Abstract
Vascular dysfunction has been reported in adults who have recovered from COVID-19. To date, no studies have investigated the underlying mechanisms of persistent COVID-19-associated vascular dysfunction. Our purpose was to quantify nitric oxide (NO)-mediated vasodilation in healthy adults who have recovered from SARS-CoV-2 infection. We hypothesized that COVID-19-recovered adults would have impaired NO-mediated vasodilation compared with adults who have not had COVID-19. In methods, we performed a cross-sectional study including 10 (5 men/5 women, 24 ± 4 yr) healthy control (HC) adults who were unvaccinated for COVID-19, 11 (4 men/7 women, 25 ± 6 yr) healthy vaccinated (HV) adults, and 12 (5 men/7 women, 22 ± 3 yr) post-COVID-19 (PC, 19 ± 14 wk) adults. COVID-19 symptoms severity (survey) was assessed. A standardized 39°C local heating protocol was used to assess NO-dependent vasodilation via perfusion (intradermal microdialysis) of 15 mM NG-nitro-l-arginine methyl ester during the plateau of the heating response. Red blood cell flux was measured (laser-Doppler flowmetry) and cutaneous vascular conductance (CVC = flux/mmHg) was expressed as a percentage of maximum (28 mM sodium nitroprusside + 43°C). In results, the local heating plateau (HC: 61 ± 20%, HV: 60 ± 19%, PC: 67 ± 19%, P = 0.80) and NO-dependent vasodilation (HC: 77 ± 9%, HV: 71 ± 7%, PC: 70 ± 10%, P = 0.36) were not different among groups. Neither symptom severity (25 ± 12 AU) nor time since diagnosis correlated with the NO-dependent vasodilation (r = 0.46, P = 0.13; r = 0.41, P = 0.19, respectively). In conclusion, healthy adults who have had mild-to-moderate COVID-19 do not have altered NO-mediated cutaneous microvascular function.
NEW & NOTEWORTHY Healthy young adults who have had mild-to-moderate COVID-19 do not display alterations in nitric oxide-mediated cutaneous microvascular function. In addition, healthy young adults who have COVID-19 antibodies from the COVID-19 vaccinations do not display alterations in nitric oxide-mediated cutaneous microvascular function.
Keywords: COVID-19, intradermal microdialysis, microvascular function, skin blood flow
INTRODUCTION
There have been over 45 million cases of COVID-19 in the United States and 244 million worldwide (1). The clinical spectrum of COVID-19 disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is wide (2, 3). Although ∼97% of infected individuals recover from the initial infection, the short- and long-term implications of SARS-CoV-2 infection are unknown (1). Cardiovascular complications have emerged as a key determinant of poor prognosis for full recovery from COVID-19 (4). Reports indicate that 40%–80% of American and European COVID-19 recovered patients display cardiovascular complications including arrhythmia, myocarditis, myocardial infarction, and venous thromboembolisms (5–8). These cardiovascular complications have occurred in a wide range of demographics and have persisted as far as 12 mo postdiagnosis. Discovering the underlying mechanisms linking SARS-CoV-2 infection and cardiovascular consequences are important for identifying additional treatment strategies in SARS-CoV-2-infected adults.
SARS-CoV-2 infects the host through the angiotensin-converting enzyme 2 receptor (ACE-2) which is ubiquitously expressed throughout several tissues, including vascular endothelial cells (9, 10). Early preclinical data and case reports demonstrate endothelial cell involvement across different organ systems and vascular beds in patients with COVID-19 and profound systemic endothelialitis (11, 12). Consequently, microvascular endothelial dysfunction has emerged as a potential unifying mechanism underlying the sequelae of persistent cardiovascular complications arising from SARS-CoV-2 infection.
Although preclinical data suggest endothelial dysfunction as a primary mechanism underlying COVID-19 and cardiovascular complications, only two cross-sectional reports in young adults have investigated persistent vascular dysfunction following COVID-19 (13, 14). Both studies used brachial artery flow-mediated dilation (FMD) and reactive hyperemia as a primary measure of peripheral vascular function. Collectively, these studies found that young adults have blunted FMD responses postacute mild-to-moderate COVID-19, and those reduced responses continue to persist in individuals with lingering COVID-19 symptoms. However, reactive hyperemia results were equivocal. Ratchford et al. also found blunted femoral artery blood flow responses to single passive leg movement (sPLM) postacute COVID-19 in young adults. Because full expression of FMD and sPLM responses are nitric oxide (NO)-dependent (15–17), impairments in those responses suggest reduced NO signaling in the macrovasculature and microvasculature, respectively. However, no study has directly assessed endothelial NO-dependent vasodilation with appropriate pharmacological blockade.
Therefore, the purpose of this study was to quantify NO-dependent cutaneous microvascular endothelial function in 1) otherwise healthy young adults who have been infected with SARS-CoV-2, 2) those who had SARS-CoV-2 antibodies induced through vaccination, and 3) those had neither been infected with SARS-CoV-2 or yet received the vaccine. Using the skin as a model circulation (18), we hypothesized that 1) young adults who have been infected with SARS-CoV-2 will have impaired microvascular endothelial function compared with appropriately matched adults who have not had COVID-19 and 2) those who have been vaccinated for COVID-19 but have not had COVID-19 will not have impaired microvascular endothelial function.
METHODS
The Institutional Review Board at The Pennsylvania State University approved all experimental procedures and protocols. Verbal and written informed consent were voluntarily obtained from all participants before participation and in accordance with the guidelines set forth by the Declaration of Helsinki.
Participants
All participants underwent a complete medical screening, including a blood chemistry analysis (Quest Diagnostics, Pittsburgh, PA). Participants were nonobese, did not use tobacco products, and were not taking any prescription medications with primary or secondary cardiovascular effects (e.g., antihypertensives, statins, anticoagulants, etc.). Ten women were on birth control (oral contraceptives HC: n = 2, PC: n = 2, HV: n = 2; intrauterine devices HC: n = 0, PC: n = 2, HV: n = 2). All women were premenopausal; thus, a urine pregnancy test confirmed the absence of pregnancy before experimental visits. The phase of menstrual cycle was not controlled among women participants (19).
Participants were classified into one of three groups: 1) healthy control (never diagnosed with COVID-19, no self-reported COVID-19 symptoms since January 2020, not vaccinated for COVID-19 and negative COVID-19 antibody test), 2) post-COVID-19 (tested positive for SARS-CoV-2, positive COVID-19 antibody test), and 3) healthy vaccinated control (never diagnosed with COVID-19, no reported COVID-19 symptoms since January 2020, fully vaccinated for COVID-19, and positive COVID-19 antibody test).
COVID-19 Antibody Quantification
The protocol for the quantitative estimation of antibodies against SARS-CoV-2 has been previously described in detail (20–22) and is deposited in protocols.io https://doi.org/10.17504/protocols.io.bivgke3w. Briefly, SARS-CoV-2 antibodies in serum samples were detected and quantified against purified recombinant SARS-CoV-2 spike receptor-binding domain (S/RBD) proteins using in-house indirect Fab antibody-based or isotype-specific ELISA assays.
Symptom Survey
During the screening visit, vaccinated and post-COVID-19 participants completed a COVID-19 symptoms severity survey, which lists the top 18 most common symptoms to COVID-19 (14, 23). Participants rated each symptom on a scale of 0–100 of increasing severity. The values for each symptom were totaled and averaged. Post-COVID-19 participants were asked to recall the severity of their symptoms during peak COVID-19 illness and for current symptoms at time of testing. Healthy vaccinated adults were asked to recall peak symptoms post-complete vaccination.
Physical Activity Assessment
To characterize participant physical activity among the groups, physical activity was assessed using accelerometry (ActiGraph GT9X, LLC, Pensacola, FL). Participants wore the accelerometer around their waist aligned with the midline of their dominant thigh (24). Participants were instructed to wear the accelerometer during waking hours for 7 days before the study and to remove them nightly at bedtime. Participants also removed the accelerometer if they were in water (e.g., bathing, swimming) or if they were engaged in activity that could result in damage of the device (e.g., full contact sports). A minimum of 4 days wearing the accelerometer for ≥10 h/day were required for inclusion in these analyses (24). A total of 24 out of 33 participants (HC n = 9, PC n = 8, and VAC n = 7) had sufficient data for analysis. Awake activity was then classified into sedentary, light, and moderate-to-very vigorous activity and calculated as the percentage of awake time spent in each of these categories (ActiLife, ActiGraph). The participants were blinded (i.e., the screen of the device was turned off) to the ongoing recording of data (e.g., steps taken, active minutes, etc.) to eliminate behavioral reactivity.
Cutaneous Microvascular Function
Before each experimental session, participants were instructed to abstain from caffeine, alcohol, and strenuous physical activity for at least 12 h before arrival at the laboratory. An intradermal microdialysis fiber (CMA Linear 31 probe, 55 kDa, Harvard Apparatus, Holliston, MA) was inserted into the ventral forearm skin for the local delivery of pharmacological agents, as previously described (25–27). Cutaneous red blood cell flux was continuously measured directly over the microdialysis site with an integrated laser Doppler flowmetry probe placed in a local heating unit (VP12 and VHP2; Moor Instruments, Wilmington, DE).
Pharmacological agents were mixed just before use, dissolved in lactated Ringer’s solution, sterilized using syringe microfilters (Acrodisc; Pall, Port Washington, NY), and wrapped in foil to prevent degradation due to light exposure (FDA IND No. 120058). All solutions were perfused through microdialysis fibers at a rate of 2 µL/min (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems, West Lafayette, IN).
After placement of microdialysis fiber, 60–90 min were allowed for hyperemia associated with fiber placement to resolve. Baseline data were then collected (∼10 min) before beginning a standardized local heating (39°C) protocol, as described previously (28). This local heating protocol elicits an initial axon reflex-mediated peak skin blood flow response, followed by a brief nadir, after which there is a gradual rise and eventual blood flow plateau (after ∼40 min). After observing a stable local heating plateau, 15 mM NG-nitro-l-arginine methyl ester (l-NAME; NO synthase inhibitor) was perfused, allowing for quantification of NO-dependent vasodilation (%NO) (25–28). After observing a stable l-NAME plateau, 28 mM sodium nitroprusside (SNP; USP, Rockland, MD) was perfused and local temperature was increased to 43°C to elicit maximal vasodilation (29, 30). Automated brachial BP (Cardiocap; GE Healthcare, Milwaukee, WI; Connex Spot Monitor, Welch Allyn, Skaneateles Falls, NY) was measured at each stage (e.g., baseline, initial axon reflex, local heating plateau, l-NAME plateau, and maximal vasodilation) throughout the protocol.
Data and Statistical Analysis
Data were recorded at 40 Hz and stored for offline analysis (Powerlab/LabChart, ADInstruments, Bella Vista, NSW, Australia; WINDAQ, DATAQ Instruments, Akron, OH). Average values for red cell flux (perfusion units) were obtained during baseline and at each phase of the local heating protocol. Cutaneous vascular conductance (CVC) was calculated as red cell flux divided by mean arterial pressure. Because of the heterogeneity of capillary density at each microdialysis site, CVC was normalized as a percentage of the site-specific maximum (CVC%max) (31–35).
Based on previously published data (27, 36), conservatively assuming an effect size of d = 0.6, an a priori power analysis (power = 0.80, α = 0.05) confirmed a sample size of n = 10/group was needed to detect a between-group difference in the NO contribution to local heating response. Participant characteristics were analyzed using a one-way ANOVA (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, v. 26.0. Armonk, NY; GraphPad Prism v. 9.0.0 for Windows, GraphPad Software, San Diego, CA). Responses to local heating were analyzed using a two-way mixed-measures ANOVA to evaluate group (healthy control, post-COVID-19, and healthy vaccinated) and phase (baseline, plateau, l-NAME, and maximum) effects. The NO contributions were analyzed using a one-way ANOVA. Linear regression analyses were performed using a Pearson’s correlation coefficient. Significance was set a priori at α < 0.05. All results are presented as means ± SD, and range is displayed as minimum to maximum.
RESULTS
Participants
A total of 33 young adults participated in the study (healthy controls n = 10, post-COVID-19 n = 12, vaccinated n = 11). Participant characteristics did not differ among groups (all P > 0.05, Table 1). Time spent in sedentary and light physical activity were not different among groups, regardless of whether it was expressed as minutes per week or percent of awake time (both P > 0.05, Table 1). Post-COVID-19 adults had lower amounts of moderate-to-very vigorous activity compared with the healthy control group when expressed as minutes per week (P = 0.04, Table 1), but not when expressed as percent of awake time (P = 0.11). All healthy control participants had a confirmed negative COVID-19 antibody test, whereas all healthy vaccinated and post-COVID-19 participants had positive COVID-19 antibody tests. A breakdown of vaccine status within groups is presented in Fig. 1. One post-COVID-19 participant was fully vaccinated (Johnson & Johnson’s Janssen) before testing positive for COVID-19; two post-COVID-19 participants had received the first dose of the Pfizer-BioNTech before testing positive for COVID-19, but were fully vaccinated before the experimental visit; and five post-COVID-19 were fully vaccinated after testing positive for COVID-19, but before the experimental visit. Removing the post-COVID-19 who were either partially or fully vaccinated when getting COVID-19 did not alter the results (all P > 0.05).
Table 1.
Participant characteristics
| Healthy Control | Post-COVID-19 | Healthy Vaccinated | |
|---|---|---|---|
| Participants, n (men/women) | 10 (5/5) | 12 (5/7) | 11 (4/7) |
| Age, yr | 24 ± 4 [19–31] | 22 ± 3 [19–27] | 25 ± 6 [19–35] |
| BMI, kg/m2 | 25 ± 3 [20–29] | 24 ± 3 [16–28] | 24 ± 4 [20–32] |
| SBP, mmHg | 114 ± 8 [100–128] | 112 ± 13 [95–132] | 108 ± 11 [89–122] |
| DBP, mmHg | 68 ± 8 [50–78] | 74 ± 7 [64–82] | 66 ± 6 [54–74] |
| HR, beats/min | 62 ± 7 [47–75] | 69 ± 12 [52–91] | 71 ± 9 [55–84] |
| HDL, mg/dL | 59 ± 10 [46–79] | 60 ± 7 [46–80] | 51 ± 11 [39–69] |
| LDL, mg/dL | 91 ± 16 [65–119] | 87 ± 27 [26–109] | 87 ± 16 [47–104] |
| Total CHO, mg/dL | 169 ± 18 [136–183] | 163 ± 29 [104–195] | 159 ± 13 [135–176] |
| HbA1c, % | 4.9 ± 0.3 [4.4–5.4] | 4.7 ± 0.3 [4.3–5.2] | 4.8 ± 0.2 [4.4–5.1] |
| Race/ethnicity, n | |||
| Asian/Indian | 1 | 1 | 2 |
| NH Black | 1 | 0 | 2 |
| NH White | 7 | 9 | 6 |
| Latino | 0 | 2 | 1 |
| Mixed (2+ listed above) | 1 | 0 | 0 |
| Physical activity, %awake time | |||
| Sedentary | 64 ± 5 [57–71] | 70 ± 10 [53–83] | 67 ± 3 [64–73] |
| Light | 28 ± 5 [22–35] | 25 ± 10 [16–44] | 27 7 ± [16–44] |
| Moderate-to-very vigorous | 8 ± 4 [2–15] | 4 ± 3 [1–8] | 6 ± 3 [1–9] |
| Days since… | Diagnosis | Vaccine | |
| 131 ± 97 [12–357] | 81 ± 36 [43–139] |
Values are means ± SD and ranges [minimum–maximum]. BMI, body mass index; CHO, cholesterol; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; NH, non-Hispanic; SBP, systolic blood pressure.
Figure 1.
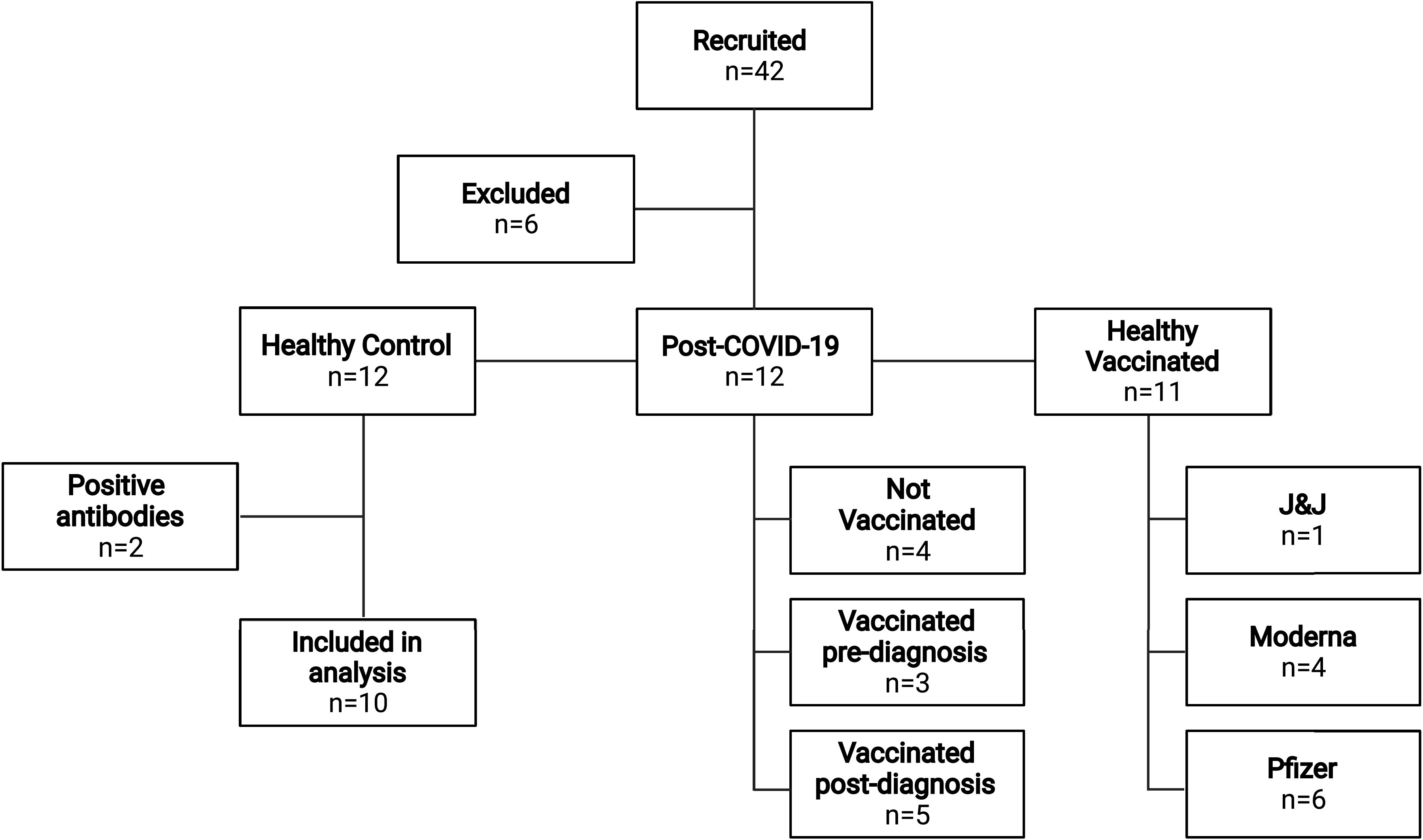
Flow chart representation of participant recruitment and group classification. Out of the 42 recruited individuals, 6 were excluded for not meeting inclusion/exclusion criteria. Within the n = 12 healthy controls, two participants tested positive for COVID-19 antibodies and were excluded from data analysis. Four post-COVID-19 participants were not vaccinated. Three post-COVID-19 participants were either fully (n = 1) or partially (n = 2) vaccinated before testing positive for COVID-19, but were fully vaccinated before the experimental visit. Five post-COVID-19 were fully vaccinated after testing positive for COVID-19, but before the experimental visit. Within the healthy vaccinated group: six received Pfizer-BioNTech (Pfizer), four received Moderna, and one received Johnson & Johnson’s Janssen (J&J). This image was created using BioRender and published with permission.
Blood Flow Responses
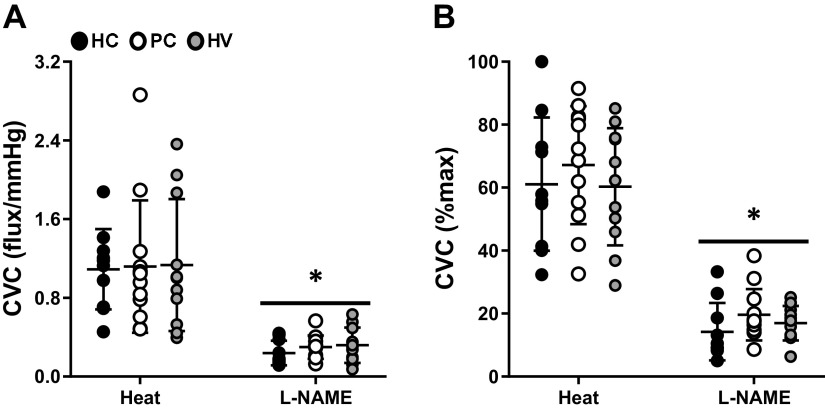
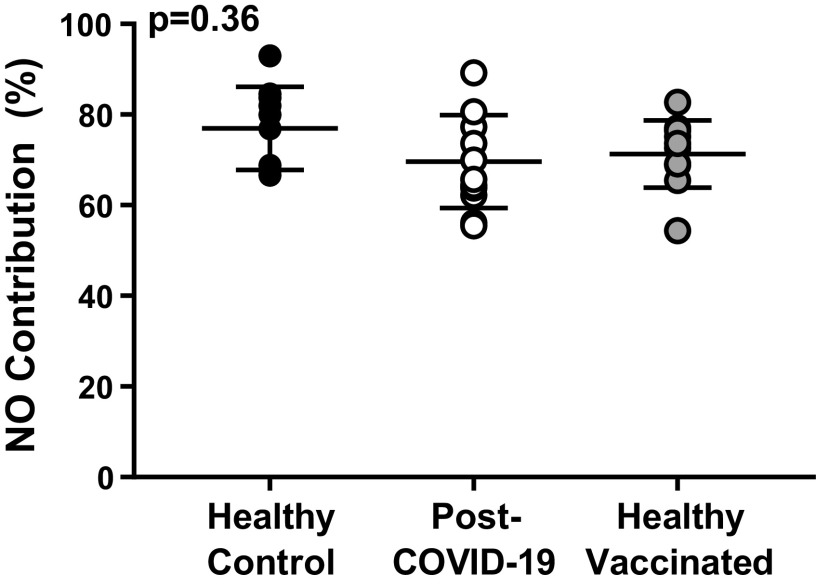
Baseline, initial axon reflex, and maximum values CVC, when expressed as absolute (flux/mmHg) or relative (CVC%max), were not different among groups (Table 2). The remaining cutaneous microvascular responses to the local heating protocol are presented in Fig. 2. There were no differences among groups in the local heating plateau or the post-l-NAME plateau, regardless of whether those responses were presented as absolute CVC (Fig. 2A) or normalized to a percentage of maximum CVC (Fig. 2B). Similarly, the NO-contribution to the local heating response did not differ among groups (Fig. 3).
Table 2.
Cutaneous blood flow responses to local heating
| Healthy Control | Post-COVID-19 | Healthy Vaccinated | P Values | |
|---|---|---|---|---|
| Absolute CVC, flux/mmHg | ||||
| Baseline | 0.3 ± 0.2 [0.1–0.5] | 0.4 ± 0.3 [0.1–0.8] | 0.3 ± 0.2 [0.1–0.7] | Phase, P < 0.01Group, P = 0.99Phase × group, P = 0.82 |
| Axon reflex | 1.0 ± 0.6 [0.4–2.0] | 0.9 ± 0.4 [0.3–1.4] | 0.8 ± 0.4 [0.2–1.4] | |
| Maximal vasodilation | 1.8 ± 0.57 [1.3–2.9] | 1.7 ± 0.7 [0.7–3.6] | 1.9 ± 0.9 [0.9–3.7] | |
| Relative CVC, %maximal | ||||
| Baseline | 14.5 ± 9.7 [4.6–36.8] | 21.3 ± 9.4 [9.8–40.3] | 16.6 ± 13.5 [4.3–45.1] | Phase, P < 0.01Group, P = 0.41Phase × group, P = 0.79 |
| Axon reflex | 54.4 ± 22.6 [28.6–97.1] | 58.2 ± 15.0 [23.3–69.9] | 47.6 ± 21.8 [23.3–87.5] |
Values are means ± SD and ranges [minimum–maximum]. CVC, cutaneous vascular conductance.
Figure 2.
Cutaneous vascular conductance (CVC) responses during the local heating plateau (Heat) and NG-nitro-l-arginine methyl ester plateau (l-NAME) in healthy controls (HC, closed circles, n = 10), post-COVID-19 adults (PC, open circles, n = 12), and healthy vaccinated adults (HV, gray circles, n = 11). There are no differences among groups when presented as absolute CVC (A) or a percentage of maximum CVC (B). Two-way mixed ANOVA results are presented in Table 1. *P < 0.01 vs. local heating plateau.
Figure 3.
The nitric oxide (NO) contribution to the local heating response in healthy controls (closed circles, n = 10), post-COVID-19 adults (open circles, n = 12), and healthy vaccinated adults (gray circles, n = 11) did not differ among groups (one-way ANOVA).
Timeline
The time from COVID-19 diagnosis (Table 1) was not related to the magnitude of the local heating plateau (r = 0.34, P = 0.27) or the NO-contribution (Fig. 4A). In the healthy vaccinated group, the time from COVID-19 vaccine (Table 1) was not related to the magnitude of the local heating plateau (r < 0.01, P = 0.99) or the NO-contribution (r = 0.02, P = 0.95).
Figure 4.
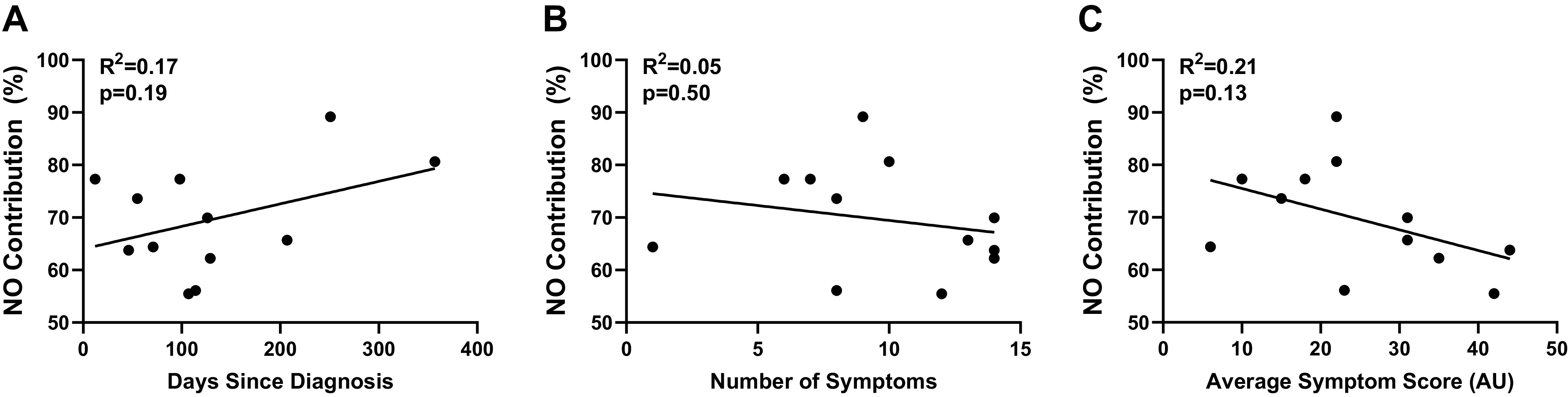
The nitric oxide (NO) contribution to the local heating response in post-COVID-19 adults (n = 11) was not correlated with time since diagnosis (A), number of symptoms during illness (B), or average symptom score during illness (C; linear regression via Pearson’s correlation coefficient).
Symptomology
All post-COVID-19 and healthy vaccinated adults were symptom-free at the time of testing. Individual data for peak symptom-severity recall of post-COVID-19 adults are presented in Supplemental Table S1; all Supplemental Tables are available at https://doi.org/10.6084/m9.figshare.17207282.v2. The number of COVID-19 symptoms (10 ± 4 symptoms, 1–14) and the average symptom severity (25 ± 12 AU, 6–44) were not related to the magnitude of the local heating plateau (number of symptoms: r = 0.41, P = 0.18; average symptom severity: r = 0.28, P = 0.37) or the NO-contribution (Fig. 4, B and C). In the healthy vaccinated group, the number of vaccine-related symptoms (4 ± 4 symptoms, 0–10) and the average symptom severity (4 ± 6 AU, 0–17) were not related to the magnitude of the local heating plateau (number of symptoms: r = 0.37, P = 0.25; average symptom severity: r = 0.54, P = 0.10) or the NO-contribution (number of symptoms: r = 0.10, P = 0.79; average symptom severity: r = 0.17, P = 0.61).
DISCUSSION
The major findings of this study include: 1) the NO contribution to local heating was not different among groups who have had COVID-19, the COVID-19 vaccine, and those who have not and 2) the time from diagnosis and symptom severity were not correlated with microvascular function in otherwise healthy, young adults’ post-COVID-19 illness or post-COVID-19 vaccination.
Blood Flow Responses
In this study, we demonstrate that there were no differences in NO-mediated cutaneous microvascular function in young, otherwise healthy adults who have had COVID-19 compared with vaccinated or unvaccinated control subjects (confirmed with antibody testing). Previous studies have investigated the impact of COVID-19 on microvascular function via brachial artery reactive hyperemia to cuff inflation. Early in the pandemic (December 2020), Ratchford et al. (14) reported that young adults acute postinfection did not display alterations in reactive hyperemia responses compared with a historic cohort of healthy-matched controls. Nandadeva et al. (38) 45subsequently reported blunted reactive hyperemia responses only in adults who had persistent symptoms following COVID-19. The underlying mechanisms of blood flow responses to cuff inflation include the release of NO, adenosine, endothelium-derived hyperpolarizing factors, and adenosine triphosphate-sensitive potassium channels (37, 38). Ratchford et al. (15) also reported decrements in sPLM responses, a stimulus that induces a predominantly NO-dependent response, in acute post-COVID-19 adults. However, neither of these studies used appropriate pharmacological blockade/intervention approaches to assess NO- versus non-NO-, or endothelium-independent vasodilation. By using intradermal microdialysis in this study, we were able to pharmacodissect the NO-mediated response to an eNOS-specific stimulus (18, 30, 39).
In this study young otherwise healthy adults who had mild-to-moderate COVID-19 did not exhibit impaired cutaneous microvascular function. Despite experiencing a cardiovascular stressor, healthy young adults oftentimes do not display impairments in vascular function because they have multiple, redundant mechanisms to maintain vascular homeostasis (40). This vascular resilience decreases with increasing cardiovascular disease factors such as sedentary lifestyle, obesity, hypertension, and aging (35, 41–44). Furthermore, adults with preexisting cardiovascular disease risk factors were at a greater risk for experiencing severe COVID-19 as well as post-COVID-19 (45–47). The primary goal of this study was to investigate the impact of COVID-19 on microvascular function in young, otherwise healthy adults. Thus, all participants were nonobese, normotensive, and young. Unlike the two previous studies (13, 14), the current study only assessed microvascular function and therefore we cannot speculate whether macrovascular function would be altered in this cohort. Future investigations are needed to further elucidate the impacts of COVID-19 on micro and macrovascular function in older demographics, as well as those who have preexisting cardiovascular disease risk factors.
Timeline
Although Ratchford et al. (14) characterized vascular function in post-COVID-19 adults 3–4 wk post-infection, Nandadeva et al. (13) did so in adults on average 13 wk post infection (range: 4–21 wk). In this study, participants were tested in a range of 12 days to 357 days post-diagnosis. These participants were, on average, farther out from diagnosis than those in the Ratchford study; thus, the equivocal findings may be explained based on the differences in time between diagnosis and experimental testing across studies. However, our regression analysis indicated there was no relation between time since diagnosis and eNOS-dependent vasodilation in the cutaneous microcirculation.
Symptomology
Although there was a wide range of symptomology, all post-COVID-19 participants were considered as having mild-to-moderate symptoms (48). To characterize symptomology, we used the same survey as Ratchford et al. (14) to ensure adequate comparison across studies. We recognize the average symptom severity scores appear mild, but this may be a limitation in the analysis of the symptom severity survey. The survey includes 18 unique symptoms. Therefore, in order for a participant to indicate experiencing the greatest severity survey score, a severity of “100/100” would be required for all 18 symptoms. Half of the post-COVID-19 adults had 10 or more symptoms (Supplemental Table S1) indicating a rather intense illness; however, because of the diverse list of symptoms, their average scores remained 22–44 out of 100. A potential limitation of both studies is that these data were self-reported, and thus subject to recall bias. Of note, our average symptoms severity scores were greater than that of Ratchford et al. (15 ± 12 AU). However, none of our participants were hospitalized for COVID-19-related illness. The current investigation is limited to this specific demographic who have experienced mild-to-moderate COVID-19 symptoms. Whether decrements in cutaneous microvascular function occur in young adults who have experienced severe COVID-19 is unknown. A participant cohort that includes those who experienced severe COVID-19 symptomology (e.g., hospitalization, respiratory failure) may be needed to observe a significant relation between symptomology and microvascular function following COVID-19. In addition, none of the post-COVID-19 participants had long-haul symptoms at the time of testing. The longevity of symptoms may be an important moderator in understanding the underlying mechanisms of COVID-19 long-haul vascular complications. In this context, Nandadeva, et al. (13) subsequently reported blunted FMD and reactive hyperemia only in adults who had persistent symptoms following COVID-19.
Vaccination Status
With the introduction of the COVID-19 vaccines, there was hesitancy among the general population in receiving it due to initial lack of full FDA-approval and potential unknown side effects (49, 50). Although the implications of the COVID-19 vaccines are still being elucidated, in this study we demonstrate that there is no evidence of vascular dysfunction in young adults who have received the vaccine ∼11.5 wk before testing. Therefore, having the COVID-19 antibodies independent of the viral infection does not impact microvascular dysfunction. As this was a secondary study objective, we are not appropriately powered to determine differences among the different types of COVID-19 vaccines.
SARS-CoV-2 vaccinations are effective in preventing severe COVID-19 symptoms and COVID-19-related hospitalization (51, 52). However, the impact on SARS-CoV-2 vaccination on preexisting long-haul COVID-19 symptoms is unknown. Anecdotal evidence is limited to self-report recall surveys. One case series consisting of 28 adults with persistent symptoms 8 mo following COVID-19-related hospitalization found ∼23% of symptoms improved following vaccination, ∼6% worsened, and an overwhelming majority of ∼71% were not different (53). In contrast, Wanga et al. (54) reported that those with long-haul symptoms (n = 100) at time of vaccination, ∼40% reported symptoms getting better, ∼37% got worse, and ∼23% did not change. To our knowledge, prospective trials investigating this have not been conducted and mechanistic investigations suggesting physiological evidence of this potential phenomenon are limited (55). In this study, 3 out of the 12 post-COVID-19 participants were either partially or fully vaccinated at the time of being infected with SARS-CoV-2; 5 out of the 12 post-COVID-19 participants became fully vaccinated after SARS-CoV-2 infection, but before the experimental visit. Therefore, the symptomology of the post-COVID-19 group may be dampened because of the heterogeneity of vaccination status within the group. Whether receiving the COVID-19 vaccine before or after SARS-CoV-2 infection improves COVID-19 associated microvascular dysfunction is unknown. Removing the post-COVID-19 individuals who were either partially or fully vaccinated when getting COVID-19 did not change the conclusions of study; however, we recognize that a limitation of this study is that we are underpowered to cross-sectionally observe the impact of COVID-19 vaccination before or after SARS-CoV-2 infection on COVID-19 symptomology and microvascular function.
Conclusions
In conclusion, young, otherwise healthy adults who have had a mild-to-moderate SARS-CoV-2 infection do not display impaired NO-mediated microvascular function. Adults who have COVID-19 antibodies from the vaccine also do not show alterations in cutaneous microvascular function. Despite the range in symptomology, none of the post-COVID-19 participants had to be hospitalized for severe COVID-19 or had long-haul COVID-19 impacts. As such, investigations in young adults, as well as other demographics, who have had more severe COVID-19 cases are warranted.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2: https://doi.org/10.6084/m9.figshare.17207282.v2.
GRANTS
This work was funded by National Institutes of Health Grant T-32-5T32AG049676 (to G.A.D.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.A.D. and L.M.A. conceived and designed research; G.A.D. and S.T.W. performed experiments; G.A.D. and S.T.W. analyzed data; G.A.D., S.T.W., and L.M.A. interpreted results of experiments; G.A.D. prepared figures; G.A.D. drafted manuscript; G.A.D., S.T.W., and L.M.A. edited and revised manuscript; G.A.D., S.T.W., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sue Slimak, Faith Swanger, and Zach Lichter for technical support, as well as the Kapur Laboratory for antibody testing assistance and Dr. David Conroy for accelerometer testing assistance.
REFERENCES
- 1.Centers for Disease Control and Prevention. COVID Data Tracker, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home [2022 Jan 11].
- 2.Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF. COVID-19: discovery, diagnostics and drug development. J Hepatol 74: 168–184, 2021. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azevedo RB, Botelho BG, Hollanda JVG, Ferreira LVL, Junqueira de Andrade LZ, Oei SSML, Mello TS, Muxfeldt ES. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens 35: 4–11, 2021. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397: 220–232, 2021. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 17: 543–558, 2020. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 5: 1265–1273, 2020. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 6: 116–118, 2021. [Erratum in JAMA Cardiol 6: 123, 2021]. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 116: 1097–1100, 2020. [Erratum in Cardiovasc Res 116: 1994, 2020]. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosse JH, Haraldsen G, Falk K, Edelmann R. Endothelial cells in emerging viral infections. Front Cardiovasc Med 8: 619690, 2021. doi: 10.3389/fcvm.2021.619690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandadeva D, Young BE, Stephens BY, Grotle A-K, Skow RJ, Middleton AJ, Haseltine FP, Fadel PJ. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am J Physiol Heart Circ Physiol 321: H479–H484, 2021. doi: 10.1152/ajpheart.00368.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, Bobo LK, Stickford ASL. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 320: H404–H410, 2021. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD, Richardson RS. Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol (1985) 123: 1468–1476, 2017. doi: 10.1152/japplphysiol.00533.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. doi: 10.1161/01.CIR.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 17.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 19.Stanhewicz AE, Wong BJ. Last word on point: counterpoint: investigators should not control for menstrual cycle phase when performing studies of vascular control that include women. J Appl Physiol (1985) 129: 1117–1119, 2020. doi: 10.1152/japplphysiol.00860.2020. [DOI] [PubMed] [Google Scholar]
- 20.Gontu A, Srinivasan S, Salazar E, Nair MS, Nissly RH, Greenawalt D, Bird IM, Herzog CM, Ferrari MJ, Poojary I, Katani R, Lindner SE, Minns AM, Rossi R, Christensen PA, Castillo B, Chen J, Eagar TN, Yi X, Zhao P, Leveque C, Olsen RJ, Bernard DW, Gollihar J, Kuchipudi SV, Musser JM, Kapur V. Limited window for donation of convalescent plasma with high live-virus neutralizing antibody titers for COVID-19 immunotherapy. Commun Biol 4: 267, 2021. doi: 10.1038/s42003-021-01813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuno T, Kondelis N. Evaluation of dithiothreitol (DTT) for inactivation of IgM antibodies. J Clin Pathol 31: 1152–1155, 1978. doi: 10.1136/jcp.31.12.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadlbauer D, Amanat F, Chromikova V, Jiang K, Strohmeier S, Arunkumar GA, Tan J, Bhavsar D, Capuano C, Kirkpatrick E, Meade P, Brito RN, Teo C, McMahon M, Simon V, Krammer F. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol 57: e100, 2020. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stute NL, Stickford JL, Province VM, Augenreich MA, Ratchford SM, Stickford ASL. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol 599: 4269–4285, 2021. doi: 10.1113/JP281888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc 30: 777–781, 1998. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clin Sci (Lond) 131: 2777–2789, 2017. doi: 10.1042/CS20171292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension 70: 382–389, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf ST, Jablonski NG, Ferguson SB, Alexander LM, Kenney WL. Four weeks of vitamin D supplementation improves nitric oxide-mediated microvascular function in college-aged African Americans. Am J Physiol Heart Circ Physiol 319: H906–H914, 2020. doi: 10.1152/ajpheart.00631.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol (1985) 117: 277–283, 2014. doi: 10.1152/japplphysiol.01397.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 30.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 31.Craighead DH, Wang H, Santhanam L, Alexander LM. Acute lysyl oxidase inhibition alters microvascular function in normotensive but not hypertensive men and women. Am J Physiol Heart Circ Physiol 314: H424–H433, 2018. doi: 10.1152/ajpheart.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, Alexander LM. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension 69: 902–909, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts KA, van Gent T, Hopkins ND, Jones H, Dawson EA, Draijer R, Carter HH, Atkinson CL, Green DJ, Thijssen DHJ, Low DA. Reproducibility of four frequently used local heating protocols to assess cutaneous microvascular function. Microvasc Res 112: 65–71, 2017. doi: 10.1016/j.mvr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Roustit M, Blaise S, Millet C, Cracowski JL. Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc Res 79: 102–108, 2010. doi: 10.1016/j.mvr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurr C, Patik JC, Kim K, Christmas KM, Brothers RM. Tempol augments the blunted cutaneous microvascular thermal reactivity in healthy young African Americans. Exp Physiol 103: 343–349, 2018. doi: 10.1113/EP086776. [DOI] [PubMed] [Google Scholar]
- 37.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res 113: 1023–1032, 2013. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ, (With the Technical Assistance of Darrell Loeffler and Tammy Eickhoff). Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol (1985) 81: 1807–1814, 1996. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- 39.Kenney WL, Edward F. Adolph distinguished lecture: skin-deep insights into vascular aging. J Appl Physiol (1985) 123: 1024–1038, 2017. doi: 10.1152/japplphysiol.00589.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Y, Galis ZS. Exploring the role of endothelial cell resilience in cardiovascular health and disease. Arterioscler Thromb Vasc Biol 41: 179–185, 2021. doi: 10.1161/ATVBAHA.120.314346. [DOI] [PubMed] [Google Scholar]
- 41.de Jongh RT, Serné EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation 109: 2529–2535, 2004. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 42.Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol 589: 4787–4797, 2011. doi: 10.1113/jphysiol.2011.212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurley DM, Williams ER, Cross JM, Riedinger BR, Meyer RA, Abela GS, Slade JM. Aerobic exercise improves microvascular function in older adults. Med Sci Sports Exerc 51: 773–781, 2019. doi: 10.1249/MSS.0000000000001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol (1985) 93: 1644–1649, 2002. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 45.Harrison SL, Buckley BJR, Rivera-Caravaca JM, Zhang J, Lip GYH. Cardiovascular risk factors, cardiovascular disease, and COVID-19: an umbrella review of systematic reviews. Eur Heart J Qual Care Clin Outcomes 7: 330–339, 2021. doi: 10.1093/ehjqcco/qcab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahman A, Sathi NJ. Risk factors of the severity of COVID-19: a meta-analysis. Int J Clin Pract 75: e13916, 2021. doi: 10.1111/ijcp.13916. [DOI] [PubMed] [Google Scholar]
- 47.Treskova-Schwarzbach M, Haas L, Reda S, Pilic A, Borodova A, Karimi K, Koch J, Nygren T, Scholz S, Schönfeld V, Vygen-Bonnet S, Wichmann O, Harder T. Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence. BMC Med 19: 212, 2021. doi: 10.1186/s12916-021-02058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.COVID-19 Treatment Guidelines Panel. Coronavirus Disease. 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ [2021 Dec 13]. [PubMed] [Google Scholar]
- 49.King WC, Rubinstein M, Reinhart A, Mejia R. COVID-19 vaccine hesitancy January-May 2021 among 18-64 year old US adults by employment and occupation. Prev Med Rep 24: 101569, 2021. doi: 10.1016/j.pmedr.2021.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King WC, Rubinstein M, Reinhart A, Mejia RJ. Time trends and factors related to COVID-19 vaccine hesitancy from January-May 2021 among US adults: findings from a large-scale national survey. medRxiv, 2021. doi: 10.1101/2021.07.20.21260795. [DOI]
- 51.Chung H, He S, Nasreen S, Sundaram ME, Buchan SA, Wilson SE, Chen B, Calzavara A, Fell DB, Austin PC, Wilson K, Schwartz KL, Brown KA, Gubbay JB, Basta NE, Mahmud SM, Righolt CH, Svenson LW, MacDonald SE, Janjua NZ, Tadrous M, Kwong JC; Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ 374: n1943, 2021. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 397: 1819–1829, 2021. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: a case series. Ann Intern Med 174: 1334–1336, 2021. doi: 10.7326/M21-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanga V, Chevinsky JR, Dimitrov LV, Gerdes ME, Whitfield GP, Bonacci RA, Nji MAM, Hernandez-Romieu AC, Rogers-Brown JS, McLeod T, Rushmore J, Lutfy C, Bushman D, Koumans E, Saydah S, Goodman AB, Coleman King SM, Jackson BR, Cope JR. Long-term symptoms among adults tested for SARS-CoV-2-United States, January 2020-April 2021. MMWR Morb Mortal Wkly Rep 70: 1235–1241, 2021. doi: 10.15585/mmwr.mm7036a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra PK, Bruiners N, Ukey R, Datta P, Onyuka A, Handler D, Hussain S, Honnen W, Singh S, Guerrini V, Yin Y, Dewald H, Choudhary A, Horton Db, Barrett Es, Roy J, Weiss SH, Fitzgerald-Bocarsly P, Blaser MJ, Carson JL, Panettieri RA, Lardizabal A, Chang TL-Y, Pinter A, Gennaro ML. Vaccination boosts protective responses and counters SARS-CoV-2-induced pathogenic memory B cells. medRxiv, 2021. doi: 10.1101/2021.04.11.21255153. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S2: https://doi.org/10.6084/m9.figshare.17207282.v2.