Abstract
Aim: The Dietary Approaches to Stop Hypertension (DASH) diet is recommended for lowering blood pressure (BP). Our previous single-arm trial revealed that the Japanese cuisine-based DASH (J-DASH) diet (supplying NaCl 8.0 g per day) reduced BP and improved cardiometabolic biomarkers. The present study’s primary objective was to test the feasibility of the J-DASH diet based on its effects on the BP and BP variability of subjects with untreated high-normal BP or stage 1 hypertension.
Methods: The 6-month study period was held from December 2015 to August 2016. The participants were recruited through advertisements in local newspapers and our website and from among randomized participants at Yamaguchi University Hospital. The 2-month treatments included the following: the J-DASH-1 diet 1×/day or the J-DASH-2 diet providing a fish hamburger-patty 2×/day (5 days/week respectively). The control group consumed their usual diets. For the subsequent 4 months, all participants consumed their usual diets. The main outcome measure was the feasibility of the J-DASH diet. We also collected the data of clinic BP and home BP (by automatic BP monitor), cardiometabolic biomarkers, and lifestyle and psychosocial parameters during the intervention phase. We examined behavior changes throughout the study period, and the diets’ safety.
Results: Fifty-one participants were recruited; following screening, 48 met the inclusion criteria and were randomized by central allocation. Eight participants were eliminated based on exclusion criteria, and the 40 participants were randomly allocated to the J-DASH 1 and J-DASH 2 groups ( n =13 each) and the usual-diet group ( n =14). The participants’ mean age was 50 years, and 44% were women. The three groups’ clinic BP values were not significantly different, but the home BP values were lower in the J-DASH 1 group and lowest in the J-DASH 2 group compared to the usual-diet group and differed significantly among the three groups throughout the study period ( p <0.0001). The home BP variability was significantly lower in the J-DASH groups compared to the usual-diet group throughout the study period ( p <0.01). The other indices including fish oil showed little differences among the groups throughout the study period.
Conclusions: The J-DASH diet was feasible to improve home BP and stabilize its variability, and it did so more effectively than the participants’ usual diets.
Keywords: DASH diet, Blood pressure, Variability, Sodium, Japanese cuisine
See editorial vol. 29: 141-142
Introduction
In some regions and countries, including Japan, an excessive intake of salt is one of the causes of the high prevalence of hypertension, which increases the risk of stroke independently of vascular risk factors 1 - 4) . The Dietary Approaches to Stop Hypertension (DASH) diet is recommended for lowering an individual’s lower blood pressure (BP) 5 - 7) . However, as the DASH diet was designed for western populations, it is difficult for Japanese people to follow the original DASH diet because of the significant differences in dietary patterns and taste preferences of the Japanese people. It is also difficult to design a menu that matches the nutrient composition of the DASH diet by using Japanese ingredients, and it has been difficult for Japanese people to consume the DASH diet on a regular basis.
Japanese cuisine or ‘’Washoku’’ is registered by United Nations Educational, Scientific and Cultural Organization (UNESCO) 8) . We conducted an open-label single-arm trial to assess the effects of the Japanese cuisine-based DASH (J-DASH) diet, a modified DASH diet which is rich in fruits, vegetables, and low-fat dairy foods and provides 8.0 g salt per day and almost double the quantities of potassium, calcium, magnesium, and fiber that are consumed in the average Japanese diet 9) . The trial enrolled individuals with untreated high-normal BP or stage 1 hypertension. The results of that trial demonstrated that the J-DASH diet when consumed 3×/day for 2 months significantly enhanced the reduction of BP and improved cardiometabolic biomarkers in both the participants with untreated high-normal BP and those with stage 1 hypertension, and these effects of the J-DASH diet continued for 4 months after the end of the intervention 9) .
Although the adverse cardiovascular consequences of hypertension depend largely on the absolute BP values 10) , assessments of the variability of BP must take into account the targeting of antihypertensive treatment toward stabilizing an individual’s long-term BP variability 11) . The effects of inter-individual BP variation may also influence the differences in the effects of antihypertensive drugs on the risk of stroke, independently of the effects on the mean systolic BP 12 , 13) .
The consumption of fish is associated with improved cardiovascular health, but the clinical and physiologic benefits of lowering BP with the typical western levels of fish intake may require years of fish consumption 14 - 17) . An antihypertensive effect of the n-3 polyunsaturated fatty acids in fish oil was observed in hypertensive patients who were treated with an increased intake of this oil 18 , 19) . However, fish oil is not part of the original DASH diet 5) or the original J-DASH diet 9) . In the present study, we also tested the effects of the addition of fish oil to the J-DASH diet.
Study Aims
We conducted the present feasibility randomized controlled study of participants with untreated high normal BP or stage 1 hypertension to compare the effects of a normal usual-diet, the J-DASH diet alone, or the J-DASH diet with fish oil on the participants’ BP and the variability of their BP.
Participants and Methods
The J-DASH Diet Study was a single-center, pragmatic, open-label, triple-arm parallel-group randomized single-masking controlled-feeding trial comparing the effects on BP of the participants’ ‘usual’ diet and two J-DASH diets among adults with normal-high BP or stage 1 hypertension as defined by the Japanese Society of Hypertension 2014 Guidelines for the Management of Hypertension (JSH2014) 20) . This study was conducted from December 2015 to August 2016. The participants were recruited via advertisements in local newspapers and the home page of the Coordinate and Data Center, Ube, Yamaguchi, Japan. The recruitment ended in January 2016.
Ethical Considerations
The study was conducted in accord with the Declaration of Helsinki. Its protocol was reviewed and approved by the institutional review board of Yamaguchi University Hospital, Ube, Japan. Before participating in the study, all participants were fully informed about the study by the investigators, and written informed consent was given by each participant. After the randomization of the participants, all participants were followed-up for 6 months until the study was terminated.
Study Participants
The study participants were men and women aged 40–74 years who had never taken antihypertensive medication. Each participant had an average clinic BP value that indicated normal-high BP or stage 1 hypertension at two screening visits at Onaka Hospital, Ube, Japan during the 1-month screening and run-in phase.
The major criteria for exclusion from the study were as follows: secondary hypertension 20) ; a history of chronic kidney disease with an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73m 2 20) ; a previous history of cardiovascular disease; being treated with post-menopausal hormone replacement therapy <3 months before study entry; dementia; diabetes mellitus requiring insulin or oral hypoglycemic treatment; liver dysfunction (>3 times the normal ranges of liver enzymes); allergies to food ingredients; pregnancy or potential pregnancy during the study; and being away from home for ≥ 1 week in total during the intervention phase.
Outcome Measurements
Laboratory Measurements
All of the blood and urine samples were collected from fasting participants during their medical examinations at Onaka Hospital. Urine specimens were collected in a clean, plastic, dry urine container. Blood and urine samples from the participants were evaluated by a central laboratory (SRL, Tokyo) at baseline, at the end of the intervention phase, and at the end of the follow-up phase. Each participant’s estimated salt intake (g per day) was calculated by Tanaka’s formula 21) . We used the ratio of eicosapentaenoic acid ethyl ester (EPA) to arachidonic acid (AA) as a marker of the clinical efficacy of fish oil in this study 22 - 24) , because the ratio of EPA to AA was described as a useful marker for the clinical efficacy of fish oil in clinical trials 23 , 24) .
Office and Home Blood Pressure Measurements
At each visit, the measurement of each participant’s standard clinic BP values was performed two or more times at intervals of 1–2 min by specially trained nurses, maintaining the arm-cuff position at the participant’s heart level as he or she rested in a seated position. The mean value of two measurements that provided stable values (i.e., the difference in the values was <5 mmHg) was used for the study analyses in accord with the JSH2014 guideline 20) . Smoking was prohibited 30 min before the measurement.
The diagnoses of normal-high BP and stage 1 hypertension were made according to the JSH2014 20) based on the participant’s clinic BP values measured on two different occasions during the screening and run-in phase at an interval of ≥ 1 month. The clinic BP was measured at two screening visits while the participant was seated, twice during the run-in phase, twice in a single visit during the last week of the 2-month intervention phase, and twice in a single visit during the last week of the 4-month follow-up phase.
The home BP monitoring was performed using a cuff oscillometric device equipped with a mobile network communication function (HEM-7252G-HP; Omron Healthcare, Kyoto, Japan). The data obtained by the device were transmitted automatically to a cloud-based remote monitoring system, the MedicalLink ® program provided by Omron Healthcare. In keeping with the JSH2014 guideline 20) , after randomization, all participants were instructed to measure their morning home BP (measured 2× within 1 hr after waking; both measurements taken after urination, before taking morning medications and after 1–2 min of seated rest) and their evening home BP (measured 2× before bedtime after 1–2 min of seated rest) every day by themselves throughout the intervention and the follow-up phase. The mean of the morning or evening BP values of the last 5 days (five times) was taken as the home morning or evening BP value of the day, respectively 25) , and we used these values for the analyses.
Day-to-Day BP Variability
For each participant, the home mean systolic BP (SBP) and diastolic BP (DBP) values measured during the intervention phase and the follow-up phase were selected for analysis. In addition to the standard deviation (SD) and the coefficient of variation (CV) of the mean BP, the day-to-day variability in SBP and DBP were analyzed separately for the three diet groups. The mean of the two stable measurements taken each morning and evening of a single day was used as the BP value for that occasion. The mean of the morning or evening BP values of the last 5 days (five times) was taken as the home morning or evening BP value of the day, respectively 25) , and we used these values for the analyses. For each patient, the SD was divided by the corresponding mean BP value to express the day-to-day variability as a CV or, in other words, as a normalized value. The individual values were averaged to obtain the mean values for each group considered.
Lifestyle Modification Lecture
All participants (including those in the usual-diet group) were given a 1-hr dietary and behavioral lecture about lifestyle modifications based on nonpharmacological interventions including healthy eating habits (such as the DASH diet) for reducing BP in people with hypertension and high-normal BP according to the JSH2014 20) . This lifestyle modification lecture was given twice: at the start of the intervention phase before randomization and at the start of the follow-up phase.
The J-DASH Diets and Controlled Feeding
For the two J-DASH diets, a 5-day menu cycle was developed for each respective diet: five meals without a fish hamburger-patty (the J-DASH 1 group) and 10 meals with a fish hamburger-patty (the J-DASH 2 group) for each week; these two J-DASH diets provide the same calorie level (average approx. 600 kcal per meal, Supplemental Table 1 ). The J-DASH diet menus were created essentially in accord with the nutritional composition of the original DASH diet 5 , 6) . However, as revealed by the measurement of the nutritional composition of the menu items after cooking, the J-DASH diets contained less total fat and saturated fatty acids than the original DASH diet, as reported 9) .
Supplemental Table 1. The average nutrient composition of five frozen packs of the J-DASH 1 diet and ten frozen packs of the J-DASH 2 diet.
| J-DASH 1 diet | J-DASH 2 diet (Included fish hamburger-patty) | |
|---|---|---|
| Total energy, kcal | 603 | 609 (124) |
| Total fat, g | 15 | 17 (5.0) |
| Carbohydrate, g | 88 | 85 (10) |
| Protein, g | 28 | 26 (11) |
| Salt, g | 1.9 | 1.8 (0.71) |
| Potassium, mg | 1,115 | 1,095 (242) |
| Calcium, mg | 293 | 306 (40) |
| Magnesium, mg | 119 | 111 (33) |
| Saturated fatty acid, mg | 2.9 | 3.6 (1.0) |
| Cholesterol, mg | 59 | 45 (22) |
| Fiber, g | 7.7 | 7.3 (1.8) |
| Eicosapentaenoic acid, mg | 16 | 189 (140) |
| Docosahexaenoic acid, mg | 35 | 355 (300) |
Please note that eicosapentaenoic acid and docosahexaenoic acid contentsare lower in the J-DASH 1 diet without a fish hamburger-patty compared to the J-DASH 2 diet with a fish hamburger-patty. J-DASH 2 diet includes 80g of fish hamburger-patty.
The J-DASH 1 group was given one pack of the J-DASH diet 1×/day for lunch or dinner, and the remaining meal was the participant’s usual meal or choice. The J-DASH 2 group was given two packs of the J-DASH diet per day that each contained a fish hamburger-patty as fish oil; this was not contained in the original DASH diet 5) or the original J-DASH diet 9) . For the standardization of the two J-DASH diets used herein, the J-DASH meals were prepared by Maruha Nichiro (Tokyo), and frozen packages with 10-day frozen packs were home-delivered to each participant to be consumed at lunch and/or dinner each weekday, every week for 2 months ( Supplemental Fig.1 ) . For the weekend, the participants were allowed to eat anything they wanted. The participants were allowed to drink beverages (including caffeinated beverages and alcoholic beverages) as usual throughout the study period. For each day of controlled feeding, the participants recorded their intake of discretionary items (i.e., beverages and salt). They recorded whether they ate any non-study foods and whether they did not eat all of the study foods.
Supplemental Fig.1.
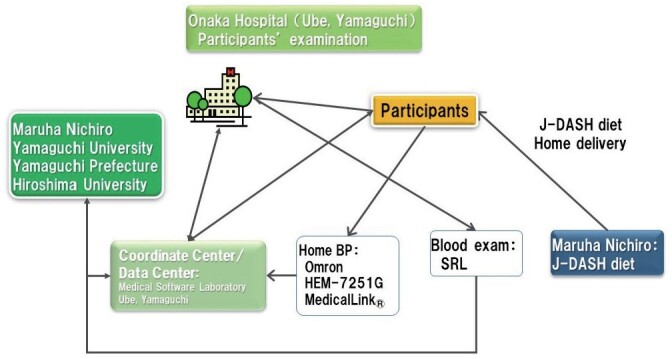
The J-DASH Diet Study implementation system
For all participants (including the usual-diet participants), 50 g of the same commercial cereal (Calbee Frugra ® ) was provided 1×/day for breakfast, 5 days/week for 2 months, and the lifestyle modification lectures were provided by a hypertension specialist as incentive to promote adherence to the study. Reimbursements were also given for the participants’ travel expenses after the study was finished.
Study Protocol
Since this study was a feasibility study designed primarily to assess the methods including those to reduce bias, a sample size calculation was not performed. This study was designed to determine whether our findings in the previous study 9) are feasible, and to test a surrogate outcome. The clinic staff and research personnel who collected the data were all blinded to the participants’ allocation.
This study consisted of three phases ( Supplemental Fig.2 ) : the screening run-in phase (1 month), the intervention phase (2 months), and the follow-up phase (4 months). In the screening run-in phase, the clinic BP values, fasting blood and urine samples, and information about the medical history of each participant and his or her family members were obtained. The participants then ate their assigned diet for the next 2 months. Each participant’s energy intake from their diets was constant throughout the intervention phase. As the follow-up phase, the participants were followed for 4 months with their usual diets until the study was terminated.
Supplemental Fig.2.
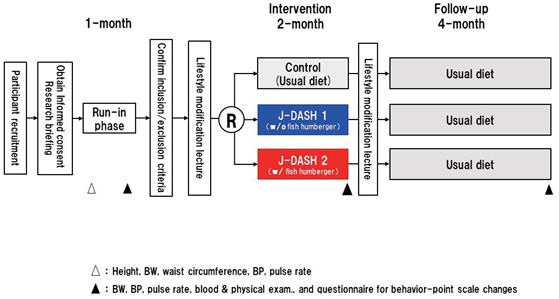
The J-DASH Study flow. w/: with, w/o: without
A pair of clinic BP values was obtained at each visit during four separate weeks. At the first visit, the participant’s body weight and height were also measured. At the second visit, the following information was obtained from all participants who met the inclusion criteria: medical histories, laboratory tests at a fasting state, physical examination and vital signs, and other information including the results of a questionnaire about symptoms.
Randomization
The computer-generated randomization was done centrally at Yamaguchi University Hospital. The participants were randomly assigned to one of the following arms in a 1:1:1 ratio: the usual diet as a control group, the J-DASH 1 diet group, or the J-DASH 2 diet group (See Supplemental Data). Participants suspected of having secondary hypertension (especially primary aldosteronism) 20) were excluded from the study. No one directly involved in the study had access to the participant allocation codes.
Diet Treatments
We assessed the participants’ adherence to the diet by reviewing their daily food diaries and by having them eat their weekday lunches or dinners on-site. Side effects were monitored by means of questionnaires regarding symptoms and illnesses. During the last 2 weeks of the 2-month intervention phase, a pair of clinic BP measurements was obtained, and we administered a symptom/behavior change scale questionnaire and a physical-activity-recall questionnaire. All participants were then given the 1-hr lifestyle modification lecture.
During the last 2 weeks of the 4-month follow-up phase, a pair of clinic BP measurements was obtained and the symptom questionnaire and physical-activity-recall questionnaire were administered.
Further details of the study protocol can be found in the Supplemental Data.
Outcomes
This was a feasibility study to examine the differences in the following pre-specified primary and secondary outcome measures among the control and intervention groups. The two primary outcomes of this study were (i) the changes in the participants’ systolic and diastolic clinic BP at rest, and (ii) the changes in the participants’ home morning and evening BP values at rest throughout the study period. The secondary outcomes were the home variability in the participants’ SBP and DBP, and the changes in body weight, estimated sodium intake, and other biochemical markers including fish oil, and the safety of the two J-DASH diets.
Statistical Methods
The results of our analyses are expressed as the mean±standard deviation (SD), median (interquartile range [IQR]), or the number of participants with the characteristic (percentage). Analyses were performed in compliance with the full analysis set. The demographics and the clinical characteristics of the three diet groups at baseline are reported using descriptive statistics and were compared using either a one-way analysis of variance (ANOVA), the Kruskal-Wallis test, or Fisher’s exact test as appropriate. Changes in each of the primary and secondary outcomes during the study were analyzed by generalized linear mixed models (GLMMs) with the main effects of treatment (control, J-DASH 1, and J-DASH 2 groups) and time (baseline, end of intervention, and 4-month follow-up), treatment×time interactions, and participant-level random intercepts.
The mean of the participant’s morning or evening BP values of the last 5 days (five times) was used as the home morning or evening BP value of the day, respectively 25) , and we used these values for the analyses. Differences in the mean, SD, and coefficient of variation (CV) of the BP values were analyzed by a multiple comparison (Dunnett), and the number of home BP measurements in the three treatment groups during the intervention phase and the follow-up phase were analyzed by an ANOVA. All data were analyzed using SAS ® System Release 9.4 software (SAS Institute, Cary, NC, USA). All p -values were two-sided, and a p -value <0.05 was considered significant.
Results
The Participants’ Demographic and Baseline Characteristics
Of the original number of 51 participants, 48 underwent randomization based on the criteria in the screening run-in phase in this study. We excluded one of the usual-diet participants, three J-DASH 1 participants, and three J-DASH 2 participants due to their diagnoses of primary aldosteronism, and we excluded one participant who did not meet the inclusion criteria from the analyses. A final total of 40 eligible participants underwent the analyses: the usual-diet group ( n =14), the J-DASH 1 group ( n =13), and the J-DASH 2 group ( n =13) ( Fig.1 ) . All of the participants completed the study and provided home BP measurements during the intervention and follow-up phases. The participants’ baseline and demographic characteristics are summarized in Tables 1 and 2 .
Fig.1. Study flow diagram of the J-DASH Diet Study.
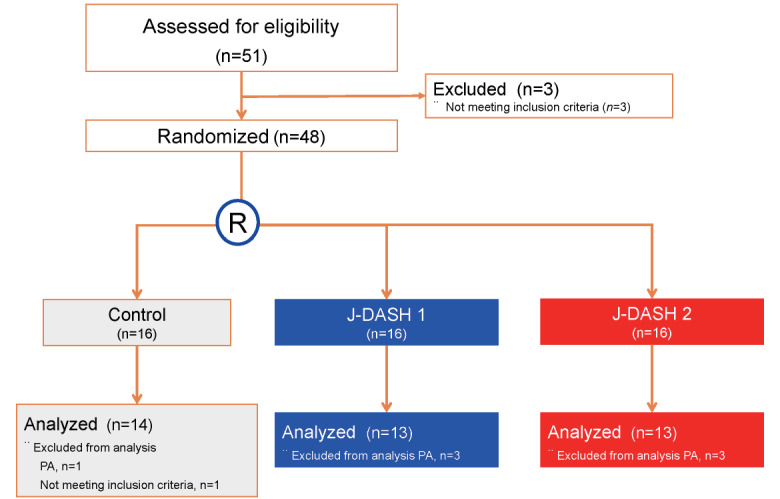
The diagnosis of primary aldosteronism (PA) was based on the following criteria: a plasma aldosterone concentration to plasma renin activity ratio >200 and a plasma aldosterone concentration >120 pg/mL according to the JSH2014 20) . R: randomization.
Table 1. Demographic and baseline characteristics of the participants according to the assigned diet.
| Variable |
Control n = 14 |
J-DASH 1 n = 13 |
J-DASH 2 n = 13 |
p -value |
|---|---|---|---|---|
| Age, yrs | 48.6±7.6 | 51.9±6.2 | 50.0±9.5 | 0.569 |
| Age > 65 yrs, n | 1 | 0 | 1 | – |
| Female, n (%) | 7 (50.0) | 5 (38.5) | 6 (46.2) | 0.922 |
| Menopause, n (%) | 6 (85.7) | 4 (100.0) | 2 (40.0) | 0.142 |
| Family history of hypertension, n (%) | 1 (7.1) | 3 (25.0) | 2 (15.4) | 0.400 |
| Hypertension, % | 0.541 | |||
| High-normal BP | 5 (35.7) | 3 (23.1) | 2 (15.4) | – |
| Stage 1 hypertension | 9 (64.3) | 10 (76.9) | 11 (84.6) | – |
| SBP, mmHg | 142.1±8.2 | 142.4±7.0 | 141.9±8.3 | 0.990 |
| DBP, mmHg | 85.8±8.0 | 87.6±7.4 | 90.9±5.5 | 0.186 |
| BMI, kg/m 2 | 24.5±3.1 | 25.2±2.5 | 24.4±2.5 | 0.705 |
| Waist circumference, cm | 85.9±7.4 | 86.8±6.8 | 84.8±7.5 | 0.783 |
| Metabolic syndrome, n (%) * | 3 (21.4) | 3 (23.1) | 3 (23.1) | 0.705 |
| Type 2 diabetes mellitus, n (%) | 1 (7.1) | 0 (0.0) | 1 (7.7) | 1.000 |
| Dyslipidemia, n (%) | 9 (64.3) | 8 (61.5) | 9 (69.2) | 1.000 |
| Smoking habit, n (%) | 2 (14.3) | 4 (33.3) | 3 (23.1) | 0.507 |
| Alcohol habit, n (%) | 6 (42.9) | 5 (41.7) | 8 (61.5) | 0.563 |
| Exercise habit, n (%) | 8 (57.1) | 8 (66.7) | 8 (61.5) | 0.919 |
| Estimated daily salt intake, g/day ** | 10.4±2.0 | 10.9±2.3 | 9.5±1.8 | 0.200 |
| Behavior change scale | 4.5±1.6 | 4.7±2.0 | 4.1±1.7 | 0.659 |
Data are mean±SD or percentage. Hypertension was defined during the two screening visits, and baseline BP was the average of two screening measurements during the run-in phase according to the JSH2014 20) . The body mass index (BMI) is the weight in kg divided by the square of the height in meters. Baseline urinary sodium was determined from a spot urine collection during the screening phase, when the participants were eating their customary, self-selected diets. Waist circumference was measured, and * the diagnosis of metabolic syndrome was based on the criteria for metabolic syndrome 40) . ** The estimated daily salt intake was determined by Tanaka’s formula 21) . DBP: diastolic blood pressure, SBP: systolic blood pressure.
Table 2. Baseline characteristics of the participants; body weight, lipid, creatinine, eGFR, uric acid, serum and urinary concentrations of mineral and albumin according to assigned diet.
| Variable |
Control n = 14 |
J-DASH 1 n = 13 |
J-DASH 2 n = 13 |
p -value |
|---|---|---|---|---|
| Total cholesterol, mg/dL | 216±39 | 223±32 | 217±26 | 0.866 |
| Triglyceride, median (IQR), mg/dL | 98 (61–159) | 87 (64–153) | 136 (59–174) | 0.977 |
| Creatinine, mg/dL | 0.66±0.10 | 0.72±0.16 | 0.74±0.13 | 0.294 |
| eGFR, mL/min/1.73 m 2 | 93.7±14.3 | 78.6±7.7 | 82.0±15.3 | 0.049 |
| Urea nitrogen, mg/dL | 12.7±3.5 | 12.7±2.2 | 13.3±3.9 | 0.871 |
| Uric acid, mg/dL | 4.9±1.2 | 5.5±1.2 | 5.1±1.0 | 0.329 |
| Serum sodium, mEq/L | 141±1.5 | 141±1.4 | 141±1.3 | 0.958 |
| Serum potassium, mEq/L | 4.1±0.3 | 4.2±0.2 | 4.2±0.3 | 0.588 |
| Serum calcium, mg/dL | 9.4±0.3 | 9.4±0.3 | 9.3±0.5 | 0.833 |
| Serum magnesium, mg/dL | 2.4±0.1 | 2.3±0.2 | 2.3±0.2 | 0.214 |
| PRA, median (IQR), ng/mL/hr | 1.1 (0.7–1.5) | 1.2 (0.9–1.7) | 0.8 (0.7–1.1) | 0.151 |
| PAC, pg/mL | 162.6±64.0 | 148.3±58.3 | 173.8±41.3 | 0.510 |
| Urinary sodium, mEq/day | 137±62 | 157±63 | 147±44 | 0.657 |
| Urinary potassium, mEq/day | 55±24 | 51±29 | 64±33 | 0.515 |
| Urinary albumin, mg/L | 7.1±5.7 | 8.7±7.3 | 12.4±10.4 | 0.229 |
| EPA/AA ratio | 0.32±0.2 | 0.28±0.2 | 0.30±0.2 | 0.814 |
* Data are mean±SD or median (IQR). AA: arachidonic acid, eGFR: estimated glomerular filtration rate, EPA: eicosapentaenoic acid ethyl ester, HDL: high-density lipoprotein, IQR: interquartile range, PAC: plasma aldosterone concentration, PRA: plasma renin activity.
The participants’ mean age was 50 years, and 18 (44%) were female. The baseline demographic characteristics of the two DASH-diet groups and the usual-diet group were well-balanced except for the eGFR, which differed slightly among the groups ( p =0.049). The participants’ estimated daily salt intake was approx. 10 g per day.
The Rates of DASH Diet Consumption during the Study Period
In the intervention phase, the mean rate of consuming the DASH diet was 85.6% in the J-DASH 1 group and 83.1% in the J-DASH 2 group. The weekly food consumption rate gradually declined in both J-DASH diet groups ( p <0.05). There was little difference in the diet-consumption rate between the two J-DASH diet groups ( p =0.687), and there was no difference in the decrease in the diet-consumption rate between the J-DASH 1 and J-DASH 2 groups ( p =0.669) ( Supplemental Fig.3 ) .
Supplemental Fig.3. Weekly changes in the J-DASH diet consumption rate.
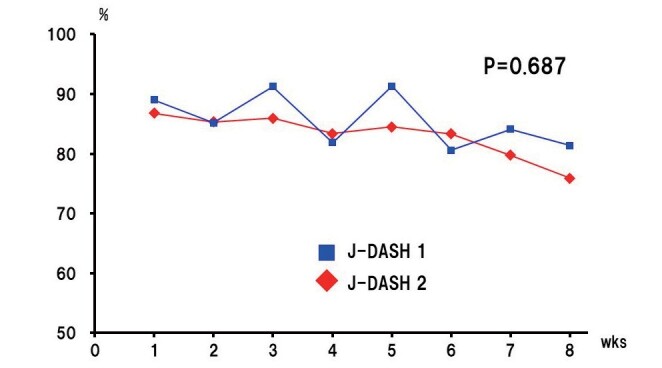
All participants were offered the same cereal for breakfast and five types of J-DASH diet without the fish hamburger-patty at dinner (J-DASH 1) or with a fish hamburger-patty at lunch and dinner (J-DASH 2). The diet consumption rate decreased (time variation p <0.05). However, there was no significant difference in the decrease in the diet consumption rate between the J-DASH 1 and 2 groups (time × group interaction p =0.669). The J-DASH 1 group maintained a mean diet consumption of 85.6%. In the J-DASH 2 group, although the food consumption rate gradually declined, its average was 83.1%. A two-factor mixed design with repeated measures on one factor was used for the analysis.
The Effects of the J-DASH Diets on Clinic BP Values
Fig.2 provides the data of the J-DASH diets’ effects on the participants’ clinic SBP and DBP at baseline, at the end of the intervention phase, and at the end of the follow-up phase. The clinic SBP values of all three groups tended to decrease during the intervention phase and rose during the follow-up phase, but these values of all three groups were still significantly lower than the baseline SBP levels ( p =0.0004, respectively). However, the clinic SBP values were not significantly different among the three assigned groups at baseline, at the end of the intervention phase, or at the end of the follow-up phase ( p =0.303).
Fig.2. Changes from baseline in clinic systolic and diastolic BP in the J-DASH Diet Study.
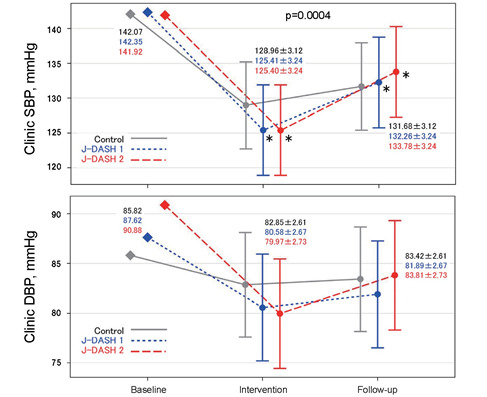
The data are shown as least squares means with 95% confidence. * p <0.05 vs. baseline.
The clinic DBP values of all three groups tended to decrease during the intervention phase and remained lower than the baseline BP levels during the follow-up phase, but they were not significantly different compared to the baseline DBP values ( p =0.105). The clinic DBP data were also not significantly different among the three groups at baseline, at the end of the intervention phase, or at the end of the follow-up phase ( p =0.482).
The mean difference in clinic SBP after the intervention was smaller by only −3.55 mmHg in the J-DASH 1 group and −3.56 mmHg in the J-DASH 2 group compared to the baseline ( p <0.05, respectively).
The Effects of the J-DASH Diets on Home BP
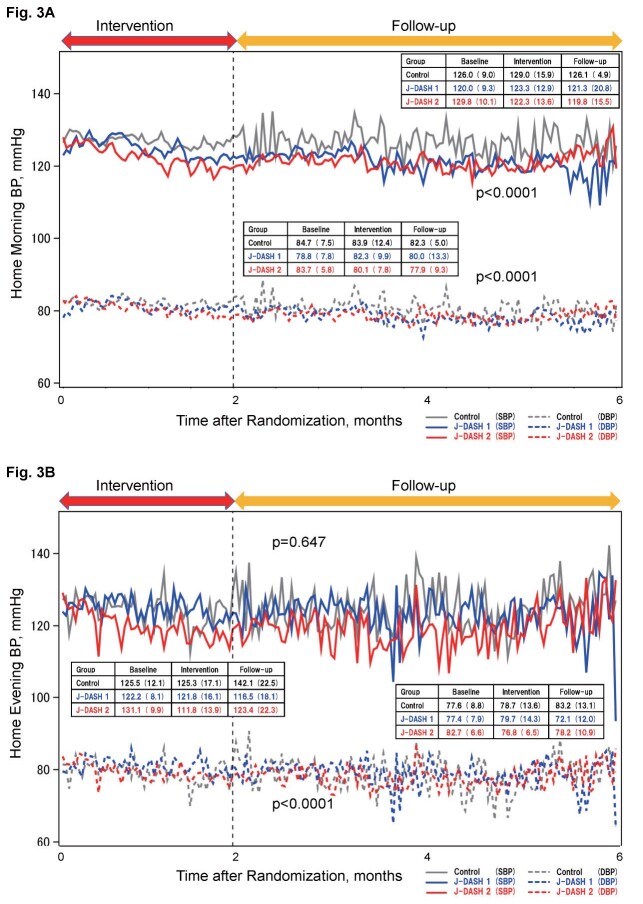
Fig.3 demonstrates the home BP trends measured by the participants during the intervention and follow-up phases. There were no difference on the numbers of measuring of home BP among the three assigned diet groups ( Supplemental Table 2 ) . In contrast to the clinic BP values, the home morning SBP and DBP values were lower in the J-DASH 1 group and lowest in the J-DASH 2 group compared to the usual-diet group, and they were significantly different among the three diet groups throughout the study period ( Fig.3A , p <0.0001).
Fig.3. Time trend of mean home BP values throughout the study period.
Data are expressed as the mean of the assigned groups and the mean±SD in the boxes. Each point of the BP curve in each assigned group is indicated by the average value of the measured values on 5 days (5 times) 25) . A: Home morning blood pressure; p <0.0001 among the three diet groups throughout the study period. B: Home evening blood pressure; p <0.0001 among the groups throughout the study period.
Supplemental Table 2. The total numbers of home BP measurements in the three diet groups.
| Total no. of home BP measurement occasions | Control n = 14 | J-DASH 1 n = 13 | J-DASH 2 n = 13 | p -value |
|---|---|---|---|---|
| Morning | 117.9±13.2 | 129.4±13.7 | 129.2±13.7 | 0.786 |
| Evening | 65.4±12.1 | 83.1±12.6 | 78.5±12.6 | 0.579 |
After randomization, all participants were instructed to measure their morning home BP (measured twice within 1 hr after waking; both measurements taken after urination, before taking morning medications and after 1–2 min of seated rest), and evening home BP (measured twice before bedtime after 1–2 min of seated rest) everyday by themselves throughout the intervention and follow-up phase. The mean of morning or evening BP values of the last 5 days (five times) was taken as the home morning or evening BP values of the day, respectively 6) . Data are the number of participants BP measurements occasions. Differences in the number of BP measurement occasions among the three diet groups were analyzed by an ANOVA.
On the other hand, although the home evening SBP values were not significantly different among the three groups throughout the study period ( Fig.3B , p =0.647), the home evening DBP values were significantly lower in the J-DASH 1 group and lowest in the J-DASH 2 group compared to the usual-diet group, and they were significantly different among the groups throughout the study period ( Fig.3B , p <0.0001).
The mean difference in the morning home SBP and DBP values after the intervention were smaller by −5.7 and −6.7 mmHg in the J-DASH 1 group and by −1.6 and −3.8 mmHg in the J-DASH 2 group compared to the usual-diet group.
The Effects of the J-DASH Diets on the Day-to-Day Home BP Variability
Fig.4 illustrates the effects of the J-DASH diets on the participants’ day-to-day home BP variability during the intervention and follow-up phases. The values of day-to-day morning home SBP variability and DBP variability that we assessed by obtaining the CV values were significantly lower in the two J-DASH diet groups compared to the usual-diet group throughout the study period ( p <0.01) ( Fig.4A ) .
Fig.4. Morning and evening day-to-day BP variability throughout the study period.
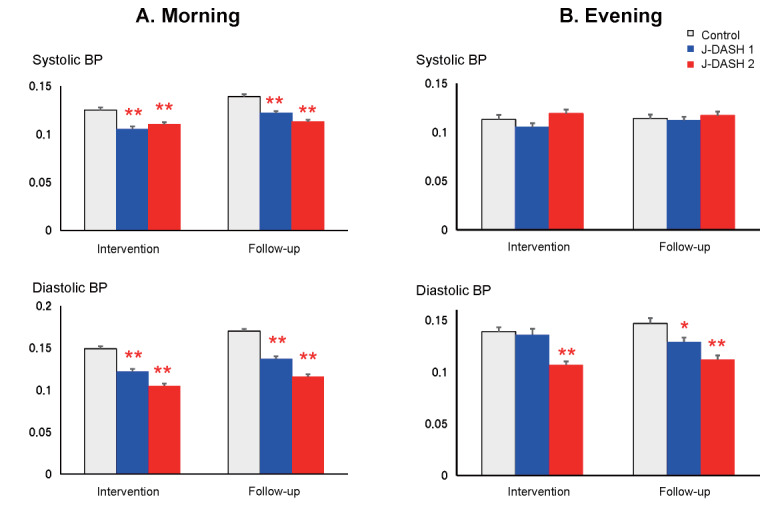
The data are mean±SD. * p <0.05, ** p <0.01 vs. the usual-diet group.
Regarding the day-to-day evening home BP variability, when assessed by the CV compared to the control group, only the day-to-day evening diastolic BP variability of the J-DASH 1 group was significantly lower in the follow-up phase ( p <0.01). In the J-DASH 2 group, the participants’ day-to-day evening diastolic BP variability was also lower in the intervention phase ( p <0.05) and follow-up phase ( p <0.01) ( Fig.4B ) .
The Effects of the BMI and Biochemical, Serum, and Urinary Biomarkers, and the Safety of the J-DASH Diets
The body mass index (BMI) was not significantly different among the three diet groups throughout the study period ( p =0.607), but the BMI of each of the three groups gradually decreased during the study period ( p =0.040). The serum sodium values and the urinary sodium and albumin concentrations were significantly increased after the intervention in all three groups (serum sodium concentration; p <0.0001, urinary sodium concentration; p =0.0013, and urinary albumin concentration; p =0.0357) with little difference among the groups throughout the study period (serum urinary concentration; p =0.795, urinary sodium concentration; p =0.795, and urinary albumin concentration; p =0.357).
Negligible differences were observed among the groups in the other biochemical, serum, and urine biomarkers throughout the study period, including the abdominal waist circumference ( p =0.858), behavior change scale ( p =0.141), estimated daily salt intake ( p =0.610), and EPA/AA ratio ( p =0.157) ( Supplemental Table 3 and Supplemental Fig.4 ) . We did not observe any adverse effects or events in the two J-DASH diet groups compared to the usual-diet group throughout the study. Change of other variables are shown in Supplemental Tables 3 and 4 , and Supplemental Fig.4 .
Supplemental Table 3. Two-factor mixed design with repeated measures on each factor with baseline values as covariates for each variable.
| Variable | Factor | F-value | p -value |
|---|---|---|---|
| BMI | Group | 1.75 | 0.188 |
| Time variation | 4.51 | 0.040 | |
| Time x group interaction | 0.51 | 0.607 | |
| Waist circumference | Group | 0.30 | 0.745 |
| Time variation | 0.82 | 0.372 | |
| Time x group interaction | 0.15 | 0.858 | |
| SBP | Group | 0.07 | 0.936 |
| Time variation | 15.07 | 0.0004 | |
| Time x group interaction | 1.23 | 0.303 | |
| DBP | Group | 0.16 | 0.856 |
| Time variation | 2.76 | 0.105 | |
| Time x group interaction | 0.75 | 0.482 | |
| Serum sodium | Group | 1.91 | 0.163 |
| Time variation | 24.47 | <.0001 | |
| Time x group interaction | 0.58 | 0.564 | |
| Serum potassium | Group | 0.32 | 0.726 |
| Time variation | 1.87 | 0.180 | |
| Time x group interaction | 0.52 | 0.599 | |
| Serum calcium | Group | 0.31 | 0.737 |
| Time variation | 0 | 0.988 | |
| Time x group interaction | 0.22 | 0.800 | |
| Serum magnesium | Group | 0.59 | 0.560 |
| Time variation | 0.12 | 0.726 | |
| Time x group interaction | 0 | 1.000 | |
| Creatinine | Group | 0.45 | 0.638 |
| Time variation | 1.65 | 0.207 | |
| Time x group interaction | 0.44 | 0.650 | |
| eGFR | Group | 0.48 | 0.624 |
| Time variation | 4.18 | 0.052 | |
| Time x group interaction | 0.18 | 0.833 | |
| Plasma aldosterone | Group | 0.03 | 0.967 |
| Time variation | 0 | 0.973 | |
| Time x group interaction | 0.55 | 0.580 | |
| Plasma renin activity | Group | 0.48 | 0.621 |
| Time variation | 0.10 | 0.750 | |
| Time x group interaction | 1.59 | 0.217 | |
| EPA to AA ratio | Group | 0.21 | 0.814 |
| Time variation | 0.38 | 0.539 | |
| Time x group interaction | 1.95 | 0.157 | |
| Urinary sodium | Group | 2.36 | 0.109 |
| Time variation | 12.2 | 0.001 | |
| Time x group interaction | 0.23 | 0.795 | |
| Urinary potassium | Group | 2.67 | 0.083 |
| Time variation | 1.44 | 0.238 | |
| Time x group interaction | 0.68 | 0.514 | |
| Urinary albumin | Group | 0.47 | 0.628 |
| Time variation | 4.75 | 0.036 | |
| Time x group interaction | 1.06 | 0.357 | |
| Estimated daily salt intake | Group | 0.90 | 0.415 |
| Time variation | 0.11 | 0.746 | |
| Time x group interaction | 0.50 | 0.610 | |
| Behavior change scale | Group | 1.42 | 0.255 |
| Time variation | 0.13 | 0.722 | |
| Time x group interaction | 2.07 | 0.141 |
Generalized linear mixed models with effects of diet (control, J-DASH 1, and J-DASH 2 groups) and time (baseline, end of intervention, and 4-month follow-up), treatment by time interactions, and participant-level random intercepts for each variable was used. AA: arachidonic acid, BMI: body mass index, DBP: diastolic blood pressure, EPA: eicosapentaenoic acid ethyl ester, SBP: systolic blood pressure.
Supplemental Fig.4. The least squares mean (LSM) with 95% confidence of each factor.
A two-factor mixed design with repeated measures on each factor with baseline values as covariates was used for each factor. The numbers in the lower panel are mean±SD.
Supplemental Table 4. Changes in lifestyle based on the weekend questionnaire in the three diet groups.
| Variable |
Control n = 14 |
J-DASH 1 n = 13 |
J-DASH 2 n = 13 |
p -value |
|---|---|---|---|---|
| Avg. physical condition, median (IQR) | 2.00 (1.88–2.00) | 2.00 (2.00–2.13) | 2.00 (1.94–2.00) | 0.687 |
| Avg. mood, median (IQR) | 2.00 (2.00–2.25) | 2.00 (2.00–2.06) | 2.00 (1.94–2.06) | 0.709 |
| Avg. bowel movement, median (IQR) | 2.00 (1.88–2.13) | 2.00 (2.00–2.19) | 1.81 (1.56–2.00) | 0.163 |
| Avg. sleep condition, median (IQR) | 2.00 (1.88–2.25) | 2.00 (2.00–2.31) | 2.00 (1.94–2.00) | 0.440 |
During the 2-month intervention phase, we asked about lifestyle changes (average [Avg.] physical condition, average mood, average bowel movement, and average sleep condition) for 1 week each, and the average of the numerical values were calculated for each individual. Based on the results of the questionnaire on each weekend, the differenc in these indices were analyzed by the Kruskal-Wallis test for each variable. Condition from the baseline condition in each variable was evaluated as follows; 1: better, 2: no change, and 3: worse. IQR: interquartile range.
Discussion
Our present findings demonstrated that in a population of participants with untreated high-normal BP and in participants with stage 1 hypertension who had never taken antihypertensive medication, the clinic BP values were not significantly different among three assigned diet groups, whereas compared to the usual-diet group, the home BP values were lower in the J-DASH 1 group and lowest in the J-DASH 2 group, and these values differed significantly among the three participant groups in the intervention phase; these effects continued another 4 months without the consumption of the J-DASH diet. In addition, the home BP variability was significantly lower in the two J-DASH diet groups (to the same extent) compared to the usual-diet group throughout the study period.
Our results are compatible with those of a study 9) which demonstrated that (i) the J-DASH diet (3×/day for 2 months) significantly enhanced the reduction of BP in participants with untreated high-normal BP or stage 1 hypertension, and (ii) the BP-lowering effects of the J-DASH diet continued for 4 months after the intervention. The differences between this previous study and our present investigation are as follows. In the present study, (i) there were only slight differences in the cardiometabolic biomarkers among the three assigned groups; (ii) the J-DASH diet 1×/day, 5 days/week was also effective in lowering BP throughout the study period; and (iii) the comprehensive education including salt restriction with the participants’ usual diet also effectively reduced clinic and home BP values, but its BP lowering effect was less than that of the J-DASH diet. Our data also agree with those of reports suggesting that the greatest and most sustained BP benefit is obtained when multiple lifestyle interventions are incorporated simultaneously 27) , and that the magnitude of the lowering of BP achieved with sodium reduction showed a dose-response relationship 28) .
Although the adverse cardiovascular consequences of hypertension depend largely on absolute BP values, it was reported that these outcomes might also depend on increased BP variability 11) . We reported that a dihydropyridine calcium channel blocker combined with a thiazide appeared to be more effective for reducing the risk of stroke 29) , probably due to the greater reduction of the participants’ visit-to-visit BP variability 30) , suggesting that BP variability may also depend on salt sensitivity. Our present BP variability data are in agreement with these reports 29 , 30) , demonstrating that the J-DASH diet reduced BP variability more than the lifestyle intervention alone.
In our cohort, the estimated daily salt intake remained unchanged at approx. 10 g per day, and the participants’ behavior change scale values also remained unchanged in the three diet groups; the BMI values were reduced equally in the three groups after the intervention and follow-up phase. Although the J-DASH diet provides 8.0 g of salt per day and our participants were allowed to eat anything they wanted on the weekends and to drink beverages as usual throughout the study period, the clinic and home BP values were significantly lower in the two J-DASH diet groups compared to the usual-diet group, indicating that the J-DASH diet may be the only key factor for the BP reduction observed in this study.
Although the consumption of fish is associated with improved cardiovascular health, the clinical and physiologic benefits of lowering BP by the typical Western levels of fish intake may require years of fish consumption 14 - 17) . It was also reported that the consumption of tuna or other broiled or baked fish reduced the study participants’ BP, whereas the consumption of fried fish and fish burgers raised their BP 31) . In the present study, we selected a fish hamburger-patty as the source of fish oil for the J-DASH 2 diet, and this was not contained in the original DASH diet 5) or the original J-DASH diet 9) . However, the EPA/AA ratio was unchanged not only in the present usual-diet group but also in the J-DASH 1 and J-DASH 2 groups even during the intervention phase, indicating that the amount of fish oil content in the J-DASH diet or the duration of the intervention phase may not be enough to increase the EPA/AA ratio, or it may be that the fish hamburger itself may not be suitable as a source of fish oil 31) .
It was reported that the magnitude of BP-lowering achieved with sodium reduction was greater for older populations, non-white populations, and those with higher BP, and that short-term studies underestimate the effect of sodium reduction on BP 28) . Another report described significant increases in plasma renin, aldosterone, cholesterol, and triglyceride and a decrease in BP among Asian participants with hypertension: the mean differences in systolic and diastolic BP were −7.75 mmHg and −2.68 mmHg, respectively with low sodium intake compared to high sodium intake 32) . In our present study, unlike a previous report 33) , there were insignificant differences in the estimated daily salt intake assessed by spot urine tests 21) among the three diet groups, and the plasma aldosterone concentration, renin activity, lipids, and serum and urinary mineral concentrations remained unchanged by the usual diet and the J-DASH diets with a lifestyle intervention. However, the mean difference in clinic SBP and home SBP and DBP values at the end of the intervention phase were significantly lower in the J-DASH diet groups compared to the usual-diet group. Because the J-DASH diet contains almost double the quantities of potassium, calcium, and magnesium and less salt content (~8.0 g per day) than the average Japanese diet 9) , these characteristics of the mineral content of the J-DASH diet may cause BP lowering synergistically and effectively similar to the original DASH diet, especially in Japanese populations 34) because of the differences in intrinsic/extrinsic racial factors between Western countries and Japan such as dietary habits, including the higher salt intake in Japan 35 , 36) . It was suggested that even a modest reduction in salt intake across an entire population can lead to a major improvement in public health and cost savings, such as that provided by the J-DASH diet 37) .
Study Limitations
Some limitations of our study should be considered. First, relatively few participants were included ( n =40), and therefore the statistical power of the study was low. The study was designed to explore whether the J-DASH diets are feasible or not and was randomized and conducted for a future definitive randomized controlled study; the results of the study could ultimately be combined in a systematic review and meta-analysis 38) . However, the mean effect of the J-DASH diet on the participants’ BP was similar to that provided by a modest salt intake reduction 7) .
Second, we used the PROBE design, which might have induced bias in assessing the outcomes. Although the primary outcomes were obtained in a blinded fashion by using a mobile network communication function and by the personnel who collected the outcome data (who were unaware of the participants’ diet assignments), the non-blinded treatment allocation could potentially have affected the participants’ behaviors. In addition, we did not survey the dietary or lifestyle record in the usual diet follow-up phase, and thus we could not clearly differentiate the effects of the J-DASH diets on BP in the follow-up phase, although the participants’ behavior change scale scores remained unchanged among the three groups throughout the study.
Third, because the J-DASH diet was prepared by the research team, the feasibility of the diet for use in the general population is unknown due to its relatively high cost and long preparation time 39) . However, our present results demonstrated that 2 months of the J-DASH diet was enough to lower the participants’ BP during the intervention phase, and this effect continued in the 4-month follow-up phase. In addition, our participants were recruited via local newspapers and the Coordinate and Data Center’s website, and thus mainly relatively low-risk younger participants without cardiovascular complications who are highly motivated to maintain their health were recruited. This may have affected the study results, but all of the participants were free to eat and drink without any lifestyle restrictions, and even in the usual-diet group, the participants’ BP values were decreased only by the lifestyle modification lecture, whereas the J-DASH diet decreased the participants’ BP more compared to the usual diet, indicating that the J-DASH diet itself may be effective for lowering BP. Because salt reduction is often necessary for high-risk patients who have moderate to severe hypertension, cardiac morbidities, or diabetes, the effectiveness of the J-DASH diet in these patients should be investigated in future research toward the goal of reducing their cardiovascular risk.
Conclusion
The J-DASH diet was feasible to improve home BP and stabilize its variability better than the usual diet in Japanese participants with untreated high-normal BP or stage 1 hypertension. The use of the J-DASH diet may help control BP and reduce cardiovascular risk among the Japanese.
Acknowledgements
We are indebted to the study participants and cooperators (especially the nurses and staff of Onaka Hospital) for their sustained commitment to the J-DASH diet study.
Conflicts of Interest
The sponsor of the study had no role in the data collection, data analysis, data interpretation, or writing of the report. The trial statistician (R.K.) had full access to all data, and S.U. had full access to all of the data in the study and had overall responsibility for the decision to submit the manuscript for publication.
This study was conducted with a Yamaguchi Industrial Strategy R&D Grant and as Collaborative Research among Yamaguchi University, Yamaguchi Prefecture, Hiroshima University, and the sponsor (Maruha Nichiro Corp.). This study was also funded by a grant from the Japan Society for the Promotion of Science (KAKENHI), no. 15K09123. S.U. reports grants and a consultancy fee from the sponsor. S.U. and A.K. also received grants for previous collaborative research between Yamaguchi University and the sponsor. R.K. and U.O. declare no competing interests.
SUPPLEMENTAL DATA
Principal Study Coordinator - Seiji Umemoto
Study Statistician - Reo Kawano
Coordinating and Data Center - Hidetoshi Yagi (Medical Software Laboratory, Ltd. Co., Ube, Yamaguchi, Japan)
Contributors: Yoshiaki Matsumoto (Yamaguchi Prefectural Industrial Technology Institute, Ube, Yamaguchi, Japan)
Methods
Lifestyle Modification Lecture
All participants including the usual-diet group were given a 1-hr dietary and behavioral lecture concerning lifestyle modifications and nonpharmacological interventions including healthy eating (such as the DASH diet) for reducing the BP of individuals with hypertension or high-normal BP according to the JSH2014 guideline 1) . These modifications included weight loss, sodium reduction, stress management, and nutritional supplements (calcium, magnesium, potassium, and fish oil) and recommendations regarding how to identify sodium in the diet, self-monitor intake, and select or prepare lower-sodium foods and condiments suited to personal preferences, provided by a study investigator as a hypertension specialist.
The J-DASH Diets and Controlled Feeding
In this study, a five-day menu cycle with five meals without a fish hamburger-patty (J-DASH 1 group) or 10 meals with a fish hamburger-patty (J-DASH 2 group) for each week (average approx. 600 kcal per meal) was developed for each diet. These two J-DASH diets provide the same amount of calories. The J-DASH diet menu was created to be essentially in accord with the nutritional composition of the original DASH diet 2-4) , but the measurements of the nutritional composition of the menu items after cooking revealed that the J-DASH diet contained less total fat and saturated fatty acids than the original DASH diet, as reported 5) . Briefly, menus were designed that used commonly available foods in a variety of forms (fresh, frozen, canned, and dried).
The J-DASH diet is rich in fruits, vegetables, and low-fat dairy foods, and it provides almost double the quantities of potassium, calcium, magnesium, and fiber that are consumed in the average Japanese diet 5) . The salt content of the J-DASH diet is ~8.0 g per day 5) . The average nutrient composition of five frozen packs of the J-DASH 1 diet and ten frozen packs of the J-DASH 2 diet are shown in Supplemental Table 1 .
The J-DASH 2 group was given two packs of the J-DASH diet per day. Each pack contained a fish hamburger-patty as a source of fish oil, which was not part of the original DASH diet or the original J-DASH diet 5) . We selected a fish hamburger-patty as the source of fish oil. It was easy to fortify the diets with nutrients such as fish oil and potassium.
To standardize the J-DASH diets, the meals were prepared by Maruha Nichiro (Tokyo), and frozen packages of 10-day frozen packs for 2 weeks were home-delivered every 2 weeks to each participant to be consumed at lunch and/or dinner each weekday, every week for 2 months ( Supplemental Fig.1 ) . All of the participants were allowed to eat anything they wanted on the weekends. The participants were allowed to drink (including caffeinated beverages and alcoholic beverages) as usual throughout the study period. For each day of the controlled feeding, the participants recorded their intake of discretionary items (beverages and salt). They recorded whether they ate any non-study foods and whether they did not eat all of the study foods.
The same commercial cereal, Calbee Frugra ® , was provided as the breakfast meal to all participants, including the usual-diet group. Calbee Frugra ® is granola made from oats, rye, and other grains in combination with several different types of fruit. Fifty grams (219 kcal, protein 4.1 g, lipids 7.6 g, sugar 31.4 g, dietary fiber 4.5 g, salt 0.2 g, K 137 mg, and phosphate 98 mg) of the cereal were prepared for all participants, once a day for breakfast, 5 days/week for 2 months during the intervention phase.
Randomization
During the 1-month screening and run-in phase, the eligible participants consumed their normal diets. If an individual satisfied the inclusion criteria and did not violate any exclusion criteria based on the average BP values and other screening information including blood chemistry, the matched participants were then randomly assigned to one of the three diets. The participants learned of their diet assignments on the day of the first lifestyle modification lecture, before entering the intervention phase.
The randomization was done centrally at Yamaguchi University Hospital. The treatment random allocation sequence was generated by the study programmer. The randomization was stratified with minimization by using a dynamic allocation method for key prognostic factors: hypertension stage 1) , diabetes, and smoking habit. If the eligibility of the individual was confirmed, he or she was notified of the allocated treatment, and the participant was randomly assigned to one of the following arms in a 1:1:1 ratio: the usual control diet, the J-DASH 1 diet, or the J-DASH 2 diet. The allocation was essentially concealed from the investigators and staff of Onaka Hospital until the study was finished.
According to the study protocol, a systolic BP value >160 mmHg or a diastolic BP value >90 mmHg at a single visit was considered to necessitate a second measurement; if the reading was sustained, the participant was referred to his or her physician for further evaluation and treatment. The participants suspected of having secondary hypertension, especially primary aldosteronism based on a blood sampling (plasma aldosterone concentration to plasma renin activity [ng/mL/hr] ratio >200 and a plasma aldosterone concentration >120 pg/mL) 1) were also referred to a hypertension specialist for further examination and treatment, and they were excluded from the study.
Diet Treatments
The participants and the dietary staff were unaware of the outcome data; the personnel involved in the collection of the outcome data were unaware of the participants’ diet assignments. We assessed the participants’ adherence to the diets by reviewing their daily food diaries, and by having them eat their weekday lunches or dinners on-site. Side effects were monitored by means of questionnaires regarding symptoms and illnesses.
During the 2-month intervention phase, the participants followed their assigned diets. During the last 2 weeks of the intervention phase, a pair of clinic BP measurements was obtained, and the symptom and behavior change scale questionnaire and physical-activity-recall questionnaire were administered once each. All of the participants were then given a 1-hr lifestyle modification lecture before randomization.
During the 4-month follow-up phase, the participants followed their usual diets. During the last 2 weeks of the follow-up phase, staff members who were blinded to the diet assignments measured the clinic BP of each participant. A pair of clinic BP measurements was obtained, and the symptom questionnaire and physical-activity-recall questionnaire were administered.
References
1)Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S and Japanese Society of Hypertension Committee for Guidelines for the Management of H: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-390
2)Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH and Karanja N: A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med, 1997; 336: 1117-1124
3)Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH and Group DA-SCR: Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med, 2001; 344: 3-10
4)Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM and American Heart A: Dietary approaches to prevent and treat hypertension: A scientific statement from the American Heart Association. Hypertension, 2006; 47: 296-308
5)Kawamura A, Kajiya K, Kishi H, Inagaki J, Mitarai M, Oda H, Umemoto S and Kobayashi S: Effects of the DASH-JUMP dietary intervention in Japanese participants with high-normal blood pressure and stage 1 hypertension: An open-label single-arm trial. Hypertens Res, 2016; 39: 777-785
6)Imai Y, Kario K, Shimada K, Kawano Y, Hasebe N, Matsuura H, Tsuchihashi T, Ohkubo T, Kuwajima I, Miyakawa M and Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at H: The Japanese Society of Hypertension Guidelines for Self-monitoring of Blood Pressure at Home (Second Edition). Hypertens Res, 2012; 35: 777-795
References
- 1).Miura K, Nakagawa H, Ohashi Y, Harada A, Taguri M, Kushiro T, Takahashi A, Nishinaga M, Soejima H, Ueshima H and Japan Arteriosclerosis Longitudinal Study G: Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: a meta-analysis of 16 cohort studies. Circulation, 2009; 119: 1892-1898 [DOI] [PubMed] [Google Scholar]
- 2).Asayama K, Ohkubo T, Yoshida S, Suzuki K, Metoki H, Harada A, Murakami Y, Ohashi Y, Ueshima H, Imai Y and Japan Arteriosclerosis Longitudinal Study g: Stroke risk and antihypertensive drug treatment in the general population: the Japan arteriosclerosis longitudinal study. J Hypertens, 2009; 27: 357-364 [DOI] [PubMed] [Google Scholar]
- 3).Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, Sugihara H, Morita Y, Hirose K, Okayama A, Miura K and Ueshima H: Ischemic stroke subtypes in a Japanese population: Takashima Stroke Registry, 1988-2004. Stroke, 2010; 41: 1871-1876 [DOI] [PubMed] [Google Scholar]
- 4).Gardener H, Rundek T, Wright CB, Elkind MS and Sacco RL: Dietary sodium and risk of stroke in the northern Manhattan study. Stroke, 2012; 43: 1200-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH and Group DA-SCR: Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med, 2001; 344: 3-10 [DOI] [PubMed] [Google Scholar]
- 6).Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM and American Heart A: Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension, 2006; 47: 296-308 [DOI] [PubMed] [Google Scholar]
- 7).He FJ, Li J and Macgregor GA: Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ, 2013; 346: f1325 [DOI] [PubMed] [Google Scholar]
- 8).(UNESCO) UNESaCO: Washoku, Traditional Dietary Cultures of the Japanese, Notably for the Celebration of New Year. [Google Scholar]
- 9).Kawamura A, Kajiya K, Kishi H, Inagaki J, Mitarai M, Oda H, Umemoto S and Kobayashi S: Effects of the DASH-JUMP dietary intervention in Japanese participants with high-normal blood pressure and stage 1 hypertension: an open-label single-arm trial. Hypertens Res, 2016; 39: 777-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Turnbull F and Blood Pressure Lowering Treatment Trialists C: Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet, 2003; 362: 1527-1535 [DOI] [PubMed] [Google Scholar]
- 11).Parati G, Ochoa JE, Lombardi C and Bilo G: Assessment and management of blood-pressure variability. Nat Rev Cardiol, 2013; 10: 143-155 [DOI] [PubMed] [Google Scholar]
- 12).Rothwell PM: Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet, 2010; 375: 938-948 [DOI] [PubMed] [Google Scholar]
- 13).Webb AJ, Fischer U, Mehta Z and Rothwell PM: Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet, 2010; 375: 906-915 [DOI] [PubMed] [Google Scholar]
- 14).de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J and Mamelle N: Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications After Myocardial Infarction: Final Report of the Lyon Diet Heart Study. Circulation, 1999; 99: 779-785 [DOI] [PubMed] [Google Scholar]
- 15).Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM and OmniHeart Collaborative Research G: Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA, 2005; 294: 2455-2464 [DOI] [PubMed] [Google Scholar]
- 16).Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA and Investigators PS: Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med, 2013; 368: 1279-1290 [Google Scholar]
- 17).Mozaffarian D and Rimm EB: Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA, 2006; 296: 1885-1899 [DOI] [PubMed] [Google Scholar]
- 18).Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR and Kok FJ: Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens, 2002; 20: 1493-1499 [DOI] [PubMed] [Google Scholar]
- 19).Minihane AM, Armah CK, Miles EA, Madden JM, Clark AB, Caslake MJ, Packard CJ, Kofler BM, Lietz G, Curtis PJ, Mathers JC, Williams CM and Calder PC: Consumption of Fish Oil Providing Amounts of Eicosapentaenoic Acid and Docosahexaenoic Acid That Can Be Obtained from the Diet Reduces Blood Pressure in Adults with Systolic Hypertension: A Retrospective Analysis. J Nutr, 2016; 146: 516-523 [DOI] [PubMed] [Google Scholar]
- 20).Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S and Japanese Society of Hypertension Committee for Guidelines for the Management of H: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-390 [Google Scholar]
- 21).Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H and Hashimoto T: A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens, 2002; 16: 97-103 [DOI] [PubMed] [Google Scholar]
- 22).Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K and Japan EPAlisI: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 23).Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y and Investigators J: Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb, 2011; 18: 99-107 [DOI] [PubMed] [Google Scholar]
- 24).Ohnishi H and Saito Y: Eicosapentaenoic acid (EPA) reduces cardiovascular events: relationship with the EPA/arachidonic acid ratio. J Atheroscler Thromb, 2013; 20: 861-877 [DOI] [PubMed] [Google Scholar]
- 25).Imai Y, Kario K, Shimada K, Kawano Y, Hasebe N, Matsuura H, Tsuchihashi T, Ohkubo T, Kuwajima I, Miyakawa M and Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at H: The Japanese Society of Hypertension Guidelines for Self-monitoring of Blood Pressure at Home (Second Edition). Hypertens Res, 2012; 35: 777-795 [DOI] [PubMed] [Google Scholar]
- 26).Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS and Walker A: A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change. Health Technol Assess, 2002; 6: 1-231 [DOI] [PubMed] [Google Scholar]
- 27).Frisoli TM, Schmieder RE, Grodzicki T and Messerli FH: Beyond salt: lifestyle modifications and blood pressure. Eur Heart J, 2011; 32: 3081-3087 [DOI] [PubMed] [Google Scholar]
- 28).Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NRC, Li Q, Lackland DT, Leung AA, Anderson CAM, MacGregor GA and He FJ: Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ, 2020; 368: m315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Umemoto S, Ogihara T, Rakugi H, Matsumoto M, Kitagawa K, Shimada K, Higaki J, Ito S, Suzuki H, Ohashi Y, Saruta T, Matsuzaki M and Combination Therapy of Hypertension to Prevent C: Effects of a benidipine-based combination therapy on the risk of stroke according to stroke subtype: the COPE trial. Hypertens Res, 2013; 36: 1088-1095 [DOI] [PubMed] [Google Scholar]
- 30).Umemoto S, Ogihara T, Matsuzaki M, Rakugi H, Ohashi Y, Saruta T and Combination Therapy of Hypertension to Prevent Cardiovascular Events CTG: Effects of calcium channel blocker-based combinations on intra-individual blood pressure variability: post hoc analysis of the COPE trial. Hypertens Res, 2016; 39: 46-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Mozaffarian D, Gottdiener JS and Siscovick DS: Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol, 2006; 97: 216-222 [DOI] [PubMed] [Google Scholar]
- 32).Graudal NA, Hubeck-Graudal T and Jurgens G: Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev, 2017; 4: CD004022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).He FJ, Li J and Macgregor GA: Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev, 2013; CD004937 [DOI] [PubMed] [Google Scholar]
- 34).Chen J, Gu D, Jaquish CE, Chen CS, Rao DC, Liu D, Hixson JE, Hamm LL, Gu CC, Whelton PK, He J and GenSalt Collaborative Research G: Association between blood pressure responses to the cold pressor test and dietary sodium intervention in a Chinese population. Arch Intern Med, 2008; 168: 1740-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Yamori Y, Nara Y, Mizushima S, Sawamura M and Horie R: Nutritional factors for stroke and major cardiovascular diseases: international epidemiological comparison of dietary prevention. Health Rep, 1994; 6: 22-27 [PubMed] [Google Scholar]
- 36).Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T and Kimura G: Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet, 1997; 350: 1734-1737 [DOI] [PubMed] [Google Scholar]
- 37).He FJ and MacGregor GA: Role of salt intake in prevention of cardiovascular disease: controversies and challenges. Nat Rev Cardiol, 2018; 15: 371-377 [DOI] [PubMed] [Google Scholar]
- 38).Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA and group Pc: CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ, 2016; 355: i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Nakamura M and Ojima T: A modified DASH diet is one possible solution for overcoming the unfavorable link between vegetable and salt intake in the Japanese diet. Hypertens Res, 2016; 39: 756-757 [DOI] [PubMed] [Google Scholar]
- 40).Yamagishi K and Iso H: The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol Health, 2017; 39: e2017003 [DOI] [PMC free article] [PubMed] [Google Scholar]