Abstract
α-Synuclein is a key protein in the pathogenesis of Parkinson’s disease as it accumulates in fibrillar form in affected brain regions. Misfolded α-synuclein seeds recruit monomeric α-synuclein to form aggregates, which can spread to anatomically connected brain regions, a phenomenon that correlates with clinical disease progression. Thus, downregulating α-synuclein levels could reduce seeding and inhibit aggregate formation and propagation. We previously reported that microRNA-7 (miR-7) protects neuronal cells by downregulating α-synuclein expression through its effect on the 3′-untranslated region of SNCA mRNA; however, whether miR-7 blocks α-synuclein seeding and propagation in vivo remains unknown. Here, we induced miR-7 overexpression in the mouse striatum unilaterally by infusing adeno-associated virus 1 (AAV-miR-7) followed by inoculation with recombinant α-synuclein preformed fibrils (PFF) a month later. Compared with control mice injected with non-targeting AAV-miR-NT followed by PFF, AAV-miR-7 pre-injected mice exhibited lower levels of monomeric and high-molecular-weight α-synuclein species in the striatum, and reduced amount of phosphorylated α-synuclein in the striatum and in nigral dopamine neurons. Accordingly, AAV-miR-7-injected mice had less pronounced degeneration of the nigrostriatal pathway and better behavioral performance. The neuroinflammatory reaction to α-synuclein PFF inoculation was also significantly attenuated. These data suggest that miR-7 inhibits the formation and propagation of pathological α-synuclein and protects against neurodegeneration induced by PFF. Collectively, these findings support the potential of miR-7 as a disease modifying biologic agent for Parkinson’s disease and related α-synucleinopathies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01130-6.
Keywords: Parkinson’s disease, α-Synucleinopathies, α-Synuclein, Pre-formed fibrils, microRNA-7
Introduction
Parkinson’s disease (PD) is characterized by progressive degeneration of various neuronal populations, including prominently dopaminergic neurons in the substantia nigra pars compacta (SNpc), and accumulation of α-synuclein (α-syn) in Lewy bodies and Lewy neurites [1, 2]. The initial oligomerization followed by fibrillization of α-syn is believed to be critical steps leading to neuronal dysfunction and death [3, 4]. Growing body of evidence suggests that seeds of misfolded α-syn can recruit endogenous monomeric α-syn to form aggregates that can propagate across interconnected neural networks [5, 6], a phenomenon that correlates with the progressive nature of the disease and the emergence of additional clinical manifestations over time [7]. These pathologic aggregates are typically hyperphosphorylated at serine 129 (pS129), and antibodies raised against pS129-α-syn are commonly used to detect these aggregates in studies of postmortem human brains and animal models of synucleinopathies [8, 9].
A number of factors have been identified to play a role in the formation and propagation of α-syn aggregates, including increased expression of α-syn, impaired dephosphorylation, decreased clearance, and factors that promote oxidative stress [10–14]. The identification of families with duplication or triplication of the SNCA gene encoding α-syn linked to dominantly inherited PD and dementia with a gene dosage effect is a powerful evidence for the role of α-syn levels in its pathologic aggregation and neurotoxicity as a gain of function [15]. Additionally, normal dopaminergic neurons differentiated from human embryonic stem cells (hESCs) form pS129-α-syn-positive aggregates when challenged with recombinant α-syn preformed fibrils (PFF), while heterozygous and homozygous deletion of the SNCA gene in these neurons using CRISPR/Cas9 technology renders these neurons markedly resistant to the formation of such aggregates, with SNCA−/− neurons not exhibiting any [16]. Thus, reducing endogenous α-syn levels is a viable strategy to prevent the formation and transmission of pathologic α-syn aggregates.
MicroRNAs (miRNAs) are a class of highly conserved small RNAs that regulate diverse cellular processes by base pairing with the 3′-untranslated regions (UTRs) of their target mRNAs, which leads to inhibition of protein translation or degradation of the target mRNA transcript [17]. Emerging evidence from postmortem brain analyses and animal model studies has suggested that dysfunction of miRNAs contributes to neurodegenerative disorders including PD [18–20]. The first miRNA identified to regulate α-syn levels is microRNA-7 (miR-7) [21], which is evolutionarily conserved among vertebrates and enriched in the brain. miR-7 directly downregulates α-syn expression by binding to the SNCA mRNA 3′-UTR, reducing α-syn levels and toxicity in cultured dopaminergic cells [21]. In addition to repressing α-syn levels, miR-7 targets other mRNAs that contribute to neuronal homeostasis including oxidative stress, mitochondrial health, glycolysis, apoptosis, and inhibition of inflammasome activation [22–26]. Conversely, miR-7 is decreased in the SNpc of patients with PD, and loss of miR-7 regulation leads to α-syn accumulation and dopaminergic neuronal loss in vitro and in vivo [27]. All these findings suggest that miR-7 has potential for slowing PD progression. However, whether miR-7 inhibits seeding, fibrillization, and propagation of α-syn in vivo, and consequently, protects against the neurodegeneration induced by pathologic α-syn aggregates remains unexplored.
In the present study, we report that transgenic overexpression of miR-7 using an AAV vector in the mouse striatum attenuates the formation and propagation of hyperphosphorylated and high-molecular-weight (HMW) α-syn, reduces the inflammation, and protects nigrostriatal dopaminergic neurons against the toxicity of recombinant α-syn preformed fibrils (PFF). These neuropathological markers are associated with better behavioral performance. Our findings collectively demonstrate for the first time the ability of miR-7 to mitigate the phenotype of a progressive α-synucleinopathy model in vivo.
Methods
Animals
Male C57BL/6 J mice (4 weeks) were obtained from the Jackson Laboratories (Bar Harbor, ME). Animals were housed with food and water ad libitum in 12-h light and dark cycles. All the experimental procedures and postoperative care were carried out in accordance with the NIH Guide for the Care and Use of Experimental Animals and approved by the Rutgers-Robert Wood Johnson Medical School Institutional Animal Care and Use Committee.
Preparation of Recombinant α-Synuclein Fibrils
pT7-7 plasmid containing mouse α-syn cDNA [28, 29] was used to generate α-syn. Purification of α-syn protein and preparation of PFF were done as detailed previously [9, 10].
Stereotaxic Surgery
AAV1-EF1α-hsa-miR-7–1-eGFP (AAV-miR-7) and AAV1-EF1α-ctl-miR-eGFP (AAV-miR-NT) were ordered from Vector Biolabs (Malvern, PA). This AAV vector expresses human pre-miRNA hsa-mir-7–1 (which is identical to the mouse miR-7 sequence) or a scrambled pre-miR sequence under an EF1α promoter, which also drives eGFP reporter expression (Fig. 1A). Mice were randomly divided into three groups: group 1 received AAV-miR-NT followed by phosphate buffered saline injection (NT + PBS), group 2 received AAV-miR-NT followed by α-syn PFF inoculation (NT + PFF), and group 3 received AAV-miR-7 followed by α-syn PFF inoculation (miR-7 + PFF). Studies began for all mice at 2 months of age with unilateral injection of AAV-miR-NT or AAV-miR-7. α-syn PFF or PBS was injected 1 month after AAV injection. Mice were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 my/kg, i.p.) before each stereotactic surgical procedure. Both AAV-miR-NT and AAV-miR-7 were diluted to 2.6 × 1013 GC/mL before use. One microliter of each AAV vector was injected into the right dorsal striatum (A–P: 0.2 mm; M–L: − 2.0 mm; D–V: − 2.6 mm) at a rate of 0.2 µL per min using a 10-µL Hamilton Neuros syringe equipped with a 33-gauge needle and attached to a Quintessential Stereotaxic Injector (Stoelting, Wood Dale, IL). To prevent reflux, after each injection, the needle was left in place for 5 min before removal. All of the animals recovered on a heating pad at 37 °C and were monitored regularly. One month later, α-syn PFF (5 µg) or 2.5 µL PBS was injected stereotaxically into the same coordinates as the virus injection. The procedures for PFF and PBS injections were the same as those for virus injection. Six months following α-syn PFF or PBS injection, behavior assessments were performed before mice were euthanized and brains were recovered for histological and molecular analyses.
Fig. 1.
Overexpression of miR-7 in striatal neurons of mice 1 month after AAV injection. A Map of AAV-miR-7 vector. B Timeline of in vivo experiments. C Representative images of miR-7 and GFP expression in the dorsal striatum of AAV-miR-7 or non-targeting AAV-miR-NT-injected mice. For miR-7 detection, in situ hybridization utilized a specific or scrambled probe. Scale bar = 100 μm. D Representative images of co-localization of GFP and NeuN immunofluorescence in the dorsal striatum of AAV-injected mice. Scale bar = 25 μm. E Western blot and quantitative analysis of monomeric α-syn protein in striatal lysates. Data shown are means ± SEM (n = 3 per group; * p < 0.05; two-tailed unpaired t-test)
In Situ Hybridization of miR-7
In situ hybridizations were performed as described [30] with 30-μm cryosections of mouse brains. The sections were dried at room temperature for 30 min, followed by fixation in 10% formalin (Sigma-Aldrich, St. Louis, MO) for 10 min. After washes in PBS, sections were acetylated in acetic anhydride/triethanolamine. After proteinase K treatment (10 mg/mL) for 10 min, sections were pre-hybridized in a hybridization solution (50% formamide, 5 × saline-sodium citrate buffer (SSC), 0.5 mg/mL yeast tRNA, 1 × Denhardt’s solution) at room temperature for 4–5 h. DIG-labeled miR-7 and scrambled probe (5 pmol) (LNA miRCURY probe; Exiqon) were denatured in a denaturing buffer by heating at 80 °C for 5 min and then hybridized to the sections at 55 °C overnight. After post-hybridization washes in 0.2 × SSC at 60 °C, the in-situ hybridization signals were detected using nitro-blue-toluidine/5-bromo-4-chloro-3-indolyl-phosphate (Sigma, St. Louis, MO) developer solution for 6 h, with light blue cytoplasmic staining being positive. The images were taken by a Leica DMi8 microscopy.
Immunohistochemistry and Immunofluorescence
Mice were perfused transcardially with PBS followed by 10% formalin (Sigma, St. Louis, MO), and the brains were removed and post-fixed in 10% formalin at 4 °C overnight. After dehydration by 30% sucrose, brains were sectioned using a cryostat at 30-μm thickness, and slices were collected as sets with the same interval. For immunohistochemistry, free-floating sections were washed in PBS containing 0.05% Triton X-100 (PBST) and then incubated for 30 min with 0.3% H2O2 to quench endogenous peroxidase activity. The sections were soaked with a blocking buffer (5% goat serum and 0.05% Tween-20 in PBS) and then incubated with primary antibodies dissolved in a dilution buffer (1% serum in 0.05% Tween-20 in PBS) at 4 °C overnight. Vectastain elite ABC kit (Vector Laboratories, Burlingame, CA) and 3.3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO) were used for amplifying and color development. For striatal TH staining, an elliptical area of interest (AOI) of the same size that encompasses the striatum was applied to all the sections. For pS129-α-syn stains, four striatal sections and six SNpc sections per brain were counted. For Iba-1 staining in the striatum, four sections per brain were analyzed. Staining intensity and pS129-α-syn-positive cell counts were obtained by Image Pro Plus as previously described [10]. Intensity calibration was set to the level of a blank area in each image. Hue, saturation, and intensity (HSI) was used for color selection with the standard parameters of H: 0–30, S: 0–255, and I: 0–160. To count only positively stained cells, color selection was adjusted according to the antibody used and background intensity. Stains that were smaller than 4 pixels were excluded from the analysis. For immunofluorescence staining of NeuN, free-floating sections were washed in PBST, and then soaked with a blocking buffer at room temperature for 1 h. Sections were incubated with a primary antibody overnight at 4 °C and fluorescent secondary antibody for 1 h at room temperature. Images were captured by a Leica DMi8 microscopy. The primary antibodies used were anti-pS129-α-syn (#015–25,191, WAKO, Richmond, VA; 1:10,000), anti-Iba-1 (#019–19,741, WAKO, Richmond, VA; 1:100), anti-TH (T2928, Sigma, St. Louis, MO, 1:3000 in striatum, and 1:5000 in the substantia nigra), and anti-NeuN (MAB377A5, Sigma, St. Louis, MO; 1:100).
Automated Analysis of TH-Positive Neuron Counting
For each mouse brain, six sections through the rostral–caudal extent of the SNpc at 150-μm intervals were analyzed. Sections were imaged using a Leica DMi8 microscope with a 10 × objective. Stitched SNpc digitized images with a resolution of 0.22 μm per pixel were then uploaded to the Aiforia™ Cloud Version 4.8 image processing and management platform (Aiforia Inc., Cambridge, USA) for processing with deep learning convolutional neural networks (CNNs) and supervised learning. This computer-assisted cell counting method based on supervised machine learning and automated image recognition is described in detail and validated [9, 31, 32]. A supervised, multi-layered CNN was trained on annotations from digitized TH-stained coronal slices from 3 cohorts to quantify high-quality tissue and count TH-positive cell bodies. The algorithm was trained on both diverse and representative whole-slide images in the dataset (using 72.22% of the total dataset) to create a generalizable machine-learning model. The TH neuron algorithm was trained on a total of 1563 object annotations across 65 images and resulted in a total verification error rate 1.86% (False positive = 1.09%; False negative = 0.77%) compared to the input annotations. Known artifacts of the staining procedure were identified and trained into the artificial intelligence (AI) model to exclude them from the analysis as background. Final AI model analysis was applied to manual ROIs that were provided in the Aiforia™ Cloud platform.
Western Blot Analysis
Cultured cells and striatal tissue samples were extracted in 2% SDS in PBS containing phosphatase inhibitor cocktail set II (Calbiochem, La Jolla, CA). Samples were sonicated and cleared at 140,000 × g for 10 min. Protein concentrations were determined using the BCA assay (Thermo Fisher Scientific, Waltham, MA). Lysates (20 µg) were mixed with 4 × protein loading buffer (Li-Cor, Lincoln, NE), separated on a NuPage 4–20% (GenScript, Piscataway, NJ), and transferred onto a nitrocellulose blotting membrane (Cytiva, Marlborough, MA). Blots were blocked in an Intercept (TBS) blocking buffer (Li-Cor, Lincoln, NE) for 1 h. Primary antibodies were diluted in Intercept T20 (TBS) antibody diluent and incubated with the membranes at 4 °C overnight. Membranes were washed three times in TBS-Tween and incubated in diluted IRDye 800CW or IRDye 680RD secondary antibody (Li-Cor, Lincoln, NE) for 1 h at room temperature. Following washing, membranes were scanned by a Li-Cor Odyssey CLx infrared imaging system. Images were analyzed by using Image Studio Lite Software (Li-Cor, Lincoln, NE). For detection, immunoblots were performed using antibodies against: α-syn (ab21284, Abcam, Cambridge, MA; 1:1000), GFP (#2955, Cell Signaling Technology, Danvers, MA; 1:2000), and β-actin (A5441, Sigma-Aldrich, St. Louis, MO; 1:10,000).
Generation of SH-SY5Y Cells Stably Overexpressing miR-7
Lentivirus production, including lenti-miR-NT and lenti-miR-7 were packaged as described previously [23]. Lentiviral vector pLemiR encodes human pri-miR-7–2 and Turbo Red fluorescent protein (tRFP) within a bicistronic transcript, which allows easy tracking of miR expressing cells. SH-SY5Y cells with stable integration of each construct (tRFP positive) were selected using 2 μg/mL of puromycin (Sigma, St. Louis, MO) after 48 h of virus transduction. Western blot analysis (Fig. S1A, B) shows SH-SY5Y cells stably overexpressing miR-7 reduces α-syn levels by 46% compared to negative control lenti-miR-NT transduced cells.
Cell Viability Assay
Cell viability was measured using a CellTiter 96 aqueous 3-(4,5-dimethylazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent (Promega, Madison, WI), according to the manufacturer’s instructions.
Behavior Assessments
Behavioral assessments were performed at 6 months post-α-syn PFF injections. To test nesting behavior, each mouse was housed separately in a cage with a 5-cm square cotton nestlet (Ancare, Bellmore, NY, USA) for 24 h. Each cage was then scored blindly depending on the condition of nesting material on a scale ranging from 1 (non-shredded) to 5 (maximally shredded) [33]. For the rotarod test, mice were placed on a rotating cylinder (diameter = 4.5 cm) with a coarse surface for firm grip and were trained for four trials for the first four days: the first two trials are acquisition trials with an increasing speed from 4 to 20 rpm in 180 s, and the last two trials are probe trials with an accelerating speed of 0.2 rpm/s, increasing from 4 to 40 rpm in 180 s. On the fifth day, mice were tested for 3 actual probe trials, latency on the rod before falling was measured, and the average of 3 trials taken for analysis.
Statistical Analysis
Data are presented as means ± S.E.M. and analyzed by either one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test or unpaired t test unless otherwise stated. Significance was determined at p < 0.05.
Results
Validation of miR-7 Overexpression in the Mouse Striatum via AAV Delivery
To verify that miR-7 overexpression protects against α-syn PFF in a cellular model ahead of in vivo studies, we first demonstrated that human neuroblastoma SH-SY5Y cells stably transduced with a lenti-miR-7 vector indeed have reduced α-syn protein levels detected by Western blotting and are protected against α-syn PFF-induced cell death (Fig. S1). In vivo studies were then carried out by infusing AAV-miR-NT or AAV-miR-7 unilaterally into the right striatum of 2-month old mice. One month after viral transduction, miRNA expression was evaluated by in situ hybridization (Fig. 1C). Both AAV-miR-NT and AAV-miR-7 yielded strong GFP expression, whereas only AAV-miR-7-infused brain sections showed a robust miR-7 signal using a miR-7 specific probe. Scrambled in situ probes showed no signal in either AAV-miR-7 or AAV-miR-NT-infused brain sections. These results indicate that AAV-miR-7 infused into the mouse striatum expresses miR-7 robustly, and that 1 month is sufficient time for its expression, which supports the design of the present in vivo experiments (Fig. 1B).
AAV1 is commonly used to target local populations of neurons after direct injection. To check the type of cells transduced with the AAV vectors used in the present study, immunohistochemical staining for the neuronal marker NeuN was done. Our data showed that all GFP-positive cells stained for NeuN, indicating that AAV vectors infected only striatal neurons (Fig. 1D; Fig. S2A) as reported previously [34]. It is noted that overexpression of miR-7 in the striatum does not affect the number of striatal neurons (Fig. S2B). Western blot analysis of striatal lysates from AAV-injected mice showed that overexpression of miR-7 significantly downregulates α-syn protein levels by 30% (Fig. 1E). These data support the ability of miR-7 delivered via viral vector to reduce α-syn expression in the mouse brain.
miR-7 Prevents the Nucleation and Propagation of pS129- α -syn in α-syn PFF-Inoculated Mice
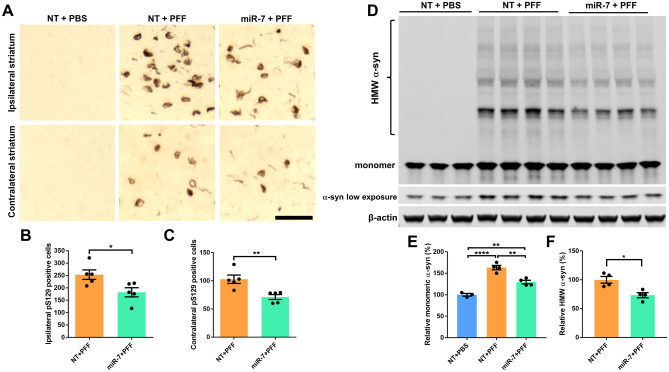
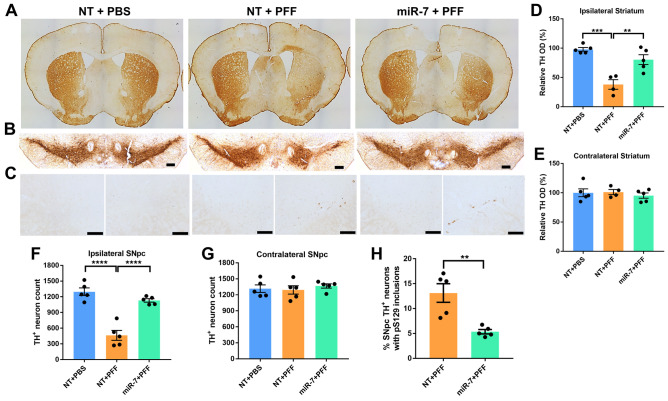
To examine whether miR-7 impacts pS129-α-syn nucleation and propagation in the α-syn PFF model, striatal and SNpc tissue sections were stained with p-α-syn antibody. As described previously with this model [5], abundant pS129-α-syn-labeled cells were found in the striatum and SNpc ipsilateral to control AAV-miR-NT pre-injected and α-syn PFF-inoculated side, and fewer pS129-α-syn immunoreactive neurons were detected in the contralateral hemisphere (Figs. 2A–C and 4C, H). On the other hand, compared with AAV-miR-NT pre-injected mice, AAV-miR-7 pre-injected and PFF-inoculated brains had less abundant pS129-α-syn immunoreactivity in both the ipsilateral and contralateral striata (Fig. 2A–C) as well as in the ipsilateral nigral neurons (Fig. 4C, H). Compared with AAV-miR-NT pre-injected and α-syn PFF-inoculated brains, in which 13% of residual tyrosine hydroxylase (TH)-positive SNpc neurons contained pS129-α-syn, this figure was only 5.4% in AAV-miR-7 pre-injected and PFF-inoculated brains (Fig. 4H). As expected, brains that were injected with PBS instead of PFF had no pS129-α-syn immunoreactive neurons.
Fig. 2.
AAV-miR-7 prevents the formation of pS129-α-syn and high molecular weight (HMW) α-syn. A Representative images of pS129-α-syn staining in the striatum ipsilateral and contralateral to the injected side 6 months post-α-syn PFF injections. Scale bar = 50 μm. B, C Quantification of pS129-α-syn-positive cells in the ipsilateral (B) and contralateral (C) striatum of mice inoculated with α-syn PFF following AAV injection. Data shown are means ± SEM (n = 5 per group; * p < 0.05, ** p < 0.01; two-tailed unpaired t-test). D Western blot of striatal lysates from all 3 groups. E, F Quantitative analysis of monomeric α-syn (E) and HMW α-syn (F) in panel D. Data shown are means ± SEM; * p < 0.05, ** p < 0.01, **** p < 0.0001; one-way ANOVA or two-tailed unpaired t-test)
Fig. 4.
AAV-miR-7 protects nigral dopaminergic neurons and their terminals against intrastriatal α-syn PFF injections. A Representative immunohistochemistry images of tyrosine hydroxylase (TH) staining of the striatum. Injections were made in the right striatum. B Representative immunohistochemistry images of TH staining of the SNpc. C Representative images of pS129-α-syn staining in the ipsilateral and contralateral substantia nigra pars compact (SNpc). Scale bar in panels B and C = 200 μm. D, E Quantification of TH staining intensity in the ipsilateral (D) and contralateral (E) striatum for all 3 groups. Data shown are means ± SEM (n = 4–5 per group; ** p < 0.01, *** p < 0.001; one-way ANOVA). F, G Quantification of TH-positive neurons in the ipsilateral (F) and contralateral (G) SNpc for all 3 groups. Data shown are means ± SEM (n = 4–5 per group; **** p < 0.0001; one-way ANOVA). H Percentage of TH-positive SNpc neurons containing pS129-α-syn-immunoreactive inclusions in α-syn PFF-inoculated groups. Data shown are means ± SEM (n = 5 per group; ** p < 0.01; two-tailed unpaired t-test)
To examine whether miR-7 impacts α-syn protein levels in vivo, striatal lysates from the 3 mouse groups were examined by Western blotting. Monomeric α-syn was detected in all groups but increased by 63% in PFF-inoculated and AAV-miR-NT pre-injected mice compared to control PBS-injected animals. PFF inoculation also resulted in the accumulation of HMW oligomers (Fig. 2D–F). On the other hand, AAV-miR-7 pre-injection resulted in 21% reduction of monomeric α-syn and 27% reduction of HMW compared with AAV-miR-NT pre-injected mice, substantiating our previous in vitro observations using differentiated human neuron-like ReNcell VM cells [35]. These findings suggest that by reducing endogenous mouse α-syn protein levels, miR-7 prevents seeding initiated by injected PFF and reduces the accumulation of pathologic α-syn.
miR-7 Attenuates the Neuroinflammation in α-syn PFF-Inoculated Mice
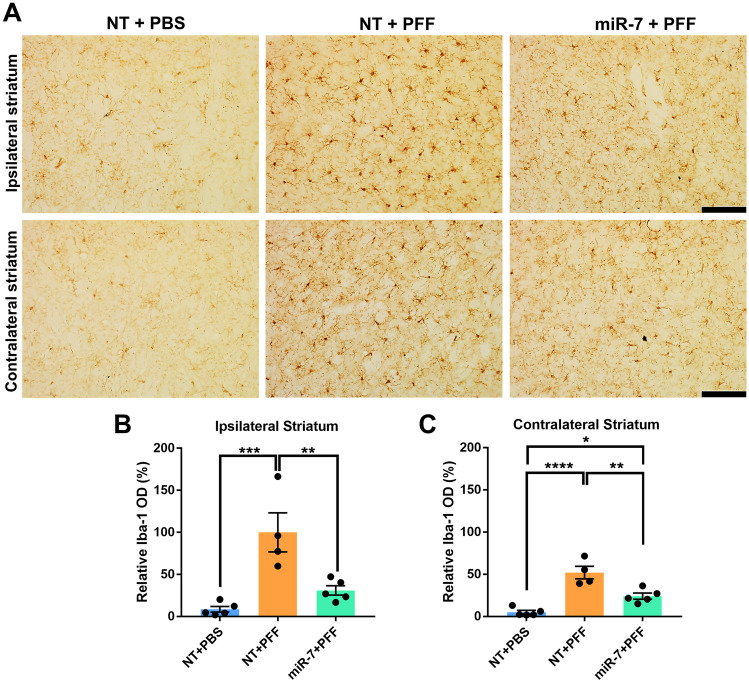
α-Syn is known to activate microglia in various experimental paradigms including human microglial cells isolated from surgically resected human temporal lobe tissue, mouse primary microglial cultures, and in mice overexpressing α-syn [36, 37]. α-Syn PFF inoculation of the mouse brain also induces a robust microglial activation [38, 39]. To examine the impact of miR-7 on the neuroinflammation induced by α-syn PFF inoculation, striatal sections were stained with the microglial marker Iba-1. Compared with PBS injection, α-syn PFF inoculation significantly increased the number of reactive microglia both in the striatum ipsilateral to the injection site (12-fold) and to a lesser extent in the contralateral striatum (sixfold) (Fig. 3). AAV-miR-7 pre-injection resulted in a marked repression of the microglial reaction to PFF injections, reducing the number of these inflammatory cells by 70% in the ipsilateral striatum and by 54% in the contralateral side. These findings indicate that miR-7 attenuates the neuroinflammatory response to α-syn PFF in vivo.
Fig. 3.
AAV-miR-7 suppresses the microglial activation in the striatum of mice inoculated with α-syn PFF. A Representative immunohistochemistry images of the microglial marker Iba-1 in the ipsilateral and contralateral striatum. Scale bar = 100 μm. B, C Quantification of immunohistochemical staining of Iba-1 in the ipsilateral B and contralateral C sides. Data shown are means ± SEM (n = 4–5 per group; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; one-way ANOVA)
miR-7 Protects Dopaminergic Neurons in α-syn PFF-Inoculated Mice
To study whether miR-7 protects nigral dopaminergic neurons from PFF-induced death, we first assessed the integrity of nigrostriatal terminals in the 3 mouse groups. TH immunostaining in the striatum revealed that α-syn PFF injections reduced dopaminergic terminals in the ipsilateral striatum by 63% in AAV-miR-NT pre-injected mice compared to only 20% in AAV-miR-7 pre-injected mice, representing a 43% protection (Fig. 4A, D). As described previously [5, 10], no changes in TH immunoreactivity were detected in the contralateral striatum in any mouse group (Fig. 4A, E). Similarly, immunohistochemical staining of the SNpc for TH and counting these neurons using AI model analysis showed marked loss of ipsilateral nigral dopaminergic neurons in α-syn PFF-inoculated mice. Compared with a 65% loss of these neurons in AAV-miR-NT pre-injected mice, only a 13% decrease was seen in AAV-miR-7 pre-injected mice, representing a 52% protection (Fig. 4B, F). No changes in the number of TH-positive neurons in the contralateral SNpc occurred with PFF or AAV injections (Fig. 4B, G). These results collectively indicate that miR-7 protects against degeneration of dopamine neurons and their terminals caused by α-syn PFF inoculation and seeding.
miR-7 Protects the Behavioral Impairment Induced by α-syn PFF Inoculation
To determine whether the preservation of nigrostrial dopaminergic neurons seen in AAV-miR-7 pre-injected and α-syn PFF-inoculated mice translates to their behavioral performance as well, nest building and latency to fall from the rotarod test were assessed 6 months after α-syn PFF inoculations as described previously shortly prior to sacrificing the animals [10, 40]. Mice inoculated with α-syn PFF showed significantly impaired nesting behavior and ability to stay on the rotarod compared to PBS-injected mice, whereas AAV-miR-7 pre-injected animals exhibited markedly improved performance (Fig. 5). For nesting behavior, compared with AAV-NT + PBS group, PFF inoculation reduced scores by 38%, while this figure was only 5% in AAV-miR-7 + PFF animals, yielding a 33% protection. And for the rotarod test, latency to fall was shortened by 42% in PFF-inoculated mice, and by 14% in AAV-miR-7 pre-injected + PFF mice, resulting in a 28% protection. These behavioral improvements in AAV-miR-7 pre-injected mice are consistent with the histopathologic data shown above.
Fig. 5.

AAV-miR-7 improves behavioral performance of α-syn PFF-inoculated mice. A Performance on the nest building test. B Performance on the rotarod. Assessments were made 6 months post-PBS or α-syn PFF injection. Data shown are means ± SEM (n = 11–12 per group; * p < 0.05, ** p < 0.01, *** p < 0.001; one-way ANOVA)
Discussion
The present findings demonstrate that AAV-miR-7 can prevent the neurodegeneration induced by exogenously administered α-syn PFF in the brains of wild-type mice. Overexpression of miR-7 in the striatum of mice markedly reduced monomeric and HMW α-syn, attenuated the accumulation and propagation of pS129-α-syn, repressed neuroinflammation, and preserved the integrity and function of nigrostriatal dopaminergic neurons. This improved the neuropathological profile resulting from miR-7 overexpression translated to mitigation of the behavioral impairment induced by α-syn PFF inoculation.
Aggregates of α-syn in Lewy bodies and Lewy neurites are the main pathological hallmark of PD and related synucleinopathies [41]. The oligomerization and fibrillization of the normally intrinsically disordered protein α-syn lead to neuronal dysfunction and death [42]. α-Syn aggregates produced in one neuron can be transmitted to neighboring neurons by sequential events of exocytosis and endocytosis [43]. This cell-to-cell transmission of pathological α-syn seeds is increasingly thought to underlie the progression of these neurodegenerative disorders [44–47]. In primary neuron cultures, application of sonicated α-syn fibrils can induce endogenously expressed α-syn to form aggregates [6]. Additionally, intrastriatal injection of sonicated mouse PFF initiates the aggregation of endogenous α-syn and spread to remote brain regions [5]. And this process can be accelerated by elevated levels of intraneuronal α-syn [48]. These findings support the hypothesis that exogenous aggregates act as seeds that recruit endogenous α-syn to form pathologic aggregates. Meanwhile, genetic reduction or deletion of the SNCA gene in human dopaminergic neurons renders these neurons markedly resistant to the formation of α-syn aggregates induced by PFF [16]. Thus, reducing endogenous α-syn level is a viable strategy to repress the formation and propagation of α-syn aggregates and consequent neurotoxicity.
miR-7 is a brain-enriched miRNA that plays critical roles in brain development and neurological disorders, including PD [49]. It directly downregulates α-syn expression in dopaminergic neurons leading to cytoprotection [21], and its levels have been found to be reduced in serum and mesencephalic samples from animal models and patients with PD [26, 27]. In addition, a study using in vitro and in vivo models of PD supports that loss of miR-7 impacts α-syn accumulation, loss of dopaminergic cells, and reduction of striatal dopamine [27]. Based on these studies, we submit that miR-7, by lowering α-syn production, would be beneficial to patients with synucleinopathies by inhibiting the formation and transmission of pS129-α-syn and its toxicity.
Since our initial discovery about the relevance of miR-7 to PD linked to repressing α-syn levels [21], a growing body of evidence has uncovered additional target mRNAs of miR-7 that lead to neuroprotection in PD as well. For example, miR-7 protects neuronal cells by downregulating RelA through promoting glycolysis [22, 24], upregulating the mTOR pathway [50], protecting mitochondria by preventing opening of the permeability transition pore through downregulating voltage dependent anion channel 1 (VDAC1) [23], and inhibiting neuronal apoptosis by targeting Bax and Sirt2 [25]. miR-7 also reduces oxidative stress by repressing Kelch-like ECH-associated protein 1 (Keap1) expression and activating Nuclear factor E2-related factor 2 (Nrf2) [51]. Additionally, in α-syn PFF challenged cellular model, miR-7 facilitates the clearance of α-syn aggregates by promoting autophagy [35]. The protective effects of downregulating α-syn levels can also be achieved using antisense oligonucleotides (ASOs) or shRNA delivered by AAV [52], as demonstrated in AAV-mediated α-syn overexpression, PFF transmission, and the rotenone model of PD in rodents [53–55]. It is notable that the level of α-syn knockdown in the present study (30%) is similar to the degree of knockdown found to be efficacious in previous studies using ASO or shRNA, which may serve as a reference value for dosage setting in future clinical trials. The decreased expression of miR-7 in the substantia nigra of PD patients [27] and its multi-faceted neuroprotective actions [22–25, 35, 50, 51] point to added therapeutic value of miR-7 gene therapy in this disease.
Considerable interest has been generated in recent years in neuroinflammation as an important contributor to the pathogenesis of PD. α-Syn and its aggregates are well known to activate microglia [39, 56–58], while microglia in turn exacerbate α-syn-mediated neurotoxicity and increase oxidative stress [59]. In an inflammatory environment, the formation and propagation of α-syn aggregates are accelerated [60]. Besides α-syn, miR-7 targets Nod-like receptor protein 3 (NLRP3) expression and modulates NLRP3 inflammasome activation [26]. Thus, it is conceivable that miR-7 may exert additional beneficial effects by directly regulating NLRP3 expression in microglia while regulating α-syn in neurons. In the present study, AAV-miR-7 infected only neurons, suggesting that the effects of miR-7 in neurons are sufficient to mitigate neuroinflammation. A plausible possibility is that miR-7 reduces oxidative stress through two pathways. First, miR-7 reduces α-syn levels and attenuates its aggregation, which is known to induce reactive oxygen species (ROS) generation and consequent inflammation [61–64]. And second, miR-7 increases Nrf2 activity with a consequent upregulation of antioxidant genes, heme oxygenase 1 (HO-1), and glutamate-cysteine ligase modifier subunit (GCLM) by suppressing Keap1 [51].
Some studies using the shRNA approach to knock down SNCA have raised questions about possible toxicity in the form of decreased TH expression and even loss of TH neurons [65, 66]. However, long-term SNCA knockdown using shRNA delivered by AAV in the substantia nigra did not cause significant functional deficits in the ascending dopaminergic projection or neurodegeneration [52]. The present study showed no indication of any toxicity of miR-7 as the number of striatal NeuN-positive neurons was no different between AAV-miR-7 and control AAV-NT-injected brains. The safety profile of miR-7 is also supported by a study whereby stereotactic injection of miR-7 mimics into the striatum had no significant effect on TH+ cell number in A53Ttg/tg mice [26]. Considering that miR-7 has other mRNA targets besides SNCA that contribute to neuronal homeostasis including oxidative stress, mitochondrial health, glycolysis, apoptosis, and inhibition of inflammasome activation [22–26], it is likely that these functions provide added means of neuroprotection.
In the present study, AAV-miR-7 was delivered prior to α-syn PFF inoculation, thus demonstrating the protective effect of this therapeutic approach against future seeding and subsequent neurodegeneration. Whether this approach can also reverse existing pathology is supported by a recent study showed that knocking down α-syn using a single intracerebroventricular injection of SNCA-targeted ASO at 14 or 21 days following intrastriatal α-syn PFF injection can remove established α-syn pathology and prevent dopaminergic cell loss [53]. Thus, strategies that reduce α-syn protein levels might modulate the equilibrium between the soluble and misfolded forms of the protein, leading to gradual clearance of existing pathology. The clinical implications of this notion are clear since considerable pathology is already present when Parkinson symptoms first emerge, and innovative therapeutics such as discussed here will be used in symptomatic individuals for the foreseeable future until pre-symptomatic identification of at-risk individuals becomes routine and the safety profile of these therapeutics is well established.
In conclusion, the present study provides the first demonstration that overexpression of miR-7 in the mouse brain through an AAV vector reduces pS129-α-syn propagation and attenuates the neurodegeneration and behavioral deficits induced by PFF inoculation. These findings suggest the translational potential of AAV-miR-7 and support developing this biologic as a disease-modifying therapeutic agent for PD and related synucleinopathies.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author Contribution
J. Z., E. J., and M. M. M conceptualized and designed the study. J. Z. performed the experiments. M. Z. analyzed the immunostaining data. R. Y., J. L., and S. M. contributed to the experimental work and data interpretation. J. Z., E. J., and M. M. M. interpreted the data and wrote the manuscript. All the authors approved the final version of the manuscript.
Funding
The study was supported in part by the New Jersey Health Foundation and the Nicholson Foundation. E. J. was supported by the National Institutes of Health (NIH) (NS070898). M. M. M. is the William Dow Lovett Professor of Neurology and received funding from the National Institutes of Health (NIH) (AT006868, NS101134, NS096032, NS116921), the Michael J. Fox Foundation for Parkinson’s Research (MJFF-12350, MJFF-001006), and the American Parkinson Disease Association.
Declarations
Conflict of Interest
M. M. M and E. J. have an issued patent (“RNA Targeting in alpha-synucleinopathies”). M. M. M. is a founder of MentiNova, Inc., which focuses on other therapeutics.
Disclaimer
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hijaz BA, Volpicelli-Daley LA. Initiation and propagation of α-synuclein aggregation in the nervous system. Molecular Neurodegeneration. 2020;15(1):19. doi: 10.1186/s13024-020-00368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Tredici K, Braak H. Review: Sporadic Parkinson's disease: development and distribution of alpha-synuclein pathology. Neuropathology and applied neurobiology. 2016;42(1):33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- 5.Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H, Del Tredici K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson's disease: Separating the Wheat from the Chaff. J Parkinsons Dis. 2017;7(s1):S71–S85. doi: 10.3233/JPD-179001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281(40):29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 9.Yan R, Zhang J, Park HJ, Park ES, Oh S, Zheng H, et al. Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson's disease and DLB. Proc Natl Acad Sci U S A. 2018;115(51):E12053–E12062. doi: 10.1073/pnas.1813365115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Park ES, Park H-J, Yan R, Grudniewska M, Zhang X, et al. Apoptosis signal regulating kinase 1 deletion mitigates α-synuclein pre-formed fibril propagation in mice. Neurobiology of Aging. 2020;85:49–57. doi: 10.1016/j.neurobiolaging.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bingol B. Autophagy and lysosomal pathways in nervous system disorders. Mol Cell Neurosci. 2018;91:167–208. doi: 10.1016/j.mcn.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Luk KC, Song C, O'Brien P, Stieber A, Branch JR, Brunden KR, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ, Lee KW, Park ES, Oh S, Yan R, Zhang J, et al. Dysregulation of protein phosphatase 2A in parkinson disease and dementia with lewy bodies. Ann Clin Transl Neurol. 2016;3(10):769–780. doi: 10.1002/acn3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du TT, Wang L, Duan CL, Lu LL, Zhang JL, Gao G, et al. GBA deficiency promotes SNCA/alpha-synuclein accumulation through autophagic inhibition by inactivated PPP2A. Autophagy. 2015;11(10):1803–1820. doi: 10.1080/15548627.2015.1086055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Annals of neurology. 2008;63(6):743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Dolt KS, Kriek M, Baker T, Downey P, Drummond NJ, et al. Engineering synucleinopathy-resistant human dopaminergic neurons by CRISPR-mediated deletion of the SNCA gene. Eur J Neurosci. 2019;49(4):510–524. doi: 10.1111/ejn.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature Reviews Genetics. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 18.Junn E, Mouradian MM. MicroRNAs in neurodegenerative disorders. Cell cycle. 2010;9(9):1717–1721. doi: 10.4161/cc.9.9.11296. [DOI] [PubMed] [Google Scholar]
- 19.Mouradian MM. MicroRNAs in Parkinson's disease. Neurobiology of disease. 2012;46(2):279–284. doi: 10.1016/j.nbd.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 20.Juźwik CA, S. Drake S, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A, et al. microRNA dysregulation in neurodegenerative diseases: A systematic review. Progress in Neurobiology. 2019;182:101664. [DOI] [PubMed]
- 21.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106(31):13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi DC, Chae YJ, Kabaria S, Chaudhuri AD, Jain MR, Li H, et al. MicroRNA-7 protects against 1-methyl-4-phenylpyridinium-induced cell death by targeting RelA. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(38):12725–12737. doi: 10.1523/JNEUROSCI.0985-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhuri AD, Choi DC, Kabaria S, Tran A, Junn E. MicroRNA-7 Regulates the Function of Mitochondrial Permeability Transition Pore by Targeting VDAC1 Expression. J Biol Chem. 2016;291(12):6483–6493. doi: 10.1074/jbc.M115.691352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhuri AD, Kabaria S, Choi DC, Mouradian MM, Junn E. MicroRNA-7 Promotes Glycolysis to Protect against 1-Methyl-4-phenylpyridinium-induced Cell Death. J Biol Chem. 2015;290(19):12425–12434. doi: 10.1074/jbc.M114.625962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Lv X, Zhai K, Xu R, Zhang Y, Zhao S, et al. MicroRNA-7 inhibits neuronal apoptosis in a cellular Parkinson's disease model by targeting Bax and Sirt2. American journal of translational research. 2016;8(2):993–1004. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Lu M, Du R-H, Qiao C, Jiang C-Y, Zhang K-Z, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Molecular Neurodegeneration. 2016;11(1):28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillan KJ, Murray TK, Bengoa-Vergniory N, Cordero-Llana O, Cooper J, Buckley A, et al. Loss of MicroRNA-7 Regulation Leads to α-Synuclein Accumulation and Dopaminergic Neuronal Loss In Vivo. Molecular Therapy. 2017;25(10):2404–2414. doi: 10.1016/j.ymthe.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinreb PH, Zhen W Fau - Poon AW, Poon Aw Fau - Conway KA, Conway Ka Fau - Lansbury PT, Jr., Lansbury PT, Jr. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35(43):13709–15(0006–2960 (Print)). [DOI] [PubMed]
- 29.Wu KP, Kim S, Fela DA, Baum J. Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: implication for aggregation. Journal of molecular biology. 2008;378(5):1104–1115. doi: 10.1016/j.jmb.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obernosterer G, Martinez J, Alenius M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat Protoc. 2007;2(6):1508–1514. doi: 10.1038/nprot.2007.153. [DOI] [PubMed] [Google Scholar]
- 31.Becker G, Michel A, Bahri MA, Mairet-Coello G, Lemaire C, Deprez T, et al. Monitoring of a progressive functional dopaminergic deficit in the A53T-AAV synuclein rats by combining 6-[(18)F]fluoro-L-m-tyrosine imaging and motor performances analysis. Neurobiol Aging. 2021;107:142–152. doi: 10.1016/j.neurobiolaging.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Sucunza D, Rico AJ, Roda E, Collantes M, Gonzalez-Aseguinolaza G, Rodriguez-Perez AI, et al. Glucocerebrosidase Gene Therapy Induces Alpha-Synuclein Clearance and Neuroprotection of Midbrain Dopaminergic Neurons in Mice and Macaques. Int J Mol Sci. 2021;22(9). [DOI] [PMC free article] [PubMed]
- 33.Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1(3):1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 34.Haery L, Deverman BE, Matho KS, Cetin A, Woodard K, Cepko C, et al. Adeno-Associated Virus Technologies and Methods for Targeted Neuronal Manipulation. Frontiers in Neuroanatomy. 2019;13(93). [DOI] [PMC free article] [PubMed]
- 35.Choi DC, Yoo M, Kabaria S, Junn E. MicroRNA-7 facilitates the degradation of alpha-synuclein and its aggregates by promoting autophagy. Neuroscience letters. 2018;678:118–123. doi: 10.1016/j.neulet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, et al. α-Synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiology of Aging. 2008;29(5):739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiology of Aging. 2008;29(11):1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumenstock S, Rodrigues EF, Peters F, Blazquez-Llorca L, Schmidt F, Giese A, et al. Seeding and transgenic overexpression of alpha-synuclein triggers dendritic spine pathology in the neocortex. EMBO Mol Med. 2017;9(5):716–731. doi: 10.15252/emmm.201607305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boza-Serrano A, Reyes JF, Rey NL, Leffler H, Bousset L, Nilsson U, et al. The role of Galectin-3 in alpha-synuclein-induced microglial activation. Acta Neuropathol Com. 2014;2:156. doi: 10.1186/s40478-014-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee KW, Woo JM, Im JY, Park ES, He L, Ichijo H, et al. Apoptosis signal-regulating kinase 1 modulates the phenotype of alpha-synuclein transgenic mice. Neurobiol Aging. 2015;36(1):519–526. doi: 10.1016/j.neurobiolaging.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 42.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nature reviews Neuroscience. 2001;2(7):492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 43.Li J-Y, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Medicine. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 44.Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, et al. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiology of disease. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu Y, Muller S, Tavares A, Barret O, Alagille D, Seibyl J, et al. Intrastriatal alpha-synuclein fibrils in monkeys: spreading, imaging and neuropathological changes. Brain : a journal of neurology. 2019;142(11):3565–3579. doi: 10.1093/brain/awz296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. Journal of Experimental Medicine. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braak H, Del Tredici K. Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70(20):1916. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 48.Thakur P, Breger LS, Lundblad M, Wan OW, Mattsson B, Luk KC, et al. Modeling Parkinson's disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proc Natl Acad Sci U S A. 2017;114(39):E8284–E8293. doi: 10.1073/pnas.1710442114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horsham JL, Ganda C, Kalinowski FC, Brown RAM, Epis MR, Leedman PJ. MicroRNA-7: A miRNA with expanding roles in development and disease. The international journal of biochemistry & cell biology. 2015;69:215–224. doi: 10.1016/j.biocel.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Fragkouli A, Doxakis E. miR-7 and miR-153 protect neurons against MPP+-induced cell death via upregulation of mTOR pathway. Frontiers in cellular neuroscience. 2014;8(182). [DOI] [PMC free article] [PubMed]
- 51.Kabaria S, Choi DC, Chaudhuri AD, Jain MR, Li H, Junn E. MicroRNA-7 activates Nrf2 pathway by targeting Keap1 expression. Free Radical Bio Med. 2015;89:548–556. doi: 10.1016/j.freeradbiomed.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zharikov A, Bai Q, De Miranda BR, Van Laar A, Greenamyre JT, Burton EA. Long-term RNAi knockdown of α-synuclein in the adult rat substantia nigra without neurodegeneration. Neurobiology of disease. 2019;125:146–153. doi: 10.1016/j.nbd.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole TA, Zhao H, Collier TJ, Sandoval I, Sortwell CE, Steece-Collier K, et al. α-Synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson's disease. JCI Insight. 2021;6(5). [DOI] [PMC free article] [PubMed]
- 54.Alarcón-Arís D, Pavia-Collado R, Miquel-Rio L, Coppola-Segovia V, Ferrés-Coy A, Ruiz-Bronchal E, et al. Anti-α-synuclein ASO delivered to monoamine neurons prevents α-synuclein accumulation in a Parkinson's disease-like mouse model and in monkeys. EBioMedicine. 2020;59:102944. [DOI] [PMC free article] [PubMed]
- 55.Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, et al. shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. The Journal of clinical investigation. 2015;125(7):2721–2735. doi: 10.1172/JCI64502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69(4):337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated α-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. The FASEB Journal. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 58.Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, et al. Triggering of Inflammasome by Aggregated α–Synuclein, an Inflammatory Response in Synucleinopathies. PLOS ONE. 2013;8(1):e55375. [DOI] [PMC free article] [PubMed]
- 59.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 60.Wilms H, Rosenstiel P, Romero-Ramos M, Arlt A, Schafer H, Seegert D, et al. Suppression of MAP kinases inhibits microglial activation and attenuates neuronal cell death induced by alpha-synuclein protofibrils. Int J Immunopathol Pharmacol. 2009;22(4):897–909. doi: 10.1177/039463200902200405. [DOI] [PubMed] [Google Scholar]
- 61.Junn E, Mouradian MM. Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett. 2002;320(3):146–150. doi: 10.1016/s0304-3940(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 62.Jiang H, Wu YC, Nakamura M, Liang Y, Tanaka Y, Holmes S, et al. Parkinson's disease genetic mutations increase cell susceptibility to stress: mutant alpha-synuclein enhances H2O2- and Sin-1-induced cell death. Neurobiol Aging. 2007;28(11):1709–1717. doi: 10.1016/j.neurobiolaging.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. alpha-Synuclein implicated in Parkinson's disease catalyses the formation of hydrogen peroxide in vitro. Free Radic Biol Med. 2001;30(10):1163–1170. doi: 10.1016/s0891-5849(01)00513-5. [DOI] [PubMed] [Google Scholar]
- 64.Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VM, et al. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci. 2013;33(24):10154–10164. doi: 10.1523/JNEUROSCI.5311-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorbatyuk OS, Li S, Nash K, Gorbatyuk M, Lewin AS, Sullivan LF, et al. In vivo RNAi-mediated alpha-synuclein silencing induces nigrostriatal degeneration. Mol Ther. 2010;18(8):1450–1457. doi: 10.1038/mt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benskey MJ, Sellnow RC, Sandoval IM, Sortwell CE, Lipton JW, Manfredsson FP. Silencing Alpha Synuclein in Mature Nigral Neurons Results in Rapid Neuroinflammation and Subsequent Toxicity. Front Mol Neurosci. 2018;11:36. doi: 10.3389/fnmol.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.