Abstract
Background
The thrombin generation (TG) assay, which measures global coagulation, has mainly been used as a research tool to investigate thrombotic and bleeding disorders. Recently, Diagnostica Stago launched the ST Genesia, a fully automated system to perform “routine version” of this assay. The objectives of this study were to evaluate the imprecision compared with the previous method, Thrombinoscope CAT, and to establish reference intervals.
Methods
Thrombin generation was measured in platelet‐poor citrated plasma from 20 normal controls (fresh and after freezing at −80°C up to 12–13 weeks) on CAT and ST Genesia in duplicate to estimate the total variation, and within and between variations. The reference intervals were estimated nonparametrically in 30 men, 30 women taking combined oral contraceptives (COCs), and 30 women not taking COCs. These were sampled in both Vacutainer and Monovette tubes (i.e., tubes with a high and minimal contact activation, respectively).
Results
Freezing had minimal effects. Imprecision was comparable between the ST Genesia and CAT, with a strong correlation between the two methods. TG was higher when sampled in Vacutainer than in Monovette. We observed a distinct difference between women taking and not taking COCs, whereas men and women not taking COC were quite similar.
Conclusions
Thrombin generation on ST Genesia showed an analytical variation similar to that of CAT. The results depended on the type of sample tubes; thus, reference intervals must be established for the collection tubes used in each laboratory. Furthermore, a considerable difference was observed between women using and not using COCs.
Keywords: CAT, contact activation, imprecision, reference intervals, ST genesia, thrombin generation
Essentials.
A new routine instrument for thrombin generation assay, the ST Genesia, was evaluated.
Imprecision and reference intervals (tubes with high and low contact activation) were estimated.
ST Genesia showed analytical variation similar to that for the CAT instrument
The reference intervals depend on the sample tube; for women, use of combined oral contraception.
1. INTRODUCTION
In the routine coagulation laboratory, the global analyses activated partial thromboplastin time (APTT) and prothrombin time (PT) have been used for almost a century and remain widely used. These tests measure the time from the initiation of the coagulation process until the first fibrin formation. However, it has been shown that most of the thrombin is actually formed after the start of fibrin formation; therefore, these analyses provide only a small glimpse of coagulation activity and do not measure the activity of coagulation inhibitors. In the 1950s, MacFarlane and Biggs 1 reported measurements of the entire thrombin generation (TG) to provide a more complete picture of the coagulation process. The method was greatly improved by Hemker at the advent of the 20th century 2 , 3 , 4 with the introduction of a fluorogenic substrate for thrombin to allow measurements in plasma with fibrinogen.
This methodology has been widely used as a research tool on various platforms: calibrated automated thrombin (CAT) generation performed on the Thrombinoscope CAT (Diagnostica Stago; Asnieres sur Seine, France); on Technothrombin (Technoclone; Vienna, Austria); or Innovance ETP (Siemens; Erlangen, Germany). Patients with thromboses show a higher level of TG, whereas those with bleeding show lower TG, 5 , 6 , 7 , 8 although there is a substantial between‐subject variation. 9 Furthermore, several new causes of bleeding have been identified and described that include other mechanisms than APTT and PT measure. 10 , 11 However, this methodology has not been introduced in routine laboratories because the current equipment was mainly developed for research. Recently a new generation of analyser the ST Genesia was launched by Diagnostica Stago to introduce the TG assay to routine laboratory investigations. The ST Genesia is based on the CAT platforms but was developed with full automation with improved temperature control. This TG platform is further enhanced by the inclusion of specialized kits, including samples for quality control and for specific clinical conditions, namely Bleedscreen (BS) with a low concentration of tissue factor (TF) sensitive for measuring factor deficiencies (reportedly all factors included in APTT and PT 12 ); Thromboscreen (TS), with a medium concentration of TF sensitive for measuring thrombophilia (reportedly the natural anticoagulants 12 and possibly also Factor V Leiden and Prothrombin G20210A 13 ); and Drugscreen with a high concentration of TF sensitive for the measurement of anticoagulants. However, the exact concentrations of TF and phospholipids are not available. The calibration also differs between CAT and Genesia: in the CAT measurements, a calibrator is added to all samples, whereas for the ST Genesia, calibration is only performed once daily with a solution of thrombin and a fluorogenic substrate, and each plasma sample is spiked with a fluorophore to correct for plasma color.
We comprehensively investigated analytical variation and practicability of this new coagulation instrument compared with the previous model CAT. We have also described reference intervals for both BS and TS reagents.
2. MATERIALS AND METHODS
2.1. Blood collection
Venous blood was collected from the antecubital fossa using a 21‐gauge needle in 20 healthy volunteers into 3.2% (w/v) trisodium citrate Monovette tubes (Sarstedt, Nümbrecht, Germany). For estimation of reference intervals, blood was obtained from blood donors (healthy people without any apparent illness) using a 16‐gauge needle and both Monovette and Vacutainer tubes (BD, Plymouth, UK) containing 3.2% (w/v) trisodium citrate. The first tube was discarded. Platelet‐poor plasma (PPP) was obtained by centrifugation twice at 2500g for 15 min at 20°C immediately after collection in accordance with current guidelines. 14 After centrifugation, plasma samples from each individual and type of tube were pooled and stored at −80°C until analysis, except the fresh samples were analyzed within 2–3 h. For the variation study, one‐half of the plasma was diluted 9 + 1 in HEPES buffer, pH 7.35 (containing 300 mM HEPES and 5 mg/ml bovine serum albumin) as described previously. 15
All samples were obtained after obtaining informed consent. According to Danish legislation, this kind of measurement is classified as quality control; therefore, approval from the local ethical committee was not required.
2.2. Thrombin generation
TG was measured using the ST Genesia (Diagnostica Stago, Asnieres sur Seine, France) and the CAT method (Thrombinoscope BV, Maastricht, The Netherlands). For the imprecision study, fresh and frozen samples were measured, and the frozen samples were thawed for 5 min at 37°C and analyzed after 2–3, 5–6, and 12–13 weeks as duplicates each day. In the CAT system, the PPPLow reagent (1 pM TF, 4 µM PL) (Thrombinoscope BV) was used. For the ST Genesia, the BS kit was used. All validation measures were performed using the same batch of reagents. For estimation of the reference intervals, all plasma samples were frozen before analysis with the BS and TS used on the ST Genesia. When using TS, the TG was measured with and without the addition of thrombomodulin (TM).
2.3. The calibrated automated thrombogram
Thrombin generation measurements were conducted according to the manufacturer's instructions using 80 µl PPP mixed with 20 µl of trigger solution denoted PPPLow; subsequently coagulation was initiated by the addition of 20 µl FluCa buffer containing a fluorescent substrate (Z‐Gly‐Gly‐Arg‐AMC) and CaCl2 (all reagents from Thrombinoscope BV). All measurements were performed in duplicates. To correct for differences in plasma color, each plasma measurement was calibrated against the same plasma mixed with 20 µl thrombin calibrator (Thrombinoscope BV), also in duplicate. The fluorescence of AMC (7‐amino‐4methylcoumarin) was measured using a Fluoroskan Ascent (Thermo Scientific, Waltham, MA, USA) equipped with a 390 nm excitation and 460 nm emission filter set. The dedicated Thrombinoscope software (Thrombinoscope BV) was used to calculate: lagtime, endogenous thrombin potential (ETP), peak, and time to peak (ttPeak).
2.4. ST Genesia
The ST Genesia (Diagnostica Stago) measures the development of fluorescence of AMC to detect thrombin activation as in the CAT system but differs from the CAT system by performing one overall calibration representing the activity of a known thrombin concentration once daily rather than a plasma‐specific calibration. A separate fluoSet containing a fixed amount of the AMC fluorophore was added to each plasma to correct for the plasma color. The ST Genesia performs four measurements for each sample, namely, TG in samples in duplicate and fluoSet in duplicate. The samples are preheated at 37°C with precise temperature control and the fluorescence registered with a 377/440 filter set (excitation/emission). The kits contain a standardized STG‐QualiTest, Low and Normal control set, and reference plasma (STG‐RefPlasma) for normalization of the TG value.
2.5. Routine assays
APTT (seconds), international normalized ratio (INR) (ratio, [no unity]), fibrinogen (µmol/L), and antithrombin (IU/ml) assays were performed on the ACL Top 500 (Instrumentation Laboratory, Milano, Italy) with dedicated reagents for INR, fibrinogen, and antithrombin, and the reagent Pathromtin (Siemens, Erlangen, Germany) for APTT.
2.6. Statistical analysis
Calculations of the total variation (CVT), within variation (CVW), and the variation between runs (CVB) were performed essentially as described before. 9 It is also described in the Supporting Information.
Values are generally described as mean ± SEM. When appropriate, differences were tested with Student's t test and were considered significant at p < 0.05. The associations between tests were assessed using Pearson's correlation coefficients and are described as R 2.
The reference intervals were determined as 2.5 and 97.5 percentiles (nonparametrically) for each group as recommended. 16 Furthermore, the 25th and 75th percentiles were calculated and are included in the figures.
3. RESULTS
The imprecision of the methods was calculated by measuring fresh samples and samples frozen for 2–3, 5–6, and 12–13 weeks from 20 volunteers. The measurements were performed in duplicates on both CAT and the ST Genesia. First, the effect of freezing was examined by comparing the results obtained from the fresh samples to the mean of the three frozen samples and to each of the days. All differences and SEM results are shown in Table S1. The samples measured on CAT showed no differences in ETP and Peak, whereas there was a small, but significantly shorter lagtime and ttPeak in fresh samples. The ST Genesia showed slightly different results, with a trend toward a higher ETP. Peak and lagtime were not affected, whereas ttPeak was slightly reduced (i.e., only ttPeak was slightly shorter for both methods in fresh samples). However, because the differences between fresh and frozen samples were very small, although significant, we chose to use all these results, fresh and frozen samples, for the calculation of variations. The total (CVT), between (CVB), and within assay (CVW) variations were measured as described in the Methods section, and as shown in Table 1.
TABLE 1.
Coefficients of variation for TG performed on CAT and ST Genesia, as well as normalized values on the ST Genesia
| Lagtime | ttPeak | Peak | ETP | |
|---|---|---|---|---|
| CAT | ||||
| CVW (%) | 2.7 | 2.2 | 8.3 | 6.8 |
| CVB (%) | 5.5 | 3.3 | 5.6 | 5.9 |
| CVT (%) | 6.1 | 4.0 | 10.0 | 9.0 |
| ST Genesia | ||||
| CVW (%) | 3.3 | 2.6 | 5.1 | 3.4 |
| CVB (%) | 2.9 | 3.8 | 9.5 | 7.1 |
| CVT (%) | 4.4 | 4.6 | 10.8 | 7.9 |
| Normalized | ||||
| CVW (%) | 3.3 | 2.6 | 5.3 | 3.6 |
| CVB (%) | 3.6 | 3.2 | 10.2 | 9.1 |
| CVT (%) | 4.9 | 4.1 | 11.5 | 9.8 |
Abbreviations: CAT, calibrated automated thrombin; ETP, endogenous thrombin potential; TG, thrombin generation; ttPeak, time to peak.
We previously reported that pH in frozen samples may increase during the storage, 15 which can be avoided by adding HEPES buffer to the sample. We also tested this procedure in the present study by mixing some of the fresh samples with HEPES buffer, which were analyzed as fresh samples and after storage for 2–3 and 12–13 weeks. This reduced the small difference in ttPeak between the fresh and frozen samples. Table S2 shows the CVT, CVB, and CVW of these samples. Although the TG level was higher in these samples, the variation was comparable to that in samples without HEPES.
The ST Genesia has a reference plasma that can be used for normalization of the samples to reduce the variation. The variations calculated based on these values are also shown in Table 1. CVW was unchanged, but CVT and CVB were slightly higher when the normalized values are used.
Finally, we included controls at the beginning and at the end of each series of analyses. We observed no trend of change in the level during each of the series or from the start to the end of all the series. Moreover, the imprecision for the ST Genesia and CAT was similar to the other samples.
The results for CAT and ST Genesia were compared by calculating the correlations between the means of each of the 20 samples (four duplicates of each) on each system (Figure 1). Generally, there was a rather strong correlation between the two measurements with R 2 values between 0.67 and 0.81. However, lagtime and ttPeak were considerable shorter on the ST Genesia with BS, indicating that the TF concentration is higher in BS than in PPPLow. The peak was quite similar, whereas the ETP was slightly lower on the ST Genesia.
FIGURE 1.
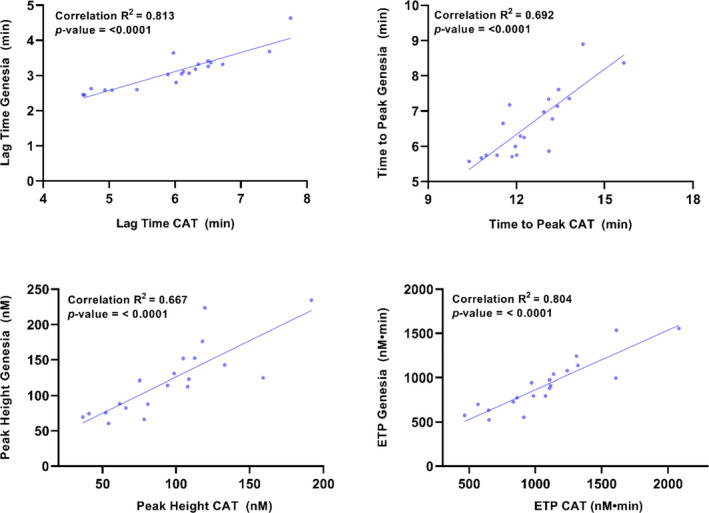
Comparison between TG performed on CAT (PPPLow reagent) and on ST Genesia using the Bleedscreen reagent. The correlations between the mean of eight values (four duplicates) of each sample measured on each instrument are shown. TG, thrombin generation
We calculated reference values for the reagents: BS (low TF), TS (medium‐high TF), and TS with the addition of TM. We included 30 men (age [M ± SD] 46 ± 15 years, range 22–69 years) and 60 women, 30 of whom were taking combined oral contraceptives (COCs) (age: 26 ± 5 years, range 19–35 years) and 30 women who were not taking COCs (age: 36 ± 12 years, range 19–55 years), because COCs may affect TG, 17 , 18 based on limited numbers of individuals in the previous reports. Sample tubes also reportedly had a substantial effect on TG 19 ; therefore, we used two types of tubes, one with a high degree of contact activation (i.e., activation via surface activation of Factor XII/Kallikrein) (Vacutainer) and one with a minimal contact activation (Monovette). If coagulation is initiated through contact activation in addition to TF in the reagents, the coagulation response will be higher than that without contact activation, especially when the TF concentration is low. 19 The absolute results are shown as box‐whisker plots in Figure 2 (BS) and 3 (TS ± TM). The results of the normalized values are shown in Figures S1 and S2, respectively. The ETP and peak values were higher and the ttPeak values shorter in samples in Vacutainer for all participants and analyses compared with samples in Monovette tubes. The differences were more pronounced using BS than for TS. The reference intervals for women taking COCs differed from the reference intervals for women not taking COCs, with higher ETP and peak and shorter ttPeak, whereas lagtime was only slightly shorter in women taking COCs. The differences between Vacutainer and Monovette tubes were less distinct for women using COCs than for men and women not taking COCs.
FIGURE 2.
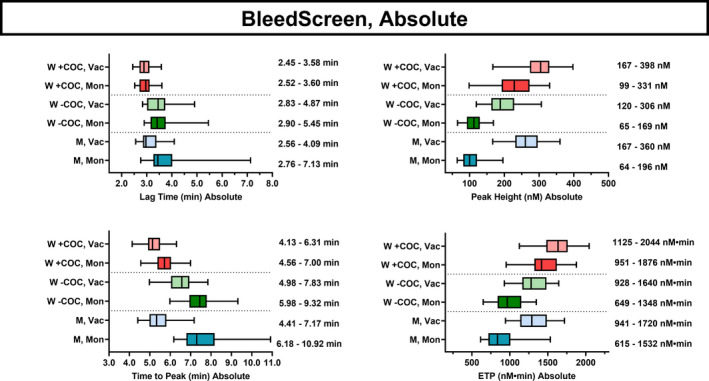
Reference intervals for the absolute values for women (W) taking COCs, women not taking COCs, and men (M) sampled in the tubes Monovette (Mon) or Vacutainer (Vac) tubes and measured using the Bleedscreen reagent. Each line shows the 95% central values, whereas the bars indicate the 50% central values and the median for lagtime, peak, ttPeak, and ETP. The values are shown to the right of each group. COCs, combined oral contraceptives; ttPeak, time to peak; ETP, endogenous thrombin potential
FIGURE 3.
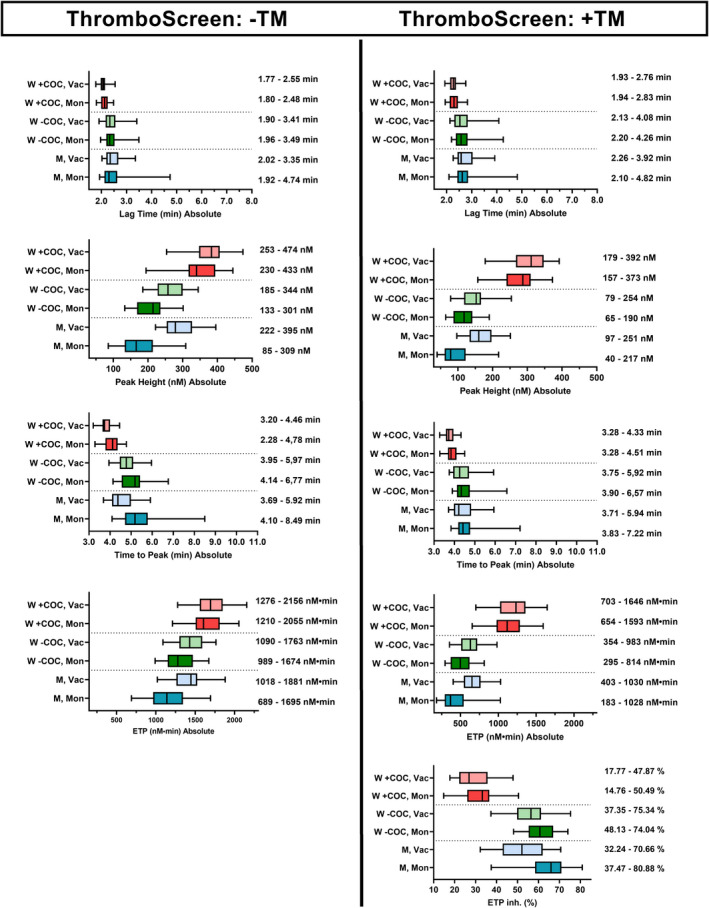
Reference intervals for the absolute values for women (W) taking COCs, women not taking COCs, and men (M) sampled in the tubes Monovette (Mon) or Vacutainer (Vac) tubes and measured with the Thromboscreen reagent without and with the addition of thrombomodulin. Each line shows the 95% central values, whereas the bars indicate the 50% central values and the median for lagtime, peak, ttPeak, ETP, and also ETP inhibition (the degree of inhibition after the addition of thrombomodulin). The values are shown to the right of each group. COCs, combined oral contraceptives; ttPeak, time to peak; ETP, endogenous thrombin potential
We observed a strong correlation between the results using BS and TS (without TM) (Table 2). We examined the correlation with age, which generally was very weak (R 2 < 0.05) for peak and ETP, whereas it was slightly higher for lagtime and ttPeak (R 2 = 0.003–0.13), with unchanged to slightly higher values for the elder persons, but the differences were minor (Table S3).
TABLE 2.
Correlations between BS and TS
| R 2 values | ETP | Peak | ttPeak | Lagtime |
|---|---|---|---|---|
| Monovette | ||||
| All | 0.88 | 0.92 | 0.92 | 0.94 |
| Men | 0.86 | 0.90 | 0.96 | 0.96 |
| Women – COCs | 0.79 | 0.83 | 0.85 | 0.92 |
| Women + COCs | 0.85 | 0.88 | 0.85 | 0.83 |
| Vacutainer | ||||
| All | 0.86 | 0.76 | 0.72 | 0.76 |
| Men | 0.72 | 0.50 | 0.66 | 0.83 |
| Women – COCs | 0.77 | 0.76 | 0.81 | 0.90 |
| Women + COCs | 0.94 | 0.77 | 0.86 | 0.83 |
Abbreviations: BS, Bleedscreen; COCs, combined oral contraceptives; ETP, endogenous thrombin potential; TS, Thromboscreen; ttPeak, time to peak.
We investigated correlations of these analyses with the routine analyses, APTT, INR, fibrinogen, and antithrombin (Figure S3). The correlations with INR and fibrinogen were very weak to weak and slightly higher in Vacutainer than in Monovette tubes. Correlations with APTT were higher and more marked in Vacutainer tubes than in Monovette tubes. On the contrary, the correlations to antithrombin were moderate in Monovette tubes, but lower in Vacutainer tubes.
4. DISCUSSION
The present study shows that the new equipment for TG assays, the ST Genesia, is comparable to the Thrombinoscope CAT regarding variation. We have presented suggestions for reference intervals and highlighted that the TG levels are highly dependent on the tubes that are used for sampling. Furthermore, we show that the TG levels are substantially different between women taking COCs and women not taking COCs.
CAT has been used for many years, and is well‐known to show a considerable variation, especially between laboratories. 20 The present findings (Table 2) are consistent with previous results. 9 We observed a similar variation in the ST Genesia; that is, this more automated instrument did not perform better than the older one. The manufacturer has introduced a reference sample to allow “normalization” of the results. However, we did not observe any improvement in variation after using this system; in fact, the variation may be slightly higher. A plausible explanation is probably that two analyses are needed for these results, and, therefore, you have variation on both. Thus, it is not an improvement in the daily practice using a specific lot, but it is probably an advantage when lots are changed, as shown by Cornette et al. 7 Also in cases where results from different laboratories are compared, this normalization may reduce the substantial inter‐laboratory variation. 20 , 21 However, it is also crucial that the standardization material (i.e., STG RefPlasma) is robust and validation of new lots is performed thoroughly to maintain the level (cf use of material for the calculation of international sensitivity index values to maintain a stable INR calculation).
We previously demonstrated that pH changes of plasma may affect TG results 15 ; therefore, we also measured the variation in plasma with HEPES added. Although this slightly improved the imprecision, the difference was not marked. This addition always resulted in higher TG, although the difference between the results with and without HEPES was much smaller using ST Genesia. The reason for this is not known; however, because of this difference, if HEPES is used, it must be used in all samples in a specific project, even though it is smaller on the ST Genesia. When samples are stored for shorter periods, this addition is not beneficial but can be considered if samples must be stored for longer periods although this is a time‐consuming step in a routine laboratory. We previously recommended filling vials to the maximum volume to avoid pH changes. 15
Our estimation of the reference intervals showed that reference intervals must be determined for the respective sampling tubes used in the laboratory. The difference between Vacutainer and Monovette tubes was quite large, and more marked when using BS with a lower TF concentration. Contact activation in the Vacutainer tubes probably caused this difference. When measuring TG in women, it is important to know whether they use COCs because this substantially impacts TG. Therefore, separate reference intervals for these groups are necessary. The difference between Vacutainer and Monovette was smaller in women taking COCs, which may be caused by some contact activation from COCs 22 reducing the effect of the tube. Thrombomodulin also had a lower effect in women taking COCs, likely because COCs reduce protein S levels. Therefore, this analysis may be a tool to measure (some of) the effect of COCs on the coagulation system.
We observed a reasonably high correlation between PPPLow and BS; however, BS obviously had a higher TF concentration, and results using BS were not directly interchangeable with results from CAT using PPPLow. The correlation between BS and TS was strong; consequently, the results showing a high or a low TG will probably be revealed from both kits, and the reference values (Figures 2 and 3) are not very different. Thus, the results from the two reagents are not very different on samples from healthy individuals. However, we did not test patients with different types of coagulopathies; therefore, we cannot offer a conclusion on their applicability to pathological samples. Cornette et al. 7 found that CAT (PPPLow) and Genesia (BS) were almost equal in diagnosing patients with a bleeding tendency encompassing one‐third of patients with a plasma coagulation defects and two‐thirds without a diagnosis after routine bleeding investigations. We previously showed that the between‐subject variation was higher for PPPLow with a low TF concentration compared with the PPP‐reagent with a higher TF concentration. 9 However, using TS with and without TM will probably be an advantage because the Protein C:Protein S system is included in the determination. 13 Roullet et al. 23 only observed minor differences between results using BS and TS on samples from patients who had undergone liver transplantation, whereas they observed some differences from the results of CAT that the authors could not explain.
Finally, we investigated the correlations between routine coagulation tests and TG levels. Generally, the correlations were quite low; however, interestingly, the correlations between APTT and TG were higher for Vacutainer tubes, likely indicating a higher degree of contact activation in these. On the other hand, the correlations between AT and TG were higher in the Monovette tubes, indicating that it is easier to detect the coagulation inhibitors in tubes with a low degree of contact activation. Therefore, tubes with a minimal contact activation may provide better information on global coagulation.
Calzavarini et al. 17 reported variation and reference intervals for the ST Genesia. However, the variation was calculated from only a few samples. The reference intervals were only described as the normalized central 50% of the population (25th–75th percentile). The levels were comparable to our results: lagtime and ttPeak were nearly identical, whereas the peak and ETP were consequently 10%–20% lower than our values. They also reported a higher TG in women using COCs compared with women not using COCs in a limited number of individuals, as well as in elderly women, whereas we observed a minor effect of age and in the opposite direction. Ninivaggi et al. 18 also created reference values for BS and TS using Greiner Bio‐One sampling tubes with results intermediate between those of the Monovette and Vacutainer tubes in the present study. They included a few women using COCs and reported an increased TG; however, excluding these women from the population did not change the reference values. They found similar levels for men and women without COCs, and no influence of age. This is in accordance with our findings: although we found some differences between men and women, the reference intervals were not very different. Douxfiles et al. 24 examined variation and sample stability but only for Drugscreen. Variation was only calculated based on controls, and they generally found smaller variation than in the present study; however, Drugscreen has a higher TF concentration. They found no effect of freezing with minimal nonsignificant differences between fresh and frozen samples for up to 11 months except for ttPeak, similar to the findings in the present study.
4.1. Practicability
The new equipment is designed for use in a routine laboratory test, whereas CAT is a research instrument. Therefore, the ST Genesia is in some ways easier to use because it is more automated with no pipetting, and samples can be measured one by one, whereas a series is usually analyzed on CAT. It can, therefore, probably be part of routine coagulation laboratory tests in the future. In this way, it functions well. However, the software in the present model is relatively slow, and the screen can frequently freeze, which slowed the analysis. This must be improved to fulfill the requirements for use in a routine laboratory setting.
4.2. Limitations
The reference intervals determined in this study were based on only 30 individuals in each group rather than the recommended 120 individuals. However, the main issue here is to describe the important effects of the type of sampling tube and use of COC in women; thus, the proposed reference intervals are suggestive of the levels. We have used tubes with a high and a minimal contact activation, but each laboratory must develop its own reference intervals based on the type of tubes used for sample collection and should recommend the use of the same tubes and the same material when samples are delivered from other laboratories. Although the range of ages in the group of women using COCs was smaller than that in women not using COCs, age is not important for the level of intervals, and COC users are generally younger women.
5. CONCLUSION
The new instrument for measuring TG, the ST Genesia, showed comparable imprecision compared with that for the CAT method, and the kits contain samples for quality control and for normalization of the results, which may be an advantage. We have suggested reference intervals for men, and women using and not using COCs, and sampled in Monovette and Vacutainer tubes. The reference intervals depend on contact activation in the tubes used.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest. Reagents kits for estimating variation in this project were bought with a 50% discount from Diagnostica Stago.
AUTHOR CONTRIBUTIONS
Søren Risom Kristensen designed the study, interpreted data, and wrote the paper. Jette Nybo contributed to the design and performed the analyses and some data analysis. Shona Pedersen performed data analysis and interpretation. All authors contributed to the critical revision of the manuscript and approved the final version to be published.
Supporting information
Supplementary Material
Figure S1
Figure S2
Figure S3
Kristensen SR, Nybo J, Pedersen S. Thrombin generation measured on ST Genesia, a new platform in the coagulation routine lab: Assessment of analytical and between‐subject variation. Res Pract Thromb Haemost. 2022;6:e12654. doi: 10.1002/rth2.12654
Handling Editor: Dr Michelle Sholzberg
REFERENCES
- 1. Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. 1953;6(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4‐15. [DOI] [PubMed] [Google Scholar]
- 3. Hemker HC, Wielders S, Kessels H, Béguin S. Continuous registration of thrombin generation in plasma, its use for the determination of the thrombin potential. Thromb Haemost. 1993;70(4):617‐624. [PubMed] [Google Scholar]
- 4. Hemker HC, Willems GM, Béguin S. A computer assisted method to obtain the prothrombin activation velocity in whole plasma independent of thrombin decay processes. Thromb Haemost. 1986;56(1):9‐17. [PubMed] [Google Scholar]
- 5. van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142(6):889‐903. [DOI] [PubMed] [Google Scholar]
- 6. Tripodi A. Thrombin generation assay and its application in the Clinical Laboratory. Clin Chem. 2016;62(5):699‐707. [DOI] [PubMed] [Google Scholar]
- 7. Cornette M, Monteyne T, De Kesel PM, Devreese KMJ. Thrombin generation measured by two platforms in patients with a bleeding tendency. J Thromb Haemost. 2021;19(6):1460‐1471. [DOI] [PubMed] [Google Scholar]
- 8. Liestøl S, Sandset PM, Mowinckel MC, Wisløff F. Activated protein C resistance determined with a thrombin generation‐based test is associated with thrombotic events in patients with lupus anticoagulants. J Thromb Haemost. 2007;5(11):2204‐2210. [DOI] [PubMed] [Google Scholar]
- 9. Kristensen AF, Kristensen SR, Falkmer U, Münster AB, Pedersen S. Analytical and between‐subject variation of thrombin generation measured by calibrated automated thrombography on plasma samples. Scand J Clin Lab Invest. 2018;78(3):175‐179. [DOI] [PubMed] [Google Scholar]
- 10. Vincent LM, Tran S, Livaja R, Bensend TA, Milewicz DM, Dahlbäck B. Coagulation factor V(A2440G) causes east Texas bleeding disorder via TFPIα. J Clin Invest. 2013;123(9):3777‐3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunha ML, Bakhtiari K, Peter J, Marquart JA, Meijers JC, Middeldorp S. A novel mutation in the F5 gene (factor V Amsterdam) associated with bleeding independent of factor V procoagulant function. Blood. 2015;125(11):1822‐1825. [DOI] [PubMed] [Google Scholar]
- 12. "Stago" (Hemker HC, Lecompte LT) . An Introduction to the Concise Measurement of Thrombin Forming Potential in Plasma. Diagnostica Stago; 2017. [Google Scholar]
- 13. Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/‐ thrombomodulin to recognise the prothrombotic phenotype. Thromb Haemost. 2006;96(5):562‐567. [PubMed] [Google Scholar]
- 14. Lacroix R, Judicone C, Poncelet P, et al. Impact of pre‐analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10(3):437‐446. [DOI] [PubMed] [Google Scholar]
- 15. Kristensen SR, Nybo J, Pedersen S. The effect of pH on thrombin generation‐An unrecognized potential source of variation. Res Pract Thromb Haemost. 2020;4(2):224‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute Document EP28‐A3c. Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 17. Calzavarini S, Brodard J, Quarroz C, et al. Thrombin generation measurement using the ST Genesia Thrombin Generation System in a cohort of healthy adults: normal values and variability. Res Pract Thromb Haemost. 2019;3(4):758‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ninivaggi M, de Laat‐Kremers RMW, Carlo A, de Laat B. ST Genesia reference values of 117 healthy donors measured with STG‐BleedScreen, STG‐DrugScreen and STG‐ThromboScreen reagents. Res Pract Thromb Haemost. 2021;5(1):187‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loeffen R, Kleinegris MC, Loubele ST, et al. Preanalytic variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. 2012;10(12):2544‐2554. [DOI] [PubMed] [Google Scholar]
- 20. Dargaud Y, Luddington R, Gray E, et al. Effect of standardization and normalization on imprecision of calibrated automated thrombography: an international multicentre study. Br J Haematol. 2007;139(2):303‐309. [DOI] [PubMed] [Google Scholar]
- 21. Perrin J, Depasse F, Lecompte T. Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. 2015;136(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 22. Glintborg D, Sidelmann JJ, Altinok ML, Mumm H, Andersen M. Increased thrombin generation in women with polycystic ovary syndrome: a pilot study on the effect of metformin and oral contraceptives. Metabolism. 2015;64(10):1272‐1278. [DOI] [PubMed] [Google Scholar]
- 23. Roullet S, Labrouche S, Freyburger G. Comparison of two thrombin generation methods, CAT and ST‐Genesia, in liver transplant patients. Thromb Haemost. 2019;119(6):899‐905. [DOI] [PubMed] [Google Scholar]
- 24. Douxfils J, Morimont L, Bouvy C, et al. Assessment of the analytical performances and sample stability on ST Genesia system using the STG‐DrugScreen application. J Thromb Haemost. 2019;17(8):1273‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Figure S1
Figure S2
Figure S3


