Abstract
Background
Self-medication is the first option and response to most illness episodes. Use of antimicrobials without health care professionals’ guidance may result in greater probability of inappropriate use, missed diagnosis, delays in appropriate treatment, pathogen resistance and increased morbidity. There is no sector in the health care community which is immune to drug abuse or misuse of which the worst offenders include physicians, nurses and pharmacy professionals. Self-medication among health care professionals may be an indicator that the health professional is neglecting his or her own health. This represents serious issues for both patients and the professionals.
Objective
To assess self-medication practices with antibiotics among health care professionals in selected hospitals of Addis Ababa, Ethiopia.
Method
Facility based cross-sectional study was conducted from April to May, 2017 among 317 health care professionals. Convenient sampling technique was used to select study participants. Data were collected through self-administered questionnaire and analyzed using Statistical Package for Social Sciences software version 20. Binary logistic regression analysis was done to check the relationship between the dependent variable (antibiotic self-medication) and selected independent variables (sex, age, marital status, income, professional qualification and work experience).
Results
The prevalence of self-medication with antibiotics among health care professionals in one month recall period was found to be 72 (22.7%). The main reasons given for this practice were being familiar with the treatment options, 31 (43.1%) and need for rapid relief, 25 (34.7%). Respiratory problems, 29 (40.3%) and gastro intestinal problems, 28 (38.9%) were the most common illnesses for which self-medication with antibiotics was practiced while penicillins, 30 (41.6%) and fluoroquinolones, 29 (40.3%) constituted the two most commonly used antibiotics for the same. None of the variables had significant association with the practice of self-medication with antibiotics.
Conclusion
Self-medication with antibiotics was common among the study participants. Efforts should be made by health authorities including Drug and Therapeutics Committee, Drugs Regulatory Authority, Hospitals’ management and other stakeholders to ensure safe usage of antibiotics.
Keywords: Self-medication, Antibiotics, Health care professionals, Public hospitals, Addis Ababa
Self-medication, Antibiotics, Health Care Professionals, Public hospitals, Addis Ababa.
1. Introduction
World Health Organization (WHO) defined self-medication as the selection and use of medicines to manage self-diagnosed illnesses or symptoms (WHO, 1998). Self-medication is performed all across the globe, despite limits and effective regulation in some developed countries (Arikpo et al., 2009). This practice, particularly with antibiotics, is widespread around the world, with up to 68 percent in 20 European nations (Bretagne et al., 2006). A survey conducted in Lithuania revealed that one quarter (24.9%) of respondents had engaged in self-Medication with Antibiotics (SMA), despite the national rule that designated antibiotics as prescription-only medicines (Pavyde et al., 2015). The total prevalence of SMA in the Middle East ranged from 19% to 82%, with higher rates observed in Yemen, Oman, Saudi Arabia, United Arab Emirates, Syria, Iran, Jordan, Lebanon and Turkey (Alhomoud et al., 2017). A survey conducted on Mongolian children showed that 42.3% children used non-prescribed antibiotics for upper respiratory tract infection symptoms such as cough, fever or nasal and throat problems (Togoobaatar et al., 2010). Higher prevalence of SMA was also reported by a study conducted in Kuwait (92%) and Karachi (47.6%) (Abahussain et al., 2005; Jawad et al., 2014).
Studies from Africa showed that SMA was a common in various areas of the continent. It was found to be 48% among adult patients in Kenya (Kiragu et al., 2016) and 38.6% in Nigeria (Tamuno and Mohammed, 2011). Higher prevalence was also observed in Sudan (73.9%) (Awad et al., 2005). Coming to Ethiopia, study done in Mekelle showed that 44.5% of the participants practiced SMA (Eticha et al., 2014). The same practice was found in 18% and 26.4% of respondents in studies conducted in Bahir Dar and Addis Ababa, respectively (Andualem and Gebre-Mariam, 2004; Gebeyehu et al., 2015). Lack of access to health care, being a cheaper alternative, availability of numerous medicines Over The Counter (OTC) and poor drug regulatory practices are some of the reasons for the commonness of self-medication practice (including antibiotics) in many countries (Vaananen et al., 2006). Low satisfaction with medical practitioners as well as misunderstanding about the efficacy of medications given by health facilities also play their part (Abay and Amelo, 2010; Grigoryan et al., 2008; Radyowijati and Haak, 2003; Saradamma et al., 2000).
Although responsible self-medication is not discouraged, there are potential risks related to this practice including misdiagnosis, over dosage, extended exposure to medicines, medicines interactions, poly-pharmacy, risk of dependence, risk of abuse which may lead to toxicity and pharmacological risks associated with improper use of medicines (Khan et al., 2014; Gholap and Mohite, 2013; WHO, 2000). In developing countries self-medication usually leads to inadequate medicine use and is especially concerning when it involves prescription medicine such as antibiotics (Hussain et al., 2011). Problems related to SMA in these countries are complex as they are intertwined with other issues such as poverty, non-availability of medicines and lack of access to medicine related information, low quality of health facilities and weak implementation of medicines' regulations (Widayati et al., 2011). The consequences of SMA are severe; infections produced by treatment resistant pathogens, resulting in prolonged sickness and greater risk of mortality. Treatment failure also leads to longer period of infectivity which increases the number of infected individuals in the community and thus expose the general public to the risk of contracting resistant bacterial strains (Abasaeed et al., 2009). Despite being aware of the consequences and potential risks of medicines, the prevalence of self-medication practices with these agents is high among Health care Professionals (HCPs). Several studies revealed that the prevalence of self-medication practice among HCPs ranges between 24% and 96% (Ali et al., 2012; Gholap and Mohite, 2013; Harris et al., 2013; Hem et al., 2005; Pankaj et al., 2015). Most often HCPs find it difficult to enter into patient's role due to various reasons such as limited time, nature of illness, high drug expertise, concern about confidentiality, high ego and ease of access to medications and hence rely on self-medication (Ali et al., 2012). This practice was found to be much more common among physicians, nurses, and pharmacists than among the general population (Sharif et al., 2015). There is no sector of the health care community that is immune to medicine abuse or misuse; the worst offenders including physicians and pharmacists. A study from Malaysia revealed that physicians and pharmacists were the worst offenders as they have easy access to medications, have medical background and high self-confidence (Ali et al., 2012). Nursing workers, who frequently work many jobs, coupled with the intricacy of the work performed in hospitals, leads to the conclusion that these professionals confront difficult moments or crisis, making medication use a means to make their life easier (Reisrocha et al., 2009).
Self-medication by HCPs represents serious issues for both patients and the professionals. For the professionals, there is an obvious threat to their health of inappropriate subjective or delayed objective treatment. For the patient, a health care professional whose health is impaired is at risk of not being able to deliver care of the expected quality (Montgomery et al., 2011). In Ethiopia, there are indications on the misuse of antibiotics by HCPs, unskilled practitioners and drug consumers (DACA and MSH, 2009). Literatures and the practice showed that most antibiotics are easily available as OTC medicines in most retail pharmacies in the country (Beedemariam and Kaba, 2016). Eticha et al. (2014) found in a study done in Mekelle town, Ethiopia that amoxicillin was the most commonly used antibiotic for self-medication (43.3%), followed by ampicillin and ciprofloxacin. Since HCPs have knowledge about medicines, they might be expected to behave differently than the general public. Even though there is malpractice of SMA among HCPs in health facilities, studies conducted on this issue so far are limited and the prevalence of SMA among HCPs in Ethiopia is yet unknown. As a result, the goal of this study was to examine antibiotic-based self-medication among HCPs, with the findings potentially assisting in the development of appropriate regulatory and administrative solutions in Ethiopian hospitals in general and Addis Ababa in particular.
2. Methods
2.1. Study area and period
A Hospital based cross sectional study was conducted in Addis Ababa, capital city of Ethiopia from April to May, 2017 in four selected hospitals under Addis Ababa City Health Bureau (AACHB). The city is located at the center of the country and covers area of about 540 km2. The population size of Addis Ababa in 2018 was estimated to be more than 3.5 million (CSA, 2013). During the study period, the city had a total of 14 public hospitals and 37 private and Non-Governmental Organization hospitals (FMOH, 2014). Out of the 14 public hospitals, four were under the Federal Ministry of Health (FMOH), one University Hospital (Tikur Anbesa Specialized Hospital under Addis Ababa University) and three are Armed Force, Federal Prison and Federal Police hospitals. The remaining six hospitals were managed under AACHB. The total number of HCPs (physicians, nurses and pharmacists) working in the 6 hospitals managed under the city's health bureau was 1354 (FMOH, 2014).
2.2. Source and study population
The source population was all HCPs working in public hospitals under AACHB. The HCPs who were working in selected hospitals and who met the inclusion criteria were study population.
2.3. Inclusion and exclusion criteria
Physicians, pharmacists and nurses working in the selected hospitals, available during data collection period and volunteer to participate were included in the study. The remaining HCPs were excluded from the study.
2.4. Sample size determination and sampling procedure
2.4.1. Sample size determination
Sample size (ni) required for the study was calculated by using single population proportion formula (Kirkwood and Sterne, 2003). Since there is no study conducted regarding SMA among HCPs in Ethiopia previously the prevalence of SMA among HCPs (p) was taken as 0.5. The sample size ni was then calculated as:
Where, ni = initial sample size
z = statistic for 95% level of confidence = 1.96
d = precision/margin of error = 0.05
Based on estimation of the proportion from a finite population of size N, the final sample size was calculated as follows:
| nf = ni /(1+ ((ni -1)/N)) = 300 HCPs |
Where: nf = final sample size and
N = total number of HCPs working in public hospitals under AACHB (sampling frame) which was 1354.
Adding 10% for incomplete and non-responses, the total sample size required for this study was found to be 330. The number of HCPs included in the study from each hospital was decided based on proportionate to size method.
2.4.2. Sampling procedure
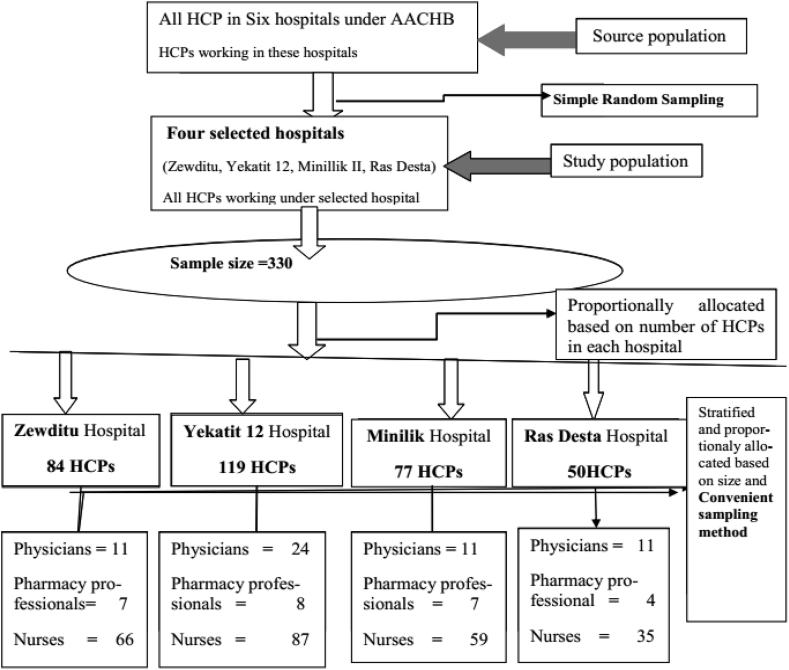
Multi-stage sampling technique was applied in the study (Figure 1). First, all the six hospitals under AACHB were listed and among them four hospitals (Zewditu, Yekatit 12, Ras Desta Damitew and Minillik II Memorial Hospital) were selected by using a lottery method. The total number of HCPs was retrieved from human resource department of the six hospitals and this data was used as a sampling frame. Then, the total sample size was calculated. The number of HCPs to be taken from each hospital was determined based on proportionate to size of the staff. After this, HCPs were further stratified into physicians, nurses and pharmacy professionals and the final sample size was allocated proportionally based on respective number of HCPs from each department in each hospital. Convenient sampling method was used to select the final sampling units.
Figure 1.
Sampling procedure for SMA among HCPs in public hospitals of Addis Ababa, April 2017.
2.5. Variables of the study
2.5.1. Dependent variable
Antibiotic self-medication
2.5.2. Independent variables
Sex, Age, Marital status, Income, Professional qualification, Work experience
2.6. Data collection method and instrument
Data were collected using a pre-tested self-administered questionnaire consisting 9 open and 18 closed ended questions. The tool was prepared in English language after reviewing studies done on the same issue (Abasaeed et al., 2009; Abdulraheem et al., 2016; Ali et al., 2012; Fadare and Tamuno, 2011; Pankaj et al., 2015). The tool included questions on socio-demographic characteristics of respondents, practice of self-medication, reasons for self-medication, type of antibiotics used and common indications for SMA. All items in the questionnaire were related to the recall period of one month. The duration of one month was chosen because of the belief that recall of medication use for HCPs is very reliable within this time frame. Other similar studies also utilized this same recall period (Awad et al., 2005; Sado and Gedif, 2014; Widayati et al., 2011; Worku and G/Mariam, 2003).
Two health professionals (one pharmacist and one nurse) were recruited for data collection. The principal investigator was also involved in data collection. The questionnaire was handed over to respondents in person. Some of the questionnaires were filled and returned on the same day and others after few days.
2.7. Data quality management
Before the start of actual data collection, the questionnaire was pre-tested on 15 HCPs working in one hospital in Addis Ababa and this facility was excluded from the actual study. A slight modification (word change) was made to some questions of data collection tool in light of the pretest made and the tool was then applied to data collection. Data collectors were trained by principal investigator for half day on how to give standard instruction, clarify questions, approach respondents, how to obtain informed consent and how to secure confidentiality during data collection. The collected data was checked by principal investigator on daily basis for accuracy and completeness.
2.8. Data processing and analysis
The collected data was checked for completeness and consistency and then cleaned, coded, entered and analyzed using Statistical Package for Social Sciences (SPSS) version 20. Descriptive statistics such as frequency and percentages were used to present the findings. Responses to open ended questions were grouped, coded, analyzed and presented with frequencies of similar responses.
Cross-tabulations and bivariate logistic regression analysis were performed to select variables for multivariable analysis. Variables with p-value ≤ 0.25 in the bivariate analysis were taken as candidates for multivariable logistic regression analysis. Finally, multivariable logistic regression was conducted to identify the independent determinants of SMA. Variables with a p-value ≤ 0.05 in the multivariable logistic regression were considered statistically significant predictors of SMA.
2.9. Ethical consideration
Ethical approval was obtained from Ethics Review Committee of School of Pharmacy, Addis Ababa University and AACHB. The selected hospitals were communicated with formal letter written from AACHB ethical review committee and data collection commenced after obtaining permission from the administrators of each hospital. Participants were asked for written consent before participating in the study. During the consent process, each participant was provided information regarding the purpose of the study, why and how they were selected for the involvement in the study, what was expected of them and the confidentiality of information acquired. Confidentiality was assured by not using any personal identifier in the questionnaire and analyzing data in aggregate. They were also informed about their full right to refuse participation in the study.
3. Results
A total of 330 questionnaires were distributed in four selected public hospitals managed under AACHB. Three hundred seventeen respondents, 81 (10 physicians, 6 pharmacy professionals and 65 nurses) from Zewditu Hospital, 114 (23 physicians, 7 pharmacy professionals and 84 nurses) from Yekatit 12 Hospital, 74 (9 physicians, 7 pharmacy professionals and 58 nurses) from Menelik II Hospital and 48 (11 physicians, 4 pharmacy professionals and 33 nurses) from Ras Desta Hospital properly filled and returned the questionnaire making a response rate of 96%.
3.1. Socio-demographic characteristics of the participants
One hundred sixty four (51.7%) of the 317 respondents were females. The mean age of the participants was 29.0 years (SD = 6.7, range: 20–55 years). The age of 223 (70.3%) participants was between 20 and 30 years. One hundred eighty nine (59.6%) patients were single and majority were nurses in occupation, 236 (74.4%). Majority, 263 (82.9%) were holders of first degree and above and had less than 5 years of professional work experience, 206 (65%). One hundred thirty nine participants (43.8%) had monthly income between 4001 and 7000 ETB (Table 1).
Table 1.
Socio-demographic characteristics of respondents in selected public hospitals of Addis Ababa, April 2017.
| Variables | Frequency | Percentage | |
|---|---|---|---|
| Sex | Male | 153 | 48.3 |
| Female | 164 | 51.7 | |
| Age (in years) | 20–29 | 223 | 70.3 |
| 30–39 | 65 | 20.5 | |
| 40–49 | 22 | 6.9 | |
| 50–59 | 7 | 2.2 | |
| Marital status | Single | 189 | 59.6 |
| Married | 120 | 37.8 | |
| Others∗ | 8 | 2.5 | |
| Monthly income (in ETB) | 1001–4000 | 82 | 25.9 |
| 4001–7000 | 139 | 43.8 | |
| 7001–10000 | 81 | 25.6 | |
| Above 10000 | 15 | 4.7 | |
| Professional qualification | Medical specialist | 143 | 45.1 |
| General Practitioner | 41 | 12.9 | |
| Pharmacist | 18 | 5.7 | |
| Druggist | 8 | 2.5 | |
| Nurse (BSc) | 183 | 57.7 | |
| Nurse (Diploma) | 46 | 14.5 | |
| Nurses (MSc) | 7 | 2.2 | |
| Work experience (in years) | ≤5 | 206 | 65.0 |
| 6–10 | 68 | 21.5 | |
| >10 | 43 | 13.6 | |
∗Divorced, widowed and separated ETB = Ethiopian Birr.
BSc = Bachelor of Science MSc = Master of Science.
3.2. Health care seeking behavior of respondents
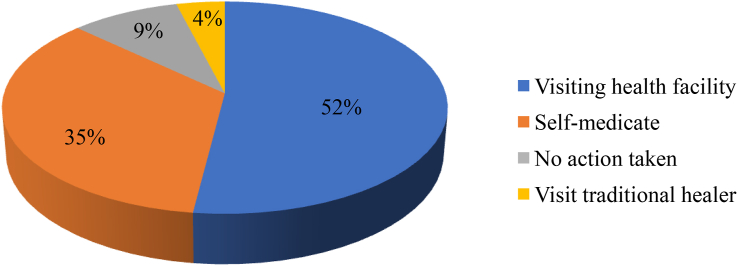
More than half of the study participants, 164 (51.7%) preferred visiting modern health facility as the first option when encountered illness while 111 (35.0%) preferred self-medication as the first choice for the same (Figure 2).
Figure 2.
Actions taken by respondents in case of illness in selected public hospitals of Addis Ababa, April 2017.
Of the total 317 respondents, 166 (52.4%) believed that they could successfully treat common infectious diseases by themselves. Regarding the safety of SMA, 148 (46.7%) of the participants believed that SMA is a safe practice. Eighty five (50.3%) of those who mentioned SMA is unsafe reported that the practice leads to the risk of developing resistance while 33 (19.5%) said it has risks of misdiagnosis. Believing that SMA is unethical, 20 (11.8%) and the need for laboratory investigation for most infectious diseases, 22 (13%) were also the claimed reasons for not considering SMA a safe practice. The respondents were also asked regarding the term ‘antibiotic resistance’; 204 (64.4%) claimed that they have knowledge about it and elaborated it in words, 61 (19.2%) said they have knowledge but could not elaborate and 52 (16.4%) reported they did not know about antibiotic resistance.
3.3. Prevalence of self-medication practices
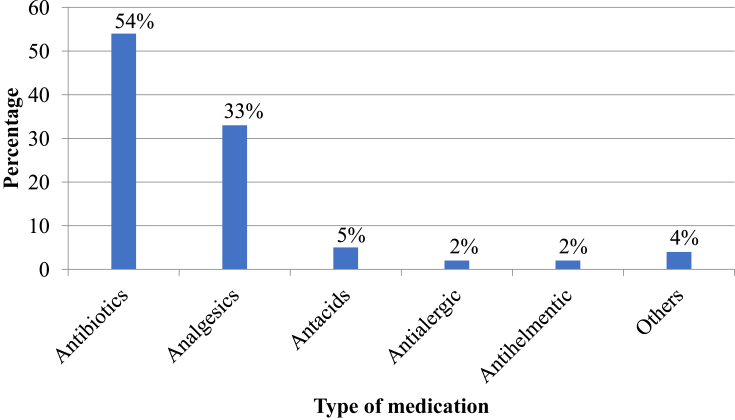
All study participants were asked whether they had taken any medicine without prescription within one month before the study. One hundred thirty three (41.9%) reported that they had self-medicated with modern medicines, the most common being antibiotics, 72 (54%) and analgesics, 44 (33%) (Figure 3).
Figure 3.
Category of medicines used for self-medication by HCPs in selected public hospitals of Addis Ababa, April 2017. Others: Oral Rehydration Salt, Ferrous sulphate + Folic acid, Sildenafil citrate.
The overall prevalence of SMA among HCPs in the current study was found to be 72(22.7%); 51(70.8%)for nurses, 13 (18.1%) for physicians and 8 (11.1%) for pharmacy professionals.
3.4. Patterns of antibiotics use for self-medication
Among those who practiced SMA, 67 (93.0%) self-medicated once and the remaining did it twice with different antibiotics in one month recall period. However, for better recall only the second time antibiotic use was analyzed to describe the patterns of SMA. About 19 (26.4%) of the respondents claimed to have experienced side effects such as of those who preferred self-medication, 71 (63.9%) reported to commonly use analgesics while 46 (41.4%) used antibiotics (Table 2). Diarrhea, vomiting, gastritis, nausea, dizziness and rush after taking antibiotics and 6 of those who experienced side effects said they have stopped taking antibiotics while one reported to have reduced dose in response to the side effects experienced. Among all respondents who self-medicated with antibiotics, 33 (45.8%) said they did not take full dose mentioning feeling better in few days, 21 (63.6%) and encountering side effects, 12 (36.4%) as the main reasons.
Table 2.
Types of medicines used in self-medication in selected public hospitals of Addis Ababa, April 2017.
| Category of drugs | Frequency | Percentage |
|---|---|---|
| Analgesics | 71 | 63.9 |
| Antibiotics | 46 | 41.4 |
| Anti-acids | 10 | 9.0 |
| Others∗∗ | 4 | 3.6 |
| Could not specify | 2 | 1.8 |
NB: Multiple responses are possible.
∗∗anti-helminthics and anti-protozoals.
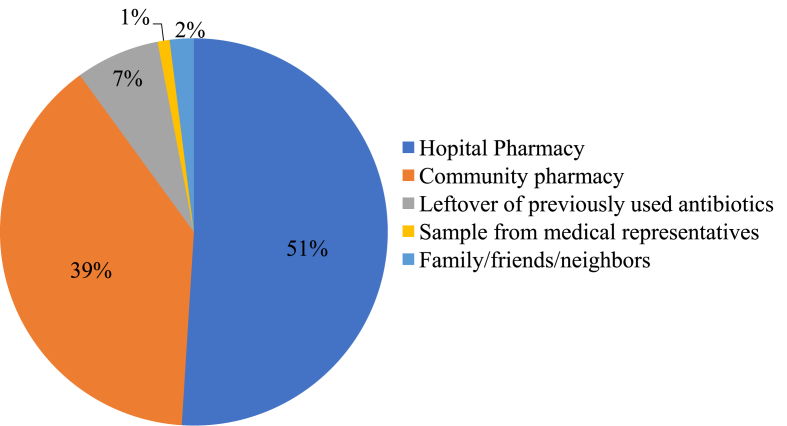
Regarding sources of antibiotics used for self-medication, majority of respondents, 37 (51.4%) reported that they obtained the medicines from hospital pharmacies and 28 (38.9%) said they got from community pharmacies (Figure 4).
Figure 4.
Sources of antibiotics used for self-medication among HCPs in selected public hospitals of Addis Ababa, April 2017.
3.5. Reasons for antibiotic self-medication
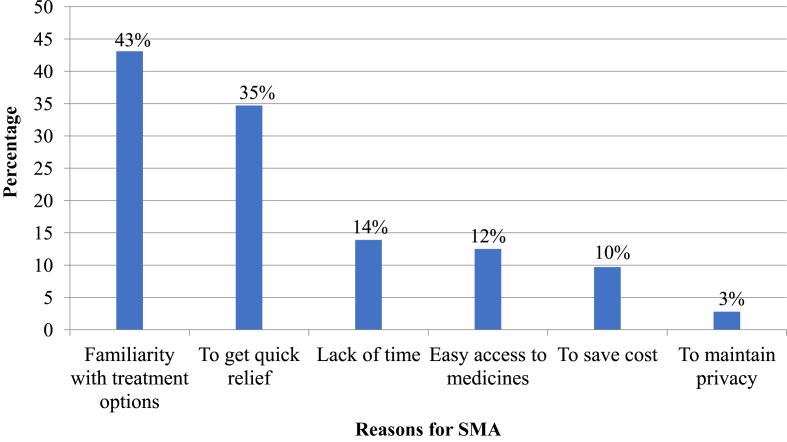
Being familiar with treatment, 31 (43.1%) and need for quick relief, 25 (34.7%) were the most common reasons for HCPs to practice SMA (Figure 5).
Figure 5.
Reasons for self-medication with antibiotics (SMA) among HCPs in selected public hospitals of Addis Ababa, April 2017. NB: Multiple responses are possible.
3.6. Perceived illnesses for which antibiotics are used for self-medication
The main perceived illnesses against which SMA were practiced were respiratory problems, 29 (40.3%) and gastro intestinal problems, 28 (38.9%) (Table 3).
Table 3.
Perceived illnesses against which HCPs self-medicated with antibiotics in selected public hospitals of Addis Ababa, April 2017.
| Perceived illnesses | Frequency | Percentage |
|---|---|---|
| Respiratory problems | 29 | 40.3 |
| Gastro intestinal problems | 28 | 38.9 |
| Skin problems | 3 | 4.2 |
| Urinary tract problems | 6 | 8.3 |
| Unidentified cases | 6 | 8.3 |
| Total | 72 | 100.0 |
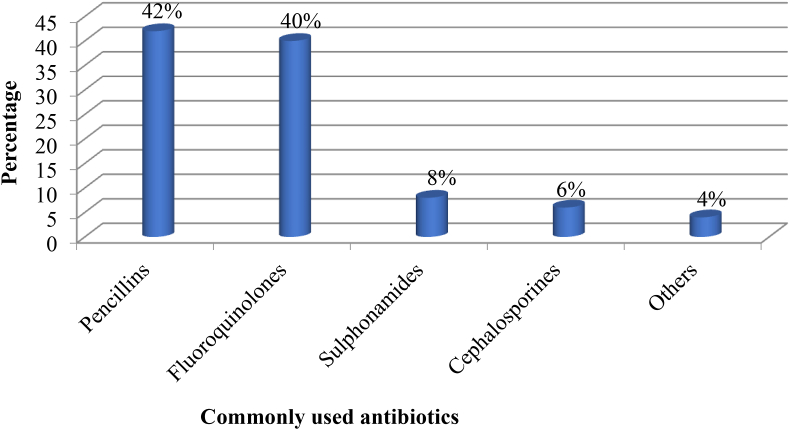
Penicillins, 30 (41.6%) and fluoroquinolones, 29 (40.3%) were the most commonly used antibiotics without prescription (Figure 6). Thirty five (48.6%) of the participants practicing SMA reported that they got cured from their illness/s and 22 (30.5%) said there was improvement in their illness/s. The remaining respondents, 15 (20.8%) said they were neither cured nor improved after SMA.
Figure 6.
Antibiotics commonly used for self-medication by HCPs in selected public hospitals of Addis Ababa, April 2017. Others: Tetracycline, Aminoglycosides and Macrolides.
3.7. Suggested solutions to reduce antibiotics self-medication
All of the study participants were asked open ended questions to suggest the possible solution and recommendations in order to tackle or reduce SMA. More than half the study participants, 165 (52%) said that creating and increasing awareness about Anti-Microbial Resistance (AMR) is essential. One hundred thirty six (43%) respondents mentioned that enforcing strict rules and regulations on the sale of antibiotics is necessary. Thirty eight (12%) respondents said pharmacists should not dispense self-prescribed antibiotics while 25 (8%) pointed all medical college and university students should be given intensive course on AMR before graduation.
3.8. Factors associated with self-medication practices with antibiotics
None of the socio-demographic factors tested in multivariable logistic regression were found to be associated with HCPs SMA (Table 4).
Table 4.
Binary logistic regression analysis result on SMA among HCPs in selected public hospitals of Addis Ababa, April 2017.
| Variables | Self-Medication with Antibiotics |
95%CI |
|||
|---|---|---|---|---|---|
| Yes | No | COR | AOR | ||
| Sex | Male | 33 (45.8%) | 25 (41.0%) | 1.00 | 1.00 |
| Female | 39 (54.2%) | 36 (59.0%) | 0.82 [0.41–1.63] | 1.50 [0.70–3.30] | |
| Age (in years) | 20–29 | 56 (77.8%) | 39 (63.9%) | 1.00 | 1.00 |
| 30–39 | 13 (18.1%) | 14 (22.9%) | 0.26 [0.06–1.05] | 1.90 [0.50–6.70] | |
| 40–49 | 3 (4.1%) | 8 (13.1%) | 0.40 [0.09–1.86] | 0.76 [0.90–6.40] | |
| Marital status | Single | 43 (59.7%) | 32 (52.4%) | 1.00 | 1.00 |
| Married | 29 (40.3) | 29 (47.5%) | 1.30 [0.70–2.70] | 1.00 [0.40–2.40] | |
| Monthly income (ETB) | 1,001–4,000 | 14 (19.4%) | 16 (26.2%) | 1.14 [0.24–5.44] | 4.80 [0.30–7.87] |
| 4,001–7,000 | 36 (50.0%) | 22 (36.1) | 0.61 [0.14–2.69] | 2.80 [0.20–33.1] | |
| 7,001–10,000 | 18 (25%) | 19 (31.1%) | 1.06 [0.23–4.87] | 2.20 [0.30–15.6] | |
| Above 10,000 | 4 (5.5%) | 4 (6.5%) | 1.00 | 1.00 | |
| Profession | Physicians | 13 (18.0%) | 14 (22.9%) | 1.00 | 1.00 |
| Pharmacy professionals | 8 (11.1%) | 4 (6.5%) | 1.28 [0.54–3.01] | 3.00 [0.60–15.50] | |
| Nurses | 51 (70.8%) | 43 (70.5%) | 0.59 [0.17–2.10] | 0.80 [0.20–3.40] | |
| Education | Diploma | 6 (8.3%) | 12 (19.7%) | 1.00 | 1.00 |
| First degree | 60 (83.3%) | 44 (61.1%) | 0.37 [0.13–1.05] | 0.30 [0.10–1.30] | |
| Above first degree | 6 (8.3%) | 5 (8.2%) | 0.42 [0.09–1.94] | 0.30 [0.04–2.30] | |
| Experience (in years) | 0–5 | 51 (70.8%) | 36 (59.0) | 1.00 | 1.00 |
| 6–10 | 13 (18.0%) | 15 (24.6%) | 0.56 [0.20–1.57] | 1.60 [0.50–5.50] | |
| Above 10 | 8 (11.1) | 10 (16.4%) | 0.92 [0.28–3.03] | 1.10 [0.20–7.30] | |
ETB: Ethiopian Birr COR: Crude Odds Ratio.
CI: Confidence Interval AOR: Adjusted Odds Ratio.
4. Discussion
Self-medication is common all over the world (Phalke and Durgawale, 2006). Taking non-prescription medicines is the initial response in almost half of all illness episodes, particularly for symptoms viewed as non-serious (Lau et al., 2000). In this study, the prevalence of SMA among HCPs (physicians, nurses and pharmacy professionals) in public hospitals under AACHB was investigated. Accordingly, 111 (35.0%) of the study participants reported they would prefer self-medication before taking any other action in case of illness. This finding is lower compared to a study from China where 45.4% selected self-medication as the first action in case of discomfort (Lei et al., 2018).
The current study revealed that respondents would prefer to commonly use analgesics and antibiotics for self-medication. This finding is similar to the results from other studies (Abay and Amelo, 2010; Baruzaig and Bashrahil, 2008; Belachew et al., 2011; Sajith et al., 2017; Sawalha, 2007). It is understood that majority of analgesics are available as Over The Counter (OTC) medicines in retail outlets. However, the common use of antibiotics for self-medication is alarming in the face of increasing threat of antibiotics resistance (Gebrekidan et al., 2015; Mekuria et al., 2017; Terfassa and Jida, 2018).
The prevalence of SMA among HCPs was found to be 22.7% in the current study. This is lower compared to the result of studies done in India (88.6%) and Nigeria (38.6%) (Fadare and Tamuno, 2011; Pankaj et al., 2015). It is also lower than the finding of a study from Nekemte town, Western Ethiopia that showed a prevalence of 33% (Sado and Gedif, 2014). But it is higher than a study from Malaysia which showed a prevalence of 6.7% (Ali et al., 2012). The variation between the finding of the current study and the comparatives could be due to the difference in methodology and recall period used.
When seen profession wise, the prevalence of SMA among pharmacy professionals was 30.8%, 23.6% among physicians and 21.6% among nurses similar to the findings from India (Pankaj et al., 2015). Pharmacists and Physicians are among the HCPs with the greatest access to medications and have impressive knowledge of prescription drugs and their use in the treatment of various drug therapies which increase the potential of self-medication (Ali et al., 2012; Balbisi and Ambizas, 2005). Other studies from India (with six months recall period) revealed SMA prevalence of 53.5% among Nurses and 53% among physicians (Gholap and Mohite, 2013; Nalini, 2010); finding of the current study is lower compared to these literatures and the difference might be related to short recall period used in the present study.
The current study revealed that 37 (51.4%) of the respondents obtained antibiotics used in self-medication from hospital pharmacies dissimilar to the other studies that showed most respondents obtaining their drugs from community pharmacies (Afolabi et al., 2014; Donkor et al., 2012; Heidarifar et al., 2013; Kiragu et al., 2016). The dissimilarity might be due to the difference in study populations between the current study and the comparative studies.
Self-medication among HCPs in this study appeared to be more driven by knowledge of diagnosis and treatment options congruent to other findings (Ali et al., 2012; Gholap and Mohite, 2013; Harris et al., 2013; Pankaj et al., 2015). The professionals have imposed their subjective judgment in determining both their own diagnosis and treatment when they get sick. Lack of time was also the other reason for practicing SMA. High patient load and absence of functional separate system for HCPs may predispose them to self-medicate instead of consulting a doctor for it. Easy access to medicines was also reported as a reason for SMA (Abdulraheem et al., 2016; Donkor et al., 2012; Harris et al., 2013). The present study also reflected the same although not to a significant proportion.
Regarding disease conditions treated with SMA, similarities were observed between the current study and other studies (Abasaeed et al., 2009; Pant et al., 2015; Shankar et al., 2002; Widayati et al., 2011). Respiratory problems and gastro intestinal problems were the main illnesses for which SMA was practiced in the current study similar to study from Indonesia and Iran (Heidarifar et al., 2013; Sarahroodi et al., 2010; Widayati et al., 2011). Respiratory problems are mostly due to viral infections, which do not require antibiotics as they can be managed by home remedies and some OTC drugs but such self-treatment may contribute to antibiotic resistance (Linder and Stafford, 2001).
Penicillins and fluoroquinolones (ciprofloxacin in particular) were the most commonly used antibiotics for self-medication in the current study. This finding is consistent with studies from Europe, India, Iran and Sierra Leone (Afolabi et al., 2014; Eticha et al., 2014; Grigoryan et al., 2008; Pankaj et al., 2015). Amoxicillin was also reported to be widely used in this study similar to the findings reported by studies from other countries (Abasaeed et al., 2009; Donkor et al., 2012; Eticha et al., 2014; Gunawardhana et al., 2015). Study from Ethiopia also reported amoxicillin to be among the commonly sold antibiotics in community pharmacies for self-medication (Beedemariam and Kaba, 2016). Such antibiotics utilization opens doors for antibiotics resistance (Mekuria et al., 2017) and therefore health care professionals are expected to carry out their responsibility for eliminating or reducing AMR rather than contributing for the same.
The current study revealed that 45.8% of respondents did not complete full course of antibiotics. This finding is higher than the result of study conducted in Iran (Sarahroodi et al., 2010). It is widely believed that human malpractices such as inadequate dosing, incomplete courses and indiscriminate drug use have contributed to the emergence and spread of AMR. The consequence of this is the switch from relatively cheap to new drugs. Resorting to the new and more expensive drugs to fight microbial resistance leads to additional problems for resource poor countries (WHO, 2001). Thus, rational use of antibiotics is of utmost importance to limit the emerging AMR prevalence. As HCPs are highly likely to affect the behavior of their patients towards rational use of medicines, they should be model and teach the public about self-medication and AMR.
Previous studies reported mixed results about the association of SMA and gender. While some found significant and positive association between SMA and gender (Abasaeed et al., 2009; Abdulraalheem et al., 2016; Awad et al., 2005), other studies didn't show significant association (Donkor et al., 2012; Fadare and Tamuno, 2011). Reports also revealed that participant's age and level of education were significantly associated with the practice of SMA (Abasaeed et al., 2009; Abdulraheem et al., 2016; Awad et al., 2005; Kiragu et al., 2016; Osemene and Lamikanra, 2012). Unlike the above findings, the current study showed that none of the socio-demographic variables were significantly associated with the practice of SMA just in line with the findings from Indonesia, Pakistan and United Arab Emirates (Jawad et al., 2014; Shehnaz et al., 2013; Widayati et al., 2011).
5. Conclusion
Self-medication with antibiotics was common among the study participants. Respiratory tract problems, gastro-intestinal problems, skin problems and urinary tract infections were the major illnesses treated with self-medication using antibiotics, mainly penicillins and floroquinolones. There was no significant association between SMA and socio-demographic characteristics of the study participants. In general, potentially dangerous effects of SMA seem to be underestimated by HCPs. Efforts should be made by health authorities including Drug and Therapeutic Committee, drug regulatory authority, hospitals’ management and other stakeholders to ensure safe usage of antibiotics.
5.1. Study limitations
The study was conducted in a specific context (health care professionals working in hospitals under AACHB only). Hence, more studies in different contexts with larger numbers would be helpful to further validate the findings. The analysis was also based on self-reporting of health care professionals resulting in the possibility of over and under-reporting. There might also be under or over representation of the population due to the type of the sampling technique used (i.e convenience sampling).
Declarations
Author contribution statement
Tsehay Kassa: Conceived and designed the study; Performed the study; Analyzed and interpreted the data; Wrote the paper.
Teferi Gedif and Tenaw Andualem: Analyzed and interpreted the data; Contributed materials, analysis tools or data; Wrote the paper.
Temesgen Aferu: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the graduate program of Addis Ababa University.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are very much grateful to all study participants for their kind cooperation/participation in the study. We are also grateful to AACHB staffs and the administration of each studied Hospital for the contributions they made to this study.
References
- Abahussain E., Matowe K., Nicholls P. Self- reported medication use among adolescents in Kuwait. Med. Princ. Pract. 2005;14(3):161–164. doi: 10.1159/000084633. [DOI] [PubMed] [Google Scholar]
- Abasaeed A., Vicek J., Abuelkhair M., Kubena A. Self-medication with antibiotics by the community of Abu Dhabi Emirate, United Arab Emirates. J. Infect. Dev. Ctries. 2009;3(7):491–497. doi: 10.3855/jidc.466. [DOI] [PubMed] [Google Scholar]
- Abay S., Amelo W. Assessment of self-medication practices among medical, pharmacy and health science students in gondar university. Ethiopia. J. Young Pharm. 2010;2(3):306–310. doi: 10.4103/0975-1483.66798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulraheem I., Adegboye A., Fatiregun A. Self-medication with Antibiotics: empirical evidence from Nigerian rural population. BJPR. 2016;11(5):1–13. [Google Scholar]
- Afolabi M., Macarthy L., Osemene K. Use of antimicrobial medicines among university students in Sierra Leone. BJPR. 2014;4(1):101–112. [Google Scholar]
- Alhomoud F., Aljamea Z., Almahasnah R., Alkhalifah K., Basalelah L., Alhomoud F. Self-medication and self-prescription with antibiotics in the Middle East do they really happen? A systemic review of the prevalence, possible reasons and outcomes. IJID. 2017:1–44. doi: 10.1016/j.ijid.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Ali A.N., Tiong J., Kai T.K., Keat C.C., Dhanaraj S.A. Self-medication practices among health care professionals in a Private University, Malaysia. Int. Curr. Pharmaceut. J. 2012;1(10):302–310. [Google Scholar]
- Andualem T., Gebre-Mariam T. Self-medication practices in Addis Ababa: a prospective study. Ethiop. J. Health Sci. 2004;14(1):1–11. [Google Scholar]
- Arikpo G., Eja M., Idoh K. Self medication in rural Africa: the Nigerian experience. Internet J. Health. 2009;11(1):1–7. [Google Scholar]
- Awad A., Eltayeb I., Matowe L., Thalib L. Self-medication with antibiotics and antimalarials in the community of Khartoum state, Sudan. J. Pharm. Pharmaceut. Sci. 2005;8(2):326–331. [PubMed] [Google Scholar]
- Balbisi E., Ambizas E. Self- prescribing of non-controlled substances among pharmacists. Am. J. Health Syst. Pharm. 2005;62(23):2508–2511. doi: 10.2146/ajhp050007. [DOI] [PubMed] [Google Scholar]
- Baruzaig A., Bashrahil K. Self-medication: concept, prevalence & risks in Mukalla City (Yemen) 2004-2005. Andalus Stud. Res. 2008;2:1–3. [Google Scholar]
- Beedemariam G., Kaba M. Exploration of over the counter sales of antibiotics in community pharmacies of Addis Ababa, Ethiopia: pharmacy professionals’ perspective. Antimicrob. Resist. Infect. Control. 2016;5(2):1–7. doi: 10.1186/s13756-016-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew G., Alemayehu D., Abebe Z., Fikadu D., Hadgu A., Ghezu M., Solomon G., Gebre Samuel N., Yarlagadda R., Wondimu A. Self-medication practices among health sciences students: the case of Mekelle University. JAPS. 2011;1(10):183–189. [Google Scholar]
- Bretagne J., Molyoivd B., Honnorat C. Gastroesophageal reflux in the French general population: national survey of 8000 adults. Med. J. 2006;35:23–31. doi: 10.1016/s0755-4982(06)74515-8. [DOI] [PubMed] [Google Scholar]
- Central Statistical Agency . 2013. Population Projections for Ethiopia (2007-2037). Federal Democratic Republic of Ethiopia Population Census Commission, Addis Ababa, Ethiopia. [Google Scholar]
- DACA and MSH . USAID; Addis Ababa: 2009. Antimicrobial Use, Resistance and Containment Baseline Survey Synthesis of Findings; p. 1. [Google Scholar]
- Donkor E., Tetteh-Quarcoo P., Nartey P., Agyeman I. Self-medication practices with antibiotics among tertiary level students in Accra, Ghana: a Cross-Sectional Study. Int. J. Environ. Res. Publ. Health. 2012;9:3519–3529. doi: 10.3390/ijerph9103519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha T., Araya H., Alemayehu A., Solomon G., Ali D. Prevalence and predictors of self-medication with antibiotics among Adi-haqi campus students of Mekelle University, Ethiopia. IJPSR. 2014;5:975–992. [Google Scholar]
- Fadare J., Tamuno I. Antibiotic self-medication among university medical undergraduates in Northern Nigeria. J. Publ. Health Epidemiol. 2011;3:217–220. [Google Scholar]
- FMOH . 2014. Health and Health Related Indicators (2012/2013). Addis Ababa; p. 49. [Google Scholar]
- Gebeyehu E., Bantie L., Azage M. Inappropriate use of antibiotics and its associated factors among urban and rural communities of Bahir dar city administration, Northwest Ethiopia. PLoS One. 2015 doi: 10.1371/journal.pone.0138179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrekidan A., Asmelash T.D., Kahsay G., Gebreysus A.W. Prevalence and antimicrobial susceptibility patterns of Shigella among acute diarrheal outpatients in Mekelle hospital, Northern Ethiopia. BMC Res. Notes. 2015;8(611):1–7. doi: 10.1186/s13104-015-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholap M., Mohite V. Assess the self-medication practices among staff nurses. Indian JL Sci. 2013;4(1):81–84. [Google Scholar]
- Grigoryan L., Burgerhof J., John E., Degener J. Determinants of self-medication with anti biotics in Europe: the impact of beliefs, country wealth and the healthcare system. J. Timicrob. Chemother. 2008;61:1172–1179. doi: 10.1093/jac/dkn054. [DOI] [PubMed] [Google Scholar]
- Gunawardhana C., Sakeena M., Sivayoganthan C. Awareness of rational medication use and antibiotic self-medication practices among undergraduate students in a university in Sri Lanka. Trop. J. Pharmaceut. Res. 2015;14(4):723–729. [Google Scholar]
- Harris M., Ismail R., Anjum F., Saeed L., Ghayas S., Ali T., Ahmed S., Alam S., Rizvi M., Zafar S., Ahmed M., Nisa Z. Survey based study on the use of non-prescription drugs among pharmacists and non-pharmacists. Afr. J. Pharm. Pharmacol. 2013;7(38):2652–2656. [Google Scholar]
- Heidarifar R., Koohbor M., Mansourabad K., Mikaili P., Sarahroodi S. Self -medication with antibiotics among Iranian population in Qom State. J. Sci. Ind. Res. 2013;2(4):785–789. [Google Scholar]
- Hem E., Stokke G., Tyssen R., Gronvold N., Vaglum P., Ekeberg O. Self-prescribing among young Norwegian doctors : a nine-year follow-up study of a nationwide sample. BMC Med. 2005;7:1–7. doi: 10.1186/1741-7015-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Malik F., Muhammad K., Parveen G., Hameed A., Ahmad S., Riaz H., Akhtar P,Saeed T. Prevalence of self-medication and health-seeking behavior in a developing country. Afr. J. Pharm. Pharmacol. 2011;5(7):972–978. [Google Scholar]
- Jawad S., Ahmad H., Binte R., Najeeb S., Mumtaz M., Hashim M., Sharoz M., Zakariya M., Farooq S., Kadir M. Self-medication with antibiotics among non-medical university students of Karachi: across-sectional study. BMC Pharmacol. Toxicol. 2014;15(74):1–7. doi: 10.1186/2050-6511-15-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T., Tajuddin M., Krishna S., Kondal S., Kumar S. Evaluation of self-medication practice among tribal district students of south India. J. Contemp. Med. A. 2014;2(3):1–4. [Google Scholar]
- Kiragu C., Mwagiru P., Wala J. Self-medication with antibiotics prior to seeking treatment among adult patients attending outpatient department at Gatundu sub-county hospital, Kiambu country, Kenya. IJIR. 2016;2(8):609–616. [Google Scholar]
- Kirkwood B., Sterne J. second ed. 2003. Essential Medical Statistics; p. 413. USA. [Google Scholar]
- Lau J., Yu A., Cheung J., Leung S. Studies on common illness and medical care utilization patterns of adolescents. J. Adolesc. Health. 2000;27(6):443–452. doi: 10.1016/s1054-139x(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Lei X., Jiang H., Liu C., Ferrier A., Mugavin J. Self-medication practice and associated factors among residents in Wuhan, China. Int. J. Environ. Res. Publ. Health. 2018;15(68):1–10. doi: 10.3390/ijerph15010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J., Stafford R. Antibiotic treatment of adults with sore throat by community prima ry care physicians: a national survey, 1989-1999. JAMA. 2001;286:1181–1186. doi: 10.1001/jama.286.10.1181. [DOI] [PubMed] [Google Scholar]
- Mekuria B., Gedif T., Beedemariam G., Bekele T., Negussie M. 2017. Assessment of Bacterial Resistance Trend and Contributing Factors to Fluoroquinolone Among Patients’ Specimens Analyzed at International Clinical Laboratories in Addis Ababa. Master’s thesis. Addis Ababa. [Google Scholar]
- Montgomery A., Bradley C., Rochfort A., Panagopoulou E. A review of self-medication in physicians and medical students. Occup. Med. 2011;61:490–497. doi: 10.1093/occmed/kqr098. [DOI] [PubMed] [Google Scholar]
- Nalini G.K. Self medication among allopathic medical Doctors in Karnataka, India. BJMP. 2010;3(2):325. [Google Scholar]
- Osemene K., Lamikanra A. A study of the prevalence of self-medication practice among university students in southwestern Nigeria. TJPR. 2012;11(4):683–689. [Google Scholar]
- Pankaj C., Arvind M., Atul J., Neha S., Ajitesh M. Self medication practices with antibiotics among health care professional in Uttar Pradesh, India: a questionnaire based study. IAJPR. 2015;5(2):752–759. [Google Scholar]
- Pant N., Sagtani R., Pradhan M., Bhattarai A., Sagtani A. Self-medication with antibiotics among dental students of Kathmandu- prevalence and practice. Nepal Med. Coll. J. 2015;17(1-2):47–53. [Google Scholar]
- Pavydė E., Veikutis V., Mačiulienė A., Mačiulis V., Petrikonis K., Stankevičius E. Public knowledge, beliefs and behavior on antibiotic use and self-medication in Lithuania. Int. J. Environ. Res. Publ. Health. 2015;12:7002–7016. doi: 10.3390/ijerph120607002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalke V., Durgawale M. Self-medication practices in rural Maharashtra. Indian J. Community Med. 2006;31:1. [Google Scholar]
- Radyowijati A., Haak H. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Soc. Sci. Med. 2003;57:733–744. doi: 10.1016/s0277-9536(02)00422-7. [DOI] [PubMed] [Google Scholar]
- Reisrocha A., Harter R., Rotenberg L. Self-medication among nursing workers from public hospitals. Rev. Latino-Am. Enferm. 2009;17(6):1015–1022. doi: 10.1590/s0104-11692009000600014. [DOI] [PubMed] [Google Scholar]
- Sado E., Gedif T. Drug utilization at household level in Nekemte Town and surrounding rural areas, Western Ethiopia: a cross-sectional study. Open Access Libr. J. 2014;1:1–9. [Google Scholar]
- Sajith M., Suresh S.M., Roy N.T., Pawar A. Self-medication practices among health care professional students in a tertiary care hospital, Pune. Open Publ. Health J. 2017;10:63–68. [Google Scholar]
- Saradamma R., Higginbotham N., Nichter M. Social factors influencing the acquisition of antibiotics without prescription in Kerala State, south India. Soc. Sci. Med. 2000;50:891–903. doi: 10.1016/s0277-9536(99)00380-9. [DOI] [PubMed] [Google Scholar]
- Sarahroodi S., Arzi A., Sawalha, Ashtarinezhad A. Antibiotic self-medication among southern Iranian university students. Int. J. Pharmacol. 2010;6(1):48–52. [Google Scholar]
- Sawalha A. Assessment of self-medication practice among university students in Palestine: therapeutic and toxicity implications. The Islamic Univ. J. (Series of Natural Studies and Engineering) 2007;15(2):67–82. [Google Scholar]
- Shankar P., Partha P., Shenoy N. Self-medication and non-doctor prescription practices in Pokhara valley, Western Nepal: a questionnaire-based study. BMC Fam. Pract. 2002;3:1–7. doi: 10.1186/1471-2296-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S., Bugaighis L., Sharif R. Self-medication practice among pharmacists in UAE. Pharmacol. Pharm. 2015;6:428–435. [Google Scholar]
- Shehnaz S., Khan N., Sreedharan J., Issa K., Arifulla M. Self-medication and health related complaints among expatriate high school students in the United Arab Emirates. Pharm. Pract. 2013;11(4):211–218. doi: 10.4321/s1886-36552013000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamuno I., Mohammed I. Self medication with antibiotics amongst students of a Nige rian tertiary institution. J. Basic Appl. Sci. Res. 2011;1(10):1319–1326. [Google Scholar]
- Terfassa A., Jida M. Prevalence and antibiotics susceptibility pattern of Salmonella and Shigella species among diarrheal patients attending Nekemte referral hospital, Oromia, Ethiopia. Int. J. Microbiol. 2018:1–6. doi: 10.1155/2018/9214689. Article ID 9214689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togoobaatar G., Ikeda N., Ali M., Sonomjamts M., Dashdemberel S., Mori R., Shibuya K. Survey of non-prescribed use of antibiotics for children in an urban community in Mongolia. Bull. World Health Organ. 2010;88:930–936. doi: 10.2471/BLT.10.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaananen M., Pietila K., Airaksinen M. Self-medication with antibiotic- does it really happen in Europe. Health Pol. 2006;77:166–171. doi: 10.1016/j.healthpol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- WHO . Report of the 4th WHO, Role of the Pharmacist. Geneva. 1998. The role of pharmacist in self- care and self-medication; pp. 26–28. [Google Scholar]
- WHO . World Health Organization Geneva; 2000. WHO Drug Information; p. 9. [Google Scholar]
- WHO . WHO; Geneva, Switzerland: 2001. WHO Global Strategy for Containment of Antimicrobial Resistance. [Google Scholar]
- Widayati A., Suryawati S., Crespigny C., Hiller J. Self medication with antibiotics in Yogyakarta City Indonesia: a cross sectional population-based survey. BMC Res. Notes. 2011;4:491. doi: 10.1186/1756-0500-4-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worku S., G/Mariam A. Practice of self-medication in Jimma town, Ethiopia. Ethiop. J. Health Dev. 2003;17(2):111–116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.