon dioxide and water vapor, with the net effect of driving surface heating, or global warming (17). Human activities have resulted in ∼1.0°C of global warming above preindustrial levels, with warming currently increasing at 0.2°C per decade (18). Warming above the global average is occurring more over land than water and in the Arctic region (18). Global warming is likely to reach 1.5°C above preindustrial levels between 2030 and 2050 if it continues to increase at the current rate (18).
The United Nations Intergovernmental Panel on Climate Change (IPCC) has adopted representative concentration pathways (RCPs)—scenarios that include emissions and concentrations of GHGs, aerosols, and chemically active gases as well as changes in land use over time—for climate modeling. There were 4 original RCPs, with RCP2.6 representing low GHG emissions and high mitigation that gives a 2 out of 3 chance to limit global warming below 2°C by 2100, and RCP8.5 representing a high GHG scenario with the absence of policies to combat climate change, leading to continued and sustained growth in GHG concentrations (19). RCP4.5 and RCP6.0 represented intermediate scenarios. The RCPs have been used to model the effects of climate change on crop yields and nutrient quality and to estimate future trajectories and effects on climate change, as noted further below.
Effects of rising carbon dioxide and temperature on major crops
As carbon dioxide concentrations rise, most plants respond by growing faster (20). The impacts of elevated atmospheric carbon dioxide, as an isolated factor, on plants were originally studied using open top chambers (OTCs). Free-air carbon dioxide enrichment (FACE), a technique in which plants are grown under elevatedcarbon dioxide in natural and fully open-air conditions, gradually replaced OTCs for most crop studies (21). Plants can be distinguished into 2 groups, C3 and C4, based on the type of photosynthesis. The first stable products of photosynthesis are either three-carbon molecules in C3 plants or four-carbon molecules in C4 plants. C3 plants constitute 95% of species on the planet (22) and include most food crops such as wheat, rice, barley, potato, legumes, nuts, fruit, and vegetables (23). The few food crops that are C4 species include maize, sugarcane, sorghum, and millet. In general, C3 food crops have modest increases in yield when grown under elevated atmospheric carbon dioxide using FACE. In contrast, elevated atmospheric carbon dioxide, as an isolated factor, has no direct effect upon carbon uptake by C4 plants (24), but some studies suggest that yield of C4 crop plants may increase under drought conditions (25, 26).
Elevated carbon dioxide, as an isolated factor, has been shown to increase the agricultural yield for crops such as wheat, rice, potato, and legumes (21, 27–29). Plants increase the synthesis of carbohydrates when exposed to higher levels of atmospheric carbon dioxide. The increase in carbohydrate content with elevated carbon dioxide appears to dilute the overall mineral content of plant tissues by 8% (28). Elevated carbon dioxide increased the carbon content of plants but reduced the nitrogen, potassium, phosphorus, calcium, sulfur, magnesium, iron, zinc, and copper content (28). The reduction in mineral content has been observed regardless of whether determined by FACE or non-FACE studies, by geographic location, and in plant groups or tissues (28). Elevated atmospheric carbon dioxide also reduces canopy transpiration and decreases nutrient uptake by the roots (30).
In contrast to elevated carbon dioxide, elevated temperature is expected to have a detrimental effect upon cereal, vegetable, and legume production. For each 1°C increase in temperature, the yield of wheat and barley is reduced by 5–6% (31). Rice is sensitive to changes in temperature (32), and grain yield of rice is reduced by global warming (33). In Africa, rice yields are projected to drop by 24% in 2070 compared with 2000 under RCP8.5 (34), and global warming of 2.0°C would reduce maize yield (35). In general, most vegetable crops require low temperature for their cultivation, and even slight increases in temperature will reduce crop productivity (36). Legumes are sensitive to heat stress (37) and increasing temperatures will reduce crop yields (38).
In order to study the effect of both elevated carbon dioxide and elevated temperature on cereals and other crops, which is more consistent with future climate change projections, a more recent platform has been developed known as temperature FACE (T-FACE). This experimental setup involves FACE combined with temperature-controlled infrared heaters to simulate conditions of both elevated carbon dioxide and elevated temperature that are anticipated by the middle and end of the 21st century. OTC, FACE, and T-FACE platforms are illustrated in Figure 2. The experimental evidence for the effects of elevated carbon dioxide and elevated temperature, as isolated factors, elevated carbon dioxide and elevated temperature combined, and other stressors on major crops is discussed in greater detail in the following sections on rice, wheat, legumes, vegetables and fruit, and nuts. .
FIGURE 2.
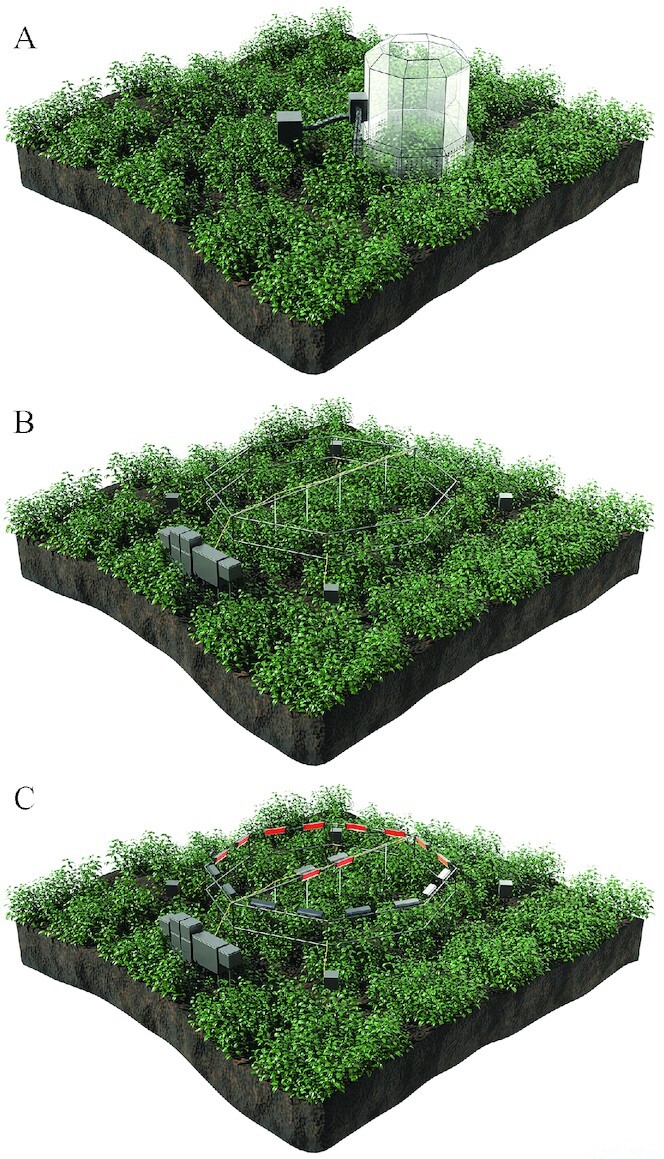
Platforms for assessing plant growth and yield in controlled environments: (A) open-top chamber (OTC), (B) free-air carbon dioxide enrichment (FACE), (C) temperature free-air carbon dioxide enrichment (T-FACE).
Rice
Rice is the staple food of ∼3.5 billion people worldwide (39). Milled rice production worldwide in 2019 was 755 million tons (40). The 10 countries with the largest consumption of rice as a fraction of total available calories are Bangladesh, Cambodia, China, Indonesia, Lao People's Democratic Republic, Madagascar, Myanmar, Philippines, Thailand, and Vietnam (41). Rice provides up to 50% of the dietary caloric supply and a substantial part of protein intake for >500 million people living in poverty in Asia (39). Rice consumption is steadily growing in sub-Saharan Africa, the Caribbean, and Latin America (39). The leading producers of rice worldwide are China and India, followed by Indonesia, Bangladesh, Vietnam, Myanmar, Thailand, and the Philippines (40).
The impact of elevated carbon dioxide on overall grain yield and upon zinc and iron concentrations in rice grains has been studied in OTC, FACE, and T-FACE studies conducted in Asia, Europe, and Australia (42–58). There is considerable heterogeneity in the type of study, number of replicates, number of growing seasons, cultivars utilized, and geographic location of the testing sites (Supplemental Table 1). Most studies showed that elevated temperature reduced the yield of rice, with a range of 7–21% reductions observed compared with ambient temperature (43, 47, 49, 50). The combination of elevated carbon dioxide and temperature generally reduced the yield of rice (47–52) (Table 1). A meta-analysis suggested that the combination of elevated carbon dioxide and temperature may increase the yield of rice by 10.1% (59); however, the analysis was limited to only 5 studies and did not include 1 OTC study (48) and 2 more recent T-FACE studies (43, 44).
TABLE 1.
Effect of elevated carbon dioxide, elevated temperature, and other conditions on the zinc and on content and yield of rice1
| Location | Study | Growing conditions | Micronutrients | Yield | Reference |
|---|---|---|---|---|---|
| China | T-FACE | CO2: 384 vs. 574 ppm Temperature: ambient vs. +1.4°C | Zn ↑ ∼42% (eCO2 + eT)Fe ↑ ∼23% (eCO2 + eT) | Not reported | 42 |
| China | T-FACE | CO2: ambient vs. 510 ppm Temperature: ambient vs. +1.8°C | Zn ↓ 8% (eCO2)Zn ↑ 7% (eT)Fe ↓ 20% (eCO2)Fe ↑ 30% (eT) | ↑ 11% (eCO2)↓ 12% (eT) | 43 |
| China | T-FACE | CO2: 390 vs 590 ppm Temperature: ambient vs. +1.5°C | Not reported | ↓ 5% (eCO2 + eT) in 2015↓ 12% (eCO2 + eT) in 2016 | 44 |
| Japan | FACE | CO2: 370 vs 700 ppm; 9 cultivars | Zn ↓ 6% (eCO2)Fe ↔ (eCO2) | Not reported | 45 |
| Japan and China | FACE | CO2: 395 vs. 568-590 ppm; 18 cultivars | Zn ↓ 5% (eCO2) overallZn ↓ 10–15% (eC02), with some cultivarsFe ↓ 8% (eCO2), overallFe ↓ 10–20% (eCO2), with some cultivars | Not reported | 46 |
| China | T-FACE | CO2: ambient vs. 590 ppm; Temperature: ambient vs. +1.0°C | Not reported | ↑ 15% (eCO2), in 2015↑ 13% (eCO2), in 2016↓ 8% (eT), in 2015↓ 21% (eT), in 2016↓ 4% (eCO2 + eT) in 2015↓ 14% (eCO2 + eT) in 2016 | 47 |
| India | OTC | CO2: 396 vs. 596 ppm; Heat stress: ambient vs. +3–4°C; 2 cultivars | Zn ↓ 11–16% (eCO2)Zn ↓ 10–23% (eCO2 + eT)Fe ↓ 12–20% (eCO2)Fe ↓ 14–23% (eCO2 + eT) | ↑ 14–20% (eCO2)↓ 9–15% (eCO2 + eT) | 48 |
| China | T-FACE | CO2: ambient vs. 590 ppm; Temperature: ambient vs. +1.0°C | Not reported | ↑ 6% (eCO2), in 2013↑ 10% (eCO2), in 2014↓ 25% (eT), in 2013↓ 11% (eT), in 2014↓ 12% (eCO2 + eT) in 2013↓ 7% (eCO2 + eT) in 2014 | 49 |
| China | T-FACE | CO2: ambient vs. 500 ppm; Temperature: ambient vs. +2.0°C | Not reported | ↑ 8% (eCO2)↓ 10% (eT)↓ 5% (eCO2 + eT) | 50 |
| Japan | FACE | CO2: 382 vs. 574 ppm Water and soil temperature: ambient vs. +2.0°C | Not reported | ↑ 14% (eCO2)↔ (eT) | 51 |
| China | T-FACE | CO2: ambient vs. 500 ppm; Temperature: ambient vs. +1.5°C, +2.0°C | Not reported | ↓ 17–35% (eCO2 + eT) | 52 |
| Portugal | OTC | CO2: 375 vs. 550 ppm | Zn ↑ 8% (eCO2)Fe ↑ 14% (eCO2) | Not reported | 53 |
| China | Glass house | CO2: ambient vs. 600 ppm; plus eO3 | Zn ↔ (eCO2 + ambient O3)Zn ↔ (eCO2 + eO3) | Not reported | 54 |
| Japan | FACE | CO2: ambient vs. 577 ppm; 8 cultivars | Not reported | ↑ 17% (eCO2) | 55 |
| China | FACE | CO2: ambient vs. 572 ppm; range of nitrogen fertilizer | Fe ↔ (eCO2) | ↑ 11% (eCO2) | 56 |
| Japan | FACE | CO2: ambient vs. 625 ppm or 570 ppm | Zn ↔ (eCO2, 625 ppm)Fe ↔ (eCO2, 625 ppm)Zn ↔ (eCO2, 570 ppm)Fe ↔ (eCO2, 570 ppm) | ↑ 14% (eCO2) | 57 |
| Australia | OTC | CO2: 350 vs. 700 ppm plus range of P supply | Zn ↓ 14% (eCO2 + 0 P)Zn ↓ 16% (eCO2 + 240 mg/kg P)Fe ↔ (eCO2 + 0 P)Fe ↓ 18% (eCO2 + 240 mg/kg P) | ↑ 58% (eCO2) | 58 |
eCO2, elevated carbon dioxide; eO3, elevated ozone; eT, elevated temperature; FACE, free-air carbon dioxide enrichment; OTC, open-top container; T-FACE, temperature free-air carbon dioxide enrichment, arrow up denotes increase, arrow down denotes decrease, arrow side-to-side denotes no change .
Elevated carbon dioxide concentrations have been shown to decrease (45, 46, 48, 54, 58), increase (53), or have no effect (57) on zinc concentrations, and to decrease (46, 48), increase (53), or have no effect (45, 56, 58) on iron concentrations in rice grains (Table 1). Elevated carbon dioxide concentrations have been shown to increase the yield of rice grains (43, 47–51, 55–58). Two independent meta-analyses show that elevated carbon dioxide reduces zinc concentrations by 3% and iron concentrations by 5% in rice grains compared with ambient carbon dioxide levels (60, 61). It is not possible to conclude on the basis of these meta-analyses that climate change will have an adverse impact upon zinc and iron concentrations in rice in the future, since these meta-analyses only addressed the effect of elevated carbon dioxide alone. T-FACE experiments show that the combination of elevated carbon dioxide and temperature will increase rice grain concentrations of both iron and zinc when compared with current conditions (42, 43). Although elevated carbon dioxide reduces zinc and iron concentrations in rice grains, elevated temperatures increase zinc and iron concentrations, offsetting the reductions due to elevated carbon dioxide (43). The combination of elevated carbon dioxide and temperature increased the concentrations of cadmium and lead in rice grains, raising potential issues about metal toxicity in rice in the future with climate change (43).
Wheat
Wheat (Triticum aestivum) is the second most important energy source for humans and has an annual global production of ∼762 million tons (40). In some micronutrient-deficient populations, wheat is the dominant staple in the diet, providing >50% of daily energy intake (62). The impacts of elevated carbon dioxide and elevated temperature on wheat yield and on zinc and iron concentrations in wheat grains have been studied in Europe, Turkey, China, and Australia (43, 63–70) (Table 2), with considerable heterogeneity between studies (Supplemental Table 2). Elevated carbon dioxide concentrations, as an isolated factor, have been shown to increase (43, 64, 65, 71, 72) or to have no effect (50, 63, 69) on the yield of wheat. Elevated carbon dioxide concentrations have been shown to decrease (66–68, 71, 73, 72, 74) or have no effect (69, 75, 76) on zinc concentrations, and to decrease (67, 69, 71, 73, 75, 74) or have no effect (66, 77, 76) on iron concentrations in wheat grains (Table 2). An early review concluded that elevated carbon dioxide reduced wheat grain zinc and iron by 13% and 18%, respectively (78). Two independent meta-analyses showed that elevated carbon dioxide, as an isolated factor, reduces zinc concentrations by 9% and iron concentrations by 5–7% in wheat grains (60, 79).
TABLE 2.
Effect of elevated carbon dioxide, elevated temperature, and other conditions on the zinc and iron content and yield of wheat1
| Location | Study | Growing conditions | Micronutrients | Yield | Reference |
|---|---|---|---|---|---|
| China | T-FACE | CO2: ambient vs. 510 ppm Temperature: ambient vs. +1.8°C | Zn ↓ 14% (eCO2)Zn ↑ 4% (eT)Fe ↓ 3% (eCO2)Fe ↑ 18% (eT) | ↑ 11% (eCO2)↓ 12% (eT) | 43 |
| Germany, Italy, China, Australia, USA | FACE | CO2: 400 vs. 550 ppm | Not reported | ↔ (eCO2) Germany ↔ (eCO2) Italy↔ (eCO2) China↔ (eCO2) Australia↑ ∼25% (eCO2) USA | 63 |
| India | FACE | CO2: 390 vs. 550 ppm | not reported | ↑ 16% (eCO2) | 64 |
| Turkey | OTC | CO2: 400 vs. 700 ppmTemperature: ambient vs. +3°CSoil: adequate vs. low NSoil: adequate vs. low Zn | Zn ↑ 15% (eCO2 + eT) overall | ↓ 12% (eCO2 + eT) overall | 65 |
| Australia | FACE | CO2: 390 vs. 550 ppm | Zn ↓ 10% (eCO2)Fe ↔ (eCO2) | Not reported | 66 |
| Italy | FACE | CO2: 400 vs. 570 ppm | Zn ↓ 22% (eCO2)Fe ↓ 27% (eCO2) | Not reported | 67 |
| Turkey | OTC | CO2: 400 vs. 700 ppmWater: watered vs. droughtSoil: adequate vs. low Zn | Zn ↓ 10% (eCO2) wateredZn ↓ 23% (eCO2) droughtZn ↓ 22% (eCO2) adequate soil ZnZn ↓ 26% (eCO2) low soil Zn | ↑ 21% (eCO2) watered↑ 47% (eCO2) drought↑ 27% (eCO2) adequate soil Zn↑ 38% (eCO2) low soil Zn | 68 |
| China | T-FACE | CO2: ambient vs. 500 ppmTemperature: ambient vs. +2.0°C | Not reported | ↔ (eCO2)↓ 26% (eT)↔ (eCO2 + eT) | 50 |
| China | T-FACE | CO2: ambient vs. 500 ppmTemperature: ambient vs. +1.5°C, +2.0°C | Not reported | ↓ 10–12% (eCO2 + eT) | 52 |
| Germany | FACE | CO2: ambient vs. 571 ppm | Zn ↔ (eCO2)Fe ↓ 6% (eCO2) | ↔ (eCO2) | 69 |
| Australia | Tunnel house | CO2: ambient vs. 700 ppmTemperature: ambient vs. +2°C, +4°C, or +6°CWater: watered vs. droughtTwo cultivars: Janz and 38–19 | Not reported | ↓ (eCO2 + eT) both cultivars both watered and drought conditions | 70 |
| Australia | FACE | CO2: 384 vs. 550 ppm | Zn ↓ 22% (eCO2)Fe ↓ 10% (eCO2) | ↑ 48–61% (eCO2) | 71 |
| Germany | FACE | CO2: 375 vs. 550 ppm | Zn ↓ 9% (eCO2)Fe ↓ 15% (eCO2) | Not reported | 72 |
| China | OTC | CO2: 350 vs. 700 ppmWater: watered vs. drought | Zn ↓ 33% (eCO2) | ↑ 166% (eCO2) watered↑ 78% (eCO2) drought | 73 |
| Spain | Temperature gradient funnel | CO2: 350 vs. 700 ppm, Temperature: ambient vs. +4°C | Fe ↔ (eCO2) | Not reported | 74 |
| UK, Germany, Belgium, Denmark, Ireland, the Netherlands | OTC | CO2: 360 vs. 680 ppm | Zn ↔ (eCO2)Fe ↓ 18% (eCO2) | Not reported | 75 |
| Germany | OTC | CO2: 361, 523, or 639 ppmSoil N: 50 vs. 270 kg/h | Zn ↓ 13% (eCO2) at 523 ppm, N 150 kg/hZn ↓ 23% (eCO2) at 523 ppm, N 270 kg/hZn ↓ 13% (eCO2) at 639 ppm, N 150 kg/hZn ↓ 18% (eCO2) at 639 ppm, N 270 kg/hFe ↓ 18% (eCO2) at 523 ppm, N 150 kg/hFe ↓ 28% (eCO2) at 523 ppm, N 270 kg/hFe ↓ 25% (eCO2) at 639 ppm, N 150 kg/hFe ↓ 35% (eCO2) at 639 ppm, N 270 kg/h | Not reported | 76 |
| Germany | OTC | CO2: 384, 551, 718 ppm | Zn ↔ (eCO2)Fe ↔ (eCO2) | Not reported | 77 |
eCO2, elevated carbon dioxide; eT, elevated temperature; FACE, free-air carbon dioxide enrichment; OTC, open-top container; T-FACE, temperature free-air carbon dioxide enrichment, arrow up denotes increase, arrow down denotes decrease, arrow side-to-side denotes no change.
When the combined effects of both elevated carbon dioxide and temperature were studied together, elevated temperature offset the reductions of zinc and iron concentrations in wheat grains (43, 77). The combination of elevated carbon dioxide and temperature increased the concentrations of cadmium and lead in wheat grains, raising similar issues about metal toxicity as with rice with regard to climate change (43). Elevated carbon dioxide and temperature reduced the yield of wheat (43, 52, 65, 70), suggesting that the main effect of climate change on zinc and iron deficiencies will be to limit the yield of wheat rather than alter the zinc and iron content of wheat grains alone.
Legumes
Legumes, an important source of iron, zinc, B-complex vitamins, and protein, are generally consumed worldwide, with the highest consumption in Latin America and the Caribbean, sub-Saharan Africa, and South Asia (80). Soybean accounts for nearly three-quarters of global legume production; however, most soybean is used as animal feed and cooking oil, and only ∼6% is used directly as food for humans (80). Other legumes of importance in the human diet include peanut, common bean, field pea, chickpea, cowpea, fava bean, lentil, pigeon pea, lupin, and Bambara bean (80). Both chickpea and lentil are cool-season legumes that are more sensitive to the effects of global warming (81, 82).
The impacts of elevated carbon dioxide, elevated temperature, and other conditions upon zinc and iron concentrations and total yield of legumes have been examined in studies conducted in the United States, Brazil, Asia, and Australia (64, 83–94) (Table 3). These studies have been limited to a small number of legume species (Supplemental Table 3). Elevated carbon dioxide concentrations, as an isolated factor, increase the yield of soybean (92), chickpea (64), lentil (88), and common bean (93, 94). Elevated temperature alone decreases the yield of peanuts (95). In chickpeas, elevated carbon dioxide alone reduced zinc concentration but had no impact upon iron concentration (90). Elevated carbon dioxide alone reduced zinc and iron concentrations in both lentils and fava beans (85). Zinc concentrations were reduced by elevated carbon dioxide alone in some soybean cultivars (86). A meta-analysis showed that elevated carbon dioxide reduced zinc and iron concentrations in both soybeans and field peas (61). Elevated carbon dioxide and elevated temperatures combined had no impact upon zinc and iron concentrations in soybean (83). The overall yield of legumes is threatened by climate change, as elevated carbon dioxide and elevated temperatures combined have been shown to reduce the yield of soybean (84), lentil (88), peanut (91), and common bean (94). Currently, the common bean is cultivated by smallholder farmers in Zambia, Zimbabwe, Tanzania, western Malawi, and northern Mozambique. A study using the EcoCrop model developed by United Nations FAO predicts that most of this region will no longer be suitable for cultivation of the common bean by 2050 given climate change and predicted drought conditions (96).
TABLE 3.
Effect of elevated carbon dioxide, elevated temperature, and other conditions on the zinc and iron content and yield of legumes1
| Location | Study | Growing conditions | Micronutrients | Yield | Reference |
|---|---|---|---|---|---|
| India | FACE | CO2: 390 vs. 550 ppm | Not reported | ↑ 22% (eCO2) chickpea | 64 |
| USA | T-FACE | CO2: 400 vs. 600 ppmTemperature: ambient vs. +2.7°C day and +3.4°C night | Zn ↔ (eCO2 + eT) soybeanFe ↔ (eCO2 + eT) soybean | ↔ (eCO2 + eT) soybean | 83 |
| Brazil | OTC | CO2: 380 vs. 800 ppmTemperature: ambient vs. +4°C | Not reported | ↓ 11% (eCO2 + eT) soybean | 84 |
| Australia | FACE | CO2: 400 vs. 550 ppm Water: dry vs. wet | Zn ↓ 6% (eCO2), wet, lentilZn ↓ 24% (eCO2), dry, lentilFe ↓ 4% (eCO2) wet, lentilFe ↓ 20% (eCO2) dry, lentilZn ↓ 9% (eCO2) wet, fava beanZn ↓ 25% (eCO2) dry, fava beanFe ↓ 8% (eCO2) wet, fava beanFe ↓ 22% (eCO2) dry, fava bean | Not reported | 85 |
| China | OTC | CO2: 390 vs. 550 ppm, 4 soybean cultivars: ZK-1, ZK-2, ZK-3, HD | Zn ↓ 10% (eCO2) ZK-1Zn ↑ 14% (eCO2) ZK-2Zn ↓ 22% (eCO2) ZK-3Zn ↑ 14% (eCO2) HDFe ↓ 10% (eCO2) ZK-1Fe ↓ 8% (eCO2) ZK-2Fe ↓ 6% (eCO2) ZK-3Fe ↓ 13% (eCO2) HD | Not reported | 86 |
| Australia | FACE | CO2: ambient vs. 550 ppmTemperature: ambient vs. 38–40°C × 3 d to mimic heat wave | Not reported | ↓ 33% (ambient CO2 + eT) lentil↓ 33% (eCO2 + eT) lentil | 87 |
| Australia | FACE | CO2: ambient vs. 550 ppm | Not reported | ↑ 18–138% (eCO2) lentil | 88 |
| USA | OTC | CO2: 360 vs. 700 ppmTemperature: 26°C day, 16°C night vs. 45°C day, 35°C night | Zn ↓ 20% (eCO2 + eT) soybeanFe ↓ 36% (eCO2 + eT) soybean | Not reported | 89 |
| India | OTC | CO2: ambient vs. 580 ppm | Zn ↓ 36–41% (eCO2) chickpeaFe ↔ (eCO2) chickpea | Not reported | 90 |
| USA | Growth chamber | CO2: 380 vs. 550 ppmTemperature: ambient vs. +2.5°C, +5°CPeanut (Georgia Green, Pronto cultivars) | Not reported | ↓ 82% (eCO2 + eT + 2.5°C), Georgia Green↓ 92% (eCO2 + eT + 5°C), Georgia Green↓ 46% (eCO2 + eT + 2.5°C), Pronto↓ 91% (eCO2 + eT + 5°C), Pronto | 91 |
| India | OTC | CO2: 370 vs. 550 ppm | Not reported | ↑ 15% (eCO2), chickpea | 92 |
| USA | OTC | CO2: 385 vs. 565 ppm4 cultivars, common bean | Not reported | ↑ 17% (eCO2) overall for common bean, large variation by cultivar | 93 |
| USA | OTC | CO2: 350 vs. 700 ppmTemperature: ambient vs. range 28–40°C day and 18–30°C night | Not reported | ↑ 22% (eCO2) common bean↓ 30–100% (eCO2 + eT) common bean | 94 |
eCO2, elevated carbon dioxide; eT, elevated temperature; FACE, free-air carbon dioxide enrichment; HD, Hei-maodou; OTC, open-top container; T-FACE, temperature free-air carbon dioxide enrichment; ZK, Zhongke-maodou; arrow up denotes increase, arrow down denotes decrease, arrow side-to-side denotes no change.
Vegetables and fruit
Dark-green leafy vegetables and orange and yellow fruits are important plant sources of provitamin A carotenoids. Vegetables provide a substantial amount of iron and zinc among populations with a high intake of plant-based foods. The impact of elevated carbon dioxide upon overall yield and zinc, iron, and carotenoid concentrations in vegetables and fruit has been studied in Asia, Europe, the United States, and Australia, mostly using OTCs, growth chambers, or greenhouses (45, 97–113) (Table 4). The effect of climate change has been studied only in a limited number of vegetables and fruit species and cultivars (Supplemental Table 4). Meta-analyses and systematic reviews show that elevated carbon dioxide alone increases the yield of vegetables by 34% (114) and fruit by 38% (95); however, elevated temperature attenuates the stimulation and yield by elevated carbon dioxide (95). Elevated carbon dioxide, as an isolated factor, generally reduces the iron and zinc content of vegetables, although there can be differences by cultivar and soil conditions (Table 4). The impact of elevated carbon dioxide alone upon the carotenoid content of crops has not been consistent. Elevated carbon dioxide had no effect upon carotenoid concentrations in sweet pepper (100), spinach (105), and sweet potato (113), but reduced carotenoid concentrations in lettuce (108) and canola (111). A meta-analysis suggests that elevated carbon dioxide, as an isolated factor, reduces the carotenoid concentration of plants by 15% (115). This meta-analysis was based upon a diversity of plants, including non-crop plants, and it is unclear if these results can be generalized to vegetables and fruits that are important sources of provitamin A carotenoids.
TABLE 4.
Effect of elevated carbon dioxide, elevated temperature, and other conditions on the zinc, iron, and carotenoids content and yield of fruit and vegetables1
| Location | Type | Crop | Conditions | Micronutrients | Yield | Reference |
|---|---|---|---|---|---|---|
| Japan | Semi-closed chamber | Mustard spinach, bok choy | CO2: 390 vs. 700 ppm | Zn ↓ 20% (eCO2)Fe ↓ 20% (eCO2) | Not reported | 45 |
| China | OTC | Cucumber | CO2: 400 vs. 800 or 1200 ppmSoil: low or high N | Zn ↑ 16% (eCO2 800 ppm) low NFe ↓ 14% (eCO2 800 ppm) low NZn ↓ 14% (eCO2 800 ppm) high NFe ↓ 15% (eCO2 800 ppm) high NZn ↑ 30% (eCO2 1200 ppm) low NFe ↓ 17% (eCO2 1200 ppm) low NZn ↓ 12% (eCO2 1200 ppm) high NFe ↓ 34% (eCO2 1200 ppm) high N | ↑ 36% (eCO2 800 ppm) low N↑ 34% (eCO2 800 ppm) low N↑ 107% (eCO2 1200 ppm) high N↑ 71% (eCO2 1200 ppm) high N | 97 |
| Australia | Growth chamber | Strawberry (Albion, San Andreas cultivars) | CO2: 400 vs. 650, 950 ppmTemperature: 25°C vs. 30°C | Not reported | ↑ 12% (eCO2 650 ppm + eT), Albion↓ 49% (eCO2 950 ppm + eT), Albion↓ 56% (eCO2 650 ppm + eT), San Andreas↓ 50% (eCO2 950 ppm + eT), San Andreas | 98 |
| China | OTC | Cucumber | CO2: 400 vs. 625 or 1200 ppmSoil: low, medium, or high N | Zn ↓ 63% (eCO2) low NFe ↓ 57% (eCO2) low NZn ↓ 15% (eCO2) medium NFe ↓ 33% (eCO2) medium NZn ↓ 25% (eCO2) high NFe ↓ 3% (eCO2) high N | Not reported | 99 |
| Spain | Greenhouse | Sweet pepper | CO2: 380 vs. 800 ppmSoil: nitrate vs. ammonium nitrate | Lycopene ↔ (eCO2)β-Carotene ↔ (eCO2)Zn ↔ (eCO2)Fe ↓ 13% (eCO2) | Not reported | 100 |
| Netherlands | Climate-controlled room | Chinese cabbage | CO2: 420 vs. 800 ppmTemperature: 15°C day and 12°C night vs. 21°C day and 18°C nightSoil: nitrate vs. ammonium nitrate | Zn ↓ 18% (eCO2) nitrateZn ↔ (eCO2) ammonium nitrateZn ↔ (eCO2 + eT) nitrateZn ↔ (eCO2 + eT) ammonium nitrateFe ↔ (eCO2) nitrateFe ↔ (eCO2) ammonium nitrateFe ↔ (eCO2 + eT) nitrateFe ↔ (eCO2 + eT) ammonium nitrate | Not reported | 101 |
| USA | Growth chamber | Lettuce, spinach | CO2: 400 vs. 700 ppm | Zn ↓ 29% (eCO2) lettuceZn ↓ 18% (eCO2) spinachFe ↔ (eCO2) lettuceFe ↓ 30% (eCO2) spinach | Not reported | 102 |
| Spain | Growth chamber | Lettuce, (green and red cultivars) | CO2: 400 vs. 700 ppm | Zn ↔ (eCO2) green lettuceZn ↔ (eCO2) red lettuceFe ↓ 55% (eCO2) green lettuceFe ↓ 40% (eCO2) red lettuceCarotenoids ∼↓ 6% (eCO2) green lettuceCarotenoids ↔ (eCO2) red lettuce | Not reported | 103 |
| India | OTC | Potato | CO2: 382 vs. 570 ppmO3: 50 (ambient) vs. 70 ppb | Zn ↓ 2% (eCO2)Fe ↓ 25% (eCO2)Zn ↓ 15% (eCO2 + eO3)Fe ↓ 11% (eCO2 + eO3) | ↑ 92% (eCO2)↑ 69% (eCO2 + eO3) | 104 |
| India | OTC | Spinach | CO2: 382 vs. 570 ppmO3: 50 (ambient) vs. 70 ppb | Carotenoids ↔ (eCO2)Carotenoids ↑ 70% (eCO2 + eO3)Carotenoids ↓ 55% (eO3) | ↑ 73% (eCO2)↓ 25% (eO3)↑ 16% (eCO2 + eO3) | 105 |
| Pakistan | Greenhouse | Carrot, radish, turnip | CO2: 400 vs. 1000 ppm | Zn ↓ 17% (eCO2) carrotFe ↓ 8% (eCO2) carrotZn ↓ 17% (eCO2) radishFe ↑ 3% (eCO2) radishZn ↓ 15% (eCO2) turnipFe ↓ 19% (eCO2) turnip | ↑ 69% (eCO2) carrot↑ 139% (eCO2) radish↑ 72% (eCO2) turnip | 106 |
| Pakistan | Greenhouse | Tomato (Astra and Eureka cultivars) | CO2: 400 vs. 1000 ppm | Zn ↓ 14% (eCO2) AstraFe ↑ 3% (eCO2) AstraZn ↓ 28% (eCO2) EurekaFe ↑ 13% (eCO2) Eureka | ↑ 74% (eCO2) Astra↑ 84% (eCO2) Eureka | 107 |
| Spain | Greenhouse | Lettuce (red-green cultivars) | CO2: 395 vs. 710 ppmSoil: with or without arbuscular mycorrhizal fungi (AMF) | Zn ↑ 40% (eCO2) no AMF, red outer leavesZn ↑ 19% (eCO2) no AMF, red inner leavesZn ↑ 84% (eCO2) with AMF, red outer leavesZn ↑ 3% (eCO2) with AMF, red inner leavesFe ↑ 510% (eCO2) no AMF, red outer leavesFe ↓ 23% (eCO2) no AMF, red inner leavesFe ↑ 548% (eCO2) with AMF, red outer leavesFe ↑ 148% (eCO2) with AMF, red inner leavesZn ↓ 46% (eCO2) no AMF, green outer leavesZn ↓ 19% (eCO2) no AMF, green inner leavesZn ↑ 16% (eCO2) with AMF green outer leavesZn ↓ 33% (eCO2) with AMF green inner leavesFe ↑ 758% (eCO2) no AMF, green outer leavesFe ↑ 309% (eCO2) no AMF, green inner leavesFe ↑ 2% (eCO2) with AMF green outer leavesFe ↓ 48% (eCO2) with AMF green inner leavesCarotenoids ↓ ∼40% (eCO2) no AMF, red outer leavesCarotenoids, ↓ ∼16% (eCO2) no AMF, red inner leavesCarotenoids ↓ ∼18% (eCO2) with AMF, red outer leavesCarotenoids ↓ ∼24% (eCO2) with AMF, red inner leavesCarotenoids ↓ ∼7% (eCO2) no AMF, green outer leavesCarotenoids ↓ ∼9% (eCO2) no AMF, green inner leavesCarotenoids ↓ ∼55% (eCO2) with AMF, green outer leavesCarotenoids ↓ ∼22% (eCO2) with AMF, green inner leaves | Not reported | 108 |
| Germany | OTC | Potato | CO2: 380 vs. 550 ppm | Zn ↔ (eCO2)Fe ↔ (eCO2) | Not reported | 109 |
| India | OTC | Spinach, fenugreek | CO2: 350 vs. 600 ppm | Fe ↓ 55% (eCO2) spinachFe ↓ 38% (eCO2) fenugreek | Not reported | 110 |
| Canada | Growth chamber | Canola | CO2: 370 vs. 740 ppmTemperature: 22°C day and 18°C night vs. 28°C day and 14°C nightSoil: watered vs. drought | Carotenoids ↓ ∼8% (eCO2) wateredCarotenoids ↑ ∼17% (eCO2) droughtCarotenoids ↔ (eCO2 + eT) wateredCarotenoids ↔ (eCO2 + eT) drought | Not reported | 111 |
| Norway | Field chamber | Onion, carrot, lettuce, parsley, leek, Chinese cabbage, celery, celery root | CO2: 350 vs. 850 ppm | Not reported | ↑ ∼23% (eCO2) onion↑ ∼8% (eCO2) carrot↑ ∼18% (eCO2) lettuce↑ ∼17% (eCO2) parsley↔ (eCO2) leek↔ (eCO2) Chinese cabbage↔ (eCO2) celery↔ (eCO2) celery root | 112 |
| USA | Growth chamber | Sweet potato | CO2: 354 vs. 431, 506, 659 ppm | Carotenoids ↔ (eCO2 431 ppm)Carotenoids ↔ (eCO2 506 ppm)Carotenoids ↓ 24%, (eCO2 649 ppm) | Not reported | 113 |
AMF, arbuscular mycorrhizal fungi; eCO2, elevated carbon dioxide; eO3, elevated ozone; eT, elevated temperature; FACE, free-air carbon dioxide enrichment; OTC, open-top container; T-FACE, temperature free-air carbon dioxide enrichment; arrow up denotes increase, arrow down denotes decrease, arrow side-to-side denotes no change.
Mango, an important source of vitamin A in many LMICs, is sensitive to rainfall and temperature (116). Climate change is already affecting the cultivation and production of mango, with shifts to higher elevations and higher latitudes that are more amenable to flowering and fruit development (116, 117). Potato yields are expected to decline across India with climate change (118).
Nuts
The potential impact of elevated carbon dioxide and elevated temperature upon micronutrient quality and yield of nuts has not been well characterized (95). Temperate nut species require exposure to chilling conditions in the winter in order to break dormancy and to produce higher yields (119). Climate change is affecting winter chill and is expected to adversely affect nut production in some regions. Analysis of historic chill records and modeling of future chill estimates show that the production of almonds and pistachios in central Tunisia may no longer be viable in the future because of insufficient chilling (120). These findings suggest that temperate nut production in other parts of the Mediterranean region may be similarly affected. Other areas of temperate nut production that may be affected by chill losses include southern Brazil and South Africa (119). Tropical nut production is also likely to be affected by climate change. Cashew is sensitive to rainfall and temperature, particularly during flowering, and is considered highly susceptible to climate change (121). Elevated carbon dioxide and temperature are expected to adversely impact coconut production in areas of India (122).
Effects of ground-level ozone on major crops
In preindustrial times, the concentration of ground-level ozone was <20 ppb. Ground-level ozone is formed through photochemical reactions with products of anthropogenic emissions such as carbon monoxide, nitrogen oxides, methane, and non-methane volatile organic compounds. The rate of ground-level ozone formation increases with elevated atmospheric temperature (123). Current ground-level ozone concentrations in the northern mid-latitudes are ∼30 to 50 ppb (124). Since the 1950s, ground-level ozone levels have increased in the Northern Hemisphere by 1–5 ppb per decade and in the Southern Hemisphere by 1–2 ppb per decade (124). Geographic regions with higher ground-level ozone include northeastern India, eastern China, west and southern Africa, and the eastern United States (125). Ozone adversely affects plants by reducing photosynthesis and yield (124, 126).
A meta-analysis of wheat quality showed that elevated ground-level ozone, as an isolated factor, increases the zinc concentration of wheat grains but has no effect on the iron concentration (127). There has been a paucity of studies examining the impact of elevated carbon dioxide and elevated ozone combined upon micronutrient concentrations in crops. Elevated carbon dioxide and elevated ozone had no effect on the zinc content of rice (54), reduced the zinc and iron content of potato (104), and increased the carotenoid content of spinach (105). The impact of ozone on crop yields is more well established. Current levels of ground-level ozone are already causing yield loss in major food crops. In a meta-analysis that compared the yield of 6 major food crops grown at ozone levels of ≤26 ppb compared with the current 31–50 ppb, elevated ozone reduced the yield of rice, wheat, and barley by 17.5%, 9.7%, and 8.9%, respectively (128). Elevated ozone reduced the yield of soybean, common bean, and potato by 7.7%, 19%, and 5.3%, respectively (128). Elevated ozone threatens the food supply in the Indo Gangetic Plain of South Asia and the eastern coastal region of China (124). In a study that modeled global data on ozone and wheat production, elevated ground-level ozone reduced wheat yields by an estimated 9.9% in the Northern Hemisphere and 6.2% in the Southern Hemisphere in 2010–2012 (129). In China, during 2015–2018, elevated ground-level ozone concentrations reduced the production of winter wheat by an estimated 20–33%, maize by 5–6%, and rice by 4–9%, representing a total economic loss of US$23–45 billion (130).
Future changes in crop yields due to elevation in temperature and ground-level ozone conditions have been modeled for 2050 compared with 2000 using the Community Earth System Model (CESM) (131). CESM includes a climate change model to simulate the Earth's system consisting of atmosphere, atmospheric chemistry, ocean, ice, land surface, carbon cycle, and other components (132). The production of wheat, maize, and soybean under the RCP8.5 scenario is projected to have a global decrease while rice production is unchanged in 2050 compared with 2000 (131). Ozone regulation under the RCP4.5 scenario has the potential to reduce the warming impact and increase wheat production in China and the United States, but reduce wheat production in South Asia by up to 40% (131). Under RCP4.5, maize in the United States, Europe, and South America, and soybean in South America, would decrease by 40–50% mostly due to higher and more frequent extreme temperatures regardless of trends in ozone (131). A systematic review showed that a 25% increase in ozone was associated with an 8.9% reduction in the yield of vegetables and legumes (133).
Limitations of crop modeling studies
There is considerable heterogeneity between studies (Supplemental Tables 1–4), which limits the generalizability of the findings from experimental studies of the effects of carbon dioxide, temperature, and other conditions on crop yield and micronutrient content. Major limitations of the agronomic studies have been the lack of standardization in study design, experimental setup, number of replicates, and reporting of results. There are differences in cultivars, soils, and agronomic practices that may limit the generalizability of findings. The field may benefit from establishing standards of study design and reporting to facilitate interpretation and comparison of the results across studies. In addition, power calculations are lacking from nearly all agronomic studies reported in this review.
Pollinator loss
Climate change is associated with the phenological disruption of plant–pollinator mutualisms (134). A recent comprehensive review of the impact of climate change on pollinator decline shows that increasing ambient temperature, extended length of growing season, frequent extreme weather events, and winter weather fluctuations drive the homogenization of pollinator biodiversity, leading to long-term loss of resilience and greater susceptibility to collapses of pollinator communities (135). Rising temperature can cause changes in the development rates of plants such that first flowering comes at a different time than the emergence of their pollinators. Historical modeling shows that global change over the last 120 y degraded the interaction network between flowering plants and their pollinators, leading to a loss of bee species (136). Climate-driven shifts in plant ranges, habitat loss and fragmentation due to land-use changes, and high use of pesticides have further reduced pollinator populations (134, 137). Large declines in bumble bee populations have been recorded in North America and Europe (138). The global expansion of agricultural monoculture is associated with a limited pollinator supply and reduced pollination (139).
Of 150 of the world's leading crops, the proportions of vitamin C, vitamin A, folate, and iron in the food supply that are estimated to come from animal-pollinated crop plants are 98%, 70%, 55%, and 29%, respectively (140). The top pollinator-dependent crops that are sources of vitamin A include carrot, sweet potato, spinach, pumpkin, melon, and mango in many areas where vitamin A deficiency is highly prevalent, and in specific regions, such as okra in India; tropical fruits such as guava, jackfruit, passionfruit in India and Thailand; apricot and sour cherry in Iran; and peach in Mexico (141). A modeling analysis of food supplies and 156 countries suggests that complete removal of pollinators would cause a global decrease in the supply of fruit, vegetables, and nuts and seeds by 22.9%, 16.3%, and 22.1%, respectively (142). There would be a large decline in the vitamin A supply, with 71 million people in low-income countries becoming newly deficient in vitamin A (142).
Extreme weather events
Climate change is leading to the increased frequency, magnitude, and duration of extreme weather events such as drought, tropical cyclones, and heat waves (143). The risk of drought is predicted to increase for the entire African continent, with greater severity in central African countries (144). Climate change is predicted to increase the frequency of high-intensity tropical cyclones, with the most damage in North America, East Asia, and the Caribbean–Central American region (145). Extreme weather events can adversely impact agriculture. High groundwater levels in river flows can persist, increasing the susceptibility of flooding with later storms (143). Reduction in soil moisture can amplify the effects of heat waves (143). Extreme weather events have direct impacts upon agriculture and global food production, disrupt the food supply chain, and reduce the access to food (146).
Rising sea level and crops
Rising sea level has great potential to alter coastal ecosystems and destroy productive agricultural land. Ice sheets or glaciers cover ∼10% of the Earth's surface and are affected by climate change (19). The Greenland ice sheet and the ice sheets of Antarctica contain enough water to raise global mean sea level (GMSL) by 7.4 m (147) and 58 m (148), respectively. Glaciers, exclusive of Greenland and Antarctica, cover ∼706,000 km² worldwide and have the potential to raise GMSL by 0.4 m (149). The ocean contains 97% of water on Earth and currently covers 71% of the Earth's surface (19).
Surface air temperature is projected to increase at the average rate of 0.3°C per decade (19). Atmospheric warming is the main driver of glacier recession and ice sheet loss worldwide (19). GMSL is rising and accelerating from 1.4 mm/y in 1901–1990, to 2.1 mm/y in 1993–2015, and to 3.6 mm/y in 2006–2015 (19). The dominant source of GMSL is the sum of melt water from glaciers and the Greenland and Antarctic ice sheets. Between 1992 and 2018, Greenland has lost 3800 billion tons of ice (150). Satellite observations show that, from 1992 to 2017, the Antarctic ice sheet lost 2720 billion tons of ice, raising mean sea level by 7.6 mm (151). Mountain glaciers have lost ∼250 billion tons of ice per year between 1901 and 2009, or a loss of 21% of the glaciated volume of glaciers worldwide, excluding Antarctica (152). GMSL has risen by 0.43 to 0.84 m by 2010 relative to 1986–2005 (19). Sea-level rise by the end of the century is projected to be faster under all RCP emissions scenarios, including those compatible with the temperature goal of the Paris Agreement (19). Extreme sea-level events (episodic coastal inundations caused by a combination of high tides, waves, and storm surges) that are historically rare are expected to become commonplace by 2100 (19).
Sea-level rise has the greatest impact on the low elevation coastal zone (LECZ), defined as the contiguous and hydrologically connected zone of land along the coast and below 10 m of elevation. The LECZ comprises 2.3% of the total land area of all coastal countries (153). Currently, ∼680 million people, or 10% of the 2010 global population, inhabit the LECZ, and this population is projected to reach >1 billion people by 2050 (19). The populations in the LECZ with the greatest exposure to sea-level rise are mostly in low-income countries (154). The countries with the largest number of rural poor in the LECZ are India, Bangladesh, Myanmar, Cambodia, Nigeria, Pakistan, Iraq, Mozambique, Senegal, Brazil, China, Indonesia, the Philippines, Vietnam, and Thailand (154).
Rising sea level alters coastal ecosystems, agriculture, and the habitability of coastal communities. Erosion, flooding, and salinization accompany rising sea level in low-lying coastal areas. Sea water intrusion is a major cause of salinization and soil degradation. Rice production in coastal areas is especially susceptible to rising sea level and increasing soil salinity (155, 156). Sea-level rise is predicted to reduce global rice production by 1.6% to 2.7% accompanied by global rice price increases of 7.1% to 12.8% by 2080 (157). Rice production in Bangladesh, Japan, Taiwan, Egypt, Myanmar, and Vietnam is especially vulnerable to sea-level rise (157).
Impact of climate change on fisheries
Marine fish are rich in micronutrients and have been suggested as a potential solution to prevent micronutrient deficiencies in coastal populations of LMICs (158). Realizing such potential in the face of degradation of fisheries and climate change may be a challenge. Global fisheries peaked at 130 million tons in 1996 and have been steadily declining since that time (159). Ocean warming and declining oxygen levels in ocean and coastal waters are having an adverse impact upon global fisheries and shifting viable habitats and species ranges (160, 161). Industrial fishing is dominated by wealthy countries (162). Fishing fleets from the European Union, Russia, and East Asia, which have paid for access to fish in the exclusive economic zone (EEZ) of poorer countries, have led to local fish scarcity (163). In addition, countries such as China have built industrial fish meal–processing plants that convert local fresh fish primarily into animal feed (163). If the decline in the marine fish supply continues, an estimated 845 million people, or an additional 11% of the current global population, will become deficient in micronutrients such as zinc, iron, and vitamin A (164).
Another potential consequence of climate change is an adverse effect upon local coastal fisheries in low-income countries. Coral reefs are found in at least 150,000 km of coastline in >100 countries (19). Coral reefs are an important source of fish for poor communities relying upon small-scale, artisanal, or subsistence fishing. Worldwide, there are an estimated 6 million reef fishers in 99 countries and territories with coral reefs (7). Sea-level rise is predicted to elevate water depths beyond the vertical growth capacity of coral reefs, thus jeopardizing local fish supplies for coastal communities (165). Mangrove forests cover 138,000 to 152,000 km2 in tropical and subtropical coasts of ∼120 countries (19) and provide an ecosystem that supports near-shore subsistence, artisanal, and small-scale commercial fisheries (9). There are an estimated 4.1 million mangrove-associated fishers locally, with the highest number found in Indonesia, India, Bangladesh, Myanmar, and Brazil (9). Large-scale mortality of mangroves has occurred since the 1960s, with ∼70% of reported mangrove loss from natural causes occurring due to low-frequency, high-intensity weather events such as heat waves and tropical cyclones (166). Satellite mapping shows that natural causes, such as extreme weather, are currently a greater driver than human causes, such as farming and aquaculture, in causing mangrove loss (167). Sea-level rise is altering the distribution of mangroves inland and poleward (19). Small-scale fisheries that rely upon mangroves are important in providing protein and micronutrients to local populations, and degradation of mangrove-associated fisheries may jeopardize this dietary source of micronutrients.
Inland capture fisheries and aquaculture contribute >40% to global finfish production (168). The total inland fisheries catch was 11.5 million tons in 2015, or >12% of global capture fishery production (169). Most of this catch comes from LMICs in Africa and Asia; a large proportion of these fish are destined for local human consumption and are an important source of micronutrients for developing world populations (8). Climate change and freshwater warming are predicted to alter freshwater ecosystems, including species composition, number, and geographic range (169). Freshwater ecosystems are sensitive to climate-related shocks and variability (169). A vulnerability assessment of Africa's freshwater fishes showed that nearly 40% of fish species were vulnerable to climate change due to the highly specialized habitat and life-cycle requirements, especially in the African rift valley lakes, the Congo River drainage, and coastal rivers of West Africa (170).
Animal agriculture and climate change
Meat consumption is projected to increase in LMICs due to large population sizes, high population growth rates, and rising incomes (171). Global consumption of meat is expected to grow between 2020 and 2029 by 12%, with developing regions accounting for ∼80% of the growth in meat production (171). Meat production is a major source of GHG emissions, water use, and deforestation (172–174). In addition, arable land that produces plant foods for human consumption is converted to produce animal feed (175). Some LMICs are adopting the industrial food animal production (IFAP) model, which requires intensive use of feed and water (176). In some countries with scarce resources, IFAP may be reducing food security by producing grains for animal feed rather than humans (176). Meat can contribute micronutrients such as iron, zinc, and vitamin B-12 to the diet, but health and environmental concerns have been raised about excessive consumption of meat if LMICs adopt the dietary pattern of industrialized countries (177).
Mitigation and adaptation strategies
The most fundamental mitigation strategy to prevent declines in crop yields and ensure food security is to reduce GHG emissions, the main driver of climate change (19). Short of this goal, which requires international cooperation and compliance, a variety of adaptive strategies have been proposed to improve the micronutrient quality of plants or increase crop yields in the face of climate change. Iron and zinc biofortification of rice may be achieved through agronomic practices such as applying zinc or iron to soil or foliage (178–180). Plant-breeding approaches, whether conventional breeding approaches or genetic engineering, may be used to obtain higher zinc and iron concentrations in rice (178, 179, 181). Challenges to zinc fortification of rice include low uptake, transport, and loading of zinc into the rice grain (182). Both rice and wheat have extremely low iron concentrations, and most iron is present mainly in the outer bran layers (183). Conventional breeding may be difficult as an approach to increase iron in rice or wheat, since iron-rich rice and wheat germplasms are not available (183). Iron and zinc biofortification of wheat is also being developed using fertilizers and plant breeding (59, 184). Stable isotope studies show that zinc absorption from agronomically biofortified wheat is similar to post-harvest fortified wheat in humans (185). Plant-breeding approaches have been proposed to make legumes more resistant to elevated temperatures using insights from proteomic studies (37).
Climate change is altering environmental factors that affect both the geographical range of plants and growing season. Adaptive approaches to ensure crop productivity include shifting the location and elevation where crops are grown and increasing the length of the growing season where possible (186). The adverse impact of ozone upon rice and wheat yields may be countered by breeding plants that are more ozone-resistant (129, 187, 188). Similarly, the development of rice cultivars that are more salt tolerant may help adapt to salt intrusion in areas of coastal rice production (189). Policy changes are needed to ensure sustainability and better management of marine fisheries (162). Countries that have coastal fisheries that provide an important source of micronutrients for populations in LMICs could enact more protective legislation to regulate foreign fleets in their EEZ (163). Greater efforts could be taken to protect inland fisheries from competition with other water uses (168). The characterization of sustainable healthy diets, defined by FAO and WHO as “dietary patterns that promote all dimensions of individuals’ health and wellbeing; have low environmental pressure and impact; are accessible, affordable, safe and equitable; and are culturally acceptable” (190), with low to modest amounts of animal-source foods may help limit GHGs and other environmental impacts of animal agriculture in LMICs (191). The development of crops that are adapted to climate change may take a considerable amount of time and investment. More immediate approaches such as national micronutrient supplementation programs, post-harvest fortification of staple foods, and initiatives to increase dietary diversity and promote micronutrient-rich foods are a major priority.
Conclusions
Climate change has great potential to increase the risk of micronutrient malnutrition in vulnerable populations through many different pathways (Figure 1), with impacts as summarized in Table 5. The combination of increasing atmospheric carbon dioxide and rising temperature is predicted to reduce the overall yield of major staple crops, fruits, vegetables, and nuts, rather than affect the micronutrient content of the foods themselves. In addition, the yield of crops is reduced by increased ground-level ozone and an increase in extreme weather events. Pollinator loss is expected to reduce the yield of many pollinator-dependent crops such as fruits, vegetables, and nuts. Sea-level rise resulting from melting of ice sheets and glaciers is predicted to result in coastal inundation, salt intrusion, and loss of coral reefs and mangrove forests, with an adverse impact upon coastal rice production and coastal fisheries. Global ocean fisheries catch is predicted to decline because of ocean warming and declining oxygen. Freshwater warming is also expected to alter ecosystems and reduce inland fisheries catch. A decrease in the availability of micronutrient-rich foods (i.e., major staple crops, fruits, vegetables, nuts, and fish) may lead to global increases in food prices and increased vulnerability of poor families to micronutrient malnutrition (11, 12, 192). The impacts of climate change on the availability of micronutrient-rich foods are likely to vary by region and capacity for adaptation.
TABLE 5.
Potential impacts of climate change on food sources of micronutrients
| Climate change factor | Biological consequences | Impact on food sources of micronutrients |
|---|---|---|
| Increasing carbon dioxide and rising temperature combined | Reduced photosynthesisAltered plant mineral metabolismLoss of suitable growing region | Decreased yield of rice, wheat, barley, legumes, vegetables, fruits, nuts |
| Ground-level ozone | Reduced photosynthesisAltered plant mineral metabolism | Reduced yield of rice, wheat, barley, corn, legumes, potato |
| Increased extreme weather events | Water scarcityWater excessWind damageSoil loss | Reduced yield of cropsLoss of crops |
| Pollinator loss | Reduced pollination | Reduced yield of pollinator-dependent crops: fruits, vegetables, nuts |
| Sea-level rise | Coastal inundationSalt intrusionCoral reef lossMangrove loss | Loss of coastal rice productionLoss of coastal fisheries |
| Ocean warming and declining oxygen | Shifts in range of fishDecline in suitable habitat | Reduced ocean fisheries catch |
| Freshwater warming | Altered freshwater ecosystemsDecline in suitable habitat | Reduced inland fisheries catch |
In addition to limiting GHG production, a variety of mitigation strategies will be required to ensure reduction in the risk of micronutrient malnutrition, such as post-harvest fortification of foods; micronutrient supplementation; biofortification of staple crops with zinc and iron; plant breeding or genetic approaches to increase zinc, iron, and provitamin A carotenoid content of plant foods; and developing staple crops that are tolerant of abiotic stressors such as elevated CO2, elevated temperature, and increased soil salinity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Michael Milli for the figures of the plant growth platforms. The authors’ responsibilities were as follows—RDS, SA, and KK: designed the research; RDS: conducted research, analyzed data, and had primary responsibility for final content; RDS, SA, MWB, SG, and KK: wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Children's Investment Fund Foundation, London, UK, and the Santa Barbara Foundation.
Author disclosures: The authors report no conflicts of interest.
Disclaimer: The funders had no role in the design, implementation, analysis, or interpretation of the data.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CESM, Community Earth System Model; EEZ, exclusive economic zone; FACE, free-air carbon dioxide enrichment; GHG, greenhouse gas; GMSL, global mean sea level; IFAP, industrial food animal production; LECZ, low elevation coastal zone; LMIC, low- and middle-income country; OTC, open-top chamber; RCP, representative concentration pathway; SSP, shared socioeconomic pathway; T-FACE, temperature free-air carbon dioxide enrichment.
Contributor Information
Richard D Semba, Johns Hopkins Center for a Livable Future, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Sufia Askari, Children's Investment Fund Foundation, London, United Kingdom.
Sarah Gibson, Children's Investment Fund Foundation, London, United Kingdom.
Martin W Bloem, Johns Hopkins Center for a Livable Future, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Klaus Kraemer, Sight and Life, Basel, Switzerland; Center for Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
References
- 1. Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl 2):22–33. [DOI] [PubMed] [Google Scholar]
- 2. United Nations, Department of Economic and Social Affairs, Population Division . World population prospects 2019: highlights (ST/ESA/SER.A/423). New York, United Nations; 2019. [Google Scholar]
- 3. Kc S, Lutz W. The human core of the shared socioeconomic pathways: population scenarios by age, sex and level of education for all countries to 2100. Global Environ Change. 2017;42:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fanzo J, Bellows AL, Spiker ML, Thorne-Lyman AL, Bloem MW. The importance of food systems and the environment for nutrition. Am J Clin Nutr. 2021;113(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myers SS, Smith MR, Guth S, Golden CD, Vaitla B, Mueller ND, Dangour AD, Huybers P. Climate change and global food systems: potential impacts on food security and undernutrition. Annu Rev Public Health. 2017;38:259–77. [DOI] [PubMed] [Google Scholar]
- 6. Solomons NW. Plant-based diets are traditional in developing countries: 21st century challenges for better nutrition and health. Asia Pac J Clin Nutr. 2000;9(Suppl 1):S41–54. [DOI] [PubMed] [Google Scholar]
- 7. Mark HE, Houghton LA, Gibson RS, Monterrosa E, Kraemer K. Estimating dietary micronutrient supply and the prevalence of inadequate intakes from national food balance sheets in the South Asia region. Asia Pac J Clin Nutr. 2016;25(2):368–76. [DOI] [PubMed] [Google Scholar]
- 8. Teh LS, Teh LC, Sumaila UR. A global estimate of the number of coral reef fishers. PLoS One. 2013;8(6):e65397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooke SJ, Allison EH, Beard TD Jr, Arlinghaus R, Arthington AH, Bartley DM, Cowx IG, Fuentevilla C, Leonard NJ, Lorenzen K et al. On the sustainability of inland fisheries: finding a future for the forgotten. Ambio. 2016;45(7):753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ermgassen PSE, Mukherjee N, Worthington TA, Acosta A, Araujo ARR, Beitl CM, Castellanos-Galindo GA, Cunha-Lignon M, Dahdouh-Gueba FS, Diele K et al. Fishers who rely on mangroves: modelling and mapping the global intensity of mangrove-associated fisheries. Estuarine Coastal Shelf Sci. 2020;247:106975. [Google Scholar]
- 11. Bloem MW, Semba RD, Kraemer K. Castel Gandolfo workshop: an introduction to the impact of climate change, the economic crisis, and the increase in the food prices on malnutrition. J Nutr. 2010;140(1):132S–5S. [DOI] [PubMed] [Google Scholar]
- 12. Lake IR, Hooper L, Abdelhamid A, Bentham G, Boxall AB, Draper A, Fairweather-Tait S, Hulme M, Hunter PR, Nichols G et al. Climate change and food security: health impacts in developed countries. Environ Health Perspect. 2012;120(11):1520–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eggleton T. A short introduction to climate change. Port Melbourne, Victoria: Cambridge University Press; 2013. [Google Scholar]
- 14. IPCC, 2014: Climate Change 2014: impacts, adaptation, and vulnerability . Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, et al, editors]. Cambridge (UK) and New York: Cambridge University Press; 2014. [Google Scholar]
- 15. Keeling RF, Keeling CD. Atmospheric monthly in situ CO2 data—Mauna Loa Observatory, Hawaii, 2017. [Internet]. In: Scripps CO2 Program Data. UC San Diego Library Digital Collections. Available from: 10.6075/J08W3BHW (accessed 14 August 2020). [DOI] [Google Scholar]
- 16. National Oceanic and Atmospheric Administration. Earth System Research Laboratories. Global Monitoring Laboratory . Trends in atmospheric carbon dioxide. [Internet]. Available from: https://www.esrl.noaa.gov/gmd/ccgg/trends (accessed 25 July 2020). [Google Scholar]
- 17. Anderson TR, Hawkins E, Jones PD. CO2, the greenhouse effect and global warming: from the pioneering work of Arrhenius and Callendar to today's Earth system models. Endeavour. 2016;40(3):178–87. [DOI] [PubMed] [Google Scholar]
- 18. Hoegh-Guldberg O, Jacob D, Taylor M, Bindi M, Brown S, Camilloni IA, Diedhiou A, Djalante R, Ebi K et al. The human imperative of stabilizing global climate change at 1.5°C. Science. 2019;365(6459):eaaw6974. [DOI] [PubMed] [Google Scholar]
- 19. IPCC, 2019 : IPCC special report on the ocean and cryosphere in a changing climate [Internet] [Pörtner HO, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A et al. et al., editors]. Available from: https://www.ipcc.ch/srocc/ (accessed 25 July 2020). [Google Scholar]
- 20. Ainsworth EA, Lemonnier P, Wedow JM. The influence of rising tropospheric carbon dioxide and ozone on plant productivity. Plant Biology. 2020;22(S1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–72. [DOI] [PubMed] [Google Scholar]
- 22. Still CJ, Berry JA. Global distribution of C3 and C4 vegetation: carbon cycle implications. Glob Biogeochem Cycles. 2003;17:6–14. [Google Scholar]
- 23. Sage RF, Zhu XG. Exploiting the engine of C4 photosynthesis. J Exp Bot. 2011;62:2989–3000. [DOI] [PubMed] [Google Scholar]
- 24. Long SP, Ainsworth EA, Leakey AD, Morgan PB. Global food insecurity. Treatment of major food crops with elevated carbon dioxide or ozone under large-scale fully open-air conditions suggests recent models may have overestimated future yields. Philos Trans R Soc Lond B Biol Sci. 2005;360(1463):2011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot. 2009;60:2859–76. [DOI] [PubMed] [Google Scholar]
- 26. Van der Kooi CJ, Reich M, Löw M, De Kok LJ, Tausz M. Growth and yield stimulation under elevated CO2 and drought: a meta-analysis on crops. Environ Exp Bot. 2016;122:150–57. [Google Scholar]
- 27. Wang L, Feng Z, Schjoerring JK. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): a meta-analytic test of current hypotheses. Agric Ecosyst Environ. 2013;178:57–63. [Google Scholar]
- 28. Loladze I. Hidden shift of the ionome of plants exposed to elevated CO₂ depletes minerals at the base of human nutrition. Elife. 2014;3:e02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singer SD, Chatterton S, Soolanayakanahally RY, Subedi U, Chen G, Archarya SN. Potential effects of a high CO2 future on leguminous species. Plant-Environ Interact. 2020;1:67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGrath JM, Lobell DB. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 2013;36:697–705. [DOI] [PubMed] [Google Scholar]
- 31. Jacott CN, Boden SA. Feeling the heat: developmental and molecular responses of wheat and barley to high ambient temperatures. J Exp Bot. 2020; 71:5740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arshad MS, Farooq M, Asch F, Krishna JSV, Prasad PVV, Siddique KHM. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol Biochem. 2017;115:57–72. [DOI] [PubMed] [Google Scholar]
- 33. Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, Centeno GS, Khush GS, Cassman KG. Rice yields decline with higher night temperature from global warming. Proc Natl Acad Sci. 2004;101(27):9971–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Oort PAJ, Zwart SJ. Impacts of climate change on rice production in Africa and causes of simulated yield changes. Global Change Biol. 2018;24(3):1029–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhai R, Tao F, Lall U, Elliott J. Africa would need to import more maize in the future even under 1.5°C warming scenario. Earth's Future. 2021;9:e2020EF001574 [Google Scholar]
- 36. Naik PS, Singh M, Ranjan JK. Impact of climate change on vegetable production and adaptation measures. In Minhas PS, Rane J, Pasala RKeditors. Abiotic stress management for resilient agriculture. Singapore: Springer; 2017. p. 413–28. [Google Scholar]
- 37. Sita K, Sehgal A, HanumanthaRao B, Nair RM, Prasad PVV, Kumar S, Gaur PM, Farooq M, Siddique KHM, Varshney RK et al. Food legumes and rising temperatures: effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front Plant Sci. 2017;8:1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prasad PVV, Allen LH Jr, Boote KJ. Crop responses to elevated carbon dioxide and interaction with temperature. J Crop Improve. 2005;13:113–55. [Google Scholar]
- 39. Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. An overview of global rice production, supply, trade, and consumption. Ann N Y Acad Sci. 2014;1324:7–14. [DOI] [PubMed] [Google Scholar]
- 40. Food and Agricultural Organization of the United Nations . FAOSTAT. Food and agriculture data. [Internet]. Available from: https://www.fao.org/faostat/en(accessed 16 March 2021). [Google Scholar]
- 41. Zhu C, Kobayashi K, Loladze I, Zhu J, Jiang Q, Xu X, Liu G, Seneweera S, Ebi KL, Drewnowski A et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci Adv. 2018;4(5):eaaq1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei L, Wang W, Zhu J, Wang Z, Wang J, Li C, Zeng Q, Ziska LH. Responses of rice qualitative characteristics to elevated carbon dioxide and higher temperature: implications for global nutrition. J Sci Food Agric. 2021;101:3854–61. [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Li L, Lam SK, Liu X, Pan G. Responses of wheat and rice grain mineral quality to elevated carbon dioxide and canopy warming. Field Crops Res. 2020;249:107753. [Google Scholar]
- 44. Wang W, Cai C, He J, Gu J, Zhu G, Zhang W, Zhu J, Liu G. Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res. 2020;248:107605. [Google Scholar]
- 45. Ujiie K, Ishimaru K, Hirotsu N, Nagasaka S, Miyakoshi Y, Ota M, Tokida T, Sakai H, Usui Y, Ono K et al. How elevated CO2 affects our nutrition in rice, and how we can deal with it. PLoS One. 2019;14(3):e0212840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu C, Kobayashi K, Loladze I, Zhu J, Jiang Q, Xu X, Liu G, Seneweera S, Ebi KL, Drewnowski A et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci Adv. 2018;4:eaaq1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang W, Cai C, Lam SK, Liu G, Zhu J. Elevated CO2 cannot compensate for japonica grain yield losses under increasing air temperature because of the decrease in spikelet density. Eur J Agron. 2018;99:21–9. [Google Scholar]
- 48. Chaturvedi AK, Bahuguna RN, Pal M, Shah D, Maurya S, Jagadish KSV. Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crops Res. 2017;206:149–57. [Google Scholar]
- 49. Li C, Zhu JG, Sha LN, Zhang JS, Zeng Q, Liu G. Rice (Oryza sativa L.) growth and nitrogen distribution under elevated CO2 concentration and air temperature. Ecol Res. 2017;32:405–11. [Google Scholar]
- 50. Wang J, Liu X, Zhang, Smith P, Li L, Filley TR, Cheng K, Shen M, He Y, Pan G. Size and variability of crop productivity both impacted by CO2 enrichment and warming—a case study of 4 year field experiment in a Chinese paddy. Agric Ecosyst Environ. 2016;221:40–9. [Google Scholar]
- 51. Usui Y, Sakai H, Tokida T, Nakamura H, Nakagawa H, Hasegawa T. Rice grain yield and quality responses to free-air CO2 enrichment combined with soil and water warming. Global Change Biol. 2016;22:1256–70. [DOI] [PubMed] [Google Scholar]
- 52. Cai C, Yin X, He S, Jiang W, Si C, Struik PC, Luo W, Li G, Xie Y, Xiong Y et al. Response of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Global Change Biol. 2016;22:856–74. [DOI] [PubMed] [Google Scholar]
- 53. Goufo P, Falco V, Brites C, Wessel DF, Kratz S, Rosa EAS, Carranca C, Trindade H. Effect of elevated carbon dioxide concentration on rice quality: nutritive value, color, milling, cooking, and eating qualities. Cereal Chem. 2014;91:513–21. [Google Scholar]
- 54. Wang Y, Song Q, Frei M, Shao Z, Yang L. Effects of elevated ozone, carbon dioxide, and the combination of both on the grain quality of Chinese hybrid rice. Environ Pollut. 2014;189:9–17. [DOI] [PubMed] [Google Scholar]
- 55. Hasegawa T, Sakai H, Tokida T, Nakamura H, Zhu C, Usui Y, Yoshimoto M, Fukuoka M, Wakatsuki H, Katayanagi N et al. , Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct Plant Biol. 2013;40:148–59. [DOI] [PubMed] [Google Scholar]
- 56. Yang L, Wang Y, Dong G, Gu H, Huang J, Zhu J, Yang H, Liu G, Han Y. The impact of free-air CO2 enrichment (FACE) and nitrogen supply on grain quality of rice. Field Crops Res. 2007;102:128–40. [Google Scholar]
- 57. Lieffering M, Kim HY, Kobayashi K, Okada M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crops Res. 2004;88:279–86. [Google Scholar]
- 58. Seneweera SP, Conroy JP. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition. Soil Sci Plant Nutr. 1997;43:1131–6. [Google Scholar]
- 59. Al-Hadeethi I, Li Y, Odhafa AKH, Al-Hadeethi H, Seneweera S, Lam SK. Assessment of grain quality in terms of functional group response to elevated [CO2], water, and nitrogen using a meta-analysis: grain protein, zinc, and iron under future climate. Ecol Evol. 2019;9(13):7425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey AD, Bloom AJ, Carlisle E, Dietterich LH, Fitzgerald G, Hasegawa T et al. Increasing CO2 threatens human nutrition. Nature. 2014;510:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hu S, Wang Y, Yang L. Response of rice yield traits to elevated atmospheric CO2 concentration and its interaction with cultivar, nitrogen application rate and temperature: a meta-analysis of 20 years FACE studies. Sci Total Environ. 2021;764:142797. [DOI] [PubMed] [Google Scholar]
- 62. Cakmak I, Pfeiffer WH, McClafferty B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010;87:10–20. [Google Scholar]
- 63. Tcherkez G, Mariem SB, Larraya L, García-Mina JM, Zamarreño AM, Paradela A, Cui J, Badeck FW, Mea D, Rizza F et al. Elevated CO2 has concurrent effects on leaf and green metabolism but minimal effects on yield in wheat. J Exp Bot. 2020;71:5990–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chakrabarti B, Singh SD, Bhatia A, Kumar V, Harit RC. Yield and nitrogen uptake in wheat and chickpea grown under elevated carbon dioxide level. Natl Acad Sci Lett. 2020;43:109–13. [Google Scholar]
- 65. Asif M, Tunc CE, Yazici MA, Tutus Y, Rehman R, Rehman A, Ozturk L. Effect of predicted climate change on growth and yield performance of wheat under varied nitrogen and zinc supply. Plant Soil. 2019;434:231–44. [Google Scholar]
- 66. Jin J, Armstrong R, Tang C. Impact of elevated CO2 on grain nutrient concentration varies with crops and soils—a long-term FACE study. Sci Total Environ. 2019;651(Pt 2):2641–47. [DOI] [PubMed] [Google Scholar]
- 67. Beleggia R, Fragasso M, Miglietta F, Cattivelli L, Menga V, Nigro F, Pecchioni N, Fares C. Mineral composition of durum wheat grain and pasta under increasing atmospheric CO2 concentrations. Food Chem. 2018;242:53–61. [DOI] [PubMed] [Google Scholar]
- 68. Asif M, Yilmaz O, Ozturk L. Elevated carbon dioxide ameliorates the effect of Zn deficiency and terminal drought on wheat grain yield but compromises nutritional quality. Plant Soil. 2017;411:57–67. [Google Scholar]
- 69. Högy P, Brunnbauer M, Koehler P, Schwadorf K, Breuer J, Franzaring J, Zhunusbayeva D, Fangmeier A. Grain quality characteristics of spring wheat (Triticum aestivum) as affected by free-air CO2 enrichment. Environ Exp Bot. 2013;88:11–8. [Google Scholar]
- 70. Dias de Oliveira D, Bramley H, Siddique KHM, Henty S, Berger J, Palta JA. Can elevated CO2 combined with high temperature ameliorate the effect of terminal drought in wheat?. Funct Plant Biol. 2013;40:160–71. [DOI] [PubMed] [Google Scholar]
- 71. Fernando N, Panozzo J, Tausz M, Norton R, Fitzgerald G, Seneweera S. Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain. Food Chem. 2012;133(4):1307–11. [DOI] [PubMed] [Google Scholar]
- 72. Wu DX, Wang GX, Bai YF, Liao JX. Effects of elevated CO2 concentration on growth, water use, yield and grain quality of wheat under two soil water levels. Agric Ecosyst Environ. 2004;104:493–507. [Google Scholar]
- 73. Erbs M, Manderscheid R, Jansen G, Seddig S, Pacholski A, Weigel HJ. Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agric Ecosyst Environ. 2010;136(1-2):59–68. [Google Scholar]
- 74. De La Puente LS, Pérez PP, Martínez-Carrasco R, Morcuende RM, Del Molino IMM. Action of elevated CO2 and high temperatures on the mineral chemical composition of two varieties of wheat. Agrochim. 2000;44:221–30. [Google Scholar]
- 75. Fangmeier A, De Temmerman L, Mortensen L, Kemp K, Burke J, Mitchell R, van Oijen M, Weigel HJ. Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment “ESPACE-wheat.”. Eur J Agron. 1999;10:215–29. [Google Scholar]
- 76. Fangmeier A, Grüters U, Högy P, Vermehren B, Jäger HJ. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat—II: nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ Pollut. 1997;96:43–59. [DOI] [PubMed] [Google Scholar]
- 77. Manderscheid R, Bender J, Jäger HJ, Weigel HJ. Effects of season long CO2 enrichment on cereals. II. Nutrient concentrations and grain quality. Agric Ecosyst Environ. 1995;54:175–85. [Google Scholar]
- 78. Högy P, Fangmeier A. Effects of elevated atmospheric CO2 on grain quality of wheat. J Cereal Sci. 2008;48(3):580–91. [Google Scholar]
- 79. Broberg MC, Högy P, Pleijel H. CO2-induced changes in wheat grain composition: meta-analysis and response functions. Agronomy. 2017;7(2):32. [Google Scholar]
- 80. Semba RD, Ramsing R, Rahman N, Kraemer K, Bloem MW. Legumes as a sustainable source of protein in human diets. Global Food Security. 2021;28:100520. [Google Scholar]
- 81. Guar PM, Jukanti AK, Samineni S, Chaturvedi SK, Basu PS, Babbar A, Jayalakshmi V, Nayyar H, Devasirvatham V, Mallikarjuna N et al. Climate change and heat stress tolerance in chickpea. In: Tuteja N, Gill SS, editors. Climate change and plant abiotic stress tolerance. Weinheim, Germany: Wiley-Blackwell; 2013. p. 837–56. [Google Scholar]
- 82. Kumar J, Kant R, Kumar S, Basu PS, Sarker A, Singh NP. Heat tolerance in lentil under field conditions. Legume Genomics Genet. 2016;7:1–11. [Google Scholar]
- 83. Köhler IH, Huber SC, Bernacchi CJ, Baxter IR. Increased temperatures may safeguard the nutritional quality of crops under future elevated CO2 concentrations. Plant J. 2019;97:872–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Palacios CJ, Grandis A, Carvalho VJ, Salatino A, Buckeridge MS. Isolated and combined effects of elevated CO2 and high temperature on the whole-plant biomass and the chemical composition of soybean seeds. Food Chem. 2019;275:610–17. [DOI] [PubMed] [Google Scholar]
- 85. Parvin S, Uddin S, Tausz-Posch S, Armstrong R, Fitzgerald G, Tausz M. Grain mineral quality of dryland legumes as affected by elevated CO2 and drought: a FACE study on lentil (Lens culinaris) and faba bean (Vicia faba). Crop Pasture Sci. 2019;70:244–53. [Google Scholar]
- 86. Li Y, Yu Z, Jin J, Zhang Q, Wang G, Liu C, Wu J, Wang C, Liu X. Impact of elevated CO2 on seed quality of soybean at the fresh edible and mature stages. Front Plant Sci. 2018;9:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bourgault M, Löw M, Tausz-Posch S, Nuttall JG, Delahunty AJ, Brand J, Panozzo JF, McDonald L, O'Leary GJ, Armstrong RD et al. Effect of a heatwave on lentil grown under free-air CO2 enrichment (FACE) in a semi-arid environment. Crop Sci. 2018;58:803–12. [Google Scholar]
- 88. Bourgault M, Brand J, Tausz-Posch S, Armstrong RD, O'Leary GJ, Fitzgerald GJ, Tausz M. Yield, growth and grain nitrogen response to elevated CO2 and six lentil cultivars grown under free air CO2 enrichment (FACE) in a semi-arid environment. Eur J Agron. 2017;87:50–8. [Google Scholar]
- 89. Bellaloui N, Hu Y, Mengistu A, Abbas HK, Kassem MA, Tigabu M. Elevated atmospheric carbon dioxide and temperature affect seed composition, mineral nutrition, and 15N and 13C dynamics in soybean genotypes under controlled environments. Atlas J Plant Biol. 2016:56–65. [Google Scholar]
- 90. Saha S, Chakraborty D, Sehgal VK, Pal M. Potential impact of rising atmospheric CO2 on quality of grains in chickpea (Cicer arietinum L.). Food Chem. 2015;187:431–36. [DOI] [PubMed] [Google Scholar]
- 91. Bannayan M, Tojo Soler CM, Garcia y Garcia A, Guerra LC, Hoogenboom G. Interactive effects of elevated [CO2] and temperature on growth and development of a short- and long-season peanut cultivar. Clim Change. 2009;93:389–406. [Google Scholar]
- 92. Pal M, Talawar S, Deshmukh PS, Vishwanathan C, Khetarpal S, Kumar P, Luthria D. Growth and yield of chickpea under elevated carbon dioxide concentration. Indian J Plant Physiol. 2008;13:367–74. [Google Scholar]
- 93. Bunce JA. Contrasting responses of seed yield to elevated carbon dioxide under field conditions within P haseolus vulgaris. Agric Ecosyst Environ. 2008;128:219–24. [Google Scholar]
- 94. Prasad PVV, Boote KJ, Allen LH Jr, Thomas JMG. Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Global Change Biol. 2002;8:710–21. [Google Scholar]
- 95. Alae-Carew C, Nicoleau S, Bird FA, Hawkins P, Tuomisto HL, Haines A, Dangour AD, Scheelbeek PFD. The impact of environmental changes on the yield and nutritional quality of fruits, nuts and seeds: a systematic review. Environ Res Lett. 2020;15:023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hummel M, Hallahan BF, Brychkova G, Ramirez-Villegas J, Guwela V, Chataika B, Curley E, McKeown PC, Morrison L, Talsma EF et al. Reduction in nutritional quality and growing area suitability of common bean under climate change induced drought stress in. Sci Rep. 2018;8(1):16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dong J, Xu Q, Gruda N, Chu W, Li X, Duan Z. Elevated and super-elevated CO2 differ in their interactive effects with nitrogen availability on fruit yield and quality of cucumber. J Sci Food Agric. 2018;98(12):4509–16. [DOI] [PubMed] [Google Scholar]
- 98. Balasooriya HN, Dassanayake KB, Seneweera S, Ajlouni S. Interaction of elevated carbon dioxide and temperature on strawberry (Fragaria x ananassa) growth and fruit yield. World Acad Sci Eng Tech Int J Agric Biosys Eng. 2018;12:279–87. [Google Scholar]
- 99. Dong J, Xun L, Gruda N, Duan Z. Interactive effects of elevated carbon dioxide and nitrogen availability on fruit quality of cucumber (Cucumis sativus L.). J Integrat Agric. 2018;17:2438–46. [Google Scholar]
- 100. Piñero MC, Otálora G, Porras ME, Sánchez-Guerrero MC, Lorenzo P, Medrano E, Del Amor FM. The form in which nitrogen is supplied affects the polyamines, amino acids, and mineral composition of sweet pepper fruit under an elevated CO2 concentration. J Agric Food Chem. 2017;65:711–17. [DOI] [PubMed] [Google Scholar]
- 101. Reich M, van den Meerakker AN, Parmar S., Hawkesford MJ, De Kok LJ. Temperature determines size and direction of effects of elevated CO2 and nitrogen form on yield quantity and quality of Chinese cabbage. Plant Biol. 2016;18(Suppl 1):63–75. [DOI] [PubMed] [Google Scholar]
- 102. Giri A, Armstrong B, Rajashekar C. Elevated carbon dioxide level suppresses nutritional quality of lettuce and spinach. Am J Plant Sci. 2016;7:246–58. [Google Scholar]
- 103. Pérez-López U, Miranda-Apodaca J, Lacuesta M, Mena-Petite A, Muñoz-Rueda A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Scientia Hort. 2015;195:56–66. [Google Scholar]
- 104. Kumari S, Agrawal M. Growth, yield and quality attributes of a tropical potato variety (Solanum tuberosum L. cv Kufri chandramukhi) under ambient and elevated carbon dioxide and ozone and their interactions. Ecotoxicol Environ Saf. 2014;101:146–56. [DOI] [PubMed] [Google Scholar]
- 105. Kumari S, Agrawal M, Tiwari S. Impact of elevated CO2 and elevated O3 on Beta vulgaris L.: pigments, metabolites, antioxidants, growth and yield. Environ Pollut. 2013;174:279–88. [DOI] [PubMed] [Google Scholar]
- 106. Azam A, Kahn I, Mahmood A, Hameed A. Yield, chemical composition and nutritional quality responses of carrot, radish and turnip to elevated atmospheric carbon dioxide. J Sci Food Agric. 2013;93:3237–44. [DOI] [PubMed] [Google Scholar]
- 107. Khan I, Azam A, Mahmood A. The impact of enhanced atmospheric carbon dioxide on yield, proximate composition, elemental concentration, fatty acid and vitamin contents of tomato (Lycopersicon esculentum). Environ Monit Assess. 2013;185:205–14. [DOI] [PubMed] [Google Scholar]
- 108. Baslam M, Garmendia I, Goicoechea N. Elevated CO2 may impair the beneficial effect of arbuscular mycorrhizal fungi on the mineral and phytochemical quality of lettuce. Ann Appl Biol. 2012;161:180–91. [Google Scholar]
- 109. Högy P, Fangmeier A. Atmospheric CO2 enrichment affects potatoes: 2. Tuber quality traits. Eur J Agron. 2009;30:85–94. [Google Scholar]
- 110. Jain V, Pal M, Raj A, Khetarpal S. Photosynthesis and nutrient composition of spinach and fenugreek grown under elevated carbon dioxide concentration. Biologia plantarum. 2007;51:559–62. [Google Scholar]
- 111. Qaderi MM, Kurepin LV, Reid DM. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: temperature, carbon dioxide and drought. Physiol Plant. 2006;128:710–21. [Google Scholar]
- 112. Mortensen LM. Effects of elevated CO2 concentrations on growth and yield of eight vegetables species in a cool climate. Scientia Hort. 1994;58:177–85. [Google Scholar]
- 113. Lu JY, Biswas PK, Pace RD. Effect of elevated CO2 growth conditions on the nutritive composition and acceptability of baked sweet potatoes. J Food Sci. 1986;51:358–59. [Google Scholar]
- 114. Dong J, Gruda N, Li X, Tang Y, Zhang P, Duan Z. Sustainable vegetable production under changing climate: the impact of elevated CO2 on yield of vegetables and the interactions with environments—a review. J Cleaner Prod. 2020;253:119920. [Google Scholar]
- 115. Loladze I, Nolan JM, Ziska LH, Knobbe AR. Rising atmospheric CO2 lowers concentrations of plant carotenoids essential to human health: a meta-analysis. Mol Nutr Food Res. 2019;63(15):1801047. [DOI] [PubMed] [Google Scholar]
- 116. Makhmale S, Bhutada P, Yadav L, Yada BK. Impact of climate change on phenology of mango—a case study. Eco Env Cons. 2016;22:S127–32. [Google Scholar]
- 117. Normand F, Lauri PE, Legave JM. Climate change and its probable effects on mango production and cultivation. Acta Horticulturae. 2015;1075:21–32. [Google Scholar]
- 118. Ayyogari K, Sidhya P, Pandit MK. Impact of climate change on vegetable cultivation—a review. Int J Agric Environ Biotech. 2014;7:145–55. [Google Scholar]
- 119. Luedeling E. Climate change impacts on winter chill for temperate fruit and nut production: a review. Scientia Hort. 2012;144:218–29. [Google Scholar]
- 120. Benmoussa H, Luedeling E, Ghrab M, Ben Mimoun M. Severe winter chill decline impacts Tunisian fruit and nut orchards. Clim Change. 2020;162:1249–67. [Google Scholar]
- 121. Rupa TR, Rejani R, Bhat MG. Impact of climate change on cashew and adaptation strategies. In: Singh HCP, Rao NKS, editors. Climate-resilient horticulture: adaptation and mitigation strategies. New Delhi, India: Springer; 2013. p. 189–98. [Google Scholar]
- 122. Hebbar KB, Balasimha D, Thomas GV. Plantation crops response to climate change: coconut perspective. In: Singh HCP, Rao NKS, editors. Climate-resilient horticulture: adaptation and mitigation strategies. New Delhi, India: Springer; 2013. p. 177–87. [Google Scholar]
- 123. Orru H, Ebi KL, Forsberg B. The interplay of climate change and air pollution on health. Curr Envir Health Rpt. 2017;4:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Emberson L. Effects of ozone on agriculture, forests and grasslands. Philos Trans R Soc A. 2020;378:20190327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fishman J, Wozniak AE, Creilson JK. Global distribution of tropospheric ozone from satellite measurements using the empirically corrected trophospheric ozone residual technique: identification of the regional aspects of air pollution. Atmos Chem Phys. 2003;3:893–907. [Google Scholar]
- 126. Wilkinson S, Mills G, Illidge R, Davies WJ. How is ozone pollution reducing our food supply?. J Exp Bot. 2012;63:527–36. [DOI] [PubMed] [Google Scholar]
- 127. Broberg MC, Feng Z, Xin Y, Pleijel H. Ozone effects on wheat grain quality—a summary. Environ Pollut. 2015;197:203–13. [DOI] [PubMed] [Google Scholar]
- 128. Feng Z, Kobayashi K. Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos Environ. 2009;43:1510–19. [Google Scholar]
- 129. Mills G, Sharps K, Simpson D, Pleijel H, Broberg M, Uddling J, Jaramillo F, Davies WJ, Dentener F, Van den Berg M et al. Ozone pollution will compromise efforts to increase global wheat production. Global Change Biol. 2018;24:3560–74. [DOI] [PubMed] [Google Scholar]
- 130. Zhao H, Zheng Y, Zhang Y, Li T. Evaluating the effects of surface O3 on three main food crops across China during 2015-2018. Environ Pollut. 2020;258:113794. [DOI] [PubMed] [Google Scholar]
- 131. Tai APK, Martin MV, Health CL. Threat to future global food security from climate change and ozone air pollution. Nat Clim Change. 2014;4:817–21. [Google Scholar]
- 132. Hurrell JW, Holland MM, Gent PR, Ghan S, Kay JE, Kushner PJ, Lamarque J-F, Large WG, Lawrence D, Lindsay K et al. The Community Earth System Model: a framework for collaborative research. Bull Am Meteorol Soc. 2013;94:1339–60. [Google Scholar]
- 133. Scheelbeek PFD, Bird FA, Tuomisto HL, Green R, Harris FB, Joy EJM, Chalabi Z, Allen E, Haines A, Dangour AD. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc Natl Acad Sci. 2018;115:6804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Settele J, Bishop J, Potts SG. Climate change impacts on pollination. Nature Plants. 2016;2:16092. [DOI] [PubMed] [Google Scholar]
- 135. Vasiliev D, Greenwood S. The role of climate change in pollinator decline across the Northern Hemisphere is underestimated. Sci Total Environ. 2021;775:145788. [DOI] [PubMed] [Google Scholar]
- 136. Burkle LA, Marlin JC, Knight TM. Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science. 2013;339:1611–15. [DOI] [PubMed] [Google Scholar]
- 137. Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J et al. Safeguarding pollinators and their values to human well-being. Nature. 2016;540:220–29. [DOI] [PubMed] [Google Scholar]
- 138. Soroye P, Newbold T, Kerr J. Climate change contributes to widespread declines among bumble bees across continents. Science. 2020;367:685–88. [DOI] [PubMed] [Google Scholar]
- 139. Aizen MA, Aguiar S, Biesmeijer JC, Garibaldi LA, Inouye DW, Jung C, Martins DJ, Medel R, Morales CL, Ngo H et al. Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Global Change Biol. 2019;25:3516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Eilers EJ, Kremen C, Smith Greenleaf S, Garber AK, Klein AM. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS One. 2011;6:e21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chaplin-Kramer R, Dombeck E, Gerber J, Knuth KA, Mueller ND, Mueller M, Ziv G, Klein AM. Global malnutrition overlaps with pollinator-dependent micronutrient production. Proc Biol Sci. 2014;281:20141799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Smith MR, Singh GM, Mozaffarian D, Myers SS. Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis. Lancet North Am Ed. 2015;386(10007):1964–72. [DOI] [PubMed] [Google Scholar]
- 143. IPCC . Managing the risks of extreme events and disasters to advance climate change adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change[Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner GK, Allen SK et al., editors]. Cambridge (UK) and New York: Cambridge University Press; 2012. [Google Scholar]
- 144. Ahmadalipour A, Moradkhani H, Castelletti A, Magliocca N. Future drought risk in Africa: integrating vulnerability, climate change, and population growth. Sci Total Environ. 2019;662:672–86. [DOI] [PubMed] [Google Scholar]
- 145. Mendelsohn R, Emanuel K, Chonabayashi S, Bakkensen L. The impact of climate change on global tropical cyclone damage. Nature Climate Change. 2012;2:205–9. [Google Scholar]
- 146. Beer T. The impact of extreme weather events on food security. In: Mal S, Singh RB, Huggel C, editors. Climate change, extreme events and disaster risk reduction. Cham (Switzerland): Springer; 2018. p. 121–33. [Google Scholar]
- 147. Morlighem M, Williams CN, Rignot E, An L, Arndt JE, Bamber JL, Catania G, Chauché N, Dowdeswell JA et al. BedMachine v3: complete bed topography and ocean bathymetry mapping of Greenland from multibeam echo sounding combined with mass conservation, Geophys Res Lett. 2017;44:11051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Fretwell P, Pritchard HD, Vaughan DG, Bamber JL, Barrand NE, Bell R, Bianchi C, Bingham RG, Blankenship DD, Casassa G et al. Bedmap2: improved ice bed, surface and thickness datasets for Antarctica. The Cryosphere. 2013;7:375–93. [Google Scholar]
- 149. Zemp M, Huss M, Thibert E, Eckert N, McNabb R, Huber J, Barandun M, Machguth H, Nussbaumer SU, Gärtner-Roer I et al. Global glacier mass changes and their contributions to sea-level rise from 1961 to 2016. Nature. 2019;568:382–86. [DOI] [PubMed] [Google Scholar]
- 150. IMBIE Team . Mass balance of the Greenland ice sheet from 1992 to 2018. Nature. 2020;579:233–39. [DOI] [PubMed] [Google Scholar]
- 151. IMBIE Team . Mass balance of the Antarctic ice sheet from 1992 to 2017. Nature. 2018;558:219–22. [DOI] [PubMed] [Google Scholar]
- 152. Wunderling N, Willeit M, Donges JF, Winkelmann R. Global warming due to loss of large ice masses and Arctic summer sea ice. Nat Commun. 2020;11:5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Neumann B, Vafeidis AT, Zimmermann J, Nicholls RJ. Future coastal population growth and exposure to sea-level rise and coastal flooding—a global assessment. PLoS One. 2015;10:e0118571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Barbier EB. Climate change impacts on rural poverty and low-elevation coastal zones. Estuarine Coastal Shelf Sci. 2015;165:A1–13. [Google Scholar]
- 155. Genua-Olmedo A, Alcaraz C, Caiola N, Ibáñez C. Sea level rise impacts on rice production: the Ebro Delta as an example. Sci Total Environ. 2016;571:1200–10. [DOI] [PubMed] [Google Scholar]
- 156. Vu DT, Yamada T, Ishidaira H. Assessing the impact of sea level rise due to climate change on seawater intrusion in Mekong Delta, Vietnam. Water Sci Technol. 2018;77:1632. [DOI] [PubMed] [Google Scholar]
- 157. Chen CC, McCarl B, Chang CC. Climate change, sea level rise and rice: global market implications. Clim Change. 2012;110:543–60. [Google Scholar]
- 158. Hicks CC, Cohen PJ, Graham NAJ, Nash KL, Allison EH, D'Lima C, Mills DJ, Roscher M, Thilsted SH, Thorne-Lyman AL et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature. 2019;574:95–8. [DOI] [PubMed] [Google Scholar]
- 159. Pauly D, Zeller D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat Commun. 2016;7:10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Deutsch C, Ferrel A, Seibel B, Pörtner HO, Huey, RB. Ecophysiology. Climate change tightens a metabolic constraint on marine habitats. Science. 2015;348:1132–35. [DOI] [PubMed] [Google Scholar]
- 161. Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K et al. Declining oxygen in the global ocean and coastal waters. Science. 2018;359:eaam7240. [DOI] [PubMed] [Google Scholar]
- 162. McCauley DJ, Jablonicky C, Allison EH, Golden CD, Joyce FH, Mayorga J, Kroodsma D. Wealthy countries dominate industrial fishing. Sci Adv. 2018;4:eaau2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pauly D. Micronutrient richness of global fish catches. Nature. 2019;574:41–2. [DOI] [PubMed] [Google Scholar]
- 164. Golden CD, Allison EH, Cheung WW, Dey MM, Halpern BS, McCauley DJ, Smith M, Vaitla B, Zeller D, Myers SS. Nutrition: fall in fish catch threatens human health. Nature. 2016;534:317–20. [DOI] [PubMed] [Google Scholar]
- 165. Perry CT, Alvarez-Filip L, Graham NAJ, Mumby PJ, Wilson SK, Kench PS, Manzello DP, Morgan KM, Slangen ABA, Thomson DP et al. Loss of coral reef growth capacity to track future increases in sea level. Nature. 2018;558:396–400. [DOI] [PubMed] [Google Scholar]
- 166. Sippo JZ, Lovelock CE, Santos IR, Sanders CJ, Maher DT. Mangrove mortality in a changing climate: an overview. Estuarine Coastal Shelf Sci. 2018;215:241–49. [Google Scholar]
- 167. NASA Earth Observatory. Mapping the roots of mangrove loss. August 27, 2020 [Internet]. Available from: https://coastalcare.org/2020/08/mapping-the-roots-of-mangrove-loss/ (accessed 12 June 2021). [Google Scholar]
- 168. Lynch AJ, Cooke SJ, Deines AM, Bower SD, Bunnell DB, Cowx IG, Nguyen VM, Nohner J, Phouthavong K, Riley B et al. The social, economic, and environmental importance of inland fish and fisheries. Environ Rev. 2016;24:115–21. [Google Scholar]
- 169. Harrod C, Ramírez A, Valbo-Jørgensen J, Funge-Smith S. How climate change impacts inland fisheries. In: Barange M, Bahri T, Beveridge MCM, Cochrane KI, Funge-Smith S, Poulain F, editors. Impacts of climate change on fisheries and aquaculture: synthesis of current knowledge, adaptation and mitigation options. FAO Fisheries and Aquaculture Technical Paper 627. Rome: FAO; 2018. p. 375–91. [Google Scholar]
- 170. Nyboer EA, Liang C, Chapman LJ. Assessing the vulnerability of Africa's freshwater fishes to climate change: a continent-wide trait-based analysis. Biol Conserv. 2019;236:505–20. [Google Scholar]
- 171. OECD/FAO . OECD-FAO Agricultural Outlook 2020-2029. [Internet]. Paris: FAO, Rome/OECD Publishing. Available from: 10.1787/1112c23b-en. [DOI] [Google Scholar]
- 172. Machovina B, Feeley KJ, Ripple WJ. Biodiversity conservation: the key is reducing meat consumption. Sci Total Environ. 2015;536:419–31. [DOI] [PubMed] [Google Scholar]
- 173. Godfray HCJ, Aveyard P, Garnett T, Hall JW, Key TJ, Lorimer J, Pierrehumbert RT, Scarborough P, Springmann M, Jebb SA. Meat consumption, health, and the environment. Science. 2018;361:eaam5324. [DOI] [PubMed] [Google Scholar]
- 174. Zu Ermgassen E, Godar J, Lathuillière MJ, Löfgren P, Gardner T, Vasconcelos A, Meyfroidt P. The origin, supply chain, and deforestation risk of Brazil's beef exports. Proc Natl Acad Sci. 2020;117:31770–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Shepon A, Eshel G, Noor E, Milo R. The opportunity cost of animal based diets exceeds all food losses. Proc Natl Acad Sci. 2018;115:3804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lam Y, Fry JP, Nachman KE. Applying an environmental public health lens to the industrialization of food animal production in ten low- and middle-income countries. Global Health. 2019;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Salter AM. The effects of meat consumption on global health. Rev Sci Tech. 2018;37:47–55. [DOI] [PubMed] [Google Scholar]
- 178. Zaman QU, Aslam Z, Yaseen M, Ihsan MZ, Khaliq A, Fahad S, Bashir S, Ramzani PMA, Naeem M. Zinc biofortification in rice: leveraging agriculture to moderate hidden hunger in developing countries. Arch Agron Soil Sci. 2018;64:147–61. [Google Scholar]
- 179. Kok ADX, Yoon LL, Sekeli R, Yeong WC, Yusof ZNB, Song LK. Iron biofortification of rice: progress and prospects. In: Shah F, Kahn ZH, Iqbal A, editors. Rice crop: current developments. London: IntechOpen; 2018. p. 25–44. [Google Scholar]
- 180. Prom-u-thai C, Rashid A, Ram H, Zou C, Guilherme LRG, Corguinha APB, Guo S, Kaur C, Naeem A, Yamuangmorn S et al. Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front Plant Sci. 2020;11:589835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rao DS, Neeraja CN, Babu PM, Nirmala B, Suman K, Rao LVS, Surekha K, Raghu P, Longvah T, Surendra P et al. Zinc biofortified rice varieties: challenges, possibilities, and progress in India. Front Nutr. 2020;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Nakandalage N, Nicolas M, Norton RM, Hirotsu N, Milham PJ, Seneweera S. Improving rice zinc biofortification success rates through genetic and crop management approaches in a changing environment. Front Plant Sci. 2016;7:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ludwig Y, Slamet-Loedin IH. Genetic biofortification to enrich rice and wheat grain iron: from genes to product. Front Plant Sci. 2019;10:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Liu D, Liiu Y, Zhang W, Chen X, Zou C. Agronomic approach of zinc biofortification can increase zinc bioavailability in wheat flour and thereby reduce zinc deficiency in humans. Nutrients. 2017;9:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Signorell C, Zimmermann MB, Cakmak I, Wegmüller R, Zeder C, Hurrell R, Aciksoz SB, Boy E, Tay F, Frossard E et al. Zinc absorption from agronomically biofortified wheat is similar to post-harvest fortified wheat and is a substantial source of bioavailable zinc in humans. J Nutr. 2019;149:840–46. [DOI] [PubMed] [Google Scholar]
- 186. Bisbis MB, Gruda N, Blanke M. Potential impacts of climate change on vegetable production and product quality—a review. J Cleaner Prod. 2018;170:1602–20. [Google Scholar]
- 187. Frei M. Breeding of ozone resistant rice: relevance, approaches and challenges. Environ Pollut. 2015;197:144–55. [DOI] [PubMed] [Google Scholar]
- 188. Ainsworth EA. Understanding and improving global crop response to ozone pollution. Plant J. 2017;90:886–97. [DOI] [PubMed] [Google Scholar]
- 189. Solis CA, Yong MT, Vinarao R, Jena K, Holford P, Shabala L, Zhou M, Shabala S, Chen ZH. Back to the wild: on a quest for donors towards salinity tolerant rice. Front Plant Sci. 2020;11:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. FAO; WHO . Sustainable healthy diets—guiding principles. [Internet]. Rome: 2019. Available from: 10.4060/CA6640EN (accessed 28 March 2021). [DOI] [Google Scholar]
- 191. De Pee S, Hardinsyah R, Jalal F, Kim BF, Semba RD, Deptford A, Fanzo JC, Ramsing R, Nachman KE, McKenzie S et al. Balancing a sustained pursuit of nutrition, health, affordability and climate goals: exploring the case of Indonesia. Am J Clin Nutr. 2021:nqab258. doi: 10.1093/ajcn/nqab258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. West KP Jr, Mehra S. Vitamin A intake and status in populations facing economic stress. J Nutr. 2010;140:201S–7S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.