Abstract
Objectives:
Individual kidney tubule biomarkers are associated with CKD risk in persons living with HIV (PLWH). Whether a combination of kidney biomarkers can be integrated into informative summary scores for PLWH is unknown.
Methods:
We measured 8 urine biomarkers of kidney tubule health at 2 visits over a 3-year period in 647 women living with HIV in the Women’s Interagency Health Study. We integrated biomarkers into factor scores using exploratory factor analysis. We evaluated associations between CKD risk factors and factor scores, and used generalized estimating equations to determine associations between factor scores and risk of incident CKD.
Results:
Factor analysis identified 2 unique factor scores: a tubule reabsorption score comprising α-1-microglobulin, beta-2-microglobulin, and trefoil factor-3 and a tubule injury score comprising interleukin-18 and kidney injury molecule-1. We modeled the 2 factor scores in combination with urine epidermal growth factor (EGF) and urine albumin. Predominantly HIV-related CKD risk factors were independently associated with worsening tubule reabsorption scores and tubule injury scores. During a median follow-up of 7 years, 9.7% (63/647) developed CKD. In multivariable time-updated models that adjusted for other factor scores and biomarkers simultaneously, higher tubule reabsorption scores (RR=1.27, 95% CI: 1.01, 1.59 per 1-SD higher time-updated score), higher tubule injury scores (RR=1.36, 95% CI: 1.05, 1.76), lower urine EGF (RR=0.75, 95% CI: 0.64, 0.87), and higher urine albumin (RR=1.20, 95% CI: 1.02, 1.40) were jointly associated with risk of incident CKD.
Conclusions:
We identified 2 novel and distinct dimensions of kidney tubule health that appear to quantify informative metrics of CKD risk in PLWH.
Keywords: biomarkers, chronic kidney disease, exploratory factor analysis, HIV
Introduction
In the era of combination antiretroviral therapy, people living with HIV (PLWH) have had dramatic gains in life expectancy.(1,2) At the same time, PLWH are experiencing an increasing burden of age-related comorbidities, including chronic kidney disease (CKD).(3) CKD is more common and occurs earlier in PLWH compared to the general population.(4-6) CKD in PLWH also has strong associations with end-stage kidney disease, cardiovascular disease, heart failure, and early death.(7,8) As the HIV population continues to survive to older ages, the monitoring of kidney disease will become an increasingly important requirement for HIV care.
Despite the kidney tubules comprising over 90% of the kidney’s cortical mass and having a central role in CKD pathogenesis, clinical assessment of kidney disease relies upon two measures that primarily reflect glomerular health: estimated glomerular filtration rate (eGFR) and urine albumin. Although eGFR is often used as a surrogate for overall kidney function, it lacks sensitivity for detecting kidney tubule injury or early reductions in kidney function in PLWH.(9,10) Urine albumin is an established marker of glomerular damage, but cannot adequately identify tubulointerstitial disease, which is a hallmark finding in all forms of CKD.(11)
Over the past decade, several novel urine biomarkers reflecting kidney tubule health have shown promise in improving the characterization of kidney disease in PLWH. These urine biomarkers represent a wide array of functions and pathophysiologic mechanisms specific to the kidney tubules, including: injury (kidney injury molecule-1 [KIM-1] and interleukin-18 [IL-18]); reabsorption (alpha-1-microglobulin [α1m], beta-2-microglobulin [β2m], and trefoil factor-3 [TFF3]); fibrosis and repair (epidermal growth factor [EGF] and chitinase 3-like protein 1 [YKL-40]); and loop of Henle synthetic function (uromodulin). Several of these urine biomarkers are higher in PLWH compared to uninfected individuals and associate with longitudinal kidney function decline and mortality.(12-17)
Given the diverse mechanisms underlying the development of kidney disease in PLWH, CKD risk may be optimally assessed using a combination of biomarkers that provide complementary information on glomerular and tubular health. However, combining information from several inter-correlated biomarkers using standard statistical approaches can pose problems in modeling and interpretation. Factor analysis is a variable reduction method that can identify underlying patterns within the data by combining measured biomarkers into latent variables, also known as factors.(18) Each factor is a linear combination of multiple inter-correlated variables, and the resulting factors can be evaluated in regression analyses as composite scores. Factor analysis is commonly used in the social sciences, but has been rarely applied in kidney biomarker research.(19)
Our study had 3 objectives. First, to use exploratory factor analysis to partition and summarize the information from repeated measures of 8 kidney tubule biomarkers into factor scores among women living with HIV in the Women’s Interagency HIV Study (WIHS) cohort. Second, to evaluate the associations of CKD risk factors with each factor score. Third, to evaluate the associations of factor scores with risk of incident CKD. We hypothesized that exploratory factor analysis could separate the 8 kidney tubule biomarkers into distinct factor scores; that CKD risk factors would have different associations with each factor score; and that baseline and repeated factor scores would be independently associated with incident CKD.
Methods
Study design
The WIHS study design and methods have been described previously.(20) In brief, a total of 3,766 HIV-infected and uninfected women of similar backgrounds were enrolled in 1994-1995 and 2001-2002 from six sites (Bronx/Manhattan, Brooklyn, Chicago, Los Angeles, San Francisco and Washington, DC). The WIHS protocol comprises a baseline visit and follow-up visits every six months; each visit includes an interviewer-administered questionnaire, a physical examination, and collection of laboratory specimens.
Among WIHS participants living with HIV, we designed a nested study to investigate the trajectory of kidney injury and function in the setting of HIV. We included 647 women living with HIV who had available urine and serum specimens collected twice and had eGFR ≥60 ml/min/1.73m2 at the time of the first specimen collection. The first urine specimen was collected between October 2009 and March 2011, and the second urine specimen was collected a median (interquartile range [IQR]) 2.5 (2.4–2.5) years later. Follow-up started at the first urine specimen collection and was truncated in April 2017. There were few losses to follow-up (35/647 =5.4%).
WIHS and associated protocols were approved by the institutional review boards of all participating institutions, and informed consent was obtained from all study participants. This kidney biomarker ancillary study was also approved by the Committee on Human Research of the University of California San Francisco (UCSF).
Urine biomarker measurements
Urine specimens were in continuous storage at −80°C until biomarker measurement. Urine KIM-1, IL-18, α1m, β2m, TFF3, EGF, YKL-40, uromodulin, albumin, and creatinine were all measured at the UCSF Kidney Health Research Collaboration (KHRC) Biomarker Laboratory. Baseline and follow-up urine biomarkers were measured on the same plates to minimize assay drift. Biomarker intra-assay coefficients of variation were <15% and are shown along with analytic ranges and assays in Supplementary Table S1. Laboratory personnel performing the biomarker assays were blinded to participants’ clinical information.
Factor score derivation
Robust maximum likelihood estimation was used to derive factor scores using only the kidney biomarkers. Factor derivation was agnostic to all covariates and incident CKD events. The scree test and proportion criteria were used to determine the optimal number of factor scores, comparing different oblique and orthogonal rotation methods to identify solutions with the best structure. We derived factor scores using the baseline urine biomarker measurements and then used the same biomarker weights to estimate factor scores for the second set of urine biomarker measurements. Because our goal was to determine whether factor scores derived from kidney tubule biomarkers had associations with incident CKD independent of urine albumin, we included urine albumin in the final multivariable models as a separate measure. From the 8 kidney tubule biomarkers, uromodulin and YKL-40 were excluded because these biomarkers had small factor loadings (<0.4), meaning they were not prominent contributors to any factor score. We also excluded urine EGF from the factor analysis because its factor loading was in the opposite hypothesized direction from its CKD association. However, because of the strength of association of urine EGF with CKD in our prior work, we retained it in the final multivariable models as a separate measure.(21,22) A model with 2 factors was determined to be the best solution for the remaining 5 biomarkers. We then used the regression scoring method to derive standardized factor scores.
In summary, this process resulted in the construction of 2 factor scores from kidney tubule biomarkers, which we modeled together with urine EGF and urine albumin to represent 4 dimensions of kidney health.
CKD risk factors
Demographics, clinical characteristics, and HIV-related characteristics were assessed at each examination. Consistent with our prior studies, the following covariates were included in all multivariable models: age, race/ethnicity (self-reported Black, White, or other), diabetes (defined using confirmatory criteria for fasting glucose ≥126 mg/dL, self-reported diabetes, self-reported diabetes medication use, or hemoglobin A1c [HbA1c] ≥6.5%), systolic and diastolic blood pressure, hypertension (defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or self-reported history of hypertension and antihypertensive medication use), self-reported history of cardiovascular disease, statin use, low- and high-density lipoprotein cholesterol levels, body mass index, cigarette smoking status (current, past, or never), serum albumin level, self-reported current intravenous drug use, hepatitis C virus (HCV) infection (confirmed by detectable HCV RNA following a positive HCV antibody result), current and nadir CD4 lymphocyte count, current and peak plasma HIV-1 RNA level, history of clinical acquired immunodeficiency syndrome (AIDS) diagnosis, duration of HIV infection, and duration of antiretroviral therapy (tenofovir disoproxil fumarate [TDF], ritonavir, or combination antiretroviral therapy [ART] regimens defined in accordance with US Department of Health and Human Services treatment guidelines).(23) Undetectable HIV viral load was defined as plasma HIV-1 RNA <80 copies/mL. The percentage of participants with missing covariate data was <5%.
Incident CKD
Incident CKD was defined as an eGFR <60 ml/min/1.73m2 measured at two consecutive six-month visits and an average annual eGFR decline of ≥3% per year. Serum creatinine was measured in local laboratories for each study site with assays using the modified Jaffe method traceable to isotope dilute mass spectrometry. We calculated creatinine-based eGFR using the corresponding CKD Epidemiology Collaboration estimating equation.(24)
Statistical analysis
We summarized clinical characteristics and urine biomarker levels at the baseline and follow-up urine biomarker collection visits for all participants. We analyzed urine biomarkers as log-transformed continuous variables due to their right-skewed distributions. All models included urine creatinine as a separate covariate at each of the two urine collections to account for variations in urine concentration. Biomarkers were log-transformed and standardized to the same scale (mean 0, standard deviation [SD] 1) prior to analysis.
We first examined all pairwise Spearman correlations of baseline and follow-up levels between the 2 factor scores, urine EGF, urine albumin, and eGFR. Next, we evaluated associations between baseline and changes in CKD risk factors with baseline and changes in each of the 4 kidney health dimensions (the 2 factor scores, urine EGF, and urine albumin). All CKD risk factors and dimensions of kidney health were modeled in combination using the multivariable sparse group least absolute shrinkage and selection operator (MSG-LASSO) method for variable selection.(25) This method is appropriate for settings involving both multiple predictors and multiple outcomes, and can produce a sparse solution by removing unimportant variables. After retaining variables selected by MSG-LASSO, we then used multivariable simultaneous linear equations (constructed with three-stage least squares) to account for correlations between the dimensions of kidney health. This method is more appropriate than individual regression models given the relatedness among the biomarker measurements. To facilitate comparisons of the magnitude of each CKD risk factor’s association with each kidney health dimension, we reported standardized regression coefficients with 95% confidence intervals (CIs), such that an estimate of 0.2 indicates that a 1 standard deviation (SD) higher level of the CKD risk factor is associated with a 0.2 SD higher level of the kidney health dimension.
Next, we modeled associations of each dimension of kidney health with incident CKD with three approaches: (1) using the baseline value; (2) time-updating the baseline value with the follow-up value; and (3) using the absolute change from baseline, with adjustment for the baseline value as a separate predictor. For each approach, we calculated relative risks using modified Poisson regression from generalized estimating equation models to account for repeated measures within subjects. To determine whether each dimension of kidney health was independently associated with incident CKD, multivariable models additionally adjusted for demographics, CKD risk factors, baseline urine albumin (in models without urine albumin as the main predictor), and baseline eGFR. Time-updated and change from baseline analyses included covariates from both baseline and follow-up visits.
MSG-LASSO was performed using the R package MSGLasso. All other analyses were performed using the SAS system, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Study population characteristics
The 647 women living with HIV included in our study had a median (interquartile range [IQR]) duration of follow-up of 7.0 (6.8–7.1) years from the baseline biomarker measurements to the end of our study period. The median (IQR) age at baseline was 45 (40–51) years; 67% were African American; and 20% had diabetes. The median (IQR) duration of HIV infection was 14 (8-15) years; 75% were receiving ART; median (IQR) CD4 count was 518 cells/mm3; and 60% had undetectable viral levels (Table 1).
Table 1.
Summary of clinical characteristics and urine biomarkers of women living with HIV in WIHS at the baseline and second biomarker measurements
| Parameter | Baseline N=647 |
Follow-up N=647 |
P |
|---|---|---|---|
| Calendar year | 2009 | 2012 | |
| Age, y | 45 (40, 51) | 48 (43, 53) | |
| Race | |||
| African American | 432 (67%) | 432 (67%) | |
| Other | 100 (15%) | 100 (15%) | |
| White | 115 (18%) | 115 (18%) | |
| Hispanic | 133 (21%) | 133 (21%) | |
| Smoking | |||
| Current | 249 (38%) | 229 (35%) | 0.05 |
| Past | 210 (32%) | 232 (36%) | |
| Never | 188 (29%) | 186 (29%) | |
| Diabetes | 130 (20%) | 147 (23%) | <0.01 |
| Hypertension | 229 (35%) | 267 (41%) | <0.01 |
| LDL, mg/dL | 93 (76, 118) | 97 (74, 117) | 0.59 |
| Statin use | 93 (14%) | 109 (17%) | 0.03 |
| BMI, kg/m2 | 29 (25, 34) | 29 (25, 34) | 0.55 |
| Current ART | |||
| HAART use | 487 (75%) | 548 (85%) | <0.01 |
| NRTI use | 483 (75%) | 537 (83%) | <0.01 |
| NNRTI use | 202 (31%) | 221 (34%) | 0.03 |
| PI use | 274 (42%) | 302 (47%) | <0.01 |
| TDF use | 396 (61%) | 451 (70%) | 0.54 |
| Current CD4 | 518 (343, 730) | 537 (365, 756) | <0.01 |
| Lifetime Nadir CD4 | 213 (113, 307) | 200 (98, 290) | <0.01 |
| History of AIDS | 233 (36%) | 250 (39%) | <0.01 |
| Plasma HIV RNA < 80 | 386 (60%) | 447 (69%) | <0.01 |
| Peak HIV RNA > 10,000 | 510 (79%) | 521 (81%) | 0.01 |
| Hepatitis C virus infection | 124 (19%) | 130 (20%) | 0.03 |
| Heroin use | 8 (1%) | 9 (1%) | 0.74 |
| α1m, mg/dL | 0.89 (0.54, 1.79) | 1.01 (0.54, 2.02) | <0.01 |
| β2m, ng/mL | 149.6 (72.7, 333.9) | 156.9 (71.3, 358.8) | 0.31 |
| TFF3, pg/mL | 106.1 (32.1, 313.1) | 103.7 (33.5, 303.8) | 0.61 |
| IL-18, pg/mL | 63.3 (33.6, 127.3) | 63.0 (33.1, 127.4) | 0.91 |
| KIM-1, pg/mL | 535.1 (271.2, 1025.7) | 588.0 (300.7, 1166.4) | 0.05 |
| EGF, ng/mL | 13.9 (9.0, 20.3) | 13.1 (8.7, 19.5) | 0.32 |
| ACR, mg/g | 4.6 (2.1, 11.2) | 4.2 (2.0, 9.8) | 0.38 |
α1m, α-1 microglobulin; ACR, albumin-to-creatinine ratio; AIDS, acquired immunodeficiency syndrome; β2m, β-2 microglobulin; BMI, body mass index; EGF, epidermal growth factor; eGFR, estimated glomerular filtration rate; HAART, highly active antiretroviral therapy; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; LDL, low density lipoprotein cholesterol; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate; TFF3, trefoil factor 3
Notes: Data are presented as median (IQR) or numbers (percent). P-values are from Wilcoxon signed-rank test for continuous variables and McNemar’s test for categorical variables.
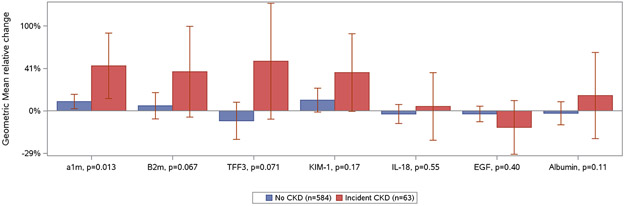
The median (IQR) eGFR and urine albumin-to-creatinine ratio at baseline were 104 (89–117) ml/min/1.73m2 and 4.6 (2.1–11.2) mg/g, respectively. During follow-up, 9.7% (63/647) of participants developed CKD. All CKD events developed after the second urine biomarker measurement (range, 0.4–4.7 years). The median (IQR) eGFR at time of CKD diagnosis was 53 (46–57) ml/min/1.73m2. Urine biomarker measurements at the baseline and follow-up visits are shown in Table 1, and changes in urine biomarker levels among persons who did and did not develop CKD are shown in Figure 1.
Figure 1. Relative percentage change in urine biomarker concentrations among women living with HIV in WIHS stratified by incident CKD status.
Geometric mean relative change in urine biomarker concentrations measured at the baseline and follow-up biomarker visits with 95% confidence intervals displayed as error bars. P values for difference in relative changes by CKD status calculated with Wilcoxon rank sum test. Biomarker concentrations are indexed to urine creatinine. Abbreviations: α1m, alpha-1-microglobulin; β2m, beta-2-microglobulin; EGF, epidermal growth factor; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; TFF3, trefoil factor 3; UAlb, urine albumin.
Exploratory factor analysis
Our standardized factor loading matrix for the 2 factor scores identified in exploratory factor analysis is shown in Table 2. Factor 1 was dominated by urine α1m, β2m, and TFF3, whereas factor 2 was dominated by urine IL-18 and KIM-1. Based on the biomarkers’ presumed pathophysiologic mechanisms, we labeled factor 1 as the tubule reabsorption score and factor 2 as the tubule injury score.
Table 2.
Standardized factor loading matrix from exploratory factor analysis of kidney tubule biomarkers
| Urine biomarker | Standardized Factor loadings | |
|---|---|---|
| Tubule reabsorption score |
Tubule injury score |
|
| α-1 microglobulin | 0.61 | 0.32 |
| β-2 microglobulin | 0.81 | 0.15 |
| Trefoil factor 3 | 0.78 | −0.06 |
| Interleukin-18 | −0.07 | 0.77 |
| Kidney injury molecule-1 | 0.11 | 0.65 |
Spearman correlations were overall weak to moderate between the factor scores, urine EGF, urine albumin, and eGFR at baseline and follow-up (Supplementary Table S2). At baseline, there were weak inverse correlations between eGFR and the factor scores (−0.10 for both reabsorption and injury scores), and moderate correlations between urine albumin and the factor scores (0.35 for both reabsorption and injury scores). Correlations between changes in factor scores were also moderate (0.41; Supplementary Figure S1).
CKD risk factor associations with kidney health dimensions
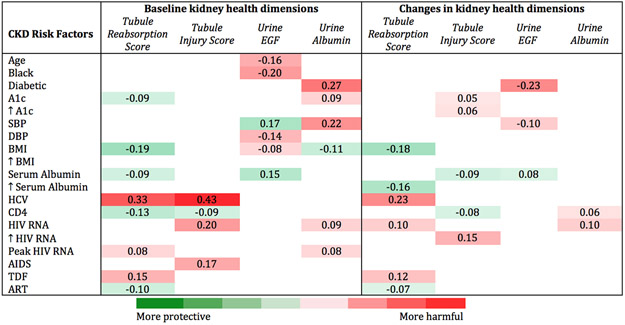
The CKD risk factors selected from penalized regression and their associations with baseline levels and changes in the tubule reabsorption score, the tubule injury score, urine EGF, and urine albumin are shown in Figure 2. Predominantly HIV-related CKD risk factors were associated with baseline tubule reabsorption and tubule injury scores and subsequent changes in both scores. In contrast, predominantly vascular and metabolic CKD risk factors were associated with baseline urine EGF levels and changes in urine EGF, and a combination of vascular, metabolic, and HIV-related CKD risk factors associated with baseline urine albumin levels and changes in urine albumin.
Figure 2. Simultaneous multivariable adjusted associations of baseline and change in CKD risk factors with baseline and change in dimensions of kidney health among women living with HIV in WIHS.
Standardized regression coefficients from simultaneous linear equations using 3-stage least squares to account for correlations between outcomes with 95% confidence intervals displayed. Numbers within each cell can be interpreted like correlation coefficients (scaled from − 1 to + 1). e.g., a 1 standard deviation (SD) higher SBP is associated with 0.27 SD higher urine albumin. Red shaded cells indicate harmful associations between CKD risk factors and dimensions of kidney health, and green shaded cells indicate protective associations. The degree of shading denotes the magnitude of the standardized beta coefficients. ↑ corresponds to a 1 SD increase in the CKD risk factor from baseline. Models simultaneously adjusted for traditional and HIV-related risk factors, urine creatinine, tubule reabsorption score, tubule injury score, urine EGF, and urine albumin. Abbreviations: A1c, hemoglobin A1c; AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; BMI, body mass index; DBP, diastolic blood pressure; EGF, epidermal growth factor; HCV, hepatitis C virus infection; SBP, systolic blood pressure; TDF, tenofovir disoproxil fumarate.
Kidney health dimension associations with incident CKD
Baseline measures of each dimension of kidney health were significantly associated with incident CKD in models adjusted for urine creatinine and CKD risk factors (Table 3). In models that additionally adjusted for baseline urine albumin (when urine albumin was not the main predictor) and eGFR, higher baseline tubule injury scores and urine albumin remained significantly associated with risk of incident CKD.
Table 3.
Baseline and time-updated associations of each kidney health dimension with risk of incident CKD among women living with HIV in WIHS
| Measure (per 1 SD) |
Model 1* RR (95% CI) |
Model 2† RR (95% CI) |
Model 3‡ RR (95% CI) |
|---|---|---|---|
| Baseline | |||
| Tubule reabsorption score | 2.06 (1.56, 2.72) | 1.60 (1.19, 2.15) | 1.12 (0.84, 1.49) |
| Tubule injury score | 2.54 (1.93, 3.34) | 2.04 (1.39, 3.00) | 1.53 (1.05, 2.25) |
| Urine EGF | 0.61 (0.50, 0.75) | 0.64 (0.50, 0.81) | 0.85 (0.63, 1.15) |
| Urine albumin | 2.67 (2.12, 3.35) | 2.14 (1.51, 3.03) | 2.11 (1.48, 3.02) |
| Time-updated | |||
| Tubule reabsorption score | 2.40 (1.92, 3.00) | 2.18 (1.73, 2.74) | 1.48 (1.20, 1.82) |
| Tubule injury score | 2.84 (2.15, 3.74) | 2.27 (1.70, 3.02) | 1.62 (1.30, 2.01) |
| Urine EGF | 0.50 (0.41, 0.61) | 0.55 (0.45, 0.67) | 0.70 (0.60, 0.81) |
| Urine albumin | 2.53 (1.98, 3.23) | 2.02 (1.53, 2.67) | 1.78 (1.37, 2.31) |
EGF, epidermal growth factor; RR, risk ratio; SD, standard deviation.
Note: Tubule reabsorption score comprises urine α-1 microglobulin, β-2 microglobulin, and trefoil factor 3. Tubule injury score comprises urine interleukin-18 and kidney injury molecule-1. Factor scores and urine biomarkers are modeled individually, not jointly.
Model 1: adjusted for urine creatinine.
Model 2: adjusted for model 1 plus demographics, traditional kidney risk factors, and infection-related risk factors.
Model 3: adjusted for model 2 plus baseline eGFR. Models without urine albumin as the main predictor additionally adjusted for urine albumin.
When modeling time-updated associations, each dimension of kidney health was individually associated with risk of incident CKD after multivariable adjustment including baseline urine albumin and eGFR (Table 3). When modeling change from baseline associations, increased factor scores for tubule reabsorption (RR: 1.47 per 2-fold increase from baseline, 95% CI: 1.06, 2.04) and tubule injury (RR: 1.45, 95% CI: 1.12, 1.87), and decreasing concentrations of urine EGF (RR: 0.64, 95% CI: 0.50, 0.84) were significantly associated with higher risk of incident CKD in fully adjusted models. Change in urine albumin was not significantly associated with incident CKD in the fully adjusted model (RR: 1.37, 95% CI: 0.87, 2.34).
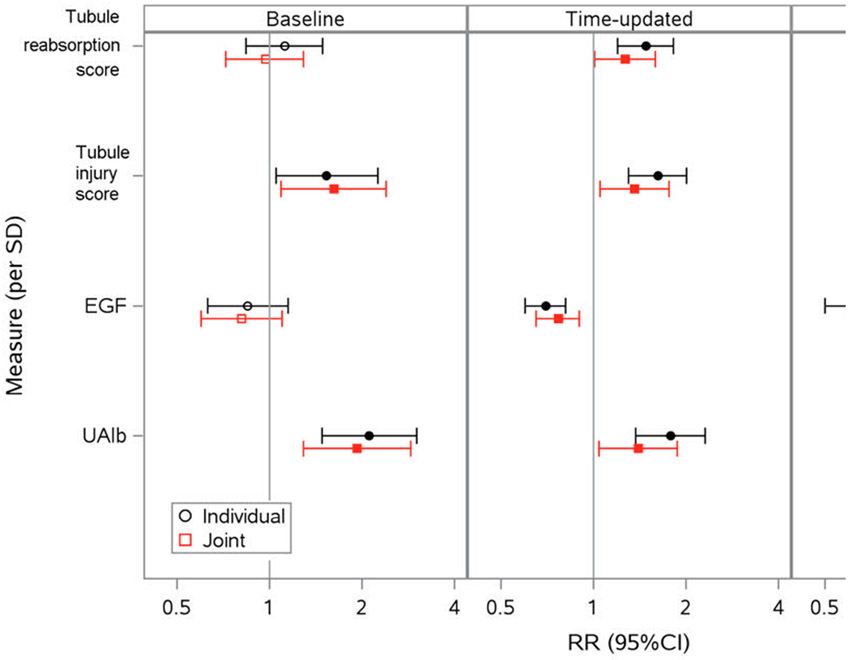
Lastly, we modeled the 4 kidney health dimensions in combination to evaluate whether they were jointly associated with incident CKD after mutual adjustment (Figure 3). In the baseline model, higher tubule injury scores and urine albumin were jointly associated with risk of CKD after adjustment for the other factor scores and biomarkers. In the time-updated model, higher tubule reabsorption scores (RR=1.27, 95% CI: 1.01, 1.59 per 1-SD higher time-updated score), higher tubule injury scores (RR=1.36, 95% CI: 1.05, 1.76), lower urine EGF (RR=0.75, 95% CI: 0.64, 0.87), and higher urine albumin (RR=1.20, 95% CI: 1.02, 1.40) were jointly associated with increased risk of incident CKD. In the change from baseline model, only increases in urine EGF reached the significance threshold.
Figure 3. Multivariable adjusted individual and joint associations of kidney health dimensions with risk of incident CKD among women living with HIV in WIHS.
Associations of baseline, time-updated, and change from baseline measures of the tubule reabsorption score, tubule injury score, urine EGF, and urine albumin with risk of incident CKD modeled individually (black) and jointly (red) with 95% confidence intervals displayed. Associations estimated from generalized estimating equation models that adjusted for demographics, CKD risk factors, urine creatinine, baseline urine albumin (when urine albumin was not the main predictor), and baseline eGFR. Filled symbols denote statistically significant associations. Abbreviations: CI, confidence interval; CKD, chronic kidney disease; EGF, epidermal growth factor; eGFR, estimated glomerular filtration rate; RR risk ratio; SD, standard deviation; UAlb, urine albumin.
Discussion
In this study of women living with HIV in the WIHS cohort, we used exploratory factor analysis to derive 2 novel factor scores from multiple urine biomarkers reflecting kidney tubule health. These factor scores were generated agnostic to their relationship with CKD risk factors and incident CKD events, but produced 2 factors that were easily recognizable based on their pathological correlates within the kidney: all biomarkers reflecting tubule reabsorption loaded onto a single factor, while those marking tubule injury distinguished a second factor. We found that several CKD risk factors were independently associated with both baseline values and changes in factor scores, urine EGF, and urine albumin. In addition, baseline and repeated factor score measures were associated with risk of incident CKD independent of CKD risk factors, urine albumin and eGFR. These findings suggest that integrating multiple kidney tubule biomarkers into summary scores can create new, informative metrics of kidney health.
To our knowledge, this is the first study in PLWH to summarize multiple biomarkers of kidney health with factor analysis. A previous study among CKD participants in the Systolic Blood Pressure Intervention Trial (SPRINT) used factor analysis to combine 10 kidney biomarkers into 4 distinct factors, and found that each factor score had independent associations with cardiovascular disease, heart failure, and death, and also improved event prediction.(19) Over the previous decade, we have demonstrated that several kidney tubule biomarkers are elevated in PLWH compared to uninfected individuals; reflect different CKD risk factor profiles; predict eGFR decline following TDF initiation; independently associate with eGFR decline and mortality risk; and have stronger associations with CKD risk when the biomarker measures are repeated over time.(12-17,21,22,26-28) However, interpreting a large number of novel kidney biomarkers would be challenging for the clinician.
To create parsimonious kidney biomarker panels for clinical practice, an important next step requires methods of prioritizing and combining biomarkers. In the present study, exploratory factor analysis selected 5 kidney tubule biomarkers and combined them into 2 factor scores that were independently associated with CKD risk. The factor scores in our study grouped similar biomarkers together despite being agnostic to CKD risk factors and incident CKD, which highlights the potential of factor analysis to uncover relevant biological mechanisms. Factor 1 was dominated by urine α1m, β2m, and TFF3, which are freely filtered low-molecular-weight proteins that reflect proximal tubule dysfunction when levels are increased in the urine.(29-32) Factor 2 was dominated by urine IL-18 and KIM-1, which are markers of proximal tubule injury.(33,34) The factor scores were modeled with urine EGF, a biomarker of kidney repair, and urine albumin, which reflects glomerular damage.(35,36) EGF and albumin had minimal correlation with either factor score, suggesting that they also represent different and distinct biological processes. We envision that our biomarker-based factor scores can serve as scaffolding for future kidney biomarker research in PLWH as they allow for a more direct assessment of the incremental value of new candidate biomarkers. However, our findings first warrant validation in additional cohorts of PLWH to determine the ideal number of dimensions that fully capture kidney health.
There were multiple significant CKD risk factor associations with baseline and repeated measures of the factor scores and urine EGF independent of urine albumin and eGFR, which underscores how current measures of kidney health do not adequately capture kidney tubule dysfunction and injury. The preponderance of HIV-related CKD risk factors associated with worsening tubule reabsorption and tubule injury scores reinforces the importance of the tubulointerstitial pathology that is often seen in HIV-associated nephropathy and antiretroviral nephrotoxicity.(37,38) In contrast, vascular and metabolic CKD risk factors primarily associated with urine EGF and albumin.
The associations of time-updated and changes in factor scores, urine EGF, and urine albumin with incident CKD highlight the potential of kidney biomarkers to detect biologic changes in the kidneys over time. As in this study, our previous work in WIHS demonstrated that repeated measures of individual urine biomarkers appear to have stronger associations than their baseline measures.(21) We hypothesize that dynamic biomarker changes may capture kidney tubule damage that progresses over time and lead to the tubulointerstitial fibrosis often seen in CKD.(11) In addition, we suspect that urine biomarker patterns within individual PLWHs may need to be evaluated periodically to remain reflective of the changing kidney injury patterns and to capture changes in CKD risk that occur following risk factor modification or switching ART regimens.
Strengths of our study include a contemporary and diverse cohort of women living with HIV; repeated urine biomarker measurements; evaluation of multiple CKD risk factors; and use of urine biomarkers that localize to different regions of the nephron. We were also able to analyze baseline and follow-up biomarker measurements on the same plates with low intra-assay coefficients of variation.
Our study also has important limitations. First, our biomarker measurements were only performed at two study visits, which limited the detail of our characterization of longitudinal changes in kidney tubule health. Second, urine albumin was not measured between biomarker collection visits, which limited our definition of incident CKD to longitudinal changes in eGFR only. Third, although we studied 8 urine biomarkers of kidney tubule health and urine albumin, they are a selection from a larger set of candidate kidney biomarkers that warrant investigation, including serum biomarkers, which may capture new dimensions of kidney health that were not identified in our study. Fourth, the biomarkers were measured before the use of more recent antiretroviral therapies, such as integrase strand transfer inhibitors and tenofovir alafenamide fumarate, and therefore may not reflect the nephrotoxicity profile of current antiretroviral regimens. Finally, our results may not be generalizable to individuals with significant albuminuria, to men living with HIV, or to kidney disease in individuals without HIV.
In summary, we combined 5 kidney tubule biomarkers into 2 novel factor scores, which were modeled together with urine EGF and urine albumin to reflect 4 dimensions of kidney health. We found that distinct sets of CKD risk factors were associated with each dimension of kidney health, and that repeated measures of all 4 kidney health dimensions were associated with risk of incident CKD. Summary scores of kidney tubule health may help capture information about CKD risk distinct from standard measures of kidney health.
Supplementary Material
Acknowledgements:
Data in this manuscript were collected by the Women’s Interagency HIV Study, now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Connie Wofsy Women’s HIV Study, Northern California CRS (Bradley Aouizerat and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Funding:
WIHS Kidney Study is funded by grant R01 AG034853-01A2 (PI, M.G.S.), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. M.G.S. and R.S. are supported by grant R01 AG034853-08. M.M.E. is supported by grant 5R01Dk103574.
Footnotes
Conflicts of interest: Dr. Shlipak is a scientific advisor and holds stock options TAI Diagnostics, and has received personal compensation from Cricket Health, Inc. All other authors declare no relevant conflicts of interest.
References
- 1.Lohse N, Hansen A-BE, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007. Jan 16;146(2):87–95. [DOI] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014. Dec 15;59(12):1787–97. [DOI] [PubMed] [Google Scholar]
- 4.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011. Dec;53(11):1120–6. [DOI] [PubMed] [Google Scholar]
- 5.Wong C, Gange SJ, Buchacz K, et al. First Occurrence of Diabetes, Chronic Kidney Disease, and Hypertension Among North American HIV-Infected Adults, 2000–2013. Clin Infect Dis. 2017. Feb 15;64(4):459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities Among US Patients With Prevalent HIV Infection-A Trend Analysis. J Infect Dis. 2017. 19;216(12):1525–33. [DOI] [PubMed] [Google Scholar]
- 7.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010. Feb 9;121(5):651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk Factors for ESRD in HIV-Infected Individuals: Traditional and HIV-Related Factors. Am J Kidney Dis. 2012. May;59(5):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odden MC, Scherzer R, Bacchetti P, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007. Nov 12;167(20):2213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driver TH, Scherzer R, Peralta CA, et al. Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. AIDS Lond Engl. 2013. Sep 10;27(14):2291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis Off J Natl Kidney Found. 1992. Jul;20(1):1–17. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Scherzer R, Abraham A, et al. Urinary Markers of Kidney Injury and Kidney Function Decline in HIV-Infected Women: JAIDS J Acquir Immune Defic Syndr. 2012. Dec;61(5):565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jotwani V, Scherzer R, Abraham A, et al. Does HIV infection promote early kidney injury in women? Antivir Ther. 2013;19(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jotwani V, Scherzer R, Abraham A, et al. Association of urine α1-microglobulin with kidney function decline and mortality in HIV-infected women. Clin J Am Soc Nephrol CJASN. 2015. Jan 7;10(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jotwani V, Scherzer R, Estrella MM, et al. HIV Infection, Tenofovir, and Urine α1-Microglobulin: A Cross-sectional Analysis in the Multicenter AIDS Cohort Study. Am J Kidney Dis Off J Natl Kidney Found. 2016. Oct;68(4):571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jotwani V, Scherzer R, Estrella MM, et al. Association of HIV infection with biomarkers of kidney injury and fibrosis in the Multicenter AIDS Cohort Study. Antivir Ther. 2017. Jan 5; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ascher SB, Scherzer R, Estrella MM, et al. Associations of Urine Biomarkers with Kidney Function Decline in HIV-Infected and Uninfected Men. Am J Nephrol. 2019. Sep 25;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Rourke N, Larry Hatcher. O’Rourke N, & Hatcher L (2013). A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling (2nd Ed.). Cary, NC: SAS Press. 2013. [Google Scholar]
- 19.Lee AK, Katz R, Jotwani V, et al. Distinct Dimensions of Kidney Health and Risk of Cardiovascular Disease, Heart Failure, and Mortality. Hypertens Dallas Tex 1979. 2019;74(4):872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005. Sep;12(9):1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ascher SB, Scherzer R, Estrella MM, et al. Associations of repeated urine biomarkers of kidney tubule health with incident CKD in women living with HIV, Submitted. [Google Scholar]
- 22.Muiru AN, Shlipak MG, Scherzer R, et al. Kidney disease risk factors associate with urine biomarkers concentrations in HIV-positive persons; a cross-sectional study. BMC Nephrol [Internet]. 2019. Jan 3 [cited 2020 Mar 16];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6318986/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed [June 24, 2020]. [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009. May 5;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Nan B, Zhu J. Multivariate sparse group lasso for the multivariate multiple linear regression with an arbitrary group structure. Biometrics. 2015. Jun;71(2):354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WR, Scherzer R, Estrella MM, et al. Tenofovir disoproxil fumarate initiation and changes in urinary biomarker concentrations among HIV-infected men and women. AIDS. 2019. Mar 15;33(4):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ascher SB, Scherzer R, Estrella MM, et al. Association of Urinary Biomarkers of Kidney Injury with Estimated GFR Decline in HIV-Infected Individuals following Tenofovir Disoproxil Fumarate Initiation. Clin J Am Soc Nephrol CJASN. 2018. Sep 7;13(9):1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muiru AN, SCHERZER R, Ascher SB, et al. Associations of CKD risk factors and longitudinal changes in urine biomarkers of kidney tubular health among women living with HIV, Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber MH, Verwiebe R. Alpha 1-microglobulin (protein HC): features of a promising indicator of proximal tubular dysfunction. Eur J Clin Chem Clin Biochem J Forum Eur Clin Chem Soc. 1992. Oct;30(10):683–91. [PubMed] [Google Scholar]
- 30.Gauthier C, Nguyen-Simonnet H, Vincent C, Revillard JP, Pellet MV. Renal tubular absorption of beta 2 microglobulin. Kidney Int. 1984. Aug;26(2):170–5. [DOI] [PubMed] [Google Scholar]
- 31.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003. Sep;4(9):721–32. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, Jin H, Holder D, et al. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol. 2010. May;28(5):470–7. [DOI] [PubMed] [Google Scholar]
- 33.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis Off J Natl Kidney Found. 2004. Mar;43(3):405–14. [DOI] [PubMed] [Google Scholar]
- 34.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002. Jul 1;62(1):237–44. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013. May;83(5):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int. 2006. Feb 1;69(3):440–9. [DOI] [PubMed] [Google Scholar]
- 37.Ross MJ, Bruggeman LA, Wilson PD, Klotman PE. Microcyst formation and HIV-1 gene expression occur in multiple nephron segments in HIV-associated nephropathy. J Am Soc Nephrol JASN. 2001. Dec;12(12):2645–51. [DOI] [PubMed] [Google Scholar]
- 38.Sise ME, Hirsch JS, Canetta PA, Herlitz L, Mohan S. Nonalbumin proteinuria predominates in biopsy-proven tenofovir nephrotoxicity. AIDS Lond Engl. 2015. May 15;29(8):941–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.