Abstract
This study aims to investigate the association between thymectomy and the risk of generalization in patients with ocular myasthenia gravis (MG). Data on patients with ocular MG from seven neurological centers in China were retrospectively reviewed. Ocular MG naïve to immunotherapy was categorized according to whether thymectomy was performed (thymectomized group vs. nonsurgical group). Patients in the thymectomized group all underwent surgery within 2 years since ocular symptom onset. The main outcome measure was the generalization. The follow-up period was defined from the date of ocular symptom onset to the date of generalization confirmation, immunotherapy initiation, or last follow-up (defined as 60 months). Of 519 eligible patients (mean [SD] age, 48.7 [15.2] years, 46.6% women), 31 (23.7%) of 131 generalized in the thymectomized group and 122 (31.4%) of 388 did in the nonsurgical group during a median follow-up of 19 months (IQR 8.0–50.0). Thymectomy was independently associated with reduced generalization risk (adjusted HR 0.41, 95% CI 0.25–0.66, P < 0.001). Multivariable stratified analysis also verified this association across the subgroups. Kaplan–Meier curves showed that the 5-year cumulative rate was significantly lower in the thymectomized group than in the nonsurgical group. To conclude, thymectomy may be considered effective in modifying the progression from ocular to generalized MG irrespective of thymoma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01129-z.
Keywords: Ocular myasthenia gravis, Generalized myasthenia gravis, Thymectomy, Generalization, Proportional hazards model
Introduction
Myasthenia gravis (MG) is an autoimmune disorder mediated mainly by antibodies to the acetylcholine receptor (AChR), with ocular MG being a subset defined as weakness limited to the orbicularis, levator, and extraocular muscles. Usually, approximately 50% of cases with ocular MG will develop systemic muscular weakness and convert to generalized MG within 6 months, and the rate will increase to 80% within 2 years since ocular symptoms onset [1–3].
Thymectomy, as a therapeutic surgery, is indicated for nearly all patients with thymoma and in many other nonthymoma patients with MG. A multicenter randomized study conducted in 2016 provided conclusive evidence that nonthymoma generalized MG patients (< 50 years of age) undergoing thymectomy had significantly improved clinical outcomes and were less dependent on immunosuppressive treatment [4]; however, the study excluded patients with ocular MG. Currently, for nonthymoma ocular myasthenia, thymectomy has been used to a limited extent and in highly selected patients unresponsive to traditional immunotherapy [5–7] mainly because evidence for the efficacy of thymectomy in ocular MG is lacking, especially evidence from randomized, prospective, controlled clinical trials. In addition, few studies have considered generalization as the primary outcome. Thus, the efficacy of thymectomy on preventing generalization has not yet been clarified in patients with ocular MG.
Whether thymectomy reduces the generalization risk of ocular MG remains controversial. The results varied in different studies. Several retrospective studies reported that patients undergoing thymectomy were less likely to progress to generalized MG and more likely to receive remission [8–16], while other studies suggested thymectomy had no apparent advantage over medical treatment alone in reducing the risk of generalization or ocular symptom improvement [17–20]. The heterogeneity of the above studies may be attributed to the small sample size, inconsistent inclusion criteria, different outcome measurements, the presence of confounding factors, and variable statistical methods. It is noted that most available studies included patients on immunosuppression, likely confounding the outcome evaluation [20–25].
Here, we retrospectively reviewed the data of patients with ocular MG from seven neurological centers and investigated the association between thymectomy and the risk of generalization in patients with ocular MG naïve to immunotherapy.
Methods
Study Design and Participants
This multicenter retrospective cohort study was based on the MG database of Tangdu Hospital and electronic medical records from other research centers. All patients diagnosed with ocular MG were reviewed between January 1, 2015, and May 1, 2019. The MG database of Tangdu Hospital was created in 2019, and the collected data were from electronic medical records of outpatients and inpatients and from telephone and face-to-face interviews. Patients included in the database usually had regular visits at 1–3-month intervals. Ten neurologists and three nurses participated in data collection, and data on 103 items were collected, including demographic, clinical, immunological, therapeutic, and thymectomy-related characteristics.
Ocular MG was diagnosed by two neurologists based on the presence of relevant symptoms (ptosis and/or diplopia) together with one or more of the following criteria: (a) seropositive AChR antibody; (b) positive neostigmine testing; and (c) abnormal repetitive nerve stimulation (RNS) findings (more than 10% decreased amplitude of the compound muscle action potential in the RNS). A minimum of 3 months was the limit for purely ocular symptoms before classifying a patient as having ocular MG [26]. The included patients were categorized according to whether thymectomy was performed (thymectomized group vs. nonsurgical group). Patients in the thymectomized group all underwent thymic surgery within 2 years after ocular symptom onset.
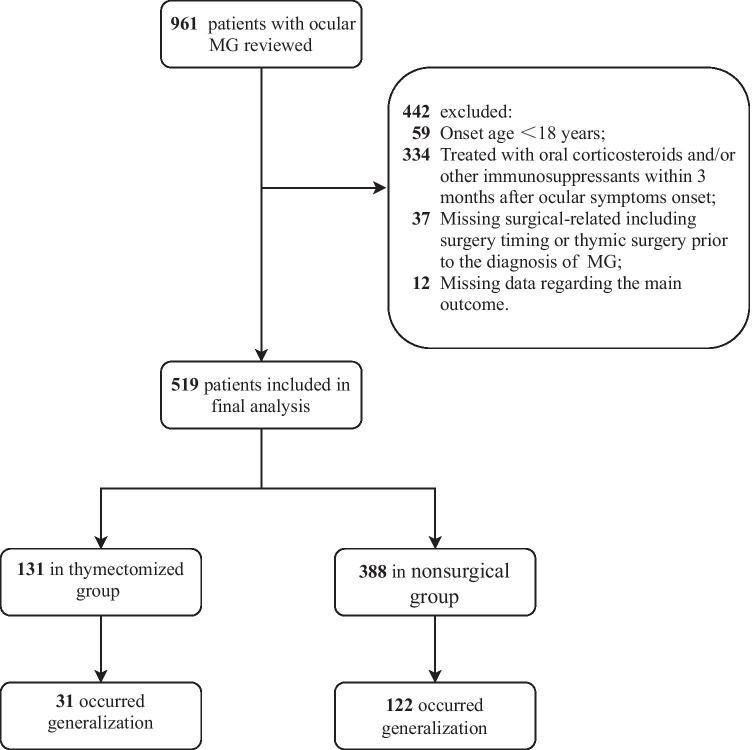
The exclusion criteria were as follows: (a) onset age < 18 years; (b) patients treated with oral corticosteroids and/or other immunosuppressants within 3 months after ocular symptom onset; (c) patients with missing surgery-related data, including surgery timing or thymic surgery prior to the diagnosis of MG; and (d) missing data regarding the main outcome (generalization occurrence). Details regarding participant recruitment are provided in Fig. 1.
Fig. 1.
Study flowchart
Data Collection
The main clinical characteristics of ocular MG were recorded. Onset age as a categorical variable was based on the generally accepted cutoff value (< 50 years, ≥ 50 years). Concurrent autoimmune disease (e.g., systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, Hashimoto’s thyroiditis, and optic neuromyelitis spectrum disease) and RNS findings were recorded. AChR antibody was measured by radioimmunoassay or enzyme-linked immunosorbent assay. Thoracic computed tomography (CT) or magnetic imaging (MRI) was routinely performed to detect thymic features. Postoperative thymic features were determined on histopathological findings. Associated clinical information at the time of outpatient visit or hospitalization was also assessed, including sex, onset symptoms, and neostigmine testing.
Main Outcome Measures
The occurrence of generalization was the main outcome measure, which was defined as the presence of generalized symptoms other than ocular symptoms, including weakness of the jaw, bulbar, neck, arm, leg, or respiratory muscles. Generalization was evaluated by a neuromuscular specialist at each visit or hospitalization. The follow-up period was defined from the date of ocular symptom onset to the date of generalization confirmation, immunotherapy initiation, or last follow-up (defined as 60 months). The data prior to immunotherapy were treated as censored data. Patients who developed generalized symptoms and signs were defined as OMG-G (OMG-generalization), while patients remaining with only ocular manifestation were defined as OMG-R (OMG-remaining) during the entire follow-up period.
Statistical Analysis
Onset age is presented as the mean (standard deviation, SD), and the follow-up period is presented as the median (interquartile range, IQR). Categorical variables are reported as counts and percentages. Differences in baseline characteristics between the 2 groups were evaluated with a 2-tailed chi-square or Fisher exact test for categorical variables, and Student’s t test or Wilcoxon rank sum test was used for quantitative variables.
The differences in generalization development between the 2 groups were compared through Kaplan–Meier curves and univariate (crude) and multivariable (adjustment for onset age, sex, AChR antibody, RNS findings, and thymic feature) hazard ratios (HRs) from Cox proportional hazards regression. Risk factors with a P value lower than 0.1 in univariate analyses were included in multivariable models. If a factor was removed from the model, resulting in a 10% or greater change in the HR estimate for thymectomy, it was significantly associated with thymectomy and included in the final model. The proportional hazards assumption was verified for individual factors included in the final model. Subgroup analyses were conducted to assess the association between thymectomy and generalization, and interaction tests were performed to assess the heterogeneity of outcomes across the subgroups. To maximize statistical power and minimize bias, multiple imputation with chained equations was used to impute missing values [27]. All P values presented are two-sided, and P values less than 0.05 were considered significant. All analyses were performed with the statistical software package R (version 4.0.0).
Protocol Approvals Standard, Registrations, and Patient Consents
The study was approved by the Ethics Committee of the Fourth Military Medical University (202009–15). The institutional review board at each participating center approved this study. Informed consent was waived by the ethics committee for this retrospective observational study due to the use of deidentified clinical information. The study was reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [28].
Results
Study Population
A total of 961 patients with ocular MG were admitted between January 2015 and May 2019, and 519 patients were eligible, as shown in Fig. 1. Of the 519 patients, 131 (25.2%) underwent thymectomy. Among these thymectomized patients, the mean (SD) onset age was 45.4 (11.6) years old, and 55 (42.0%) were female. The nonsurgical group consisted of 388 (74.8%) patients, the mean (SD) onset age was 49.8 (16.1) years old, and 187 (48.2%) were female.
The frequency of early-onset MG was obviously higher in the thymectomized group than in the nonsurgical group (63.4% vs. 47.4%). Compared with the nonsurgical group, patients in the thymectomized group had a lower rate of diplopia at disease onset (26.7% vs. 39.4%, P = 0.01). Diplopia alone or concurrent diplopia and ptosis were found only in 26.7% of the thymectomized patients, while 73.3% presented solely with ptosis. The percentages of patients with seropositive AChR antibody in the thymectomized and nonsurgical groups were 84.7% and 78.9%, respectively. The proportion of thymoma was much higher in the thymectomized group than in the nonsurgical group (72.5% vs. 10.8%, P < 0.001). Thirty-six participants without a suspected thymoma confirmed pathologically underwent thymectomy. Preoperative thymic imaging showed that 28 patients (77.7%) had thymic hyperplasia and 8 patients (22.3%) had a normal thymus. The preoperative radiological findings and postoperative histopathological findings of these 36 patients are shown in Table S1. The median follow-up period was 14 (IQR 7.0–50.0) months in the nonsurgical group vs. 30 (IQR 11.5–49.5) months in the thymectomized group (P = 0.07). Table 1 lists the baseline clinical characteristics of the included patients. There were missing data in 44 patients. The baseline characteristics of missing and complete data are summarized in Table S2.
Table 1.
Demographic and clinical characteristics of the participants
| Thymectomy | ||||
|---|---|---|---|---|
|
All (%) (n = 519) |
No (%) (n = 388) |
Yes (%) (n = 131) |
P value | |
| Onset age, years (mean ± SD) | 48.7 (15.2) | 49.8 (16.1) | 45.4 (11.6) | 0.004 |
| Onset age, years, n (%) | 0.002 | |||
| Early onset (< 50) | 267 (51.4) | 184 (47.4) | 83 (63.4) | |
| Late onset (≥ 50) | 252 (48.6) | 204 (52.6) | 48 (36.6) | |
| Sex, n (%) | 0.26 | |||
| Male | 277 (53.4) | 201 (51.8) | 76 (58.0) | |
| Female | 242 (46.6) | 187 (48.2) | 55 (42.0) | |
| Onset symptoms, n (%) | 0.01 | |||
| Ptosis | 331 (63.8) | 235 (60.6) | 96 (73.3) | |
| Diplopia/diplopia and ptosis | 188 (36.2) | 153 (39.4) | 35 (26.7) | |
| Concurrent autoimmune disease, n (%) | 0.53* | |||
| No | 506 (97.5) | 377 (97.2) | 129 (98.5) | |
| Yes | 13 (2.5) | 11 (2.8) | 2 (1.5) | |
| AChR antibody, n (%) | 0.18 | |||
| Seronegative | 102 (19.7) | 82 (21.1) | 20 (15.3) | |
| Seropositive | 417 (80.3) | 306 (78.9) | 111 (84.7) | |
| RNS findings, n (%) | 0.10 | |||
| Normal | 252 (48.6) | 197 (50.8) | 55 (42.0) | |
| Abnormal | 267 (51.4) | 191 (49.2) | 76 (58.0) | |
| Neostigmine test, n (%) | 0.43 | |||
| Negative | 68 (13.1) | 54 (13.9) | 14 (10.7) | |
| Positive | 451 (86.9) | 334 (86.1) | 117 (89.3) | |
| Thymic features, n (%) | < 0.001 | |||
| Nonthymoma | 382 (73.6) | 346 (89.2) | 36 (27.5) | |
| Thymoma | 137 (26.4) | 42 (10.8) | 95 (72.5) | |
| Follow-up period, months, median (IQR) | 19.0 [8.0, 50.0] | 14.0 [7.0, 50.0] | 30.0 [11.5, 49.5] | 0.07a |
| Number of patients at generalization risk, n (%) | ||||
| 1 y | 346 (66.7) | 248 (63.9) | 98 (74.8) | 0.03 |
| 2 y | 242 (46.7) | 166 (42.8) | 76 (58.0) | 0.003 |
| 3 y | 192 (40.0) | 135 (34.8) | 57 (43.5) | 0.09 |
| 4 y | 152 (29.3) | 113 (29.1) | 39 (29.8) | 0.98 |
| 5 y | 102 (19.7) | 84 (21.6) | 18 (13.7) | 0.07 |
AChR acetylcholine receptor, RNS repetitive nerve stimulation, IQR interquartile range
*Fisher’s exact test
aMann–Whitney U test
Main Outcome Measures
Thirty-one (23.7%) of the 131 patients developed generalization in the thymectomized group, and 122 (31.4%) of the 388 patients developed generalization in the nonsurgical group. After adjusting for potential confounding factors, including onset age, sex, AChR antibody, RNS findings, and thymic features, thymectomy was independently associated with a reduced risk of generalization (adjusted HR = 0.41, 95% CI 0.25–0.66, P < 0.001) (Table 2).
Table 2.
Uni- and multivariate analyses on generalization in patients with ocular MG
| No. of generalization/total no. (%) | Crude HR (95% CI) | P value | Adjust HR* (95% CI) | P value | |
|---|---|---|---|---|---|
| Onset age | 1.02 (1.01,1.03) | < 0.001 | 1.01 (1.00, 1.02) | 0.03 | |
| Sex, n (%) | |||||
| Male | 81/277 (29.2) | 1 [reference] | |||
| Female | 72/242 (29.8) | 0.98 (0.71, 1.34) | 0.89 | 1.11 (0.81, 1.53) | 0.52 |
| Onset of symptoms, n (%) | |||||
| Ptosis | 87/331 (26.3) | 1 [reference] | |||
| Diplopia/diplopia and ptosis | 66/188 (35.1) | 1.43 (1.04, 1.97) | 0.03 | ||
| Concurrent autoimmune disease, n (%) | |||||
| No | 148/506 (29.2) | 1 [reference] | |||
| Yes | 5/13 (38.5) | 1.00 (0.41, 2.45) | 0.99 | ||
| AChR antibody, n (%) | |||||
| Seronegative | 9/102 (8.8) | 1 [reference] | 1 [reference] | ||
| Seropositive | 144/417 (34.5) | 4.34 (2.21, 8.50) | < 0.001 | 2.48 (1.23, 4.99) | 0.01 |
| RNS findings, n (%) | |||||
| Normal | 36/252 (14.3) | 1 [reference] | 1 [reference] | ||
| Abnormal | 117/267 (43.8) | 3.50 (2.41, 5.09) | < 0.001 | 3.07 (2.10, 4.50) | < 0.001 |
| Neostigmine test, n (%) | |||||
| Negative | 8/68 (11.8) | 1 [reference] | 0.003 | ||
| Positive | 145/451 (32.2) | 2.95 (1.45, 6.01) | |||
| Thymic features, n (%) | |||||
| Nonthymoma | 102/382 (26.7) | 1 [reference] | 1 [reference] | ||
| Thymoma | 51/137 (37.2) | 1.39 (0.99, 1.94) | 0.06 | 1.86 (1.24, 2.80) | 0.003 |
| Thymectomy, n (%) | |||||
| No | 122/388 (31.4) | 1 [reference] | 1 [reference] | ||
| Yes | 31/131 (23.7) | 0.67 (0.45, 1.00) | 0.05 | 0.41 (0.25, 0.66) | < 0.001 |
HR hazard ratios, CI confidence interval, AChR acetylcholine receptor, RNS repetitive nerve stimulation
*Adjusted for onset age, sex, AChR antibody, RNS findings, and thymic feature
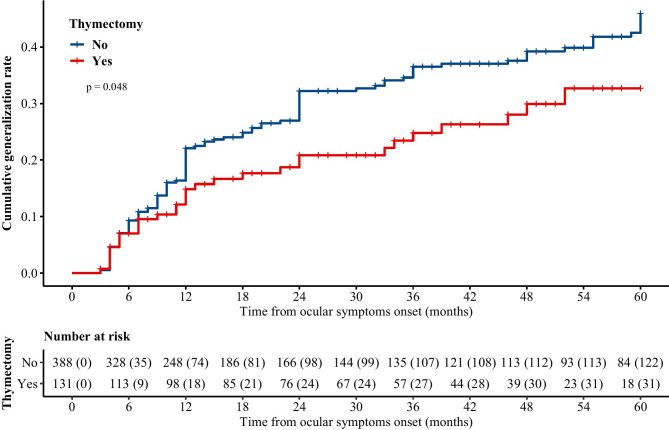
Kaplan–Meier curves of 5-year cumulative generalization rates in both groups were plotted, and a significant difference was identified by the log-rank test (Fig. 2, P = 0.048). The estimated 5-year cumulative generalization rates were 32.7% (95% CI 21.1–42.6%) in the thymectomized group and 45.9% (95% CI 38.9–52.2%) in the nonsurgical group since ocular symptom onset. We repeated all analyses with the complete data for comparison, and the results were consistent before and after multiple imputation (Tables S3 and S4).
Fig. 2.
Kaplan–Meier curve of 5-year cumulative rates in thymectomized and nonsurgical groups
Subgroup Analyses
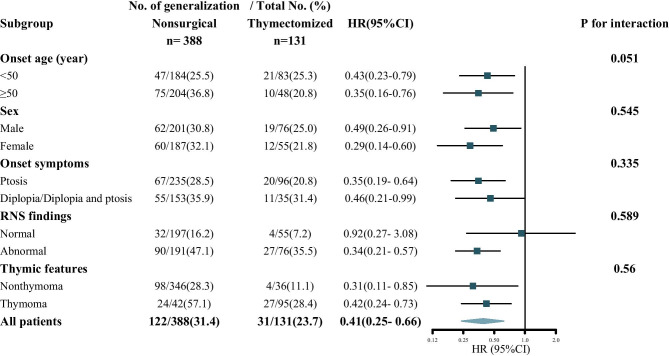
Each subgroup was adjusted for all the other variables (onset age, sex, AChR antibody, RNS findings, and thymic features) except the stratification variable itself. The result of each stratified variable was basically consistent, and no significant heterogeneity in HR was observed in any subgroup. The efficacy of thymectomy appeared to be more pronounced in patients with abnormal RNS findings, but the difference was nonsignificant (P for interaction 0.589) (Fig. 3). In the subgroup of negative AChR antibodies, 20 patients underwent thymectomy and none of them underwent generalization. The number of patients in the two groups stratified by AChR antibody is shown in Table S5.
Fig. 3.
Multivariable stratified analyses of the association between thymectomy and generalization risk. Comparison of thymectomized and nonsurgical cohorts. HR indicates hazard ratio. The square represents the effect value, the horizontal line represents the 95% CI, and the diamond corresponds to the 95% CIs for the entire group of patients
Discussion
In this retrospective cohort study, thymectomy significantly reduced the risk of generalization in ocular MG patients with or without thymoma. To our knowledge, this is the first study to investigate the efficacy of thymectomy in preventing generalization in ocular MG naïve to immunotherapy.
In assessing the efficacy of thymectomy for ocular MG, two important clinical outcomes need to be considered: the impact on the progression from ocular to generalized disease and on symptom remission, such as diplopia and ptosis. In addition to the subgroup of patients with thymoma requiring thymectomy for oncological reasons, thymectomy, as a surgical treatment, is recommended early in nonthymoma generalized MG patients with AChR antibody in updated International Consensus Guidance recently [7]. However, therapeutic thymectomy remains a rescue therapy for patients with nonthymoma ocular MG and has only been applied to patients unresponsive to conventional immunotherapy. The important reason is that the efficacy evidence of thymectomy in nonthymoma ocular MG varies in available studies. An analysis of 26 studies [29] revealed a pooled complete stable remission rate of 0.5074 for 640 patients with nonthymoma ocular MG. Liu et al. [8] reported none of 115 patients with ocular MG generalized post thymectomy, and 92.2% of them were pathologically confirmed without thymoma. A study by Mineo and Ambrogi et al. [9] demonstrated that nonthymoma ocular MG achieved more rapid remission with surgical treatment than those without surgery. Several studies demonstrated that patients without thymoma had a better response to thymectomy than those with thymoma [12–15, 20, 30], and a shorter duration of disease was associated with a better outcome from thymectomy [12, 14, 31].
Conversely, Hamedani et al. [21] failed to show a benefit of thymectomy either in symptom improvement or generalization risk reduction in nonthymoma ocular MG. Such paradoxical results might be attributed to the following factors. In their study, a logistic model was used to calculate the propensity score when considering thymectomy or not as the main outcome. A total of 9 variables were included, with 30 patients undergoing surgery, and the sample size was too small to provide enough test power to accurately calculate the propensity score. Furthermore, thymic features (normal or thymic hyperplasia) were not included as a variable in the logistic model. From a clinical point of view, we believe that patients with thymic hyperplasia are more likely to accept surgery than patients with a normal thymus. Thus, it is necessary to include thymic features as a possible confounding variable when calculating the propensity score.
In this study, consistent with most studies, we found that thymectomy significantly reduced the risk of generalization in ocular patients with or without thymoma. Our study has several strengths. First, despite its retrospective nature, a relatively large-scale sample size available to date allowed the performance of comprehensive and powerful statistical tests. For example, a multivariate Cox proportional hazards model was used to adjust for confounding and account for censored data, yielding rigorous findings and conclusions. Thymoma, as an important confounding variable, was adjusted to balance the baseline variables between the two groups. Furthermore, we first investigated the efficacy of thymectomy in nonimmunosuppressed OMG patients. Previous studies and meta-analyses conducted by us demonstrated that early immunotherapy might prevent the progression to generalized MG [32, 33], which is a strong confounding factor. To avoid the loss of sample size while excluding confounding factors, follow-up was stopped once immunotherapy was initiated, and the data prior to immunotherapy were treated as censored data. Finally, we first chose generalization as the main outcome measurement, which is the main controversy regarding thymectomy in ocular MG, especially in nonthymoma ocular MG. The aforementioned studies focused more on the efficacy of ocular symptom remission and seldom investigated the impact of surgery on the progression from ocular to generalized disease [29, 34, 35].
Notably, 36 participants confirming the absence of thymoma pathologically postsurgery underwent thymectomy, and 4 (11.1%) of them underwent generalization. Preoperative thymic imaging tests showed no suspected thymoma in these 36 participants, and the reason for surgery in these patients might not be the removal of a suspected thymic mass. We noticed that most of them had thymic hyperplasia, which mainly consisted of B cell follicles and ectopic germinal centers. It was reported that the synthesis of AChR antibody likely took place in these activated thymic regions; the presence of activated thymic tissue may coexist with a normal thymus [36–39]. In theory, removal of as much thymic parenchyma as possible and activated tissue should be an effective treatment. In view of preoperative radiological findings, we assumed that the reason for surgery in these patients might be better outcomes (progression prevention, symptom improvement, and steroid reduction).
Although thymectomy becomes safer and involves less trauma than previously with the development of thoracoscopy, surgery still carries higher risks than medical treatment. Thus, treatment decisions should be made cautiously. Moreover, the course of ocular MG varies greatly, and not all patients with ocular MG have the same risk of generalization. Performing such surgery in the higher-risk group may be more justified in exposing them to the side effects of surgery. The important questions of who may benefit most from thymectomy and when to initiate such surgery need to be answered in the design of future studies.
The limitations of our study should be mentioned. First, this study was limited by its retrospective design. To minimize selection bias, we conducted a multicenter study with a relatively large sample size to make it more representative. Moreover, multiple imputation with chained equations was adopted to minimize the impact of missing data, and further analysis was conducted before and after imputation to confirm the consistency of the main result. Second, the surgical approach was not unified in our cohort, and most patients received a transsternal thymectomy. However, this might contribute little influence on the result because the extent of thymectomy was unified and extended thymectomy was performed for all patients in our cohort. It was reported that long-term clinical efficacy was similar between thoracoscopic surgery and transsternal thymectomy [40, 41]. Third, although the inclusion of immunotherapy-naïve patients and use of multivariate analysis might minimize confounding bias, potential confounders may still exist due to unidentified variables.
Thymectomy may be considered effective in modifying the progression from ocular to generalized MG irrespective of thymoma. Well-designed prospective randomized controlled studies will be warranted to confirm this result.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant 81671233) and the Youth Talent Support Program of Tangdu Hospital. We thank the patients, their families, and all the investigators involved in this study. We are grateful to coinvestigators in the following neuroimmunology teams for their support and help with the study: West China Hospital, Sichuan University; Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University; Jiangxi Provincial People’s Hospital, Nanchang; Xianyang First People’s Hospital, Xianyang; Xi’an No.1 Hospital, Xi’an; and Xi’an Fourth Hospital, Xi’an. The authors are grateful to Kai-bin Shi at the Tianjin Medical University General Hospital for their helpful advice during the drafting of this paper. We are also grateful to Dr. Lin Zeng at the Center for Clinical Epidemiology, Third Hospital, Peking University, for constructive guidance on the statistical analysis of this work.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Data Availability
The data analyzed in the study are available from the corresponding authors on reasonable requests.
Declarations
Conflict of Interest
The authors declare no competing interests.
Protection of Human Subjects Research References
The study was approved by the Ethics Committee of the Fourth Military Medical University (202009–15). The institutional review board at each participating center approved this study. Informed consent was waived by the ethics committee for this retrospective observational study due to the use of deidentified clinical information.
Footnotes
The original online version of this article was revised to correct the affiliations.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huanhuan Li and Zhe Ruan contributed equally to this work.
Change history
11/8/2021
A Correction to this paper has been published: 10.1007/s13311-021-01144-0
Contributor Information
Zhuyi Li, Email: lizhuyiafu@163.com.
Ting Chang, Email: changting1981@163.com.
References
- 1.Kupersmith M, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Archives of neurology. 2003;60:243–248. doi: 10.1001/archneur.60.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Oosterhuis H. The natural course of myasthenia gravis: a long term follow up study. Journal of neurology, neurosurgery, and psychiatry. 1989;52:1121–1127. doi: 10.1136/jnnp.52.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bever C, Aquino A, Penn A, Lovelace R, Rowland L. Prognosis of ocular myasthenia. Annals of neurology. 1983;14:516–519. doi: 10.1002/ana.410140504. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe G, Kaminski H, Aban I, Minisman G, Kuo H, Marx A, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. The New England journal of medicine. 2016;375:511–522. doi: 10.1056/NEJMoa1602489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerty E, Elsais A, Argov Z, Evoli A, Gilhus N. EFNS/ENS Guidelines for the treatment of ocular myasthenia. European journal of neurology. 2014;21:687–693. doi: 10.1111/ene.12359. [DOI] [PubMed] [Google Scholar]
- 6.Gilhus N. Myasthenia Gravis. The New England journal of medicine. 2016;375:2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 7.Narayanaswami P, Sanders D, Wolfe G, Benatar M, Cea G, Evoli A, et al. International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology. 2021;96:114–122. doi: 10.1212/WNL.0000000000011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Feng H, Yeung S, Zheng Z, Liu W, Ma J, et al. Extended transsternal thymectomy for the treatment of ocular myasthenia gravis. The Annals of thoracic surgery. 2011;92:1993–1999. doi: 10.1016/j.athoracsur.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Mineo T, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. The Journal of thoracic and cardiovascular surgery. 2013;145:1319–1324. doi: 10.1016/j.jtcvs.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 10.Schumm F, Wiethölter H, Fateh-Moghadam A, Dichgans J. Thymectomy in myasthenia with pure ocular symptoms. Journal of neurology, neurosurgery, and psychiatry. 1985;48:332–337. doi: 10.1136/jnnp.48.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papatestas A, Genkins G, Kornfeld P, Eisenkraft J, Fagerstrom R, Pozner J, et al. Effects of thymectomy in myasthenia gravis. Annals of surgery. 1987;206:79–88. doi: 10.1097/00000658-198707000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masaoka A, Yamakawa Y, Niwa H, Fukai I, Kondo S, Kobayashi M, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. The Annals of thoracic surgery. 1996;62:853–859. doi: 10.1016/S0003-4975(96)00376-1. [DOI] [PubMed] [Google Scholar]
- 13.Roberts P, Venuta F, Rendina E, De Giacomo T, Coloni G, Follette D, et al. Thymectomy in the treatment of ocular myasthenia gravis. The Journal of thoracic and cardiovascular surgery. 2001;122:562–568. doi: 10.1067/mtc.2001.116191. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Hsu H, Huang B, Lee H, Kao K, Hsu W, et al. Factors influencing the outcome of transsternal thymectomy for myasthenia gravis. Acta neurologica Scandinavica. 2005;112:108–114. doi: 10.1111/j.1600-0404.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 15.Shrager J, Nathan D, Brinster C, Yousuf O, Spence A, Chen Z, et al. Outcomes after 151 extended transcervical thymectomies for myasthenia gravis. The Annals of thoracic surgery. 2006;82:1863–1869. doi: 10.1016/j.athoracsur.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 16.Takanami I, Abiko T, Koizumi S. Therapeutic outcomes in thymectomied patients with myasthenia gravis. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2009;15:373–377. [PubMed] [Google Scholar]
- 17.Evoli A, Batocchi A, Provenzano C, Ricci E, Tonali P. Thymectomy in the treatment of myasthenia gravis: report of 247 patients. Journal of neurology. 1988;235:272–276. doi: 10.1007/BF00314173. [DOI] [PubMed] [Google Scholar]
- 18.Hatton P, Diehl J, Daly B, Rheinlander H, Johnson H, Schrader J, et al. Transsternal radical thymectomy for myasthenia gravis: a 15-year review. The Annals of thoracic surgery. 1989;47:838–840. doi: 10.1016/0003-4975(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi N, Kuwabara S, Nemoto Y, Fukutake T, Satomura Y, Arimura K, et al. Treatment and outcome of myasthenia gravis: retrospective multi-center analysis of 470 Japanese patients, 1999–2000. Journal of the neurological sciences. 2004;224:43–47. doi: 10.1016/j.jns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Sommer N, Sigg B, Melms A, Weller M, Schepelmann K, Herzau V, et al. Ocular myasthenia gravis: response to long-term immunosuppressive treatment. Journal of neurology, neurosurgery, and psychiatry. 1997;62:156–162. doi: 10.1136/jnnp.62.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamedani A, Pistilli M, Singhal S, Shindler K, Avery R, Tamhankar M, et al. Outcomes After Transcervical Thymectomy for Ocular Myasthenia Gravis: A Retrospective Cohort Study With Inverse Probability Weighting. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2020;40:8–14. doi: 10.1097/WNO.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monsul N, Patwa H, Knorr A, Lesser R, Goldstein J. The effect of prednisone on the progression from ocular to generalized myasthenia gravis. Journal of the neurological sciences. 2004;217:131–133. doi: 10.1016/j.jns.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Zach H, Cetin H, Hilger E, Paul A, Wuschitz B, Jung R, et al. The effect of early prednisolone treatment on the generalization rate in ocular myasthenia gravis. European journal of neurology. 2013;20:708–713. doi: 10.1111/ene.12057. [DOI] [PubMed] [Google Scholar]
- 24.Yagi Y, Sanjo N, Yokota T, Mizusawa H. Tacrolimus monotherapy: a promising option for ocular myasthenia gravis. European neurology. 2013;69:344–345. doi: 10.1159/000347068. [DOI] [PubMed] [Google Scholar]
- 25.Mee J, Paine M, Byrne E, King J, Reardon K, O'Day J. Immunotherapy of ocular myasthenia gravis reduces conversion to generalized myasthenia gravis. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2003;23:251–255. doi: 10.1097/00041327-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Wong S, Huda S, Vincent A, Plant G. Ocular myasthenia gravis: controversies and updates. Current neurology and neuroscience reports. 2014;14:421. doi: 10.1007/s11910-013-0421-9. [DOI] [PubMed] [Google Scholar]
- 27.Enders C. Multiple imputation as a flexible tool for missing data handling in clinical research. Behaviour research and therapy. 2017;98:4–18. doi: 10.1016/j.brat.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Vandenbroucke J, von Elm E, Altman D, Gøtzsche P, Mulrow C, Pocock S, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. International journal of surgery (London, England). 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhu K, Li J, Huang X, Xu W, Liu W, Chen J, et al. Thymectomy is a beneficial therapy for patients with non-thymomatous ocular myasthenia gravis: a systematic review and meta-analysis. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2017;38:1753–1760. doi: 10.1007/s10072-017-3058-7. [DOI] [PubMed] [Google Scholar]
- 30.Buckingham J, Howard F, Bernatz P, Payne W, Harrison E, O'Brien P, et al. The value of thymectomy in myasthenia gravis: a computer-assisted matched study. Annals of surgery. 1976;184:453–458. doi: 10.1097/00000658-197610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seybold M. Myasthenia gravis. A clinical and basic science review. JAMA. 1983;250:2516–2521. [DOI] [PubMed]
- 32.Benatar M, Mcdermott M, Sanders D, Wolfe G, Barohn R, Nowak R, et al. Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): A randomized, controlled trial. Muscle & nerve. 2016;53:363–369. doi: 10.1002/mus.24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Ge F, Guo R, Zhe R, Chang T. Do early prednisolone and other immunosuppressant therapies prevent generalization in ocular myasthenia gravis in Western populations: a systematic review and meta-analysis. Therapeutic Advances in Neurological Disorders. 2019;12:175628641987652. doi: 10.1177/1756286419876521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng Y, Dong J, Zhou Q. Rapid improvement of muscle weakness post-thymectomy indicates good long-term neurological outcome in patients with ocular myasthenia gravis. European journal of neurology. 2019;26:1421–1423. doi: 10.1111/ene.14036. [DOI] [PubMed] [Google Scholar]
- 35.Cataneo A, Felisberto G, Cataneo D. Thymectomy in nonthymomatous myasthenia gravis - systematic review and meta-analysis. Orphanet journal of rare diseases. 2018;13:99. doi: 10.1186/s13023-018-0837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marx A, Pfister F, Schalke B, Saruhan-Direskeneli G, Melms A, Ströbel P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmunity reviews. 2013;12:875–884. doi: 10.1016/j.autrev.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Mori T, Nomori H, Ikeda K, Kobayashi H, Iwatani K, Kobayashi T. The distribution of parenchyma, follicles, and lymphocyte subsets in thymus of patients with myasthenia gravis, with special reference to remission after thymectomy. The Journal of thoracic and cardiovascular surgery. 2007;133:364–368. doi: 10.1016/j.jtcvs.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Roxanis I, Micklem K, McConville J, Newsom-Davis J, Willcox N. Thymic myoid cells and germinal center formation in myasthenia gravis; possible roles in pathogenesis. Journal of neuroimmunology. 2002;125:185–197. doi: 10.1016/S0165-5728(02)00038-3. [DOI] [PubMed] [Google Scholar]
- 39.Newsom-Davis J, Willcox N, Calder L. Thymus cells in myasthenia gravis selectively enhance production of anti-acetylcholine-receptor antibody by autologous blood lymphocytes. The New England journal of medicine. 1981;305:1313–1318. doi: 10.1056/NEJM198111263052203. [DOI] [PubMed] [Google Scholar]
- 40.Yang C, Hurd J, Shah S, Liou D, Wang H, Backhus L, et al. A national analysis of open versus minimally invasive thymectomy for stage I to III thymoma. The Journal of thoracic and cardiovascular surgery. 2020;160:555–567.e515. doi: 10.1016/j.jtcvs.2019.11.114. [DOI] [PubMed] [Google Scholar]
- 41.Kamel M, Villena-Vargas J, Rahouma M, Lee B, Harrison S, Stiles B, et al. National trends and perioperative outcomes of robotic resection of thymic tumours in the United States: a propensity matching comparison with open and video-assisted thoracoscopic approaches†. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2019;56:762–769. doi: 10.1093/ejcts/ezz111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in the study are available from the corresponding authors on reasonable requests.