Abstract
Objective
To evaluate the impact of the coronavirus disease 2019 (COVID-19) measures on the lives and psychosocial well-being of persons with epilepsy (PWE) during the third trimester of the COVID-19 pandemic.
Methods
A structured questionnaire investigating different aspects of the lives and psychosocial well-being of PWE during the COVID-19 pandemic was developed. Persons with epilepsy were invited via social media to anonymously respond to a secure web-based online questionnaire (www.icpcovid.com). Responses were collected between July 26th and December 3rd, 2020. Hospital anxiety and depression scales (HADS) were used to screen respondents for depression (HADS-D) and anxiety (HADS-A).
Results
Responses of 407 PWE were included in the analysis; 304 (74.7%) respondents were female and 245 (60.2%) living in Europe, 157 (38.6%) in South America, and 5 (1.2%) in Canada. Seventy-six (18.7%) reported a decrease of income during the COVID-19 lockdown, and 122 (30.0%) experienced difficulties in obtaining anti-seizure medication (ASM), mostly (72/122, 59.0%) due to unavailability. Seizure frequency increased in 122 (30.0%); 295 (72.5%) screened positive for anxiety, and 159 (39.1%) for depression. Hundred eighty-eight (46.2%) reported reluctance to seek medical care; 27.3% believed that epilepsy was associated with an increased risk of COVID-19 disease. Forty-six (74.2%) of 62 PWE who were followed up by telephone or video consult were satisfied with this consult. Fifty-five respondents, most (89.1%) of whom were from Europe, had also participated in a previous survey during the early months of the pandemic. In this subgroup, although there was no difference in prevalence of a positive screening for depression or anxiety, mean scores on HADS-A and HADS-D increased from 6.65 ± 3.99 to 7.27 ± 4.01 (p = 0.418), and from 5.84 ± 4.43 to 6.60 ± 4.45 (p = 0.371), respectively.
Conclusions
The COVID-19 pandemic continues to impact the psychosocial and somatic well-being of PWE. To minimize this impact, ensuring uninterrupted access to ASM is essential. Teleconsultations are valid alternatives for continued follow-up, but should include attention to psychosocial well-being. Persons with epilepsy should be more actively informed that epilepsy is not a risk factor for developing (more severe) COVID-19 disease.
Keywords: Epilepsy, COVID-19, Mental health, HADS, Telemedicine
1. Introduction
With about 50 million people affected worldwide, epilepsy is one of the most frequent neurological diseases [1]. Besides seizures, at least half of the persons with epilepsy (PWE) have one or more psychiatric, cognitive, or somatic comorbidities [2]. The prevalence of lifetime anxiety disorders or depression is 2–3 times higher in PWE than in persons without epilepsy, which significantly impacts their quality of life (QoL) and treatment outcomes [3], [4]. As a result, care for PWE must be holistic, considering both the seizures and psychosocial condition of the affected persons.
Since the end of 2019, the coronavirus disease 2019 (COVID-19) has spread over the world. In many countries, the enormous demand for (out-of-) hospital resources to treat patients with COVID-19 has forced a complete reorganization of healthcare infrastructure with the postponement of nonurgent care and follow-up visits, and the introduction of telemedicine for long distance follow-up. In order to control the COVID-19 outbreak, governments have taken several restrictive measures, ranging from social distancing to a complete lockdown, causing an enormous impact on healthcare as well as socioeconomic activities and psychosocial well-being. Knowing that PWE are vulnerable persons with higher risks of unemployment, psychological distress, and social stigma, the impact of the pandemic could entail devastating consequences for PWE worldwide [5], [6].
In a first online survey during the early months of the COVID-19 pandemic, our study group reported a high prevalence of anxiety and depression among PWE, reaching up to 50% [7]. Studies from West China, the United States, Spain, and Italy have reported similar findings and suggest more severe depressive and/or anxiety symptoms during COVID-19 pandemic among PWE compared to controls [5], [8], [9], [10].
An increase in seizure frequency during the COVID-19 outbreak has been reported in 8.6–35% of PWE [5], [9], [10], [11], [12], [13], [14]. This increase does not seem to be directly related to COVID-19 infection, but rather to other consequences of the pandemic, such as an increase in emotional distress, difficulties obtaining anti-seizure medication (ASM) or securing appointments with epilepsy care providers, and disruption of normal routine [10], [13]. Confinement at home has been reported to be an important contributor to this increase in emotional distress [13]. In Spain, Conde et al. found that almost 50% of PWE felt more anxious and depressed than usual, with more frequent seizures among 45% of this group of patients [14].
To evaluate the longer-term consequences on the lives and well-being of PWE during the ongoing COVID-19 pandemic, we conducted a follow-up multi-country online survey. Moreover, we assessed the evolution of these consequences over time in 55 PWE by comparing their responses in this survey with those in a first survey early in the pandemic.
2. Methods
2.1. Study design
An online questionnaire was distributed among PWE and their caretakers between July 26th and December 3rd, 2020. During this period, second wave COVID-19 outbreaks were observed in many countries worldwide and confinement measures were tightened in some places.
Persons with epilepsy were invited via social media to anonymously respond to a secure web-based online questionnaire (www.icpcovid.com). Official organizations for PWE supported dissemination on their networks such as the Epilepsy Liga Flanders, the Brazilian Federation of Epilepsy (Epibrasil), and the Brazilian Association of Epilepsy (ABE). Persons with epilepsy who filled in the first survey and gave permission to be re-contacted, were actively sent an e-mail with an invitation to fill in the second survey [7]. An e-consent was mandatory, as well as the confirmation to be a PWE or caretaker/parent of a PWE. Persons with epilepsy under 18 years of age were excluded, except when the survey was completed by their adult caretakers/parents. Caretakers/parents of PWE were asked to complete the questionnaire from the perspective of the PWE. We excluded PWE from continents with less than five respondents.
If respondents filled in both the first and second surveys, results of these PWE were compared by matching their encrypted email addresses used in both questionnaires. The study was approved by the ethics committee of the Antwerp University Hospital (ethical committee number: 20/14/168) and by the National Research Ethics Commission from Brazil (Protocol number: 30343820.9.0000.0008, dated April 01, 2020).
2.2. Questionnaire
The questionnaire used in the survey consisted of six parts, namely: (1) respondents’ characteristics; (2) screening for anxiety and depression; (3) questions related to flu-like symptoms and COVID-19 testing; (4) questions related to the impact of COVID-19 on daily life and economic situation; (5) epilepsy-related information; and (6) healthcare seeking behavior. Most questions were similar to those asked in the first survey, only the parts about the impact of COVID-19 on daily life and economic situation, and healthcare seeking behavior were added. The previously validated Hospital Anxiety and Depression Scale (HADS) was used to screen for anxiety and depression in PWE [15], [16]. Each subscale contained seven questions, each question with a 4‐point Likert scale, ranging from 0 to 3 [15]. The maximum score of each scale was 21 and the higher the score, the greater the severity of psychological problems [15]. A score higher than 7 on the subscales for anxiety (HADS-A) and depression (HADS-D) were considered as screened positive for the respective condition. Previous research has suggested that this cutoff point had the best performance in screening for anxiety and depression among adult PWE [16]. Satisfaction of telemedicine was assessed using a 5-point Likert scale (0 = not satisfied at all, 5 = completely satisfied). Persons with epilepsy who scored more than 3 were classified as satisfied. The questionnaire was available in English, Dutch, French, Portuguese, and Spanish. The questionnaire is displayed as supplementary material 1.
2.3. Statistical analyses
The statistical program Statistical Product and Service Solutions (SPSS) version 27.0 was used for data analysis. Continuous data, reported as mean with standard deviation (mean ± SD), were compared across categories using a Student t-test or Mann–Whitney U test as appropriate. Categorical variables were compared using a chi-square or Fisher’s exact test. Multiple logistic regression analysis was used to identify factors associated with anxiety, depression and increased seizure frequency. Only variables with a p-value <0.100 in bivariate analysis were included in multivariate analyses. Throughout the analyses, all statistical tests were two-sided. P-values <0.05 were considered as statistically significant.
3. Results
3.1. Respondent characteristics
A total of 424 responses were collected, 17 of which were excluded because the respondent was not a PWE nor caretaker of a PWE (n = 4), because the respondent was younger than 18 years (n = 7), or because PWE were from a continent with less than 5 respondents (n = 6). Four hundred and seven (96.0%) responses from 19 different countries (Supplementary Table 1) were thus included in the analysis. The majority (74.7%) of PWE was female (Table 1 ). Results from male and female PWE were similar, and therefore pooled together for analysis (Supplementary Table 2). The mean age was 34.52 ± 14.03 years. A total of 157 (38.6%) lived in South America, 245 (60.2%) in Europe and, 5 (1.2%) in Canada. Hundred thirty-five PWE (33.2%) reported financial difficulties and 58 (14.3%) difficulties to pay for ASM. Financial difficulties were significantly more often reported by PWE from South America than from Europe (51.6% vs 21.6%; p < 0.001) (Supplementary Table 3). Responses were provided by PWE themselves for 337 (82.8%) participants, and by parents/caretakers of PWE for 70 (17.2%) participants. Given that the responses provided by PWE and parents/caretakers were similar, they were pooled together for reporting and analysis in this paper. The responses of the parents/caretakers sub-group can be found in Supplementary Table 4.
Table 1.
Characteristics of persons with epilepsy.
| Total n | 407 |
|---|---|
| Age (years) | |
| Mean (±SD) | 34.52 ± 14.03 |
| Gender | |
| Male (%) | 102 (25.1%) |
| Female (%) | 304 (74.7%) |
| Country of residence | |
| Europe (%) | 245 (60.2%) |
| South America (%) | 157 (38.6%) |
| Canada (%) | 5 (1.2%) |
| Relationship status | |
| Single (%) | 198 (48.6%) |
| In a relationship/married (%) | 209 (51.4%) |
| Maximum educational level | |
| Primary (%) | 36 (8.8%) |
| Secondary (%) | 159 (39.1%) |
| University undergraduate degree (%) | 129 (31.7%) |
| University postgraduate degree (%) | 73 (17.9%) |
| Housemates | |
| Parents (%) | 138 (33.9%) |
| Spouse/partner (%) | 173 (42.5%) |
| Child(ren) (%) | 122 (30.0%) |
| Siblings or other family relatives (%) | 56 (13.8%) |
| Friend(s) (%) | 7 (1.7%) |
| None, i.e. living alone (%) | 63 (15.5%) |
| Job status | |
| Self-employed (%) | 33 (8.1%) |
| Employee (%) | 169 (41.5%) |
| Retired (%) | 28 (6.9%) |
| Unemployed (%) | 85 (20.9%) |
| Student (%) | 75 (18.5%) |
| Other (%) | 16 (3.9%) |
| Financial situation | |
| Financial difficulties | 135 (33.2%) |
| To feed properly (%) | 54 (13.3%) |
| To pay for housing/bills (%) | 81 (19.9%) |
| To pay for ASM (%) | 58 (14.3%) |
| No financial difficulties (%) | 272 (66.8%) |
ASM: anti-seizure medication; N: number; SD: standard deviation.
When considering the overall cohort, patient characteristics of the follow-up survey significantly differed from those of the first survey, with more PWE from South America participating in the second survey (157/407, 38.6%) compared to the first (67/399,15.0%; p < 0.001) [7]. Therefore, we opted not to compare answers at the group level. We did however compare results of 55 PWE who filled in both surveys, and whose answers to both surveys could be linked using their encrypted email addresses.
3.2. COVID-19 symptoms and testing
Two hundred and seventy PWE (66.3%) experienced flu-like symptoms since the start of the COVID-19 pandemic. The most frequently reported symptoms were headache (207/270; 76.7%), stuffy and/or running nose (134/270; 49.6%), muscle or body pain (120/270; 44.4%), and sore throat (120/270; 44.4%) (Supplementary Fig. 1). One hundred and nine PWE (26.8%) had been tested for COVID-19, 23 of whom (21.1%) tested positive. The most important reasons for being tested were flu-like symptoms (45/109; 41.3%) and close contact with someone who had tested positive for COVID-19 (25/109; 22.9%). The main symptoms reported by COVID-19 positive PWE included headache (19/23; 86.4%), general weakness (18/23; 81.8%), and muscle or body pain (18/23; 81.8%). Only one PWE (4.3%) who tested positive for COVID-19 was asymptomatic.
3.3. Impact of COVID-19 on daily life and finances
The majority of PWE (86.2%) reported that COVID-19 measures had an impact on their daily life with the most frequently reported impact being the prohibition to see people other than housemates (207; 50.9%). Seventy-six (18.7%) reported a decrease of income during the COVID-19 lockdown. This was more frequently reported by respondents from South America (41/157, 26.1%) compared to European countries (33/245, 13.5%; p = 0.002). One hundred and twenty-four PWE (30.5%) experienced an increase in expenditure, while 81 (19.9%) reported a decrease.
3.4. Epilepsy characteristics
Three-hundred and ninety two PWE (96.3%) reported taking ASM, 257 of whom (65.6%) were taking more than one ASM. Anti-seizure medication therapy was adapted during the pandemic in 114 (28.0%); 60 (14.5%) reported an increase in dose and 24 (5.9%) switched to other ASM. One hundred and twenty-two (30.0%) respondents experienced difficulties in obtaining ASM; 115 (28.3%) between January and June 2020 and 81 (19.9%) since July 2020. Reasons mentioned for the difficulties to obtain ASM during these two periods were, respectively: unavailability of ASM (56.5% and 58.0%), mobility restrictions (19.1% and 14.8%), and less or no income to buy ASM due to COVID-19 (10.4 % and 16.0%). Problems to obtain ASM were more frequently reported by PWE from South America than those from European countries (45.2% vs 18.0%; p < 0.001). Of the 72 PWE who mentioned that ASM were unavailable in their locality, 43 (59.7%) indicated that such ASM shortages did not occur prior to the COVID-19 outbreak and associated restrictions. Of the 407 respondents, 122 (30.0%) reported an increase in seizure frequency (107 [26.3%] during a lockdown period) and 24 PWE (5.8%) a decrease in seizure frequency. In a multivariate regression model, increase in seizure frequency was associated with younger age (Odds ratio (OR): 0.970, 95% confidence interval (CI) 0.951–0.989) and taking more than one ASM (OR: 2.130, 95% CI: 1.238–3.664). We did not observe an association between difficulties to obtain ASM and increase in seizure frequency. Nevertheless, 23.8% (29/122) of PWE who had problems obtaining ASM, did report that this situation had led to an increase in seizure frequency.
3.5. Anxiety and depression among PWE
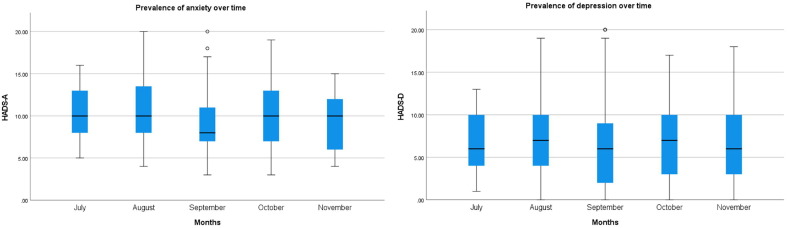
A total of 295 (72.5%) and 159 (39.1%) PWE screened positive for anxiety and depression, respectively, according to the HADS-A and HADS-D. Moreover, 11 (2.7%) scored over 16 points on HADS-D, which is associated with severe depressive symptoms. Hundred and five (25.8%) PWE were taking anti-depressants or anxiolytics. Depression (OR: 5.015, 95% CI: 2.657–9.466), problems to obtain ASM between January and June (OR: 4.565, 95% CI: 1.196–17.419), and increased expenditure (OR: 2.255, 95% CI: 1.134–4.425) were factors associated with a higher risk of anxiety in multivariate regression modeling. Living alone lowered the odds of anxiety (OR: 0.438, 95% CI 0.220–0.873). Anxiety (OR: 5.889, 95% CI: 1.614–21.488) was associated with higher odds for a positive screening for depression. To evaluate responses to the psychological assessment scales over time, mean scores of HADS-A and HADS-D per month were compared (Fig. 1 ). No significant difference was seen in the prevalence of anxiety (p = 0.185) and depression (p = 0.984) during the different months responses of the follow-up questionnaire were collected.
Fig. 1.
HADS scores of anxiety and depression among PWE during the different months of follow-up survey.
3.6. Healthcare seeking behavior and telemedicine
Of the 407 PWE, 188 (46.2%) reported reluctance to seek medical care. Most important reasons reported were fear of getting infected with COVID-19 (106/188; 56.4%), less accessible healthcare (67/188; 35.6%), the assumption that healthcare providers were occupied (47/188; 25.4%), and the assumption that seizure symptoms were not considered a priority during the pandemic (37/188; 19.7%). Two hundred and fifty PWE (61.4%) considered that it was not safe to go to the hospital, and 18.2% preferred telephone or video consultations. Indeed, 111 (27.3%) respondents were convinced that as a PWE, they had a higher risk of developing COVID-19 disease. This line of thought was more frequently reported by participants from South America than by those from Europe (40.1% vs 18.8%; p = <0.001). Two hundred and thirty PWE (56.5%) were considering vaccination against seasonal flu for the coming season.
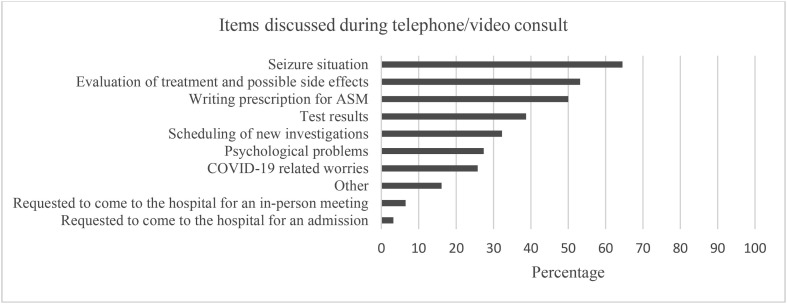
During the lockdown period, 220 (54.1%) of the PWE had a planned doctor’s visit, 65 (29.5%) of which were changed to a video or telephone consult, and 62 had already taken place at the time of filling in the survey. Forty-six (74.2%) of these 62 respondents were satisfied and 34 (54.8%) found the telephone/video consult as satisfactory as an in-person meeting with the healthcare provider. Persons who screened positive for depression were less satisfied with the telephone or video consult compared with nondepressed PWE, but this difference was not significant (61.1% vs 79.5%, p = 0.183). Items most often discussed during the telephone or video consult were the seizure situation (64.5%) and seizure treatment including possible side effects (53.2%) (Fig. 2 ). Psychological problems were discussed in only 27.4% of the teleconsultations.
Fig. 2.
Overview of reported items discussed during telephone or video consult.
3.7. Trends over time among a cohort of PWE who participated in the two surveys
Answers of 55 respondents in the current survey could be linked to their answers in our first survey using their encrypted email addresses. Almost all PWE in this follow-up cohort (49/55; 89.1%) were living in Europe, and majority (39/55; 70.9%) were female. Financial problems were more frequently reported during the first survey when compared to the second (29.1% vs 12.7%; p = 0.039) (Table 3 ). There were no significant differences in access to ASM and in seizure frequency nor in prevalence of a positive screening for anxiety or depression. However, 24 PWE (41.8%) scored higher points on HADS-A during the second survey and the mean score increased from 6.65 ± 3.99 to 7.27 ± 4.01 (p = 0.418). In a multivariate regression model, there was an association between reporting an increased seizure frequency in round 2 and a higher score on HADS-A in the follow-up survey compared to the score of the first survey (OR: 7.357, 95% CI: 1.349–40.107). Moreover 29 PWE (52.7%) scored higher on HADS-D during the second survey and the mean score increased from 5.84 ± 4.43 to 6.60 ± 4.45 (p = 0.371). Taking more than one ASM reduced the odds of scoring higher on HADS-D in the follow-up survey (OR: 0.121, 95% CI: 0.026–0.568). Three PWE (5.5%) started taking antidepressants or anxiolytics between the first and second surveys.
Table 2.
Impact of COVID-19 on daily life and finances.
| Total n | 407 |
|---|---|
| Impact of COVID-19 on daily life | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Impact of COVID-19 on income | |
|
|
|
|
|
|
|
|
| Impact of COVID-19 on expenditure | |
|
|
|
|
|
|
|
|
N: number.
Table 3.
Comparisons between the first and second online surveys among PWE.
| Second respondents | Round 1 (n = 55) | Round 2 (n = 55) | p-value | |
|---|---|---|---|---|
| Financial problems | Yes; n(%) | 16 (29.1%) | 7 (12.7%) | 0.039 |
| Problems to obtain ASM | Yes; n(%) | 9 (16.4%) | 5 (9.1%) | 0.259 |
| Taking ASM | Yes; n(%) | 54 (98.2%) | 53 (96.4%) | 0.491 |
| Number of ASM | >1; n(%) | 27 (49.1%) | 33 (60.0%) | 0.202 |
| Seizure frequency | Increased; n(%) | 8 (14.5%) | 10 (18.2%) | 0.607 |
| HADS-Anxiety | Positive; n(%) | 21 (38.2%) | 25 (45.5%) | 0.440 |
| HADS-A score | Mean (±SD) | 6.65 ± 3.99 | 7.27 ± 4.01 | 0.418 |
| HADS-Depression | Positive; n(%) | 15 (27.3%) | 19 (34.5%) | 0.410 |
| HADS-D score | Mean (±SD) | 5.84 ± 4.43 | 6.60 ± 4.45 | 0.371 |
ASM: anti-seizure medication; HADS: Hospital anxiety and depressions scale; N: number; SD: standard deviation.
4. Discussion
In this study, we evaluated different aspects of lives and well-being of PWE during the first eight months of the COVID-19 pandemic. We documented that a high proportion of PWE screened positive for anxiety (72.5%) and depression (39.1%), which is two to three times higher than what has been reported in the general population during the COVID-19 pandemic, and 1.5 times higher when compared with previous studies conducted among PWE during the pandemic, including our own first survey [5], [7], [9], [17], [18].
It is tempting to conclude that there is an increase in prevalence of depression and anxiety in PWE with prolonged COVID-19 measures, but comparison of studies is problematic because characteristics of respondents might significantly differ. Indeed, no significant difference in prevalence of anxiety or depression was observed when comparing the sub-group of respondents who filled both the first and follow-up surveys. We did see an increase in the mean HADS-A and HADS-D score in this subgroup though, suggesting that the degree in psychological distress does increase in vulnerable people. This concurs with recent findings from a Dutch cohort which revealed that in persons who had mental health problems prior to the COVID-19 outbreak, symptoms increased mildly, although healthy controls experienced an even greater increase in psychological symptoms during the pandemic [19]. Problems to obtain ASM, and increased expenditure were associated with higher odds to have anxiety. The explanation why increased expenditure was associated with anxiety is not clear, but a hypothesis could be that respondents tended to spend more on necessities such as groceries, household supplies, and home entertainment because they were anxious and worried about the future. We found that PWE who lived alone had lower odds to develop anxiety, which could possibly be explained by the fact that those people already had developed coping mechanisms to live in social isolation.
More than half of respondents reported no change of income, whereas almost a fifth reported a decrease of income. It has been reported that the negative economic impact of the COVID-19 pandemic has affected mainly those with a lower income prior to the pandemic and made them more prone to mental health problems [20], [21]. Our study confirms this finding as decrease of income was significantly more frequently reported by respondents from South America, most likely to have a lower income than Europeans.
One third of the PWE reported financial difficulties, and 14.3% even had difficulties to pay for ASM; however, our research methods do not enable us to confirm whether these financial difficulties were directly related to the COVID-19-pandemic. The 55 respondents who participated in the two surveys reported significantly more financial problems in the first survey compared with the follow-up survey. In contrast, comparing all responses from both surveys, more financial difficulties were reported during the second survey, but this was most likely because more PWE from South America participated in the second survey (38.6%) compared to the first survey (15.0%).
An increase in seizure frequency was observed in 30.0% of PWE, which was associated with younger age and taking more than one ASM. One third of PWE reported difficulties in obtaining ASM, and one fourth of them reported this led to increase in seizure frequency, underlining the importance of ensuring access to ASM. Difficulties in obtaining ASM were caused by unavailability of ASM in more than half of the cases, and around 40% of them indicated they already encountered such difficulties prior to the pandemic. The pandemic seems to exacerbate longerstanding problems of drug shortages, which is a particular risk for PWE since sudden discontinuation of ASM can lead to breakthrough seizures and even status epilepticus [22]. Our study also shows that experiencing difficulties to obtain ASM is associated with a positive anxiety screening on the HADS-A. The experiences of PWE during the COVID-19 pandemic should therefore serve as lessons for policy makers about the impact of drug shortages, and to urge pharmaceutical companies to provide more adequate communication to both health care provider and organizations for PWE on foreseen drug shortages and their expected duration. This can enable PWE to timely order refills or discuss alternatives with their treating neurologist. Finally, when comparing results of PWE filling in the first and second surveys, an association between increased seizure frequency and an increasing score on HADS-A between the two study time points was seen, illustrating the relationship between seizure control and psychological distress. Similarly, other studies, mostly conducted early during the confinement period, also showed an increase in seizure frequency in 8.6–35% of cases, with worsening of stress, and mood and sleep disorders as associated factors [5], [9], [11], [13], [14].
Almost half of PWE presented reluctance to seek medical care and 61.4% felt unsafe about going to the hospital. Delayed arrival at the emergency department due to reluctance to seek medical care has been reported in several studies [23], [24], [25], [26]. Therefore, it should be emphasized to PWE that measures are in place to assure safety in the hospital and that non-COVID-19-related healthcare should not be postponed. Remarkably, 27.3% of PWE thought that having epilepsy was associated with higher odds of getting infected with COVID-19. This assumption is unfounded since epilepsy itself neither increases the risk of getting infected with COVID-19, nor the severity of the disease [27]. In order to overcome these erroneous assumptions, PWE need to be informed correctly by reliable sources, like healthcare providers or associations that support PWE. Respondents who thought that they were more likely to get infected with COVID-19 because they had epilepsy also showed significantly more symptoms of anxiety or depression. Fortunately, several studies have shown that implementation of telemedicine as a tool for follow-up of PWE appears feasible nowadays [5], [14], [28]. In our study, almost three-quarters of PWE were satisfied with a telephone or video consult. Importantly, only 27.4% of PWE reported that psychological problems were discussed. In conclusion, telemedicine could be used as an add-on service rather than a substitute for in-person consultations, and healthcare workers should pay specific attention to potential psychological problems of PWE.
Several limitations of our study need to be acknowledged. First, the web-based nature of the survey induces a sampling bias, as some participants like elderly or persons living in lowerincome countries, may not have internet access or may be less active on web platforms. The response bias is illustrated by the fact that 89.1% of the 55 PWE who participated in the two surveys were from Europe. Moreover, PWE with symptoms of depression or anxiety might be more prone to participate in online surveys. In this study, the majority of PWE were female. Nevertheless, results from male and female PWE were similar, and consequently, female gender did not influence responses and was not an explanation for the high incidences of depression and anxiety. Second, we cannot verify whether the answers were entered truthfully and whether all respondents had epilepsy according to the International League Against Epilepsy (ILAE) diagnostic criteria [29]. Third, the survey link was freely accessible to anyone, making it impossible to estimate the reached sample. Fourth, given that the sampling occurred over a period of several months and we did not collect information about the precise location of the respondents, responses couldn’t be linked to the actual situation of infection rate, measures taken by the government, and healthcare situation in that region at the time of filling in the survey. Fifth, no control group was included, hampering comparison of the impact of COVID-19 on PWE versus the general population.
5. Conclusion
In conclusion, the COVID-19 pandemic continues to challenge PWE in terms of reduced access to ASM, psychological distress, and worsened seizure-control. To minimize this impact, ensuring uninterrupted access to ASM and providing sufficient psychosocial support to reduce levels of anxiety and depression are essential. Given their reluctance to seek medical care at health facilities, and the overall satisfaction with telemedicine, telehealth can be introduced to ensure follow-up of PWE. Improved communication strategies are needed to inform PWE that there is no evidence that epilepsy is a risk factor for developing (more severe) COVID-19 disease.
Funding
R. Colebunders received funding from the European Research Council (ERC 671055) and VLIRUOS.
S. Weckhuysen receives funding from FWO-FKM (1861419N).
C. Millevert receives funding from BOF-UA (DOCPRO4 FFB200262).
A. Gil-Nagel received speaker and advisory board honoraria and research grants from Bial, Biocodex, Eisai, Esteve, GWPharmaceutical, Stoke and, Zogenix.
Author contributions
Study conception and design: Colebunders Robert (C.R.), Weckhuysen Sarah (W.S.)
Collection of data: Faria de Moura Villela Edlaine (F.M.V.E.), Gil-Nagel Antonio (G.N.A.), Rosso Barbara (R.B.), C.R. and W.S.
Analysis and interpretation of data: Millevert Charissa (M.C.), Van Hees Stijn (V.H.S.), Siewe Fodjo Joseph Nelson (S.F.J.N.), Wijtvliet Veerle (WV), C.R., and W.S.
Writing of the paper: M.C., V.H.S., S.F.J.N., W.V., C.R., and W.S.
Critical vision: M.C., V.H.S., W.V., S.F.J.N., C.R., and W.S.
All authors read and approved the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yebeh.2021.107800.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organisation. Epilepsy fact sheet; 2019. Available at https://www.who.int/news-room/fact-sheets/detail/epilepsy [accessed 4 December 2020].
- 2.Keezer M.R., Sisodiya S.M., Sander J.W. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15:106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 3.Tellez-Zenteno J.F., Patten S.B., Jetté N., Williams J., Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 4.Josephson C.B., Jetté N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry. 2017;29:409–424. doi: 10.1080/09540261.2017.1302412. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca E., Quintana M., Lallana S., Luis Restrepo J., Abraira L., Santamarina E., et al. Epilepsy in time of COVID-19: A survey-based study. Acta Neurol Scand. 2020 doi: 10.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza J.L., Faiola A.S., Miziara C., de Manreza M.L.G. The Perceived Social Stigma of People with Epilepsy with regard to the Question of Employability. Neurol Res Int. 2018;2018:4140508. doi: 10.1155/2018/4140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Hees S., Siewe Fodjo J.N., Wijtvliet V., Van den Bergh R., Faria de Moura Villela E., da Silva C.F., et al. Access to healthcare and prevalence of anxiety and depression in persons with epilepsy during the COVID-19 pandemic: A multicountry online survey. Epilepsy Behav. 2020;112:107350. doi: 10.1016/j.yebeh.2020.107350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao X., Zhou D., Li Z., Zeng G., Hao N., Li E., et al. Severe psychological distress among patients with epilepsy during the COVID-19 outbreak in southwest China. Epilepsia. 2020;61:1166–1173. doi: 10.1111/epi.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assenza G., Lanzone J., Brigo F., Coppola A., Di Gennaro G., Di Lazzaro V., et al. Epilepsy Care in the Time of COVID-19 Pandemic in Italy: Risk Factors for Seizure Worsening. Front Neurol. 2020;11:737. doi: 10.3389/fneur.2020.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller W.R., Von Gaudecker J., Tanner A., Buelow J.M. Epilepsy self-management during a pandemic: Experiences of people with epilepsy. Epilepsy Behav. 2020;111 doi: 10.1016/j.yebeh.2020.107238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S., Wu C., Jia Y., Li G., Zhu Z., Lu K., et al. COVID-19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia. 2020 doi: 10.1111/epi.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aledo‐Serrano Á., Mingorance A., Jiménez‐Huete A., Toledano R., García‐Morales I., Anciones C., et al. Genetic epilepsies and COVID-19 pandemic: Lessons from the caregiver perspective. Epilepsia. 2020;61(6):1312–1314. doi: 10.1111/epi.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Larsen A., Gonzalez-Villar E., Díaz-Maroto I., Layos-Romero A., Martínez-Martín Á., Alcahut-Rodriguez C., et al. Influence of the COVID-19 outbreak in people with epilepsy: Analysis of a Spanish population (EPICOVID registry) Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conde Blanco E., Manzanares I., Centeno M., Khawaja M., Betrán O., Donaire A., et al. Epilepsy and lockdown: A survey of patients normally attending a Spanish center. Acta Neurol Scand. 2020 doi: 10.1111/ane.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smarr K.L., Keefer A.L. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S454–S466. doi: 10.1002/acr.20556. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira G.N., Lessa J.M.K., Gonçalves A.P., Portela E.J., Sander J.W., Teixeira A.L. Screening for depression in people with epilepsy: comparative study among neurological disorders depression inventory for epilepsy (NDDI-E), hospital anxiety and depression scale depression subscale (HADS-D), and Beck depression inventory (BDI) Epilepsy Behav. 2014;34:50–54. doi: 10.1016/j.yebeh.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Shevlin M., McBride O., Murphy J., Miller J.G., Hartman T.K., Levita L., et al. Anxiety, depression, traumatic stress and COVID-19-related anxiety in the UK general population during the COVID-19 pandemic. BJPsych Open. 2020;6 doi: 10.1192/bjo.2020.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Yang Z., Qiu H., Wang Y., Jian L., Ji J., et al. Anxiety and depression among general population in China at the peak of the COVID-19 epidemic. World Psychiatry. 2020;19:249–250. doi: 10.1002/wps.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan K.Y., Kok A.A.L., Eikelenboom M., Horsfall M., Jörg F., Luteijn R.A., et al. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: a longitudinal study of three Dutch case-control cohorts. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hossain M.M., Tasnim S., Sultana A., Faizah F., Mazumder H., Zou L., et al. Epidemiology of mental health problems in COVID-19: a review. F1000Res. 2020;9:636. doi: 10.12688/f1000research.24457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottan N., Hoffmann B., Vera-Cossio D. The unequal impact of the coronavirus pandemic: Evidence from seventeen developing countries. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt D., Löscher W. Uncontrolled epilepsy following discontinuation of antiepileptic drugs in seizure-free patients: a review of current clinical experience. Acta Neurol Scand. 2005;111:291–300. doi: 10.1111/j.1600-0404.2005.00408.x. [DOI] [PubMed] [Google Scholar]
- 23.Lazzerini M., Barbi E., Apicella A., Marchetti F., Cardinale F., Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:e10–e11. doi: 10.1016/S2352-4642(20)30108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kugelman N., Lavie O., Assaf W., Cohen N., Sagi-Dain L., Bardicef M., et al. Changes in the obstetrical emergency department profile during the COVID-19 pandemic. J Matern Fetal Neonatal Med. 2020:1–7. doi: 10.1080/14767058.2020.1847072. [DOI] [PubMed] [Google Scholar]
- 25.Zorzi A., Vio R., Rivezzi F., Falzone P.V., Giordani A.S., Condello C., et al. Characteristics and hospital course of patients admitted for acute cardiovascular diseases during the coronavirus disease-19 outbreak. J Cardiovasc Med (Hagerstown) 2020 doi: 10.2459/JCM.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 26.Claeys M.J., Argacha J.F., Collart P., Carlier M., Van Caenegem O., Sinnaeve P.R., et al. Impact of COVID-19-related public containment measures on the ST elevation myocardial infarction epidemic in Belgium: a nationwide, serial, cross-sectional study. Acta Cardiol. 2020:1–7. doi: 10.1080/00015385.2020.1796035. [DOI] [PubMed] [Google Scholar]
- 27.Adan G.H., Mitchell J.W., Marson T. Epilepsy care in the COVID-19 era. Clin Med (Lond) 2020;20(4):e104–e106. doi: 10.7861/clinmed.2020-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Wrede R., Moskau-Hartmann S., Baumgartner T., Helmstaedter C., Surges R. Counseling of people with epilepsy via telemedicine: Experiences at a German tertiary epilepsy center during the COVID-19 pandemic. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.