Abstract
Depression is a common psychiatric illness affecting over 300 million people globally. Acupuncture has been reported to be a safe complementary treatment for depression. This study is aimed to investigate the efficacy and mechanism of combining acupuncture with antidepressants in treating depression compared to the sole use of antidepressants. Seventy depression patients were randomly assigned to the treatment group (n = 50) and control group (n = 20). The treatment group received acupuncture combined antidepressants treatment for 3 weeks, while the control group took antidepressants monotherapy for 3 weeks. Among the 70 patients, 40 participants (20 control; 20 treatment) were randomized for studying functional connectivity (FC) of the dorsolateral prefrontal cortex (DLPFC) measured by the functional near-infrared spectroscopy. The primary outcome was HAMD-17 and secondary outcomes were PHQ-9, and the relationships of resting-state FC (rsFC) with the depression severity. PHQ-9 and HAMD-17 scores in the treatment group were significantly lower than those in the control group at Week 3 (p = 0.01) with effect sizes of −0.4 and −0.61 respectively. The rsFC in F1, F3, AF3, AF7, FC3, FC5 (left DLPFC, 10–20 system), AF8, and F6 (right DLPFC) in the treatment group had significant temporal correlation (p < 0.05, FDR corrected) in DLPFC compared to the channels in the control group. No significant correlation was found between the changes of rsFC and depression severity. In conclusion, depressed patients receiving acupuncture combined with antidepressants have improvement of depressive symptoms and the stronger rsFC in the DLPFC compared to those using antidepressants alone.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01098-3.
Keywords: Acupuncture, Antidepressant, Depression, Functional near-infrared spectroscopy, Clinical trial
Introduction
Depression is a common psychiatric illness affecting more than 300 million people around the world, causing huge social and financial burdens to society and lowering the quality of life of those affected [1]. It is also identified as the second leading cause of disability and a major cause of death worldwide [2, 3]. Antidepressants (AD) are used widely in treating depression; however, it has also been reported that 50% to 60% of the patients cannot achieve adequate response, where some may also experience an undesirable adverse effect, potential risks of dependence, and delayed onset of therapeutic action [4]. These poor responses pose a great challenge to patients and clinicians in the treatment of depression [4].
Acupuncture is a traditional Chinese Medicine treatment that involves the insertion of fine needles in certain sites on the body. Over recent years, a large number of studies and reviews have investigated acupuncture’s clinical safety and efficacy in treating depression [5–10]. Acupuncture is now recognized as a safe and effective therapy in treating depression [3]. A recent study reported that the combined usage of AD with acupuncture can improve the tolerance of side effects caused by AD and also shows an early onset of therapeutic action compared to the sole use of AD for the first 6 weeks of treatment [6]. Although the clinical efficacy of acupuncture is gaining increased attention, its underlying mechanism remains unclear and its augmented effect when combined with AD compared to the monotherapy of AD is still debatable [10]. To better understand the neuromodulation effects and clinical efficacy of acupuncture, there is an urge to further investigate its fundaments, such as the cerebral communications and neuronal activities among depression subjects.
Functional connectivity measures the temporal dependency of neuronal activation patterns of anatomically separated brain regions and reveals the functional connections of specific brain regions and local networks [11], which may provide new insights in understanding the functional communication in the brain network from an overall view [11]. Recently, the resting-state functional magnetic resonance imaging (rs-fMRI) studies have suggested that disrupted brain communication is pivotal to the cognitive and emotional dysfunctions in Major Depression Disorder (MDD) [12, 13]. It has been identified that emotional and cognitive dysregulation in MDD attributes to the functional abnormalities in the cognitive control network (CCN), where the dorsolateral prefrontal cortex (DLPFC) together with the dorsal anterior cingulate cortex (dACC) and dorsal/posterior parietal cortex (DPC) constitutes the CCN and plays an important role in the pathophysiology of MDD and emotional regulation [14]. In addition, the MRI studies revealed MDD patients have the reduction in resting-state functional connectivity (rsFC) between right DLPFC and left cuneus, and the low rsFC within the CCN characterizes late-life depression and predicts persistence of depressive symptoms and signs, low remission rate, apathy, and dysexecutive behavior compared to healthy subjects [14, 15]. A recent fMRI study found that verum acupuncture plus fluoxetine significantly increases the rsFC at the dorsal portion of the temporal cortex and visual areas in MDD patients [16]. The increased rsFC change of the cortico-striatum was positively associated with clinical improvement indicating the potential mechanism of acupuncture in treating MDD.
Functional near-infrared spectroscopy (fNIRS) is a non-invasive imaging tool that has been widely used to study tissue hemodynamics, and connections between brain regions via measuring changes of oxyhemoglobin/deoxyhemoglobin concentrations in the brain [17]. It is a safe, portable, and quiet neuroimaging modality which does not require participants to stay still in a confined and noisy space like functional magnetic resonance imaging (fMRI). In recent years, fNIRS has been gaining popularity in cognition, psychological, and acupuncture studies [18–23]. To better understand the anti-depressive mechanism of acupuncture, we employed the fNIRS to investigate the resting-state functional connectivity (rsFC) changes in the DLPFC when the sole use of antidepressants or the combined treatment of acupuncture plus antidepressants was given to depression patients. We hypothesized that in contrast to the waiting list (control group), the group who received acupuncture would have significant changes in their DLPFC-rsFC, and the changes would be positively correlated to the improvement of clinical depressive symptoms.
Materials and Methods
Participants
The study protocol was reviewed and approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB). The study was registered at ClinicalTrials.gov (identifier: NCT02711527). A total of 70 eligible patients were recruited from the Queen Mary Hospital, The Mental Health Association of Hong Kong, Dance with Depression Association, Hong Kong Depression Disorder Support, and through community postings from January 2017 to 2019 April. All eligible participants had to meet the inclusion criteria and provide written informed consents before participation.
Inclusion Criteria
Patients who were (1) aged between 18 and 65 years old, (2) right-handed, (3) met the criteria of depression in DSM-V, (4) regular intake of antidepressants at a fixed dose for at least 3 months before baseline and during the study period, (5) 17-item Hamilton Depression Rating Scale (HAMD-17) score ≥ 8 and Patients Health Questionnaire (PHQ-9) score ≥ 10 at baseline, and (6) provided a voluntary informed consent.
Exclusion Criteria
(1) Any history of psychosis or mania; (2) conditions that may make it difficult to conclusively determine that depressive symptoms are the result of depression and not some other condition (e.g., substance abuse or relevant medical conditions including epilepsy, and history of an abnormal EEG); (3) with a bleeding disorder or receiving anti-coagulants; (4) had infection or malignancy overlapping with the acupuncture sites; (5) had sensory neuropathies; (6) had serious uncontrolled medical conditions; (7) pregnant, breast-feeding, or childbearing potential but not using adequate contraception; (8) had received Electroconvulsive therapy (ECT) in the past year; (9) received acupuncture 3 months before baseline; (10) change in antidepressant treatment within 4 weeks before baseline or during the study; (11) were current active suicidal or self-injurious potential that necessitated immediate treatment.
Intervention
All participants were randomly allocated to the control group (CG) or treatment group (TG) according to the block randomization generated by MS Excel. Only the accessor was blinded throughout the whole study. Treatment group subjects received acupuncture treatment right after group allocation and questionnaire assessment. Control group subjects had a 3-weeks waiting period before receiving acupuncture to avoid the nocebo effect. All subjects received 6 sessions of acupuncture (twice per week for 3 consecutive weeks). In each treatment session, all needles were retained for 30 min.
Acupuncture Administration
The acupuncture points were exposed and disinfected with alcohol swabs (70%, Sugama). An acupuncturist with >3 years of experience performed all acupuncture treatments. Needles (0.25 mm × 40 mm, Huanqiu) were inserted through a short plastic tube to a depth of 15-20 mm. After insertion, a “De-qi” sensation (a tingling, numbness, and heaviness feelings) was triggered by the to-and-fro rotation of the needles for 10 s, and the needles were then left in situ for 30 min.
“Liver Qi stagnation (LQS)” and “Heart and Spleen deficiency (HSD)” are considered to be the two most common and contrasted syndromes observed in TCM clinical diagnostics [24]. Therefore, 2 sets of acupuncture-point combinations (See Table 1, each set consists of 14 points) were applied according to the patients’ TCM syndrome. The acupoints were chosen based on the TCM treatment principle “Smoothen the Liver Qi” for LQS syndrome and “Tonify and Invigorate the Heart and Spleen Yang” for HSD syndrome respectively.
Table 1.
Acupoints used in the study
| Combination | TCM theory | Acupoint combination | Location |
|---|---|---|---|
| A | Soothing liver-qi stagnation (疏肝解鬱) | Taichong太衝 (LR 3)*2 | On the dorsum of the foot, in the depression distal to the junction of the 1st and 2nd metatarsal bones. |
| Ligou蠡沟 (LR 5)*2 | 5 cun above the apex of the medial malleolus, in the depression between the medial border of the tibia and the gastrocnemius. | ||
| Zhongdu 中都(LR6)*2 | 7 cun above the apex of the medial malleolus, in the depression between the medial border of the tibia and the gastrocnemius. | ||
| Xiguan膝關 (LR 7)*2 | 1 cun posterior to Yinlingquan(SP9), in the depression inferior and posterior to the medial condyle of the tibia. | ||
| Ququan曲泉 (LR 8)*2 | Superior to the medial end of the popliteal crease, in the depression anterior to the tendons of semitendinosus and semimembranosus, posterior to the medial condyle of the tibia, about 1 cun anterior to Yingu(KI10). | ||
| Zhangmen章門(LR13)*2 | Below the tip of the 11th rib. | ||
| Qimen期門 (LR14)*2 | Below the nipple, in the 6th intercostal space. | ||
| B | Tonify and invigorate the heart and the spleen Yang (補養心脾) | Taichong太衝 (LR 3)*2 | On the dorsum of the foot, in the depression distal to the junction of the 1st and 2nd metatarsal bones. |
| Sanyinjiao 三陰交 (SP6)*2 | 3 cun directly above the tip of the medial malleolus, on the posterior border of the tibia. | ||
| Xuanzhong懸鐘 (GB 39)*2 | 3 cun above the tip of the external malleolus, on the posterior border of the fibula. | ||
| Zusanli 足三里(ST 36)*2 | 3 cun below Dubi, one finger-breadth from the anterior crest of the tibia. 2 cun above the transverse crease of the wrist, between the tendons of m.palmaris longus and m.flexor carpi radialis. | ||
| Shenmen 神門 (HT 7)*2 | At the ulnar end of the transverse crease of the wrist, in the depression on the radial side of the tendon of m. flexor carpiulnaris. | ||
| Baihui 百會(DU 20) | At the vertex of the head, 5 cun posterior to the midpoint of the anterior hairline and 7 cun directly above the midpoint of the posterior hairline, or directly above the apex of the auricles on the midline of the head. | ||
| Shenting神庭(DU24)*2 | At the front of the head, 0.5 cun posterior to the midpoint of the anterior hairline. | ||
| Guanyuan 關元(RN4) | 3 cun below the center of the umbilicus |
Remarks: *2 regards to acupoints on the both sides of the body
Clinical Outcomes
HAMD-17 and PHQ-9 were used to assess the clinical outcomes. The primary outcomes were the changes in the HAMD-17 score before the first treatment and after the last treatment. PHQ-9 served as a secondary outcome was assessed on baseline, a week, 2 weeks, and 3 weeks after the baseline. Participants were required to fill out the PHQ-9 questionnaire, followed by an assessment of HAMD-17 by a trained research assistance before acupuncture administration.
fNIRS Data Acquisition
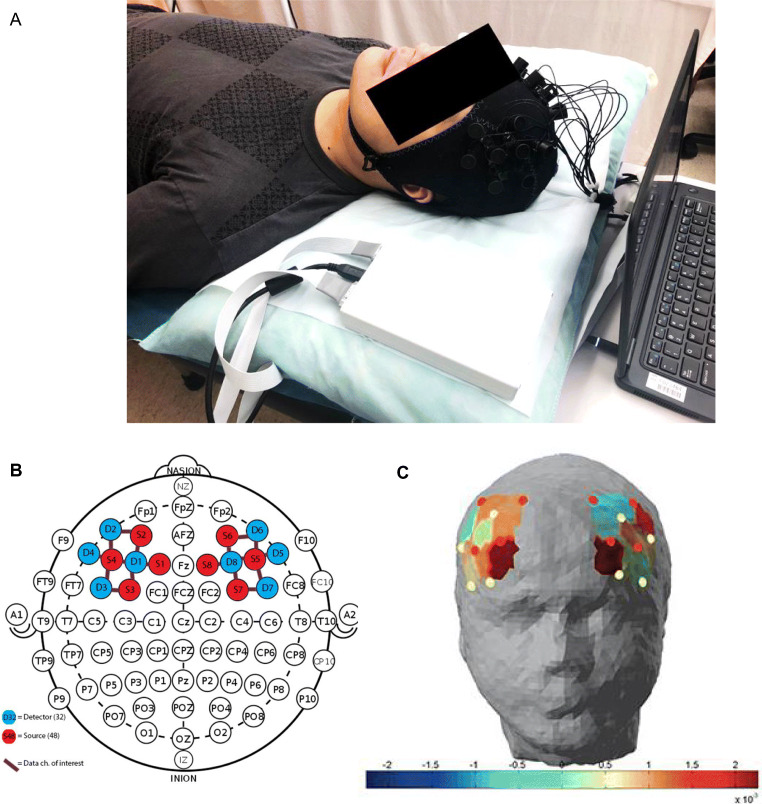
A multichannel portable Functional Near-Infrared Spectroscopy (fNIRS) system, NIRSport (NIRx Medical Technologies LLC, Glen Head, NY, USA) was used to detect the attenuated dual wavelength signals (760 nm and 850 nm) of the DLPFC. As shown in Fig. 1, head measurements were taken to ensure the placement accuracy of the fNIRS cap over the prefrontal region. The cap contains 8 source and 8 detector LEDs (18 channels) to record changes in blood oxygenation levels with a sampling rate of 7.81 Hz. Distance between sources and detectors were at approximately 3 cm, with the arrangement of the channels compatible with that of the international 10–5 system [22]. Data from the detectors were first calibrated and synchronized, then transferred directly to a laptop computer. Participants were asked to lie down in a supine position for 3–5 min for the resting-state recording before receiving acupuncture treatments. They were reminded to keep their eyes closed and relax but not to fall asleep or think of anything in particular. Then, the hemodynamic measurements were recorded for 1–3 min. After the recording was completed, the device was turned off and the acupuncturist then came in to begin the acupuncture treatment. The recordings for the treatment group were taken before their 1st and last acupuncture treatment session prior to the needling. While for participants in the wait-list group, their first measurements were taken on their first visit to the clinic, which is 3 weeks prior to their first compensation acupuncture treatment. Their end-point measurements were taken after completing their 3-weeks-wait.
Fig. 1.
fNIRS device recording of DLPFC hemodynamic changes. A An illustration of fNIRS device. B Locations of sources, detectors, and channels. C Graphic representation of resting-state hemodynamic concentration changes in the DLPFC. DLPFC: dorsolateral prefrontal cortex; fNIRS: Functional near-infrared spectroscopy
Statistical Analysis
Clinical Data Analysis
The outcomes were analyzed based on the intent-to-treat (ITT) principle. We used the linear mixed model to compare the primary outcomes between- and within-group. Missing data were not replaced because the mixed model can tolerant missing data under the assumption of missing at random. Assessments conducted at each time point were included as outcomes in the mixed model. The measurements at each time point were set as the dependent variable, treatment as the fixed effect, the baseline values as covariates, and the subjects as the random effects. The group-by-time interaction, which indicates the difference for a given outcome between interventions over time, was considered our primary measure of the intervention effect. The secondary outcomes with longitudinal data received the same approach as the primary outcome. All statistical analyses were performed using SAS version 9.4 (SAS Institute) with a 2-sided p value of less than 0.05 considered significant.
Resting-State fNIRS Data Analysis
fNIRS data were processed and analyzed with the Homer2 Matlab Source Code (homer2_src_v2_8_11022018homer2_src_v2_8_11022018) based in Matlab (Mathworks.com). Participants’ raw data were first converted to HomER script compatible format (*nirs) then pre-processed.
For pre-processing: (1) The raw NIRS light intensity was converted to optical density signal; (2) Filtration: identify, remove or recalibrate channels with bad signals and long periods of motion artifacts, then using HomER2 built-in function (parameters set as tMotion = 3 s; tMAsk = 1.0) a band-pass filter was applied to reduce physiological artifacts (0.01–0.2 Hz cut-off frequency); (3) The filtered optical density data were converted into oxy-Hb, deoxy-Hb, and total-Hb concentration by applying the modified Beer-Lambert law [25]. Total-Hb was defined as the sum of oxy-Hb and deoxy-Hb concentration.
After pre-processing, the hemodynamic changes between the pre- and post-treatment were converted into correlation coefficients which indicated the strength of functional connectivity among the 18 channels. Because the t-test was performed for each channel, Bonferroni correction [*] was used to correct for the increased probability of an incorrect t-test result (familywise errors: type I, false-positives) because of the large number of channels. Before the correction, we used a p < 0.05, uncorrected threshold.
Results
Clinical Characteristics of Participants
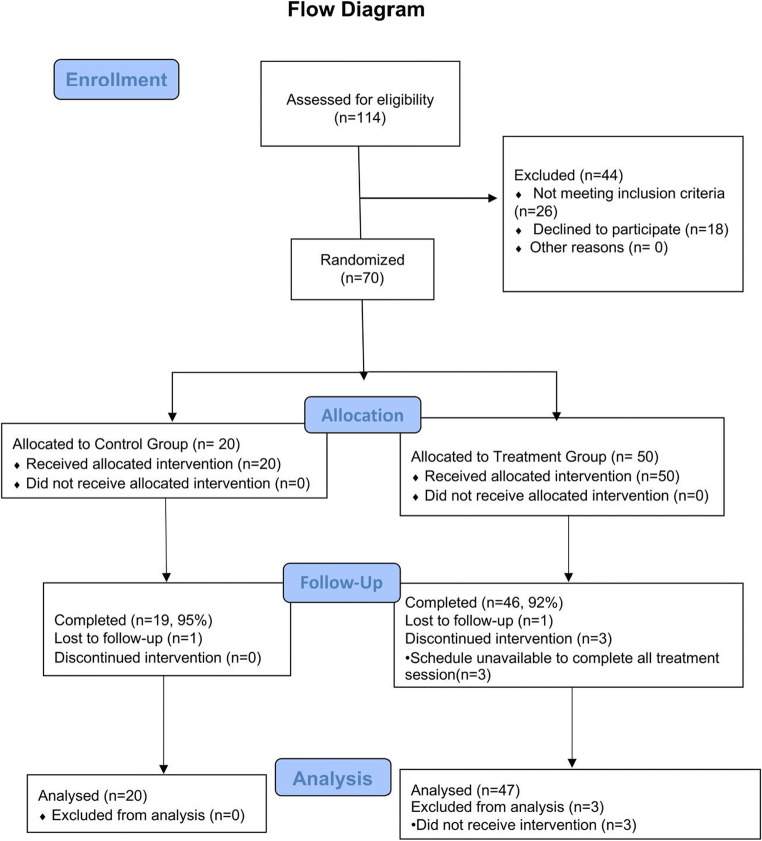
A total of 144 patients were screened, 70 eligible participants were randomized into two groups (20 in control group CG, 50 in treatment group TG); 67 (95.7%) completed all treatments, 3 participants from TG dropped out (due to schedule conflict) and 4 participants (1 from CG, 3 from TG) were lost in contact after receiving the last acupuncture treatment (Week 3) (Fig. 2). However, since all participants’ baseline data was present, all participants were included in baseline data analysis. The missing at random (MAR) approach was used in handling missing data. No significant differences were observed between the two groups in all baseline variables (Table 2).
Fig. 2.
Flow chart
Table 2.
Baseline characteristics
| Variable | All participants (n = 70) | Control (n = 20) | Treatment (n = 50) | p value | |
|---|---|---|---|---|---|
| Age, y | 49.2 ± 11.2 | 44.8 ± 10.3 | 50.9 ± 11.1 | 0.063 | |
| Female gender, % | 49 (70.0) | 14 (70.0) | 34 (68.0) | 0.987 | |
| Marital status, | 0.228 | ||||
| Single | 29 (41.4) | 7 (35.0) | 22 (44.0) | ||
| Married/cohabiting | 31 (44.3) | 9 (45.0) | 22 (44.0) | ||
| Divorced/widowed | 10 (14.3) | 4 (20.0) | 6 (12.0) | ||
| Education level, % | 0.430 | ||||
| Never received education | 1 (1.43) | 0 (0.00) | 1 (2.00) | ||
| Primary School level | 2 (2.86) | 0 (0.00) | 2 (4.00) | ||
| Secondary School level | 33 (47.1) | 9 (45.0) | 24 (48.0) | ||
| University level or higher | 34 (48.6) | 11 (55.0) | 23 (46.0) | ||
| Occupation % | 0.308 | ||||
| Professional | 21 (30.0) | 6 (30.0) | 15 (30.0) | ||
| Non-professional | 32 (45.7) | 12 (60.0) | 20 (40.0) | ||
| Technician | 3 (4.29) | 0 (0.00) | 3 (6.0) | ||
| Non-technician | 3 (4.29) | 0 (0.00) | 3 (6.0) | ||
| Housewife | 8 (11.4) | 1 (5.00) | 7 (14.0) | ||
| Student | 1 (1.43) | 0 (0.00) | 1 (2.00) | ||
| Other | 2 (2.86) | 1 (5.00) | 1 (2.00) | ||
| Refuse to answer | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Depression duration, y | 10.8 ± 10.4 | 10.3 ± 8.48 | 12.4 ± 12.1 | 0.576 | |
| Antidepressant, % | 0.365 | ||||
| Serotonin and norepinephrine reuptake inhibitors, SSRI | 48 (68.6) | 14 (70.0) | 34 (68.0) | ||
| Serotonin and norepinephrine reuptake inhibitors, SNRI | 9 (12.9) | 3 (15.0) | 6 (12.0) | ||
| Dopamine reuptake blocker | 3 (0.04) | 0 (0.00) | 3 (0.06) | ||
| Othera | 10 (0.14) | 2 (0.10) | 8 (0.16) | ||
| Alcohol drinker, % | |||||
| Never | 46 (65.7) | 9 (45.00) | 37 (74.0) | 0.069 | |
| Occasional | 24 (34.3) | 11(55.0) | 13 (26.0) | ||
| Frequent | 0 (0.00) | 0(0.00) | 0 (0.00) | ||
| Smoker, % | |||||
| Never | 67 (95.7) | 17 (85.0) | 50 (100.00) | 0.110 | |
| Occasional | 2 (2.86) | 2 (10.0) | 0 (0.00) | ||
| Frequent | 1 (1.43) | 1 (5.00) | 0 (0.00) | ||
| Drug abuser, % | 0 (0.00) | 0 (0.00) | 0 (0.00) | / | |
| TCM diagnosis | 0.102 | ||||
| Yang-Deficiency | 7 (10.00) | 1 (5.00) | 6 (12.0) | ||
| Liver-Qi Stagnation | 24 (34.3) | 7 (35.0) | 17 (34.0) | ||
| Both | 39 (55.7) | 12 (60.0) | 27 (54.0) | ||
a Other antidepressants such as noradrenergic antagonists, monoamine oxidase inhibitors, or tricyclic antidepressants
Changes in Clinical Assessment before and after Treatment
Changes in PHQ-9 and HAMD-17 scores over time were shown in Table 3 and 4 respectively. Between-group comparisons on PHQ-9 scores showed that TG had significantly lower scores than CG at Week 3 (p = 0.01) and Week 4 (p = 0.04) (Table 3), with effect sizes of −0.4 and −0.5, respectively. HAMD-17 scores also observed a significant decrease in TG compared to CG after receiving acupuncture for 3 weeks (p = 0.01), with an effect size of −0.61 (Table 4). No adverse events were reported for both groups during the study period.
Table 3.
PHQ-9 score outcome analysis of depression severity in patients
| Variables a | Control (n = 20) | p value b | Treatment (n = 40) | p value b | Between-group difference | Between-group effect Size | p value c |
|---|---|---|---|---|---|---|---|
| PHQ-9 | |||||||
| Baseline | 14.5 (12.1 to 16.8) | <0.0001 | 15.8 (14.3 to 17.3) | <0.0001 | −1.3 (−0.2 to 0.7) | 0.3 | 0.35 |
| Week 1 | 14.7 (12.3 to 17.1) | <0.0001 | 11.1 (9.6 to 12.6) | <0.0001 | 3.6 (−1.1 to −0.1) | −0.6 | 0.33 |
| Week 2 | 12.2 (9.8 to 14.5) | <0.0001 | 10.1 (8.6 to 11.6) | <0.0001 | 2.1 (−0.8 to 0.1) | −0.4 | 0.01* |
| Week 3 | 11.7 (9.3 to 14.0) | <0.0001 | 8.7 (14.3 to 17.3) | <0.0001 | 3 (−1.0 to 0.03) | −0.5 | 0.04* |
aValues are adjusted means and 95%CI
bCompared to baseline value within group
cCompared between the two groups at the same time point
Table 4.
HAMD-17 score outcome analysis of depression severity in patients
| Variables | Control a (n = 20) |
SD | Treatment a (n = 40) |
SD | Between-group difference | Between-group effect Size |
p value b |
|---|---|---|---|---|---|---|---|
| Baseline | 21.9 | 5.7 | 21.92 | 6.97 | 0.02 (−0.5 to 0.5) | 0.003 | 0.991 |
| Week 3 | 18.4 | 5.5 | 14.6 | 6.6 | 3.8 (−1.1 to −0.1) | −0.61 | 0.015* |
a Mean score within group
b Compared between the two groups at the same time point
Functional Near-Infrared Spectroscopy Observations
Among the 70 eligible participants, 40 subjects in the treatment group (n = 20) and control group (n = 20) received the fNIRS recording. A total of 36 subjects (4 dropouts, 2 dropouts from both groups) completed the recording. In the 36 recordings taken, 3 raw data (all from the control group) were unable to convert into HomER2 format (*.nirs) and 13 subjects (3 from the control group, 10 from the treatment group) had incomplete data set. The analysis of FC requires a complete set of pre-and post-treatment data, hence any loss or incompletion in either one of the data would cause inconsistency to the data-pair and hence cannot be used for further analysis. 20 participants were excluded from the analysis, resulting in data being available from 12 in the control group and 8 in the treatment group, a total of 20 participants.
Resting-State Functional Connectivity
The change in resting-state functional connectivity (rsFC) was measured on baseline and week 3 before receiving the last acupuncture treatment. The intragroup rsFC connectivity was compared between the two groups in terms of correlations.
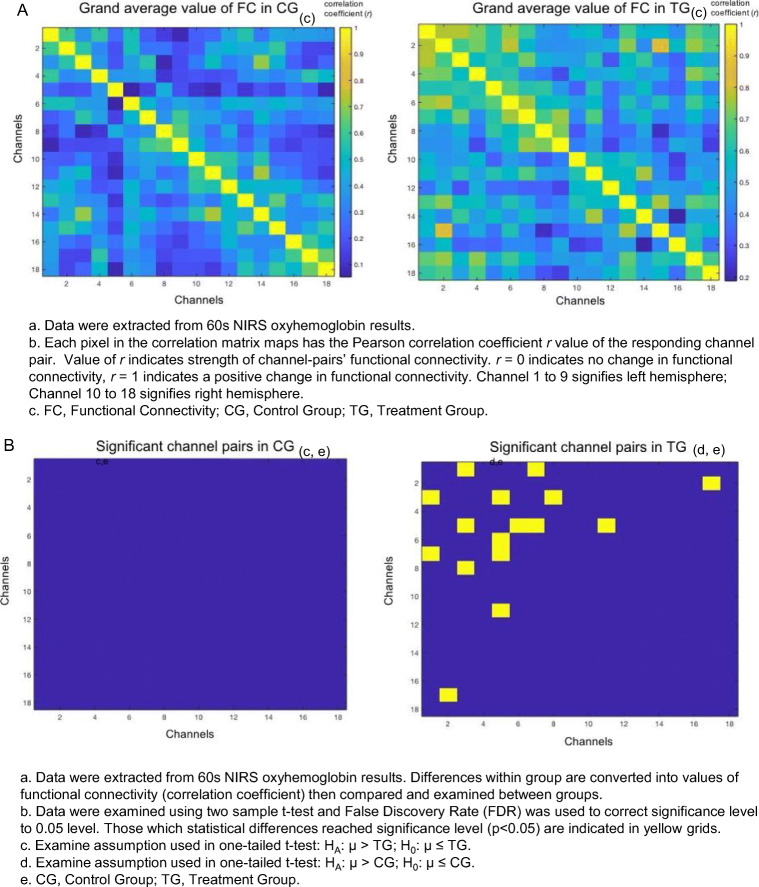
Oxyhemoglobin (OHb)
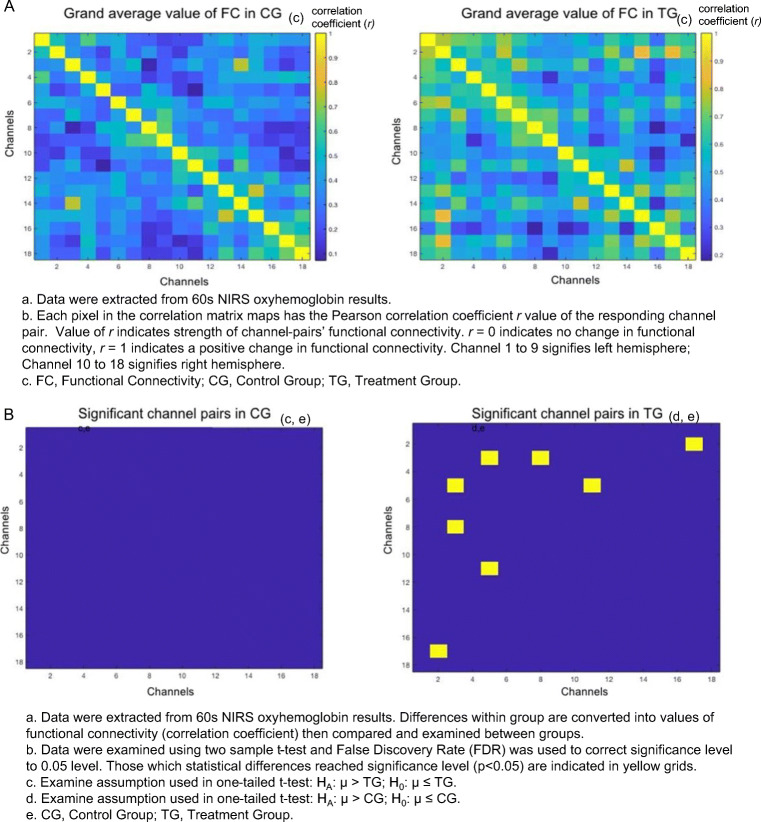
As shown in Fig. 3, the OHb correlation matrix maps within groups in the control group (CG) and treatment group (TG) respectively. It demonstrated that the channel pairs had a significant temporal correlation in functional connectivity (FC) in the DLPFC during resting-state. Resting-state FC in channel pairs (1,3), (1,7), (2,17), (3,5), (3,8), (5,6), (5,7), and (5,11) in TG was significantly stronger (r = 0.5 to 0.8, p < 0.05, FDR corrected) than those in CG (r = 0.05 to 0.4). No pairs in CG were found to be significantly stronger than TG. The channels 1, 2, 3, 5, 6, 7, and 8 were all located on the left hemisphere, where Channel 11 and 17 were located on right. A positive correlation was found in all significant pairs (Fig. 3).
Fig. 3.
The resting-state functional connectivity in oxyhemoglobin. A Grand average value of functional connectivity in oxyhemoglobin. B Significant channel pairs for oxyhemoglobin
Deoxyhemoglobin (HHb)
Similarly, the channel pairs had significant HHb rsFC temporal correlation (Fig. 4). Only one channel pair (12,18) in TG was significantly stronger (r = 0,4785, p < 0.05, FDR corrected) than CG (r = −0.1619). No pairs in CG were found to be significantly stronger than TG.
Fig. 4.
The resting-state functional connectivity in deoxyhemoglobin. A Grand average value of functional connectivity in deoxyhemoglobin. B Significant channel pair for deoxyhemoglobin
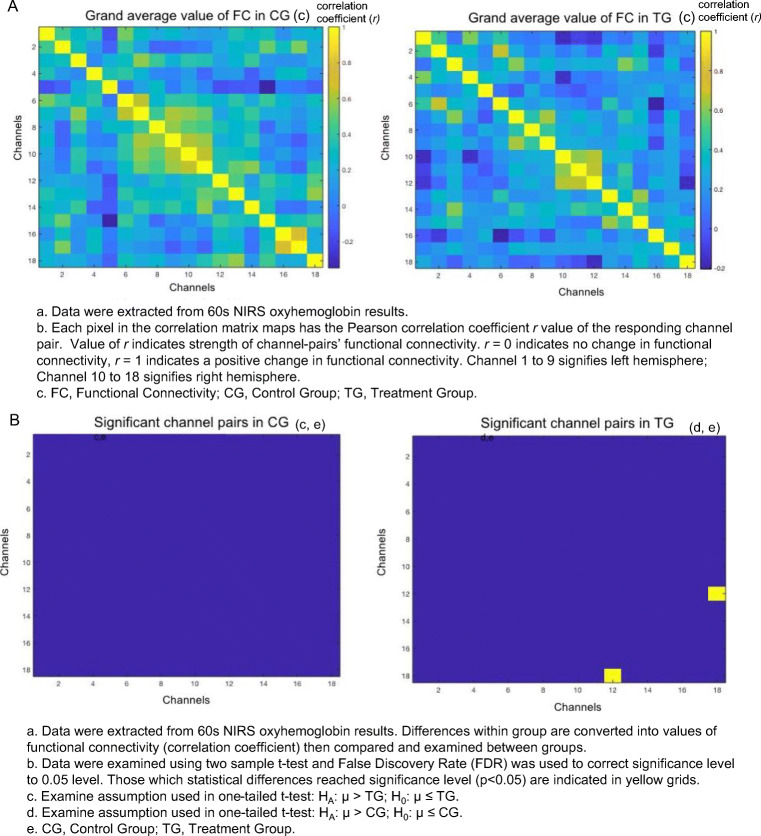
Total Hemoglobin (tHb)
As shown in Fig. 5, the channel pairs (2,17), (3,5), (3,8), and (5,11) in TG were significantly stronger (r = 0.6 to 0.8, p < 0.01, FDR corrected) than CG (r = 0.07 to 0.2). No pairs in CG were found to be significantly stronger than TG. All significant pairs had a positive correlation (Fig. 5). Patients that received acupuncture combined with antidepressants had greater rsFC in the DLPFC compared to those who only used antidepressants.
Fig. 5.
The resting-state functional connectivity for total hemoglobin. A Grand average value of functional connectivity in total hemoglobin. B Significant channel pairs for total hemoglobin
Hemodynamic Changes and Severity of Depression
The demands of OHb and HHb during resting-state in the DLPFC had no significant difference between both groups in pre- and post-treatment (data not shown). No correlation between the rsFC and HAMD-17/PHQ score changes was observed.
Discussion
This major finding of this study is that acupuncture combined with antidepressants compared to the sole use of antidepressants had a more significant reduction in depressive symptoms and a significantly stronger resting-state functional connectivity (rsFC) in the DLPFC after 3 weeks of treatment. HAMD-17 is a golden standard commonly used by clinicians to assess the remission and response in MDD patients [26], where PHQ-9 as a self-assessment tool is commonly used in the initial screening and follow-ups to monitor treatment responses [27]. In our study, both HAMD-17 and PHQ-9 scores reduced significantly, with a medium effect size of −0.4 to −0.6, indicating a reduction in depression severity. This finding is consistent with the previous study [5] and provides more evidence on acupuncture’s promising effects in treating depression.
Non-invasive brain imaging studies are made possible by the implementation of near-infrared light. The concentration changes in oxy- and deoxyhemoglobin were measured by the differences between the traversed and returned light received because of the distinct absorption spectra of oxyhemoglobin and deoxyhemoglobin [22]. Activated neurons increase the demand for oxygen and manifest a rise in hemodynamic responses. However, due to the imbalanced nature between the consumption rate of oxygen and the corresponding increase in localized cerebral blood flow, classic hemodynamic response function incorporates both an increase in oxyhemoglobin concentration and a decrease in deoxyhemoglobin concentration [28]. Resting-state functional connectivity (rsFC) examines the statistical relationship between the measures of activity in discrete brain regions during rest [29].
Two regions show functional connectivity if an increased activity in one region is associated above chance with activity in another [30]. In MDD patients, the functional connectivity in the central executive network decreases compared to healthy controls. Whereas the dorsolateral prefrontal cortex (DLPFC), which plays an important role in emotion processing [31], was reported to have shown the most significant decline with other nodes in the network [32, 33] and is closely related to patient’s depression symptoms and maladaptive mood regulations [33]. It is also a neuroanatomical site commonly targeted by Transcranial Magnetic Stimulation (TMS) therapy in treating depressive disorders, and many fMRI studies have confirmed the effectiveness of stimulating this location [34].In our study, although there was no precise stimulation, the rsFC observed in the DLPFC still showed a neuromodulation change when acupuncture was combined with antidepressants. An explanation for this phenomenon may be that functional correlation does not interpret any causal relationship or direct connection between the regions due to the relayed processes by transmitting via cascades of several intermediates or through cortical-subcortical loops. Strong functional connectivity may still be observed even if the structural connections, such as fibers or blood vessels, running between the two areas are weak or absent [35]. For instance, depression is also identified to be associated with increased limbic activity in response to emotional information processing and decreased relationships between the amygdala and DLPFC activity, potentially signifying decreased functional relationships among these structures [36].
Although it remains unclear whether acupuncture reduces depressive symptoms by modulating the connectivity in the dorsolateral prefrontal cortex or the modulated connectivity is simply a reflection of the acupuncture’s clinical effect, it still does not undermine acupuncture’s potential in reducing depressive symptoms. Moreover, observation in this region may provide an objective measure for future acupuncture-depression-related studies to overcome the problems of single-blinding [33, 37].
There are a few limitations in the study that should be addressed. First, the study was open-label in nature without a sham control. A wait-list control group was applied; however, we could not exclude the possibilities that bias from subjects might affect the results in the treatment group and therefore reveals a greater reduction in depression severity compared to studies that applied a sham control [8]. This design may undermine the evidential power of the efficacy of acupuncture combined with antidepressants [38]. Second, the timeframe of the fNIRS recording was not homogeneous. Despite the consistency in setup and procedures, hemoglobin concentrations may have changed at different times of the day. The blood flow of the skin itself (Sakakibara et al., 2016), and peripheral perfusion enhanced by emotional or stress responses before receiving acupuncture treatments (Ehlis, Schneider, Dresler, & Fallgatter, 2014) may also affect the accuracy in hemodynamic measurements. Finally, the selection of acupoints in this study was mostly based on empirical evidence. However, we managed to limit the acupuncture treatment regimens to two settings, to reduce the variations in treatment outcomes caused by differences in acupuncture protocols among trials.
Future studies should include sham-control and increase the sample size to replicate and further explore the efficacy and underlying mechanisms of acupuncture therapy’s antidepressant effects. To our best knowledge, this study was the first to apply the fNIRS in measuring the resting-state functional connectivity and investigate the mechanism of adjunctive acupuncture therapy with an antidepressant in depression patients.
Conclusion
In conclusion, we found that 3 weeks of acupuncture combined with antidepressants treatment has a stronger rsFC in the DLPFC in depression patients compared with those using antidepressants alone. Significant clinical improvements were also observed in the combined treatment group. Our results may at least partially explain some of the underlying mechanism; however, the neuromodulation effect of acupuncture in treating MDD when combined with antidepressants warrants further investigation in the future.
Supplementary Information
(PDF 497 kb)
(PDF 498 kb)
(PDF 497 kb)
(PDF 498 kb)
(PDF 499 kb)
(PDF 500 kb)
(PDF 501 kb)
(PDF 503 kb)
(PDF 504 kb)
(PDF 505 kb)
Acknowledgments
The authors acknowledge the generous advice and equipment support from Dr. Susan M. Bridges (GRF ref. 17100514). It is thankful to Dr. Lo lo Yam and Mr. Chi Wing Tam for providing acupuncture treatment, and to Miss Mei Yan Chan for performing clinical assessment and data collection. This study is funded by the HKU SEED Funding (ref: 201505159005).
Abbreviations
- MDD
Major Depressive Disorder
- ANOVA
Analysis of variance
- DLPFC
Dorsolateral prefrontal cortex
- rfFC
Resting-state functional connectivity
- fNIRS
Functional near-infrared spectroscopy
- IRB
Institutional Review Board
- TG
Treatment group
- CG
Control group
- PHQ-9
Patient Health Questionnaire 9
- HAMD-17
Hamilton Depression Rating Scale 17 items
- MADRS
Montgomery-Asberg Depression Rating Scale
- PSQI
Pittsburgh Sleep Quality Index
- SF-36
36-Item Short Form Survey
- FDR
False Discovery Rate
- AD
Antidepressants
- CCN
Cognitive Control Network
- dACC
dorsal Anterior Cingulate Cortex
- DPC
dorsal/posterior Parietal Cortex
- ECT
Electroconvulsive therapy
- LQS
Liver Qi stagnation
- HSD
Heart and Spleen deficiency
- ITT
Intention To Treat
- MAR
Missing at random
- OHb
Oxyhemoglobin
- HHb
Deoxyhemoglobin
- tHb
Total hemoglobin
Authors’ Contribution
ZJZ, JMW, FZ, NZ, QZ, and HYC were involved in the conception, design of the study, and critical comments on the manuscript. YKW, GDZ, and ZSQ conducted data analysis. ZZJ, ZSQ, and YKW conducted re-examination and re-analysis of the data set. JMW and HYC carried out acupuncture treatment. YKW drafted the manuscript. ZJZ, HYC, XJY, FZ, and JMW revised manuscript.
Declarations
Competing Interests
The authors declare that they have no competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yat Kwan Wong and Jun Mei Wu contributed equally to this work.
Contributor Information
Haiyong Chen, Email: haiyong@hku.hk.
Zhang-Jin Zhang, Email: zhangzj@hku.hk.
References
- 1.World Health Organization. Depression and other common mental disorders: global health estimates. Geneva: World Health Organization, 2017;1-24.
- 2.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. Journal of Psychiatric Research. 2020;126:134–40. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Yeung AS, Schnyer R, Wang Y, Mischoulon D. Acupuncture for depression: a review of clinical applications. Can J Psychiatry. 2012;57(7):397–405. doi: 10.1177/070674371205700702. [DOI] [PubMed] [Google Scholar]
- 4.Fava M. Diagnosis and definition of treatment-resistant depression. Biological Psychiatry. 2003;53(8):649–59. doi: 10.1016/S0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 5.Armour M, Smith CA, Wang L-Q, Naidoo D, Yang G-Y, MacPherson H, et al. Acupuncture for Depression: A Systematic Review and Meta-Analysis. J Clin Med. 2019;8(8):1140. doi: 10.3390/jcm8081140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan YY, Lo WY, Yang SN, Chen YH, Lin JG. The benefit of combined acupuncture and antidepressant medication for depression: A systematic review and meta-analysis. Journal of Affective Disorders. 2015;176(91):106–17. doi: 10.1016/j.jad.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Ernst E, Lee MS, Choi TY. Acupuncture for depression?: A systematic review of systematic reviews. Evaluation and the Health Professions. 2011;34(4):403–12. doi: 10.1177/0163278710386109. [DOI] [PubMed] [Google Scholar]
- 8.Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi: 10.1002/14651858.CD004046.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang WJ, Yang XB, Zhong BL. Combination of acupuncture and fluoxetine for depression: A randomized, double-blind, sham-controlled trial. Journal of Alternative and Complementary Medicine. 2009;15(8):837–44. doi: 10.1089/acm.2008.0607. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZJ, Chen HY, Yip KC, Ng R, Wong VT. The effectiveness and safety of acupuncture therapy in depressive disorders: Systematic review and meta-analysis. Journal of Affective Disorders. 2010;124(1-2):9–21. doi: 10.1016/j.jad.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20(8):519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev. 2012;22(3):229–51. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Yang S, Sun WL, Shi YZ, Duan HF. Altered functional interaction hub between affective network and cognitive control network in patients with major depressive disorder. Behavioural Brain Research. 2016;298:301–9. doi: 10.1016/j.bbr.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Wang X, Liu J, Chen J, Liu X, Nie G, et al. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J Psychiatr Res. 2017;84:18–26. doi: 10.1016/j.jpsychires.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X, Bray S, Reiss AL. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage. 2010;49(4):3039–46. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu CM, Zhang YJ, Biswal BB, Zang YF, Peng DL, Zhu CZ. Use of fNIRS to assess resting state functional connectivity. Journal of Neuroscience Methods. 2010;186(2):242–9. doi: 10.1016/j.jneumeth.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H, Xu J, Li J, Peng H, Cai T, Li X, et al. Decreased functional connectivity and disrupted neural network in the prefrontal cortex of affective disorders: A resting-state fNIRS study. J Affect Disord. 2017;221:132–44. doi: 10.1016/j.jad.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Han Y, Yuan B, Zhang Y, Wang X, Lang W, Yan X. Role of fNIRS technology in observing the effect of needling Hegu (LI 4) on the functions of prefrontal cortex in healthy volunteers. Journal of Acupuncture and Tuina Science. 2017;15(2):94–8. doi: 10.1007/s11726-017-0982-2. [DOI] [Google Scholar]
- 21.Xu L, Wang B, Xu G, Wang W, Liu Z, Li Z. Functional connectivity analysis using fNIRS in healthy subjects during prolonged simulated driving. Neuroscience Letters. 2017;640:21–8. doi: 10.1016/j.neulet.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Perry S, Bridges SM, Zhu F, Leung WK, Burrow MF, Poolton J, et al. Getting to the root of fine motor skill performance in Dentistry: Brain activity during dental tasks in a virtual reality haptic simulation. Journal of Medical Internet Research. 2017;19(12):e371. doi: 10.2196/jmir.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Gao L, Chen C, Li J, He S. Resting-state functional connectivity in prefrontal cortex investigated by functional near-infrared spectroscopy: A longitudinal and cross-sectional study. Neuroscience Letters. 2018;683:94–9. doi: 10.1016/j.neulet.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Liu LY, Zhang HJ, Luo LY, Pu JB, Liang WQ, Zhu CQ, et al. Blood and urinary metabolomic evidence validating traditional Chinese medicine diagnostic classification of major depressive disorder. Chin Med. 2018;13:53. doi: 10.1186/s13020-018-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33(12):1433–42. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- 26.Leucht S, Fennema H, Engel R, Kaspers-Janssen M, Lepping P, Szegedi A. What does the HAMD mean? Journal of Affective Disorders. 2013;148(2):243–8. doi: 10.1016/j.jad.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Plemmons G. Depression and Suicide Screening. In: Morelli VBT, editor. Adolescent Health Screening an Update in the Age of Big Data: Elsevier; 2019. p. 135-49.
- 28.Cooper RJ, Boas DA. Functional Near-Infrared Spectroscopy. In: Toga AW, editor. Brain Mapping. Waltham: Academic Press; 2015. p. 143-8.
- 29.Tachtsidis I, Scholkmann F. False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics. 2016;3(3):039801. doi: 10.1117/1.NPh.3.3.039801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, et al. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacology. 2016;41(7):1822–30. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci. 2015;9:58. doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord. 2017;207:86–94. doi: 10.1016/j.jad.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai L, Zhou H, Xu X, Zuo Z. Brain structural and functional changes in patients with major depressive disorder: a literature review. PeerJ. 2019;7:e8170. doi: 10.7717/peerj.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luborzewski A, Schubert F, Seifert F, Danker-Hopfe H, Brakemeier EL, Schlattmann P, et al. Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression. Journal of Psychiatric Research. 2007;41(7):606–15. doi: 10.1016/j.jpsychires.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Eickhoff SB, Müller VI. Functional Connectivity. In: Toga AW, editor. Brain Mapping. Waltham: Academic Press; 2015. p. 187-201.
- 36.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased Amygdala and Decreased Dorsolateral Prefrontal BOLD Responses in Unipolar Depression: Related and Independent Features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 37.Röschke J, Wolf C, Müller MJ, Wagner P, Mann K, Grözinger M, et al. The benefit from whole body acupuncture in major depression. J Affect Disord. 2000;57(1):73–81. doi: 10.1016/S0165-0327(99)00061-0. [DOI] [PubMed] [Google Scholar]
- 38.Witt CM, Schützler L. The gap between results from sham-controlled trials and trials using other controls in acupuncture research-the influence of context. Complement Ther Med. 2013;21(2):112–4. doi: 10.1016/j.ctim.2012.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 497 kb)
(PDF 498 kb)
(PDF 497 kb)
(PDF 498 kb)
(PDF 499 kb)
(PDF 500 kb)
(PDF 501 kb)
(PDF 503 kb)
(PDF 504 kb)
(PDF 505 kb)