Abstract
Background
Mindfulness-based intervention (MBI) as a psychological treatment is adopted in the sports field, but its effect during competition has not been explored. This study investigated the acute effect of a brief MBI on athletes’ cognitive function after a 45-min, lab-based soccer protocol.
Methods
In a single-blind randomized counter-balanced crossover design, 17 male soccer players completed two main trials—an MBI trial and a control trial. The MBI trial was provided with a brief MBI after 45-min exercise; the control trial was instead assigned a travel-related audio to listen to at that time. In each main trial, cognitive function (i.e., Stroop task for inhibition; Corsi-block tapping task for working memory), salivary cortisol, blood lactate and mental fatigue were measured at baseline (pretest) and after the intervention (posttest). The cerebral oxygenation status was recorded using functional near-infrared spectroscopy during the cognitive function test.
Results
The brief MBI improved working memory performance in terms of both reaction time (pre vs. post, P = 0.02, d = 0.71) and accuracy (pre vs. post, P = 0.009, d = 0.58), supported by eliciting increased oxyhemoglobin concentration in the prefrontal cortex of the brain. Whereas a slightly better cognitive performance for MBI trial than control trial at posttest (P = 0.37, d = 0.32) accompanied by a lower oxyhemoglobin concentration. A lower mental fatigue level (P = 0.05, d = 0.6) and lower cortisol concentration (P = 0.04, d = 0.65) were observed in the MBI trial than in the control trial after the intervention at posttest. The decreased cortisol concentration correlated with increased inhibition performance in the MBI trial.
Conclusion
The acute effect of MBI on athletes’ mental fatigue and cortisol concentration was detected, and the beneficial effect on working memory was preliminarily supported. In general, MBI is recommended to be adopted at half-time of a soccer game.
Keywords: Mindfulness, Soccer, Half-time, Cognition, Functional near-infrared spectroscopy, Hemodynamics
1. Introduction
The competitive demands of many intermittent team sports require two consecutive stages of play to be split at half-time. Half-time can be viewed as the recovery period after the first half, the preparation period for the second half, or the transition period between the two halves. During the half-time period, acid-base balance and glycemic response changes may further affect performance in the initial stage of the second half.1 The total distance covered and the distance covered at high speed were shown to be reduced in the first 15-min of the second half when compared with the corresponding period of the first half.2 In addition to reporting diminished physical output, studies have observed impaired cognitive performance between the first and second halves. Specifically, the increase in response accuracy observed during the first 30-min of intermittent exercise was attenuated in the first 15-min of the second half.1,3 Therefore, half-time practices appear to influence subsequent performance during the initial stages of the second half.1 Adopting appropriate recovery strategies at half-time to help players service their cognitive needs and restore their physical strength could help maintain or even improve performance in the initial stage of the second half.
Although strategies such as nutrient-rich fluid intake, tactical discussion, and re-warm-up are widely used during the half-time break in team sports, psychological interventions such as mindfulness-based interventions (MBIs) have received increasing attention. In contrast to most forms of psychological interventions (e.g., goal setting, imagery, and self-talk) which directly aim to change dysfunctional thoughts and emotions,4 MBI can be grouped under the concept of mindfulness aiming to change the relationship between physiological and psychological states. Mindfulness is defined as “the awareness that emerges through paying attention on purpose, in the present moment, and nonjudgmentally to the unfolding of experience moment by moment."5 It involves close observation of all experiences that arise, with an attitude of acceptance, openness, and willingness and without impulsive attempts to change or escape them, even if they are unpleasant or unwanted.6 MBI includes an assortment of meditative practices, including mindful breathing and body scan, with the essential elements involving open-minded attention, awareness, nonjudgment and experiential acceptance.7 The MBI programs typically last for 8–12 weeks, with the effects of reducing stress, anxiety, burnout, and aggression.7, 8, 9 Because of the great intervention demands, short-term or brief MBIs have been designed and verified. In general, a short-term MBI lasted for 5–20 min per session and was reported to reduce negative mood and various types of anxiety in a review study.10 Other benefits of such interventions have also been documented. Randomized controlled trials have revealed that a 10–20 min MBI involving a body scan contemplation improved performance in pain coping.11,12 In addition, May et al.13 observed that a 15-min MBI positively affected cardiovascular modulation in a randomized controlled trial.
Accordingly, MBIs offer a promising psychological recovery strategy for cognitive performance that can be provided during the half-time break. The acute positive effects of such interventions on cognitive function were demonstrated.14 Kozasa et al.15 found that when comparing brain activation (i.e., by fMRI) in the inhibition-related task (i.e., Stroop task) between MBI practitioners and non-MBI practitioners, MBI practitioners increased the efficiency of networks recruited during the attention and impulse control demands of the task. Mrazek et al.16 observed that MBI improved the working memory capacity by reducing mind wondering. Van and Jha17 found that MBI influences information processing in the working memory task via improved information quality and reduction response conservativeness but no changes in decisional factors. Hölzel et al.18 proposed four mechanisms through which mindfulness mediates its effects: attention regulation, body awareness, emotion regulation (including reappraisal and exposure, extinction, and reconsolidation), and change in perspective on the self. Among them, attention regulation was theorized to promote sustained and selective attention and executive control of cognitive resources. In the sports context, Zhu et al.19 adopted a brief MBI coupled with carbohydrate intake during the half-time break to improve athletes' cognitive function in the second half of a simulated soccer-specific field test. Although beneficial effects have been observed, effects unique to brief MBIs have yet to be investigated in team sports. In team sports, cognitive performance plays a crucial role in sports performance because motor actions must be constantly monitored and controlled amid rapidly occurring changes (e.g., ball and player positions) in the field.20 Additionally, excellent visuospatial working memory in space is required to cooperate with teammates, choose positions, and anticipate possible game options.21 Therefore, the first objective of this study was to investigate the acute effect of a brief MBI during half-time on soccer players' cognitive function.
In addition to verifying the influence of an MBI on cognitive function, understanding the mechanism underlying such an effect is vital. Changes in oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) levels in the prefrontal cortex may be a mechanism explaining cognitive performance changes. Regarding MBIs, a functional magnetic resonance imaging (fMRI) study indicated that MBIs regulate prefrontal cortex activity and that this area is closely associated with cerebral oxygenation.22 Deepeshwar et al.23 observed significantly increased O2Hb concentrations in the right prefrontal cortex accompanied by shorter reaction times during the Stroop task after overseeing a 20-min MBI. Exercise also plays a role in cerebral oxygenation changes.24 One meta-review determined that prefrontal oxygenation levels, measured through functional near-infrared spectroscopy (fNIRS), exhibited a quadratic response to incremental exercise.25 Such levels (i.e., O2Hb) increased as the exercise intensity increased from moderate to strenuous and then decreased at extremely high-intensity levels.25 Therefore, the second objective of this study was to examine the mechanisms associated with brief MBI effects on cognitive performance during the initial stage of the second half in soccer games. As MBI is a psychological intervention that potentially affects mental parameters, mental fatigue and salivary cortisol were included to explore the effect of MBI on mental fatigue and/or the potential role of cortisol on mental fatigue and cognition. We hypothesized that an MBI during half-time would benefit cognitive function in the initial stage of the second half and be associated with higher O2Hb concentrations in the prefrontal cortex.
2. Methods
2.1. Participants
Twenty right-handed male student-athletes from a university soccer team, with 17 completing the entire trial (age: 20.7 ± 3.2 years; height: 175.1 ± 5.0 cm; weight: 65.8 ± 7.8 kg; maximal oxygen consumption [VO2max]: 59.3 ± 5.0 mL/kg/min; heart rate maximum [HRmax]: 183.4 ± 12.6 beats/min; body mass index [BMI]: 21.4 ± 2.2 kg/m2). Participants were instructed to complete a medical history questionnaire before the trial, and those with a history of injury within two months of the study or taking medication were excluded. All participants were informed of the experimental procedures and potential risks and voluntarily signed the consent forms. No participant reported MBI-related experience. G∗power (version 3.1.2; Universität Düsseldorf, Germany) was used to calculate the required sample size in accordance with one previous study.14 Specifically, a sample size that could enable the detection of a moderate to large effect size with 90% power and a two-tailed probability level of 0.05 while assuming a 20% dropout rate. This study was approved by a university human research ethics committee (Ref. no. 2019-2020-0384).
2.2. Measures
2.2.1. Behavioral outcomes
A test battery of cognitive function comprising the Stroop effect task (ST)26 and Corsi block-tapping task (CBT)27 in an event design was administered from one computer. Before taking the test, participants were asked if they could remember the practice (i.e., ST and CBT) from the preliminary trial. If participants answered that they were unfamiliar with it, they could practice the tasks once and then take a 1-min break before the formal test. The task order was performed consistently to prevent the carry-over effect of emotional state and regulation on domain-general cognitive control.28,29 The tests were conducted in a room with silence and darkness, and the computer screen was set bright and comfortable. The distance and angle at which participants looked at the screen were approximately 50–70 cm and approximately 15° below the horizontal line, adjusted for each participant's vision and height. In addition, the light was dimmed to minimize external interference and improve the view of the screen. One researcher sat at the participants' back to remind the cognitive tasks began and set markers in fNIRS simultaneously (one marker at the beginning and another marker at the end of each task, including ST congruent, ST incongruent and CBT). The response of each participant was recorded by the battery automatically, with the accuracy and corrected reaction time were obtained for data analysis. The battery has been described and applied successfully in several studies24,29,30 and briefly summarized as follows.
The ST is a commonly used cognitive test; both congruent and incongruent conditions were included in the test in this study. The congruent condition involved presenting a color word in the same color as the color it described. The incongruent condition involved presenting the color word in a different color to the color it described. Instead of reading the word itself, the participants were asked to click on the color of the font. The congruent condition contained 14 stimuli and the incongruent condition contained 20 stimuli, and the choices remained on the screen until the participants responded with an inter-stimulus interval of 1-sec. In the CBT, nine identical, spatially separated blocks were randomly tapped in a specific sequence by the computer. The participants were required to remember and tap these blocks in the same sequence shown by the computer. There were 12 stimuli in the task, and the choices remained on the screen until the participants responded with an inter-stimulus interval of 1-sec.
2.2.2. Physiological outcomes
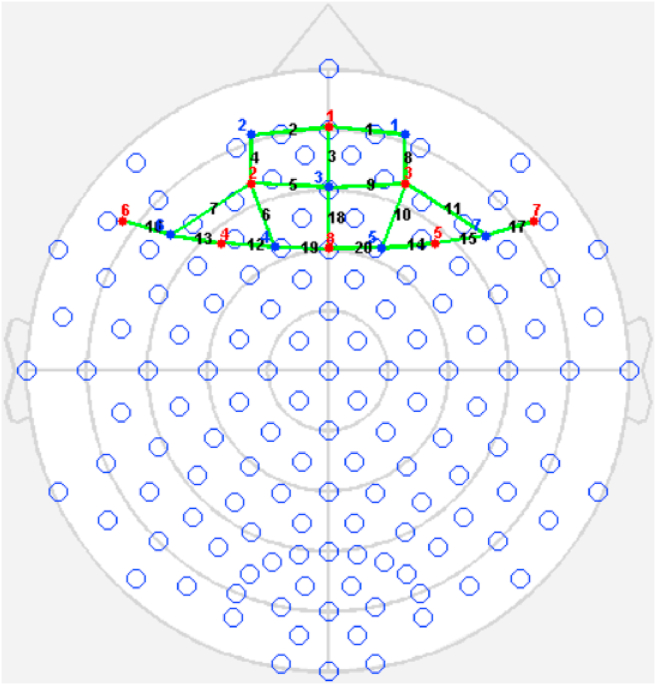
Cerebral oxygenation was monitored using a continuous-wave fNIRS system with dual-wavelength (760 and 850 nm) and 3-cm source-detector separation comparison channels (NIRx Medical Technologies LLC, New York, US). The detectors were located across the dorsal and anterior prefrontal cortex. Data were collected at 7.81 Hz. The system provided eight light sources and eight detectors, of which eight sources and seven detectors were used, amounting to a 16-channel arrangement (see Fig. 2). On the basis of the international 10–20 system,31 the cap was positioned on each participant's forehead by centering the bottom of the probe at the Fpz position. The NIRStar acquisition software (NIRx Medical Technologies, LLC, New York, USA) was used to acquire fNIRS data and evaluate the signal-to-noise ratio.
Fig. 2.
Probe setup.
Salivary cortisol. Oral swabs (Cortisol-Savivette, SARSTEDT AG & Co, Germany) specially designed for cortisol determination from saliva were used to collect salivary samples. The swabs were positioned under the front of the tongue to collect saliva and then removed from the mouth and inserted into a syringe barrel. After centrifuged (Sorvall ST 8 R, Thermo-fisher Scientific, Pittsburgh, USA), the saliva sample was stored at −80 °C in an ultra-low-temperature freezer (MDF-682, Panasonic, Osaka, Japan) and analyzed within 30 days. According to the manufacturer's instructions, salivary cortisol levels were assessed using commercial enzyme-linked immunosorbent assay kits (HePeng Biotech., Ltd, Shanghai, China).
Blood lactate concentration was used to monitor the exercise intensity in two main trials and measured via a portable lactate analyzer (Lactate Scout, SensLab GmbH, Leipzig, Germany).
2.2.3. Perception outcomes
State mindfulness. Similar to a previous study,19 the participants were asked to respond to two survey items (“I felt in touch with my body” and “I focused on my breathing”) with ratings on a Likert scale ranging from 0 (“very slightly or not at all”) to 6 (“extremely”) after the intervention as a manipulation check. Mindfulness states were computed by averaging the two ratings. A higher score was considered to represent a superior mindfulness state.
Mental fatigue was assessed through subjective responses based on a visual analog scale ranging from 0 (“no pain at all”) to 10 (“extremely unbearable”). The scale has been used previously.19
2.3. Procedure
2.3.1. Experimental design
This study adopted a single-blind randomized counter-balanced crossover design. Each participant visited the lab on three separate days (i.e., one day for the preliminary trial, two days for the control trial and MBI trial) within one month. After consent, participants were randomly assigned to one of the two conditions (i.e., MBI or control) using a random number-producing algorithm. To prevent the carry-over effect and the effect of individual differences in recovery, washout periods of more than 72-h were instituted.32 The participants were required to refrain from consuming alcohol or caffeine and from any strenuous or unfamiliar physical activities for the 24-h prior to each visit. In each main trial, the participants were asked to ingest 500 mL of plain water 2-h before arriving at the lab so as to be ordinarily hydrated. They were asked to intake the same diet in days and hours before each main trial to minimize the variation from baseline tests and were instructed to empty their bladders entirely directly before the main trials. This was to ensure that the test results were not affected by the participants' nutritional status before the main experimental trials. The trials were performed in lab with constant temperatures of 22 °C. Regarding the blinding strategy, the participants were not informed of the intervention purpose until they had concluded the entire trial.
2.3.2. Preliminary trial
In the preliminary trial, the participants were instructed to complete demographic information forms. Each participant's body weight and BMI were measured using a composition analyzer (In Body 570, Cerritos, CA, USA). Subsequently, they performed a 5-min warm-up on a treadmill, followed by a VO2max test using the Astrand treadmill test protocol.33 Briefly, the participants started by running on the treadmill at 8 km/h for 3-min, with the gradient at 0%. The gradient was then increased by 2.5% every 2-min until participant exhaustion. The expired air was collected and measured using metabolic carts (Cortex Metamax 3B, Leipzig, Germany). After the VO2max test, all participants were instructed to be familiar with the main trial protocol on a treadmill. In addition, the participants were required to undergo cognitive function tests at least twice to avoid a learning effect.
2.3.3. Main trial
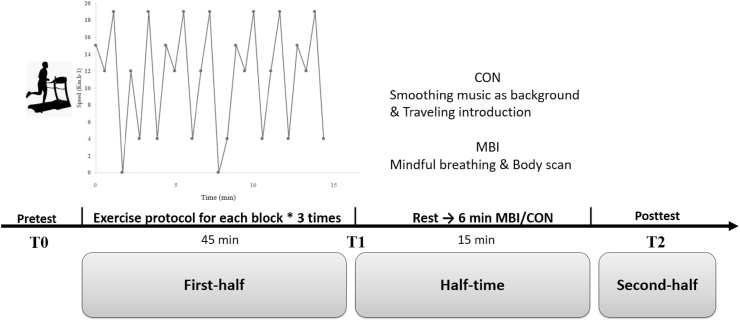
A soccer-specific intermittent exercise protocol, designed by Drust et al.34 and previously adopted,35,36 was used in the main trial. The protocol included various training intensities common to 90-min soccer matches (including walking, jogging, cruising, and sprinting). The total duration of the protocol was 90-min, divided into two identical 45-min periods separated by a 15-min rest interval, which represented the half-time break. Each 45-min included three 15-min blocks. Participants have completed the first three blocks (i.e., 45-min) on a motorized treadmill (H/P/Cosmos Sports & Medical GmbH, Nussdorf-Traunstein, Germany) to simulate the exercise intensity of the first half of a soccer match. The remaining three blocks simulating the second half were not performed, as the study was interested in the performance of the athletes after half-time break (i.e., after intervention).
On the main trial day, the participants arrived at the lab 5-min early to finalize preparations, such as reporting their recent food and drink consumption. After a 10-min warm-up (8.5 km/h running and strength exercise), baseline data (pretest)—including mental fatigue, blood lactate (BL; i.e., T0), and cognitive function (monitored by fNIRS) were recorded. After a 10-min rest, salivary samples were collected to circumvent the interference effect caused by cognitive tasks. After the baseline test (pretest), the 45-min exercise protocol was performed. The BL was collected immediately after the exercise (i.e., T1). A 15-min break was arranged thereafter as a simulation of a half-time break. After the first 5-min of the half-time break, the participants were instructed to listen to a 6-min introductory travel audio (as the control condition) or an audio-based MBI (as the intervention condition). After the listening activity, state mindfulness and mental fatigue were reported, and salivary samples and BL were collected (i.e., T2). After the simulated half-time break, the cognitive tasks were performed again (monitored through fNIRS) (see Fig. 1).
Fig. 1.
The protocol of main trials. MBI, 6-min mindfulness-based intervention; CON, Control condition. T0, baseline test (Pretest); T1, after the exercise protocol; T2, after the Intervention (Posttest).
2.3.4. Intervention
MBI condition: Participants in the MBI condition received a 6-min mindful induction, including two parts: mindful breathing and body scan. In mindful breathing practice, the attention rests on the physical sensations associated with breathing. When the mind wanders to something other than the breath, the individual nonjudgmentally takes note of the thought, allows the thought to pass, and gently returns the focus to the sensations of breathing. In the present study, mindful breathing was played at the beginning and the end of the audio for about 2 min (transcripts e.g., focus on your body and pay attention to your breathing; slowly, take a deep breath and breathe in, breathe out). The body scan meditation is a somatically oriented, attention-focusing practice designed to increase interoceptive awareness and acceptance.37 The practice of the body scan enhances the felt sense of being localized within one's physical body; references the lived, immediate experience of one's own body; and cultivates a subtle distinction between thinking about the body and perceiving the body.38 In the present study, the middle of the audio was a body scan for 4 min (transcripts e.g., feel the weight on the soles of the feet, the pressure, the slight tremor of the muscles, the warmth or coolness of the skin …). The practitioner audio was referred to a clinical psychologist in department of health, Hong Kong SAR (www.studenthealth.gov.hk/english/emotional_health_tips/eht_re/eht_re.html). Participants were guided by the audio to pay attention to their body sensations, in particular to those associated with the breathing procedure, feeling them without trying to alter them. When participants' minds wandered to something, they would be guided to notice it with an accepting, non-judgmental manner and tenderly return to breathing. The practice is consistent with the main feature of MBI: consciously using an accepting attitude to pay attention to the experience of each moment.7
Control condition: The time of the control condition is the same as that of the MBI condition (6 min), but the content is only an audio commentary introducing the scenery of the Hong Kong suburban park.
2.4. Statistical analysis
After checking the baseline (pretest) data, a 2 (conditions: MBI and control) × 2 (times: pretest and posttest) repeated-measures analysis of variance (ANOVA) was conducted to examine the between- and within-condition effects. The mental fatigue, BL, salivary cortisol, and behavioral data (i.e., ST and CBT) were analyzed separately. The post hoc test with Bonferroni correction was conducted if a significant effect was observed. A paired sample t-test was used to measure mindfulness state. The Spearman correlation test was used to examine the association between salivary cortisol concentration and cognitive performance. Effect size is presented as partial eta squared (η2 P) for ANOVA and Cohen's d for post hoc comparison. The significance level was set to 0.05. Statistical analyses were performed using SPSS (version 26, IBM SPSS, Armonk, NY, USA).
The nirsLAB package (version 2019.4, NIRx Medical Technologies LLC, New York, US) was used to analyze the fNIRS data. The bad channels were removed, and spike artifacts were filtered before analysis. A bandpass filter (i.e., 0.01 Hz for low-pass filter, and 0.2 Hz for the high-pass)39 was employed to remove physiological artifacts. The discontinuity data were removed if they exceeded the threshold of 5 standard deviations. The specific task-evoked O2Hb variations from the baseline (2 s before the onset of the task, 3 s after the onset of each task; during task-switching: 4–20 s after the onset of each task) were computed using the general linear model for each participant, channel, and condition. Statistical parametric mapping, with a P < 0.05 as the statistical threshold, was used to compare the within- and between-condition effects for conditions on specific tasks, with only those having significant variations represented in the image. With consideration of the reliability, the synthetic hemodynamic responses on O2Hb were analyzed and reported.40, 41, 42
3. Result
3.1. State mindfulness
At the posttest, a significantly higher mindfulness state was observed in the MBI condition compared with the control condition (2.29 ± 1.31 vs. 4.38 ± 1.1, t (16) = −5.85, P < 0.001, d = 1.78). Hence, the intervention was successful.
3.2. Blood lactate
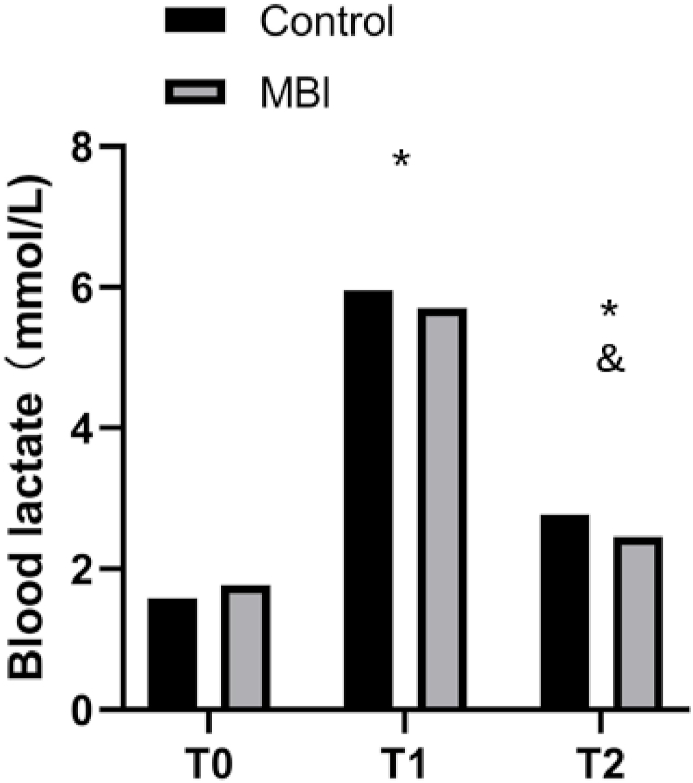
No significant interaction effect or group effect was observed for BL. A significant time effect was detected for BL (F(1,19) = 90.81, P < 0.001, η2P = 0.85). Specifically, the observed BL reached its highest level at T1 (after the exercise protocol) compared with those at T0 (baseline, P < 0.001) and T2 (after the intervention, P < 0.001). The BL level at T2 was lower than that at T1 (P < 0.001) but higher than that at T0 (P < 0.001; see Fig. 3).
Fig. 3.
Blood lactate. MBI, 6-min mindfulness-based intervention. ∗P < 0.05 vs. T0 (baseline). & P < 0.05 vs. T1(after the exercise protocol).
T2 (after the Intervention).
3.3. Behavioral outcomes
3.3.1. Stroop tasks
For the Stroop (congruent and incongruent conditions) task, no significant interaction effect was observed for reaction time or accuracy (all P > 0.05, Table 1). A significant time effect was observed for reaction time in both the congruent and incongruent tasks (F(1,32) = 8.1, P = 0.008, η2P = 0.2 for congruent; F(1,32) = 29.34, P < 0.001, η2P = 0.48 for incongruent). A significant time effect was also observed for accuracy in the incongruent task (F(1,32) = 4.85, P = 0.04, η2P = 0.13).
Table 1.
Reaction time (ms) and accuracy on Stroop task (Congruent/incongruent) and Corsi block tapping task.
| Reaction time |
Interaction | Accuracy |
Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control |
MBI |
Control |
MBI |
|||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||
| ST Congruent |
806.29± 148.94 |
753.33± 134.84 |
926.48± 368.49 |
787± 165.5∗ |
P = 0.21 η2P = 0.05 |
98.74± 2.81 |
96.22± 5.13 |
98.74± 2.81 |
97.48± 4.33 |
P = 0.5 η2P = 0.01 |
| ST Incongruent | 991.39± 222.92 |
906.51± 185.36∗ |
1085.53± 285.25 |
945.45± 189.6∗ |
P = 0.19 η2P = 0.05 |
95.29± 7.17 |
96.91± 3.91 |
93.82± 5.46 |
96.76± 4.98∗ |
P = 0.53 η2P = 0.01 |
| CBT | 581.01± 111.91 |
575.52± 133.18 |
648.92± 192.22 |
533.22± 140.98∗ |
P = 0.05 η2P = 0.11 |
67.65± 5.00 |
67.16± 4.63 |
66.18± 4.63 |
69.12± 5.72∗ |
P = 0.047 η2P = 0.12 |
Note. MBI: Brief mindfulness-based intervention; ST: Stroop task; CBT: Corsi block tapping task.
∗P < 0.05 for pretest vs. posttest comparison.
Exploring post hoc tests for time effect revealed that the MBI condition responded faster at the posttest than at the pretest in both the congruent (P = 0.04, d = 0.5) and incongruent (P < 0.001, d = 0.6) tasks. The control condition responded faster at the posttest than at the pretest in the incongruent task (P = 0.008, d = 0.43) but not in the congruent task (P = 0.06, d = 0.38). Additionally, the accuracy in the incongruent task improved at the posttest relative to the pretest in the MBI condition (P = 0.046, d = 0.58); however, this improvement was not observed in the control condition (P = 0.08, d = 0.63).
3.3.2. Corsi-block tapping task
For the CBT, significant interaction and time effects were observed for reaction time (F(1,32) = 4.13, P = 0.05, η2P = 0.11 for interaction effect; F(1,32) = 5, P = 0.03, η2P = 0.14 for time effect; Table 1)). Post hoc tests showed that the participants reacted faster at the posttest than at the pretest (P = 0.02, d = 0.71) in the MBI condition, and this improvement was not observed in the control condition (P = 0.87, d = 0.05). The MBI condition responded faster than the control condition at posttest, although no statistical difference was observed (P = 0.37, d = 0.32).
For accuracy, a significant interaction effect was observed (F(1,32) = 4.26, P = 0.047, η2P = 0.12). Post hoc tests indicated that accuracy was higher at the posttest than at the pretest (P = 0.009, d = 0.58) in the MBI condition but not in the control condition (P = 0.72, d = 0.11). The MBI condition performed better than control condition at posttest (P = 0.36, d = 0.39), although no statistical difference was detected.
3.4. fNIRS outcomes
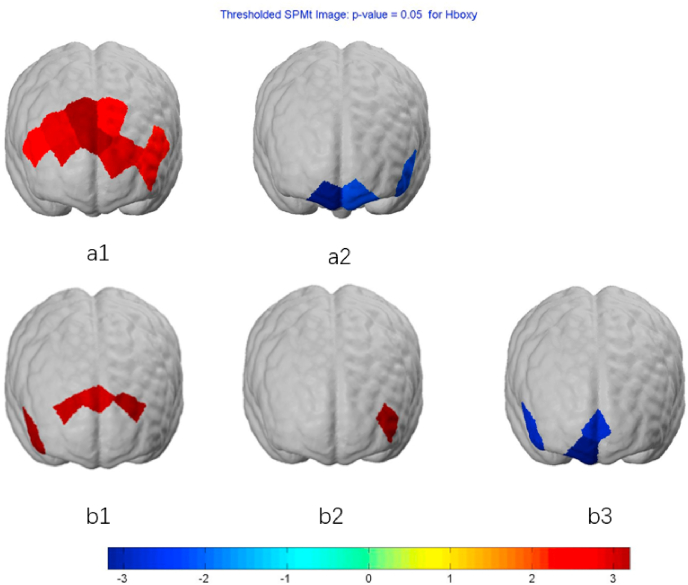
Regarding the Stroop congruent task, the pretest–posttest differences were significant in the control condition (P < 0.05) but not in the MBI condition. For the incongruent task, no significant activation was observed in the control condition, but a lower O2Hb concentration was noted in the MBI condition (P < 0.05) at the posttest compared with that at the pretest. No significant group difference was observed for either task at the posttest.
Regarding the CBT, the pretest–posttest differences were significant in both the control and MBI conditions (P < 0.05). Additionally, the MBI condition demonstrated a lower O2Hb concentration than did the control condition at the posttest (P < 0.05). (see Fig. 4).
Fig. 4.
Hemodynamic responses (O2Hb) during (a) Stroop congruent/incongruent task and (b) Corsi-block tapping task. Within comparison for pre vs. post significant changes was observed on (a1) for Control condition during congruent task, (a2) for MBI condition during incongruent task, (b1) for Control condition, (b2) for MBI condition. Between comparison for two conditions on posttest was observed on (b3), namely, MBI condition was lower than Control condition. MBI, 6-min mindfulness-based Intervention.
3.5. Salivary cortisol
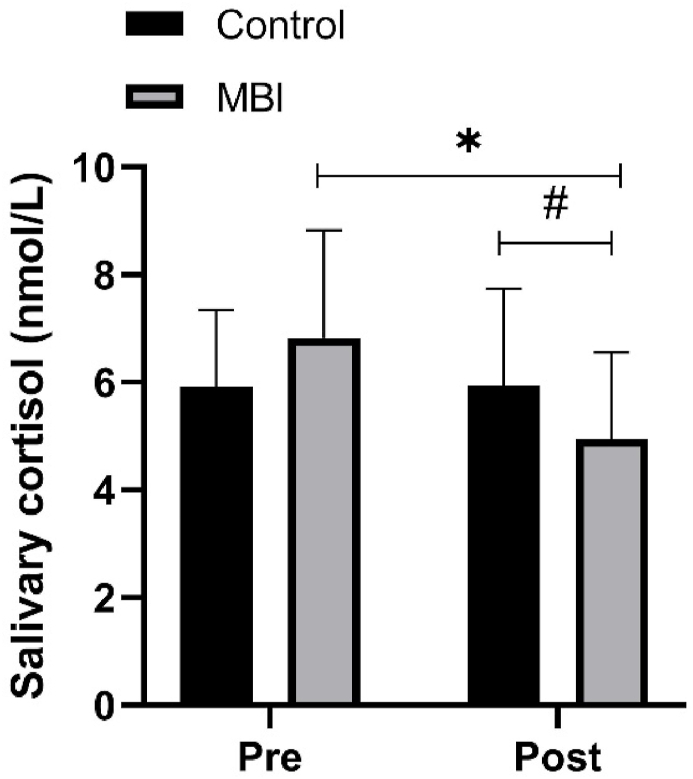
Significant interaction and time effects were observed for salivary cortisol (F(1,16) = 10.95, P < 0.01, η2P = 0.41 for interaction effect; F(1,16) = 8.14, P = 0.01, η2P = 0.34 for time effect). Post hoc tests indicated that the pretest–posttest difference was significant in the MBI condition (6.82 ± 2 vs. 4.95 ± 1.6 μg/dL, P < 0.01, d = 1.06) but not in the control condition (P = 0.96, d = 0.02). Furthermore, the MBI condition had a lower salivary cortisol concentration than did the control condition at the posttest (5.94 ± 1.8 vs. 4.95 ± 1.6 μg/dL, P = 0.05, d = 0.6). (see Fig. 5).
Fig. 5.
Salivary cortisol. MBI, 6-min mindfulness-based intervention. ∗P < 0.05 for pretest vs. posttest comparison.
#
P <
0.05 for between comparison on posttest.
Cortisol concentration in the MBI condition was associated with a shorter reaction time in the Stroop congruent task (r = 0.66, P < 0.01) and in the Stroop incongruent task (r = 0.48, P = 0.049) at the posttest. No significant association was observed in the control condition. No significant association was observed for CBT.
3.6. Mental fatigue
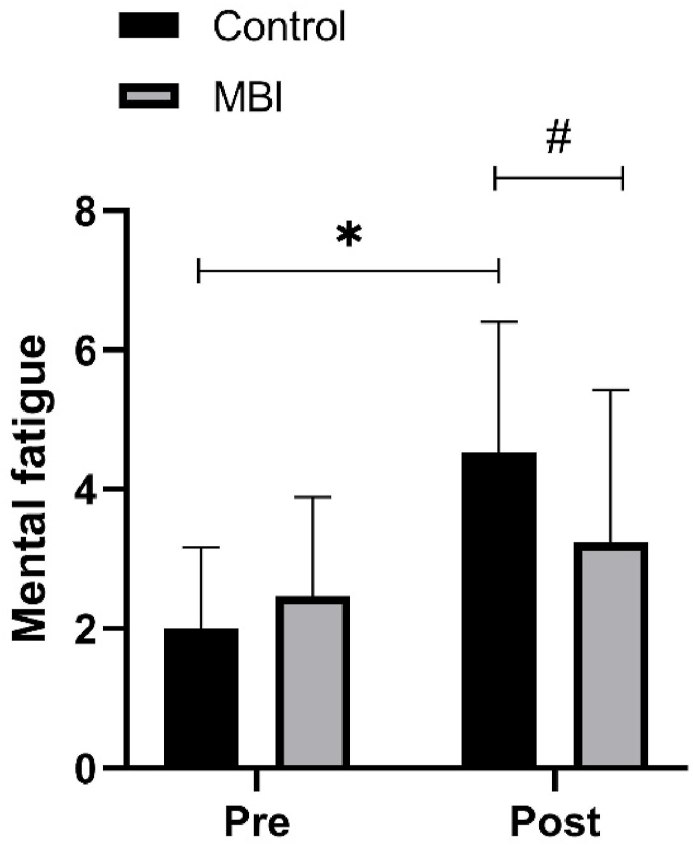
Significant interaction and time effects were observed for mental fatigue (F(1,16) = 6.67, P = 0.02, η2P = 0.29 for interaction effect; F(1,16) = 16.82, P < 0.01, η2P = 0.51 for time effect). Post hoc tests indicated that the level of mental fatigue increased significantly in the control condition (2 ± 1.17 vs. 4.53 ± 1.87, P < 0.001, d = 1.67) but not in the MBI condition (P = 0.17, d = 0.24). At the posttest, the level of mental fatigue in the MBI condition was lower than that in the control condition (3.24 ± 2.19 vs. 4.53 ± 1.87, P = 0.04, d = 0.65). (see Fig. 6).
Fig. 6.
Mental fatigue. MBI, 6-min mindfulness-based intervention. ∗P < 0.05 for pretest vs. posttest comparison.
#
P <
0.05 for between comparison on posttest.
4. Discussion
This study explored the acute effect of a brief MBI on cognition and perception after a 45-min simulated, soccer-specific, high-intensity intermittent protocol. The key finding of this study is that adopting the brief MBI during a simulated half-time break may potentially benefit athletes' working memory in terms of both reaction time and accuracy, as the fNIRS results indicated significant changes in O2Hb concentration within specific brain areas after the intervention. In addition, the concentration of salivary cortisol and level of mental fatigue decreased after the MBI compared with those in the control condition. The decreased cortisol potentially correlated with inhibition and attention capacity (i.e., ST).
Supplementing to the adequately documented beneficial effects of prolonged MBI,43 this study provides preliminary evidence of the acute effect of the brief MBI on cognitive retention and relaxation in the sports field. As hypothesized, the participants' cognitive performance (i.e., working memory) was improved after receiving the brief MBI following the exercise protocol, compared with the pretest. However, the significant between-group difference at posttest was not detected. The considerable effect size (d = 0.32 for reaction time and 0.39 for accuracy) and significant change in O2Hb concentration indicated a potential effect of brief MBI on cognition. This finding is in line with a recent study that investigated the effectiveness of short-term MBIs on attention.14 In the mentioned study, the MBI group showed a significant improvement in cognition in the ST, but the study did not include a control group. By contrast, in the present study, an improvement in the ST (in the incongruent task) was observed for both the control and MBI conditions, a finding that extends the previous study's findings. The varied O2Hb concentration observed in two conditions after the intervention may be because MBI immediately generated a cognitive control state that specifically influenced focused attention.43 The capability to achieve intentional control of both biological–somatic activities and conscious–unconscious processes contributed to the potential positive effects of the MBI on cognitive retention and relaxation.44
4.1. fNIRS results
Neural activity induced by cognitive processing increases oxygen metabolism, manifesting as a relatively high O2Hb concentration in specific prefrontal cortex areas.42 In the present study, the fNIRS results were mixed. Specifically, in the Stroop congruent task, the O2Hb concentration in the dorsolateral prefrontal cortex (DLPFC) was higher at the posttest than at the pretest in the control condition; nevertheless, this increase in concentration was not observed in the MBI condition. By contrast, in incongruent task, the O2Hb concentration was lower at the posttest than at the pretest in the MBI condition. No difference was observed between the control and MBI conditions in terms of the congruent or incongruent test results at the posttest. In the Stroop congruent task, the participants' O2Hb concentration in the DLPFC increased after exercise, accompanied by improved cognitive function (as indicated by the behavioral results). This finding is consistent with the results of previous studies, which have suggested that areas of high neural activity tend to indicate increased oxygen consumption and enhanced blood supply that ensures the requisite supply of O2Hb.45 However, the same activation was not observed in the incongruent task. A possible explanation is that the incongruent task was performed immediately after the congruent task (i.e., less than 15-sec); thus, the activation level at the beginning of the incongruent task was somehow biased.
In the CBT task, both the control and MBI conditions exhibited increased O2Hb concentrations at the posttest than at the pretest, indicating that exercise could yield an increase in O2Hb concentration, which is consistent with the Stroop congruent task findings as well as the findings of Ichinose et al.45 However, the O2Hb concentration observed in the MBI condition was lower than that in the control condition at the posttest. Furthermore, a potentially improved cognitive performance was revealed in the MBI condition compared with the control condition in the posttest. These results are not consistent with our hypothesis. Specifically, improved cognitive performance may not be accompanied by a higher O2Hb concentration in the prefrontal cortex. Previous studies have reported similar findings. For example, Ando et al.46 observed that participants' reaction time (i.e., in a flanker task) was shorter after 60% VO2max exercise compared with that at the pretest, but the participants' behavioral results were not accompanied by a cerebral oxygenation change. Ferreri et al.47 conducted a non-sport study involving a music-based intervention and observed that improved cognitive performance (i.e., verbal memory) was associated with a decreased O2Hb concentration in the DLPFC. In addition, Ramasubbu et al.48 noted that participants treated with a central nervous system drug (i.e., methylphenidate) exhibited superior working memory (i.e., N-back task) along with a reduced O2Hb concentration in the prefrontal cortex. According to Tomasi et al.,49 the inconsistency may be explained by the task-dependent balance of activation and deactivation that maximizes the activated brain network resources.
Another potential explanation for observed inconsistency is cerebral blood flow (CBF), which plays a critical role in cognitive performance.50 In the present study, the fNIRS results were different among cognitive tasks, which may be caused by the varied CBF across tasks.49 According to the cardiovascular hypothesis, a higher cardiac output (i.e., heart rate [HR]) typically results in higher CBF, implying that a higher cardiovascular work rate positively influences cognitive performance.51 However, higher HR does not always result in higher cognitive performance. According to Wang et al.,52 cognition is impaired during high-intensity exercise (80% HRmax) but not during moderate- (50% HRmax) to low-intensity (30% HRmax) exercise. Similarly, Soga et al.53 reported that working memory accuracy decreased during a continuous 70% HRmax exercise session. However, HR was not monitored during the cognitive function tasks in the present study. Future studies should consider doing so to confirm this possible explanation.
The inconsistency between cognitive performance and O2Hb concentration in the present study suggests that improved cognitive function may not be directly related to increased cerebral oxygenation. Brain neurotransmitters modulator, such as brain-derived neurotrophic factor (BDNF), potentially influence cognitive performance. BDNF is a growth factor that stimulates neural plasticity and synaptic growth and transmission; it enhanced cognition due to its upregulation and role in angiogenesis.54 A previous meta-review suggested that MBIs could effectively increase BDNF expression.55 Therefore, future research may consider investigating this biomarker.
4.2. Salivary cortisol
Existing evidence confirms the acute positive effects of MBI on salivary cortisol concentration reduction.56 In the sports field, Macdonald and Minahan57 observed that an 8 weeks MBI attenuated the increase in salivary cortisol concentrations induced by competition. John et al.58 conducted a 4 weeks MBI program among shooters and revealed a reduction in cortisol concentrations immediately before competition. The present study suggests that brief MBIs during half-time breaks might also benefit cortisol modulation.
In the present study, the reduced cortisol concentration in the MBI condition was associated with a shorter reaction time in the Stroop congruent and incongruent tasks at the posttest, which is consistent with Lee et al.,59 who suggested that higher cortisol concentrations are associated with worsening cognition. In competitive sports, cortisol is viewed as an indicator of psychological stressors that may influence an athlete's performance60,61; hence, an MBI may reduce the negative effect of stress on sports performance by attenuating cortisol concentrations in athletes.
4.3. Mental fatigue
The present findings suggest that a brief MBI is beneficial in relieving adverse psychological statuses such as mental fatigue. This is essential because mental fatigue could impair soccer-specific decision-making accuracy and response time.62 An accepting mood and relaxed breathing among brief MBI participants may influence the biological system, thus regulating the onset of mental fatigue.44 A neuroimaging review study indicated that during an MBI, the subgenual and adjacent ventral anterior cingulate cortex were activated, controlling parasympathetic activity.63 Decreased parasympathetic activity was reported to induce sympathetic hyperactivity associated with mental fatigue that is induced by prolonged cognitive load.64 Therefore, the elevated activation of parasympathetic activity may benefit mental fatigue recovery. Chen et al. reported that an MBI could modulate brain activities in multiple emotion-processing systems, contributing to the regulation of mental fatigue.65 Coimbra et al.66 observed that an MBI effectively attenuated the mental fatigue caused by competition in volleyball athletes. Therefore, adopting an MBI to curb mental fatigue appears feasible.
This study has several limitations. First, we included only male athletes. The lack of female athletes may limit the generalizability of the results. Future studies should consider involving female participants. Second, our findings were obtained in the context of a lab-based soccer match simulation. The nature of lab-based experiments may differ from actual competition, influencing athletes' psychological status differently. Future studies should be conducted in a real game situation. Third, athlete HR was not monitored during cognitive function tasks. Given that HR may impact cognitive performance, future research should involve HR monitoring (or cardiovascular index) during cognitive tasks. Fourth, the failure of detecting a significant group difference in working memory at posttest may be because the sample size was relatively small.67 A larger sample size should be adopted in future studies. Fifth, the VAS measured mental fatigue may be confounded by several response biases, given that the method has been adopted in various soccer-specific mental fatigue studies.68 Further studies may benefit from combining objective measures to assess psychophysiological (e.g., galvanic skin response) responses to mental fatigue. Sixth, changes in extracerebral oxygenation may lead to false positive or false negative results in fNIRS detection after exercise.69 The additional short channels to monitor extracerebral oxygenation changes is warranted,69 but due to the nature of device, the procedure was not performed in the current study.
5. Conclusion
In conclusion, the potential beneficial effects of brief MBI on cognitive performance (i.e., working memory) were preliminarily detected, and the hemodynamic responses in prefrontal brain area partially interpret the changes in cognitive function. The present study also provided evidence for the acute beneficial effects of brief MBI on mental fatigue and cortisol concentration during the half-time period in a simulated soccer protocol. More efforts are still needed in future investigations to clarify the benefits of MBI on cognitive function in soccer players.
Author contribution statement
Z.Y, S.F and L.C conceived and designed research. Z.Y, W.K, H.S and W.J conducted experiments. H.J and H.M contributed experimental and analytical tools. Z.Y analyzed data. Z.Y drafted the manuscript with the support from S.F, L.C, H.J and H.M. All authors read and approved the manuscript.
Funding details
This work was supported by The Education University of Hong Kong under Grant [No. FLASS/DRF/IRS-2 04540].
References
- 1.Russell M., West D.J., Harper L.D., Cook C.J., Kilduff L.P. Half-Time strategies to enhance second-half performance in team-sports players: a review and recommendations. Sports Med. 2015;45(3):353–364. doi: 10.1007/s40279-014-0297-0. [DOI] [PubMed] [Google Scholar]
- 2.Lovell R., Midgley A., Barrett S., Carter D., Small K. Effects of different half-time strategies on second half soccer-specific speed, power and dynamic strength. Scand J Med Sci Sports. 2013;23(1):105–113. doi: 10.1111/j.1600-0838.2011.01353.x. [DOI] [PubMed] [Google Scholar]
- 3.Greig M., Marchant D., Lovell R., Clough P., McNaughton L. A continuous mental task decreases the physiological response to soccer-specific intermittent exercise. Br J Sports Med. 2007;41(12):908–913. doi: 10.1136/bjsm.2006.030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bühlmayer L., Birrer D., Röthlin P., Faude O., Donath L. Effects of mindfulness practice on performance-relevant parameters and performance outcomes in sports: a meta-analytical review. Sports Med. 2017;47(11):2309–2321. doi: 10.1007/s40279-017-0752-9. [DOI] [PubMed] [Google Scholar]
- 5.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin Psychol Sci Pract. 2003;10(2):144–156. doi: 10.1093/clipsy.bpg016. [DOI] [Google Scholar]
- 6.Baer R.A. Self-focused attention and mechanisms of change in mindfulness-based treatment. Cognit Behav Ther. 2009;38(SUPPL.1):15–20. doi: 10.1080/16506070902980703. [DOI] [PubMed] [Google Scholar]
- 7.Creswell J.D. Mindfulness interventions. Annu Rev Psychol. 2017;68:491–516. doi: 10.1146/annurev-psych-042716-051139. [DOI] [PubMed] [Google Scholar]
- 8.Tao S., Li J., Zhang M., et al. The effects of mindfulness-based interventions on child and adolescent aggression: a systematic review and meta-analysis. Mindfulness (N Y) 2021;12(6):1301–1315. doi: 10.1007/s12671-020-01570-9. [DOI] [Google Scholar]
- 9.Kee Y.H., Li C., Kong L.C., Tang C.J., Chuang K.-L. Scoping review of mindfulness research: a topic modelling approach. Mindfulness. 2019;10(8):1474–1488. doi: 10.1007/s12671-019-01136-4. [DOI] [Google Scholar]
- 10.Howarth A., Smith J.G., Perkins-Porras L., Ussher M. Effects of brief mindfulness-based interventions on health-related outcomes: a systematic review. Mindfulness (N Y) 2019;10(10):1957–1968. doi: 10.1007/s12671-019-01163-1. [DOI] [Google Scholar]
- 11.Sollgruber A., Bornemann-Cimenti H., Szilagyi I.S., Sandner-Kiesling A. Spirituality in pain medicine: a randomized experiment of pain perception, heart rate and religious spiritual well-being by using a single session meditation methodology. PLoS One. 2018;(9):13. doi: 10.1371/journal.pone.0203336. e0203336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ussher M., Spatz A., Copland C., et al. Immediate effects of a brief mindfulness-based body scan on patients with chronic pain. J Behav Med. 2014;37(1):127–134. doi: 10.1007/s10865-012-9466-5. [DOI] [PubMed] [Google Scholar]
- 13.May R.W., Bamber M., Seibert G.S., et al. Understanding the physiology of mindfulness: aortic hemodynamics and heart rate variability. Stress. 2016;19(2):168–174. doi: 10.3109/10253890.2016.1146669. [DOI] [PubMed] [Google Scholar]
- 14.Izzetoglu M., Shewokis P.A., Tsai K., Dantoin P., Sparango K., Min K. Short-term effects of meditation on sustained attention as measured by fNIRS. Brain Sci. 2020;10(9):1–16. doi: 10.3390/brainsci10090608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozasa E.H., Sato J.R., Lacerda S.S., et al. Meditation training increases brain efficiency in an attention task. Neuroimage. 2012;59(1):745–749. doi: 10.1016/j.neuroimage.2011.06.088. [DOI] [PubMed] [Google Scholar]
- 16.Mrazek M.D., Franklin M.S., Phillips D.T., Baird B., Schooler J.W. Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychol Sci. 2013;24(5):776–781. doi: 10.1177/0956797612459659. [DOI] [PubMed] [Google Scholar]
- 17.Van Vugt M.K., Jha A.P. Investigating the impact of mindfulness meditation training on working memory: a mathematical modeling approach. Cognit Affect Behav Neurosci. 2011;11(3):344–353. doi: 10.3758/s13415-011-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hölzel B.K., Lazar S.W., Gard T., Schuman-Olivier Z., Vago D.R., Ott U. How does mindfulness meditation work? proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y., Sun F., Li C., Chow D.H.K., Wang K. Acute effect of brief mindfulness-based intervention coupled with fluid intake on athletes' cognitive function. J Sports Sci Med. 2020;19(4):753–760. [PMC free article] [PubMed] [Google Scholar]
- 20.Gray R. A model of motor inhibition for a complex skill: baseball batting. J Exp Psychol Appl. 2009;15(2):91–105. doi: 10.1037/a0015591. [DOI] [PubMed] [Google Scholar]
- 21.Verburgh L., Scherder E.J.A., Van Lange P.A.M., Oosterlaan J. Executive functioning in highly talented soccer players. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091254. e91254–e91254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F., Lv X., Fang J., et al. The effect of body-mind relaxation meditation induction on major depressive disorder: a resting-state fMRI study. J Affect Disord. 2015;183:75–82. doi: 10.1016/j.jad.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Deepeshwar S., Vinchurkar S.A., Visweswaraiah N.K., Nagendra H.R. Hemodynamic responses on prefrontal cortex related to meditation and attentional task. Front Syst Neurosci. 2015;8(2):252. doi: 10.3389/fnsys.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y., Sun F., Chiu M.M., Siu A.Y.-S. Effects of high-intensity interval exercise and moderate-intensity continuous exercise on executive function of healthy young males. Physiol Behav. 2021;239:113505. doi: 10.1016/j.physbeh.2021.113505. [DOI] [PubMed] [Google Scholar]
- 25.Rooks C.R., Thom N.J., McCully K.K., Dishman R.K. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol. 2010;92(2):134–150. doi: 10.1016/j.pneurobio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Stroop J.R. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 27.Berch D.B., Krikorian R., Huha E.M. The Corsi block-tapping task: methodological and theoretical considerations. Brain Cognit. 1998;38(3):317–338. doi: 10.1006/brcg.1998.1039. [DOI] [PubMed] [Google Scholar]
- 28.Giles G.E., Cantelon J.A., Eddy M.D., et al. Habitual exercise is associated with cognitive control and cognitive reappraisal success. Exp Brain Res. 2017;235(12):3785–3797. doi: 10.1007/s00221-017-5098-x. [DOI] [PubMed] [Google Scholar]
- 29.Hatch L.M., Williams R.A., Dring K.J., et al. The Daily MileTM: acute effects on children's cognitive function and factors affecting their enjoyment. Psychol Sport Exerc. 2021;57:102047. doi: 10.1016/j.psychsport.2021.102047. [DOI] [Google Scholar]
- 30.Sun F.-H., Cooper S.B., Chak-Fung Tse F. Effects of different solutions consumed during exercise on cognitive function of male college soccer players. J Exerc Sci Fit. 2020;18(3):155–161. doi: 10.1016/j.jesf.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasper H. The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- 32.Ispirlidis I., Fatouros I.G., Jamurtas A.Z., et al. Time-course of changes in inflammatory and performance responses following a soccer game. Clin J Sport Med. 2008;18(5):423–431. doi: 10.1097/JSM.0b013e3181818e0b. [DOI] [PubMed] [Google Scholar]
- 33.Astrand P. Experimental studies of physical working capacity in relation to sex and age. Copenhagen: Munksgaard. 1952;1:171. [Google Scholar]
- 34.Drust B., Reilly T., Cable N.T. Physiological responses to laboratory-based soccer-specific intermittent and continuous exercise. J Sports Sci. 2000;18(11):885–892. doi: 10.1080/026404100750017814. [DOI] [PubMed] [Google Scholar]
- 35.Clarke N.D., Maclaren D.P.M., Reilly T., Drust B. Carbohydrate ingestion and pre-cooling improves exercise capacity following soccer-specific intermittent exercise performed in the heat. Eur J Appl Physiol. 2011;111(7):1447–1455. doi: 10.1007/s00421-010-1771-5. [DOI] [PubMed] [Google Scholar]
- 36.Travers G.J.S., Nichols D.S., Farooq A., Racinais S., Périard J.D. Validation of an ingestible temperature data logging and telemetry system during exercise in the heat. Temperature. 2016;3(2):208–219. doi: 10.1080/23328940.2016.1171281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreeben S.J., Mamberg M.H., Salmon P. The MBSR body scan in clinical practice. Mindfulness. 2013;4(4):394–401. doi: 10.1007/s12671-013-0212-z. [DOI] [Google Scholar]
- 38.Mehling W.E., Gopisetty V., Daubenmier J., Price C.J., Hecht F.M., Stewart A. Body awareness: construct and self-report measures. PLoS One. 2009;4(5):e5614. doi: 10.1371/journal.pone.0005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mücke M., Andrä C., Gerber M., Pühse U., Ludyga S. Moderate-to-vigorous physical activity, executive functions and prefrontal brain oxygenation in children: a functional near-infrared spectroscopy study. J Sports Sci. 2018;36(6):630–636. doi: 10.1080/02640414.2017.1326619. [DOI] [PubMed] [Google Scholar]
- 40.Mol A., Woltering J.H.H., Colier W.N.J.M., Maier A.B., Meskers C.G.M., van Wezel R.J.A. Sensitivity and reliability of cerebral oxygenation responses to postural changes measured with near-infrared spectroscopy. Eur J Appl Physiol. 2019;119(5):1117–1125. doi: 10.1007/s00421-019-04101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshi Y. Functional near-infrared spectroscopy: current status and future prospects. J Biomed Opt. 2007;12(6):62106. doi: 10.1117/1.2804911. [DOI] [PubMed] [Google Scholar]
- 42.Herold F., Wiegel P., Scholkmann F., Müller N. Applications of functional near-infrared spectroscopy (fNIRS) neuroimaging in exercise–cognition science: a systematic, methodology-focused review. J Clin Med. 2018;7(12):466. doi: 10.3390/jcm7120466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colzato L.S., Sellaro R., Samara I., Baas M., Hommel B. Meditation-induced states predict attentional control over time. Conscious Cognit. 2015 doi: 10.1016/j.concog.2015.08.006. Published online. [DOI] [PubMed] [Google Scholar]
- 44.Creswell J.D. Handbook of Mindfulness: Theory, Research, and Practice. The Guilford Press; 2015. Biological pathways linking mindfulness with health; pp. 426–440. [Google Scholar]
- 45.Ichinose Y., Morishita S., Suzuki R., Endo G., Tsubaki A. Advances in Experimental Medicine and Biology. vol. 1232. Springer; 2020. Comparison of the effects of continuous and intermittent exercise on cerebral oxygenation and cognitive function; pp. 209–214. [DOI] [PubMed] [Google Scholar]
- 46.Ando S., Kokubu M., Yamada Y., Kimura M. Does cerebral oxygenation affect cognitive function during exercise? Eur J Appl Physiol. 2011;111(9):1973–1982. doi: 10.1007/s00421-011-1827-1. [DOI] [PubMed] [Google Scholar]
- 47.Ferreri L., Aucouturier J.J., Muthalib M., Bigand E., Bugaiska A. Music improves verbal memory encoding while decreasing prefrontal cortex activity: an fNIRS study. Front Hum Neurosci. 2013;7(11):779. doi: 10.3389/fnhum.2013.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramasubbu R., Singh H., Zhu H., Dunn J.F. Methylphenidate-mediated reduction in prefrontal hemodynamic responses to working memory task: a functional near-infrared spectroscopy study. Hum Psychopharmacol Clin Exp. 2012;27(6):615–621. doi: 10.1002/hup.2258. [DOI] [PubMed] [Google Scholar]
- 49.Tomasi D., Ernst T., Caparelli E.C., Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27(8):694–705. doi: 10.1002/hbm.20211. doi:10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ainslie P.N., Cotter J.D., George K.P., et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586(16):4005–4010. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winter B., Breitenstein C., Mooren F.C., et al. High impact running improves learning. Neurobiol Learn Mem. 2007;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Wang C.C., Chu C.H., Chu I.H., Chan K.H., Chang Y.K. Executive function during acute exercise: the role of exercise intensity. J Sport Exerc Psychol. 2013;35(4):358–367. doi: 10.1123/jsep.35.4.358. [DOI] [PubMed] [Google Scholar]
- 53.Soga K., Shishido T., Nagatomi R. Executive function during and after acute moderate aerobic exercise in adolescents. Psychol Sport Exerc. 2015;16(P3):7–17. doi: 10.1016/j.psychsport.2014.08.010. [DOI] [Google Scholar]
- 54.Bathina S., Das U.N. Brain-derived neurotrophic factor and its clinical Implications. Arch Med Sci. 2015;11(6):1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomutbutra P., Yingchankul N., Chattipakorn N., Chattipakorn S., Srisurapanont M. The effect of mindfulness-based intervention on brain-derived neurotrophic factor (BDNF): a systematic review and meta-analysis of controlled trials. Front Psychol. 2020;11:2209. doi: 10.3389/fpsyg.2020.02209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cruess D.G., Finitsis D.J., Smith A.-L., et al. Brief stress management reduces acute distress and buffers physiological response to a social stress test. Int J Stress Manag. 2015;22(3):270–286. doi: 10.1037/a0039130. [DOI] [Google Scholar]
- 57.MacDonald L.A., Minahan C.L. Mindfulness training attenuates the increase in salivary cortisol concentration associated with competition in highly trained wheelchair-basketball players. J Sports Sci. 2018;36(4):378–383. doi: 10.1080/02640414.2017.1308001. [DOI] [PubMed] [Google Scholar]
- 58.John S., Verma S.K., Khanna G.L. The effect of mindfulness meditation on HPA-Axis in pre-competition stress in sports performance of elite shooters. Mindfulness Medit HPA-Axis NJIRM. 2011;2(3):15–21. http://nicpd.ac.in/ojs-/index.php/njirm/article/view/1915 [Google Scholar]
- 59.Lee B.K., Glass T.A., McAtee M.J., et al. Associations of salivary cortisol with cognitive function in the baltimore memory study. Arch Gen Psychiatr. 2007 doi: 10.1001/archpsyc.64.7.810. Published online. [DOI] [PubMed] [Google Scholar]
- 60.Van Paridon K.N., Timmis M.A., Nevison C.M., Bristow M. The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport Exerc Med. 2017;(1):3. doi: 10.1136/bmjsem-2017-000261. e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zoccola P.M., Dickerson S.S. Assessing the relationship between rumination and cortisol: a review. J Psychosom Res. 2012;73(1):1–9. doi: 10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Smith M.R., Zeuwts L., Lenoir M., Hens N., De Jong L.M.S., Coutts A.J. Mental fatigue impairs soccer-specific decision-making skill. J Sports Sci. 2016;34(14):1297–1304. doi: 10.1080/02640414.2016.1156241. [DOI] [PubMed] [Google Scholar]
- 63.Tang Y.Y., Ma Y., Fan Y., et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;106(22):8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuno K., Tanaka M., Yamaguti K., Kajimoto O., Kuratsune H., Watanabe Y. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav Brain Funct. 2011;7(1):17. doi: 10.1186/1744-9081-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grillon C., Quispe-Escudero D., Mathur A., Ernst M. Mental fatigue impairs emotion regulation. Emotion. 2015;15(3):383–389. doi: 10.1037/emo0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coimbra D.R., Bevilacqua G.G., Pereira F.S., Andrade A. Effect of mindfulness training on fatigue and recovery in elite volleyball athletes: a randomized controlled follow-up study. J Sports Sci Med. 2021;20(1):1–8. doi: 10.52082/jssm.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sullivan G.M., Feinn R. Using effect size—or why the p value is not enough. J Grad Med Educ. 2012;4(3):279–282. doi: 10.4300/jgme-d-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson C.J., Fransen J., Skorski S., et al. Mental fatigue in football: is it time to shift the goalposts? an evaluation of the current methodology. Sports Med. 2019;49(2):177–183. doi: 10.1007/s40279-018-1016-z. [DOI] [PubMed] [Google Scholar]
- 69.Tachtsidis I., Scholkmann F. False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics. 2016;(3):3. doi: 10.1117/1.nph.3.3.031405. 031405. [DOI] [PMC free article] [PubMed] [Google Scholar]