Abstract
Aims/hypothesis
In men with diabetes, the prevalence of erectile dysfunction increases with advanced age and longer diabetes duration and is substantially higher in men with type 2 diabetes than those with type 1 diabetes. This study aimed to evaluate the prevalence of erectile dysfunction among the five novel subgroups of recent-onset diabetes and determine the strength of associations between diabetes subgroups and erectile dysfunction.
Methods
A total of 351 men with recent-onset diabetes (<1 year) from the German Diabetes Study baseline cohort and 124 men without diabetes were included in this cross-sectional study. Erectile dysfunction was assessed with the International Index of Erectile Function (IIEF) questionnaire. Poisson regression models were used to estimate associations between diabetes subgroups (each subgroup tested against the four other subgroups as reference) and erectile dysfunction (dependent binary variable), adjusting for variables used to define diabetes subgroups, high-sensitivity C-reactive protein and depression.
Results
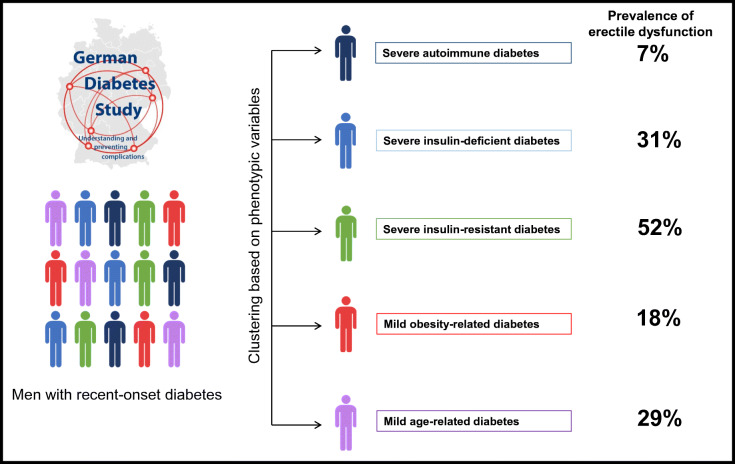
The prevalence of erectile dysfunction was markedly higher in men with diabetes than in men without diabetes (23% vs 11%, p = 0.004). Among men with diabetes, the prevalence of erectile dysfunction was highest in men with severe insulin-resistant diabetes (SIRD) (52%), lowest in men with severe autoimmune diabetes (SAID) (7%), and intermediate in men with severe insulin-deficient diabetes (SIDD), mild obesity-related diabetes (MOD) and mild age-related diabetes (MARD) (31%, 18% and 29%, respectively). Men with SIRD had an adjusted RR of 1.93 (95% CI 1.04, 3.58) for prevalent erectile dysfunction (p = 0.038). Similarly, men with SIDD had an adjusted RR of 3.27 (95% CI 1.18, 9.10) (p = 0.023). In contrast, men with SAID and those with MARD had unadjusted RRs of 0.26 (95% CI 0.11, 0.58) (p = 0.001) and 1.52 (95% CI 1.04, 2.22) (p = 0.027), respectively. However, these associations did not remain statistically significant after adjustment.
Conclusions/interpretation
The high RRs for erectile dysfunction in men with recent-onset SIRD and SIDD point to both insulin resistance and insulin deficiency as major contributing factors to this complication, suggesting different mechanisms underlying erectile dysfunction in these subgroups.
Graphical abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-021-05607-z.
Keywords: Diabetes subgroups, Erectile dysfunction, Inflammation, Insulin resistance, New-onset diabetes
Introduction
Erectile dysfunction, defined as the persistent inability to obtain or maintain a penile erection during sexual intercourse [1], is a disorder affecting mainly men older than 40 years [2] and is associated with poor relationship quality, low life satisfaction and low self-esteem [3]. In addition, erectile dysfunction is associated with an increased risk of future cardiovascular events, CHD, stroke and all-cause mortality [2, 4, 5].
Men with diabetes are three times more susceptible to develop the condition than men without diabetes [6]. The higher risk of erectile dysfunction in diabetes is associated with higher age, sedentary lifestyle and the presence of the metabolic syndrome (i.e. hyperglycaemia, hypertension and obesity) [7, 8]. Additionally, several studies demonstrated that distal sensorimotor polyneuropathy (DSPN), cardiac autonomic neuropathy (CAN), CVD, non-alcoholic fatty liver disease (NAFLD), depression and hypogonadotropic hypogonadism increase the risk of erectile dysfunction [9–13]. Available epidemiological data report a higher prevalence of erectile dysfunction in men with type 2 diabetes than those with type 1 diabetes. A systematic review and meta-analysis summarising data of 145 studies showed a prevalence of 66% in type 2 diabetes vs 37% in type 1 diabetes [14]. Of note, recent studies using data-driven cluster analyses have refined this established classification of diabetes to include five subgroups that reflect better the heterogeneity of the disease [15, 16]. Therefore, an updated assessment of the prevalence of erectile dysfunction is timely.
Indeed, the novel diabetes subgroups showed a different prevalence of diabetes-related complications. The subgroup designated severe insulin-deficient diabetes (SIDD) has the highest prevalence of retinopathy, DSPN and CAN [15, 16], suggesting, therefore, the possibility of a higher prevalence of erectile dysfunction in SIDD. In addition, the subgroup with severe insulin-resistant diabetes (SIRD) had the highest cardiovascular risk [15] and the highest levels of biomarkers of inflammation, including high-sensitivity C-reactive protein (hs-CRP) [16, 17], which may raise the hypothesis that this subgroup might also exhibit a higher erectile dysfunction prevalence.
Therefore, this study aimed: (1) to assess the prevalence of self-reported erectile dysfunction among novel subgroups of recent-onset diabetes; (2) to assess the strength of the associations between diabetes subgroups and prevalent erectile dysfunction; and (3) to investigate to what extent the clustering variables, hs-CRP and depression explain these associations. Such knowledge could help to understand the underlying mechanisms better and improve effective disease management.
Methods
Study design and study population
The German Diabetes Study (GDS) is an ongoing observational prospective study established in 2005 to evaluate the natural course of recently diagnosed diabetes and explore prognostic factors and mechanisms leading to diabetes-related complications [18]. Individuals with known diabetes duration of <1 year and aged 18–69 years at the time of the baseline examination are eligible to participate, while individuals with secondary forms of diabetes, current pregnancy and acute or severe chronic cardiac, hepatic, renal or psychiatric diseases are excluded. Diabetes diagnosis is based on the ADA criteria [19], and diabetes-related autoantibodies are measured in every participant. All participants undergo a comprehensive examination at baseline comprising standardised questionnaires, clinical examinations and detailed laboratory measurements.
The GDS is conducted according to the Declaration of Helsinki, approved by the ethics committee of Heinrich Heine University, Düsseldorf, Germany (ref. 4508) and was registered with ClinicalTrials.gov registration no. NCT01055093. All participants provided written informed consent.
This cross-sectional analysis focused on 539 consecutive men who were allocated to one of the five diabetes subgroups as part of our previously published data-driven cluster analysis [16]. From this sample, we excluded 188 men (95 men who did not have any sexual attempt during the last 4 weeks and 93 men who did not respond to one or more questions regarding their erectile function), leaving 351 men with diabetes subgroup allocation and complete data on erectile function. The study sample is illustrated in electronic supplementary material (ESM) Fig. 1. This analysis also included 124 men without diabetes from the GDS who have complete data on erectile function. Inclusion criteria for this group were age ≥18 years, normal glucose tolerance and absence of first-degree relatives with diabetes, while exclusion criteria corresponded to those defined for individuals with diabetes.
Assessment of erectile dysfunction
Erectile dysfunction was defined using questions one to five of the self-reported International Index of Erectile Function (IIEF-5) questionnaire in men who had a least one sexual attempt within the last 4 weeks [20, 21]. The IIEF-5 is a validated tool frequently used to assess erectile dysfunction in epidemiological studies or to assess treatment response in intervention trials. Participants were asked five questions to evaluate their ability to achieve and maintain an erection sufficient for satisfactory sexual intercourse without any treatment. Response options included ‘never’, ‘rarely’, ‘sometimes’, ‘mostly’ and ‘always’ and were ranked from 1 to 5. A total score ranging from 5 to 25 was calculated, with lower IIEF scores indicating poorer erectile function. Men with an IIEF score <22 are considered to have erectile dysfunction.
Data collection and measurements
The measurement of laboratory variables including HbA1c, fasting C-peptide, fasting glucose, HDL-cholesterol, LDL-cholesterol, triacylglycerols and GAD antibodies (GADA) was performed according to standardised laboratory procedures [16, 18]. HOMA2-B and HOMA2-IR were calculated with the HOMA2 calculator based on fasting C-peptide and fasting glucose (https://www.dtu.ox.ac.uk/homacalculator/, accessed 1 Dec 2020). The eGFR was calculated from serum creatinine and cystatin C using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation. Two biomarkers of systemic inflammation (hs-CRP, IL-6) and two biomarkers of vascular inflammation (soluble intercellular adhesion molecule-1 [sICAM-1], soluble E-selectin [sE-selectin]) were measured as described [22, 23]. These four biomarkers were selected because they indicate increased cardiovascular risk and potential endothelial dysfunction [24].
Information on known diabetes duration, anthropometric and lifestyle factors, and on the presence of chronic diseases and medication use (glucose-lowering drugs [insulin/metformin/none/other], non-steroidal anti-inflammatory drugs [yes/no], lipid-lowering drugs [yes/no] and phosphodiesterase-5 inhibitors [yes/no]) was obtained from questionnaires [18]. Prevalent CVD was defined as self-reported myocardial infarction, peripheral arterial occlusive disease, cerebrovascular disease and stroke. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg or use of antihypertensive medication. Peripheral nerve function was assessed with electrophysiological testing [23]. Neurological examination was performed using the Neuropathy Disability Score (NDS), while neuropathic symptoms were assessed using the Neuropathy Symptom Score (NSS) [25]. DSPN was defined according to the Toronto Consensus criteria [26] based on electrophysiological testing, NDS and NSS as described [23]. Autonomic nerve function was measured with cardiovascular autonomic reflex tests, including heart rate variability indices as described before [27]. CAN was diagnosed if three or more out of seven indices were abnormal [27]. Depressive symptoms were assessed using the Allgemeine Depressionsskala, Langversion (ADS-L), the German version of the internationally validated Center for Epidemiological Studies Depression Scale (CES-D). The ADS-L score ranges from 0 to 60, with a score ≥21 indicating depression [28].
Statistical analysis
This analysis builds on our previous work [16], which allocated GDS participants to the novel diabetes subgroups using centroids primarily identified in several Scandinavian cohorts [15]. The novel diabetes classification was based on age at diagnosis, BMI, HbA1c, HOMA2-B, HOMA2-IR and GADA [15, 16], and led to five subgroups (clusters). The first subgroup is severe autoimmune diabetes (SAID) comprising individuals with type 1 diabetes and determined by the presence of GADA. The other four subgroups, i.e. SIDD, SIRD, mild obesity-related diabetes (MOD) and mild age-related diabetes (MARD), are subtypes reflecting the heterogeneity of type 2 diabetes.
Data are presented as median (25th/75th percentiles) or percentages in descriptive statistics. Differences in characteristics according to erectile dysfunction status, diabetes status and diabetes subgroup allocation were tested with the Mann–Whitney U (Wilcoxon) test, the Kruskal–Wallis test and the χ2 test. The prevalence of erectile dysfunction is reported using percentages with 95% CIs. Differences in the subgroup distribution and clinical characteristics of included vs excluded participants from the original sample used to perform the cluster analysis were compared with the Mann–Whitney U (Wilcoxon) test and the χ2 test.
Associations between diabetes subgroups (independent variable) and erectile dysfunction (binary dependent variable) were assessed using Poisson regression models with a robust error variance. Model 1 was unadjusted. Model 2 was adjusted for the clustering variables (age at diagnosis, BMI, HbA1c, HOMA2-B, HOMA2-IR and GADA; all co-variables used as continuous variables). Model 3 was additionally adjusted for log2-transformed hs-CRP (the only tested biomarker of inflammation that showed a significant difference between men with and without erectile dysfunction). Model 4 was additionally adjusted for depression. First, each diabetes subgroup was tested against the other subgroups as a reference group. Second, the five diabetes subgroups were tested in pairs (ten pairwise associations) accounting for multiple group comparisons by applying the Tukey–Kramer correction in all models. Associations were estimated with RRs of prevalent erectile dysfunction and their corresponding 95% CIs.
Additional analyses included men without diabetes as a reference group. As a sensitivity analysis, we repeated the analysis after excluding participants with prevalent CVD because CVD may represent an intermediate step in the causal pathway between diabetes and erectile dysfunction [9, 10, 29, 30], which might bias our estimates.
All statistical analyses were carried out with SAS version 9.4 (SAS Institute, Cary, NC, USA), and p values <0.05 were considered indicators of a statistically significant difference or association.
Results
Prevalence of erectile dysfunction in the total study sample
Erectile dysfunction was present in 23% of all men with diabetes (N = 351). The characteristics of men with (n = 82) and without erectile dysfunction (n = 269) are displayed in Table 1. Men with erectile dysfunction were older, had higher BMI values, higher HOMA2-IR and higher triacylglycerols and hs-CRP but lower HDL-cholesterol levels. Also, men with and without erectile dysfunction differed in their use of glucose-lowering drugs and prevalent DSPN but were similar in their glycaemic control, smoking status, eGFR values, hypertension and prevalence of CVD, CAN and depression.
Table 1.
Clinical characteristics in the total study sample and stratified by erectile dysfunction
| Erectile dysfunction | |||||
|---|---|---|---|---|---|
| Characteristic | N | Total sample | Present: n = 82 (23%) | Absent: n = 269 (77%) | p |
| IIEF score | 351 | 25 (22, 25) | 18 (14, 20) | 25 (24, 25) | <0.0001 |
| Age (years) | 351 | 49.4 (39.1–57.6) | 55.2 (47.5–62.3) | 47.5 (36.4–55.5) | <0.0001 |
| BMI (kg/m2) | 351 | 28.1 (25.3–32.5) | 28.7 (26.6–32.0) | 27.5 (25.2–31.4) | 0.022 |
| Diabetes subgroups, % | 351 | <0.0001 | |||
| SAID | 23 | 7 | 28 | ||
| SIDD | 4 | 5 | 3 | ||
| SIRD | 7 | 17 | 5 | ||
| MOD | 25 | 20 | 26 | ||
| MARD | 41 | 51 | 38 | ||
| Diabetes duration (days) | 351 | 177 (104–262) | 185 (110–272) | 175 (104–254) | 0.587 |
| HbA1c (mmol/mol) | 351 | 44.2 (39.9–51.9) | 43.1 (39.9–48.6) | 44.2 (39.9–51.9) | 0.658 |
| HbA1c (%) | 351 | 6.2 (5.8–6.9) | 6.1 (5.8–6.6) | 6.2 (5.8–6.9) | 0.658 |
| HOMA2-B | 351 | 76.1 (52.0–110.4) | 80.0 (60.0–111.9) | 74.0 (50.7–110.4) | 0.225 |
| HOMA2-IR | 351 | 1.9 (1.2–2.8) | 2.2 (1.5–3.7) | 1.8 (1.1–2.7) | 0.0004 |
| GADA>0.9 units/ml, % | 351 | 23 | 7 | 28 | 0.0001 |
| Current smokers, % | 288 | 27 | 24 | 27 | 0.436 |
| eGFR (ml min−1 [1.73 m]−2) | 324 | 95 (85–105) | 91 (82–103) | 96 (85, 105) | 0.060 |
| Triacylglycerols (mmol/l) | 351 | 1.36 (0.89–2.02) | 1.65 (1.08–2.50) | 1.31 (0.84–1.86) | 0.0005 |
| HDL-cholesterol (mmol/l) | 345 | 1.16 (0.96–1.39) | 1.09 (0.90–1.29) | 1.19 (0.98–1.42) | 0.011 |
| LDL-cholesterol (mmol/l) | 345 | 3.13 (2.56–3.78) | 3.28 (2.64–3.85) | 3.10 (2.53–3.75) | 0.310 |
| Hypertension, % | 350 | 62 | 68 | 60 | 0.161 |
| CVD, % | 344 | 6 | 9 | 5 | 0.259 |
| DSPN, % | 324 | 16 | 26 | 13 | 0.010 |
| CAN, % | 348 | 4 | 6 | 3 | 0.274 |
| Depression, % | 351 | 10 | 13 | 9 | 0.235 |
| Glucose-lowering drugs, % | 346 | <0.0001 | |||
| None | 30 | 34 | 28 | ||
| Metformin | 32 | 52 | 26 | ||
| Insulin | 30 | 10 | 36 | ||
| Other | 9 | 4 | 10 | ||
| Lipid-lowering drugs, % | 351 | 13 | 13 | 13 | 0.854 |
| NSAIDs, % | 351 | 13 | 13 | 13 | 0.854 |
| hs-CRP (nmol/l) | 333 | 19.0 (9.5–28.6) | 19.0 (9.5–38.1) | 9.5 (9.5–28.6) | 0.046 |
| IL-6 (pg/ml) | 188 | 1.5 (1.0–2.3) | 1.8 (1.2–2.2) | 1.4 (0.9–2.3) | 0.084 |
| sICAM-1 (ng/ml) | 188 | 230 (199–272) | 223 (198–247) | 239 (200–275) | 0.148 |
| sE-selectin (ng/ml) | 188 | 41.1 (29.8–52.8) | 40.9 (31.7–53.6) | 41.2 (29.2–52.6) | 0.778 |
Continuous variables are given as median (25th percentile–75th percentile) and categorical variables are given as percentages (%)
NSAIDs, non-steroidal anti-inflammatory drugs
Men excluded from the analysis (N = 188) were older and had a higher BMI, higher HOMA2-IR, lower eGFR and higher hs-CRP than men who were included (N = 351), but there were no differences in the other characteristics (ESM Table 1).
As shown in ESM Table 2, men without diabetes (N = 124) had a lower prevalence of erectile dysfunction (11%, p = 0.004) than men with diabetes.
Prevalence of erectile dysfunction across diabetes subgroups
The prevalence of erectile dysfunction among diabetes subgroups is shown in ESM Table 3 and visually illustrated in Fig. 1. The prevalence of erectile dysfunction was highest in SIRD (52%), lowest in SAID (7%), and intermediate in SIDD (31%), MOD (18%) and MARD (29%) (p < 0.0001). Diabetes subgroups also showed differences in other baseline characteristics (ESM Table 3). Briefly, men with SAID (n = 81, 23%) were younger. Men with SIDD (n = 13, 4%) had poorer glycaemic control, lower HOMA2-B and a higher prevalence of CAN. Men with SIRD (n = 27, 7%) had pronounced insulin resistance, lower eGFR, higher triacylglycerol levels, more often hypertension and higher hs-CRP. Men with MOD (n = 87, 25%) had higher BMI values and men with MARD (n = 143, 41%) were older. The subgroups distribution did not differ between included and excluded study participants (ESM Table 1).
Fig. 1.
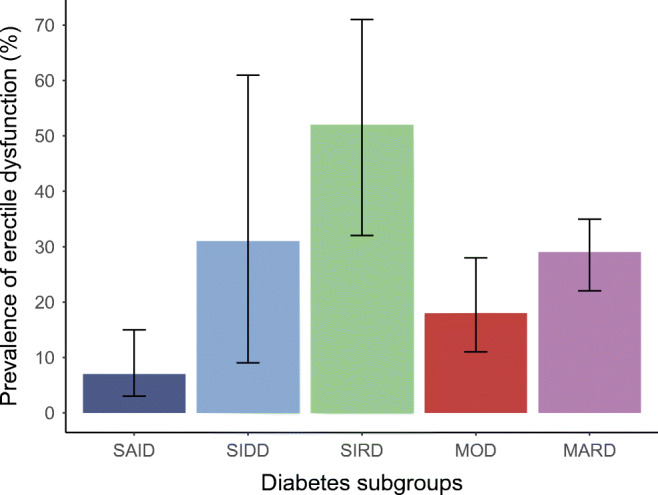
Prevalence of erectile dysfunction across subgroups of recent-onset diabetes from the GDS. Data are given as percentages and corresponding 95% CIs
Associations between diabetes subgroups and erectile dysfunction
The associations between each diabetes subgroup (using the other four subgroups as reference) and erectile dysfunction are shown in Table 2. Men with SIRD had a higher prevalence of erectile dysfunction than men with other diabetes types, and this association remained consistent and statistically significant across the four models (model 4: RR 1.93 [95% CI 1.04, 3.58], p = 0.038). Men with SIDD also had a higher prevalence of erectile dysfunction than men with other diabetes types after adjustment for clustering variables in model 2, which remained stable after further adjustment (model 4 (RR 3.27 [95% CI 1.18, 9.10], p = 0.023). The most prominent confounders increasing the RR from model 1 to model 2 were age and HOMA2-IR. Men with MARD had a higher (RR 1.52 [95% CI 1.04, 2.22], p = 0.027) and men with SAID had a lower (RR 0.26 [95% CI 0.11, 0.58], p = 0.001) prevalence of erectile dysfunction in model 1, but these associations did not remain significant in models 2, 3 and 4. No association was observed between MOD and erectile dysfunction.
Table 2.
Associations between diabetes subgroups and erectile dysfunction
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Diabetes subgroup | RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p |
| SAID | 0.26 (0.11, 0.58) | 0.001 | 0.47 (0.15, 1.41) | 0.179 | 0.44 (0.15, 1.28) | 0.132 | 0.44 (0.15, 1.26) | 0.126 |
| SIDD | 1.33 (0.57, 3.08) | 0.501 | 2.86 (1.07, 7.59) | 0.034 | 3.12 (1.14, 8.54) | 0.026 | 3.27 (1.18, 9.10) | 0.023 |
| SIRD | 2.47 (1.62, 3.76) | <0.0001 | 2.15 (1.14, 4.03) | 0.017 | 2.04 (1.10, 3.79) | 0.023 | 1.93 (1.04, 3.58) | 0.038 |
| MOD | 0.73 (0.45, 1.20) | 0.218 | 0.76 (0.41, 1.40) | 0.386 | 0.74 (0.40, 1.34) | 0.328 | 0.74 (0.40, 1.33) | 0.313 |
| MARD | 1.52 (1.04, 2.22) | 0.027 | 0.94 (0.55, 1.61) | 0.841 | 1.02 (0.59, 1.76) | 0.917 | 1.06 (0.61, 1.83) | 0.833 |
Each diabetes subgroup was tested against the four other diabetes subgroups as reference group
Model 1: unadjusted; model 2: adjusted for age, BMI, HbA1c, HOMA2-B, HOMA2-IR and GADA; model 3: adjusted for model 2 + log2 hs-CRP; model 4: adjusted for model 3 + depression
The results of the associations between each pair of diabetes subgroups and erectile dysfunction are shown in Table 3. In the unadjusted model (model 1), men with SIRD had a higher prevalence of erectile dysfunction than men with MOD. In contrast, men with SAID had a lower prevalence of erectile dysfunction than men with SIRD or MARD. After adjusting for the clustering variables (model 2), men with SIRD had a higher prevalence of erectile dysfunction than men with MOD or MARD, whereas men with SAID had a lower prevalence of erectile dysfunction than men with SIDD or SIRD. However, none of these associations in model 2 remained significant after adjustment for multiple comparisons. After additional adjustment for hs-CRP (model 3) and depression (model 4), RRs for all comparisons remained almost unaltered. In the fully adjusted model 4, men with SIRD still had a higher prevalence of erectile dysfunction than men with MOD (RR 2.21 [95% CI 1.02, 4.79], p = 0.043), and men with SAID had a lower prevalence of erectile dysfunction than men with SIDD (RR 0.18 [95% CI 0.04, 0.77], p = 0.020) or SIRD (RR 0.23 [95% CI 0.06, 0.83], p = 0.025). However, these associations were not statistically significant after adjustment for multiple comparisons.
Table 3.
Pairwise associations between diabetes subgroups and erectile dysfunction
| Subgroup comparison | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | p | p Tukey–Kramer | RR (95% CI) | p | p Tukey–Kramer | RR (95% CI) | p | p Tukey–Kramer | RR (95% CI) | p | p Tukey–Kramer | |
| SAID vs SIDD | 0.24 (0.08, 0.74) | 0.013 | 0.093 | 0.21 (0.05, 0.88) | 0.033 | 0.205 | 0.19 (0.05, 0.80) | 0.023 | 0.156 | 0.18 (0.04, 0.77) | 0.020 | 0.142 |
| SAID vs SIRD | 0.14 (0.06, 0.33) | <0.0001 | <0.0001 | 0.20 (0.05, 0.77) | 0.019 | 0.132 | 0.21 (0.06, 0.77) | 0.019 | 0.129 | 0.23 (0.06, 0.83) | 0.025 | 0.165 |
| SAID vs MOD | 0.40 (0.16, 0.98) | 0.045 | 0.262 | 0.48 (0.15, 1.55) | 0.223 | 0.740 | 0.49 (0.16, 1.51) | 0.213 | 0.725 | 0.51 (0.17, 1.54) | 0.233 | 0.755 |
| SAID vs MARD | 0.25 (0.11, 0.57) | 0.0009 | 0.008 | 0.44 (0.14, 1.40) | 0.165 | 0.636 | 0.41 (0.13, 1.28) | 0.125 | 0.539 | 0.41 (0.13, 1.27) | 0.122 | 0.533 |
| SIDD vs SIRD | 0.59 (0.24, 1.45) | 0.252 | 0.782 | 0.95 (0.26, 3.41) | 0.935 | 1.000 | 1.09 (0.29, 3.95) | 0.901 | 0.999 | 1.24 (0.33, 4.60) | 0.750 | 0.998 |
| SIDD vs MOD | 1.67 (0.66, 4.23) | 0.277 | 0.813 | 2.26 (0.78, 6.52) | 0.131 | 0.556 | 2.53 (0.88, 7.30) | 0.086 | 0.422 | 2.74 (0.93, 8.04) | 0.066 | 0.352 |
| SIDD vs MARD | 1.05 (0.44, 2.46) | 0.915 | 1.000 | 2.06 (0.72, 5.88) | 0.178 | 0.661 | 2.15 (0.73, 6.34) | 0.166 | 0.638 | 2.23 (0.74, 6.71) | 0.155 | 0.614 |
| SIRD vs MOD | 2.82 (1.59, 4.10) | 0.0004 | 0.004 | 2.38 (1.01, 5.17) | 0.028 | 0.179 | 2.33 (1.09, 5.00) | 0.029 | 0.189 | 2.21 (1.02, 4.79) | 0.043 | 0.257 |
| SIRD vs MARD | 1.76 (1.13, 2.75) | 0.012 | 0.088 | 2.17 (1.05, 4.49) | 0.037 | 0.225 | 1.98 (0.95, 4.12) | 0.068 | 0.359 | 1.80 (0.87, 3.72) | 0.113 | 0.508 |
| MOD vs MARD | 0.63 (0.37, 1.04) | 0.072 | 0.375 | 0.91 (0.43, 1.91) | 0.804 | 0.999 | 0.85 (0.39, 1.80) | 0.670 | 0.993 | 0.81 (0.38, 1.74) | 0.593 | 0.984 |
Model 1: unadjusted; model 2: adjusted for age, BMI, HbA1c, HOMA2-B, HOMA2-IR and GADA; model 3: adjusted for model 2 + log2 hs-CRP; model 4: adjusted for model 3 + depression
The results of the sensitivity analysis are shown in ESM Table 4. After excluding men with prevalent CVD (6%), the associations were consistent with the primary analysis, with the SIRD and SIDD subgroups being associated with a higher prevalence of erectile dysfunction.
The results of the additional analyses are shown in ESM Table 5. Compared with men without diabetes, men with diabetes, particularly men with SIRD and MARD, had significantly higher unadjusted RRs for erectile dysfunction. Although these associations were attenuated after adjustments, the effect estimates for all subgroups combined or analysed separately remained >1 (e.g. RR 1.53 [95% CI 0.85, 2.78] for the comparison of all men with diabetes vs those without diabetes).
Discussion
This study found a prevalence of erectile dysfunction of 23% in middle-aged men with recent-onset diabetes, which is about double that in men without diabetes. Pathophysiology-based diabetes subgroups showed differences in the prevalence of erectile dysfunction. Men with SIRD had the highest, whereas those with SAID had the lowest erectile dysfunction prevalence. The SIRD and SIDD subgroups showed higher RRs for erectile dysfunction in multivariable models, and these associations were independent of the clustering variables, hs-CRP and depression. In contrast to previous studies on erectile dysfunction in long-standing diabetes, the unique feature of this study is its focus on newly diagnosed disease and the consideration of the heterogeneity of diabetes pathophysiology reflected by the five diabetes subgroups.
The observed erectile dysfunction prevalence of 23% in men with diabetes in GDS was in line with previous reports (20–37%) in men with recent diabetes diagnosis [31–33]. In contrast, most studies conducted in men with longer-term diabetes report a higher prevalence ranging from 35 to 90% [6]. Notably, a meta-analysis of 145 studies in men with longer-term type 1 and type 2 diabetes reported an overall erectile dysfunction prevalence of 52.5% [14]. This variation in the prevalence of erectile dysfunction might be attributed to differences in the study populations (age, ethnicity, diabetes duration) and definition of erectile dysfunction (e.g. single item question or validated multi-item scale) [34, 35]. The lower prevalence of erectile dysfunction in our study may relate to the rather young age of our study population (median age 49.4 years), their short diabetes duration (<1 year), their good health status including good glucometabolic control and low prevalence of CVD and CAN [18], and the use of a multi-item questionnaire to define erectile dysfunction only in sexually active men, unlike several previous studies which used one single item question to assess erectile function regardless of sexual activity.
SIRD, the subgroup characterised by obesity and pronounced insulin resistance, was associated with higher RR for prevalent erectile dysfunction than all the other four subgroups, particularly when compared with MOD, the subgroup characterised by obesity but not by insulin resistance. These associations were robust across models when considering all four subgroups as a reference and in pairwise associations. This finding reinforces that insulin resistance increases the risk of erectile dysfunction [36] and explains the high prevalence of erectile dysfunction in the metabolic syndrome [8, 21], which is often characterised by insulin resistance as a key feature. The molecular mechanism underlying the association between insulin resistance and erectile dysfunction involves endothelial dysfunction in the penile arteries, decreasing their synthesis and release of endothelial nitric oxide. However, it is unclear whether endothelial damage in penile arteries is related to whole-body insulin resistance, which is characterised by decreased responsiveness of the liver, adipose tissue and skeletal muscle to insulin, or is instead related to decreased endothelial insulin sensitivity. Evidence from in vivo studies suggests that endothelium-specific insulin resistance can induce endothelial dysfunction independently of whole-body insulin resistance [37]. In our study, associations between diabetes subgroups and erectile dysfunction were only slightly attenuated after adjustment for HOMA2-IR, indicating that whole-body insulin resistance may only partly explain the observed associations. HOMA2-IR is primarily an index of hepatic insulin resistance but also shows a good correlation with the hyperinsulinaemic–euglycaemic clamp, the ‘gold-standard’ technique for evaluating insulin-stimulated glucose metabolism and whole-body insulin resistance [16, 38]. Of note, SIRD was the subgroup with the lowest HbA1c in our study, which indicates that glycaemic control may not be a major determinant of erectile dysfunction in adults with recent-onset diabetes.
Low-grade systemic inflammation is present in insulin resistance, obesity and type 2 diabetes suggesting that the association between insulin resistance and endothelial dysfunction in erectile dysfunction might be linked to inflammation. Accumulating evidence supports the fact that elevated circulating concentrations of biomarkers of inflammation such as hs-CRP and IL-6, and biomarkers of vascular inflammation such as sICAM-1 and sE-selectin, indicate increased cardiovascular risk and damage to the endothelium [24]. In turn, vascular damage triggers an inflammatory reaction and release of proinflammatory mediators, promoting insulin resistance and endothelial dysfunction [39]. In our study, while serum levels of IL-6, sICAM-1 and sE-selectin did not differ between men with and without erectile dysfunction, we found an increase in hs-CRP levels similar to other studies [40–43].
Because insulin resistance precedes diabetes, the development of erectile dysfunction may have started during the prediabetic stage. Studies assessing erectile dysfunction before and after diabetes diagnosis are needed to clarify these relationships. It is also worth mentioning that our findings in men with recent-onset diabetes cannot be generalised to men with long-standing diabetes who are likely to have longer exposure to chronic hyperglycaemia, dyslipidaemia and hypertension associated with greater risk of diabetes-related complications.
Our study showed higher RRs for prevalent erectile dysfunction in men with SIDD after adjustment for the clustering variables, suggesting that low insulin levels not yet compensated with insulin therapy might be involved in erectile dysfunction in men newly diagnosed with diabetes. However, because diabetes subgroup allocation can change over time as a result of treatment and disease progression, a repeated erectile dysfunction assessment is necessary to confirm this finding. Of note, SIRD and SIDD are different in their clinical presentation. Men with SIRD have a pronounced insulin resistance, but also higher BMI and the highest prevalence of hypertension and impaired kidney function based on eGFR. On the other hand, men with SIDD have the worst glycaemic control and highest prevalence of CAN. The observation that both subgroups appear to have similarly increased prevalence of erectile dysfunction suggests a different mechanism underlying their erectile dysfunction. In SIDD, the more classical concept of glucotoxicity seems to be operative, whereas in SIRD pathomechanisms related to insulin resistance such as lipotoxicity, oxidative stress and low-grade inflammation could contribute to their erectile dysfunction. Indeed, individuals with SIRD also present with a higher degree of dyslipidaemia and markers of inflammation [17]. If confirmed in other studies, these observations would have clinically relevant implications.
Differences in erectile dysfunction between diabetes subgroups reported in our study further support the evidence about differences in complications between novel diabetes subgroups. Ahlqvist et al showed a higher risk for CVD in SIRD, although this was explained by sex and age [15]. Because erectile dysfunction and CVD share endothelial dysfunction as a common feature, our findings consolidate the increased risk of SIRD for vascular diseases. Also, Zaharia et al [16] observed a higher prevalence of NAFLD in SIRD; thus, we hypothesised that a higher prevalence of erectile dysfunction might be found in this subgroup [11]. The mechanism underlying both conditions might involve insulin resistance and, to some extent, low serum testosterone [11, 36, 44–46]. Unfortunately, we did not have information on testosterone levels in our study. Thus, we cannot rule out the potential confounding effect of hypogonadism.
Moreover, findings from the GDS and the Scandinavian cohorts showed a higher prevalence of CKD in SIRD [15, 16]. Given that almost 70% of men with CKD report erectile dysfunction [47], a high prevalence of erectile dysfunction in SIRD was plausible. In addition to the higher prevalence of diabetes complications in SIRD, we previously demonstrated that a higher proinflammatory state also characterises this subgroup [17]. These characteristics make it the subgroup that might benefit from early diagnosis and treatment to prevent complications. In this context, it is interesting that a recent clinical trial showed a reduced incidence of erectile dysfunction in men with type 2 diabetes and high cardiovascular risk treated with the GLP-1 receptor agonist dulaglutide [48]. Given the phenotypic similarities between the study population of this trial and the SIRD subgroup, this drug might be of particular therapeutic benefit for individuals with SIRD.
Comparing the proportions of diabetes subgroups in included vs excluded participants did not indicate any selection bias. However, a certain degree of selection bias was evident when comparing the baseline characteristics. For example, the participants included in the present analysis had a slightly better cardiometabolic risk profile than those who were excluded, which might have attenuated the differences between diabetes subgroups.
The strengths of our study are the inclusion of men with type 1 diabetes and type 2 diabetes and the information on the clustering variables, which allowed us to investigate the prevalence of erectile dysfunction in the novel diabetes subgroups. Furthermore, the study included adults within their first year of diabetes diagnosis so that findings were not confounded by long-term hyperglycaemia and the increasing prevalence of other diabetes-related complications.
Our study also has some limitations that warrant consideration when interpreting the results. Limitations include its sample size (specifically limiting the statistical power of pairwise comparisons between diabetes subgroups and of comparisons with non-diabetic men) and its restriction to men from Germany. Therefore, our findings need validation in studies with larger sample sizes and more diverse populations. In addition, although we used a validated questionnaire to assess erectile dysfunction, our assessment is limited because the IIEF questionnaire only evaluates erectile dysfunction acquired during the last 4 weeks, whereas ‘persistent’ erectile dysfunction can only be confirmed if lasting more than 3–6 months [49], and if complemented by a physical examination. Circulating levels of sex hormones and information on primary or secondary hypogonadism were not available for the study sample, meaning that their analysis in the context of the novel diabetes subgroups was not possible. Another limitation is the potential response bias; as this study involved an outcome related to male sexuality, questions concerning sexual performance may feel too personal for some men and increase the likelihood of not answering.
Conclusion
Our study shows that novel diabetes subgroups have different prevalences of erectile dysfunction. Men with SIRD, which is characterised by pronounced insulin resistance and an increased inflammatory state, and men with SIDD, which is characterised by insulin deficiency, have the highest RRs for erectile dysfunction. This finding suggests that metabolic risk factors for erectile dysfunction may differ between diabetes subgroups. The high prevalence of erectile dysfunction in SIRD and SIDD corroborates their high risk for diabetes-related complications and calls for comprehensive screening and early treatment in these subgroups. Detailed longitudinal analyses in the GDS and future studies on therapeutic responses in the context of precision diabetes medicine will clarify whether these findings will translate into clinical benefits.
Supplementary Information
(PDF 156 kb)
Acknowledgements
We appreciate the contribution of all study participants. The authors thank the staff of the Clinical Research Center at the Institute for Clinical Diabetology, German Diabetes Center, for their excellent work. Data from this study were presented at the virtual EDEG Annual Meeting, 26-27 April 2021, the virtual Diabetes Congress of the German Diabetes Association (DDG), 12–15 May 2021 and the virtual Central European Diabetes Association (CEDA) Congress, 10–12 June 2021.
Authors’ relationships and activities
CH received a research grant from Sanofi-Aventis outside the submitted work, and is a member of the editorial board of Diabetologia. MR received fees as a member of advisory boards or speaker from Allergan, Boehringer Ingelheim Pharma, Bristol Myers Squibb, Eli Lilly, Fishawack Group, Gilead Sciences, Intercept Pharma, Inventiva, Novartis Pharma, Novo Nordisk, TARGET-NASH and Terra Firma and has been involved with clinical trial research for Boehringer Ingelheim, Danone Nutricia Research and Sanofi-Aventis, all outside the submitted work. The remaining authors declare that there is no duality of interest associated with this manuscript.
Abbreviations
- ADS-L
Allgemeine Depressionsskala, Langversion
- CAN
Cardiac autonomic neuropathy
- CES-D
Center for Epidemiological Studies Depression Scale
- CKD
Chronic kidney disease
- DSPN
Distal sensorimotor polyneuropathy
- GADA
GAD antibodies
- GDS
German Diabetes Study
- hs-CRP
High-sensitivity C-reactive protein
- IIEF
International Index of Erectile Function
- MARD
Mild age-related diabetes
- MOD
Mild obesity-related diabetes
- NAFLD
Non-alcoholic fatty liver disease
- NDS
Neuropathy Disability Score
- NSS
Neuropathy Symptom Score
- SAID
Severe autoimmune diabetes
- sE-selectin
Soluble E-selectin
- sICAM-1
Soluble intercellular adhesion molecule-1
- SIDD
Severe insulin-deficient diabetes
- SIRD
Severe insulin-resistant diabetes
Contribution statement
HM and CH designed the study. CH, OPZ, VB, JS, DZ and MR contributed data. HM drafted the analysis plan and performed the statistical analysis. CH and KS contributed to the statistical analysis. HM, CH, DZ and MR interpreted data. HM wrote the manuscript. CH, DZ and MR contributed to the draft of the manuscript. All authors reviewed and edited the manuscript and approved its submission. HM is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL. The German Diabetes Study (GDS) was initiated and financed by the German Diabetes Center (DDZ), which is funded by the German Federal Ministry of Health (Berlin, Germany), the Ministry of Culture and Science of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research (Berlin, Germany) to the German Center for Diabetes Research e.V. (DZD). The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Data availability
The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, data can be requested through an individual project agreement with the Steering Committee of the GDS (speaker: Michael Roden, michael.roden@ddz.de).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michael Roden and Dan Ziegler contributed equally to this study.
The GDS Group consists of H. Al-Hasani, V. Burkart, A. E. Buyken, G. Geerling, C. Herder, A. Icks, K. Jandeleit-Dahm, J. Kotzka, O. Kuss, E. Lammert, W. Rathmann, V. Schrauwen-Hinderling, J. Szendroedi, S. Trenkamp, D. Ziegler and M. Roden (speaker).
References
- 1.Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 3.De Berardis G, Franciosi M, Belfiglio M, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care. 2002;25:284–291. doi: 10.2337/diacare.25.2.284. [DOI] [PubMed] [Google Scholar]
- 4.Montorsi F, Briganti A, Salonia A, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–364. doi: 10.1016/S0302-2838(03)00305-1. [DOI] [PubMed] [Google Scholar]
- 5.Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378–1385. doi: 10.1016/j.jacc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6:1232–1247. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 7.Phe V, Roupret M. Erectile dysfunction and diabetes: a review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab. 2012;38:1–13. doi: 10.1016/j.diabet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu LH, Zhang T, Zhang YR, et al. Metabolic syndrome and risk for ED: a meta-analysis. Int J Impot Res. 2014;26:196–200. doi: 10.1038/ijir.2014.3. [DOI] [PubMed] [Google Scholar]
- 9.Pop-Busui R, Hotaling J, Braffett BH, et al. Cardiovascular autonomic neuropathy, erectile dysfunction and lower urinary tract symptoms in men with type 1 diabetes: findings from the DCCT/EDIC. J Urol. 2015;193:2045–2051. doi: 10.1016/j.juro.2014.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessells H, Penson DF, Cleary P, et al. Effect of intensive glycemic therapy on erectile function in men with type 1 diabetes. J Urol. 2011;185:1828–1834. doi: 10.1016/j.juro.2010.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasanain AFA, Mahdy RE, Mahran AMA, Safwat ASM, Mohamed AO, Abdel-Aal SM. Erectile dysfunction in patients with nonalcoholic fatty liver disease. Arab J Gastroenterol. 2017;18:21–24. doi: 10.1016/j.ajg.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Montorsi P, Ravagnani PM, Galli S, et al. Common grounds for erectile dysfunction and coronary artery disease. Curr Opin Urol. 2004;14:361–365. doi: 10.1097/00042307-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Corona G, Giorda CB, Cucinotta D, Guida P, Nada E. Sexual dysfunction at the onset of type 2 diabetes: the interplay of depression, hormonal and cardiovascular factors. J Sex Med. 2014;11:2065–2073. doi: 10.1111/jsm.12601. [DOI] [PubMed] [Google Scholar]
- 14.Kouidrat Y, Pizzol D, Cosco T, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34:1185–1192. doi: 10.1111/dme.13403. [DOI] [PubMed] [Google Scholar]
- 15.Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 16.Zaharia OP, Strassburger K, Strom A, et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 2019;7:684–694. doi: 10.1016/S2213-8587(19)30187-1. [DOI] [PubMed] [Google Scholar]
- 17.Herder C, Maalmi H, Strassburger K, et al. Differences in biomarkers of inflammation between novel subgroups of recent-onset diabetes. Diabetes. 2021;70:1198–1208. doi: 10.2337/db20-1054. [DOI] [PubMed] [Google Scholar]
- 18.Szendroedi J, Saxena A, Weber KS, et al. Cohort profile: the German diabetes study (GDS) Cardiovasc Diabetol. 2016;15:59. doi: 10.1186/s12933-016-0374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 20.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/S0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 21.Esposito K, Giugliano F, Martedi E, et al. High proportions of erectile dysfunction in men with the metabolic syndrome. Diabetes Care. 2005;28:1201–1203. doi: 10.2337/diacare.28.5.1201. [DOI] [PubMed] [Google Scholar]
- 22.Herder C, Schamarek I, Nowotny B, et al. Inflammatory markers are associated with cardiac autonomic dysfunction in recent-onset type 2 diabetes. Heart. 2017;103:63–70. doi: 10.1136/heartjnl-2015-309181. [DOI] [PubMed] [Google Scholar]
- 23.Schamarek I, Herder C, Nowotny B, et al. Adiponectin, markers of subclinical inflammation and nerve conduction in individuals with recently diagnosed type 1 and type 2 diabetes. Eur J Endocrinol. 2016;174:433–443. doi: 10.1530/EJE-15-1010. [DOI] [PubMed] [Google Scholar]
- 24.Tamler R, Bar-Chama N. Assessment of endothelial function in the patient with erectile dysfunction: an opportunity for the urologist. Int J Impot Res. 2008;20:370–377. doi: 10.1038/ijir.2008.13. [DOI] [PubMed] [Google Scholar]
- 25.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–154. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- 26.Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27:620–628. doi: 10.1002/dmrr.1226. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler D, Dannehl K, Mühlen H, Spüler M, Gries FA. Prevalence of cardiovascular autonomic dysfunction assessed by spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses at various stages of diabetic neuropathy. Diabet Med. 1992;9:806–814. doi: 10.1111/j.1464-5491.1992.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 28.Stein J, Luppa M. Allgemeine Depressionsskala (ADS) Psychiatr Prax. 2012;39:302–304. doi: 10.1055/s-0033-1343176. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim A, Ali M, Kiernan TJ, Stack AG. Erectile dysfunction and Ischaemic heart disease. Eur Cardiol. 2018;13:98–103. doi: 10.15420/ecr.2017.21.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raheem OA, Su JJ, Wilson JR, Hsieh TC. The Association of Erectile Dysfunction and Cardiovascular Disease: a systematic critical review. Am J Mens Health. 2017;11:552–563. doi: 10.1177/1557988316630305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Hunayan A, Al-Mutar M, Kehinde EO, Thalib L, Al-Ghorory M. The prevalence and predictors of erectile dysfunction in men with newly diagnosed with type 2 diabetes mellitus. BJU Int. 2007;99:130–134. doi: 10.1111/j.1464-410X.2006.06550.x. [DOI] [PubMed] [Google Scholar]
- 32.Corona G, Giorda CB, Cucinotta D, Guida P, Nada E, SUBITO-DE study group The SUBITO-DE study: sexual dysfunction in newly diagnosed type 2 diabetes male patients. J Endocrinol Investig. 2013;36:864–868. doi: 10.3275/8969. [DOI] [PubMed] [Google Scholar]
- 33.Junuzovic D, Hasanbegovic M, Masic I. Risk factors for erectile dysfunction in patients with newly diagnosed diabetes mellitus. Med Arh. 2010;64:345–347. doi: 10.5455/medarh.2010.64.345-347. [DOI] [PubMed] [Google Scholar]
- 34.Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7:1598–1607. doi: 10.1111/j.1743-6109.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 35.Hayes RD, Dennerstein L, Bennett CM, Fairley CK. What is the "true" prevalence of female sexual dysfunctions and does the way we assess these conditions have an impact? J Sex Med. 2008;5:777–787. doi: 10.1111/j.1743-6109.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Wu R, Huang Y, et al. Insulin resistance is an independent determinate of ED in young adult men. PLoS One. 2013;8:e83951. doi: 10.1371/journal.pone.0083951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan ER, Crossey PA, Walker S, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Phys. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 39.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Chen K, Zhang J, et al. Association between serum high-sensitivity C-reactive protein levels and erectile dysfunction: a cross-sectional study of Chinese male population. Sci Rep. 2019;9:5929. doi: 10.1038/s41598-019-42342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elzanaty S, Rezanezhad B, Willenheimer R, Borgquist R. Association between erectile function and biomarkers of subclinical atherosclerosis: a study based on middle-aged healthy men from the general population. Curr Urol. 2016;9:119–123. doi: 10.1159/000442865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlachopoulos C, Aznaouridis K, Ioakeimidis N, et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur Heart J. 2006;27:2640–2648. doi: 10.1093/eurheartj/ehl341. [DOI] [PubMed] [Google Scholar]
- 43.Vlachopoulos C, Rokkas K, Ioakeimidis N, Stefanadis C. Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common links. Eur Urol. 2007;52:1590–1600. doi: 10.1016/j.eururo.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Kwon H, Park JH, et al. A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol. 2012;12:69. doi: 10.1186/1471-230X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajfer J. Relationship between testosterone and erectile dysfunction. Rev Urol. 2000;2:122–128. [PMC free article] [PubMed] [Google Scholar]
- 46.Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013;9:479–493. doi: 10.1038/nrendo.2013.122. [DOI] [PubMed] [Google Scholar]
- 47.Papadopoulou E, Varouktsi A, Lazaridis A, Boutari C, Doumas M. Erectile dysfunction in chronic kidney disease: from pathophysiology to management. World J Nephrol. 2015;4:379–387. doi: 10.5527/wjn.v4.i3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajaj HS, Gerstein HC, Rao-Melacini P, et al. Erectile function in men with type 2 diabetes treated with dulaglutide: an exploratory analysis of the REWIND placebo-controlled randomised trial. Lancet Diabetes Endocrinol. 2021;9:484–490. doi: 10.1016/S2213-8587(21)00115-7. [DOI] [PubMed] [Google Scholar]
- 49.Pastuszak AW. Current diagnosis and Management of Erectile Dysfunction. Curr Sex Health Rep. 2014;6:164–176. doi: 10.1007/s11930-014-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 156 kb)
Data Availability Statement
The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, data can be requested through an individual project agreement with the Steering Committee of the GDS (speaker: Michael Roden, michael.roden@ddz.de).