Summary
Background
Advances in breast cancer (BC) care have reduced mortality, but their impact on survival once diagnosed with metastasis is less well described. This systematic review aimed to describe population-level survival since 1995 for de novo metastatic BC (dnMBC) and recurrent MBC (rMBC).
Methods
We searched MEDLINE 01/01/1995–12/04/2021 to identify population-based cohort studies of MBC reporting overall (OS) or BC-specific survival (BCSS) over time. We appraised risk-of-bias and summarised survival descriptively for MBC diagnoses in 5-year periods from 1995 until 2014; and for age, hormone receptor and HER2 subgroups.
Findings
We identified 20 eligible studies (14 dnMBC, 1 rMBC, 5 combined). Potential sources of bias in these studies were confounding and shorter follow-up for the latest diagnosis period.
For dnMBC, 13 of 14 studies reported improved OS or BCSS since 1995. In 2005–2009, the median OS was 26 months (range 24–30), a median gain of 6 months since 1995–1999 (range 0–9, 4 studies). Median 5-year OS was 23% in 2005–2009, a median gain of 7% since 1995–1999 (range -2 to 14%, 4 studies). For women ≥70 years, the median and 5-year OS was unchanged (1 study) with no to modest difference in relative survival (range: -1·9% (p = 0.71) to +2·1% (p = 0.045), 3 studies). For rMBC, one study reported no change in survival between 1998 and 2006 and 2007–2013 (median OS 23 months). For combined MBC, 76–89% had rMBC. Three of four studies observed no change in median OS after 2000. Of these, one study reported median OS improved for women ≤60 years (1995–1999 19·1; 2000–2004 22·3 months) but not >60 years (12·7, 11·6 months).
Interpretation
Population-level improvements in OS for dnMBC have not been consistently observed in rMBC cohorts nor older women. These findings have implications for counselling patients about prognosis, planning cancer services and trial stratification.
Funding
SL was funded in part by a National Health and Medical Research Council (NHMRC) Project Grant ID: 1125433. NH was funded by the NBCF Chair in Breast Cancer Prevention grant (EC-21–001) and a NHMRC Investigator (Leader) grant (194410). BD and SAP were funded in part by the NHMRC Centre of Research Excellence in Medicines Intelligence (1196900).
Research in context.
Evidence before this study
Breast cancer survival has improved worldwide since the mid-1990s, however survival trends for metastatic breast cancer (MBC) are not routinely reported by cancer registries and are not well described at the population level. We identified one prior systematic review of post-metastasis survival from MEDLINE using a combination of search terms for breast cancer, metastasis, survival and population-based for the search period 1/1/1995 to 12/4/2021. The authors reported median overall survival improved between 1990 and 2010 for both MBC occurring de novo and recurrent after an initial diagnosis of non-metastatic disease, but studies were not restricted to population-based cohorts.
Added value of this study
Our systematic review finding of sustained population-level improvements in overall survival and breast cancer-specific survival since 1995 for de novo MBC is consistent with prior evidence from more selected populations. Our finding that at least three-quarters of new MBC diagnoses represent recurrent disease, and survival gains have not been consistently observed for this group, is unique, and adds to evidence from trial and institution-based studies that postulate an ‘adjuvant therapy-related shortening of survival’ effect. We further report consistent evidence of no to modest survival improvement over time for patients aged 70 years and older, who make up around a third of new MBC diagnoses.
Implications of all the available evidence
This evidence is valuable for counselling patients with MBC about prognosis, planning cancer services, and establishes the importance of the stratification of MBC by de novo/recurrent status and age group for clinical trial design. Given our finding of limited population-level data on recurrent MBC, we advocate for cancer registries to routinely report on distant metastases to track population-level changes in characteristics and prognosis.
Alt-text: Unlabelled box
Introduction
Breast cancer (BC) mortality rates have decreased by approximately 40% since 1995,1,2 with population-based cancer registries documenting improved survival worldwide.3 These survival gains correspond to major advances in BC multidisciplinary care, including the introduction of population BC screening programs for early diagnosis; adjuvant therapies to reduce the risk of distant metastasis; and new effective systemic therapies for metastatic BC (MBC). For early stage BC, there is consistent evidence that distant disease-free survival has improved over time.4,5 However, survival trends for patients diagnosed with distant metastasis are not well described at the population level. While registries report on survival for de novo metastatic BC (dnMBC), comprising approximately 5% of incident BC,6, 7, 8 survival for recurrent MBC (rMBC) after an initial diagnosis of early BC is recorded less often, despite being much more common.
A systematic review and meta-analysis of post-metastasis survival estimated improvements in median overall survival (OS) between 1990 and 2010 for dnMBC (20 to 31 months) and rMBC (21 to 38 months) following no improvement in the prior decade.9 However, the analysis included both population-based and cohorts receiving treatment. Survival will differ at a whole-of-population level due to differences in baseline prognostic factors, treatment eligibility and access to services and clinical trials, in particular for older women. Further, survival gains may vary substantially for those with tumours that are hormone receptor or human epidermal growth factor receptor-2 (HER2) positive versus negative, as new and effective targeted therapies are introduced.
Documenting population-level changes in post-metastasis survival is important for understanding the full impact of changes in care on life span and the evolution of MBC prognosis over time. BC stakeholders have highlighted the importance of this evidence to inform individuals with MBC as well as cancer services planning and prioritising further research.10,11 While clinical trials can provide the most valid estimates of treatment efficacy and prognosis for well-defined patient groups, population-level data are needed to assess the survival gains across an entire BC population reflecting the impact of BC services for the whole population.
This systematic review aims to assess: the extent survival has changed at a population-level for dnMBC and rMBC since 1995; and how survival has changed for age, hormone receptor and HER2 subgroups.
Methods
We performed a systematic review and report it according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.12 We used the American Joint Committee on Cancer (AJCC) 8th edition definition of distant metastasis.13 We selected overall survival (OS) and BC-specific survival (BCSS) as primary outcomes. We assessed OS as a measure of actual survival (includes BC and non-BC deaths) most relevant to clinicians and patients14; and BCSS to estimate changes in MBC prognosis over time (BC death only, non-BC deaths censored). Changes in BCSS can be attributed to changes in BC diagnosis and treatment plus any changes in tumour prognostic factors unrelated to BC care. The review was not designed to assess a particular BC treatment or causal pathway. We assessed MBC cohorts since 1995, when tamoxifen was established as adjuvant therapy for those with hormone receptor-positive tumours, to capture potential changes in survival due to the introduction of treatment advances after 2000, such as trastuzumab for those with HER2-positive tumours.
Search strategy and study selection
We searched MEDLINE for peer-reviewed studies published between 1 January 1995 and 12 April 2021 using keywords and MeSH terms including: breast neoplasms/breast cancer AND metastatic/Stage IV AND survival/prognosis AND population-based/registries/time trend/temporal trend (Supplementary 1). Studies were eligible for inclusion if they: used a population-based data source to assemble cohorts of women with MBC by year of BC or MBC diagnosis; assessed survival after MBC diagnosis as OS, BCSS or relative survival for two or more cohorts since 1995, included at least one cohort with a diagnosis since 2000; reported median survival, 2-year or 5-year survival (or survival curves to extract these data); and were reported in English.
Studies that assessed a single time period were eligible if an additional publication using the same population-based data source reported on a second eligible time period. Studies that assessed a review-defined subgroup or specific distant metastatic sites were also included. To exclude superseded studies, where two studies reported survival for the same population and time periods, we included the study that reported the most complete data for the pre-specified outcomes. For studies reporting on the same survival outcome measure for the same population, we included the study that assessed the most recent time period. If two studies of the same population each reported data for different (non-overlapping) pre-specified outcomes (median survival, 5-year survival) or subgroups (eg. age or oestrogen receptor status), we included both studies in the review. Two investigators (KB, SL) reviewed search results independently by scanning titles and abstracts to identify potentially eligible articles. These were retrieved as full text articles to identify eligible studies. Discordant findings were resolved by discussion between the two investigators. We checked citations and reference lists of included studies to identify additional potentially eligible articles.
Data extraction
One investigator extracted study data into tables which were checked by a second investigator (KB, SL). Discordant findings were resolved by discussion. Extracted data included: number of women with MBC overall and within our study periods of interest (≥1995); population characteristics (age, hormone receptor status, HER2 status, adjuvant therapy use); and survival outcomes (OS, BCSS, relative survival) as median survival, 2-year and 5-year survival and hazard ratio (HR) for each MBC cohort and subgroups. If not reported, we extracted survival data from the survival curve where available using WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/).
Assessment of risk of bias and applicability
One investigator assessed the risk of bias, which was checked by a second investigator (SL, NH) based on five domains: study participation; prognostic factor measurement (time periods, age, tumour receptor status); outcome measurement; confounding; and analysis method using criteria adapted from QUIPS 201315 (Supplementary 2). ‘Risk of bias’ refers to the identification of a potential source of bias, not the magnitude. We rated risk of bias for each domain as: ‘high’ (potential source identified); ‘low’ (no potential source); or ‘unclear’ if inadequate information was reported for assessment. We rated the overall risk of bias for each study as high if one or more of the domains was rated as high; and low if all domains were rated as low risk. Studies without a high-risk rating but one or more domains with unclear ratings were classified as unclear for overall risk of bias. We assessed the applicability by appraising whether study participation, prognostic factor(s) and outcome measurement matched the review questions. We rated concerns about applicability as low, high or unclear, using the same approach. Discordant findings for risk of bias and applicability were resolved by discussion and consensus between the two investigators. In addition to the assessment of risk of bias and applicability, we considered precision of estimates and consistency of results between studies for judgements about the strength of the evidence underlying our conclusions.16
Data synthesis
We assessed survival following a dnMBC and rMBC diagnosis separately, using a descriptive approach. Where dnMBC and rMBC were not reported separately, we assessed them together as ‘combined’ MBC and reported%rMBC. For each study, we plotted median and 5-year survival for each time period reported to provide a visual summary of temporal trends. To avoid duplication of data for the same people, where studies reported on the same source population, we extracted data from the study that included the most recent time period and/or longest follow-up period for the survival outcome plotted. To summarise survival changes across studies quantitatively we reported the median and range for survival estimates for the following 5-year MBC diagnosis periods: 1995–1999, 2000–2004, 2005–2009, 2010–2014; and calculated the median absolute difference in survival estimates between these periods to describe changes over time. For this descriptive analysis, we included data from studies that reported survival in each 5-year period or in a period that covered at least 3 years of the period. We also summarised change over time from the study-estimated HR and 95% confidence interval (CI), if available.
We defined subgroups as follows: age at MBC diagnosis (<50, 50–69, ≥ 70 years), hormone receptor status (oestrogen receptor (ER)-positive and/or progesterone receptor (PR)-positive tumours, herein referred to as ER-positive; ER-negative and PR-negative tumours, herein referred to as ER-negative), HER2 status (HER2-positive, HER2-negative) and triple negative. The review was designed to inform our ongoing research program (research protocol: http://dx.doi.org/10.1136/bmjopen-2018-026414).17 The review protocol was not registered.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. SL and KB had access to the bibliographic search database. All authors had access to the included study data and all authors agreed with the final decision to submit for publication.
Results
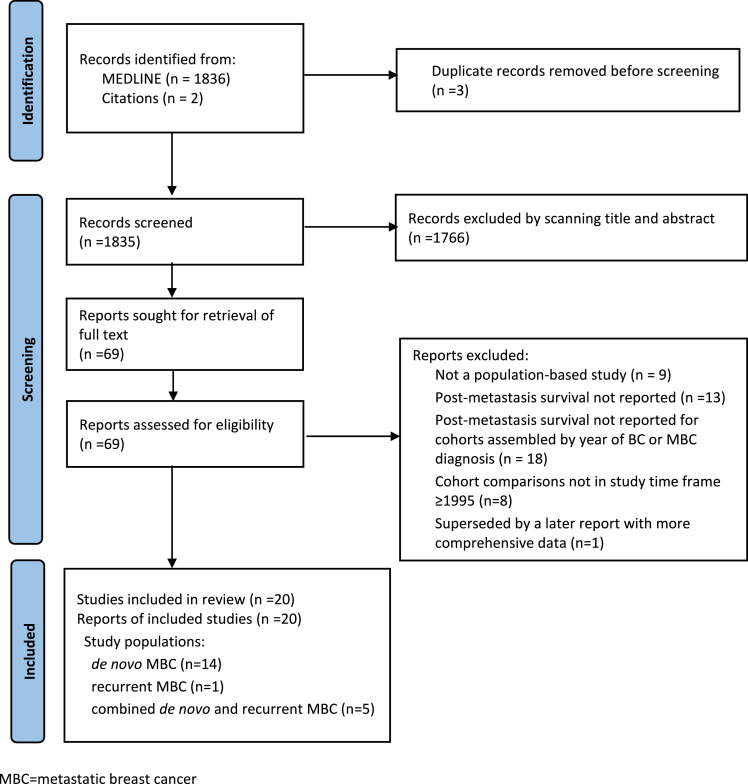
We identified 20 eligible studies assessing dnMBC (14 studies), rMBC (1 study), or ‘combined’ dnMBC and rMBC without stratification (5 studies) (Figure 1, Supplementary 3). Studies assessed city, regional or country-wide populations in North America and Europe, grouping women by their MBC diagnosis date into one to eleven-year periods to estimate OS (16 studies), BCSS (4 studies) or relative survival only (3 studies). Fourteen studies included diagnosis periods commencing from 2005; and five studies included the period 2010–2014 (Table 1, Supplementary 4).
Figure 1.
PRISMA flow diagram of study selection
MBC=metastatic breast cancer.
Table 1.
Characteristics of included studies18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37
 |
 |
 |
 |
Study design characteristics
The 14 studies of dnMBC were conducted in the US (6 studies), Netherlands (4 studies), Germany (1 study), Italy (1 study), Sweden (1 study) and one study conducted in both the US and Germany (Table 1). Of these, five studies assessed age subgroups, including one study restricted to women <40 years.18 Four studies compared ER subgroups, including one study that reported survival stratified by ER status only.19 No studies of dnMBC assessed HER2-positive or triple-negative subgroups. Sample size ranged from 51420 to 22,601.21 The six US studies used data held by the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program registries for 9 to 18 geographical regions (denoted as SEER-9, 13, 18). All six studies used data from at least 9 SEER registries in common, but time periods and eligibility criteria varied, such as: age limits18,19,22,23; exclusion of participants with >1 primary cancer21; inclusion of invasive ductal or lobular tumours only24 (Table 1).
The single study assessing rMBC included two diagnosis periods after 1995 (1998–2006 and 2007–2013, Munich Cancer Registry, N = 5700).25 Five studies of combined MBC, from British Columbia, Canada and three regions in Sweden, included 76% to 89% rMBC (Table 1). One study assessed brain metastasis alone,26 and one study was restricted to ER-positive HER2-negative tumours.28 Other differences in eligibility criteria between studies of combined MBC were exclusion of: women aged >75 years27; those with other cancer27; or contralateral BC27,28 (Table 1). One study assessed age subgroups29; and two studies assessed ER and HER2 subgroups.26,30
Risk of bias and applicability
Of 17 studies reporting adequate information for appraisal, 16 were assessed as having one or more potential sources of bias, most commonly: confounding (10 studies) eg. survival comparisons without consideration of differences in age at BC diagnosis over time; outcome assessment (9 studies) due to shorter follow-up time for women diagnosed in the most recent study period; or study participation (6 studies) eg. exclusion of older women (Supplementary 5). Five studies were assessed as high concern for applicability, most commonly due to no separate assessment of survival for rMBC for studies of combined MBC (4 studies, Supplementary 5).
Participant and treatment characteristics
Studies with no age restrictions reported at least one third of women were aged ≥70 at diagnosis (dnMBC 33–47%,24,31,32 rMBC 36%,25 combined MBC 34%29 from the most recent diagnosis period). Five studies of rMBC and combined MBC reported an increased proportion of women receiving neo/adjuvant chemotherapy or adjuvant endocrine therapy or both since 1995 (Table 1).
MBC survival
De novo MBC
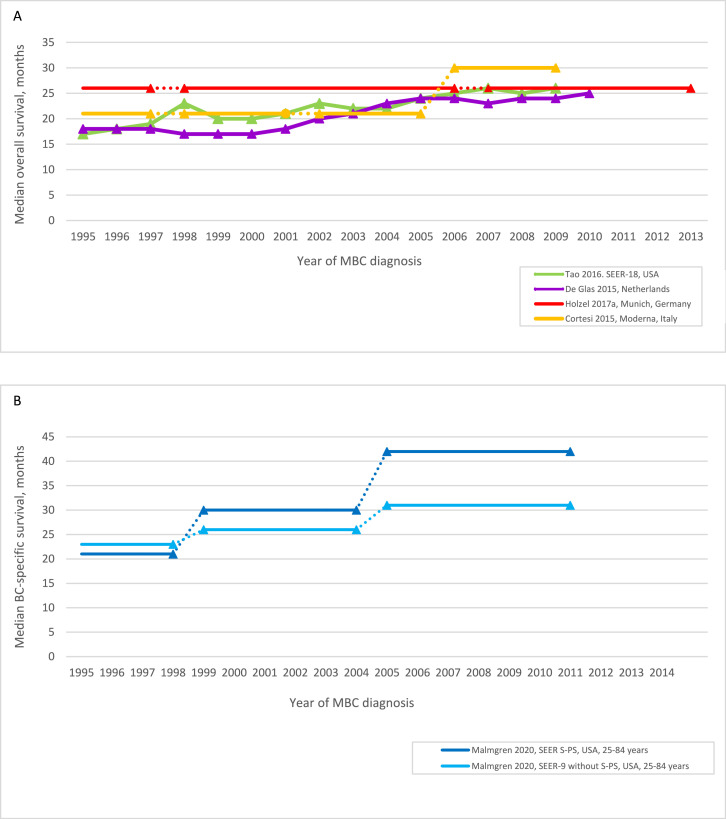
Thirteen of 14 studies of dnMBC reported improved survival (OS, BCSS or relative survival) since 1995, with 12 studies reporting 95% CIs for survival estimates and/or statistical tests for comparisons between time periods (Table 2, Figure 2A-D). Median of median OS was 20 months (range 18–26) for dnMBC diagnosed in 1995–1999; and 26 months (range 24 to 30) for dnMBC diagnosed a decade later in 2005–2009; a median OS gain of 6 months (range 0 to 9 months, 4 studies, Figure 2A).20,31,33,34 Malmgren et al. (2020) provided high quality data for women with dnMBC <85 years and estimated median BCSS gains of 5 and 12 months in two US regions between 1999 and 2004 and 2005–2011 (p<0.001 for comparison of all time periods, Table 2, Figure 2B).22
Table 2.
Changes in post-metastasis survival over time.
| Author, year,MBC diagnosis periods | N | dnMBC or rMBC | Median survival, months (95% CI) | Survival (95% CI) |
Adjusted hazard ratio (HR)(95% CI) | Co-variables for adjusted analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-year | 5-year | ||||||||||
| Malmgren et al. (2020)22 | dnMBC | Median BCSS* | 2-yr BCSS* | 5-yr OS | 5-yr BCSS | HR BCSS | |||||
| SEER 9 without Seattle-Puget Sound | |||||||||||
| 1990–1998 | 3988 | All | 23 | 49% | 16% (15–18) | 19% (18–21) | 1·00 (reference) | Age, race, ER+/PR+ status, surgery, XRT, chemotherapy | |||
| 1999–2004 | 2958 | 26 | 54% | 20% (19–21) | 23% (21–24) | 0·85 (0·80–0·91), p<0·001 | |||||
| 2005–2011 | 4094 | 31 | 60% | 23% (22–24) | 26% (24–27) | 0·72 (0·67–0·76), p<0·001 | |||||
| p<0·001 | p<0·001 | p<0·001 | |||||||||
| Seattle-Puget Sound | |||||||||||
| 1990–1998 | 608 | All | 21 | 44 | 18% (15–21) | 21% (18–24) | 1·00 (reference) | ||||
| 1999–2004 | 481 | 30 | 57 | 25% (21–28) | 27% (23–31) | 0·74 (0·63–0·87), p<0·001 | |||||
| 2005–2011 | 707 | 42 | 66 | 32% (28–35) | 35% (32–39) | 0·57 (0·49–0·66), p<0·001 | |||||
| p<0·001 | p<0·001 | p<0·001 | |||||||||
| Di Meglio et al. (2016)24 | dnMBC | Median OS | HR OS* | ||||||||
| 1990–1992 | NR | All | 18 | 1·00 (reference) | SEER registry, age, race, marital status, tumour grade, ER+/PR+ status, histology | ||||||
| 1993–1995 | 18* | 1·07 | |||||||||
| 1996–1998 | 19* | 1·05 | |||||||||
| 1999–2001 | 21* | 1·00 | |||||||||
| 2002–2004 | 23* | 0·95 | |||||||||
| 2005–2007 | 26* | 0·92 | |||||||||
| 2008–2011 | 28 | 0·85 (0·78–0·93) | |||||||||
| 2008–2011 vs 1990–1992 | p<0·001 | ||||||||||
| Per year 1990–2011 | 0·99 (0·98–0·99), p<0·05 | ||||||||||
| Per 5 years | 0·95 (0·93–0·97), p<0·05 | ||||||||||
| Tao et al. (2016)34 | dnMBC | Median OS* | 3-year relative survival* | ||||||||
| 1995 | NR | All | 17 | 31 | |||||||
| 2000 | 20 | 34 | |||||||||
| 2005 | 24 | 39 | |||||||||
| 2009 | 25 | 39 | |||||||||
| Dawood et al. (2015)21 | dnMBC | 2-yr OS | 2-yr BCSS | Odds ratio per year | |||||||
| 1990–1995 | 4215 | All | 36·2% | 40·1% |
OS >2 years 1·06 (1·05–1·07), p<0·001 BCSS >2 years 1·06 (1·05–1·07), p<0·001 |
Age, race, marital status, tumour grade, MBC site, ER, PR status, surgery, XRT, inflammatory subtype, SEER registry | |||||
| 1996–2000 | 5108 | 40·1% | 44·0% | ||||||||
| 2001–2007 | 13,278 | 44·2% | 48·1% | ||||||||
| p<0·001 | p<0·001 | ||||||||||
| Holleczek et al. (2012)35 | 5-yr relative survival (SE), age standardised | ||||||||||
| SEER-13 USA | dnMBC | ||||||||||
| 1993–1996 | 4926 | All | 20·2 (0·7) | ||||||||
| 1997–2000 | 5386 | 22·5 (0·7) | |||||||||
| 2001–2004 | 5626 | 25·2 (0·7) | |||||||||
| 2005–2008 | 6016 | 26·7 (0·7) | |||||||||
| 2005–2008 vs 1993–1996 | 6·5%, p<0·001 | ||||||||||
| Saarland, Germany | dnMBC | ||||||||||
| 1993–1996 | 228 | All | 20·3 (3·2) | ||||||||
| 1997–2000 | 262 | 22·1 (3·0) | |||||||||
| 2001–2004 | 271 | 19·0 (2·6) | |||||||||
| 2005–2008 | 242 | 23·7 (3·0) | |||||||||
| 2005–2008 vs 1993–1996 | 3·3%, p = 0·11 | ||||||||||
| van der Meer et al. (2021)36 | dnMBC | 5-yr Relative survival (95% CI), age standardised | |||||||||
| 1989–1999 | NR | All | 12·3 (10·1, 14·8) | ||||||||
| 2000–2009 | 20·8 (18·0, 23·8) | ||||||||||
| 2010–2016 | 33·2 (29·4, 37·2) | ||||||||||
| Vonderling et al. (2018)37 | dnMBC | 2-yr OS | 5-yr OS | ||||||||
| 1989–1992 | 1990: 543 | All | 40% | 14% | |||||||
| 1993–1998 | 1995: 493 | 44% | 16% | ||||||||
| 1999–2002 | 2000: 562 | 43% | 17% | ||||||||
| 2003–2009 | 2005: 612 | 53% | 22% | ||||||||
| 2010–2013 | 2010: 573 | 58% | NR | ||||||||
| de Glas et al. (2015)33 | dnMBC | Median OS* | 5-year relative survival | HR OS |
Age at diagnosis, ER/PR status, morphology, grade and number of metastatic sites |
||||||
| 1995 | NR | All | 18 | 17 | 0·99 (0·99–1·00), p<0·001 per year 1990–2010 | ||||||
| 2000 | 17 | 16 | |||||||||
| 2005 | 24 | 22 | |||||||||
| 2010 | 25 | 21 | Adjusted relative survival‡ | ||||||||
| 0·98 (0·98–0·99), p<0·001 | |||||||||||
| Ruiterkamp et al. (2011)32 | dnMBC | Median OS | 2-yr OS* | 5-yr OS* | HR OS | ||||||
| 1995 – 1999 | 2688 | All | 17·0 (16·0–18·4) | 41% | 15% | 1·00 (reference) | Age, primary tumour size, surgery, XRT, systemic therapy | ||||
| 2000 – 2004 | 2916 | 19·3 (18·1–20·4) | 44% | 17% | 0·95 (0·89–1·01), p = 0·081 | ||||||
| 2005 – 2008 | 2427 | 23·4 (21·6–25·0) | 49% | 21% | 0·83 (0·77–0·89), p<0·001 | ||||||
| p<0·001 | |||||||||||
| Holzel et al. (2017a)31 | dnMBC | Median OS* | 2-yr OS* | 5-yr OS /relative survival | HR OS |
Age, primary tumour size, grade, ER+/PR+ status, lymph node involvement, ≥ 2 metastasis site, systemic therapy |
|||||
| 1978–1987 | 376 | All | 25 | 52% | 17·6% /18·9 | 1·10 (0·95–1·26) | |||||
| 1988–1997 | 875 | 26 | 54% | 22·5% /24·7 | 1·05 (0·95–1·15) | ||||||
| 1998–2006 | 1702 | 26 | 54% | 24·7% /27·1 | 1·00 (reference) | ||||||
| 2007–2013 | 1803 | 26 | 53% | 20·5% /22·7 | 1·01 (0·93–1·10) | ||||||
| p = 0·52 | p = 0·77 | ||||||||||
| Nordenskjold et al. (2019)23 | dnMBC | 5-yr relative survival* | 5-year excess mortality rate ratio | ||||||||
| 1989–1993 | 224 | All | 1989–1991: 12 1992–1994: 12 1995–1997: 9 1998–2000: 20 2001–2003: 12 2004–2006: 20 2007–2009: 23 |
1.00 (reference) | Age | ||||||
| 1994–1998 | 200 | 0.98 (0.79–1.21), p = 0.87 | |||||||||
| 1999–2003 | 185 | 0.91 (0.73–1.13), p = 0.40 | |||||||||
| 2004–2008 | 223 | 0.83 (0.67–1.03), p = 0.08 | |||||||||
| 2009–2013 | 260 | 0.69 (0.55–0.86), p = 0.001 | |||||||||
| p = 0.03 | |||||||||||
| Cortesi et al. (2015)20 | dnMBC | Median OS* | 2-yr OS* | 5-yr OS | HR OS | ||||||
| 1990–1993 | 73 | All | 16 | 39% | 11% | 1·00 (reference) | Age, tumour grade, ER, PR status, Ki67, MBC site, chemotherapy, hormone therapy | ||||
| 1994–1997 | 82 | 21 | 46% | 15% | 0·76 (0·42–1·36), p = 0.357 | ||||||
| 1998–2001 | 114 | 21 | 42% | 12% | 0·62 (0·36–1·09), p = 0.097 | ||||||
| 2002–2005 | 126 | 21 | 46% | 20% | 0·58 (0·33–1·00), p = 0.050 | ||||||
| 2006–2009 | 119 | 30 | 51% | 29% | 0·53 (0·30–0·95), p = 0.043 | ||||||
| p = 0·012 | |||||||||||
| Holzel et al. (2017b)25 | rMBC | Median OS* | 2-yr OS* | 5-yr OS* /relative survival | |||||||
| 1978 – 1987 | 1352 | All | 23 | 48% | 20% /22·0 | ||||||
| 1988 – 1997 | 2170 | 21 | 45% | 18% /22·6 | |||||||
| 1998 – 2006 | 3502 | 23 | 49% | 21% / 17·6 | |||||||
| 2007 – 2013 | 3172 | 23 | 48% | 21% /11·8 | |||||||
| p = 0.029 | |||||||||||
| MBC and BC diagnosis within same study period | 4-yr OS* | HR OS | |||||||||
| 1978 – 1987 | 1352 | 23 | 49% | 26% | 0·91 (0·82–0·99) | Age at BC diagnosis, primary tumour size, tumour grade, lymph node involvement, ER status | |||||
| 1988 – 1997 | 1429 | 18 | 39% | 20% | 1·11 (1·03–1·20) | ||||||
| 1998 – 2006 | 1788 | 20 | 43% | 22% | 1·00 (reference) | ||||||
| 2007 – 2013 | 1187 | 14 | 32% | 16% | 1·24 (1·13–1·35) | ||||||
| Le et al. (2020)28 | rMBC | Median OS | 5-yr OS | ||||||||
| 2003 – 2005 | 755 | 77·9% | 24·4 (21·6–27·3) | 18·1% | |||||||
| 2007 – 2009 | 772 | 76·8% | 23·1 (20·7–25·5) | 17·7% | |||||||
| 2011 - 2013 | 905 | 74·1% | 23·1 (20·6–25·6) | 17·3%† | |||||||
| Chia et al. (2007)27 | rMBC | Median OS | 2-yr OS | 5-yr OS* | HR OS | ||||||
| 1991–1992 | 423 | 73% | 14·3 | 33% | 13% | 1·00 (reference) | Age at MBC diagnosis, tumour grade, ER status | ||||
| 1994–1995 | 561 | 82% | 14·8 | 34% | 14% | 0·97 (0·85–1·11), p = 0·65 | |||||
| 1997–1998 | 641 | 80% | 18·5 | 44% | 15% | 0·84 (0·74–0·96), p = 0·011 | |||||
| 1999–2001 | 525 | 78% | 21·7 | 45% | NR | 0·72 (0·61–0·84), p<0·001 | |||||
| 1997–1998 vs 1999–2001, p = 0·05 | |||||||||||
| Sundquist et al. (2017)30 | rMBC | Median OS (IQR) | 2-yr OS | 5-yr OS | |||||||
| 1985 – 1989 | 124 | 76·6% | 13 (4–31) | 31% | 10% | ||||||
| 1990 – 1994 | 147 | 87·1% | 16‡ (6–35) | 38% | 13% | ||||||
| 1995 – 1999 | 160 | 85·6% | 16 (7–36) | 36% | 9% | ||||||
| 2000 – 2004 | 129 | 83·7% | 20 (7–45) | 43% | 15% | ||||||
| 2005 – 2009 | 152 | 81·6% | 23 (6–48) | 49% | 17% | ||||||
| 2010 – 2014 | 72 | 91·7% | 33 (18–55) | 64% | 27% | ||||||
| p = 0·009 | |||||||||||
| Foukakis et al. (2011)29 | rMBC | Median OS | 2-yr OS | 5-yr OS/Relative survival* | HR OS rMBC (dnMBC excluded) | ||||||
| 1979–1984 | 899 | 84% | 15·9 | 36·8% | 11·4%/13 | 1·00 (reference) | Age at MBC diagnosis, primary tumour size, ER status, recurrence-free interval, systemic neo/adjuvant therapy, metastatic site (bone only, liver/brain) | ||||
| 1985–1989 | 1078 | 92% | 15·1 | 36·1% | 12·9%/14 | 0·99 (0·89–1·11), p = 0·90 | |||||
| 1990–1994 | 1158 | 90% | 14·5 | 34·6% | 12·5%/14 | 1·00 (0·89–1·12), p = 1·00 | |||||
| 1995–1999 | 1196 | 87% | 16·1 | 36·2% | 13·6%/15 | 1·00 (0·88–1·12), p = 0·95 | |||||
| 2000–2004 | 1132 | 89% | 15·3 | 37·7% | 15·2%/17 | 0·94 (0·83–1·07), p = 0·34 | |||||
| p = 0·12 | |||||||||||
| Thulin et al. (2020), Brain metastasis26 | rMBC | Median OS | |||||||||
| 1994 – 2004 | 45 | 80% | 11·5 | ||||||||
| 2005 – 2014 | 146 | 84% | 7·2 | ||||||||
BCSS = breast cancer specific survival; CI =confidence interval; dnMBC = de novo MBC; HR = hazard ratio; IQR = Interquartile range; MBC = metastatic breast cancer; OS = overall survival; SE = standard error; rMBC = recurrent MBC; XRT = radiation therapy.
* Extracted from published survival curve or figure; †Excludes women diagnosed after Aug 2012; ‡ Results reported in text differ from published table/figure.
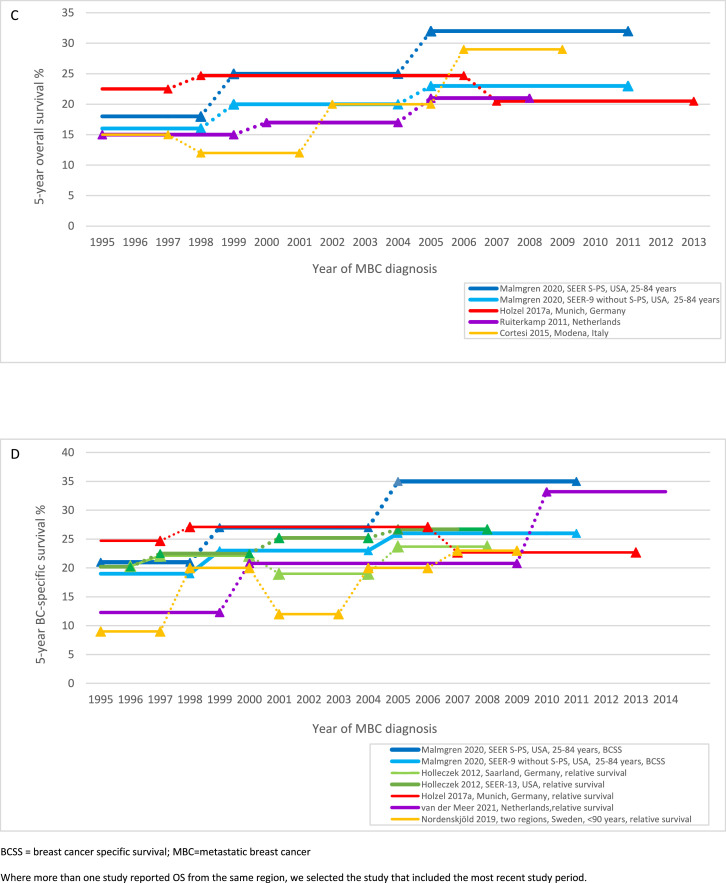
Figure 2.
Survival over time for de novo MBC: (A) median overall survival; (B) median breast cancer-specific survival; (C) 5-year overall survival; (D) 5-year breast cancer-specific survival, relative survival
BCSS = breast cancer specific survival; MBC = metastatic breast cancer
Where more than one study reported OS from the same region, we selected the study that included the most recent study period.
The median probability of OS ≥5 years for dnMBC 1995–1999 was 16% (range 15–23%); and 23% (range 21–32%) for dnMBC 2005–2009, a median gain of 7% (range −2 to 14%, 4 studies, 5 cohorts, Figure 2C).20,22,31,32 Malmgren et al. (2020) assessed both OS and BCSS and reported 5-year BCSS was 2–3 percentage points higher than 5-year OS in the two regions assessed (eg. SEER-9 without Seattle-Puget Sound 2005–2011: 5-year BCSS 26% (95% CI 24–27); 5-year OS 23% (95% CI 22–24)).22 Five-year BCSS for dnMBC increased by 3% to 8% between 1999 and 2004 and 2005–2011 across both regions assessed, similar to the 5-year OS gain of 3 to 7% during the same period (Figure 2C-D, p<0.001 for BCSS and OS comparison of all time periods).22 Another US study of overlapping SEER populations also reported similar 8% gains for 2-year OS (p<0.001) and BCSS (p<0.001).21 Three of four studies assessing relative survival for dnMBC reported 5-year relative survival gains in the US, Germany and the Netherlands between 1995 and 2009 (Figure 2D).23,31,35,36 Two studies from the Netherlands that included dnMBC diagnosed from 2010 reported a further survival gain for this later period (2-year OS 2003–2009 53%, 2010–2013 58%, no statistical test reported37; 5-year relative survival 2000–2009 20·8% (95% CI 18·0- 23·8), 2010–2016 33·2% (95% CI 29·4–37·2)36 (Table 2).
Adjusting for prognostic factors that may change over time such as age and tumour characteristics (ER status, tumour grade and histology), two studies estimated a reduction in the hazard of all-cause death of 1% per year for dnMBC in the US (HR 0·99, 95% CI 0·98–0·99 per year between 1990 and 2011, p<0·05),24 and the Netherlands (HR 0·99, 95% CI 0·99–1·00 between 1990 and 2010, p<0·001).33 An additional three studies of dnMBC reported a statistically significant reduced hazard of all-cause20,32 or BC death22 over time, although these studies were less applicable to our review question because they also adjusted for treatment factors such as surgery, radiotherapy, endocrine therapy and chemotherapy. In contrast, using data from the Munich Cancer Registry, Holzel et al. (2017a) did not find an ongoing OS or relative survival gain for dnMBC between study periods 1998–2006 and 2007–2013, nor evidence of reduced hazard for all-cause death after adjusting for age, primary tumour size, grade, ER status, lymph node involvement, multiple metastasis sites and primary systemic therapy (HR 1·01, 95% CI 0·93–1·10, Table 2).31
Two studies observed no to very small differences in OS and relative survival for women ≥70 years in three geographical regions between 1993/1995 and 2008 (5-year OS 11% for each period, 95% CIs not reported32; difference in 5-year relative survival −1·9, p = 0.71 to +2·1%, p = 0.045, Supplementary 6–7).35 Two additional studies reported relative survival did not improve for women ≥75 years in the Netherlands (relative excess risk per year 1990–2010, 1·01 (95% CI 1·00–1·02), p = 0·2;333 5-year relative survival 2000–2009 21·1% (95% CI 17·7–24·8); 2010–2016 21·1% (95% CI 17·0–25·5).36 Studies observed the largest survival improvements for women <50 years with more modest improvement for women 50–69 years (Supplementary 6–7).32,35,36 A study of women aged <40 years reported a 24% increase in 5-year BCSS between 1995 and 1999 32·2%, (95% CI 26·7–37·9) and 2010–2015 56·5% (95% CI 50·1–62·4).18
Four studies reported longer survival for ER-positive tumours than ER-negative tumours in all study periods (Supplementary 8). Of these, three US studies of overlapping SEER regions observed OS and BCSS improved over time for ER-positive dnMBC.19,21,34 Two of these studies reported 2-year21 and 5-year survival19 also improved over time for ER-negative dnMBC (percentage change per year, 5-year BCSS 1992–2006: ER-positive 2·3% (95%CI 1·0–3·6); ER-negative 3·6% (95% CI 1·3–5·9)).19 The third study reported median OS for ER-negative dnMBC remained stable at around 14 months between 1995 and 2009 (95% CI not reported).34 Holzel et al. (2017a) observed improved median survival between the periods 1988–1997 and 1998–2006 for ER-positive (31, 34 months, respectively) and ER-negative (14, 15 months respectively) but not between 1998 and 2006 and 2007–2013 (ER-positive 30 months, ER-negative 13 months), statistical tests not reported (Supplementary 8–9).31
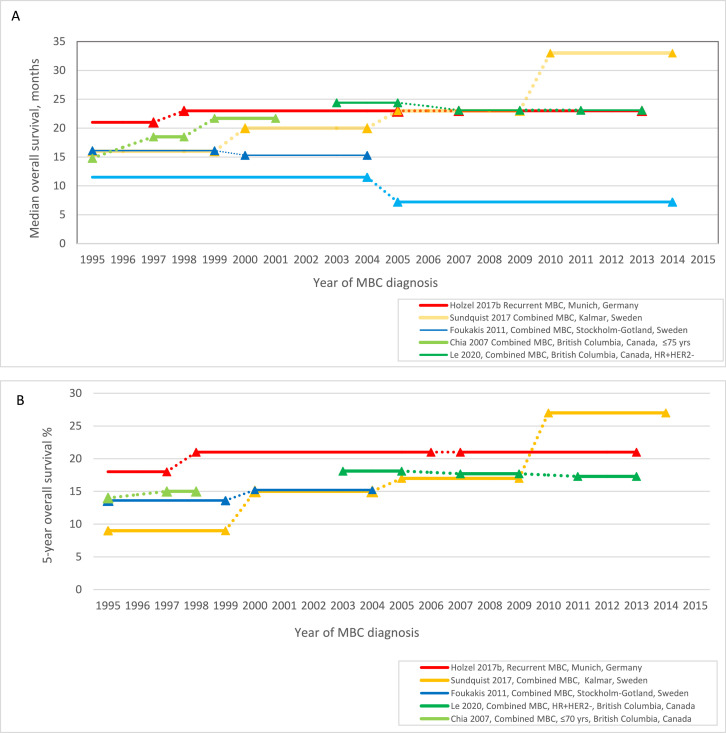
Recurrent MBC
For rMBC, using data from the Munich Cancer Registry, Holzel et al. (2017b) observed OS improved for rMBC diagnosed in 1998–2006 (median OS 23 months; 5-year OS 21%) compared to the prior period 1988–1997 (median OS 21 months; 5-year OS 18%); but no further improvement in 2007–2013 (median OS 23 months; 5-year OS 21%), p = 0.029 for comparison of all time periods, statistical tests were not reported for individual time period comparisons (Table 2, Figures 3A & 3B).25 Taking into account both the period of initial BC diagnosis and period of rMBC diagnosis, women with BC and rMBC both diagnosed 2007–2013 had a 24% higher risk of all-cause death than women with BC and rMBC both diagnosed 1998–2006, adjusting for age at BC diagnosis, tumour size, grade, lymph node involvement and ER status (HR 1·24, 95% CI 1·13–1·35, Table 2).25 As longer time to distant recurrence is associated with improved prognosis, a potential source of bias for this adjusted analysis is the 1998–2006 study period includes women with rMBC within 9 years of BC diagnosis; while the 2007–2013 period is limited to women with rMBC within 7 years.
Figure 3.
Survival over time for recurrent and combined (de novo and recurrent) MBC: (A) median overall survival, (B) 5-year overall survival.
Combined (de novo and recurrent) MBC
Estimates of median OS and 5-year OS from studies of combined MBC are illustrated in Figures 3A and 3B. Comparing MBC cohorts diagnosed between 1995 and 1999 and 2000–2004, two Swedish studies reported different temporal trends for median OS (Foukakis et al. (2011)29 1995–1999 16·1 months, 2000–2004 15·3 months, p = 0.12 for comparison of all time periods since 1979–1984, no potential source of bias identified; Sundquist et al. (2017)30 16, 20 months respectively, p = 0.009 for comparison of all time periods since 1985–1989, potential source of bias identified). For subgroup analysis by age, Foukakis et al. (2011) reported median OS for women aged ≤60 years at diagnosis of MBC improved from 19·1 months in 1995–1999 to 22·3 months in 2000–2004 (p<0.0001 for comparison of all time periods); with no improvement observed for women >60 years (12·7 months, 11·6 months respectively, and reduced survival for comparison of all time periods, p = 0.013 Supplementary 6–7).29 A third study, Chia et al. (2007) that excluded women >75 years estimated a 3 month gain in median OS for MBC diagnosed in British Columbia, Canada between 1997 and 1998 (18·5 months) and 1999–2001 (21·7 months), p = 0.05.27
Comparing MBC cohorts diagnosed after 2000, Sundquist et al. (2017) reported a 13 month gain in median OS between 2000 and 2004 (20 months) and 2010–2014 (33 months), p = 0.009 for comparison of all time periods.30 In contrast, Le et al. (2020) assessed ER-positive, HER2-negative MBC from British Columbia and reported no improvement in median or 5-year OS across three study periods between 2003 and 2005 (median OS 24.4 months (95% CI 21.6–27.3) and 2011–2013 (median OS 23.1 months (95%CI 20·6–25·6)).28 Thulin et al. (2020) reported a reduction in OS for brain metastasis (1994–2004 11·5 months; 2005–2014 7·2 months, statistical test not reported) with no difference in age at BC diagnosis.26 Le et al. (2020)28 and Sundquist et al. (2017)30 did not assess or adjust for age as a potential confounder.
For subgroup analysis by ER and HER2 status: Sundquist et al. (2017) assessed post-metastasis survival by tumour receptor status pre- versus post-2000.30 Median OS remained at 10 months for triple negative tumours but improved for other subgroups with the largest improvement observed for HER2-positive tumours (median OS 14 to 29 months; 5-year OS 2% to 31%, between 1996 and 1999 and 2000–2014, statistical test not reported Supplementary 8–9).
Discussion
This systematic review provides strong evidence that OS and BCSS following de novo MBC have continued to improve at a population-level since 1995 with a relative reduction in the risk of all-cause death of 1% per year across different populations. In contrast, the population-level survival gains observed for cohorts of women with recurrent and combined (recurrent and de novo) MBC before 2000 were not consistently observed in more recent cohorts, despite the introduction of new therapies for metastatic disease. These findings have implications for all stakeholders in advanced breast cancer, including cancer clinical services and surveillance systems, as discussed below in our interpretation of the evidence.
All included studies were conducted in North America or Europe highlighting the gap in evidence for post-metastasis survival in low- and middle-income countries. Further, our finding of variation in rMBC survival between regions demonstrates the importance of region-specific data. For high-income countries with similar health systems and available treatments, our review also provides valuable evidence about the current population characteristics of MBC. From included studies, we estimate at least three-quarters of new MBC diagnoses at a population level are recurrences following an initial diagnosis of early BC (study range 76–89%). Our review indicates that at least one third of women are 70 years or older at MBC diagnosis (de novo or recurrent). This finding is important given our review shows consistent evidence for both de novo and recurrent MBC of no to modest survival improvement over time for this older age group. Consequently, there is a widening gap in prognosis between younger versus older women with recent survival gains largely restricted to women under 70 years.
From the limited data available for subgroup analysis by ER and HER2 status, we observed population-level survival gains for MBC for both ER-positive and ER-negative subtypes. However, survival remained higher for ER-positive versus ER-negative tumours at each time period. One Swedish study provided evidence that the introduction of targeted therapies for HER2-positive MBC (of whom few rMBC received adjuvant HER2-targeted therapy) has translated to substantial population-level benefits with the median survival for this group similar to that for ER-positive MBC after 2000.30 The same study did not find evidence of improved survival for triple negative MBC.
For dnMBC, our finding of improved survival since 1995 across 13 of 14 studies is consistent with Caswell-Jin et al's estimate from a meta-analysis of an 8 month improvement in median OS from 2000 to 2010.9 The conflicting finding from one study of no survival gain from 1998 to 2006 to 2007–201331 may be explained in part by changes in the prognostic characteristics of the study dnMBC population over time that were not shared by other studies. For example, the proportion of women aged ≥70 years at dnMBC diagnosis increased from 36·4% to 46·9% during this period, whereas other studies did not observe an increase in age at dnMBC over time. Holzel et al's (2017a) finding of a survival improvement for the subgroup assembled with similar age and tumour prognostic characteristics (6 month gain in median survival) support this explanation; while their age-adjusted analysis did not, but was potentially limited by shorter follow-up time for the final cohort.31 Other included studies of dnMBC were also appraised as having one or more potential sources of bias. Nevertheless, the consistency of results for OS and BCSS across these studies support conclusions that advances in the diagnosis and management of de novo MBC have translated to incremental population-level survival gains.
Our review findings for rMBC and combined MBC (which is mostly comprised of rMBC) are more challenging to interpret. The variation in survival trends between populations is an important finding in itself. Evidence of no or limited OS gain in study periods after 2000 do not align with Caswell-Jin et al.’s (2018) review estimate of a 12 month improvement in median OS for rMBC between 2000 and 2010,9 possibly reflecting the different review approaches. Caswell-Jin et al. included trial and single institution populations, while our review focused on changes in rMBC survival in the entire MBC population, which includes older patients and those with co-morbidities who may not be eligible for clinical trials and may not tolerate standard treatment protocols.
Together with evidence of the increased use of adjuvant therapy since 1995, our finding of no to limited improvement in prognosis for rMBC in some populations provides some support for the postulation from clinical trials of an ‘adjuvant therapy-related shortening of survival’ (ATRESS) effect in pre-treated patient cohorts.38,39 Recent institution-based cohort studies of MBC treatment also provide evidence of this effect.40,41 Proposed causal pathways include the prevention of treatment sensitive metastases in pre-treated patients, leading to an apparent shift to more aggressive metastases as treatment non-sensitive tumours dominate; and potentially adjuvant therapy induces treatment resistance.38 At a molecular level, evidence of genomic differences between rMBC and treatment-naive dnMBC point to biological differences requiring further investigation to help elucidate the mechanisms for differences in prognosis.42
Our descriptive approach for evidence synthesis does not provide pooled estimates of changes in survival from MBC. However, as the diagnosis periods, exclusion criteria, and survival measures all varied between studies, only a descriptive approach was possible. The two major limitations for addressing our review questions were the paucity of published population-based contemporary data for rMBC; and the methodological limitations of the studies included in our review. The most common potential sources of bias in our included studies were: comparisons of survival over time without adjustment for age at BC diagnosis; and a shorter follow-up time, and thereby potentially more unreliable survival estimate, for the most recent study cohort. For ageing populations, the median age at BC diagnosis can be expected to increase,43 increasing the risk of both BC and non-BC death. Other sources of bias were the exclusion of older women and those with prior non-breast cancers, two groups which make up a sizeable proportion of MBC populations. Most studies assessed OS without separate assessment of BCSS. While OS is important as a measure of actual survival, changes over time reflect changes in survival from both cancer and non-cancer causes.14 Thus, assessment of BCSS is also needed to assess the impact of changes in BC care without the potential for confounding by changes in comorbidities over time. Even so, we found that where both were reported, BCSS gains were proportionally similar to OS gains. No studies assessed BCSS for our subgroup analysis of post-metastasis survival for women ≥70 years. However, we believe our finding of no to very limited survival gain for this age group is more plausibly explained by limited benefit from advances in BC therapy over time than an increase in non-cancer mortality.
Our findings have implications for clinicians, cancer service planning and the design of clinical trials. Clinicians can use this evidence to inform prognostic discussions for patients with a new diagnosis of MBC. Our finding of consistent evidence of survival improvement over time for women with de novo MBC adds to evidence from more selected BC populations and may provide hope to women diagnosed today as they are likely to live longer than published survival times which represent women often diagnosed 10 years earlier. For cancer service planning, our findings highlight the importance of tracking the prognosis and care needs of women with rMBC, as distinct from dnMBC. While the clinical benefits of adjuvant therapies to reduce the risk of rMBC and improve BC survival are well established4,5; our findings have important clinical implications for those with a diagnosis of rMBC. As new adjuvant therapies are introduced, the characteristics of the rMBC population are expected to change. For example, as practice has changed to incorporate HER2-targeted therapies in the adjuvant setting, most women with HER2-positive rMBC will have previously received HER2-targeted therapies. This contemporary population may be more resistant to further HER2-targeted therapy than a population such as assessed by Sundquist et al. (2017)30 where many did not receive HER2-targeted therapy in the adjuvant setting. The lack of survival improvement over time for older women will help inform decisions and timing on palliative care and appropriate accessible services. For clinical trial design, our findings establish the importance of stratifying randomisation by de novo/recurrent MBC status and by age group; and the need for more trials in older women to identify optimal treatment strategies.
For research implications, given rMBC represents the large majority of MBC diagnoses and thus clinical load, our finding that most population-based studies of MBC assess dnMBC alone represents an important evidence gap, and highlights the limitations of current reporting of MBC data by cancer registries. Further research is needed to understand the extent new adjuvant therapies may shift the biological profile of recurrent MBC toward more treatment resistant disease. Most MBC trials of new therapies are conducted before the treatment is tested in the adjuvant setting, thus observational data are essential to assess how new adjuvant therapies change the characteristics of rMBC. To provide high quality evidence for these investigations, we advocate for cancer registries to include and validate notifications of distant metastasis in women with an initial diagnosis of early BC. Routine reporting of rMBC by cancer registries with population-based record linkage to treatment data and death registrations would allow critical post-marketing surveillance of new therapies and outcomes in the real-world setting.
In conclusion, this review presents strong evidence of population-level improvements in OS and BCSS following dnMBC since 1995. In contrast, we found limited and conflicting evidence of changes in survival following rMBC, which represents the majority of new diagnoses of MBC. In addition, we identified no to modest survival improvement over time for women aged 70 years and older, who make up around a third of the MBC population. These findings establish the importance of the stratification of MBC by de novo/recurrent status and age group for clinical trial design; and suggest that routine reporting on distant metastases by cancer registries would support future research on the impact of new adjuvant therapies on the characteristics and prognosis of rMBC.
Declaration of interests
SJL received grant funding for research support from the National Health and Medical Research Foundation (NHMRC). BEK received funding for: advisory board participation (Roche and Gilead); an educational presentation (Novartis); meeting registration fees (Novartis). SAP received funding for post-market surveillance research (AbbVie). JB received funding for conference attendance (Novartis). NH received grant funding for research support from: the National Breast Cancer Foundation (NBCF) and NHMRC; and honoraria for journal editorial work (Elsevier).
All remaining authors declare no competing interests.
Acknowledgments
Contributors
SL and NH conceived the design of the systematic review. SL and KB screened abstracts and full texts for eligibility, conducted data extraction and drafted the original manuscript. NH checked the appraisals of risk of bias and applicability concerns. All authors contributed to the interpretation of study data, edit and review of the final manuscript.
Data sharing statement
Data extraction tables for review tables and figures are available from SL.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101282.
Appendix. Supplementary materials
References
- 1.The surveillance, epidemiology, and end results (SEER) program, national cancer institute (NCI). Cancer Stat Facts: female breast cancer https://seer.cancer.gov/statfacts/html/breast.html. Accessed 11 April 2021.
- 2.Cancer Research UK. Breast Cancer (C50), European Age-Standardised Mortality Rates per 100,000 Population, UK, 1971-2018 https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/mortality#heading-Two. Accessed 12 April 2021.
- 3.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouridsen H.T., Bjerre K.D., Christiansen P., Jensen M.B., Moller S. Improvement of prognosis in breast cancer in Denmark 1977-2006, based on the nationwide reporting to the DBCG Registry. Acta Oncol. 2008;47(4):525–536. doi: 10.1080/02841860802027009. [DOI] [PubMed] [Google Scholar]
- 5.Yerushalmi R., Woods R., Kennecke H., Speers C., Knowling M., Gelmon K. Patterns of relapse in breast cancer: changes over time. Breast Cancer Res Treat. 2010;120(3):753–759. doi: 10.1007/s10549-009-0510-2. [DOI] [PubMed] [Google Scholar]
- 6.The Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute (NCI). Breast cancer, stage distribution of SEER incidence cases, 2008-2017. https://seer.cancer.gov/statfacts/html/breast.html. Accessed 12 April 2021.
- 7.Cancer Research UK. Breast Cancer (C50), Proportion of Cases Diagnosed at Each Stage, All Ages, England 2014, Scotland 2014-2015 and Northern Ireland 2010-2014 https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive#ref-5. Accessed 12 April 2021.
- 8.Australian Institute of Health and Welfare 2019. Cancer in Australia 2019. Cancer series no.119. Cat. no. CAN 123. Canberra: AIHW.
- 9.Caswell-Jin J.L., Plevritis S.K., Tian L., et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr. 2018;2(4):pky062. doi: 10.1093/jncics/pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10."I'm still here." Insights into living – and dying – with Advanced Breast Cancer in New Zealand. Breast Cancer Foundation New Zealand. Sept 2018. Available at: https://breastcancerregister.org.nz/images/assets/3120/1/bcfnz-abc-report-2018-reprint-10.2018.pdf. Accessed 23 September 2021.
- 11.Advanced Breast Cancer (ABC) Global Alliance. ABC Global Charter 2018. Available at: https://www.abcglobalalliance.org/wp-content/uploads/2018/06/ABC-Global-Charter-Booklet-June-2018-Final.pdf. Accessed 1 October 2021.
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hortobagyi G.N., Connolly J.L., D'Orsi C.J., et al. In: American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. Amin MB, Edge S, Greene F, et al., editors. Springer; New York, NY: 2017. Breast; pp. 589–636.https://cancerstaging.org/references-tools/deskreferences/Pages/Breast-Cancer-Staging.aspx Available at. Last updated March 13, 2018. Accessed 5 July 2021. [Google Scholar]
- 14.Howlader N., Mariotto A.B., Woloshin S., Schwartz L.M. Providing clinicians and patients with actual prognosis: cancer in the context of competing causes of death. J Natl Cancer Inst Monogr. 2014;2014(49):255–264. doi: 10.1093/jncimonographs/lgu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden J.A., van der Windt D.A., Cartwright J.L., Cote P., Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 16.Iorio A., Spencer F.A., Falavigna M., et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 17.Lord S.J., Kiely B.E., Pearson S.A., et al. Metastatic breast cancer incidence, site and survival in Australia, 2001-2016: a population-based health record linkage study protocol. BMJ Open. 2019;9(2) doi: 10.1136/bmjopen-2018-026414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo F., Kuo Y.F., Shih Y.C.T., Giordano S.H., Berenson A.B. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer. 2018;124(17):3500–3509. doi: 10.1002/cncr.31638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L., Linden H.M., Anderson B.O., Li C.I. Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res Treat. 2014;147(3):609–616. doi: 10.1007/s10549-014-3112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortesi L., Toss A., Cirilli C., et al. Twenty-years experience with de novo metastatic breast cancer. Int J Cancer. 2015;137(6):1417–1426. doi: 10.1002/ijc.29503. [DOI] [PubMed] [Google Scholar]
- 21.Dawood S., Haaland B., Albaracin C., et al. Is the proportion of patients diagnosed with synchronous stage IV breast cancer who survive more than two years increasing over time? Oncology. 2015;89(2):79–87. doi: 10.1159/000371746. [DOI] [PubMed] [Google Scholar]
- 22.Malmgren J.A., Calip G.S., Atwood M.K., Mayer M., Kaplan H.G. Metastatic breast cancer survival improvement restricted by regional disparity: surveillance, Epidemiology, and End Results and institutional analysis: 1990 to 2011. Cancer. 2020;126(2):390–399. doi: 10.1002/cncr.32531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordenskjold A.E., Fohlin H., Arnesson L.G., et al. Breast cancer survival trends in different stages and age groups - a population-based study 1989-2013. Acta Oncol. 2019;58(1):45–51. doi: 10.1080/0284186X.2018.1532601. [DOI] [PubMed] [Google Scholar]
- 24.Di Meglio A., Freedman R.A., Lin N.U., et al. Time trends in incidence rates and survival of newly diagnosed stage IV breast cancer by tumor histology: a population-based analysis. Breast Cancer Res Treat. 2016;157(3):587–596. doi: 10.1007/s10549-016-3845-5. [DOI] [PubMed] [Google Scholar]
- 25.Holzel D., Eckel R., Bauerfeind I., et al. Improved systemic treatment for early breast cancer improves cure rates, modifies metastatic pattern and shortens post-metastatic survival: 35-year results from the Munich Cancer Registry. J Cancer Res Clin Oncol. 2017;143(9):1701–1712. doi: 10.1007/s00432-017-2428-0. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thulin A., Ronnerman E., Zhang C., et al. Clinical outcome of patients with brain metastases from breast cancer - A population based study over 21 years. Breast. 2020;50:113–124. doi: 10.1016/j.breast.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chia S.K., Speers C.H., D’Yachkova Y., et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 28.Le D., Speers C., Thompson L., Gondara L., Nichol A., Lohrisch C. The impact of new systemic therapies on survival and time on hormonal treatment in hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: a population-based study in British Columbia from 2003 to 2013. Cancer. 2020;126(5):971–977. doi: 10.1002/cncr.32631. [DOI] [PubMed] [Google Scholar]
- 29.Foukakis T., Fornander T., Lekberg T., Hellborg H., Adolfsson J., Bergh J. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res Treat. 2011;130(2):553–560. doi: 10.1007/s10549-011-1594-z. [DOI] [PubMed] [Google Scholar]
- 30.Sundquist M., Brudin L., Tejler G. Improved survival in metastatic breast cancer 1985-2016. Breast. 2017;31:46–50. doi: 10.1016/j.breast.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Holzel D., Eckel R., Bauerfeind I., et al. Survival of de novo stage IV breast cancer patients over three decades. J Cancer Res Clin Oncol. 2017;143(3):509–519. doi: 10.1007/s00432-016-2306-1. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiterkamp J., Ernst M.F., de Munck L., et al. Improved survival of patients with primary distant metastatic breast cancer in the period of 1995-2008. A nationwide population-based study in the Netherlands. Breast Cancer Res Treat. 2011;128(2):495–503. doi: 10.1007/s10549-011-1349-x. [DOI] [PubMed] [Google Scholar]
- 33.de Glas N.A., Bastiaannet E., de Craen A.J., et al. Survival of older patients with metastasised breast cancer lags behind despite evolving treatment strategies–a population-based study. Eur J Cancer. 2015;51(3):310–316. doi: 10.1016/j.ejca.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Tao L., Chu L., Wang L.I., et al. Occurrence and outcome of de novo metastatic breast cancer by subtype in a large, diverse population. Cancer Causes Control. 2016;27(9):1127–1138. doi: 10.1007/s10552-016-0791-9. [DOI] [PubMed] [Google Scholar]
- 35.Holleczek B., Brenner H. Trends of population-based breast cancer survival in Germany and the US: decreasing discrepancies, but persistent survival gap of elderly patients in Germany. BMC Cancer. 2012;12:317. doi: 10.1186/1471-2407-12-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Meer D.J., Kramer I., van Maaren M.C., et al. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017. Int J Cancer. 2021;148(9):2289–2303. doi: 10.1002/ijc.33417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vondeling G.T., Menezes G.L., Dvortsin E.P., et al. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer. 2018;18(1):262. doi: 10.1186/s12885-018-4158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fink M.K., Kleeberg U.R., Bartels S. Adjuvant therapy-related shortening of survival (ATRESS): an underrated phenomenon. Oncologist. 2015;20(1):88. doi: 10.1634/theoncologist.2014-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierga J.Y., Asselain B., Jouve M., et al. Effect of adjuvant chemotherapy on outcome in patients with metastatic breast carcinoma treated with first-line doxorubicin-containing chemotherapy. Cancer. 2001;91(6):1079–1089. doi: 10.1002/1097-0142(20010315)91:6<1079::aid-cncr1103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Malmgren J.A., Mayer M., Atwood M.K., Kaplan H.G. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990-2010. Breast Cancer Res Treat. 2018;167(2):579–590. doi: 10.1007/s10549-017-4529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jurrius P., Green T., Garmo H., et al. Invasive breast cancer over four decades reveals persisting poor metastatic outcomes in treatment resistant subgroup - the "ATRESS" phenomenon. Breast. 2020;50:39–48. doi: 10.1016/j.breast.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrido-Castro A.C., Spurr L.F., Hughes M.E., et al. Genomic characterization of de novo metastatic breast cancer. Clin Cancer Res. 2021;27(4):1105–1118. doi: 10.1158/1078-0432.CCR-20-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bidoli E., Virdone S., Hamdi-Cherif M., et al. Worldwide age at onset of female breast cancer: a 25-year population-based cancer registry study. Sci Rep. 2019;9(1):14111. doi: 10.1038/s41598-019-50680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.