Abstract
Lymphoma that on morphology appear blastoid or intermediate between DLBCL and BL but who lack myc and bcl-2 and/or bcl-6 rearrangements are grouped under high grade B-cell lymphoma, not otherwise specified (HGBL, NOS). Only a few studies have yet compared the outcome of HGBL, NOS treated with different chemo-immunotherapy regimens. HGBL, NOS patients were analyzed retrospectively, who were treated with CHOP or DAEPOCH regimens every 21 days for six cycles with or without rituximab. The primary clinical objective was progression free survival. One and two year PFS rates were 29.4% and 20.6% for the CHOP arm and, 65.2% and 47.8% for the DAEPOCH arm respectively. There was statistically significant difference in mean PFS between the arms (DAEPOCH vs CHOP: 19.7 months vs 12.8 months; HR = 0.44, p = 0.02, 95% CI: 0.22–0.88). One and two year OS rates were 91.1% and 20.5% for the CHOP arm and 95.6% and 60.8% for the DAEPOCH arm respectively. Mean OS was significantly better for DAEPOCH arm (28.1 months vs 20.7 months: HR = 0.43, p = 0.03, 95% CI: 0.20–0.92). Grade 3 and 4 hematological and non-hematological toxicities were more common in DAEPOCH arm. There were 2 treatment related deaths, 1 in each arm (4.3% for DAEPOCH vs 2.9% for CHOP). HGBL, NOS is a heterogeneous group of aggressive lymphoma associated with early relapse in nearly half of the cases. Intensive regimens like DAEPOCH is associated with improved outcome in terms of PFS and OS. Though toxicities are more with DAEPOCH, they are manageable and treatment related mortality is low.
Keywords: High grade B-cell lymphoma, CHOP, DAEPOCH
Introduction
High-grade B-cell lymphoma (HGBL) was introduced for the first time in the updated 2016 revision of the World Health Organization (WHO) classification, where it replaced the entity “B-cell lymphoma, unclassifiable.” HGBL is characterized by overlapping pathologic features with DLBCL, burkitt lymphoma (BL) and B-cell lymphoblastic lymphoma/leukemia (B-LBL). Currently HGBL comprises of 2 types of lymphomas: HGBL with MYC and BCL2 and/or BCL6 rearrangements and high grade B-cell lymphomas, not otherwise specified (HGBL, NOS). Former entity is also known as double/triple-hit lymphoma (DHL/THL). HGBL, NOS lymphomas do not contain MYC and BCL2 or BCL6 gene rearrangements, but present morphologies between DLBCL and BL. Also in terms of aggressiveness and prognosis, this entity is considered intermediate between DLBCL and DHL/THL. There are only a few studies which have compared the outcome of HGBL, NOS treated with different chemo-immunotherapy regimens (Fig. 1).
Fig. 1.

WHO classification of lymphoma. According to the new 2016 WHO classification of lymphoma, if myc and bcl-2 rearrangements are present, regardless of morphology, they are now categorized as high-grade B-cell lymphoma with myc and bcl-2 and/or bcl-6 rearrangements. Those cases that on morphology appear blastoid or intermediate between DLBCL and BL but who lack myc and bcl-2 and/or bcl-6 rearrangements are grouped under high grade B-cell lymphoma, not otherwise specified (HGBL, NOS) Adapted from Swerdlow et al. [1]
Methods
Study Design and Patients
Fifty-seven patients of high grade B cell lymphoma NOS (HGBL NOS) registered between Jan 2013 to Jan 2018, were retrospectively recruited for the study. Patients with minimum follow up period of 2 years or who came across an event before the minimum follow up period, had been included in the study. Eligible patients included untreated HGBL, NOS confirmed by institutional pathology review, and who were not expressing double expressor phynotype, i.e. on immunohistochemistry, tissue was negative for myc and bcl-2 or bcl-6 proteins, and on cytogenetics negative for myc and bcl-2 or 6 translocations. Eligible patients were non-randomly assigned to CHOP like regimen or DAEPOCH with or without rituximab. Glucocorticoid treatment for 7 days along with single dose vincristine as a prephase treatment were allowed before starting protocol specific chemotherapy. Other eligibility criteria included age ≥ 18 years, eastern cooperative oncology group (ECOG) performance status 0 to 2, left ventricular ejection fraction greater than 50%, absolute neutrophil count (ANC) greater than 1,500/mL, platelet count greater than 100,000/mL. Liver fuction test and renal function tests within normal limits. Patients with CNS disease or who had HIV, were excluded.
Treatment
CHOP and DAEPOCH administration was planned every 21 days for six cycles with or without rituximab. No escalation of dose was allowed but de-escalation of cyclophosphamide was planned according to the ANC and platelet nadir. Patients with elevated lactate dehydrogenase along with more than one extra-nodal sites, high CNS-IPI or involvement of sites like paranasal sinuses, breast, kidney, adrenals, testis or epidural spaces received CNS prophylaxis in the form of 2 cycles of IV HD MTX(gm/m2) and/or 4–6 cycles IT MTX(12 mg) along with the chemoimmunotherapy. Consolidation radiotherapy was given to patients with localized residual diseases. Lamivudine 100 mg per day was given to patients who were found to be positive for hepatitis B surface antigen. Pneumocystis prophylaxis was given with DAEPOCH. Filgrastim was given on days 6 through 15 of DAEPOCH or until ANC was greater than 10,000/mL after nadir. In the CHOP arm, filgrastim or pegfilgrastim was initiated if a patient experienced an ANC of less than 500/mL or febrile neutropenia with the previous cycle, or at the discretion of the treating doctor.
Response assessed according to the Lugano criteria. All the patients had undergone contrast enhanced CT scan or PET CT scan at baseline, after 3 cycles of chemotherapy and at the end of the treatment after six weeks of the last or the 6th cycle chemotherapy. Adverse events were reported according to the revised NCI Common Terminology Criteria for Adverse Events, version 5.0.
Aims and Objectives
The primary clinical end point was PFS, measured from randomization to progression, relapse or death from any cause or starting of following line of anticancer chemotherapy following detection of non-complete response to first line therapy, whichever occurred first. Secondary clinical objectives included comparisons of response rate and toxicity and overall survival (OS). The protocol required at least 2 years of follow up, if no events occurred before the minimum follow up duration.
Statistical Analysis
All analyses were performed with SPSS, version 19. The primary end point was assessed between the arms using Kaplan–Meier models, unadjusted Cox regression models and Cox models stratified by IPI. Statistical tests reported were two sided and P < 0.05 was used to declare statistical significance for the primary end point (Table 1).
Table 1.
Patient characteristics
| Characteristics | CHOP(n = 34) | DAEPOCH (n = 23) | P |
|---|---|---|---|
| Sex | 0.96* | ||
| Male | 19 (55.9%) | 13 (56.5%) | |
| Female | 15 (44.1%) | 10 (43.5%) | |
| Age (in years) | 0.28** | ||
| Mean | 54.8 | 50.1 | |
| Median | 62 | 48 | |
| Range | 20–80 | 26–75 | |
| Age group | 0.02* | ||
| 18–60 | 15 (44.1%) | 17 (73.9%) | |
| > 60 | 19 (55.9%) | 6 (26.1%) | |
| ECOG PS | 0.02* | ||
| 0 | 2 (5.9%) | 3 (13.1%) | |
| 1 | 12 (35.3%) | 11 (47.8%) | |
| 2 | 9 (26.5%) | 7 (30.4%) | |
| 3 | 11 (32.3%) | 2 (8.7%) | |
| Extranodal disease (≥ 1) | 29 (85.3%) | 11 (47.8%) | 0.02* |
| Stage | 0.76* | ||
| I | 2 (5.9%) | 2 (8.7%) | |
| II | 5 (14.7%) | 5 (21.7%) | |
| III | 6 (17.6%) | 5 (21.7%) | |
| IV | 21 (61.8%) | 11 (47.9%) | |
| RIPI risk groups | 0.34* | ||
| Very good(0) | 2 (5.9%) | 2 (8.7%) | |
| Good (1–2) | 15 (44.1%) | 14 (60.9%) | |
| Poor (3–5) | 17 (50%) | 7 (30.4%) | |
| Rituximab | 22 (64.7%) | 19 (82.6%) | 0.14* |
DAEPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; ECOG, Eastern Cooperative Oncology Group; RIPI, Revised International Prognostic Index; PS, performance status
*Chi-square test
**Independent-samples T test
Results
Patients were non-randomly assigned to CHOP like regimen or DAEPOCH with or without rituximab. Of the 57 patients 34 patients had received CHOP like regimen (CHOP = 23, COP = 3, CHOP + HDMTX = 8) and 23 had received DA-EPOCH. 22(64.7%) in the CHOP arm and 19(82.6%) in the DAEPOCH arm had received rituximab. Study arms were not balanced for the baseline characteristics. 4(7.0%), 10(17.6%), 11(19.3%) and 32(56.1%) had stage I, II, III, IV diseases respectively. 4(7%), 7(12.3%), 22(38.6%), 14(24.6%), 9(15.8%) and 1(1.8%) patients had 0, 1, 2, 3, 4 and 5 IPI score respectively. There were 25 patients above 60 yrs and 9 of them were of 70 years or older. 45(78.9%) patients received prephase treatement [26(76.5%) and 19(82.6%) in the CHOP and DAEPOCH arm respectively] with a single dose of vincristine and 7 days of corticosteroids briefly before initiating protocol specific therapy. Eight patients received consolidation radiotherapy (RT). Intrathecal methotrexate prophylaxis was administered in all 23 patients receiving DA-EPOCH. Two cycles of high dose methotrexate at 3 gm/m2 were given to 8 patients of CHOP arm, who had higher risk of CNS relapse, either alternating with CHOP, in between the first 3 cycles of chemo-immunotherapy or after the completion of the 6th cycle. In the CHOP arm, 13 (38.2%) patients received growth factor support, including 4 who initiated filgrastim with cycle one. All six treatment cycles were completed by 30 (88.2%) patients of the CHOP arm and 16(69.6%) of the DAEPOCH arm. Reasons for early discontinuation included disease progression (CHOP-1; DAEPOCH-0), adverse events (CHOP- 2; DAEPOCH- 6) and death (CHOP-1; DAEPOCH-1).
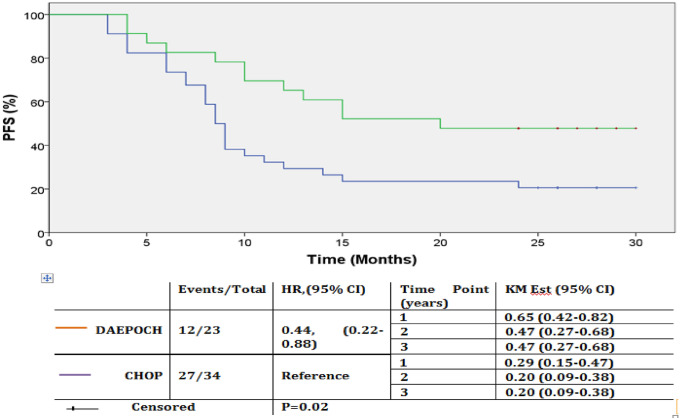
Across the arms, median follow-up period was 21 months (range: 3–37 month). During this period 39(68.4%) patients had a progression event and 36(63.2%) patients had died. One and two year PFS rates were 29.4% and 20.6% for the CHOP arm and, 65.2% and 47.8% for the DAEPOCH arm respectively. Kaplan–Meier estimation of PFS rates at 3 year were 20.5% and 47.8% for the CHOP and DAEPOCH arm respectively. Mean PFS (mPFS) for the whole cohort was 15.6 months (95% CI 12.8 months–18.3 months). There was statistically significant difference in mPFS between the arms (DAEPOCH vs CHOP: 19.7 months vs 12.8 months; HR = 0.44, 95% CI: 0.22–0.88, p = 0.02). When stratified across the R-IPI risk groups, mPFS was significantly higher for very good and good risk groups combined in comparison to poor risk group (18.1 months vs 11.7 months, p = 0.03). Within the risk groups, difference in mPFS between the DAEPOCH and CHOP arms was more pronounced in the very good risk and good risk groups combined (DAEPOCH vs CHOP: 21.5 months vs 15.0 months; HR = 0.45, p = 0.079, 95% CI: 0.18–1.15).
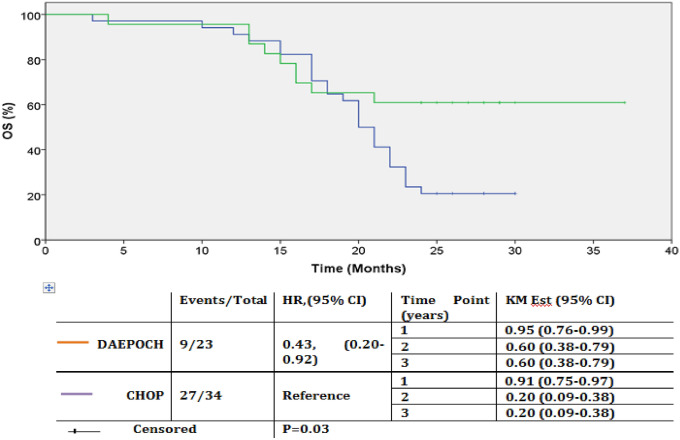
One and two year OS rates were 91.1% and 20.5% for the CHOP arm and 95.6% and 60.8% for the DAEPOCH arm respectively. Kaplan–Meier estimation of OS rates at 3 year were 20.5% and 60.8% for CHOP and DAEPOCH arm respectively. Mean OS (mOS) was significantly better for DAEPOCH arm (28.1 months vs 20.7 months: HR = 0.43, p = 0.03, 95% CI: 0.20–0.92), but when stratified across the risk groups, this difference in mean OS was only seen in very good risk and good risk groups combined (30.1 months vs 20.9 months: HR = 0.39, p = 0.06).
Addition of rituximab resulted in improvement in PFS, in the CHOP arm (14.9 months vs 8.9 months; HR = 0.47, p = 0.05, 95% CI: 0.98–4.6). Benefit in PFS in the DAEPOCH arm with the addition of rituximab, was less pronounced (19.7 months vs 17.6 months; HR = 0.99, p = 0.99). Among the patients who had received rituximab, differences in mPFS or mOS were not statistically significant, between the two chemotherapy groups (mPFS: R-DAEPOCH vs R-CHOP: 19.7 months vs 14.9 months, HR = 0.58, p = 0.185, 95% CI: 0.25–1.29; mOS: R-DAEPOCH vs R-CHOP: 28.5 months vs 21.9 months, HR = 0.52, p = 0.16, 95% CI: 0.21–1.29) (Figs. 2, 3, 4).
Fig. 2.
PFS by treatment arms. Kaplan–Meier PFS estimate of patients by treatment arms in months. There was statistically significant difference in PFS between arms (P = 0.02). Curves becoming parallel to the X axis after around 24 months, suggests that, post 2 yrs of PFS none of the patients relapsed, and these group of patients who are considered to be cured are significantly higher in number in DAEPOCH arm (47% in the DAEPOCH and 20.5% in the CHOP arm). Abbreviation: DAEPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; HR, hazard ratio; KM Est, Kaplan–Meier estimate; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone.
Fig. 3.
OS by treatment arms. Kaplan–Meier OS estimate of patients by treatment arms in months. There was statistically significant difference in OS between arms (P = 0.03). Curves becoming parallel to the X axis after 20–24 months, suggests that, a group of patients gets cured in both the arms, and their number is significantly higher in DAEPOCH arm (60.8% in the DAEPOCH and 20.5% in the CHOP arm). Abbreviation: DAEPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; HR, hazard ratio; KM Est, Kaplan–Meier estimate; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone
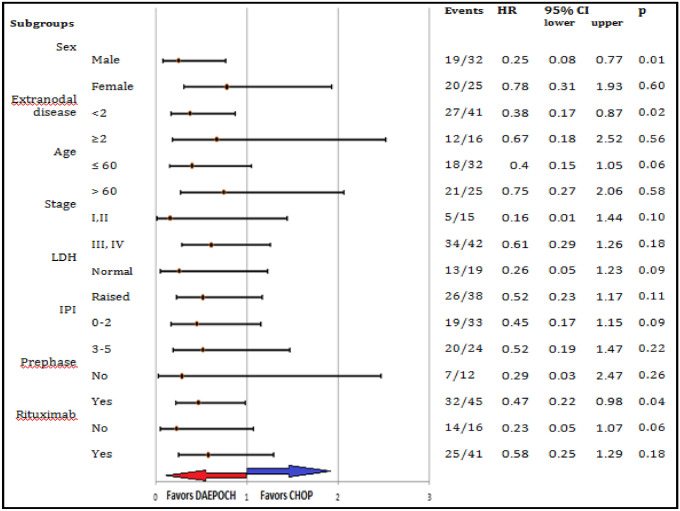
Fig. 4.
PFS by subset analysis. Comparison of PFS between the arm in subgroups of standard clinical features. PFS was significantly higher in the DAEPOCH arm in male patients (p = 0.01) and in patients with < 2 extranodal sites of involvement (p = 0.02). There was no meaningful difference in PFS between the arms for any of the other subgroups. DAEPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; HR, hazard ratio; RIPI, Revised International Prognostic Index; LDH, lactate dehydrogenase; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone
The overall response rate was 97.1% [CR = 17(50%), PR = 16(4 7.1%)] in the CHOP arm and 100% [(CR = 18(78 .3%), PR = 5(21.7%)] in the DA-EPOCH arm (P = 0.08). Overall, patients who received prephase treatment before the protocol specific chemo-immunotherapy did not differ in terms of mPFS from those who did not receive it (mPFS with no prephase vs prephase: 16.2 months vs 15.3 months; p = 0.67). But when patients were stratified according to the regimens, in the DAEPOCH arm patients who received prephase treatment had shorter PFS than who did not receive it (mPFS in DAEPOCH arm with no prephase vs prephase: 22.8 months vs 18.9 months; p = 0.36), though the difference was not statistically significant. Overall patients older than 60 years had an inferior PFS compared to younger patients (mPFS: age > 60 vs ≤ 60: 11.7 months vs 18.6 months; HR = 2.12, p = 0.02). This difference in mPFS with age was more pronounced in the DAEPOCH arm (age > 60 vs ≤ 60: 12 months vs 22.0 months; HR = 2.94, p = 0.06) in comparison to the CHOP arm (11.2 months vs 14.2 months; HR = 1.45, p = 0.33). CNS relapse occurred in 2(8.7%) patients of the DAEPOCH arm and 1(12.5%) patient of the CHOP arm, all three of them had received CNS prophylaxis in the form of IT MTX or HD MTX. There were 2 treatment related deaths, 1 in each arm (DAEPOCH vs CHOP: 4.3% vs 2.9%). Neutropenic sepsis was the cause of death in both the cases. Grade 3 or 4 hematologic toxicities occurred in all 23(100%) patients in the DAEPOCH arm and 9(26.4%) patients in the CHOP arm. Average number of episodes of grade 3 or 4 hematologic toxicity per patient was 3.04 and 0.38 for DAEPOCH and CHOP arm respectively (p = 0.00). Likewise non-hematological toxicities were also more frequent in the DAEPOCH arm. Six (26.1%) and two (5.9%) episodes of drug induced liver injury (DILI) (Transaminitis > 3 times + Total Bilirubin > 2 times of the upper limit of normal) occurred in DAEPOCH and CHOP arms respectively. One (4.3%) grade IV liver failure (severe encephalopathy) occurred in the DAEPOCH arm and none in the CHOP arm. Late cardiac events occurred in 1 (4.3%) patient in the DAEPOCH arm and in 2 (5.9%) patients in the CHOP arm (Tables 2, 3, 4).
Table 2.
PFS with respect to the treatment arms
| Treatment arm | Events/Total | HR (95% CI)* | Mean PFS at 2yrs (in months) | KM Survival Estimate** | P value*** | |
|---|---|---|---|---|---|---|
| DAEPOCH | 12/23 |
0.44, (0.22–0.88) |
19.7 | At 1 yr | 0.65(0.42–0.82) | 0.02 |
| At 2 yr | 0.47(0.27–0.68) | |||||
| At 3 yr | 0.47(0.27–0.68) | |||||
| CHOP | 27/34 | Reference | 12.8 | At 1 yr | 0.29(0.15–0.47) | |
| At 2 yr | 0.20(0.09–0.38) | |||||
| At 3 yr | 0.20(0.09–0.38) | |||||
| Over all | 39/57 | 15.6 | At 1 yr | 0.43(0.30–0.57) | ||
| At 2 yr | 0.31(0.20–0.45) | |||||
| At 3 yr | ||||||
PFS, Progression Free Survival; DAEPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone
*Cox model
**Kaplan–Meier method
***Log-rank test
Table 3.
OS with respect to the treatment arms
| Treatment arm | Events/Total | HR (95% CI)* | Mean OS at 2yrs (in months) | KM Survival Estimate** | P value*** | |
|---|---|---|---|---|---|---|
| DAEPOCH | 9/23 | 0.43, (0.20–0.92) | 28.1 | At 1 yr | 0.95 (0.76–0.99) | 0.03 |
| At 2 yr | 0.60 (0.38–0.79) | |||||
| At 3 yr | 0.60 (0.38–0.79) | |||||
| CHOP | 27/34 | Reference | 20.7 | At 1 yr | 0.91 (0.75–0.97) | |
| At 2 yr | 0.20 (0.09–0.38) | |||||
| At 3 yr | 0.20 (0.09–0.38) | |||||
| Over all | 36/57 | 24.5 | At 1 yr | 0.92 (0.82–0.97) | ||
| At 2 yr | 0.36 (0.24–0.50) | |||||
| At 3 yr | 0.36 (0.24–0.50) | |||||
OS, Overall Survival; DAEPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone
*Cox model
**Kaplan–Meier method
***Log-rank test
Table 4.
Grade 3 & 4 treatment related toxicities
| CTCAE grade 3/4 toxicities | CHOP(n = 34) | DAEPOCH (n = 23) | P* |
|---|---|---|---|
| Hematologic (Total patients) | 13 (38.2%) | 23 (100%) | 0.00 |
| Anaemia | 6 (17.6%) | 16 (69.6%) | |
| Febrile neutropenia | 9 (26.5%) | 15 (65.2%) | |
| Nonhematological (Total patients) | 8 (23.5%) | 16 (69.6%) | 0.00 |
| Mucositis | 2 (5.9%) | 9 (39.1%) | |
| CINV | 0 (0%) | 0 (0%) | |
| Diarrhoea | 4 (11.8%) | 8 (34.8%) | |
| Hepatic failure | 2 (5.9%) | 6 (26.1%) | |
| Cardiac | 1 (2.9%) | 1 (4.3%) | |
| Neuropathy | 1 (2.9%) | 5 (21.7%) |
CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0; DAEPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone
*Chi-square test
Discussion
HGBL is characterized by the overlapping pathologic features with DLBCL, burkitt lymphoma and B-cell lymphoblastic lymphoma/leukemia (B-LBL). These morphological features correlate with aggressive clinical behavior. The term high-grade B-cell lymphoma was proposed in the 2016 revision of WHO classification of lymphomas. It consists of two entities, which are DHL/THL and high-grade B-cell lymphoma NOS. Latter is a group, which do not have double-hit or triple-hit genetics by FISH or conventional karyotyping.
For DLBCL, Alliance/CALGB 50,303 study found no benefits in treating with more intensive R-DAEPOCH regimen compared to the standard R-CHOP, in terms of PFS or OS [2]. In case of burkitt lymphoma, intensive regimens achieve higher PFS and OS compared to RCHOP [3, 4]. Double/triple hit lymphomas (DHL/THL) or double expressor lymphomas (DEL) as well, are considered more aggressive than DLBCL, and are seen to have better outcome with intensive regimens [5, 6]. HGBL, NOS are more aggressive than DLBCL but are considered to have a better prognosis compared to DHL/THL/DEL. A few studies only have yet compared the outcome of HGBL, NOS treated with different chemo-immunotherapy regimens.
In a retrospective observational study by Jonathan R et al. 38 patients with with HGBL NOS received R-CHOP, R-EPOCH, or R-HyperCVAD. After a median follow-up of 18 months, there was no difference in the OS between R-CHOP and higher intensity regimens (p = 0.540). So they concluded that both standard therapy with R-CHOP and higher intensity therapies appear to be effective in the treatment of HGBL without double-hit genotype [7]. In another observational study, Jiayin Li et al. analyzed 41 cases of HGBL NOS. Median PFS was 6.0 months and the median OS was 18.0 months. Patients treated with a high-intensity chemotherapy (DA-EPOCH-R, R-CODOX-M/IVAC, or R-HyperCVAD) had superior PFS and OS (PFS: P = 0.041; OS: P = 0.023) compared with patients treated with the R-CHOP regimen. And a subgroup analysis showed that the OS for the double-expressor lymphoma (DEL) was inferior to that for the non-DEL (P = 0.033) [8]. In our study we compared the efficacy of CHOP to the more intensive, infusional DAEPOCH in patients with untreated HGBL, NOS. Though DAEPOCH was more toxic, it was associated with greater response rate and significant improvement in PFS and OS. In both the treatment arms, all the events of progression occurred in first 2 years of treatment. Patients with PFS more than 2 yrs did not relapse subsequently in the study, suggesting that a group of patients got cured in both the arms. This group was significantly larger in the DAEPOCH arm (47% vs 20.5%, p = 0.02). This may as well suggest that, the patient cohort in the study is a heterogeneous one, and a subgroup of very aggressive lymphoma associated with early relapse exists within the cohort. This aggressive subgroup could be the one who might be having myc and bcl 2 and/or bcl 6 rearrangements but who does not express respective proteins so could not be picked up in the IHC. Improvement in PFS and OS with DAEPOCH over CHOP was more pronounced in the sub group of patients with RIPI score 0 to 2 (very good risk and good risk combined). Poor risk group had only non significant benefit in PFS and no benefit in OS with DAEPOCH over CHOP.
Alliance/CALGB 50,303 observed 3 year PFS rate for DLBCL of 72% [2]. Mayo/Iowa molecular epidemiology resource cohort study found that, for patients with stage II to IV DLBCL or PMBCL treated with R-CHOP the 3-year EFS rate was 61% [9]. Patients with DLBCL with MYC rearrangement (myc + ve), especially DHL, have a worse prognosis. The British Columbia Cancer Agency reported a 5-year PFS rate of 31% with R-CHOP for patients with myc + ve DLBCL versus 66% in the MYC negative cohort. In our study 2 year PFS and OS rate of 31.6% and 36.8% respectively, suggests that HGBL, NOS is more aggressive than DLBCL and is associated with early relapse and poorer survival. Two year PFS and OS rates were significantly better in the DAEPOCH arm (PFS: 47.8% vs 20.6%, p = 0.01; OS: 60.9% vs 20.6%, p = 0.02), which suggests that these patients may benefit more from a more aggressive treatment regimen than RCHOP. Rituximab is a part and parcel of treatment regimens for DLBLCL and other CD 20 positive lymphomas. Here in India, not all our patients are covered by health insurances and not all could afford rituximab. That is why, not all patients in our study could receive rituximab. Addition of rituximab resulted in improvement in both PFS and OS irrespective of the chemotherapy back bone. But benefit was more pronounced with CHOP regimen, to the extent that, PFS and OS did not differ significantly between the two chemotherapy arms, for those who received rituximab along with the chemotherapy. This again depicts the aggressive nature of the disease and suggests that, CHOP may be inadequate as a chemotherapy regimen for HGBL, NOS.
DAEPOCH was associated with higher number of any grade or grade 3 and grade 4, hematological and non-hematological toxicities. But with supportive care most of these episodes are manageable. Treatment related mortality rates did not differ significantly between the arms (4.3% and 2.9% in the DAEPOCH and CHOP arm respectively). Alliance/CALGB 50,303 trial documented treatment related death rate of 2.1% both in DAEPOCH and CHOP arms [2]. In our study patients in the DAEPOCH arm who received prephase treatment before the chemotherapy regimen, tends to have poorer PFS than who did not receive any prephase treatment. It may suggest that, delaying the aggressive chemotherapy regimen may have an adverse effect on the PFS in HGBL, NOS.
Limitations of the Study
It was a retrospective study and patients were not randomly allotted to treatment groups, so observer bias (systematic bias) in allotment can’t be ruled out. Both the groups were not matched evenly for the baseline characteristics, with CHOP group having more patients with poor PS, older age, and with higher extranodal diseases as compared to the DAEPOCH group. For logistic issues, not all patients had received rituximab, which has proven benefits in CD 20 positive aggressive lymphomas. FISH for MYC or BCL 2 and or BCL 6 was not conducted on the patients to rule out DHL/THL. Small and heterogeneous patient population precludes us from drawing a firm conclusion from the results.
Conclusion
HGBL, NOS is a heterogeneous group of aggressive lymphoma associated with early relapse in around half of the cases. Intensive regimens like DAEPOCH is associated with improved outcome in terms of PFS and OS. DAEPOCH regimen is associated with higher hematological and non-hematological toxicities. But with supportive care, episodes of toxicities can be managed efficiently so treatment related mortality is low. Intensive regimens like R-DAEPOCH might be the chemo-immunotherapy regimen of choice in HGBL, NOS, especially in young patients with good performance status.
Authors' Contributions
LAJ conceived the idea and supervised the study and the findings. LM and KS retrieved the data from the individual patient files. LM analyzed the data, and wrote the manuscript. All the authors read and approved the final manuscript.
Funding
No external funding was taken for the study and authors have no relationships with any industry.
Availability of Data and Material
The datasets used and analyzed during the current study are available from the first or corresponding author on request.
Declaration
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
Ethical approval for the study has been taken from the institute ethical committee.
Consent to Participate
Consent for participation in clinical study was taken from all the patients.
Consent for Publication
Consent for publication of clinical data had been taken from all the patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lalatendu Moharana, Email: drlalatendu@gmail.com.
Lokanatha Dasappa, Email: drlok61@gmail.com.
Suresh Babu, Email: suresbabumc@gmail.com.
K. N. Lokesh, Email: knloki@gmail.com
Ah Rudresh, Email: rudresha.ah@gmail.com.
L. K. Rajeev, Email: lkrajeev@gmail.com
Smitha Saldanha, Email: saldanhasmitha@gmail.com.
Kanika Sharma, Email: Sharma.kanika1609@gmail.com.
Linu Abraham Jacob, Email: mannellinujacob@gmail.com.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the phase III intergroup Trial Alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790–1799. doi: 10.1200/JCO.18.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wästerlid T, Brown PN, Hagberg O, et al. Impact of chemotherapy regimen and rituximab in adult burkitt lymphoma: a retrospective population-based study from the nordic lymphoma group. Ann Oncol. 2013;24(7):1879–1886. doi: 10.1093/annonc/mdt058. [DOI] [PubMed] [Google Scholar]
- 4.Oosten LEM, Chamuleau MED, Thielen FW, et al. Treatment of sporadic burkitt lymphoma in adults, a retrospective comparison of four treatment regimens. Ann Hematol. 2018;97(2):255–266. doi: 10.1007/s00277-017-3167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124(15):2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 6.Howlett C, Snedecor SJ, Landsburg DJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol. 2015;170(4):504–514. doi: 10.1111/bjh.13463. [DOI] [PubMed] [Google Scholar]
- 7.Rush J, Sehgal AR, Roth CG. Michael Boyiadzis; The effect of therapy on high grade B cell lymphoma, not otherwise specified and outcomes in comparison with double hit lymphoma. Blood. 2016;128(22):4224. doi: 10.1182/blood.V128.22.4224.4224. [DOI] [Google Scholar]
- 8.Li J, Liu X, Yao Z, Zhang M. High-Grade B-cell lymphomas, not otherwise specified: a study of 41 cases. Cancer Manag Res. 2020;13(12):1903–1912. doi: 10.2147/CMAR.S243753.PMID:32214848;PMCID:PMC7082796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerhan JR, Link BK, Habermann TM, et al. Cohort profile: the lymphoma specialized program of research excellence (SPORE) Molecular epidemiology resource (MER) cohort study. Int J Epidemiol. 2017;46(6):1753–1754i. doi: 10.1093/ije/dyx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the first or corresponding author on request.