Abstract
Molecular abnormalities in leukemic cells are important determinants of risk stratification in Pediatric acute lymphoblastic leukemia (ALL). TCF3-PBX1 fusion is one of the common aberrations in ALL with doubtful prognostic significance. Therefore, aim of our study is to revisit the clinical characteristics and outcome of this abnormality in children with ALL treated at our institute.Demographic, Clinical and treatment related characteristics of 539 newly diagnosed ALL patients from January 2009 and December 2018, < 18 years of age treated on BFM-95 protocol, was abstracted from the medical records. Clinical characteristics and outcome of children with and without TCF3-PBX1 fusion was compared.Incidence of TCF3-PBX1 fusion was observed in 24/539(4.4%) patients with a median age of 4 years (range 1–17). None of the patients in TCF3-PBX1 group had CNS or testicular disease at presentation. Day -8 prednisolone response and morphological remission at the end of induction was similar in both study groups. 5-year overall and event free survival for those with and without fusion was 75%, 70.1% and 79.5%, 69.5% respectively.The incidence of TCF3-PBX1 fusion in the present study was 4.4% and it does not have an independent prognostic significance.
Keywords: Children, ALL, TCF3-PBX1 fusion, t (1; 19)
Introduction
Accurate risk stratification based on cytogenetic and molecular abnormality combined with response to treatment has paved way for assigning appropriate treatment to children with acute lymphoblastic leukemia (ALL) [1, 2]. The translocation t(1;19) (q23; p13), which results in the TCF3-PBX1 chimeric gene, is one of the most frequent genetic abnormality observed in B-ALL. It occurs in both adult and pediatric ALL at an overall frequency of 3 to 5% [3–8]. The t(1;19) involves E2A gene on chromosome 19p13.3, also known as TCF3 which encodes helix-loop-helix proteins involved in regulation of various lymphoid genes [9]. The homeobox gene PBX1 is located on chromosome 1q23 and has been implicated in development of leukemia. The resulting aberrant chimeric protein product appears to have oncogenic potential by affecting cell differentiation [10–12]. There is a conflicting data in literature over prognostic significance of TCF3-PBX1 fusion in children and adolescents with ALL [13, 14]. Therefore, aim of our study is to revisit clinical characteristics and outcome of this abnormality in children with ALL treated with Berlin-Frankfurt-Munster – 95 (BFM-95) protocol at our institute [15].
Material and Methods
Study Subjects
The present analysis is a single institutional study carried out in the pediatric Hematology-Oncology department of a tertiary care cancer centre. This included retrospective review of medical records of patients < 18 years of age, diagnosed with ALL from January 2009 and December 2018. For all patients at diagnosis, bone marrow aspirate were sent for karyotype and multiplex reverse transcriptase polymerase chain reaction (RTPCR) for t(12;21), t(9;22), t(4;41) and t(1;19) as a part of risk stratification work-up. All the patients who had t(1;19) (q23:p13.3) on cytogenetics or presence of TCF3:PBX1 fusion on molecular studies were included in the analysis. Previously treated or relapsed patients were excluded from the study. All patients were treated on a BFM-95 based protocol. The study was approved by the institutional review board.
Data Collection
Patient medical records were reviewed to determine demographic profile, initial disease characteristics, response to treatment, disease status and follow-up. Event free survival (EFS) was defined as time from date of diagnosis till any event which included relapse, abandonment or death from any cause. Overall survival (OS) was defined as duration from date of diagnosis till death or last follow-up visit.
Statistical Analysis
Descriptive statistics were obtained using mean with standard deviation or median with range. Categorical variables were represented with frequencies and their corresponding percentages. The chi square test was used to assess association between different variables and outcome. EFS and OS were estimated using Kaplan Meir curves by log rank test. Differences were considered to be statistically significant at P < 0.05 in all analyses. Data was analysed using SPSS software (version 21.0).
Results
During the study period, 539 of 560 consecutive patients diagnosed with ALL, were found to be eligible for analysis. Twenty four of these were noted to have TCF3-PBX1 fusion by RTPCR accounting for an incidence of 4.4% (24/539). Thirteen of these patients had t(1;19) on G-banding as well (10 had balanced; t(1;19)(q23;p13) and 3 had unbalanced translocation; der(19)t(1;19)(q23;p13). Four of these had additional cytogenetic abnormalities. Rest of the eleven patients had karyotype failure. None of patients had bcr-abl, 11q23 or t(12;21). The demographic and disease characteristics and response to treatment of these patients were comparable to rest of cohort (Table 1). Median age of this group was 4 years (range 1–17). None of the patients in the TCF3-PBX1 fusion group had central nervous system (CNS) or testicular disease at presentation. Immunophenotype revealed 23 (92%) of these patients had CD10/CD19/CyCD79a( +) and 22 (88%) had CD33/CD34 (-) expression. None of the patient with TCF3-PBX1 fusion had event during induction. All patients achieved morphological remission. Initial response to treatment (D-8 prednisolone response) and morphological remission at the end of induction was similar to rest of the patient without TCF3-PBX1 fusion.
Table 1.
Comparison of demographic and disease characteristics of children with or without TCF3-PBX1 ALL
| t(1;19) + N = 24 | t(1;19) − N = 515 | ||
|---|---|---|---|
| Characteristic | N (%) | N (%) | p- value |
| Age at Diagnosis (in years) | |||
| < 1 | 0(0) | 11(2) | 0.715 |
| > 1- 10 | 18(75) | 360(70) | |
| > 10 | 6(25) | 144(28) | |
| Gender | |||
| Male | 17(71) | 377(73) | 0.815 |
| Female | 7(29) | 138(27) | |
| WBC (cmm) at Diagnosis | |||
| < 20,000 | 12(50) | 236(46) | 0.895 |
| > 20,000–1,00,000 | 7(29) | 173(34) | |
| > 1,00,000 | 5(21) | 106(20) | |
| Immunophenotype | |||
| B | 22(92) | 425(82) | 0.490 |
| T | 2(8) | 84(16) | |
| B + T | 0(0) | 6(1) | |
| CNS status | |||
| Involved | 0(0) | 16() | 0.664 |
| Uninvolved | 24(100) | 498() | |
| Testicular involvement | 0(0) | 9(2) | |
| BFM risk | |||
| Standard | 3(12) | 90(17) | 0.698 |
| Moderate | 16(67) | 300(58) | |
| High | 5(21) | 125(24) | |
| Good prednisolone response | 21(88) | 447(87) | 0.982 |
| Poor prednisolone response | 3(12) | 68(13) | |
| End of induction BM response | |||
| CR | 24(100) | 504(98) | 0.469 |
| No CR | 0(0) | 11(2) | |
| Cranial RT | 5(21) | 88(17) | 0.585 |
WBC White blood count, BFM Berlin Frankfurt Munster, BM Bone Marrow,
CR complete remission, RT Radiotherapy
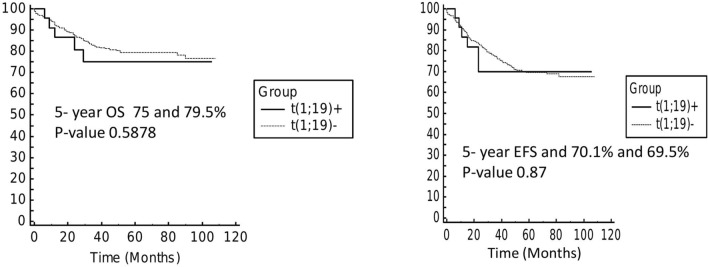
Overall there were 5 events in TCF3-PBX1 fusion group (5 relapse; 3 isolated bone marrow, and one each isolated CNS and testicular) and 5-year OS and EFS was 75% and 70.1% respectively with a median follow-up of 32 months. Median time to relapse was 15 months (range: 8–24 months). In rest of the cohort, there were 124 events (92 relapse; 49 isolated bone marrow, 16 CNS, 9 testicular, 18 combined relapses and 32 treatment related deaths). 5-year OS and EFS of this cohort was 79.5% and 69.5% which was similar to TCF3-PBX1 fusion group (p-value 0.58 and 0.87) (Fig. 1).
Fig. 1 a.
Overall survival of patients with or without TCF3-PBX1 fusion b: Event free survival of patients with or without TCF3-PBX1 fusion
Discussion
Incidence of TCF3-PBX1 fusion in childhood ALL is variable in published literature depending on ethnicity of study population and diagnostic modality to detect this genetic abnormality [5–7]. It is lower in Caucasian (1.8–3.1%) compared to Asian population (5.2–11.4%) [8, 16]. Earlier studies which had relied only on karyotype to detect t(1;19) have reported relative lower incidence. In present study, we had an incidence of 4.4%. An Indian study has reported a lower incidence of this fusion transcript (2.6% in adults and 1.8% in children) and could be due to a small sample size [17]. All patients in our study were identified to have TCF3-PBX1 fusion by RTPCR and florescent in situ hybridisation (FISH) studies were not done. Eleven patients had karyotype failure and type of translocation (balanced or unbalanced) in these patients could not be ascertained.
Pre-treatment disease characteristics and initial response to treatment of patients with TCF3-PBX1 fusion was similar to that rest of cohort. TCF3-PBX1 fusion is reported to be associated with older age (> 10 years), higher white blood cell count and high hyperdiploidy. Felice et al. have also observed higher incidence of initial CNS disease (12.5%) when compared to non-TCF3–PBX1 B-ALL (1.2%). However, this is not observed by other groups and in our cohort none of the patient had these high risk features or CNS disease at diagnosis [7, 8, 16].
Outcome of the children with TCF3-PBX1 fusion has traditionally been reported inferior compared to other ALL counterparts. However with contemporary treatment it no longer considered an independent prognostic factor. Most of the study group do not include this in risk stratification and decisions are rather guided by end of induction minimum residual disease. In addition, prognostic importance of type of translocation has also been studied. A study from the Children’s Cancer Group (CCG) showed that patients with unbalanced der(19)(1;19) had better outcome compared to those with balanced translocation [2, 17, 18]. However, more recent studies from Andersen et al. and Felice et al. did not observe significant difference in the outcome [4, 7, 19].
There are also reports of increased CNS relapses in children with TCF3-PBX1 fusion ALL. In a report from St Jude Children’s Research Hospital, children with t(1;19) had a higher risk of CNS relapse (4/41), compared to those without it. However, majority of other studies including ours have not observed increased risk of relapse in this subset [20].
Limitation of our study is that it is a retrospective study with limited sample size. However, this is the first report from India that shows epidemiology and outcome of TCF3-PBX1 fusion ALL in children and adolescents treated uniformly with BFM-95 protocol. In conclusion our data shows that incidence of TCF3-PBX1 fusion in children and adolescents with ALL is similar to published literature and it does not have an independent prognostic significance.
Declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Consent to Participate/ Consent for Publication
Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524–2539. doi: 10.3324/haematol.2020.247031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Möricke A, Zimmermann M, Reiter A, et al. Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr. 2005;217(6):310–320. doi: 10.1055/s-2005-872515. [DOI] [PubMed] [Google Scholar]
- 3.Uckun FM, Sensel MG, Sather HN, et al. Clinical significance of translocation t(1;19) in childhood acute lymphoblastic leukemia in the context of contemporary therapies: a report from the Children's Cancer Group. J Clin Oncol. 1998;16(2):527–535. doi: 10.1200/JCO.1998.16.2.527. [DOI] [PubMed] [Google Scholar]
- 4.Andersen MK, Autio K, Barbany G, et al. Paediatric B-cell precursor acute lymphoblastic leukaemia with t(1;19)(q23;p13): clinical and cytogenetic characteristics of 47 cases from the Nordic countries treated according to NOPHO protocols. Br J Haematol. 2011;155(2):235–243. doi: 10.1111/j.1365-2141.2011.08824.x. [DOI] [PubMed] [Google Scholar]
- 5.Kager L, Lion T, Attarbaschi A, et al. Incidence and outcome of TCF3-PBX1-positive acute lymphoblastic leukemia in Austrian children. Haematologica. 2007;92(11):1561–1564. doi: 10.3324/haematol.11239. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Watson N, Sartor M, McCowage G, Smith A. Fifteen cases of t(1;19)(q23;p13.3) identified in an Australian series of 122 children and 80 adults with acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2001;124(2):132–136. doi: 10.1016/s0165-4608(00)00333-2. [DOI] [PubMed] [Google Scholar]
- 7.Felice MS, Gallego MS, Alonso CN, et al. Prognostic impact of t(1;19)/ TCF3-PBX1 in childhood acute lymphoblastic leukemia in the context of Berlin-Frankfurt-Münster-based protocols. Leuk Lymphoma. 2011;52(7):1215–1221. doi: 10.3109/10428194.2011.565436. [DOI] [PubMed] [Google Scholar]
- 8.Yen HJ, Chen SH, Chang TY, et al. Pediatric acute lymphoblastic leukemia with t(1;19)/TCF3-PBX1 in Taiwan. Pediatr Blood Cancer. 2017;64(10):e26557. doi: 10.1002/pbc.26557. [DOI] [PubMed] [Google Scholar]
- 9.Mellentin JD, Nourse J, Hunger SP, Smith SD, Cleary ML. Molecular analysis of the t(1;19) breakpoint cluster region in pre-B cell acute lymphoblastic leukemias. Genes Chromosom Cancer. 1990;2(3):239–247. doi: 10.1002/gcc.2870020313. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q, Wright DD, Kamps MP. Fusion with E2A converts the Pbx1 homeodomain protein into a constitutive transcriptional activator in human leukemias carrying the t(1;19) translocation. Mol Cell Biol. 1994;14(6):3938–3948. doi: 10.1128/mcb.14.6.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Privitera E, Kamps MP, Hayashi Y, et al. Different molecular consequences of the 1;19 chromosomal translocation in childhood B-cell precursor acute lymphoblastic leukemia. Blood. 1992;79(7):1781–1788. doi: 10.1182/blood.V79.7.1781.1781. [DOI] [PubMed] [Google Scholar]
- 12.Nourse J, Mellentin JD, Galili N, et al. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- 13.Crist WM, Carroll AJ, Shuster JJ, et al. Poor prognosis of children with pre-B acute lymphoblastic leukemia is associated with the t(1;19)(q23;p13): a Pediatric Oncology Group study. Blood. 1990;76(1):117–122. doi: 10.1182/blood.V76.1.117.117. [DOI] [PubMed] [Google Scholar]
- 14.Burmeister T, Gökbuget N, Schwartz S, et al. Clinical features and prognostic implications of TCF3-PBX1 and ETV6-RUNX1 in adult acute lymphoblastic leukemia. Haematologica. 2010;95(2):241–246. doi: 10.3324/haematol.2009.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Möricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2009;113(18):4478. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 16.Pang L, Liang Y, Pan J, Wang JR, Chai YH, Zhao WL. Clinical features and prognostic significance of TCF3-PBX1 fusion gene in Chinese children with acute lymphoblastic leukemia by using a modified ALL-BFM-95 protocol. Pediatr Hematol Oncol. 2015;32(3):173–181. doi: 10.3109/08880018.2014.983625. [DOI] [PubMed] [Google Scholar]
- 17.Chopra A, Soni S, Verma D, et al. Prevalence of common fusion transcripts in acute lymphoblastic leukemia: a report of 304 cases. Asia Pac J Clin Oncol. 2015;11(4):293–298. doi: 10.1111/ajco.12400. [DOI] [PubMed] [Google Scholar]
- 18.Secker-Walker LM, Berger R, Fenaux P, et al. Prognostic significance of the balanced t(1;19) and unbalanced der(19)t(1;19) translocations in acute lymphoblastic leukemia. Leukemia. 1992;6(5):363–369. [PubMed] [Google Scholar]
- 19.Schmiegelow K, Forestier E, Hellebostad M, Heyman M, Kristinsson J, Söderhäll S, Taskinen M. Nordic society of paediatric haematology and oncology. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukaemia. Leukemia. 2010;24:345–54. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 20.Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23(8):1406–1409. doi: 10.1038/leu.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]