Abstract
A competitive enzyme-linked immunosorbent assay (cELISA), using two monoclonal antibodies (MAbs), was developed and compared with the standard virus neutralization test (VNT) for detecting antibodies against canine distemper virus (CDV) and phocine distemper virus (PDV) in sera from dogs and various species of marine mammals. The test depends on the blocking of MAb binding to solid-phase antigen in the presence of positive serum. Test conditions were optimized by using control VNT-negative and -positive sera specific for CDV and PDV. A positive cutoff value of 30% inhibition, which represents the mean cutoff of a VNT-negative population (n = 623) plus 2 standard deviations, was adopted for the test. A total of 736 serum samples were tested by the new cELISA and by the VNT as the “gold standard.” An unexpected but useful finding was the ability of this CDV- and PDV-specific cELISA to also detect antibodies against the related pair dolphin morbillivirus and porpoise morbillivirus. Based on a subpopulation of 625 sera used in statistical analyses, the overall sensitivity and specificity of cELISA relative to those of the VNT were 94.9 and 97.7%, respectively. Because the cELISA proved to be nearly as sensitive and specific as the VNT while being simpler and more rapid, it would be an adequate screening test for suspect CDV or PDV cases and would also be useful for epidemiological surveillance of morbilliviral infections in marine mammal populations.
The last 13 years have witnessed the emergence of newly recognized members of the Morbillivirus genus as significant causes of disease and mortality in marine mammals belonging in the Cetacea and Pinnipedia orders. Four morbilliviruses are now known to infect various species of marine mammals: canine distemper virus (CDV) in seals (10) and polar bears (8, 9), phocine distemper virus (PDV) in seals (18), dolphin morbillivirus (DMV) in dolphins and whales, and porpoise morbillivirus (PMV) in porpoises (13).
In 1987 to 1988, more than half of the population of bottlenose dolphins (Tursiops truncatus) along the Atlantic coast of the United States was estimated to have died during the first recognized marine morbilliviral epizootic (15). In 1987, CDV killed thousands of Siberian seals (Phoca sibirica) in Lake Baikal, Russia (10, 26). The most devastating recent morbilliviral mass mortality event occurred in 1988, when a PDV epizootic killed approximately 17,000 harbor seals (Phoca vitulina) in the North Sea (17, 18). Around the same time, an outbreak of PMV killed small numbers of harbor porpoises (Phocoena phocoena) in northwestern Europe (13, 27). Later, in 1990 to 1991, a DMV epizootic killed thousands of striped dolphins (Stenella coeruleoalba) in the western Mediterranean (1, 2, 27). In 1993 to 1994, another DMV epizootic occurred in bottlenose dolphins in the Gulf of Mexico (14). More recently, morbilliviral infection has been reported in cetaceans in the Pacific (19) and an apparently new member of the morbillivirus group in a long-finned pilot whale (Globicephalus melas) of the U.S. Atlantic coast has been described (23).
Because morbillivirus infections are now common in cetacean and pinniped populations, serological testing of marine mammals is often required prior to relocation or release into the wild following poststranding rehabilitation. The microplate virus neutralization test (VNT) has been extensively used for this purpose as well as for epidemiological studies (4–9, 12, 20, 25, 27). The VNT is both highly sensitive and highly specific; however, its use is limited to laboratories that have the necessary cell culture facilities and the appropriate live virus stocks. Furthermore, the VNT is expensive and time-consuming because of the requirement for an incubation period of at least 4 days. These limitations notwithstanding, the VNT has remained the most reliable test for marine mammal morbilliviruses because other commonly used serologic tests such as fluorescent-antibody and indirect enzyme-linked immunosorbent assay (ELISA) require specific antispecies conjugates that are not currently available. This study describes the development of a monoclonal antibody (MAb)-based competitive ELISA (cELISA) for serologic testing of sera from various species of marine mammals. The main advantage of the cELISA over those of conventional serologic assays is that a single anti-mouse immunoglobulin conjugate can be used on serum from any animal species. Additional advantages include a shorter turnaround time and lower expense.
MATERIALS AND METHODS
Viruses.
The four morbilliviruses that are established causes of disease in marine mammals were used. The viruses included the Rockborn strain of CDV, PDV strain 1-2-6A, and the Belfast strains of DMV and PMV. These viruses were used for preparation of ELISA antigen and as indicator viruses in the VNT.
Serum samples.
A total of 736 serum samples, including samples from various marine mammals belonging to five different orders or families, were used in this study. These samples were received in the diagnostic laboratory from various sources for morbillivirus serological testing. The morbillivirus antibody status of each sample was determined using the VNT, with the four viruses as indicators. Table 1 presents the animal origins and VNT antibody statuses of the serum samples. Following the VNT, all samples were frozen at −70°C until they were tested by cELISA.
TABLE 1.
Animal origins of 736 sera tested by VNT and cELISA
| Animal group | No. of sera with VNT antibody status:
|
Total | |
|---|---|---|---|
| Negative | Positive | ||
| Family Canidae | 35 | 78 | 113 |
| Family Mustelidae | 36 | 36 | |
| Order Cetacea | 104 | 14 | 118 |
| Order Pinnipedia | 389 | 16 | 405 |
| Unknown | 59 | 5 | 64 |
| Total | 623 | 113 | 736 |
Preparation of morbillivirus ELISA antigen.
Viruses were grown in African green monkey kidney (Vero) cells using the alpha modification of Eagle's minimum essential medium supplemented with Earle's salts, l-glutamine, 10% fetal bovine serum (FBS), and antibiotics (100 U of penicillin and 100 μg of streptomycin per ml). The cells, seeded into 150-cm2 cell culture flasks, were infected in suspension at a multiplicity of infection of about 0.01 50% tissue culture infective dose per cell and allowed to form monolayers at 37°C in 5% CO2. When virus-specific cytopathic effects (CPE) were observed on 80% or more of the monolayer, cells were scraped into the medium, sonicated, and clarified by low-speed centrifugation and virus was concentrated from the supernatant by centrifuging it at 125,000 × g for 1 h. Viral particles were then purified by gradient centrifugation as previously described for other morbilliviruses (22). Briefly, concentrated virus was layered onto a 20 to 60% step sucrose gradient and centrifuged at 125,000 × g for 1 h at 4°C. The virus band at the interface of the two sucrose layers was removed, pelleted at 125,000 × g for 1 h at 4°C, and layered onto a continuous 15 to 40% potassium tartrate gradient. After being centrifuged for 4 h at 4°C, the virus band was collected, diluted 1:15 in sterile phosphate-buffered saline (PBS), and centrifuged at 125,000 × g for 1 h. The resulting pellet was resuspended in sterile PBS by sonication and used as the antigen for ELISA and for MAb production.
MAbs.
Gradient purified whole viral antigens of CDV, DMV, PDV, and PMV antigens were submitted to the Hybridoma Center, Oklahoma State University, for MAb production on a contract basis. The resulting four panels of MAbs were screened in our laboratory for their reactivities against all four viruses by ELISA and VNT (see below). Indirect ELISA was used to determine their specificities, while cELISA was used to measure the ability of selected MAbs to compete with specific antisera for binding to solid-phase-gradient-purified whole viral antigen. Two CDV-induced MAbs, designated 1-1E12 and 2-5F8, were selected for development of a diagnostic cELISA for CDV and PDV on the basis of their strong indirect ELISA signals and their ability to compete with specific anti-CDV and anti-PDV sera for binding to CDV antigen. The MAb 1-1E12 was specific for CDV, while the MAb 2-5F8 reacted with both CDV and PDV by ELISA, but none of them neutralized either virus. For simplicity and didactic reasons, these MAbs will be referred to in the rest of this paper as MAb1 (1-1E12) and MAb2 (2-5F8).
VNT.
The morbilliviruses are antigenically so closely related that they cross-neutralize one another. However, serum raised against one morbillivirus will neutralize the homologous virus at a higher titer than it will other (heterologous) morbilliviruses (24). The VNT was therefore used in this study as the “gold standard” to determine the antibody specificities of diagnostic serum samples. The test was performed by following a modification of the microtiter method (21). Briefly, serial twofold dilutions of heat-inactivated sera were made in eight columns of 96-well plates using Eagle's minimum essential medium, starting at a 1:2 dilution. Equal volumes (25 μl) of the viruses containing about 100 50% tissue culture infective doses were added to duplicate columns. The virus-serum mixtures were incubated at 37°C for 1 h in 5% CO2, and a Vero cell suspension (150 μl containing 104 cells/well) was added. The plates were incubated at 37°C in 5% CO2 for 4 days. The test was read by examining cell monolayers under an inverted microscope for virus-specific CPE. Antibody titers were expressed as the reciprocals of the highest dilutions of sera that completely neutralized CPE in duplicate wells. All samples with a titer of 8 or greater were considered positive for morbillivirus antibody. For positive serum samples, the homologous virus was considered to be the one against which the serum had the highest titer (Table 2).
TABLE 2.
Comparison of VNT and cELISA results for 736 sera
| VNT antibody status | No. of sera with indicated cELISA result with:
|
|||
|---|---|---|---|---|
| MAb1
|
MAb2
|
|||
| Negative | Positive | Negative | Positive | |
| Negative | 612 | 11 | 610 | 13 |
| CDV positive | 41 | 41 | ||
| PDV positive | 2 | 11 | 1 | 12 |
| CDV-PDV positive | 33 | 33 | ||
| DMV positive | 3 | 3 | 3 | 3 |
| PMV positive | 1 | 1 | 1 | 1 |
| DMV-PMV positive | 3 | 5 | 2 | 6 |
| DMV-PDV positive | 1 | 1 | 1 | 1 |
| Undetermined | 8 | 8 | ||
| Total | 622 | 114 | 618 | 118 |
Indirect ELISA.
Indirect ELISA was used to determine MAb reactivity against the four viruses. The test was performed using a modification of established procedures (7) as previously described for another morbillivirus (22). Volumes of 100 μl/well were used unless stated otherwise. Briefly, Immulon-2 flat-bottom 96-well plates (Dynex, Alexandria, Va.) were coated with gradient-purified whole CDV at 100 μl/well containing 1 μg of total protein per ml in carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. This antigen concentration (1 or 0.1 μg/well) was selected based on previous experience with another morbillivirus (22). The following day, the plates were washed four times with PBS containing 0.05% Tween 20 (PBST) and incubated for 1 h at 37°C with MAb diluted in PBST containing 10% FBS (PBST-FBS). The plates were washed again and incubated for 1 h at 37°C with peroxidase-labeled anti-mouse immunoglobulin G (whole molecule) (Sigma Chemical Co., St. Louis, Mo.) diluted 1:1,000 in PBST-FBS. Following another wash step, the plates were reacted with a substrate-chromogen mixture consisting of 0.01% hydrogen peroxide and 0.1 mg of 3,3′,5,5′-tetramethylbenzidine (Sigma Chemical Co.) per ml in 0.05 M citrate–phosphate buffer (pH 5.0). After the plates were incubated at room temperature for 20 min on a plate shaker, color development was stopped by adding 25 μl of 2 M sulfuric acid per well. Optical density (OD) readings were taken at a wavelength of 450 nm, using a computer-interfaced ELISA plate reader.
cELISA.
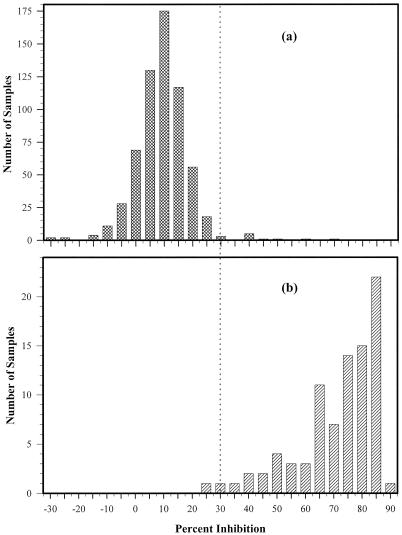
The cELISA depends upon the ability of serum antibody to compete with a MAb for binding to antigen. Competition is detected as a reduction in the OD reading of serum-MAb wells when compared to the OD of a control well with MAb alone. The gradient-purified whole CDV antigen, against which the MAbs were raised, was used as the cELISA antigen at the previously determined concentration of 0.1 μg/well. Antigen-coated Immulon-2 96-well flat-bottom plates (Dynex Laboratories) were incubated with 50 μl of serum per well diluted in PBST for 30 min at 37°C. An equal volume (50 μl/well) of MAb diluted in PBST was then added to plates without their being washed. Controls consisting of three wells without MAb or serum and three wells with MAb alone were included on each plate. The serum-MAb mixtures were allowed to react with the antigen for 1 h. The rest of the procedure was carried out exactly as described for the indirect ELISA above. The OD values were used to calculate the percent inhibition induced by each serum, using the formula percent inhibition = [1 − (ODSer/ODMAb)] × 100, where ODSer is the mean OD of wells with serum and MAb and ODMAb is the mean OD of wells with MAb alone. The percent inhibition values from a VNT-negative population (n = 623) were used to establish a negative cutoff value by adding 2 standard deviations to the mean percent inhibition (Fig. 1).
FIG. 1.
Establishment of a negative cutoff point for the MAb2 cELISA. (a) All VNT-negative sera (n = 623); (b) CDV- and PDV-specific VNT-positive sera (n = 87). The dotted line represents the cutoff point of 30% inhibition for distinguishing between negative and positive samples.
Determination of optimal MAb and serum dilutions.
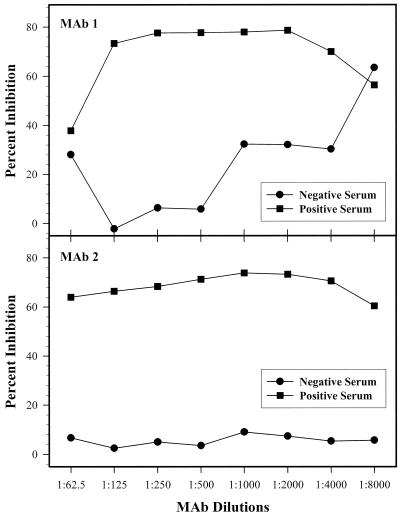
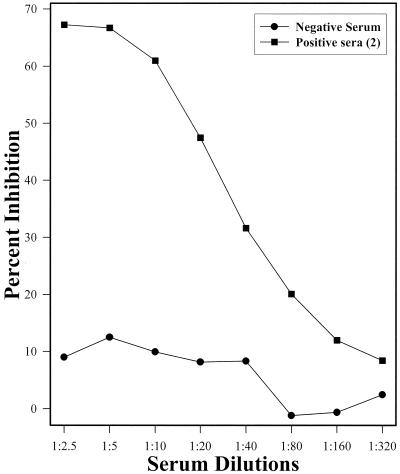
Three serum samples (two positive samples and one negative sample) were selected on the basis of their reactivities in the VNT for use in the establishment of cELISA parameters. Those sera were as follows: one polar bear sample with VN titers of 64, 12, 8, and 8 against CDV, PDV, DMV, and PMV, respectively (positive serum 1); one harbor seal serum sample with VN titers of 16, 64, 8, and 8 against the same viruses, respectively (positive serum 2); and one seal serum sample with a VN titer of <8 against all four viruses (negative serum). The cELISA using MAb1 was optimized with positive serum 1, while the test using MAb2 was optimized with positive serum 2; the same negative serum was used for optimization of both assays. It has previously been determined for another morbillivirus cELISA that serum dilutions of 1:10 to 1:20 compete well with MAb (22). Therefore, for establishment of the optimal MAb dilution, the sera were tested at a fixed dilution of 1:10 against serial twofold dilutions of MAbs (Fig. 2). Similarly, the optimal serum dilution was determined by testing serial twofold dilutions of the sera against the optimal MAb dilution (Fig. 3). The cELISA percent inhibition values thus generated were plotted against the MAb dilution (Fig. 2) or serum dilution (Fig. 3). The optimal dilution for each reagent was determined to be the highest dilution that yielded the maximum percent inhibition differential between positive and negative sera.
FIG. 2.
Determination of the optimal dilution of MAbs. Serial twofold dilutions of each MAb were reacted with a 1:10 dilution of negative and positive control sera in a cELISA. A MAb dilution of 1:500 was chosen.
FIG. 3.
Determination of an optimal dilution of serum. Serial twofold dilutions of negative and positive control sera were reacted with the optimal MAb dilution (1:500) in a cELISA. To minimize the volume of serum required, a serum dilution of 1:10 was chosen as the preferred dilution over lower dilutions that performed only slightly better.
Comparison of VNT and cELISA for titration of sera.
The 736 serum samples used in this study were tested for morbillivirus antibody using the VNT and the two newly developed cELISAs. Results of both the VNT and cELISA were expressed as positive or negative for each sample to allow a qualitative comparison of the results (Table 2).
Statistical analysis.
Using the VNT as the gold standard, the sensitivity and specificity and the exact binomial confidence intervals for these estimated parameters were calculated for each cELISA (11). Agreement between VNT and cELISA beyond chance was estimated by calculating the agreement quotient (kappa) (16). Calculations were performed using various statistical and epidemiological softwares (SPSS for Windows, version 10.0.7 [SPSS Inc., Chicago, Ill.]; StatXact-4 [Cytel Software Corp., Cambridge, Mass.]; PEPI 3.01 [Screening and Diagnostic Tests, Unicorn Software Development, Inc., Stone Mountain, Ga.]). A P value of 0.05 was used to establish the level of significance for statistical tests.
For evaluations of cELISA, sera that had positive but undetermined antibody specificity for VNT (n = 8) were excluded. Sera that had unknown family or species identification (n = 64) were also excluded. Other deletions included 36 sera from sea otters (Mustelidae), which had no VNT or cELISA positive results, and 3 sera that had unusual VNT-positive results that were not considered to be representative of marine mammals (2 serum samples that were positive for both DMV and PDV and 1 serum sample from a whale that was positive for PDV). After these exclusions were made, 625 serum samples from 109 Canidae, 117 Cetacea, and 399 Pinnipeda were used to evaluate cELISA performance (Table 3).
TABLE 3.
Estimate of cELISA performance relative to that of VNT
| MAb | VNT antibody specificity | Animal group | Sensitivity (%) | Specificity (%) | Kappa |
|---|---|---|---|---|---|
| MAb1 | CDV, PDV, and CDV-PDV | Canidae | 100.0 | 94.3 | 0.96 |
| Pinnipedia | 80.0 | 99.0 | 0.72 | ||
| Canidae-Pinnipedia | 97.6 | 98.6 | 0.94 | ||
| DMV, PMV, and DMV-PMV | Cetacea | 61.5 | 95.2 | 0.57 | |
| MAb2 | CDV, PDV, and CDV-PDV | Canidae | 100.0 | 91.4 | 0.94 |
| Pinnipedia | 90.0 | 99.0 | 0.78 | ||
| Canidae-Pinnipedia | 98.8 | 98.4 | 0.95 | ||
| DMV, PMV, and DMV-PMV | Cetacea | 69.2 | 95.2 | 0.62 |
RESULTS
Optimal dilutions of MAb and serum.
The main objective of this study was to develop cELISAs that could detect and differentiate between CDV and PDV antibodies in serum. The ability of MAbs to compete with serum antibody depends not only on the specificities of their binding to antigen but also on the relative amounts of these reagents in the reaction mix. It was thus important to titrate both of these critical reagents to determine the combination that yielded the best discrimination between positive and negative control sera. The optimal dilution of both MAbs was 1:500 (Fig. 2), while the preferred serum dilution was 1:10 (Fig. 3). This dilution was chosen for serum over apparently better-performing lower dilutions (Fig. 3) because marine mammal serum is often a limiting factor with regard to quantity received in the diagnostic laboratory.
Determination of the negative cutoff value.
The negative cutoff value was arbitrarily set by adding 2 standard deviations to the mean percent inhibition yielded by 623 sera determined to be negative for morbillivirus antibody by VNT. Using these values (37 and 30%, respectively, for MAb1 and -2), there was very good discrimination between negative sera and a population of sera positive for CDV or PDV (Fig. 1). The initial expectation was that sera positive for DMV or PMV would not react in any of the cELISAs since none of the two MAbs reacted specifically with either DMV or PMV. Surprisingly, however, both cELISAs recognized over 60% of DMV- and PMV-positive sera (Table 2). Overall, the MAb1 and MAb2 cELISAs exceeded 92% sensitivity and 97% specificity. The high overall sensitivity and specificity indicated that these cutoff values were valid.
Comparison of the cELISA and VNT.
The criterion for designation of a homologous virus was set such that a serum sample had to yield a titer of antibody to a single virus at least twofold higher than the titers induced by the other morbilliviruses before that virus would be determined to have induced the antibody in the serum. Based on this criterion, of the 118 sera that had a positive antibody titer, the homologous virus could be determined in 48% (57 of 118). Of the remainder, 28% (33 of 118) were CDV or PDV positive, 5% (6 of 118) were DMV or PDV positive, 0.85% (1 of 118) were DMV or PDV positive, and 6.8% (8 of 118) were of undetermined antibody status (Table 2). In recognition of this apparent failing of the gold standard to be more definitive, all data analyses of positive samples used the CDV-PDV and DMV-PMV pairs rather than the individual viruses.
Overall, the MAb1 cELISA was slightly less sensitive than the MAb2 cELISA (92.8 versus 94.9%), although this difference was not statistically significant. When used on CDV-PDV or DMV-PMV sera, the MAb2 cELISA was more sensitive than the MAb1 cELISA (98.8 or 69.2% versus 97.6 or 61.5%). However, the MAb1 cELISA was only slightly more specific than the MAb2 assay (97.9 versus 97.7%). None of these differences, however, between the MAb1 and MAb2 cELISAs was statistically significant. All of the values for kappa showed high agreement beyond chance (0.57 to 0.96) between VNT and corresponding cELISA results (P < 0.01). These results provided evidence that the cELISAs were valid tests.
Evaluation of the cELISAs according to animal groups produced expected results. The greatest sensitivity (100%) occurred with sera from the Canidae family. Samples from Pinnipeda provided the greatest specificity (99%). These two families involved the combination of CDV-PDV pairs from VNT. Both the sensitivity and specificity for these two families combined exceeded 98%. The results previously stated for the DMV-PVM pairs were entirely from Cetacea. The sensitivity was significantly lower for sera from this family (69.2% for MAb2) than for samples from Canidae or Pinnipeda based on an evaluation of 95% confidence intervals. The specificity (95.2% for both MAb1 and MAb2) was intermediate between the corresponding specificities for Canidae (91.4% for MAb2) and Pinnipeda (99.0% for both Mab1 and Mab2). All of the 95% confidence intervals for specificity overlapped among the different animal group or virus pair comparisons, which indicated no significant difference among these specificity estimates.
DISCUSSION
Morbillivirus infections currently occur in marine mammals in the Pacific and Atlantic oceans and the Mediterranean, Caspean, and North seas (23). Serology is a major epidemiological tool used to detect the occurrence of morbillivirus infections in marine mammal populations where clinical disease has not been observed. For example, serological evidence indicates that morbillivirus infections occur in polar bears, although clinical morbillivirus disease has not yet been described (8, 9). The availability of a simple, fast, reliable, and inexpensive test would provide a tool that can be readily used by various laboratories for diagnostic and epidemiological purposes. The cELISA described herein fits that role.
The initial goal of this study was to develop two cELISAs that could detect and differentiate between antibodies induced by CDV and PDV. This was a reasonable expectation because MAb1 was CDV specific while MAb2 reacted with CDV and PDV; both MAbs did not react with DMV and PMV by indirect ELISA. It was therefore surprising to observe that MAb1 competed against PDV-specific sera and that both MAbs competed against DMV- and PMV-specific sera for binding to solid-phase CDV antigen. A possible explanation for this cross-reactivity is that steric hindrance caused by cross-reactive serum antibody binding to epitopes close to the specific epitope recognized by the MAbs prevented them from binding. Although this outcome was not expected at the beginning, it was useful because a single MAb-based cELISA could be used to detect antibody against the four morbilliviruses.
Comparison of each cELISA with VNT for detection of CDV-PDV antibody yielded relative sensitivities and specificities of 97.6% or greater. It was observed that sample quality had an adverse effect on test results (data not shown). For example, 11 of the 13 samples that yielded false-positive results on MAb2 cELISA were of very poor quality: they were cloudy (indicating apparent microbial contamination), hemolyzed, or rich in fat. The relative sensitivity of the MAb2 cELISA for detection of DMV-PMV antibody was 69.2%. While this is a relatively low value, it is nevertheless significant for a test based on nonspecific cross-reactive binding. The MAb2 cELISA is thus be useful for quick screening of sera for all morbilliviruses. If CDV and PDV are suspect viruses, testing could end with the cELISA. If DMV or PMV is suspected under low-prevalence situations (<20%), the cELISA would still provide a negative predictive value of ≥93% as a screening test. The VNT could be performed on the positive sera if improvement in positive predictive value was needed, especially for lower-prevalence situations or in order to confirm diagnosis related to DMV or PMV.
The data from this study have confirmed previous observations by us (unpublished) and others (8) that the VNT is not always able to differentiate among antibodies from the various morbilliviruses, especially the closely related CDV-PDV and DMV-PMV pairs. From the diagnostic standpoint, we propose to use the designations pinniped virus and cetacean virus to encompass the CDV-PDV and DMV-PMV pairs, respectively. For epidemiology purposes, serology might still be used to identify the exact virus involved in a given population if mean population antibody titers against the four viruses are compared (8). In that case, the virus that yields the highest mean titer from the population of samples tested would be considered the homologous virus.
ACKNOWLEDGMENTS
This work was supported by a grant from the Morris Animal Foundation (98Z0-29).
The laboratory help of Shannon Caseltine, Julie Erbeck, and Wendy Clark is gratefully acknowledged.
REFERENCES
- 1.Domingo M L, Ferrer L, Pumarola M, Plana M A, Kennedy J, McAlisky S, Rima B K. Morbillivirus in dolphins. Nature. 1990;336:21. doi: 10.1038/348021a0. [DOI] [PubMed] [Google Scholar]
- 2.Domingo M L, Vilafranca M, Visa J, Prats N, Trudgett A L, Visser I. Evidence of chronic morbillivirus infection in the Mediterranean striped dolphin (Stenella coeruleoalba) Vet Microbiol. 1995;44:229–239. doi: 10.1016/0378-1135(95)00016-4. [DOI] [PubMed] [Google Scholar]
- 3.Duignan P J, Nielsen O, House C, Kovacs K M, Duffy N, Early G, Sadove S, St. Aubin D J, Rima B K, Geraci J R. Epizootiology of morbillivirus infection in harp, hooded, and ringed seals from the Canadian Arctic and Western Atlantic. J Wildl Dis. 1997;33:7–19. doi: 10.7589/0090-3558-33.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Duignan P J, Sadove S, Saliki J T, Geraci J R. Phocine distemper in harbor seals (Phoca vitulina) from Long Island, New York. J Wildl Dis. 1993;29:465–469. doi: 10.7589/0090-3558-29.3.465. [DOI] [PubMed] [Google Scholar]
- 5.Duignan P J, Saliki J T, St. Aubin D J, House J A, Geraci J R. Neutralizing antibodies to phocine distemper virus in Atlantic walruses (Odobenus rosmarus rosmarus) from Arctic Canada. J Wildl Dis. 1994;30:90–94. doi: 10.7589/0090-3558-30.1.90. [DOI] [PubMed] [Google Scholar]
- 6.Duignan P J, Saliki J T, St. Aubin D J, Early G, Sadove S, House J A, Kovacs K, Geraci J R. Epizootiology of morbillivirus infection in North American harbor seals (Phoca vitulina) and gray seals (Halichoerus grypus) J Wildl Dis. 1995;31:491–501. doi: 10.7589/0090-3558-31.4.491. [DOI] [PubMed] [Google Scholar]
- 7.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, ELISA-III. Quantitation of specific antibodies by enzyme-labeled antiimmunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129–135. [PubMed] [Google Scholar]
- 8.Follmann E H, Garner G W, Evermann J F, McKeirman A J. Serological evidence of morbillivirus infection in polar bears (Ursus maritimus) from Alaska and Russia. Vet Rec. 1996;138:615–618. doi: 10.1136/vr.138.25.615. [DOI] [PubMed] [Google Scholar]
- 9.Garner G W, Evermann J F, Saliki J T, Follmann E H, McKeirnan A J. Morbillivirus ecology in polar bears (Ursus maritimus) Polar Biol. 2000;23:474–478. [Google Scholar]
- 10.Grachev M A, Kumarev V P, Mamaev L V, Zorin L, Baranova L V, Denekina N N, Belikova S I, Petrov E A, Kolesnik V S, Dorofeev V N, Beim A M, Kudelin V N, Nagieva F G, Sidorov V N. Distemper virus in Baikal seals. Nature. 1989;338:209. doi: 10.1038/338209a0. [DOI] [PubMed] [Google Scholar]
- 11.Greiner M, Gardner I A. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev Vet Med. 2000;45:3–22. doi: 10.1016/s0167-5877(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 12.Henderson G A, Trudgett C, Lyons C, Ronald K. Demonstration of antibodies in archival sera from Canadian seals reactive with a European isolate of phocine distemper virus. Sci Total Environ. 1992;115:93–98. doi: 10.1016/0048-9697(92)90035-q. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy S, Smyth J A, Cush P F, McAliskey M, Moffett D, McNiven C M, Carole M. Morbillivirus infection in two common porpoises (Phocoena phocoena) from the coasts of England and Scotland. Vet Rec. 1992;131:286–290. doi: 10.1136/vr.131.13.286. [DOI] [PubMed] [Google Scholar]
- 14.Lipscomb T P, Kennedy S, Moffett D, Kraft A, Klaunberg B A, Lichy J H, Regan G T, Worthy G A J, Taubenberger J K. Morbilliviral epizootic in bottlenose dolphins of the Gulf of Mexico. J Vet Diagn Investig. 1996;8:283–290. doi: 10.1177/104063879600800302. [DOI] [PubMed] [Google Scholar]
- 15.Lipscomb T P, Schulman F Y, Moffett D, Kennedy S. Morbilliviral disease in Atlantic bottlenose dolphins (Tursiops truncatus) from the 1987–88 epizootic. J Wildl Dis. 1994;30:567–571. doi: 10.7589/0090-3558-30.4.567. [DOI] [PubMed] [Google Scholar]
- 16.Martin S W, Meek A H, Willeberg P. Veterinary epidemiology. Ames: Iowa State University Press; 1988. pp. 73–75. [Google Scholar]
- 17.Osterhaus A D M E, Groen E J, Spijkers H E M, Broeders H W J, UytdeHaag F G C M, De Vries P, Teppema J S, Visser I K G, van de Bildt M W G, Vedder E J. Mass mortality in seals caused by a newly discovered morbillivirus. Vet Microbiol. 1990;23:343–350. doi: 10.1016/0378-1135(90)90165-r. [DOI] [PubMed] [Google Scholar]
- 18.Osterhaus A D M E, Vedder E J. Identification of a virus causing recent seal deaths. Nature. 1988;335:20. doi: 10.1038/335020a0. [DOI] [PubMed] [Google Scholar]
- 19.Reidarson T H, McBain J, House C, King D P, Stott J L, Kraft A, Taubenberger J K, Heyning J, Lipscomb T P. Morbillivirus infection in stranded common dolphins from the Pacific Ocean. J Wildl Dis. 1998;34:771–776. doi: 10.7589/0090-3558-34.4.771. [DOI] [PubMed] [Google Scholar]
- 20.Ross P S, Visser I K G, Broeders H W, van De Bilt W G, Bowen W D, Osterhaus A D M E. Antibodies to phocine distemper virus in Canadian seals. Vet Rec. 1992;130:514–516. doi: 10.1136/vr.130.23.514. [DOI] [PubMed] [Google Scholar]
- 21.Rossiter P B, Jessett D M, Taylor W P. Microneutralisation systems for use with different strains of peste des petits ruminants virus and rinderpest virus. Trop Anim Health Prod. 1985;17:75–81. doi: 10.1007/BF02360775. [DOI] [PubMed] [Google Scholar]
- 22.Saliki J T, Libeau G, House J A, Mebus C A, Dubovi E J. Monoclonal antibody-based enzyme-linked immunosorbent assay for specific detection and titration of peste-des-petits-ruminants virus antibody in caprine and ovine sera. J Clin Microbiol. 1993;31:1075–1082. doi: 10.1128/jcm.31.5.1075-1082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taubenberger J K, Tsai M M, Atkin T J, Fanning T G, Kraft A E, Moeller R B, Kodsi S E, Mense M G, Lipscomb T P. Molecular genetic evidence of a novel morbillivirus in a long-finned pilot whale (Globicephalus melas) Emerg Infect Dis. 2000;6:42–45. doi: 10.3201/eid0601.000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor W P. Serological studies with the virus of peste des petits ruminants in Nigeria. Res Vet Sci. 1979;26:236–242. [PubMed] [Google Scholar]
- 25.Van Bressem M F, Van Waerebeek K, Fleming M, Barrett T. Serological evidence of morbillivirus infection in small cetaceans from the southeast Pacific. Vet Microbiol. 1998;59:89–98. doi: 10.1016/s0378-1135(97)00169-7. [DOI] [PubMed] [Google Scholar]
- 26.Visser I K G, Kumarev V P, Orvell C, de Vries P, Broeders H W J, van de Bildt M W G, Groen J, Teppema J S, Burger M C, UytdeHaag F G C M, Osterhaus A D M E. Comparison of two morbilliviruses isolated from seals during outbreaks of distemper in North West Europe and Siberia. Arch Virol. 1990;111:149–164. doi: 10.1007/BF01311050. [DOI] [PubMed] [Google Scholar]
- 27.Visser I K G, Van Bressem M F, de Swart R L, van de Bildt M W G, Vos H W, van der Heijden R W J, Saliki J T, Orvell C, Kitching P, Kuiken T, Barrett T, Osterhaus A D M E. Characterization of morbilliviruses isolated from dolphins and porpoises in Europe. J Gen Virol. 1993;74:631–641. doi: 10.1099/0022-1317-74-4-631. [DOI] [PubMed] [Google Scholar]