ABSTRACT
Consuming fat results in postprandial lipemia, which is defined as an increase in blood triglyceride (TG) concentration. According to current knowledge, an excessively elevated postprandial TG concentration increases the risk of cardiovascular disease (CVD). It is well known that meal-dependent (e.g., nutrient composition) as well as meal-independent factors (e.g., age) determine the magnitude of the lipemic response. However, there is conflicting evidence concerning the influence of fatty acid (FA) composition on postprandial TG concentration. The FA composition of a meal depends on the fat source used; for example, butter and coconut oil are rich in SFAs, while olive oil and canola oil have a high content of unsaturated FAs. To investigate the influence of meals prepared with fat sources rich in either SFAs or unsaturated FAs on postprandial lipemia, we carried out a systematic literature search in PubMed, Scopus, and the Cochrane Library. Randomized crossover studies were analyzed and the AUC of postprandial TG concentration served as the primary outcome measure. To examine the influence of health status, we differentiated between metabolically healthy individuals and those with CVD risk factors. In total, 23 studies were included. The results show that, in metabolically healthy adults, the FA composition of a meal is not a relevant determinant of postprandial lipemia. However, in individuals with CVD risk factors, SFA-rich meals (>32 g SFA/meal) often elicited a stronger lipemic response than meals rich in unsaturated FAs. The results suggest that adults with hypertriglyceridemia, an elevated BMI (≥30 kg/m2), and/or who are older (>40 y) may benefit from replacing SFA sources with unsaturated FAs. These hypotheses need to be verified by further studies in people with CVD risk factors using standardized postprandial protocols. This review was registered in PROSPERO as CRD42021214508 (https://www.crd.york.ac.uk/prospero/).
Keywords: fatty acids, SFA, MUFA, PUFA, unsaturated fatty acids, mixed meals, postprandial lipemia, triglycerides, healthy, CVD
Statement of Significance: To the best of our knowledge, this is one of the first reviews highlighting the effects of the fatty acid composition of mixed meals enriched with natural fat sources on postprandial lipemia using a food-based approach. A unique aspect of this review is the investigation of both metabolically healthy subjects and adults with CVD risk factors.
Introduction
In developed societies, the modern lifestyle is characterized by excessive and regular food intake. As a result, many individuals spend the majority of their waking hours in the postprandial state (1). This postprandial phase is characterized by increases in blood lipids (lipemia), glucose (glycemia), and insulin (insulinemia) (2). These metabolic processes are accompanied by postprandial “oxidative stress” and low-grade inflammation, which are associated with impaired endothelial function (2). Scientific interest in postprandial metabolic events as risk factors for cardiovascular disease (CVD) is therefore increasing.
Postprandial metabolic processes are dynamic, and the magnitude and duration of change are influenced by both meal-independent and -dependent factors. Age, health status, and pathological conditions (e.g., type 2 diabetes) are examples of meal-independent factors (3). Meal-dependent factors include the energy content and nutrient composition of meals, especially the fat content and composition (4, 5). Due to the intake of multiple meals, the degree of lipemia fluctuates during the day (3). Epidemiological studies have found that postprandial lipemia, particularly a high triglyceride (TG) concentration, is associated with increased CVD risk (6–10). Thus, attenuating postprandial lipemia by dietary modification may lower CVD risk.
The fatty acid (FA) composition of a meal is determined by the main source of fat. Major dietary sources of SFAs include butter and cream (both 64% of total fat as SFAs) and coconut oil (83% of total fat as SFAs) (11). Olive oil and canola oil are rich in MUFAs (73% and 63% of total fat as MUFAs, respectively), whereas other plant oils are rich in n–6 PUFAs [e.g., sunflower oil, 66% of total fat as linoleic acid (18:2n–6)] and/or n–3 PUFAs [e.g., linseed oil, 14% of total fat as linoleic acid, and 53% as ɑ-linolenic acid (18:3n–3)] (11).
The effects of different FAs on fasting lipid profiles are well described (12), and these have been incorporated into evidence-based dietary guidelines for CVD prevention (9). SFAs are commonly judged to have a negative health impact since they lead to increased concentrations of LDL cholesterol (13). By contrast, unsaturated FA intake has beneficial effects on blood lipid profile due to their role in inhibiting cholesterol synthesis and lowering LDL cholesterol by triggering the expression of hepatic LDL receptor (14). Thus, one well-accepted dietary strategy to improve the blood lipid profile is to replace food rich in SFAs with food rich in unsaturated FAs, especially MUFAs (12). However, it is essential that unsaturated FAs are mainly supplied by plant oils like canola or olive oil, and not by foods that are simultaneously rich in SFAs. A recent comprehensive meta-regression analysis demonstrated that, for each 1% of dietary energy as SFAs replaced with an equivalent amount of PUFAs or MUFAs, there was a significant decrease in fasting TGs and total and LDL cholesterol (12).
Compared with fasting lipid profiles, less is known about the importance of FA composition and different FA food sources on postprandial lipemia. Two recent meta-analyses examined the postprandial TG response after fat challenges containing different types of FAs. In contrast to fasting lipid responses, both meta-analyses found no difference in overall TG response between SFA and unsaturated FA intake in their primary analyses (15, 16). However, secondary analyses revealed that when fat tolerance tests lasted for over 8 h, there was a lower TG response to meals rich in PUFAs (15). Neither review differentiated between subjects without metabolic disorders, and therefore considered metabolically healthy, and individuals with CVD risk factors in the form of metabolic disorders (e.g., metabolic syndrome, hypertriglyceridemia). It has been shown that certain pathological conditions such as obesity, hypertriglyceridemia, and insulin resistance promote an exaggerated postprandial lipemic response (3, 17). We hypothesized that due to a more extensive metabolic reaction in subjects at risk of CVD (18–21), differences in the postprandial lipemic response after ingestion of meals with different FA compositions become more visible than in metabolically healthy participants. Thus, it might be useful to consider the metabolic health status when analyzing the metabolic reaction to different FA compositions. In addition, the primary focus of both meta-analyses was on classifying FAs according to their degree of saturation, and less on the food source of different FAs (e.g., SFAs from butter vs. SFAs from coconut oil), or on SFAs of different chain lengths (15, 16). However, these characteristics may affect the impact of SFAs on fasting lipid profile and postprandial lipemic response (12, 22, 23).
Therefore, our aim was to systematically review and critically evaluate existing evidence from acute studies comparing meals rich in SFAs and unsaturated FAs on postprandial lipemia. We chose to specifically focus on complete breakfast meals, prepared with natural, commercially available foods rather than fat tolerance tests administered as liquid meals or shakes, because the results of complete breakfast meals have a more practical relevance. In addition, we investigated whether the lipemic response differs between metabolically healthy subjects and individuals with established CVD risk factors.
Methods
A systematic literature search in the PubMed database (https://pubmed.ncbi.nlm.nih.gov) was conducted between October and December 2020. The search term “postprandial lipemia AND triglycerides AND dietary fatty acids AND meal” was used to identify suitable studies. A second literature search, using the same search term, was conducted in the Cochrane Library (https://www.cochranelibrary.com) and in the Scopus database (https://www.scopus.com). Additional studies were detected by computer-assisted manual searches. Both authors independently reviewed the identified papers and compared them with the inclusion and exclusion criteria (Table 1, Figure 1). The main inclusion criteria were as follows: studies were of a randomized, crossover design and measured postprandial responses in humans; study participants consumed at least one SFA-rich meal and one meal rich in unsaturated FAs, both prepared with natural fat sources such as plant oils or high-fat dairy products; postprandial TG concentrations were measured periodically at regular intervals; and the paper was written in English. Studies were excluded if the test meals were served as liquid meals or shakes, or if meals were enriched with isolated FAs or modified TGs (e.g., inter-esterified synthetic fats or structured TGs containing specific FAs). Different types of the AUC of postprandial TG concentration [e.g., the incremental, total, or net AUC (iAUC, tAUC, net AUC, respectively)] served as the primary outcome measure. This review was registered in PROSPERO (CRD42021214508).
TABLE 1.
Inclusion and exclusion criteria1
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
1FA, fatty acid.
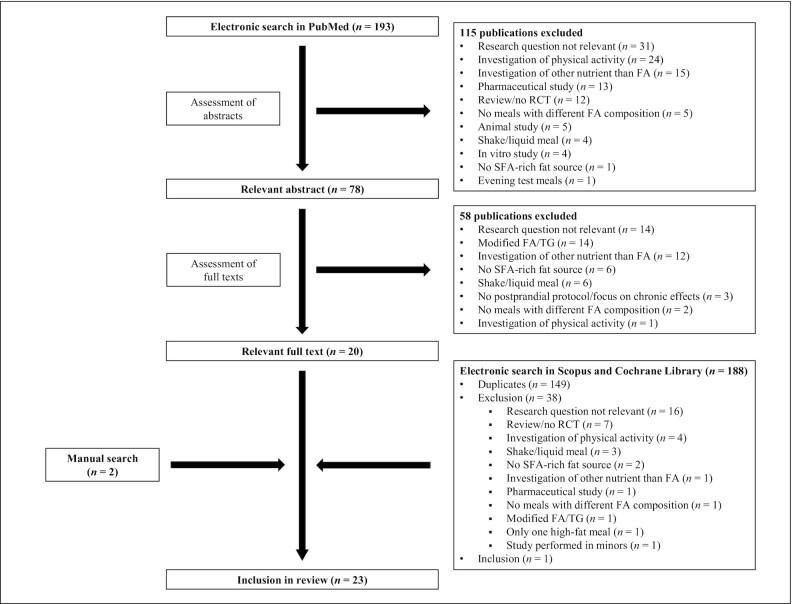
FIGURE 1.
Flowchart of article search and selection process. FA, fatty acid; RCT, randomized controlled trial.
Results
The systematic literature search in the PubMed database identified 193 publications. Of these, 115 studies were excluded after screening the abstracts because they did not fulfil the inclusion criteria and/or fulfilled at least one exclusion criterion (Table 1). After examining the full texts of the remaining 78 studies, 20 publications were rated as suitable for this review. The systematic literature searches in the Scopus database and in the Cochrane Library revealed 111 and 77 publications, respectively. After removing duplicates and screening the articles, 1 publication was included in the analysis. In addition, 2 studies were identified during the manual search. In total, 23 articles were included (Figure 1).
15 studies were performed in metabolically healthy subjects (Tables 2 and 5) and 4 studies included individuals with CVD risk factors (Table 3). In 4 studies, data from metabolically healthy subjects and individuals with CVD risk factors were obtained (Table 4). CVD risk factors included an elevated fasting TG concentration, hypercholesterolemia, being overweight, or combinations of several CVD risk factors (e.g., hypertension, elevated plasma glucose).
TABLE 2.
Acute test-meal studies comparing the effects of SFA-rich meals and meals rich in unsaturated FAs on postprandial lipemia in metabolically healthy subjects1
| Reference | Age and BMI of subject group (n) | Study design | Energy, kcal | Meal composition | Amount of fat source | Fat source/meal pattern | FA composition | Blood collection, h | Results2 |
|---|---|---|---|---|---|---|---|---|---|
| Austin et al. (30) | 54 y, 26 ± 1 kg/m2 (n = 15) | Crossover, double-blind | 667 | 34 g fat, 17 g protein, 71 g CHO | 0 g | Control (tallow for coconut oil; olive oil for fish oil) | 15 g SFA, 15 g MUFA, 2 g PUFA3 | 0, 2, 3, 3.5, 4, 4.5, 5 | AUC0-5h control > fish oil and fish oil and coconut oil (P = 0.0125 and P = 0.0186) |
| 667 | 34 g fat, 17 g protein, 71 g CHO | 6 g | Fish oil | 15 g SFA, 11 g MUFA, 6 g PUFA3 | |||||
| 667 | 34 g fat, 17 g protein, 71 g CHO | 19 g | Extra virgin coconut oil | 23 g SFA, 7 g MUFA, 1 g PUFA3 | iAUC0-5h coconut oil > fish oil and coconut oil (P = 0.0480) | ||||
| 667 | 34 g fat, 17 g protein, 71 g CHO | 6 g, 19 g | Fish oil, Extra virgin coconut oil | 22 g SFA, 3 g MUFA, 6 g PUFA3 | |||||
| Bellido et al. (36) | Age and BMI not stated (n = 8) | Crossover, 4 wk of Western diet before study | 50–66% of daily intake | 60 EN% fat, 15 EN% protein, 25 EN% CHO | 1 g/kg body mass | Butter | 35 EN% SFA, 22 EN% MUFA, 4 EN% PUFA3 | 0, 3, 6, 9 | No calculation of AUC of postprandial TG concentration. No significant difference in alternative parameter |
| Olive oil | 22 EN% SFA, 38 EN% MUFA, 4 EN% PUFA3 | ||||||||
| Walnuts | 20 EN% SFA, 24 EN% MUFA, 16 EN% PUFA3 | ||||||||
| Meikle et al. (37) | 53 ± 5 y, 30 ± 6 kg/m2 (n = 16) | Crossover | 745 | 54 g fat, 29 g protein, 37 g CHO | Not stated | Dairy products | 67 g SFA, 23 g MUFA, 5 g PUFA4 | 0, 1, 2, 3, 4 | No calculation of AUC of postprandial TG concentration. No significant difference in alternative parameter |
| 786 | 54 g fat, 29 g protein, 47 g CHO | Soy products | 37 g SFA, 40 g MUFA, 24 g PUFA4 | ||||||
| Mekki et al. (24) | 20–29 y, 22 ± 1 kg/m2 (n = 10) | Crossover | Not stated | Not stated | 0 g | No fat | Not stated | 0, 1, 2, 3, 4, 5, 6, 7 | iAUC0-7h butter < other meals (P < 0.05) |
| 40 g | Butter | 54 g/100 g SFA: 14 g/100 g C4:0–C12:0, 11 g/100 g C14:0, 30 g/100 g C16:0, 11 g/100 g C18:0, 25 g/100 g C18:14 | |||||||
| Olive oil | 11 g/100 g C16:0, 76 g/100 g C18:1, 9 g/100 g C18:24 | ||||||||
| Sunflower oil | 21 g/100 g C18:1, 67 g/100 g C18:24 | ||||||||
| Pedersen et al. (38) | 24 y, 23 kg/m2 (n = 12) | Crossover, double-blind | Breakfast, 358 | 17 g fat, 9 g protein, 43 g CHO | 15 g | Palm oil | 39% SFA, 47% MUFA, 14% PUFA4 | Every 15 min for 1.5 h after breakfast, every 30 min for 2.5 h after lunch, hourly until 9 h postprandially | No calculation of AUC of postprandial TG concentration. No significant difference in alternative parameter |
| Lunch, 1337 | 64 g fat, 32 g protein, 153 g CHO | 55 g | Canola oil | 7% SFA, 63% MUFA, 30% PUFA4 | |||||
| Sunflower oil | 11% SFA, 21% MUFA, 68% PUFA4 | ||||||||
| Perez-Martinez et al. (40) | 22 ± 2 y, 25 ± 3 kg/m2 (n = 20) | Crossover, 4 wk of diet matching the FA composition of the postprandial protocol before study | 50–66% of daily intake | 60 EN% fat, 15 EN% protein, 25 EN% CHO | 1 g/kg body mass | Butter | 35 EN% SFA, 22 EN% MUFA, 4 EN% PUFA3 | 0, 1, 2, 3, 4, 5, 6, 8.5, 11 | No calculation of AUC of postprandial TG concentration. Olive oil: greater TG concentration in early postprandial phase and earlier decrease to preprandial TG concentration (P = 0.002 and P = 0.012) |
| Olive oil | 20 EN% SFA, 36 EN% MUFA, 4 EN% PUFA3 | ||||||||
| Walnuts | 20 EN% SFA, 24 EN% MUFA, 16 EN% PUFA3 | ||||||||
| Sanders et al. (27) | 23 ± 4 y, 23 ± 3 kg/m2 (n = 9)5 | Crossover, 3 wk of diet matching the FA composition of the postprandial protocol before study | 1846 | 79 g fat, 54 g protein, 238 g CHO | Not stated | Butter | 46 wt% SFA, 33 wt% MUFA, 12 wt% PUFA4 | 0, 1, 2, 4, 6 | No significant difference |
| Olive oil | 22 wt% SFA, 55 wt% MUFA, 18 wt% PUFA4 | ||||||||
| Olive oil and fish oil | 20 wt% SFA, 52 wt% MUFA, 19 wt% PUFA4 | ||||||||
| Sciarillo et al. (32) | 24 ± 1 y, 26 ± 7 kg/m2 (n = 10) | Crossover | 13 kcal/kg body mass (mean 995 kcal) | 61 EN% fat, 7 EN% protein, 32 EN% CHO | Individual (mean 65 g) | Unsalted butter | Not stated | 0, 1, 2, 3, 4, 5, 6 | No significant difference |
| Native coconut oil | |||||||||
| Native olive oil extra | |||||||||
| Canola oil | |||||||||
| Sun et al. (28) | 27 ± 6 y, 23 ± 3 kg/m2 (n = 20) | Crossover, single-blind | Not stated | Not stated | 48 g (40 g fat) | Unsalted butter | 24 g SFA, 8 g MUFA, 8 g PUFA3 | 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4 | iAUC0-4h butter < olive oil (P < 0.01) |
| 44 g (40 g fat) | Refined olive oil | 6 g SFA, 31 g MUFA, 3 g PUFA3 | |||||||
| 40 g | Refined grape seed oil | 4 g SFA, 8 g MUFA, 28 g PUFA3 | |||||||
| Svensson et al. (29) | 34 ± 8 y, 23 ± 3 kg/m2 (n = 19) | Crossover, single-blind | 786 | 47 g fat, 23 g protein, 69 g CHO | 42 g (35 g fat) | Butter | 75 mol% SFA (C4:0–C18:0), 20 mol% MUFA, 2 mol% PUFA4 | 0, 1, 3, 5, 7 | No significant difference |
| 35 g | Native olive oil | 15 mol% SFA (C4:0–C18:0), 69 mol% MUFA, 16 mol% PUFA4 | |||||||
| 35 g | Linseed oil and canola oil | 7 mol% SFA (C4:0–C18:0), 39 mol% MUFA, 55 mol% PUFA4 | |||||||
| Tholstrup et al. (39) | 38 ± 11 y, 21 ± 1 kg/m2 (n = 10) | Crossover, single-blind | 621 kcal/100 g | 76 EN% fat, 3 EN% protein, 21 EN% CHO | 1 g/kg body mass (mean 62 g) | Cocoa butter | 48 EN% SFA, 26 EN% MUFA, 2 EN% PUFA3 | 0, 4, 6 | No calculation of AUC of postprandial TG concentration. No significant difference in alternative parameter |
| Olive oil | 12 EN% SFA, 57 EN% MUFA, 8 EN% PUFA3 | ||||||||
| Thomsen et al. (26) | 23 ± 2 y, 21 ± 2 kg/m2 (n = 10) | Crossover, single-blind | Not stated | Not stated | 0 g | No fat | Not stated | 0, 1, 2, 3, 4, 5, 6, 7, 8 | No significant difference |
| 100 g | Butter | 72% SFA4 | |||||||
| 80 g | Olive oil | 74% MUFA4 |
Age and BMI are given as mean ± SD. Numbers are rounded to whole numbers. CHO, carbohydrate; EN%, energy percentage; FA, fatty acid; iAUC, incremental AUC; wt%, weight percentage.
Referring to comparisons between AUC of postprandial TG concentration (plasma, serum, capillary blood) after SFA-rich meals and meals rich in unsaturated FAs.
Referring to the content of SFA, MUFA, and PUFA in the meal.
Referring to the content of SFA, MUFA, and PUFA in the fat source.
Age and BMI referring to all 26 study participants.
TABLE 5.
Acute test-meal studies comparing the effects of different SFA-rich meals on postprandial lipemia in metabolically healthy subjects1
| Reference | Age and BMI of subject group (n) | Study design | Energy, kcal | Meal composition | Amount of fat source | Fat source/meal pattern | FA composition | Blood collection, h | Results2 |
|---|---|---|---|---|---|---|---|---|---|
| Karupaiah et al. (41) | 30 ± 8 y, 23 ± 4 kg/m2 (n = 20) | Crossover, single blind, 1 wk before intervention 50 g/d of fat source of postprandial protocol | 960 | 50 g fat, 29 g protein, 98 g CHO | 50 g | Coconut oil and corn oil | 75% SFA, 21% MUFA, 12% PUFA3 | 0, 2, 4, 5, 6, 8 | AUC0-8h cacao butter and corn oil > other meals (P = 0.016) |
| 17 EN% SFA, 5 EN% MUFA, 3 EN% PUFA4 | |||||||||
| Cacao butter and corn oil | 59% SFA, 35% MUFA, 12% PUFA3 | ||||||||
| 14 EN% SFA, 9 EN% MUFA, 3 EN% PUFA4 | |||||||||
| Palm olein | 44% SFA, 45% MUFA, 12% PUFA3 | ||||||||
| 11 EN% SFA, 12 EN% MUFA, 3 EN% PUFA4 | |||||||||
| Panth et al. (23) | 18–45 y, 24 ± 3 kg/m2 (n = 16) | Crossover, single-blind | 666 | 41 g fat, 7 g protein, 64 g CHO | 40 g | Butter | 27 g SFA, 8 g MUFA, 1 g PUFA4 | 0, 2, 3, 4, 6 | Net AUC0-6h butter, lard > coconut oil (P < 0.05) |
| 659 | 39 g fat, 7 g protein, 66 g CHO | Lard | 20 g SFA, 14 g MUFA, 2 g PUFA4 | ||||||
| 659 | 39 g fat, 7 g protein, 66 g CHO | Coconut oil | 35 g SFA, 1 g MUFA, 1 g PUFA4 | ||||||
| Poppitt et al. (42) | 27 ± 9 y, 23 ± 2 kg/m2 (n = 18) | Crossover, single-blind | Breakfast, 792 | 52 g fat, 19 g protein, 64 g CHO | Not stated | Soft-fraction milk fat | 3 g SCT, 7 g MCT, 42 g LCT4 | 0, 0.5, 2, 3 | No significant difference |
| Tallow | 0 g SCT, 0 g MCT, 52 g LCT4 | ||||||||
| Lunch, ad libitum | Individual | Individual | |||||||
| Coconut oil | 0 g SCT, 10 g MCT, 42 g LCT4 |
Age and BMI are given as means ± SDs. Numbers are rounded to whole numbers. CHO, carbohydrate; EN%, energy percentage; FA, fatty acid; LCT, long-chain triglycerides; MCT, medium-chain triglycerides; SCT, short-chain triglycerides.
Referring to comparisons between AUC of postprandial TG concentration (plasma, serum, capillary blood) after several SFA-rich meals.
Referring to the content of SFA, MUFA, and PUFA in the fat source.
Referring to the content of SFA, MUFA, and PUFA in the meal.
TABLE 3.
Acute test-meal studies comparing the effects of SFA-rich meals and meals rich in unsaturated FAs on postprandial lipemia in subjects with risk factors for CVD1
| Reference | Health condition | Age and BMI of subject group (n) | Study design | Energy, kcal | Meal composition | Amount of fat source | Fat source/meal pattern | FA composition | Blood collection, h | Results2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Diekmann et al. (43) | Characteristics of metabolic syndrome | 70 ± 5 y, 30 ± 2 kg/m2 (n = 26) | Crossover | 1014 | 59 g fat, 26 g protein, 94 g CHO | Not stated | Western diet | 32 g SFA, 20 g MUFA, 4 g PUFA3 | 0, 1.5, 3, 4.5 | iAUC0-4.5h Western diet meal > Mediterranean diet meal (P < 0.001) |
| 1015 | 40 g fat, 26 g protein, 133 g CHO | Mediterranean diet | 5 g SFA, 20 g MUFA, 11 g PUFA3 | |||||||
| Karupaiah and Sundram (45) | Cholesterol fasting 5.26 ± 0.78 mmol/L | 36 ± 5 y, 22 ± 2 kg/m2 (n = 15) | Crossover, 1 wk before intervention 50 g/d of fat source of postprandial protocol | 1010 | 53 g fat, 32 g protein, 101 g CHO | 50 g | Palm olein | 42% SFA, 45% MUFA, 11% PUFA4 | 0, 1.5, 3.5, 5.5, 7 | No significant difference |
| 42 EN% SFA, 46 EN% MUFA, 12 EN% PUFA3 | ||||||||||
| Palm olein and soy oil | 26% SFA, 40% MUFA, 32% PUFA4 | |||||||||
| 27 EN% SFA, 41 EN% MUFA, 30 EN% PUFA3 | ||||||||||
| Palm olein and canola oil | 14% SFA, 58% MUFA, 26% PUFA4 | |||||||||
| 18 EN% SFA, 56 EN% MUFA, 24 EN% PUFA3 | ||||||||||
| Lopez et al. (25) | TG fasting >2.26 mmol/L | 33 ± 7 y, 24 ± 5 kg/m2 (n = 14) | Crossover, single-blind | 10 kcal/kg body weight (mean 800 kcal) | Not stated | 0 g | No fat | Not stated | 0, 1, 2, 3, 4, 5, 6, 7, 8 | iAUC0-8h butter > olive oil (P < 0.05) |
| 72% fat, 6% protein, 22% CHO | 50 g/m2 body surface | Butter | 65% SFA, 31% MUFA, 3% PUFA4 | |||||||
| Olive oil | 15% SFA, 81% MUFA, 4% PUFA4 | |||||||||
| Schönknecht et al. (44) | Characteristics of metabolic syndrome | 70 ± 5 y, 31 ± 3 kg/m2 (n = 60) | Crossover | 1010 | 59 g fat, 26 g protein, 94 g CHO | Not stated | High-fat Western diet | 32 g SFA, 20 g MUFA, 4 g PUFA3 | 0, 1, 2, 3, 4, 5 | iAUC0-5h high-fat Western diet meal > other meals (P < 0.001) |
| 1013 | 34 g fat, 26 g protein, 145 g CHO | Low-fat Western diet | 19 g SFA, 11 g MUFA, 2 g PUFA3 | |||||||
| 1012 | 40 g fat, 26 g protein, 133 g CHO | Mediterranean diet | 6 g SFA, 24 g MUFA, 9 g PUFA3 |
Age and BMI are given as means ± SDs. Numbers are rounded to whole numbers. CHO, carbohydrate; CVD, cardiovascular disease; EN%, energy percentage; FA, fatty acid; iAUC, incremental AUC.
Referring to comparisons between AUC of postprandial TG concentration (plasma, serum, capillary blood) after SFA-rich meals and meals rich in unsaturated FAs.
Referring to the content of SFA, MUFA, and PUFA in the meal.
Referring to the content of SFA, MUFA, and PUFA in the fat source.
TABLE 4.
Acute test-meal studies investigating the effects of high-fat meals with different FA composition on postprandial lipemia in subjects with risk factors for CVD and metabolically healthy controls1
| Reference | Health condition | Age and BMI of subject group (n) | Study design | Energy, kcal | Meal composition | Amount of fat source | Fat source/meal pattern | FA composition | Blood collection, h | Results2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bermudez et al. (31) | TG fasting >2.24 mmol/L | Age not stated, 24 ± 5 kg/m2 (n = 14) | Crossover, double-blind | Not stated | Not stated | 50 g/m2 body surface | Butter | 65% SFA, 31% MUFA, 3% PUFA3 | 0, 1, 2, 3, 4, 5, 6, 7, 8 | Both groups: iAUC0-8h butter > other meals (P < 0.05) |
| Refined olive oil | 15% SFA, 81% MUFA, 4% PUFA3 | |||||||||
| Metabolically healthy | Age not stated, 24 ± 2 kg/m2 (n = 14) | High-palmitic sunflower oil | 27% SFA, 66% MUFA, 7% PUFA3 | |||||||
| Vegetable oils and fish oils | 11% SFA, 75% MUFA, 14% PUFA3 | |||||||||
| Irawati et al. (34) | Hyper-responder | 43 ± 6 y, 29 ± 1 kg/m2 (n = 10) | Crossover, single-blind | 740 | 43 g fat, 14 g protein, 69 g CHO | 40 g | Coconut oil | 92% SFA, 8% MUFA, 0% PUFA3 | 0, 4, 8 | Hyper-responder: iAUC0-8h palm oil > other meals (P = 0.001) |
| Normo-responder | 39 ± 4 y, 24 ± 1 kg/m2 (n = 16) | Palm oil | 60% SFA, 31% MUFA, 9% PUFA3 | |||||||
| Rice bran oil | 28% SFA, 40% MUFA, 32% PUFA3 | Palm oil: iAUC0-8h hyper-responder > normo-responder (P < 0.01) | ||||||||
| Lozano et al. (33) | Lower-weight subjects | 23 ± 2 y, 26 kg/m2 (n = 21) | Crossover, 4 wk of Western diet before study | 50–66% of daily intake | 60% fat, 15% protein, 25% CHO | 1 g/kg body mass | Butter | 35% SFA, 22% MUFA, 4% PUFA4 | 0, 1, 2, 3, 4, 5, 6, 8.5, 11 | No significant difference |
| Olive oil | 22% SFA, 38% MUFA, 4% PUFA4 | |||||||||
| Higher-weight subjects | Walnuts | 20% SFA, 24% MUFA, 16% PUFA4 | ||||||||
| Øyri et al. (35) | Familial hypercholesterolemia | 25 y, 23 kg/m2 (n = 13) | Crossover, double-blind | 764 | 61 g fat, 7 g protein, 48 g CHO | Not stated | Palm oil and coconut oil | 36 g SFA, 19 g MUFA, 5 g PUFA4 | 0, 2, 4, 6 | No significant difference |
| Metabolically healthy | 25 y, 22 kg/m2 (n = 14) | 768 | 63 g fat, 6 g protein, 45 g CHO | Sunflower oil and canola oil | 8 g SFA, 21 g MUFA, 34 g PUFA4 |
Age and BMI are given as means ± SDs. Numbers are rounded to whole numbers. CHO, carbohydrate; CVD, cardiovascular disease; FA, fatty acid; iAUC, incremental AUC.
Referring to comparisons between AUC of postprandial TG concentration (plasma, serum, capillary blood) after SFA-rich meals and meals rich in unsaturated FAs.
Referring to the content of SFA, MUFA, and PUFA in the fat source.
Referring to the content of SFA, MUFA, and PUFA in the meal.
The meals of 20 studies compared SFA content with unsaturated FA content (Tables 2–4). Butter was used as the main source of SFAs, while olive oil served as the primary source of unsaturated FAs. In 3 studies, fats composed predominantly of SFAs of different origins were used to achieve a specific FA composition in meals (Table 5).
Impact of fat dose on postprandial lipemia
In 3 studies, participants received a fat-free control meal in addition to high-fat mixed meals (24–26) (Tables 2 and 3). Data revealed that fat-free meals did not increase TG concentration postprandially. By contrast, all high-fat meals provoked an increase in postprandial TG concentration. In addition to postprandial TG concentration, fat dose also influenced the time taken to reach maximum TG concentration (tmax). In protocols with very high fat doses (50 g/m2 body surface/meal, 79 g/meal), the TG concentration peaked 2 h after meal consumption (25, 27), whereas in studies with lower fat doses (35 g, 40 g), the maximum TG concentration (Cmax) was reached 3–4 h postprandially (28, 29).
Influence of FA composition on postprandial lipemia: metabolically healthy subjects
In metabolically healthy subjects, 11 of 16 studies investigated the effect of FA composition on lipemia by calculating the AUC of postprandial TG concentration (Tables 2 and 4).
SFAs vs. unsaturated FAs
In comparison with meals rich in unsaturated FAs, 2 studies reported a higher TG concentration after the consumption of a SFA-rich meal. Austin et al. (30) found that coconut oil provoked a higher iAUC0–5 h than a blend of coconut and fish oils. In addition, compared with control meals (tallow and olive oil), they reported a lower AUC0–5 h after the consumption of meals enriched with a blend of coconut and fish oils or with only fish oil (Table 2). Bermudez et al. (31) demonstrated that the consumption of butter led to a higher postprandial TG iAUC0–8 h than the consumption of oils with a larger proportion of unsaturated FAs. Reference fat sources were olive oil, high-palmitic sunflower oil, and a blend of vegetable and fish oils (Table 4).
In 2 studies at least one meal rich in unsaturated FAs provoked a significantly greater TG AUC than a SFA-rich meal (Table 2); the SFA-rich meals of both studies were prepared with butter (24, 28). Sun et al. (28) observed that olive oil triggered a stronger postprandial lipemic response than butter, whereas Mekki et al. (24) made the same observation and additionally reported a greater TG iAUC0–7 h after the consumption of a meal enriched with sunflower oil.
8 studies reported no significant differences in the AUC of postprandial TG concentration between SFA-rich meals and meals rich in unsaturated FAs (Tables 2 and 4). Most meals contained butter as the SFA source (26–29, 32, 33), whereas others were enriched with coconut oil (32, 34), palm oil (34), or with a blend of coconut and palm oil (35). Sources of unsaturated FAs were olive oil (26, 27, 29, 32, 33), canola oil (32), grapeseed oil (28), rice bran oil (34), and walnuts (33). Other meals were prepared with a blend of sunflower and canola oils (35), a blend of linseed and canola oils (29), or a blend of olive and fish oils (27).
Studies using an alternative parameter of postprandial lipemia
In 5 studies, the AUC of postprandial TG concentration was not measured (Table 2). When comparing alternative parameters of postprandial lipemia (e.g., total TG concentration, median % change from baseline), no significant differences between meals were found in 4 studies. Sources of SFAs were butter (36), dairy products (37), palm oil (38), and cocoa butter (39). Meals rich in unsaturated FAs were prepared with walnuts (36), soy products (37), olive oil (36, 39), and canola and sunflower oils (38). Only Perez-Martinez et al. (40) reported differences in the lipemic responses to high-fat meals. Compared with butter and walnuts, a meal rich in olive oil resulted in a higher TG concentration in the early postprandial phase and in an earlier decrease to the preprandial TG concentration.
Comparisons of SFAs
In 3 studies, all meals contained SFA-rich fat sources (Table 5). Metabolically healthy subjects were investigated and postprandial TG AUC was calculated. Every study reported at least one nonsignificant comparison between 2 meals with different SFA profiles. Specifically, there were no significant differences in the lipemic response between palm olein and a blend of coconut and corn oils (41), butter and lard (23), and milk fat, coconut oil, and tallow (42). 2 studies observed significant differences in the AUC of the postprandial TG concentration between meals. Karupaiah et al. (41) observed a lower lipemic response after the consumption of palm olein and a blend of coconut and corn oils than after the intake of a blend of cocoa butter and corn oil. In addition, Panth et al. (23) reported a higher net AUC0–6 h in response to butter and lard than to coconut oil.
Influence of FA composition on postprandial lipemia: individuals with CVD risk factors
In every study that included patients with increased CVD risk, the AUC of postprandial TG concentration served as the parameter for lipemia (Tables 3 and 4). When comparing meals rich in SFAs with those rich in unsaturated FAs, 5 studies reported a higher postprandial TG AUC after the consumption of a SFA-rich meal. Bermudez et al. (31) showed that, in subjects with a high fasting TG concentration, a meal enriched with butter provoked a greater TG iAUC0–8 h than meals rich in unsaturated FAs. Reference oils were high-palmitic sunflower oil, refined olive oil, and a blend of vegetable and fish oils. Likewise, Lopez et al. (25) reported a stronger lipemic response after the consumption of butter than of olive oil in subjects with hypertriglyceridemia. Irawati et al. (34) defined hyper-responders as subjects whose TG concentration exceeded 1.7 mmol/L 4 h after the consumption of a palm oil–enriched meal; the TG concentration of normal-responders remained below this threshold. Hyper-responders had a greater TG iAUC0–8 h after the consumption of palm oil than of rice bran oil. By contrast, in normal-responders, the lipemic responses to the palm oil–enriched and the rice bran oil–enriched meals were comparable. Diekmann et al. (43) and Schönknecht et al. (44) focused on dietary pattern rather than fat sources. Compared with a Mediterranean diet meal, Diekmann et al. (43) observed a greater TG iAUC0–4.5 h after consumption of a Western diet meal. Schönknecht et al. (44) observed a similar effect; a Western diet, high-fat meal provoked a stronger lipemic response than a Mediterranean diet meal. None of the studies reported a higher postprandial TG AUC after a meal rich in unsaturated FAs than after a SFA-rich meal (Tables 3 and 4).
In 4 studies, no significant differences were observed when comparing the AUC of postprandial TG concentration between a SFA-rich meal and an unsaturated FA-rich meal. Meals contained coconut or rice bran oil (34), palm olein, or a blend of palm olein with soy or canola oil (45). Other studies compared a blend of palm and coconut oils with a blend of sunflower and canola oils (35), as well as meals prepared with butter, olive oil, and walnuts (33) (Tables 3 and 4).
Discussion
The aim of this review was to investigate the influence of mixed meals enriched with fat sources with different FA compositions on postprandial lipemia. We focused on a food-based approach and distinguished between metabolically healthy adults and individuals with CVD risk factors.
Metabolically healthy subjects
Most studies in metabolically healthy subjects did not report a significant difference in the AUC of postprandial TG concentration after the consumption of fat sources rich in SFAs or unsaturated FAs (Tables 2 and 4). Therefore, for metabolically healthy humans, the SFA content of meals does not seem to be a relevant determinant of postprandial lipemia.
It should be noted that the assumption, that in a state of metabolic health the FA composition of a meal has no effect on lipemia, is based on comparisons between certain fat sources. For example, 10 studies compared the effects of meals prepared with butter or olive oil on postprandial TG concentration (24, 26–29, 31–33, 36, 40), whereas only 1 study compared pure coconut oil with canola oil (32). There is a particular lack of evidence concerning fat sources with potential health-promoting effects, such as coconut oil and hemp seed oil. Since these fat sources are increasingly used in modern kitchens, more studies are required to determine their effect on postprandial metabolism.
A lack of significant effects of SFA-rich meals on postprandial lipemia has recently been described by Yao et al. (16). Although they hypothesized a beneficial effect of unsaturated FAs on postprandial TG and cholesterol response, their meta-analysis of 17 studies, including 13 studies in metabolically healthy subjects, did not reveal any significant differences in these parameters.
However, in our analysis, 2 studies reported a significantly higher postprandial TG AUC after the consumption of a SFA-rich meal (30, 31), both of which used a blend of plant and fish oils as the reference fat source (Tables 2 and 4). In the study of Austin et al. (30), lower lipemic responses to fish oil–containing meals were found although they had a similar fat and SFA content as comparison meals (olive oil, coconut oil); thus, the addition of fish oil to a mixed meal may attenuate the postprandial lipemic response to high-fat meals. There is also well-described evidence that long-term supplementation with fish oil lowers fasting and postprandial TG in metabolically healthy, normolipidemic subjects. Brown and Roberts (46) reported that, in comparison to olive oil, 6 wk of fish oil intake led to a significantly lower postprandial TG concentration in response to a standardized high-fat meal. Park and Harris (47) confirmed this observation by showing that supplementation with marine n–3 FAs for 4 wk reduced postprandial TG concentration by 16%. It has been suggested that marine n–3 PUFAs lower postprandial lipemia by diminishing endogenous production of VLDLs (48). Additionally, EPA and DHA accelerate the clearance of chylomicrons by upregulating lipoprotein lipase activity (47). ɑ-linolenic acid from plant foods (e.g., linseed oil) may serve as an alternative source of long-chain n–3 FAs, but further studies are needed to determine its effects on lipoprotein production and clearance.
In most of the studies analyzed, participants received butter or olive oil (24, 26–29, 31–33, 36, 40); however, comparison of postprandial TG AUC between meals revealed contradictory results (Tables 2 and 4). One reason may be differences in the FA composition of the same fat source. For example, the butter in the study of Sun et al. (28) contained 50% SFAs, whereas Thomsen et al. (26) used butter with 72% SFAs. When evaluating the results of several studies, variations in FA profiles of similar fat sources should be considered.
The role of chain length of SFAs
According to Karupaiah et al. (41) and Panth et al. (23), coconut oil provokes a weaker postprandial lipemic response than cocoa butter, butter, and lard (Table 5). All of these fat sources are rich in SFAs, but differ in their SFA composition. Coconut oil is dominated by lauric acid (12:0, 42 g/100 g) and myristic acid (14:0, 17 g/100 g), whereas the content of palmitic acid (16:0, 8.6 g/100 g) and stearic acid (18:0, 2.5 g/100 g) is low (11). Compared with coconut oil, butter (24), cocoa butter, and lard (11) have a higher content of palmitic acid (30, 25, and 24 g/100 g, respectively) and stearic acid (11, 33, and 14 g/100 g, respectively). Thus, the chain length of the SFAs may influence the magnitude of the postprandial lipemic response. However, conflicting results should be noted. Poppitt et al. (42) did not find any significant differences when comparing the AUC0–3 h of postprandial TG concentration after meals enriched with coconut oil, tallow, and milk fat (Table 5). Likewise, in the study of Karupaiah et al. (41), a blend of coconut oil and corn oil did not provoke a different lipemic response than palm olein. There were also no differences between meals enriched with butter or lard (23). To better understand the impact of SFA chain length on postprandial lipemia, systematic investigations with standardized amounts of FAs are required.
Subjects with CVD risk factors
In nearly every study of subjects with CVD risk factors, a SFA-rich meal provoked a higher postprandial TG AUC than a meal rich in unsaturated FAs (Tables 3 and 4). Most meals with a high content of SFAs contained butter; thus, individuals with CVD risk factors may benefit from replacing butter with fat sources rich in unsaturated FAs such as canola or olive oil. The recent meta-analysis of Yao et al. (16) of 17 studies, including 4 studies in people with CVD risk factors, did not reveal significant differences in the AUCs of postprandial TG concentration between SFA-rich meals and meals enriched with unsaturated FAs. The authors did not differentiate between metabolically healthy subjects and individuals with a CVD risk profile. A meta-analysis including studies solely in people with CVD risk factors may provide clarity concerning the effects of FA composition on postprandial lipemia in these individuals.
The role of the type of CVD risk factors
Our analysis illustrates that investigations of postprandial lipemia require consideration of the CVD risk factors of participants. In studies with significant differences in the AUC of postprandial TG concentration, subjects had hypertriglyceridemia (25, 31), elevated postprandial TG concentrations (34), or several characteristics of the metabolic syndrome (43, 44). By contrast, investigations in subjects with mild or familial hypercholesterolemia did not reveal significant differences between meals rich in SFAs or unsaturated FAs (35, 45). In addition, subjects in studies with significant differences in the lipemic response were older and had a higher BMI than those in studies without significant differences (Tables 3 and 4). Thus, impaired TG metabolism (especially hypertriglyceridemia), advanced age (≥40 y), and elevated BMI appear to promote an exaggerated postprandial lipemic response to SFA-rich meals. It should be noted that in the study of Lozano et al. (33), meals with different FA compositions did not provoke significant differences in the postprandial TG iAUC in plasma of lower-weight subjects and higher-weight subjects; however, all subjects had a BMI (in kg/m2) <30. Therefore, a threshold of 30 may be required for detection of significant differences in lipemic responses to meals with different FA compositions.
Other investigations confirm the assumption that certain CVD risk factors increase the extent of postprandial lipemia. Jackson et al. (49) reported that, as the number of metabolic syndrome components increases, the AUC0–8 h and the iAUC0–8 h of postprandial TG concentration also increase. In several investigations, a correlation between BMI and the magnitude of the postprandial TG response was observed (32, 50). Couillard et al. (51) showed that men responded with a greater postprandial TG iAUC0–8 h than women. However, the gender difference disappeared after matching for visceral adipose tissue, since there was a significant association between visceral adipose tissue and postprandial lipemia in both genders. In the study of Madhu et al. (52), men with type 2 diabetes responded to a fat-rich meal with a greater postprandial TG AUC0–8 h and iAUC0–8 h than metabolically healthy controls. In addition, diabetic subjects showed a higher TG peak. Emerson et al. (53) observed that advanced age promotes an exaggerated postprandial lipemic response to a high-fat meal. Younger, active adults (mean age, 25 y) showed a significantly lower tAUC0–6 h of postprandial TG concentration, as well as a lower TG peak, than both older active and inactive older adults. Furthermore, older active adults (mean age, 67 y) responded to the meal with a lower lipemic response than older inactive adults (mean age, 68 y). Further studies are required to confirm the finding that older inactive subjects with characteristics of metabolic syndrome benefit from exchanging SFAs (e.g., butter) with unsaturated FAs (e.g., canola oil and olive oil).
Influence of the fat dose on magnitude and time course of postprandial lipemia
At the end of the last century, a dose–response relation between the fat content of meals and postprandial TG concentration was described (54, 55). Cohen et al. (54) demonstrated that the magnitude of lipemia was proportional to the fat content of high-fat meals. Dubois et al. (55) observed a stepwise increase in serum TG concentration after the consumption of meals with graded amounts of fat. Likewise, current reviews reported increasing lipemia with increasing fat intake (56, 57). Our analysis did not focus on the effect of fat dose on postprandial lipemia, in part because none of the included studies were performed with gradually increasing amounts of fat. Meals contained high fat doses and, in some investigations, an additional meal without fat was consumed (24–26). Comparing the lipemic responses to fat-free meals with those to high-fat meals suggests a positive dose–response relation, although this association remains to be confirmed.
It is well known that, in response to a mixed meal, TG concentration increases rapidly until Cmax, which is usually reached between the second and third hour postprandially (54, 55). After reaching a plateau between the third and fourth hour, the TG concentration remains elevated until 6 h after meal intake (57). Data indicate that, compared with a moderate fat load (e.g., 35 g), a high fat load (e.g., 79 g) triggers an earlier Cmax of TGs. However, this observation was not based on studies with graded fat loads but on comparisons between studies (Tables 2 and 3). Due to variations in study protocols, it remains uncertain whether variation in the quantity of fat in meals was responsible for differences in the TG time course. Previous investigations with graded fat loads do not clearly confirm an influence of fat dose on the tmax of postprandial TG concentration (54, 55). Thus, in addition to the fat content of meals, other factors that influence postprandial lipemia should be considered when analyzing lipemic responses.
Strengths and limitations
A strength of this analysis is the investigation of both metabolically healthy subjects and individuals with CVD risk factors. In addition, the focus on natural, commercially available fat sources means the results have a practical application. This review helps to develop nutritional recommendations to reduce postprandial lipemia. Considering that a high postprandial TG concentration is associated with increased risk of CVD, the conclusions from our analysis may contribute to lowering CVD risk, especially of individuals with CVD risk factors.
One limitation of this analysis is that meals were categorized into those rich in SFAs and those rich in unsaturated FAs. Especially in meals rich in unsaturated FAs, this categorization may not have been specific enough to capture differences between unsaturated FA composition. Fat sources dominated by MUFAs (e.g., olive oil) or PUFAs (e.g., grapeseed oil, fish oil) can have different effects on postprandial lipemia (28, 30, 58). This limitation may also affect SFAs, which include several subgroups such as medium-chain SFAs (23, 41). Therefore, it would be useful for further analysis to consider the differences in the FA profiles of SFAs and unsaturated FAs.
Due to the high heterogeneity of population, intervention, comparison, and outcome measurement of included studies, we did not perform meta-analysis. Considering the limited number of comparable studies (e.g., administering the same fat sources, or having the same length of observational period), we decided not to attempt meta-analysis with subsequent subgroup analyses. As a result, our findings have an increased risk of exaggerating effects and should be interpreted carefully. Standardization of the designs of postprandial protocols (Table 6) would enable meaningful meta-analyses verifying our findings.
TABLE 6.
Recommendations for designs of future postprandial studies1
| Recommendations | |
|---|---|
| Subject group |
|
| Study design |
|
| Behavior before intervention days |
|
| Type of meal |
|
| Fat dose |
|
| Meal consumption and postprandial period |
|
| Primary parameter of postprandial lipemia |
|
| Examples for additional parameters of postprandial lipemia |
|
1CVD, cardiovascular disease; FA, fatty acid; iAUC, incremental AUC.
Conclusions
This review revealed 3 main findings. First, in metabolically healthy subjects, the FA composition of a mixed meal is not a relevant determinant of the magnitude of postprandial lipemia. Second, in subjects with CVD risk factors, a high SFA content (>32 g SFA/meal) often provokes a greater lipemic response than unsaturated FAs. Subjects with hypertriglyceridemia, an elevated BMI (≥30 kg/m2), and/or who are older (≥40 y) may benefit from replacing SFAs with unsaturated FAs. To verify this suggestion, further postprandial protocols should concentrate on subjects with CVD risk factors rather than metabolically healthy adults. Third, because of the dose–response relation between fat load and the magnitude of postprandial lipemia, lowering the fat content of meals has a greater impact on postprandial lipemia than modifying the FA composition.
Future directions
This analysis revealed a lack of standardized procedures in postprandial protocols (Tables 2–5). Marked differences were noted in the fat dose, the length of the observational period (3–11 h), the number of postprandial blood sample collections (2–15), and the parameter of lipemia (e.g., iAUC, mmol/L × 6 h). To increase the comparability of study results, standardized procedures for postprandial protocols are required (57). To reliably induce lipemia while maintaining the physiological relevance and applicability of results, a moderate fat load (35–50 g/meal) is recommended. A postprandial observational period of 6–8 h with regular collection of blood samples (every 1–1.5 h) ensures that fluctuations in lipemia are fully captured. With regard to lipemia parameters, measuring the iAUC of postprandial TG concentration is most advisable. Because the AUC does not allow any analysis of time course, differences in lipemia may be missed when focusing only on the AUC of postprandial TG concentration. To avoid misinterpretations, further analysis should include parameters of the time course such as tmax (overview of recommendations in Table 6).
Attenuating the lipemic response is an effective strategy to lower CVD risk through nutritional recommendations. The postprandial TG concentration in blood plasma or serum is one of several parameters considered to be an independent predictor of CVD. In some studies, despite nonsignificant differences in plasma TG, meals with different FA compositions did provoke significant differences in TG concentration in specific lipoprotein fractions—for example, in small TG-rich lipoproteins (33) or in the chylomicron-rich fraction (26). Therefore, to comprehensively evaluate the influence of fat sources on cardiovascular health, it would be useful to analyze a broader spectrum of postprandial lipemia parameters. In addition, further metabolic processes, such as glycemia, insulinemia, and low-grade postprandial inflammation, should be considered when evaluating the influence of meal composition on CVD risk parameters.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—HFN and SE: conducted the search, selection, and evaluation of studies; HFN: prepared the first draft of the manuscript, which was subsequently finalized in close collaboration with SE; and both authors: declare responsibility for final content and read and approved the final manuscript.
Notes
The authors reported no specific funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: Cmax, maximum TG concentration; CVD, cardiovascular disease; FA, fatty acid; iAUC, incremental AUC; tAUC, total AUC; TG, triglyceride; tmax, time to reach the maximum TG concentration.
Contributor Information
Hannah F Neumann, Institute of Nutritional Medicine, University of Hohenheim, Stuttgart, Germany.
Sarah Egert, Institute of Nutritional Medicine, University of Hohenheim, Stuttgart, Germany.
References
- 1. Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220(1):22–33. [DOI] [PubMed] [Google Scholar]
- 2. Burton-Freeman B. Postprandial metabolic events and fruit-derived phenolics: a review of the science. Br J Nutr. 2010;104(S3):S1–S14. [DOI] [PubMed] [Google Scholar]
- 3. Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98(3):458–73. [DOI] [PubMed] [Google Scholar]
- 4. Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C, Rosenkranz SK. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: a systematic review. Adv Nutr. 2017;8(2):213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margioris AN. Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care. 2009;12(2):129–37. [DOI] [PubMed] [Google Scholar]
- 6. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–16. [DOI] [PubMed] [Google Scholar]
- 7. O'Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904. [DOI] [PubMed] [Google Scholar]
- 8. Patsch JR, Miesenböck G, Hopferwieser T, Mühlberger V, Knapp E, Dunn JK, Gotto AM Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12(11):1336–45. [DOI] [PubMed] [Google Scholar]
- 9. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al. Dietary fats and cardiovascular disease: a Presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–23. [DOI] [PubMed] [Google Scholar]
- 10. Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276(11):882–8. [PubMed] [Google Scholar]
- 11. USDA. FoodData Central. [Accessed October 2020]. Available from:https://fdc.nal.usda.gov/index.html. [Google Scholar]
- 12. Mensink RP; World Health Organization . Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva (Switzerland): World Health Organization; 2016. [Google Scholar]
- 13. Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015;(6):CD011737. [DOI] [PubMed] [Google Scholar]
- 14. Wahrburg U. What are the health effects of fat?. Eur J Nutr. 2004;43:(Suppl 1):I/6–11. [DOI] [PubMed] [Google Scholar]
- 15. Monfort-Pires M, Delgado-Lista J, Gomez-Delgado F, Lopez-Miranda J, Perez-Martinez P, Ferreira SR. Impact of the content of fatty acids of oral fat tolerance tests on postprandial triglyceridemia: systematic review and meta-analysis. Nutrients. 2016;8(9):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao Y, Pek SX, Toh DWK, Xia X, Kim JE. Effects of fatty acids composition in a breakfast meal on the postprandial lipid responses: a systematic review and meta-analysis of randomised controlled trials. Int J Food Sci Nutr. 2020;71(7):793–803. [DOI] [PubMed] [Google Scholar]
- 17. Dias CB, Moughan PJ, Wood LG, Singh H, Garg ML. Postprandial lipemia: factoring in lipemic response for ranking foods for their healthiness. Lipids Health Dis. 2017;16(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mekki N, Christofilis MA, Charbonnier M, Atlan-Gepner C, Defoort C, Juhel C, Borel P, Portugal H, Pauli AM, Vialettes B et al. Influence of obesity and body fat distribution on postprandial lipemia and triglyceride-rich lipoproteins in adult women. J Clin Endocrinol Metab. 1999;84(1):184–91. [DOI] [PubMed] [Google Scholar]
- 19. Lewis GF, O'Meara NM, Soltys PA, Blackman JD, Iverius PH, Pugh WL, Getz GS, Polonsky KS. Fasting hypertriglyceridemia in non insulin-dependent diabetes mellitus is an important predictor of postprandial lipid and lipoprotein abnormalities. J Clin Endocrinol Metab. 1991;72(4):934–44. [DOI] [PubMed] [Google Scholar]
- 20. Kolovou GD, Anagnostopoulou KK, Pavlidis AN, Salpea KD, Iraklianou SA, Tsarpalis K, Damaskos DS, Manolis A, Cokkinos DV. Postprandial lipemia in men with metabolic syndrome, hypertensives and healthy subjects. Lipids Health Dis. 2005;4(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriège P, Després JP. Postprandial triglyceride response in visceral obesity in men. Diabetes. 1998;47(6):953–60. [DOI] [PubMed] [Google Scholar]
- 22. Panth N, Abbott KA, Dias CB, Wynne K, Garg ML. Differential effects of medium- and long-chain saturated fatty acids on blood lipid profile: a systematic review and meta-analysis. Am J Clin Nutr. 2018;108(4):675–87. [DOI] [PubMed] [Google Scholar]
- 23. Panth N, Dias CB, Wynne K, Singh H, Garg ML. Medium-chain fatty acids lower postprandial lipemia: a randomized crossover trial. Clin Nutr. 2020;39(1):90–6. [DOI] [PubMed] [Google Scholar]
- 24. Mekki N, Charbonnier M, Borel P, Leonardi J, Juhel C, Portugal H, Lairon D. Butter differs from olive oil and sunflower oil in its effects on postprandial lipemia and triacylglycerol-rich lipoproteins after single mixed meals in healthy young men. J Nutr. 2002;132(12):3642–9. [DOI] [PubMed] [Google Scholar]
- 25. Lopez S, Bermudez B, Ortega A, Varela LM, Pacheco YM, Villar J, Abia R, Muriana FJ. Effects of meals rich in either monounsaturated or saturated fat on lipid concentrations and on insulin secretion and action in subjects with high fasting triglyceride concentrations. Am J Clin Nutr. 2011;93(3):494–9. [DOI] [PubMed] [Google Scholar]
- 26. Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, Hermansen K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69(6):1135–43. [DOI] [PubMed] [Google Scholar]
- 27. Sanders TA, Oakley FR, Miller GJ, Mitropoulos KA, Crook D, Oliver MF. Influence of n-6 versus n-3 polyunsaturated fatty acids in diets low in saturated fatty acids on plasma lipoproteins and hemostatic factors. Arterioscler Thromb Vasc Biol. 1997;17(12):3449–60. [DOI] [PubMed] [Google Scholar]
- 28. Sun L, Tan KWJ, Lim JZ, Magkos F, Henry CJ. Dietary fat and carbohydrate quality have independent effects on postprandial glucose and lipid responses. Eur J Nutr. 2018;57(1):243–50. [DOI] [PubMed] [Google Scholar]
- 29. Svensson J, Rosenquist A, Ohlsson L. Postprandial lipid responses to an alpha-linolenic acid-rich oil, olive oil and butter in women: a randomized crossover trial. Lipids Health Dis. 2011;10(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Austin G, Ferguson JJ, Thota RN, Singh H, Burrows T, Garg ML. Postprandial lipaemia following consumption of a meal enriched with medium chain saturated and/or long chain omega-3 polyunsaturated fatty acids: a randomised cross-over study. Clin Nutr. 2021;40(2):420–7. [DOI] [PubMed] [Google Scholar]
- 31. Bermudez B, Ortega-Gomez A, Varela LM, Villar J, Abia R, Muriana FJ, Lopez S. Clustering effects on postprandial insulin secretion and sensitivity in response to meals with different fatty acid compositions. Food Funct. 2014;5(7):1374–80. [DOI] [PubMed] [Google Scholar]
- 32. Sciarrillo CM, Koemel NA, Tomko PM, Bode KB, Emerson SR. Postprandial lipemic responses to various sources of saturated and monounsaturated fat in adults. Nutrients. 2019;11(5):1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lozano A, Perez-Martinez P, Delgado-Lista J, Marin C, Cortes B, Rodriguez-Cantalejo F, Gomez-Luna MJ, Cruz-Teno C, Perez-Jimenez F, Lopez-Miranda J. Body mass interacts with fat quality to determine the postprandial lipoprotein response in healthy young adults. Nutr Metab Cardiovasc Dis. 2012;22(4):355–61. [DOI] [PubMed] [Google Scholar]
- 34. Irawati D, Mamo JC, Slivkoff-Clark KM, Soares MJ, James AP. Dietary fat and physiological determinants of plasma chylomicron remnant homoeostasis in normolipidaemic subjects: insight into atherogenic risk. Br J Nutr. 2017;117(3):403–12. [DOI] [PubMed] [Google Scholar]
- 35. Øyri LKL, Hansson P, Bogsrud MP, Narverud I, Florholmen G, Leder L, Byfuglien MG, Veierød MB, Ulven SM, Holven KB. Delayed postprandial TAG peak after intake of SFA compared with PUFA in subjects with and without familial hypercholesterolaemia: a randomised controlled trial. Br J Nutr. 2018;119(10):1142–50. [DOI] [PubMed] [Google Scholar]
- 36. Bellido C, López-Miranda J, Blanco-Colio LM, Pérez-Martínez P, Muriana FJ, Martín-Ventura JL, Marín C, Gómez P, Fuentes F, Egido J et al. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor kappaB in peripheral blood mononuclear cells from healthy men. Am J Clin Nutr. 2004;80(6):1487–91. [DOI] [PubMed] [Google Scholar]
- 37. Meikle PJ, Barlow CK, Mellett NA, Mundra PA, Bonham MP, Larsen A, Cameron-Smith D, Sinclair A, Nestel PJ, Wong G. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. J Nutr. 2015;145(9):2012–8. [DOI] [PubMed] [Google Scholar]
- 38. Pedersen A, Marckmann P, Sandström B. Postprandial lipoprotein, glucose and insulin responses after two consecutive meals containing rapeseed oil, sunflower oil or palm oil with or without glucose at the first meal. Br J Nutr. 1999;82(2):97–104. [PubMed] [Google Scholar]
- 39. Tholstrup T, Teng KT, Raff M. Dietary cocoa butter or refined olive oil does not alter postprandial hsCRP and IL-6 concentrations in healthy women. Lipids. 2011;46(4):365–70. [DOI] [PubMed] [Google Scholar]
- 40. Perez-Martinez P, Ordovas JM, Garcia-Rios A, Delgado-Lista J, Delgado-Casado N, Cruz-Teno C, Camargo A, Yubero-Serrano EM, Rodriguez F, Perez-Jimenez F et al. Consumption of diets with different type of fat influences triacylglycerols-rich lipoproteins particle number and size during the postprandial state. Nutr Metab Cardiovasc Dis. 2011;21(1):39–45. [DOI] [PubMed] [Google Scholar]
- 41. Karupaiah T, Tan CH, Chinna K, Sundram K. The chain length of dietary saturated fatty acids affects human postprandial lipemia. J Am Coll Nutr. 2011;30(6):511–21. [DOI] [PubMed] [Google Scholar]
- 42. Poppitt SD, Strik CM, MacGibbon AK, McArdle BH, Budgett SC, McGill AT. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol Behav. 2010;101(1):161–7. [DOI] [PubMed] [Google Scholar]
- 43. Diekmann C, Huber H, Preuß M, Preuß P, Predel HG, Stoffel-Wagner B, Fimmers R, Stehle P, Egert S. Moderate postmeal walking has no beneficial effects over resting on postprandial lipemia, glycemia, insulinemia, and selected oxidative and inflammatory parameters in older adults with a cardiovascular disease risk phenotype: a randomized crossover trial. J Nutr. 2019;149(11):1930–41. [DOI] [PubMed] [Google Scholar]
- 44. Schönknecht YB, Crommen S, Stoffel-Wagner B, Coenen M, Fimmers R, Holst JJ, Simon MC, Stehle P, Egert S. Acute effects of three different meal patterns on postprandial metabolism in older individuals with a risk phenotype for cardiometabolic diseases: a randomized controlled crossover trial. Mol Nutr Food Res. 2020;64(9):1901035. [DOI] [PubMed] [Google Scholar]
- 45. Karupaiah T, Sundram K. Modulation of human postprandial lipemia by changing ratios of polyunsaturated to saturated (P/S) fatty acid content of blended dietary fats: a cross-over design with repeated measures. Nutr J. 2013;12(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown AJ, Roberts DC. Moderate fish oil intake improves lipemic response to a standard fat meal: a study in 25 healthy men. Arterioscler Thromb. 1991;11(3):457–66. [DOI] [PubMed] [Google Scholar]
- 47. Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res. 2003;44(3):455–63. [DOI] [PubMed] [Google Scholar]
- 48. Roche HM, Gibney MJ. Long-chain n-3 polyunsaturated fatty acids and triacylglycerol metabolism in the postprandial state. Lipids. 1999;34(S1):S259–65. [DOI] [PubMed] [Google Scholar]
- 49. Jackson KG, Walden CM, Murray P, Smith AM, Lovegrove JA, Minihane AM, Williams CM. A sequential two meal challenge reveals abnormalities in postprandial TAG but not glucose in men with increasing numbers of metabolic syndrome components. Atherosclerosis. 2012;220(1):237–43. [DOI] [PubMed] [Google Scholar]
- 50. Kasai M, Maki H, Nosaka N, Aoyama T, Ooyama K, Uto H, Okazaki M, Igarashi O, Kondo K. Effect of medium-chain triglycerides on the postprandial triglyceride concentration in healthy men. Biosci Biotechnol Biochem. 2003;67(1):46–53. [DOI] [PubMed] [Google Scholar]
- 51. Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriège P, Després JP. Gender difference in postprandial lipemia : importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 1999;19(10):2448–55. [DOI] [PubMed] [Google Scholar]
- 52. Madhu SV, Mittal V, Ram BK, Srivastava DK. Postprandial lipid abnormalities in type 2 diabetes mellitus. J Assoc Physicians India. 2005;53:1043–6. [PubMed] [Google Scholar]
- 53. Emerson SR, Kurti SP, Emerson EM, Cull BJ, Casey K, Haub MD, Rosenkranz SK. Postprandial metabolic responses differ by age group and physical activity level. J Nutr Health Aging. 2018;22(1):145–53. [DOI] [PubMed] [Google Scholar]
- 54. Cohen JC, Noakes TD, Benade AJ. Serum triglyceride responses to fatty meals: effects of meal fat content. Am J Clin Nutr. 1988;47(5):825–7. [DOI] [PubMed] [Google Scholar]
- 55. Dubois C, Beaumier G, Juhel C, Armand M, Portugal H, Pauli AM, Borel P, Latgé C, Lairon D. Effects of graded amounts (0–50 g) of dietary fat on postprandial lipemia and lipoproteins in normolipidemic adults. Am J Clin Nutr. 1998;67(1):31–8. [DOI] [PubMed] [Google Scholar]
- 56. Desmarchelier C, Borel P, Lairon D, Maraninchi M, Valéro R. Effect of nutrient and micronutrient intake on chylomicron production and postprandial lipemia. Nutrients. 2019;11(6):1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007;61(10):1145–61. [DOI] [PubMed] [Google Scholar]
- 58. Jackson KG, Wolstencroft EJ, Bateman PA, Yaqoob P, Williams CM. Acute effects of meal fatty acids on postprandial NEFA, glucose and apo E response: implications for insulin sensitivity and lipoprotein regulation?. Br J Nutr. 2005;93(5):693–700. [DOI] [PubMed] [Google Scholar]