Abstract
Background:
Antibiotic-resistant Acinetobacter species are a growing public health threat, yet are not nationally notifiable, and most states do not mandate reporting. Additionally, there are no standardized methods to detect Acinetobacter species colonization.
Methods:
An outbreak of carbapenem-resistant Acinetobacter baumannii (CRAB) was identified at a Utah ventilator unit in a skilled nursing facility. An investigation was conducted to identify transmission modes in order to control spread of CRAB. Culture-based methods were used to identify patient colonization and environmental contamination in the facility.
Results:
Of the 47 patients screened, OXA-23-producing CRAB were detected in 10 patients (21%), with 7 patients (15%) having been transferred from out-of-state facilities. Of patients who screened positive, 60% did not exhibit any signs or symptoms of active infection by chart review. A total of 38 environmental samples were collected and CRAB was recovered from 37% of those samples. Whole genome sequencing analyses of patient and environmental isolates suggested repeated CRAB introduction into the facility and highlighted the role of shared equipment in transmission.
Conclusions:
The investigation demonstrated this ventilated skilled nursing facility was an important reservoir for CRAB in the community and highlights the need for improved surveillance, strengthened infection control and inter-facility communication within and across states.
Keywords: Acinetobacter, Carbapenem-resistant, Outbreak, Skilled nursing facility, Infection control
BACKGROUND
Acinetobacter species (spp.) are ubiquitous in the environment and are a common source of health care-associated infections.1 These organisms are capable of surviving in dry environments for extended periods of up to 5 months, making them particularly difficult to eliminate in health care settings.2 In addition, Acinetobacter spp. are effective at acquiring genetic material from other organisms, allowing them to quickly develop resistance to antibiotics and reducing their susceptibility to cleaning and disinfecting agents.3,4
The emergence and spread of multidrug-resistant organisms (MDROs), such as carbapenem-resistant Acinetobacter baumannii (CRAB) is a major public health threat.5–7 Mortality rates of 55% for patients with CRAB bacteremia have been reported.8,9 Acinetobacter spp. are opportunistic pathogens and can colonize various body sites (eg, skin, respiratory and urinary tracts) and cause disease in susceptible hosts.10 Risk factors for Acinetobacter infection include mechanical ventilation, wound care, recent surgery, or trauma, indwelling medical devices, extended hospital stays, extensive antimicrobial treatment, and other underlying medical conditions.11–13
CRAB typically possess a carbapenemase of the OXA family of β-lactamases.14 Within this group of carbapenemase and/or β-lactamases genes, OXA-23 is one of the most frequently encountered worldwide and in the United States.15 OXA-23 transposition to conjugative plasmids has been documented16, and is concerning from an infection control perspective because this facilitates transmission of carbapenem resistance to other organisms. The combination of this transmissibility, environmental persistence, and high antibiotic resistance make this an important concern for health care settings.
This investigation was conducted in response to an outbreak at a long-term care facility in Utah. CRAB is an increasing problem in health care facilities worldwide and is considered an urgent threat,17 but is not a nationally notifiable condition in the United States. CRAB is reportable in Utah, but most states do not mandate reporting nor provide characterization of OXA genes by polymerase chain reaction or whole genome sequencing (WGS) through their public health laboratories.15 Only 1 state neighboring Utah mandated reporting of CRAB at the time of this outbreak. In addition, specific OXA carbapenemases in CRAB may not be detected or characterized by commercial polymerase chain reaction assays used in clinical laboratories.18 Lack of reporting and laboratory testing make it difficult to characterize the molecular epidemiology of OXA- carbapenemases. This investigation aimed to identify transmission modes in order to control spread of CRAB within and between facilities.
METHODS
Background
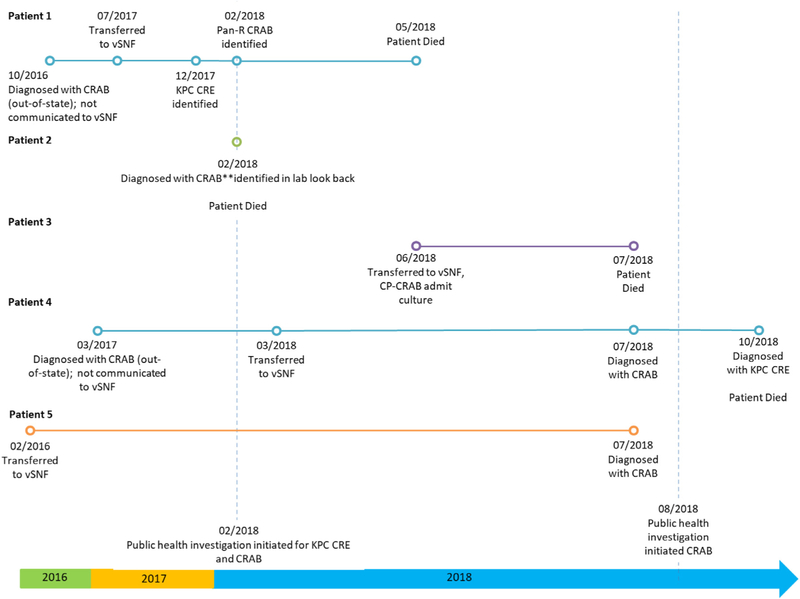
In February 2018, the Utah Department of Health (UDOH) identified patient 1, residing in a long-term care facility with a ventilator unit (vSNF, facility A). Patient 1 was diagnosed with 2 highly-resistant bacterial infections: a Klebsiella pneumoniae with a Klebsiella pneumoniae carbapenemase gene, and a CRAB. Nine patients were screened for carbapenemase-producing carbapenem-resistant Enterobacteriaceae at this time, and all were negative; CRAB colonization was not assessed. Patient 1 died in May 2018. By July 2018, UDOH identified 3 additional patients (patients 3, 4, and 5) at facility A with CRAB through routine surveillance. Patient 2 died in February 2018 and was identified posthumously through retrospective surveillance. UDOH and facility A initiated an investigation in August 2018.
Antibiotic susceptibility testing
Antimicrobial susceptibility testing was performed by broth microdilution following Clinical and Laboratory Standards Institute guidelines and breakpoints. Patient 1 was tested at the Centers for Disease Control and Prevention (CDC) and patients 2–5 at the Utah Public Health Laboratory using the Sensititre GNX2F panels (Thermo-Fisher Scientific).
WGS and bioinformatic analyses
WGS and bioinformatic analyses were performed as described by Oakeson et al.19,20 To detect the presence of antimicrobial resistance genes, de novo genome assemblies for each isolate were generated and searched against the National Center for Biotechnology Information’s Bacterial Antimicrobial Resistance Reference Gene Database using ABRicate (https://github.com/tseemann/abricate).
Case definitions
For the purpose of this investigation, confirmed cases were defined as any patient admitted to facility A with any infection or colonization with CRAB carrying an OXA-23 carbapenemase gene identified through WGS. Suspect cases were defined as any patient ever admitted to facility A with an Acinetobacter spp. infection or colonization not tested for the presence of an OXA-23 gene regardless of resistance profile.
Patient and environmental selection criteria for colonization screening
The investigation followed the CDC-recommended surveillance approach focusing on the highest risk patients in the first round of screening.21 Patients were considered high risk if they met any of the following criteria between February and August 2018: had any Acinetobacter spp. positive culture; resided in the high acuity unit (Hall A) for patients with complex health care needs; received respiratory care or mechanical ventilation; received any wound care; or transferred from an out-of-state facility to facility A. The environmental investigation focused on shared equipment and high-touch surfaces, including physical therapy equipment, nursing carts containing wound care equipment, respiratory therapy equipment, vital signs monitoring equipment, computer equipment, mechanical ventilators, and bed rails.
Patient and environmental sampling and testing
The investigation team developed a strategy for patient colonization screening for CRAB based on a literature review. This strategy focused on the collection of oropharyngeal, sputum, wound, and intact skin samples from the axilla and groin. Literature indicated that sponge collection results in higher recovery of CRAB when compared to conventional swabs22,23; therefore, intact skin in the axilla and groin region were sampled using a foam sponge collection device, EnviroMax Plus (Puritan). An Amies medium-based nylon swab system (eSwab, Copan) was used for oropharyngeal and wound specimens. If a patient had multiple wounds, the worst wound, was sampled. Sputum and/or tracheal aspirate from patients on a ventilator or with a tracheostomy site were also collected.
Patient samples were either plated directly on CHROMAgar Acinetobacter plates containing a proprietary β-lactam-supplement (for sputum samples and tracheal aspirates) or after an overnight enrichment step in tryptic soy broth (for intact skin, wound and oropharyngeal samples). Presumptive CRAB colonies from the plates were identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Environmental sampling was performed using EnviroMax Plus swabs with enrichment in water peptone. The environmental samples were processed the same as the patient samples, following the enrichment step.
Infection control observations
A CDC-recommended infection control assessment and response (ICAR) tool was used to assess infection control in the following areas: infection control infrastructure; hand hygiene; personal protective equipment (PPE); environmental cleaning; disease surveillance and reporting; and antibiotic stewardship. Investigators also performed infection control observations throughout facility A. Hand hygiene and PPE use observations were performed using CDC observation tools to quantify the proportion of appropriate hand hygiene and PPE use compared to total opportunities observed. Investigators observed a terminal cleaning of a patient room, including cleaning product choice, correct use of products, and adherence to documented cleaning protocols.
Patient data management and analysis
A form adapted from the CDC health care-associated infection outbreak investigation abstraction form was used to abstract patients’ clinical histories from the electronic health record system.24 Relevant out-of-state patient reports were obtained from respective state health departments. Information collected included: demographics; facility history; ward and room history; current precautions and status; types of services and devices; antimicrobial therapy; bacterial cultures and symptoms of infection since February 1, 2018.
Patient flow network analysis
Patient facility history, obtained from chart abstractions, was used to conduct patient flow network analysis. Network analysis was performed and plotted using the igraph (https://igraph.org.) package in R v3.4.3 (R Core Team, Vienna, Austria). The layout generator was based on Large Graph Layout (http://lgl.sourceforge.net/).
Ethical concerns
This investigation was conducted under the authority of Utah Communicable Disease Control Act (Utah Code 26–6) to investigate and control communicable diseases and epidemic infections which may affect the public. The investigation also received CDC human subject review (HSR #2018–00189) and was determined to not constitute human subjects research. Facility nursing staff obtained written informed consent for patients providing specimens. Guardians provided assent for patients unable to consent.
RESULTS
Investigation of the initial cluster
Five patients formed the initial cluster that prompted this investigation. Patients 1, 2, and 3 died before the investigation began, and patient 4 died in October 2018 (Fig 1).
Fig 1.
Investigation timeline of a carbapenem-resistant Acinetobacter baumannii (CRAB) outbreak at a long-term care facility, Utah—2018. CRAB: carbapenem-resistant Acinetobacter baumannii; CRE: carbapenem-resistant Enterobacteriaceae; KPC: Klebsiella pneumoniae carbapenemase; vSNF: ventilated skilled nursing facility; Pan-R: pan-resistant.
The characteristics of these patients are described in Table 1. The rooms of patients 1, 2, 3, and 4 were in Hall A, and patient 5 resided in Hall B. Patient 3 occupied patient 1’s former room, and patient 4 occupied patient 2’s former room. Patients 1, 2, and 3 had received respiratory care and were on ventilators.
Table 1.
Demographic and clinical characteristics of index patients and those selected for carbapenem-resistant Acinetobacter baumannii (CRAB) screening during an outbreak at a long-term care facility, Utah—2018
| Initial cluster patients | Patients selected for colonization screening† |
|||
|---|---|---|---|---|
| Patient characteristics | n = 5* No. | Total n = 47 No. (%) | Positive† n = 10 (21.3%) No. (%) | Negative n = 37 No. (%) |
|
| ||||
| Male sex | 2 | 20 (43) | 6 (60) | 14 (38) |
| Aged <65 years | 1 | 15 (32) | 5 (50) | 10 (27) |
| Former or current residence in Hall A | 4 | 10 (21) | 5 (50) | 5 (14) |
| Had been transferred from out-of-state | 3 | 7 (15) | 3 (30) | 4 (11) |
| Had been admitted in the last 6 months | 2 | 15 (32) | 2 (20) | 13 (35) |
| Recent documented clinical services received‡ | ||||
| Wound care | 5 | 30 (64) | 8 (80) | 22 (59) |
| Ventilator care | 2 | 6 (13) | 2 (20) | 4 (11) |
| Respiratory therapy | 4 | 20 (43) | 5 (50) | 15 (41) |
| Physical therapy | 4 | 15 (32) | 4 (40) | 11 (30) |
| Invasive device use | 4 | 12 (26) | 6 (60) | 6 (16) |
| Antimicrobial therapy | 5 | 17 (36) | 5 (50) | 12 (32) |
| Had symptoms consistent with an active infection | 5 | 16 (34) | 4 (40) | 12 (32) |
Includes 1 patient identified clinically posthumously after sample collection.
Includes 2 index case patients still at the facility.
Since 02/01/2018.
The antibiotic susceptibility patterns of isolates from patients 1–5 indicated high level resistance to multiple classes of antibiotics (Supplementary Appendix A). All of the initial cluster patient isolates were resistant to all of the carbapenems tested (meropenem, doripenem, and imipenem) and most of them displayed nonsusceptibility to other b-lactam antibiotics. All isolates were also resistant to fluoroquinolones and trimethoprim and/or sulfamethoxazole. Resistance to aminoglycosides and tetracyclines was also prevalent. Colistin was the only antibiotic to which all the isolates were susceptible. Overall, there were few appropriate antibiotic treatment options.
WGS revealed that isolates from the initial 5 patients clustered into 2 groups: patients 1, 3, and 4 grouped together, and patients 2 and 5 were closely related to each other (Supplementary Appendix B). WGS analysis also revealed that all isolates carried identical copies of an OXA-23 carbapenemase gene.
Characteristics of patients screened for colonization
Based on colonization screening selection criteria, 47 patients were identified for sampling. Investigators collected samples from: skin (47); wound (9); oropharyngeal (40) and tracheal aspirate (4). Twenty-one percent of patients (10 of 47) had at least 1 positive CRAB sample, with 30% (3 of 10) having multiple positive sites. CRAB with an OXA-23 gene was isolated from 21% (10 of 47) of skin, 25% (1 of 4) of tracheal aspirate, 33% (3 of 9) of wound, and 0% (0 of 40) oropharyngeal samples.
Descriptive analysis revealed important demographic differences between patients that screened positive compared to patients that screened negative (Table 1): more were male (60% vs 38%); more were < 65 years of age (50% vs 27%); more were currently residing or had previously resided in Hall A (50% vs 14%); more had been transferred from out-of-state facilities (30% vs 11%); and fewer had been admitted to facility A in the last 6 months (20% vs 35%).
A higher proportion of patients who screened positive for CRAB with an OXA-23 gene had received high-risk clinical service(s) since the beginning of February 2018, including wound care, ventilator care, respiratory therapy, physical therapy, invasive device-use, and antimicrobial therapy compared to those that screened negative (Table 1). Sixty percent of patients (6 of 10) who screened positive did not exhibit any signs or symptoms of active infection by chart review.
Environmental sampling results
A total of 38 environmental samples were collected throughout facility A, and CRAB was recovered from 37% of those samples (14 of 38). Positive sites included: handrails; physical therapy equipment shared between patient rooms and in the physical therapy gym; nursing carts containing wound or respiratory equipment; patient lifts; storage room; patient rooms; family room TV remote and coffee table (Fig 2). The positive samples collected from Hall A were identified in shared areas, from shared equipment, from a ventilator in a positive patient’s room. The positive samples in Hall B were collected in or around patient 5’s room. No positive environmental samples were identified from rooms previously occupied by initial cluster patients, following a terminal clean.
Fig 2.
Map of environmental and patient samples with carbapenem-resistant Acinetobacter baumannii (CRAB) with an OXA-23 gene during an outbreak at a long-term care facility, Utah—August 2018. PT: physical therapy.
WGS results of colonization screening and environmental isolates
WGS analysis showed that CRAB isolates obtained from the colonization screening and environmental sampling harbored identical OXA-23 genes. Phylogenetic analysis revealed isolates clustered in 3 distinct and well-supported clades (Appendix B). Clades were labeled based on the number of isolates in each clade with clade I isolates being the most abundant with 12 clinical isolates and 9 environmental isolates, followed by clade II with 7 clinical isolates and 6 environmental isolates, then clade III with 3 clinical isolates and 2 environmental isolates. Patients 1 and 3 were colonized with clade II isolates. Patient 2 was colonized with a clade I isolate. Patient 5 was colonized with both clade I and clade II isolates, and Patient 4 with both clade II and clade III isolates. Environmental samples positive for OXA-23 CRAB were isolated from shared equipment and common areas, such as the physical therapy room (Fig 2). In Hall B, clade I and clade II environmental isolates were detected in the room of Patient 5 consistently with their colonization status. Also, in Hall A, 2 clade III environmental isolates recovered near Patient 4’s room matched the colonization status of the patient.
Infection control observations
The ICAR identified significant infection control gaps in communication, isolation precautions, terminal cleaning, antibiotic stewardship, patient transport, hand hygiene, and PPE use. Table 2 shows an overview of infection control gaps identified and remediation recommendations.
Table 2.
Infection control gaps identified and remediation recommendations during a carbapenem-resistant Acinetobacter baumannii (CRAB) outbreak at a long-term care facility, Utah—2018
| Infection control gaps | Recommendations |
|---|---|
|
| |
| Lack of communication | |
| MDRO status not communicated upon transfer across facilities | Communicate MDRO status to receiving facility both verbally and by paper documentation |
| MDRO status not communicated to all staffwithin the facility | Create a multi-disciplinary committee to address infection control and communication gaps within the facility |
| Patients admitted with unknown MDRO status | Consider admission colonization screening and placing incoming patients with unknown MDRO status in isolation precautions |
| Laboratory test results not communicated from other facilities | Consider placing incoming patients with pending lab culture results in isolation precautions until final results are received |
| Low adherence to hand hygiene and PPE use | |
| Lack of monitoring | Adopt the use of a quantitative metric to measure hand hygiene and PPE use, as recommended by the CDC, and display adherence rates to provide feedback to staff |
| Low staff adherence to isolation signage, hand hygiene and PPE-use | Work toward a shared responsibility for hand hygiene and PPE use when providing care, through frequent in-service trainings and post-training observation of staff to demonstrate compliance |
| Visitors not advised to clean hands and wear PPE | Improve signage and provide formal instruction to visitors on hand hygiene and PPE use |
| Inadequate equipment cleaning | |
| No clear delineation of cleaning responsibilities | Establish clear responsibilities for cleaning between housekeeping and staff responsible for specialized medical equipment |
| Lack of documentation of cleaning | Keep records documenting periodic cleaning of equipment (eg, nursing carts carrying wound care equipment, mechanical ventilators, physical therapy equipment) and terminal cleaning of rooms |
| Shared equipment | Clean shared equipment (eg, physical therapy) before and after each use; and clean lower-risk items (eg, nursing carts) at the end of each shift |
| Use of difficult to clean furnishings (eg fabric couches, carpeting) | Implement protocols such as steam cleaning, to properly disinfect difficult-to-clean surfaces for example, carpet and fabric furniture, or consider removal and replacement |
| Improper disposal of biohazard waste | Follow current guidelines for the disposal of biohazard waste |
| Use of multiple cleaning products with different drying times | Consolidate cleaning products and use Environmental Protection Agency (EPA)-approved products containing bleach for routine cleaning |
| Lack of maintenance of individual air conditioner units | Replace individual air system filters according to manufacturer recommendations and before new room occupancy; implement tracking system |
| Lack of antibiotic stewardship | |
| Non-specific antibiotic stewardship plan | Update antibiotic stewardship plan according to CDC 7 core elements |
| Lack of standardized charting of prescription use | Improve and standardize charting of indications for antibiotic use, dosage, and start and stop |
| Overuse of last resort antibiotics | Review antibiotic prescription recommendations with a specialist |
| Movement of equipment and patients | |
| Free movement of staff, patients and visitors between low and high acuity units | Limit access to high acuity unit to individuals providing patient care or family members |
| Transport to external patient services | Ensure containment of MDRO during transport process; communicate MDRO status to transport personnel |
| Lack of dedicated equipment | Use dedicated equipment (eg, blood pressure cuffs, slings for patient lifts etc.) when possible for all patients on the high acuity unit |
| Plan for carbapenemase-producing organism colonization | |
| No long-term plan to address patient colonization | Consider instituting chlorhexidine bathing and oral care for all patients with skin, wound, or oropharyngeal colonization |
| Colonized patients not placed in isolation or isolation precautions lifted too soon | Patients should have 3 negative cultures at least 1 week apart from previously positive sites before lifting isolation precautions |
CDC, Centers for Disease Control and Prevention; MDRO, multidrug-resistant organisms; PPE, personal protective equipment.
Patients transferred from facilities (in-state and out-of-state) lacked appropriate documentation of MDRO status. Three of the 5 patients in the initial cluster had a history of CRAB upon admission identified through out-of-state medical records (Fig 1); however, the facility was not aware of this history and, therefore, did not implement the appropriate isolation protocols. The facility lacked a formal quantitative metric for monitoring hand hygiene and PPE use. Infection control observations found approximately 40% compliance with hand hygiene. Some staff were observed entering isolation rooms without wearing indicated PPE but, other staff had infection control awareness and oriented others to protocol.
Gaps identified in the terminal cleaning of equipment and patient rooms included: confusion over the use of multiple cleaning products with different dry times; unclear delineation of cleaning responsibilities between medical and housekeeping staff; lack of dedicated patient equipment (eg, slings for patient lifts); and lack of documentation of shared equipment cleaning. Lastly, the ICAR revealed gaps in antibiotic stewardship including: inappropriate use of some antibiotics; lack of a formal stewardship plan; and inconsistent charting of antibiotic prescription, use, and dosage.
Network analysis
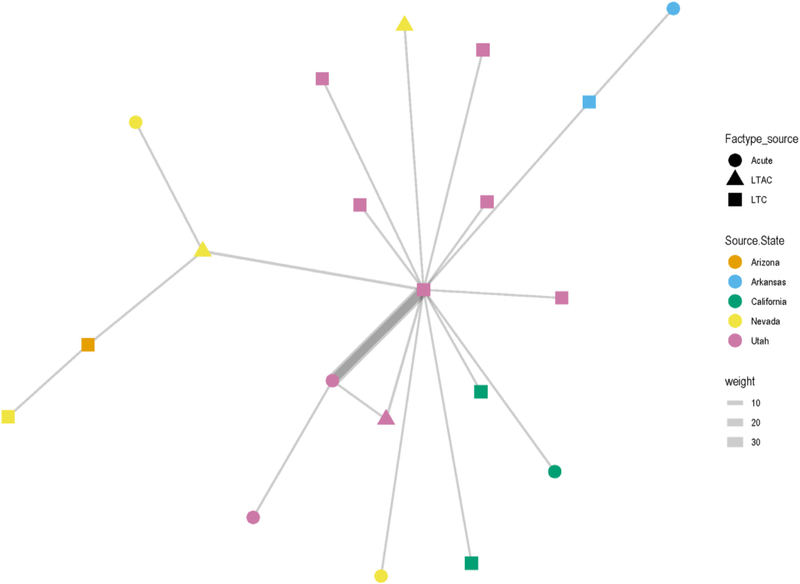
Network analysis demonstrated that initial cluster patients not screened (n = 3) and patients screened for colonization (n = 47) were highly mobile with frequent transfer between long-term care and acute-care facilities (Fig 3). Based on chart review, the majority of transfers occurred between the facility and the closest acute care hospital. Additionally, 18% (10 of 51) of patients were transferred to the facility from another state; 33% (17 of 51) of patients had not been transferred nor had any record of prior transfer.
Fig 3.
Network analysis of patient transfers between long-term, acute care, in-state, and out-of-state facilities. Facility state is represented by color and facility type is represented by shape. The central square is facility A. Line thickness represents the frequency of transfers between facilities; the thicker the line the greater the number of transfers.
DISCUSSION
The investigation helped to build a clearer picture of patients most at risk for CRAB colonization in this setting. Patients who screened positive for carbapenemase-producing carbapenem-resistant Acinetobacter baumannii were more likely to have prior antimicrobial use, invasive device use, wound care, and ventilator care compared to those that screened negative. These risk factors are often associated with high-acuity patients normally seen in intensive care settings and these findings are consistent with reports of CRAB outbreaks elsewhere.11–13,25 There was wide-spread CRAB contamination and colonization in facility A that was not localized to a specific hall or patient room. The 3 clades identified by WGS suggest a multi-source outbreak with multiple introductions and modes of transmission rather than a traditional point-source outbreak (Fig 3). Notably, of the 5 initial patients, 3 had: been transferred from out-of-state; previous documentation of CRAB prior to transfer; a clinical culture or were colonized with a clade II isolate. These findings suggest a common “out-of-state” introduction route for clade II. The current data cannot determine whether the presence of OXA-23 genes observed in all clades involves lateral gene transfer events, neither is it possible to determine whether all patients were colonized prior to admission or whether they acquired CRAB at the facility.
Infection control gaps identified in this investigation (Table 2) were consistent with those identified in other CRAB outbreaks.26–29 Poor hand hygiene and inadequate environmental cleaning likely led to the widespread dissemination of CRAB in the facility as evidenced by the environmental sampling results. In this resource-limited setting, prioritization of recommendations into a condensed intervention bundle was necessary for successful containment.21,29–32 Immediate recommendations included: training and auditing of hand hygiene; and PPE use; limiting access to the high-acuity unit; and improved cleaning of high-risk areas and shared patient equipment. Failure in communication of patient infectious status during transfers was another significant gap identified. Because MDRO status was not communicated to facility A upon transfer, appropriate infection control precautions were not implemented for these patients upon arrival. Literature shows failures in communication during patient transfer is a likely a contributor to transmission in MDRO outbreaks, and active admission screening may reduce transmission, save lives, and lower costs.33–35 Therefore, we recommended admission cultures for incoming patients transferred from another vSNF or from out-of-state, or patients on mechanical ventilation or receiving wound care, to ensure appropriate infection control measures were implemented.36
This investigation demonstrated facility A was an important reservoir for CRAB in the community and highlights the need for improved surveillance, strengthened infection control practices and inter-facility communication within and across states. The patient transfer patterns highlighted in the network analysis (Fig 3) suggest facility A remains a potential reservoir for the surrounding area and necessitates a coordinated regional containment approach amongst facilities sharing patients. Implementation of regional containment strategies that involve all health care facilities sharing patients (acute care, LTACs, and long-term care) has been shown to be more effective than single facility containment activities in reducing MDRO transmission.24
Successful control of previous CRAB outbreaks has been demonstrated in an intensive care unit (ICU) setting using an aggressive multi-modal package of infection control interventions, including; admission and weekly colonization screening and single-room occupancy for infected and colonized patients.26–29 Following Health care Infection Control Practices Advisory Committee guidelines we recommended a similar, multi-faceted intervention bundle to control this outbreak.21 Facility A, similar to other vSNFs, indicated resource restraints compared to acute care facilities in terms of, higher patient-to-nurse ratio, infection prevention staff having competing responsibilities, and limitations in dedicated equipment and existing building infrastructure (shared rooms and heating, ventilation, and air conditioning [HVAC]). Therefore, aggressive infection control tactics commonly implemented in ICU settings were not generally feasible in Facility A, necessitating prioritization of infection control recommendations. Additionally, because the patient population in the high-acuity vSNF setting is similar to those seen in ICUs, containment strategies should be modeled after infection control practices in ICU settings, while also balancing patient quality of life and available resources.
This study describes a point-prevalence analysis of CRAB colonization in a vSNF. Results are from a one-time screen; to get a more complete picture of true CRAB burden in this investigation, multiple screens are warranted to assess efficacy of recommended control interventions. Though we were able to get consent and/or assent for all patients selected for sampling, not all patients in the facility were screened. As such, the extent of colonization at Facility A and certain risk factors may have been underestimated. While literature suggests that buccal and/or pharyngeal is a high-yield site for Acinetobacter recovery,22,23 detection of CRAB from this site was likely reduced because of swab collection coinciding with resident breakfast. Generalization of risk factors is difficult in this study because only high-risk patients were sampled. Despite limitations, the infection control findings are relevant for other health care facilities, specifically long-term care settings.
CONCLUSIONS
The results of this investigation suggest that vSNFs may serve as a reservoir of highly resistant pathogens. Containment in this resource-restrained setting requires an aggressive multi-faceted infection control approach that will address the multiple gaps that have contributed to the outbreak. Resources should be prioritized to enhance basic infection control practices such as: hand hygiene and PPE use; surveillance; environmental cleaning, and communication between providers. An understanding of patient transfer patterns and increased surveillance and reporting of CRAB throughout the United States, and a regional approach focusing on inter-facility connections would improve regional containment efforts.
Supplementary Material
Footnotes
Conflicts of interest: None to report.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2020.11.012.
References
- 1.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis Off Publ Infect Dis Soc Am. 2006;42:692–699. [DOI] [PubMed] [Google Scholar]
- 2.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Cuenca F, Tomas M, Caballero-Moyano F-J, et al. Reduced susceptibility to biocides in Acinetobacter baumannii: association with resistance to antimicrobials, epidemiological behaviour, biological cost and effect on the expression of genes encoding porins and efflux pumps. J Antimicrob Chemother. 2015;70:3222–3229. [DOI] [PubMed] [Google Scholar]
- 4.Da Silva GJ, Domingues S. Insights on the horizontal gene transfer of carbapenemase determinants in the opportunistic pathogen Acinetobacter baumannii. Microorganisms. 2016;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2009;65:233–238. [DOI] [PubMed] [Google Scholar]
- 6.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. [DOI] [PubMed] [Google Scholar]
- 7.Rello J, Kalwaje Eshwara V, Lagunes L, et al. A global priority list of the TOp TEn resistant Microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2019;38:319–323. [DOI] [PubMed] [Google Scholar]
- 8.Bulens SN, Yi SH, Walters MS, et al. Carbapenem-nonsusceptible Acinetobacter baumannii, 8 US Metropolitan Areas, 2012–2015. Emerg Infect Dis. 2018; 24:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balkhair A, Al-Muharrmi Z, Al’Adawi B, et al. Prevalence and 30-day all-cause mortality of carbapenem-and colistin-resistant bacteraemia caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae: description of a decade-long trend. Int J Infect Dis. 2019;85:10–15. [DOI] [PubMed] [Google Scholar]
- 10.Bayuga S, Zeana C, Sahni J, Della-Latta P, El-Sadr W, Larson E. Prevalence and antimicrobial patterns of Acinetobacter baumannii on hands and nares of hospital personnel and patients: the iceberg phenomenon again. Heart Lung. 2002;31:382–390. [DOI] [PubMed] [Google Scholar]
- 11.Mody L, Gibson KE, Horcher A, et al. Prevalence of and risk factors for multidrug-resistant Acinetobacter baumannii colonization among high-risk nursing home residents. Infect Control Hosp Epidemiol. 2015;36:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Gethamy MM, Faidah HS, Adetunji HA, et al. Risk factors associated with multidrug-resistant Acinetobacter baumannii nosocomial infections at a tertiary care hospital in Makkah, Saudi Arabia - a matched case-control study. J Int Med Res. 2017;45:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco N, Harris AD, Rock C, et al. Risk Factors and outcomes associated with multidrug-resistant Acinetobacter baumannii upon intensive care unit admission. Antimicrob Agents Chemother. 2018;62. Available at: https://aac.asm.org/content/62/1/e01631-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans BA, Amyes SGB. OXA -Lactamases. Clin Microbiol Rev. 2014;27:241–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams-Haduch JM, Onuoha EO, Bogdanovich T, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011;49:3849–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigro SJ, Hall RM. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J Antimicrob Chemother. 2016;71:1135–1147. [DOI] [PubMed] [Google Scholar]
- 17.U.S. centers for disease control and prevention. Antibiotic Resistance Threats in the United States. 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed November 11, 2020. [Google Scholar]
- 18.Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(suppl 7):S521–S528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oakeson KF, Wagner JM, Mendenhall M, Rohrwasser A, Atkinson-Dunn R. Bioinformatic analyses of Whole-Genome sequence data in a public health laboratory. Emerg Infect Dis. 2017;23:1441–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oakeson KF, Wagner JM, Rohrwasser A, Atkinson, Dunn R Whole-genome sequencing and bioinformatic analysis of isolates from foodborne illness outbreaks of Campylobacter jejuni and Salmonella enterica. J Clin Microbiol. 2018;56:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel JD, Rhinehart E, Jackson M, Chiarello L. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35:S165–S193. [DOI] [PubMed] [Google Scholar]
- 22.Doi Y, Onuoha EO, Adams-Haduch JM, et al. Screening for Acinetobacter baumannii colonization by use of sponges. J Clin Microbiol. 2011;49:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nutman A, Lerner A, Schwartz D, Carmeli Y. uation of carriage and environmental contamination by carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect. 2016;22. 949.e6. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP). Healthcare-associated infection outbreak investigation abstraction form. 2016. [Google Scholar]
- 25.Cheng VC, Chen JH, Ng W, et al. Emergence of carbapenem-resistant Acinetobacter baumannii in nursing homes with high background rates of MRSA colonization. Infect Control Hosp Epidemiol. 2016;37:983–986. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Chetrit E, Wiener-Well Y, Lesho E, et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: long-term impact for the ICU and hospital. Crit Care Lond Engl. 2018. 21;22:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molaeb B How to successfully manage multi-drug-resistant Acinetobacter baumannii trasnmissions in intensive care unit? J Infect Public Health. 2019;12:129. [Google Scholar]
- 28.Tomczyk S, Zanichelli V, Grayson ML, et al. Control of Carbapenem-resistant enter-obacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in healthcare facilities: a systematic review and reanalysis of quasi-experimental studies. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019;68:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheon S, Kim M-J, Yun S-J, Moon JY, Kim Y-S. Controlling endemic multidrug-resistant Acinetobacter baumannii in Intensive Care Units using antimicrobial stewardship and infection control. Korean J Intern Med. 2016;31:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8:751–762. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Baño J, García L, Ramírez E, et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am J Infect Control. 2009;37:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enfield KB, Huq NN, Gosseling MF, et al. Control of simultaneous outbreaks of carba-penemase-producing enterobacteriaceae and extensively drug-resistant Acinetobacter baumannii infection in an intensive care unit using interventions promoted in the Centers for Disease Control and Prevention 2012 carbapenemase-resistant Enterobacteriaceae Toolkit. Infect Control Hosp Epidemiol. 2014;35:810–817. [DOI] [PubMed] [Google Scholar]
- 33.Kohlenberg A, Brummer S, Higgins PG, et al. Outbreak of carbapenem-resistant Acinetobacter baumannii carrying the carbapenemase OXA-23 in a German university medical centre. J Med Microbiol. 2009;58:1499–1507. [DOI] [PubMed] [Google Scholar]
- 34.Buser GL, Cassidy PM, Cunningham MC, et al. Failure to communicate: transmission of extensively drug-resistant bla OXA-237-containing Acinetobacter baumannii-multiple facilities in oregon, 2012–2014. Infect Control Hosp Epidemiol. 2017;38:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BY, McGlone SM, Doi Y, Bailey RR, Harrison LH. Economic value of Acinetobacter baumannii screening in the intensive care unit. Clin Microbiol Infect. 2011;17:1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in health care facilities. 2017. Available at: http://apps.who.int/iris/bitstream/10665/259462/1/9789241550178-eng.pdf?ua=1. Accessed November 11, 2020. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.