Abstract
Burn injuries elicit a unique and dynamic stress response which can lead to burn injury progression. Though neutrophils represent crucial players in the burn-induced immunological events, the dynamic secretion pattern and systemic levels of neutrophil-derived factors have not been investigated in detail so far. Serum levels of neutrophil elastase (NE), myeloperoxidase (MPO), citrullinated histone H3 (CitH3), and complement factor C3a were quantified in burn victims over 4 weeks post injury. Furthermore, the potential association with mortality, degree of burn injury, and inhalation trauma was evaluated. In addition, leukocyte, platelet, neutrophil, and lymphocyte counts were assessed. Lastly, we analyzed the association of neutrophil-derived factors with clinical severity scoring systems. Serum levels of NE, MPO, CitH3, and C3a were remarkably elevated in burn victims compared to healthy controls. Leukocyte and neutrophil counts were significantly increased on admission day and day 1, while relative lymphocytes were decreased in the first 7 days post burn trauma. Though neutrophil-derived factors did not predict mortality, patients suffering from 3rd degree burn injuries displayed increased CitH3 and NE levels. Accordingly, CitH3 and NE were elevated in cases with higher abbreviated burn severity indices (ABSI). Taken together, our data suggest a role for neutrophil activation and NETosis in burn injuries and burn injury progression. Targeting exacerbated neutrophil activation might represent a new therapeutic option for severe cases of burn injury.
Subject terms: Immunology, Biomarkers, Medical research
Introduction
Burn injury is an umbrella term for a trauma, most commonly affecting the skin or lung, caused by a variety of external challenges, such as thermal extremes, deleterious radiation, alkaline and acidic chemicals, or excessive friction1. The respective cause and burn degree further dictate clinical management, such as surgical intervention and moist rewarming following heat- and cold-induced injuries, respectively. In spite of the development of new therapeutic approaches, burn injuries are still associated with a high mortality rate, most commonly resulting from multiple organ failure, sepsis, and respiratory complications2. Morbidity and mortality can be increased up to 10 years after the initial insult3.
Burn trauma elicits a unique systemic stress response characterized by increased metabolism and inflammation4. The early systemic inflammatory response syndrome (SIRS) is characterized by pro-inflammatory cytokines, such as interleukin 6 (IL-6), IL-8, and tumor necrosis factor alpha (TNFα) and usually lasts for several days5,6. Later, the milieu is shifted towards an anti-inflammatory response syndrome (AIRS), where the immunosuppressant mediators TGFβ1, and IL-10 prevail6. Though inflammation is inherent and indispensable for normal wound healing7, burn injuries can induce a state of distorted inflammatory response which can persist up to several years8 and which can ultimately lead to host tissue damage and organ dysfunction. Various factors orchestrate early immune reactions, such as the amount of affected body surface area, burn depth and cause, inhalation injury, patient age, and chronic medical conditions9. Neutrophils10 and macrophages11 are early key players infiltrating the burn-injured area. Though these immune cells are readily activated by burn injury-induced damage-associated molecular patterns (DAMPs), macrophage antigen presentation and neutrophil-mediated killing of pathogens have been shown to be diminished following burn injury12,13, leading to increased susceptibility to infections. While immunological hyper-activation entails tissue damage, exaggerated immunosuppression predisposes to infections14. Hence, a delicate balance of immunomodulators determines clinical outcome in burn injuries.
A commonly observed phenomenon of burn victims is secondary burn progression15. While tissue necrosis and ischemia are immediate results of burn injury, tissue damage is often aggravated long after the initial trauma. Formation of red blood cell aggregates and microthrombi and subsequent occlusion of the microvasculature have been identified as the underlying cause of burn progression16. Though secondary burn progression substantially promotes tissue damage, its role in the post injury tissue response is often underestimated.
Neutrophils are implicated in the host defense against pathogens, whereby antimicrobial activity is exerted by phagocytosis, release of effector molecules, and formation of neutrophil extracellular traps (NETs)17. Activated protein-arginine deiminase 4 (PAD4) catalyzes citrullination of histones, which results in chromatin decondensation. Granule proteins, such as myeloperoxidase (MPO) and neutrophil elastase (NE), further promote DNA de-compaction and intracellular DNA together with granule proteins are released following plasma membrane rupture18,19. It was further demonstrated that neutrophils contain intracellular stores of C3a20, which might be self-synthesized or absorbed from the serum21. Moreover, C3a was reported to induce neutrophil activation22. A role for neutrophils in coagulation and microvascular obstruction has been described at several occasions23–27. The amount of neutrophil-derived circulating, free DNA was proposed as a predictor of mortality in severely burnt patients28 and elevated human neutrophil elastase DNA and nucleosomes were detected in burn and sepsis patients29. Though previous studies have reported a role for neutrophils and NETs in burn injury30,31, systemic surrogate markers indicating NETosis have not been comprehensively studied so far. Our group was previously able to show activation of the soluble suppressor of tumorigenicity 2 (sST2)/IL-33 axis in sepsis32 as well as thermal burn injury33 and found increased soluble ST2 to be a predictor of mortality. In the current study, we aimed to delineate the dynamics of neutrophil-derived immunomodulators to deepen our understanding of NETosis in the post-burn injury immune response.
Materials and methods
Ethical statements
This study was approved by the institutional review board of the Medical University of Vienna (Vienna, Austria) (vote 593/2011) and was conducted in accordance with the Declaration of Helsinki and applicable local regulations. Written informed consent was obtained from all donors.
Patient cohort and serum sample acquisition
Samples used in the current study have already been analyzed for sST2 and IL-3333. Patients > 18 years who were admitted within 24 h post trauma to burn intensive care unit and presented with a burn injury covering > 10% of the total body surface area (TBSA) at primary survey were included in this study (Table 1). To determine TBSA, affected areas were marked on a blank human body diagram and were subsequently quantified. Patients displaying 3rd degree burns were defined as 3rd degree-positive, irrespective of the affected area. Patients with chronic infectious diseases or autoimmune disorders were excluded. Eight healthy volunteers served as controls. Exclusion criteria for healthy donors were chronic medication and chronic conditions. All volunteers displayed an unremarkable previous medical history.
Table 1.
Study group demographics.
| Burn patients | Controls | |
|---|---|---|
| n | 32 | 8 |
| Age in years* | 51.9 (46.5) ± 21.9 [33–74] | 40.5 (36) ± 19.9 [23–56] |
| F:M ratio (%) | 10:22 (31.3:68.7) | 3:5 (37.5:62.5) |
| TBSA (%)* | 32.5 (30.0) ± 19.6 [16.3–39.5] | |
| LOH (days)* | 37.2 (27.5) ± 33.9 [10.5–61.5] | |
| LOS (days)* | 29.7 (19.5) ± 33.0 [7.3–35.3] | |
| SOFA* | 6.8 (6.5) ± 3.6 [5–9] | |
| APACHE II* | 19.7 (18.0) ± 9.3 [13.5–28.0] | |
| ABSI* | 7.91 (8.0) ± 2.8 [5–9] | |
| Deceased (%)§ | 6 (18.8) | |
| 3rd degree burn (%)§ | 22 (68.8) | |
| Inhalation trauma (%)§ | 7 (21.9) |
ABSI abbreviated burn severity index, APACHE II acute physiology and chronic health evaluation II, F:M female to male ratio, LOH length of hospitalization, LOS length of stay at intensive care unit, SOFA sequential organ failure assessment, TBSA total body surface area.
*Indicated are mean (median) ± SD [interquartile range].
§Indicated are n (SD).
The first serum sample was immediately collected upon patient admission. For all other time points, blood was routinely drawn in the morning. To assess the early dynamics in a detailed manner and to investigate potential long-term effects, sera were obtained daily within the first week after admission, and weekly up to day 28 after admission. For controls, samples were obtained in the same manner. Whole blood was incubated for thirty minutes at ambient temperature and centrifuged at 2850 relative centrifugal force for 17 min. Sera were separated and cryopreserved below -70 °C.
Intensive care management including fluid resuscitation was performed according to institutional guidelines and was not affected by this study. Inhalation trauma was assessed on primary survey and deemed present in cases of visible signs of thermal injury within the upper airway. Mortality was defined as all-cause in-hospital mortality. Depending on the time of death, the elapsed time between sample acquisition and decease varied between < 1 and 23 h.
Analyte quantifications
Serum MPO, CitH3, NE, C3a, and lactadherin (milk fat globulin protein E8, MFG-E8) were quantified by enzyme-linked immunosorbent assay (ELISA) using commercially available kits as recommended by the manufacturers (human MPO, human neutrophil elastase, human MFG-E8 Immunoassay Quantikine, all R&D Systems, Bio-Techne, Minneapolis, MN, USA; human CitH3, clone 11D3, Cayman Chemical, Ann Arbor, MI, USA; human complement C3a, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Colorimetric measurements were performed using Tecan F50 infinite microplate reader (Tecan Group, Männedorf, Switzerland) with Magellan software (version 7.2, Tecan). Analyte concentrations were determined by external standard curves. Samples were measured once and arithmetic means show averages of biological replicates.
Laboratory measurements of clinical parameters
Complete blood counts were determined during routine clinical tests and reference values were adopted from in-house reference ranges used for routine patient diagnosis. The reference ranges for absolute leukocytes were 4–10 G/L, for absolute platelets 150–350 G/L, for absolute neutrophils 2–7.5 G/L, for relative neutrophils 50–75%, for absolute lymphocytes 1–4 G/L, and for relative lymphocytes 25–40%. As controls were not hospitalized, these data were only available from burn victims. Sequential organ failure assessment (SOFA) score34, acute physiology and chronic health evaluation II (APACHE II) score35, and abbreviated burn severity index (ABSI)36 were assessed on the day of admission. Median values of severity scores were used to assign values to low and high groups. As the SOFA score of our study population displayed a median of 6.5, we chose ≤ 6 and ≥ 7 as cut-off values.
Statistical analyses
Data were statistically evaluated and visualized using SPSS Statistics (version 25, IBM, Armonk, NY, USA) and GraphPad Prism (version 5.01, GraphPad Software Inc., La Jolla, CA, USA). Continuous variables were compared by the Mann–Whitney test. One-way ANOVA and multiple comparison post hoc tests with Sidak’s or Dunnett’s correction were calculated. For correlation analysis, Pearson’s correlation coefficients were calculated. Data are presented as arithmetic means ± standard error of the mean.
Results
Neutrophil-derived factors are systemically elevated following burn injury
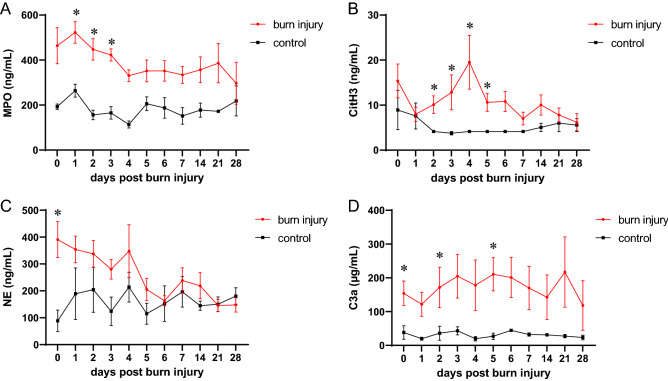
To study the role of burn injury-induced NETosis, we tracked the neutrophil-derived factors MPO, CitH3, NE, and C3a in sera of burn victims up to 4 weeks post trauma. We found that NETosis-associated factors were significantly increased compared to healthy controls (Fig. 1). MPO was remarkably increased in the early days post burn injury (Fig. 1A). While CitH3 did not differ from controls on admission day and day 1, serum CitH3 concentrations showed a delayed increase, peaking on day 4 post admission (Fig. 1B), indicating a potential ‘second burn’ hit. Intriguingly, NE levels were elevated on admission day and in the early stress response to burn trauma before approximating levels detected in healthy controls (Fig. 1C). Serum C3a was strongly elevated in burn victims compared to controls (Fig. 1D). To test a potential connection between systemic C3a levels and liver parameters in burn victims, we furthermore assessed gamma-glutamyltransferase (γ-GT) levels. γ-GT started increasing 2 weeks post injury, but did not correlate with C3a (Supplemental Figure S1). Since lactadherin promoted survival of septic rats37 and lactadherin prevented coagulopathy following traumatic brain injury38, we addressed the question whether lactadherin might also be involved in the burn injury-induced immune response. When assessing serum lactadherin levels, we observed no difference between burn victims and healthy controls (Supplemental Figure S2). We furthermore aimed to investigate the relationship between neutrophil-derived factors and found a positive correlation between MPO and NE, but not between CitH3 and NE or between CitH3 and MPO (Supplemental Figure S3). These data suggest a role for NETosis in the systemic post burn injury immune response.
Figure 1.
Elevated neutrophil-derived factors and C3a in burn sera. Systemic levels of (A) MPO, (B) CitH3, (C) NE, and (D) C3a were quantified in sera of burn victims (n = 8–19) and healthy controls (n = 4–6) up to 4 weeks post injury. Data were compared by mixed effects analysis and Sidak’s multiple comparisons post hoc test. Data are presented as means ± s.e.m. Asterisks indicate p values < .05.
Burn injury induced leukocytosis and relative lymphopenia
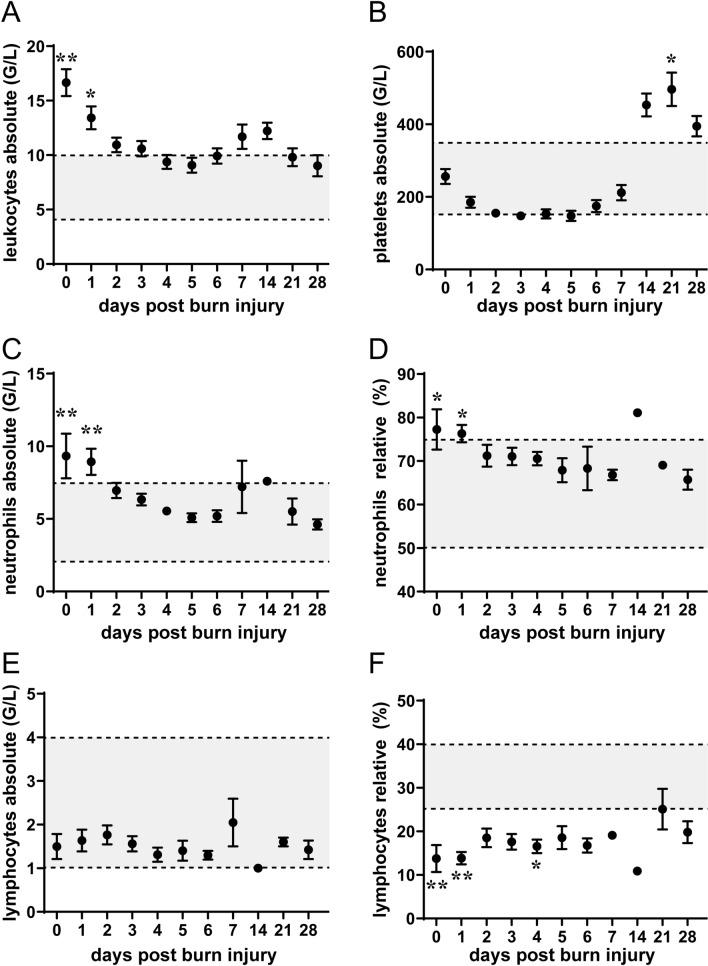
Next, we assessed immune cell status in our patient cohort. We found that absolute leukocyte and neutrophil numbers were strongly increased in burn victims (Fig. 2A, C, D). In detail, cell numbers were elevated in the early days post injury, decreased within the first week before incrementing again. While platelet counts of burn victims were below those of healthy controls within the first week post injury, we observed a remarkable increase in platelet numbers 3 weeks post trauma (Fig. 2B). These data suggest a delayed, immunological ‘second hit’ and burn injury progression even weeks after primary injury. Absolute lymphocyte counts showed no difference between controls and burn victims, while relative lymphocyte levels were strongly decreased in the first 7 days after injury (Fig. 2E, F). Together, these data indicate an intricate systemic effect of burn injury on immune cell status.
Figure 2.
Immune cell counts up to 4 weeks post injury. Patient data in relation to established in-hospital reference standards (n = 1–31). Data are presented as means ± s.e.m.
3rd degree burns are associated with increased CitH3 and NE
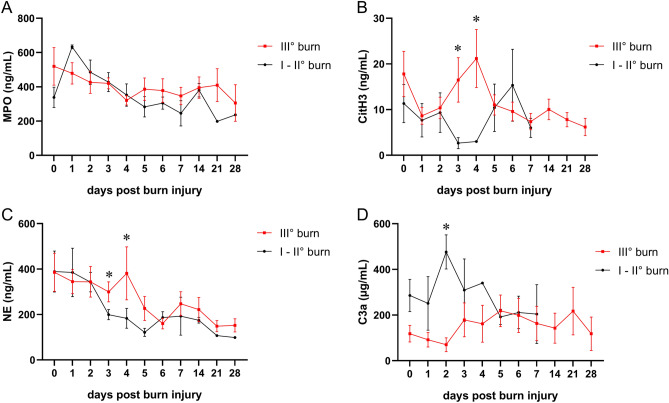
We aimed to test whether our set of serum markers might be associated with mortality, inhalation trauma, or 3rd degree burn injury. Our battery of neutrophil-derived factors did not predict mortality, and C3a was even strongly reduced in deceased burn victims (Supplemental Figure S4). We furthermore compared burn victims with inhalation trauma to those without respiratory damage and observed no effect of lung injury on serum levels of neutrophil-derived factors (Supplemental Figure S5). Similarly, serum MPO levels displayed no difference when comparing 3rd degree burn victims with patients suffering from 1st and 2nd degree burns (Fig. 3A). By comparison, we observed elevated serum CitH3 and NE levels on days 3 and 4 of 3rd degree burns compared to first and second degree burns (Figs. 3B, C). Of note, the delayed increase of serum CitH3 3 and 4 days post injury were exclusively detected in 3rd degree burns. Surprisingly, C3a levels were strongly reduced in higher degree burns on day 3 (Fig. 3D). These findings suggest that the degree of burn injury determines the extent of neutrophil activation and NETosis.
Figure 3.
Increased levels of neutrophil-derived factors and C3a in higher degree burns. Serum concentrations of (A) MPO, (B) CitH3, (C) NE, and (D) C3a in 3rd degree burn victims (n = 7–15) compared to lower degree burns (n = 1–6). Data are presented as means ± s.e.m. * indicates p values < .05.
CitH3 is elevated in patients with higher severity scores
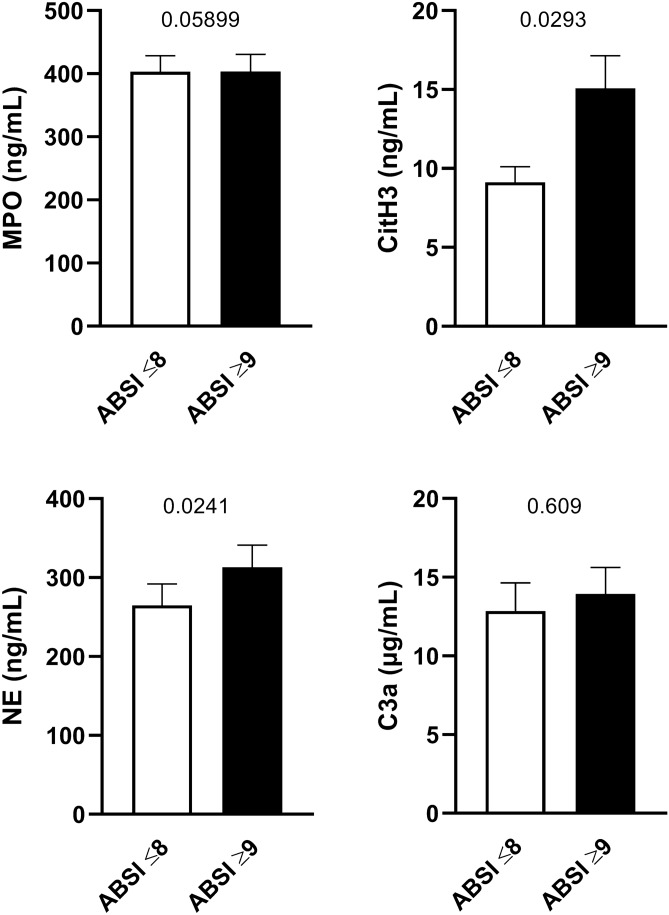
We sought to determine whether neutrophil-derived factors were associated with clinical severity scoring systems and dichotomized SOFA, APACHE II, and ABSI scores into low and high values. We observed increased MPO and CitH3 levels in patients with APACHE II scores above 18 (Supplemental Figure S6) and NE was significantly higher in SOFA scores ≥ 7 (Supplemental Figure S7). Intriguingly, CitH3 and NE levels were elevated in patients with ABSI scores above 9, while MPO and C3a showed no difference (Fig. 4). These data suggest that specific NETosis-associated factors are elevated in more severe burns.
Figure 4.
Serum concentrations of neutrophil-derived factors in low and high ABSI scores. ABSI scores were determined on admission day and categorized into low (≤ 8, n = 19) and high (≥ 9, n = 13) values. Serum concentrations of MPO, CitH3, NE, and C3a of the first 7 days post admission were compared. Data were statistically evaluated by Mann–Whitney test.
Burn injury immune cell signature is independent of severity
Finally, we investigated whether the burn injury-induced alterations in immune cell levels were associated with severity of burn injury. Therefore, cell counts were compared between 3rd degree and lower degree burn victims and furthermore between patients with higher and lower clinical severity scores. A trend towards increased leukocyte and neutrophil counts in more severe cases was found on admission day, while no difference in platelet and relative neutrophil counts was observed (Supplemental Figures S8—S11). Absolute and relative lymphopenia was more pronounced in the early days following burn injury when comparing low versus high SOFA scores (Supplemental Figure S10), indicating that immune cell counts are time- and score-dependent.
Discussion
Burn injuries trigger a complex immune response characterized by a unique cytokine secretion pattern and immune cell activation. Exaggerated burn-induced immune activation can lead to tissue damage and organ dysfunction, while immunosuppression can predispose to infectious diseases. Activation of neutrophils and secretion of neutrophil-derived factors add another piece to the complex picture of immunological reactions in response to burn injuries. We were the first to track systemic levels of neutrophil-derived immunomodulators up to 4 weeks post trauma and were able to demonstrate that the NETosis surrogate markers MPO, CitH3, NE, and C3a were increased in burn victims compared to healthy controls. Several studies have already suggested a role for neutrophils in the immune response elicited by burn injuries. Circulating DNA in plasma has been identified as a prognostic marker for mortality and an early biomarker of sepsis in burn-injured patients12,28, though free DNA does not exclusively originate from NETs39 and is therefore considered a rather indirect evidence of neutrophil involvement. Further studies investigated more neutrophil-specific factors. Bacteriostatic effects of topically administered MPO were demonstrated in Staphylococcus aureus-infected burn wounds40 and increased MPO activity was observed following burn injury in rats41. Intriguingly, burn injury reduced NETosis, indicated by diminished CitH3 levels, via pulmonary immunosuppression31. A role for NE in the proteolytic degradation of fibronectin in burn wound fluids42 and in burn-blast-induced lung injury in rats43 has been reported and inhibiting NE activity exerted cytoprotective effects43.These studies investigated local levels of MPO, CitH3, and NE in burn wounds, burn fluids, or lung tissues. Systemic concentrations of these factors have not been comprehensively described to date. Our data with a 4 week follow up time post burn injury therefore add valuable information to the role of NETosis in the burn injury-induced stress response.
We detected increased serum concentrations of complement C3a in burn-injured patients compared to controls. Elevated C3a levels have already been reported previously in plasma of burn victims44 and in subdermal tissues of second-degree thermal wounds45. Interestingly, expression of complement receptors on neutrophils was increased following burn injury46 and it was previously demonstrated that neutrophilic elastase promotes complement amplification47. Hence, an intricate crosstalk between the complement system and neutrophils occurs in the immune response to burn injury. Though intracellular stores of C3a have been found in neutrophils20, numerous other cell types and tissues, such as the liver48, are conceivable to contribute to serum C3a levels post burn injury. Our C3a levels did not correlate with γ-GT, indicating that complement activation is not associated with liver disease in our settings. Delineating the exact cellular origin of systemic C3a in burn victims will be subject of future studies.
We observed remarkably increased absolute leukocyte counts in burn victims compared to healthy controls, while absolute lymphocyte counts remained largely unaltered. In addition, absolute neutrophil levels were strongly increased post burn injury. These data are in line with data reported by Mulder et al.49 and it is tempting to speculate that the elevated neutrophil amounts detected in our patient cohort might serve as a potential cellular source for MPO, CitH3, NE, and C3a. We furthermore observed that the increase of absolute leukocyte and neutrophil counts was even more pronounced in more severe cases. Intriguingly, relative lymphocyte levels were remarkably decreased in patients with burn injury and lower in patients with higher SOFA score. Lymphopenia has already been reported in septic and burn victims, which might contribute to post-injury immunosuppression, morbidity, and mortality50–52. Platelet levels were decreased in the early stress response and re-elevated starting 14 days after burn injury. These dynamics are in accordance with previous reports, where a sustained pro-coagulant state of burn patients was described53. Together, these data connect the systemic factors with clinical blood parameters and contribute to a better understanding of the cellular and molecular processes in the concerted burn injury response.
While exaggerated NET release might be associated with deteriorated conditions, neutrophil activation and NETs might also exert beneficial effects following burn trauma. One of the primary functions of NETs is capturing pathogens17 and a reasonable level of local NETosis might help to reduce risk of infectious diseases and to prevent pathogen entry at sites with compromised barrier function due to burn injury. In addition, neutrophils are sentinels of the immune system. NETs exert immunomodulatory actions and are capable of instigating other immune cells to induce sterile inflammation54 and are involved in the early phase of wound healing55. It is therefore tempting to speculate that burn injury-induced NETs prime the immune system as part of the normal wound healing process. Whether NETs are involved in promoting post-burn trauma wound healing or whether they aggravate medical conditions remains the subject of future studies.
Microvascular obstruction was reported as the leading cause of burn injury progression and red blood cell aggregates, microthrombi, and ischemia have been described to promote secondary tissue damage16. NETs serve as a scaffold for platelets, induce platelet aggregation and coagulation23,24 and have furthermore been shown to exert pro-coagulant activities in sepsis25. Moreover, neutrophil activation and CitH3 were induced by myocardial ischemia/reperfusion injury and showed pro-thrombotic and cytotoxic features, while eliminating NETs by DNase I treatment exerted cardio-protective effects26. In addition, elevated CitH3 levels were reported in various inflammatory conditions with a role of microvascular thrombosis27,56. Though lactadherin levels remain unaltered, we observed systemic CitH3 elevation 2–5 days post burn injury and detected abnormal immune cell counts even several weeks after burn injury. These findings corroborate the concept of burn injury progression following initial damage. We hypothesize that injury-induced elevation of neutrophil counts and concomitant neutrophil activation contribute to burn injury progression. NETosis and systemic neutrophil-derived factors are induced in the course of a delayed immunological response, provoke microvascular obstruction, and thereby promote secondary tissue damage.
Our data revealed that 3rd degree burns showed higher CitH3 and NE levels and these results are in line with the clinical severity score ABSI, where cases with higher scoring displayed increased CitH3 and NE. These data suggest that the severity of burn injury dictates the level of neutrophil activation. Intriguingly, patients succumbing to burn trauma did not display elevated neutrophil-derived immunomodulators. While we found elevated CitH3 and NE levels in patients with higher degree and severity burns, future studies with larger samples sizes will be required to determine whether elevated NETosis-associated factors might serve as a prognostic factor for mortality following severe burn injury. Moreover, no difference between burn victims suffering from inhalation trauma compared to burn-injured patients without inhalation trauma was observed. Neutrophilia in the bronchoalveolar space upon inhalation trauma has been described and attenuating neutrophil recruitment improved histopathology scores and bacterial clearance57. Though NETosis markers were not related to inhalation trauma in our patient collective, a role for burn-associated lung injury in neutrophil activation cannot be entirely ruled out58.
In spite of our best efforts, this study has certain limitations. Though 32 burn victims were initially included, the number of available specimen declined over time as patients either succumbed to their injury or were discharged from the hospital. Future studies with higher sample numbers will be required to fully elucidate the role of neutrophil-derived factors in the burn injury response and for more elaborate subgroup studies, e.g. by performing patient stratification according to sepsis, pneumonia, and specific infectious diseases. Another limitation of our study is the clinical assessment of burn area and TBSA. While we employed a quantitative method which can be rather subjective, use of objective measurement tools with higher diagnostic accuracy, such as laser Doppler imaging59, might represent a preferable approach to determine burn depth. In addition, patients displaying 3rd degree burns were defined as 3rd degree-positive irrespective of the affected area. Performing more in-depth analyses of patients with severe burns considering the affected area merits future investigations. Our set of neutrophil-derived factors serves as a surrogate machinery to assess NETosis. Further investigations are necessary to provide more direct evidence of systemic NETosis in sera of burn victims, such as detection of cfDNA and specific neutrophil-derived DNAs.
Clinical management of burn victims involves fluid resuscitation, burn wound coverage, supportive care, and rehabilitation. Targeting neutrophil function in post burn injury might serve as an alternative treatment option in severe cases with exacerbated neutrophil activation. Beneficial effects of anti-NETs therapy have already been demonstrated in various neutrophil-mediated inflammatory diseases and conditions, including wound healing60, inflammatory arthritis, pulmonary fibrosis, inflammatory bowel disease, and sepsis61. Determining the full therapeutic potential of NETosis inhibition following burn trauma will merit future investigations.
Our study provides evidence of systemic, neutrophil-derived factors associated with NETosis in burn victims. As the factors tested here do not serve the ultimate proof of the exact levels of NETosis, future investigations further corroborating the existence of NETs in burn victims, e.g. by quantification of MPO- and NE-DNA together with cfDNA, are still required. More sophisticated studies will be necessary to deepen our understanding of the role of NETosis following burn injury and to determine the potential prognostic and/or therapeutic value of NETosis.
Supplementary Information
Acknowledgements
We thank the Dr. HP Haselsteiner and the CRISCAR Familienstiftung for their belief in this private public partnership to augment basic and translational clinical research.
Author contributions
Idea and Conceptualization: H.J.A. and M.L.; Data curation, M.L., M.T.L., and T.H.; Formal analysis, M.L. and M.T.L.; Funding acquisition, H.J.A.; Investigation, M.L., M.T.L., and T.H.; Project administration, H.J.A. and T.H.; Resources, H.J.A. and T.H.; Supervision, H.J.A. and T.H.; Writing—original draft, M.L., H.J.A., and T.H.; Writing—review and editing, M.T.L., D.C., M.D., K.K., D.B., A.G., B.M., C.R., S.H., and M.M.
Funding
This research project was funded by the Vienna Business Agency (Vienna, Austria; grant “APOSEC to clinic” 2343727) and by the Aposcience AG under group leader HJA. MM was funded by the Sparkling Science Program of the Austrian Federal Ministry of Education, Science and Research (SPA06/055).
Data availability
Raw data are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hendrik Jan Ankersmit and Thomas Haider.
Contributor Information
Hendrik Jan Ankersmit, Email: hendrik.ankersmit@meduniwien.ac.at.
Thomas Haider, Email: thomas.a.haider@meduniwien.ac.at.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-05768-w.
References
- 1.Association AB. National Burn Repository 2019 Update: Report of Data from 2009 to 2018. American Burn Association; 2019. [Google Scholar]
- 2.Brusselaers N, Monstrey S, Vogelaers D, Hoste E, Blot S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care. 2010;14(5):R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason SA, Nathens AB, Byrne JP, Diong C, Fowler RA, Karanicolas PJ, et al. Increased rate of long-term mortality among burn survivors: A population-based matched cohort study. Ann. Surg. 2019;269(6):1192–1199. doi: 10.1097/SLA.0000000000002722. [DOI] [PubMed] [Google Scholar]
- 4.Guillory AN, Porter C, Suman OE, Zapata-Sirvent RL, Finnerty CC, Herndon DN. Modulation of the hypermetabolic response after burn injury. Total Burn Care. 301–6 (2018).
- 5.Agay D, Andriollo-Sanchez M, Claeyssen R, Touvard L, Denis J, Roussel AM, et al. Interleukin-6, TNF-alpha and interleukin-1 beta levels in blood and tissue in severely burned rats. Eur. Cytokine Netw. 2008;19(1):1–7. doi: 10.1684/ecn.2008.0113. [DOI] [PubMed] [Google Scholar]
- 6.Ravat F, Payre J, Peslages P, Fontaine M, Sens N. Burn: An inflammatory process. Pathol. Biol. (Paris) 2011;59(3):e63–72. doi: 10.1016/j.patbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Serra MB, Barroso WA, da Silva NN, Silva SDN, Borges ACR, Abreu IC, et al. From inflammation to current and alternative therapies involved in wound healing. Int. J. Inflamm. 2017;2017:3406215. doi: 10.1155/2017/3406215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS ONE. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat. Rev. Dis. Primers. 2020;6(1):11. doi: 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lateef Z, Stuart G, Jones N, Mercer A, Fleming S, Wise L. The cutaneous inflammatory response to thermal burn injury in a Murine model. Int. J. Mol. Sci. 2019;20(3):538. doi: 10.3390/ijms20030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shallo H, Plackett TP, Heinrich SA, Kovacs EJ. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns. 2003;29(7):641–647. doi: 10.1016/s0305-4179(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 12.Hampson P, Dinsdale RJ, Wearn CM, Bamford AL, Bishop JRB, Hazeldine J, et al. Neutrophil dysfunction, immature granulocytes, and cell-free DNA are early biomarkers of sepsis in burn-injured patients: A prospective observational cohort study. Ann. Surg. 2017;265(6):1241–1249. doi: 10.1097/SLA.0000000000001807. [DOI] [PubMed] [Google Scholar]
- 13.Kupper TS, Green DR, Durum SK, Baker CC. Defective antigen presentation to a cloned T helper cell by macrophages from burned mice can be restored with interleukin-1. Surgery. 1985;98(2):199–206. [PubMed] [Google Scholar]
- 14.Moins-Teisserenc H, Cordeiro DJ, Audigier V, Ressaire Q, Benyamina M, Lambert J, et al. Severe altered immune status after burn injury is associated with bacterial infection and septic shock. Front. Immunol. 2021;12:586195. doi: 10.3389/fimmu.2021.586195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobalem M, Harder Y, Rezaeian F, Wettstein R. Secondary burn progression decreased by erythropoietin. Crit. Care Med. 2013;41(4):963–971. doi: 10.1097/CCM.0b013e318275cee7. [DOI] [PubMed] [Google Scholar]
- 16.Clark RAF, Fenner J, Sasson A, McClain SA, Singer AJ, Tonnesen MG. Blood vessel occlusion with erythrocyte aggregates causes burn injury progression-microvasculature dilation as a possible therapy. Exp. Dermatol. 2018;27(6):625–629. doi: 10.1111/exd.13518. [DOI] [PubMed] [Google Scholar]
- 17.Rios-Lopez AL, Gonzalez GM, Hernandez-Bello R, Sanchez-Gonzalez A. Avoiding the trap: Mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol. Res. 2021;243:126644. doi: 10.1016/j.micres.2020.126644. [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elvington M, Liszewski MK, Bertram P, Kulkarni HS, Atkinson JP. A C3(H20) recycling pathway is a component of the intracellular complement system. J. Clin. Invest. 2017;127(3):970–981. doi: 10.1172/JCI89412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norgauer J, Dobos G, Kownatzki E, Dahinden C, Burger R, Kupper R, et al. Complement fragment C3a stimulates Ca2+ influx in neutrophils via a pertussis-toxin-sensitive G protein. Eur. J. Biochem. 1993;217(1):289–294. doi: 10.1111/j.1432-1033.1993.tb18245.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinod K, Wagner DD. Thrombosis: Tangled up in NETs. Blood. 2014;123(18):2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16(8):887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 25.Iba T, Miki T, Hashiguchi N, Tabe Y, Nagaoka I. Is the neutrophil a 'prima donna' in the procoagulant process during sepsis? Crit Care. 2014;18(4):230. doi: 10.1186/cc13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, et al. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood. 2014;123(1):141–148. doi: 10.1182/blood-2013-07-514992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altrichter J, Zedler S, Kraft R, Faist E, Mitzner SR, Sauer M, et al. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur. J. Trauma Emerg. Surg. 2010;36(6):551–557. doi: 10.1007/s00068-010-0013-1. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman T, Magosevich D, Moreno MC, Guzman MA, D'Atri LP, Carestia A, et al. Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clin. Immunol. 2017;183:254–262. doi: 10.1016/j.clim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Korkmaz HI, Ulrich MMW, Vogels S, de Wit T, van Zuijlen PPM, Krijnen PAJ, et al. Neutrophil extracellular traps coincide with a pro-coagulant status of microcirculatory endothelium in burn wounds. Wound Repair. Regen. 2017;25(4):609–617. doi: 10.1111/wrr.12560. [DOI] [PubMed] [Google Scholar]
- 31.Sakuma M, Khan MAS, Yasuhara S, Martyn JA, Palaniyar N. Mechanism of pulmonary immunosuppression: Extrapulmonary burn injury suppresses bacterial endotoxin-induced pulmonary neutrophil recruitment and neutrophil extracellular trap (NET) formation. FASEB J. 2019;33(12):13602–13616. doi: 10.1096/fj.201901098R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner M, Krenn C, Roth G, Moser B, Dworschak M, Jensen-Jarolim E, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004;30(7):1468–1473. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 33.Hacker S, Dieplinger B, Werba G, Nickl S, Roth GA, Krenn CG, et al. Increased serum concentrations of soluble ST2 predict mortality after burn injury. Clin. Chem. Lab. Med. 2018;56(12):2079–2087. doi: 10.1515/cclm-2018-0042. [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 35.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit. Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 36.Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann. Emerg. Med. 1982;11(5):260–262. doi: 10.1016/s0196-0644(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 37.Miksa M, Dong D, Wu R, Ravikumar T, Wang P. Erhöhte Sterblichkeit von Mäusen mit Lactadherinmangel in einer Sepsis: Rolle Verminderter Phagozytose Apoptotischer Zellen. Z. Geburtshilfe Neonatol. 2007;211(S1):FV88. [Google Scholar]
- 38.Zhou Y, Cai W, Zhao Z, Hilton T, Wang M, Yeon J, et al. Lactadherin promotes microvesicle clearance to prevent coagulopathy and improves survival of severe TBI mice. Blood. 2018;131(5):563–572. doi: 10.1182/blood-2017-08-801738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019;20(8):1057–1067. doi: 10.1080/15384047.2019.1598759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo R, Li S, Xia H, Feng Z, Tang Q, Peng C. Effects of myeloperoxidase on methicillin-resistant Staphylococcus aureus-colonized burn wounds in rats. Adv. Wound Care (New Rochelle) 2019;8(7):271–280. doi: 10.1089/wound.2018.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sehirli O, Sener E, Sener G, Cetinel S, Erzik C, Yegen BC. Ghrelin improves burn-induced multiple organ injury by depressing neutrophil infiltration and the release of pro-inflammatory cytokines. Peptides. 2008;29(7):1231–1240. doi: 10.1016/j.peptides.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Grinnell F, Zhu M. Identification of neutrophil elastase as the proteinase in burn wound fluid responsible for degradation of fibronectin. J. Invest. Dermatol. 1994;103(2):155–161. doi: 10.1111/1523-1747.ep12392625. [DOI] [PubMed] [Google Scholar]
- 43.Chai JK, Cai JH, Deng HP, Zou XF, Liu W, Hu QG, et al. Role of neutrophil elastase in lung injury induced by burn-blast combined injury in rats. Burns. 2013;39(4):745–753. doi: 10.1016/j.burns.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Davis CF, Moore FD, Jr, Rodrick ML, Fearon DT, Mannick JA. Neutrophil activation after burn injury: Contributions of the classic complement pathway and of endotoxin. Surgery. 1987;102(3):477–484. [PubMed] [Google Scholar]
- 45.Machens HG, Pabst A, Dreyer M, Gliemroth J, Gorg S, Bahlmann L, et al. C3a levels and occurrence of subdermal vascular thrombosis are age-related in deep second-degree burn wounds. Surgery. 2006;139(4):550–555. doi: 10.1016/j.surg.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Moore FD, Jr, Davis C, Rodrick M, Mannick JA, Fearon DT. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. N. Engl. J. Med. 1986;314(15):948–953. doi: 10.1056/NEJM198604103141503. [DOI] [PubMed] [Google Scholar]
- 47.Fumia S, Goede JS, Fischler M, Luginbuhl A, Frick S, Fodor P, et al. Human F(ab')2-containing immune complexes together with anti-hinge natural antibodies stimulate complement amplification in vitro and in vivo. Mol. Immunol. 2008;45(10):2951–2961. doi: 10.1016/j.molimm.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 48.Thorgersen EB, Barratt-Due A, Haugaa H, Harboe M, Pischke SE, Nilsson PH, et al. The role of complement in liver injury, regeneration, and transplantation. Hepatology. 2019;70(2):725–736. doi: 10.1002/hep.30508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulder PPG, Vlig M, Boekema B, Stoop MM, Pijpe A, van Zuijlen PPM, et al. Persistent systemic inflammation in patients with severe burn injury is accompanied by influx of immature neutrophils and shifts in T cell subsets and cytokine profiles. Front. Immunol. 2020;11:621222. doi: 10.3389/fimmu.2020.621222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth G, Moser B, Krenn C, Brunner M, Haisjackl M, Almer G, et al. Susceptibility to programmed cell death in T-lymphocytes from septic patients: A mechanism for lymphopenia and Th2 predominance. Biochem. Biophys. Res. Commun. 2003;308(4):840–846. doi: 10.1016/s0006-291x(03)01482-7. [DOI] [PubMed] [Google Scholar]
- 51.Maldonado M, Venturoli A, Franco A, Nunez-Roldan A. Specific changes in peripheral blood lymphocyte phenotype from burn patients Probable origin of the thermal injury-related lymphocytopenia. Burns. 1991;17(3):188–192. doi: 10.1016/0305-4179(91)90101-l. [DOI] [PubMed] [Google Scholar]
- 52.Lebedev MY, Sholkina MN, Utkina TM, Vilkov SA, Korablev SB, Golubcova NV, et al. Immunophenotype of peripheral blood lymphocytes in patients with burns. Russ. J. Immunol. 2001;6(1):47–54. [PubMed] [Google Scholar]
- 53.Marck RE, van der Bijl I, Korsten H, Lorinser J, de Korte D, Middelkoop E. Activation, function and content of platelets in burn patients. Platelets. 2019;30(3):396–402. doi: 10.1080/09537104.2018.1448379. [DOI] [PubMed] [Google Scholar]
- 54.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 55.Liu D, Yang P, Gao M, Yu T, Shi Y, Zhang M, et al. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin. Sci. (Lond) 2019;133(4):565–582. doi: 10.1042/CS20180600. [DOI] [PubMed] [Google Scholar]
- 56.Veraar C, Koschutnik M, Nitsche C, Laggner M, Polak D, Bohle B, et al. Inflammatory immune response in recipients of transcatheter aortic valves. JTCVS Open. 2021;6:85–96. doi: 10.1016/j.xjon.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunn JLM, Kartchner LB, Stepp WH, Glenn LI, Malfitano MM, Jones SW, et al. Blocking CXCL1-dependent neutrophil recruitment prevents immune damage and reduces pulmonary bacterial infection after inhalation injury. Am J Physiol Lung Cell Mol Physiol. 2018;314(5):L822–L834. doi: 10.1152/ajplung.00272.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel PJ, Faunce DE, Gregory MS, Duffner LA, Kovacs EJ. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am. J. Respir. Cell Mol. Biol. 1999;20(6):1229–1237. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- 59.Shin JY, Yi HS. Diagnostic accuracy of laser Doppler imaging in burn depth assessment: Systematic review and meta-analysis. Burns. 2016;42(7):1369–1376. doi: 10.1016/j.burns.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Heuer A, Stiel C, Elrod J, Konigs I, Vincent D, Schlegel P, et al. therapeutic targeting of neutrophil extracellular traps improves primary and secondary intention wound healing in mice. Front. Immunol. 2021;12:614347. doi: 10.3389/fimmu.2021.614347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chirivi RGS, van Rosmalen JWG, van der Linden M, Euler M, Schmets G, Bogatkevich G, et al. Therapeutic ACPA inhibits NET formation: A potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol. Immunol. 2021;18(6):1528–1544. doi: 10.1038/s41423-020-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available from the corresponding authors upon request.