Abstract
Background
Invasive mechanical ventilation plays an important role in the prognosis of patients with sepsis. However, there are, currently, no tools specifically designed to assess weaning from invasive mechanical ventilation in patients with sepsis. The aim of our study was to develop a practical model to predict weaning in patients with sepsis.
Methods
We extracted patient information from the Medical Information Mart for Intensive Care Database-IV (MIMIC-IV) and the eICU Collaborative Research Database (eICU-CRD). Kaplan–Meier curves were plotted to compare the 28-day mortality between patients who successfully weaned and those who failed to wean. Subsequently, MIMIC-IV was divided into a training set and an internal verification set, and the eICU-CRD was designated as the external verification set. We selected the best model to simplify the internal and external validation sets based on the performance of the model.
Results
A total of 5020 and 7081 sepsis patients with invasive mechanical ventilation in MIMIC-IV and eICU-CRD were included, respectively. After matching, weaning was independently associated with 28-day mortality and length of ICU stay (p < 0.001 and p = 0.002, respectively). After comparison, 35 clinical variables were extracted to build weaning models. XGBoost performed the best discrimination among the models in the internal and external validation sets (AUROC: 0.80 and 0.86, respectively). Finally, a simplified model was developed based on XGBoost, which included only four variables. The simplified model also had good predictive performance (AUROC:0.75 and 0.78 in internal and external validation sets, respectively) and was developed into a web-based tool for further review.
Conclusions
Weaning success is independently related to short-term mortality in patients with sepsis. The simplified model based on the XGBoost algorithm provides good predictive performance and great clinical applicablity for weaning, and a web-based tool was developed for better clinical application.
Keywords: sepsis, invasive mechanical ventilation, weaning, XGBoost, simple prediction model
Introduction
Difficult weaning or prolonged invasive mechanical ventilation is more common in patients with sepsis (1, 2). Lung susceptibility to ventilatory injury is thought to be increased by sepsis (3, 4), and mechanical ventilation may also lead to the exacerbation of pulmonary infection (5). Prolonged mechanical ventilation can lead to a poor prognosis (6, 7). However, insufficient duration of mechanical ventilation is unfavorable for patients. Weaning in unprepared patients leads to increased mortality and prolonged ICU stay (8). Therefore, the choice of an appropriate weaning time is of great importance.
Previous studies on weaning have evaluated numerous methods on weaning, such as rapid shallow breathing index (RSBI) (9), spontaneous breathing experiment (SBT), and compliance, oxygenation, respiratory rate, and pressure (CROP) index. Nevertheless, weaning factors specific to patients with sepsis are scarce. Unfortunately, the accuracy of these factors in predicting weaning is unsatisfactory (10, 11). Moreover, weaning from mechanical ventilation has also been shown to be related to consciousness, diaphragmatic function, and cardiac function (12–14). Traditional prediction of weaning has several limitations. On the one hand, traditional methods of weaning as a complex process are inadequate for the use of clinical indicators utilization. On the other hand, traditional methods have deficits in predictive performance due to disease-related differences in the target population, as there are no specific target populations.
Given the rapid development of clinical medicine, a refined weaning scheme is needed to meet the demands of clinical development. A simple and reliable weaning program could not only effectively assist clinicians but also improve the patient prognosis, especially in patients with sepsis with ventilator dependence.
In this study, we aimed to develop a reliable model for predicting weaning success in patients with sepsis. To this end, we extracted data within 24 h from patients with sepsis before weaning from a large dataset. Features were selected based on their clinical availability and explained by their importance. In addition, our model was further validated using datasets from various sources.
Methods
Data Source
Our study was a retrospective cohort study based on the MIMIC-IV (version 1.0) database. This database contains over 40,000 ICU patients from Beth Israel Deaconess Medical Center between 2008 and 2019. Moreover, we used an independent external validation set called eICU-CRD Collaborative Research (eICU-CRD) Database (version 2.0), which is a multicenter database of over 200,000 ICU admissions in the United States. We carefully studied the courses and obtained permission to use the database (record ID 39691989). Because the patient privacy information was encrypted in the database, the ethics committee at the two medical centers did not require informed consent.
Patients and Definitions
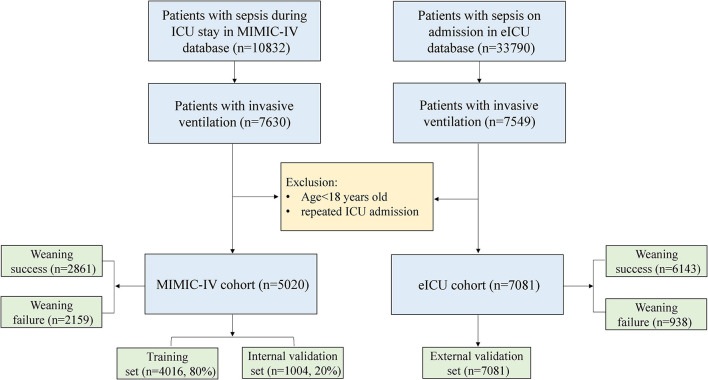
In this study, sepsis was diagnosed based on the Sepsis-3 criteria [(15); SOFA score ≥ 2, and suspicious infection]. The contents of a recent guideline had been considered before implementing the criteria for successful weaning (16). The definition of weaning success (WS) was as follows: (a) no intubation or invasive ventilation within 48 h after weaning, (b) no death within 48 h after weaning, and (c) noninvasive ventilation time was shorter than 48 h after weaning. The patients who experienced invasive mechanical ventilation and met the criteria for Sepsis-3 were included in both datasets. The exclusion criteria were as follows: (a) repeated ICU admissions and (b) age <18 years (Figure 1).
Figure 1.
The flow chart of data extraction. eICU-CRD, eICU-CRD Collaborative Research Database. MIMIC-IV, Medical Information Mart for Intensive Care-IV; ICU, intensive care unit.
Data Collection and Variable Extraction
For modeling, we preferred variables with a single measurement. Clinical importance and shapley additive explanations (SHAP) values were used to further reduce the amount of data. Patient demographics were collected, including age, sex, and body mass index (BMI). Clinical and chemical variables had been extracted within 24 h prior to weaning to create models. The extracted variables were the worst value of the day, which was as follows: arterial blood gas [pH value, arterial oxygen partial pressure (PaO2), arterial carbon dioxide partial pressure (PaCO2), base excess (BE)], full blood count [white blood cell count (WBC), hemoglobin (HB), platelet (PLT)], laboratory index (creatinine, anion gap), vital signs [heart rate, respiratory rate, mean arterial pressure (MAP), peripheral oxygen saturation (SPO2), temperature, oxygenation index (OI)], and urine output. In addition, data on therapeutic measures [days of invasive ventilation, days of antibiotic use, days of continuous renal replacement therapy (CRRT), and vasopressor therapy within 24 h]. The variables in the matched data were from the first day of ICU admission and included the variables listed above (Supplementary Table 1). To further balance the differences in baseline data between the patients with weaning failure (WF) and patients with weaning success, comprehensive indicators were extracted, such as the sequential organ failure assessment (SOFA) score, Glasgow coma scale (GCS) score, and simplified acute physiology score (SAPS II). Comorbidities, as well as infection classification (Supplementary Table 4), were also considered based on the recorded International Classification of Diseases codes (ICD-9 and ICD-10), and the Charlson comorbidity index was also calculated.
Data Analysis
Continuous variables are described as median and interquartile range (IQR). The Mann–Whitney U-test was used for statistical comparison between the two groups. Categorical variables were described as total number and percentage, and the chi-square test or Fisher's exact test was used for comparison between groups. Propensity score matching (PSM) was used to balance the differences between successful and unsuccessful weaning groups. Inverse probability weighting (IPW) (17) was used to further adjust for possible imbalances between the variables of the two groups. The Kaplan–Meier (K–M) curve was used to describe the 28-day survival rate between the two groups, and the differences in survival rates between groups were compared using the log-rank test.
After comparison, we divided the MIMIC-IV data into two parts: 80% as a training set and 20% as an internal validation set, and the integrated machine-learning algorithm eXtremely Gradient Boosting (XGBoost) to construct a weaning prediction model, which is based on multiple decision trees with gradient boost as a learning framework. The hyperparameters were optimized using a grid search (Supplementary Table 3). Other models, such as KNearest Neighbor (KNN), Multi-Layer Perceptron (MLP), Random Forest (RF), Support Vector Machine (SVM), and Logistic Regression (LR), were also derived from the training set and applied to the test set. The prediction efficiency of the models was compared using a receiver operating characteristic (ROC) curve. In addition, the model was further explained by the SHAP value, demonstrating a linear relationship through local weighted regression scatter smoothing (LOWESS).
Variables with more than 50% missing data were excluded. The missing features of the matched data and model data are shown in Supplementary Figure 1A and Supplementary Table 2. Missing values were input using the multiple imputation method. Due to the different missing datasets, we had extracted only the CROP and RSBI 24 h before weaning in MIMIC-IV, and the predictive performance was also compared with XGBoost in the internal validation set. In addition, the model was further simplified using the recursive feature elimination algorithm (RFE).
Structured query language was used to extract the data from these two databases. All statistical analyses were performed using R 3.6.2 (Chicago, Illinois) and Python (version 3.6.6), and statistical significance was set at p < 0.05.
Results
Matching Baseline and Clinical Outcomes
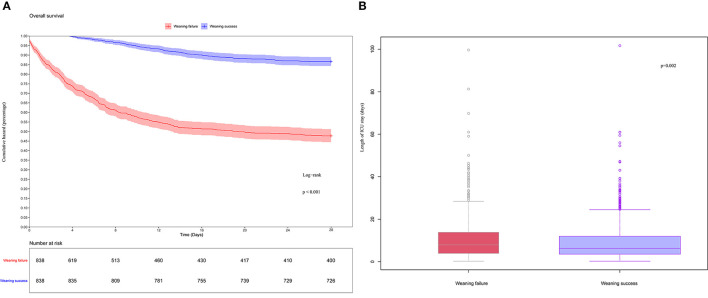
As shown in Figure 1, 5,020 patients with sepsis who received invasive mechanical ventilation were ultimately included in the MIMIC-IV database. The baseline characteristics on the first day of ICU admission are shown in Supplementary Table 1. PSM and IPW were used to better balance the differences between the two groups. A total of 1,676 patients were included (Supplementary Table 1), and the standardized mean difference (SMD) between groups was significantly reduced (Supplementary Figure 1B). In comparison, there was a significant difference in the length of stay in the ICU between the two groups (Figure 2B, p = 0.002). Similarly, the WS group had a significantly lower 28-day mortality rate (Figure 2A, p < 0.001).
Figure 2.
(A) K-M curves estimated 28-day survival probability of weaning failure and weaning success patients. (B) Box-plot of weaning failure and weaning success patients.
Baseline Characteristics of Models
In the MIMIC-IV cohort, successful weaning was associated with reductions in highest WBC, highest creatinine, highest anion gap, highest heart rate, highest respiratory rate, highest body temperature, highest PEEP level, antibiotic duration, invasive mechanical ventilation (IMV) duration, and vasopressor use 1 day before weaning (Table 1). Consequently, the performance of these indicators behaved similarly in the eICU-CRD cohort, except for age and the highest FiO2 (Table 1). Regarding comorbidity, it was observed that successful weaning benefited chronic pulmonary disease, congestive heart failure, renal disease, and diabetes in the MIMIC-IV cohort. However, except for severe liver disease, other comorbidities were inconsistent between the WS and WF groups (Table 1).
Table 1.
Baseline characteristics of the MIMIC-IV and eICU cohorts.
| Variables | MIMIC-IV cohort | eICU cohort | ||||||
|---|---|---|---|---|---|---|---|---|
|
Overall (n = 5,020) |
Weaning failure (n = 2,159) |
Weaning success (n = 2,861) |
p |
Overall (n = 7,081) |
Weaning failure (n = 938) |
Weaning success (n = 6,143) |
p | |
| n | 5,020 | 2,159 | 2,861 | 7,081 | 938 | 6,143 | ||
| Age (years) | 68 (57, 78) | 68 (56, 79) | 68 (57, 78) | 0.335 | 64 (53, 74) | 68 (57, 77) | 63 (52, 73) | <0.001 |
| Male | 2,944 (58.6) | 1,241 (57.5) | 1,703 (59.5) | 0.154 | 3,976 (56.2) | 511 (54.5) | 3,465 (56.4) | 0.283 |
| BMI (kg/m2) | 28 (24, 33) | 28 (24, 33) | 28 (24, 32) | 0.428 | 28 (23, 33) | 27 (23, 33) | 28 (23, 33) | 0.02 |
| Chronic pulmonary disease (n, %) | 1,719 (34.2) | 689 (31.9) | 1,030 (36.0) | 0.003 | 1,334 (18.8) | 175 (18.7) | 1,159 (18.9) | 0.914 |
| Congestive heart failure (n, %) | 2,155 (42.9) | 788 (36.5) | 1,367 (47.8) | <0.001 | 1,174 (16.6) | 158 (16.8) | 1,016 (16.5) | 0.852 |
| Dementia (n, %) | 239 (4.8) | 88 (4.1) | 151 (5.3) | 0.056 | 207 (2.9) | 40 (4.3) | 167 (2.7) | 0.012 |
| Severe liver disease (n, %) | 547 (10.9) | 286 (13.2) | 261 (9.1) | <0.001 | 209 (3.0) | 60 (6.4) | 149 (2.4) | <0.001 |
| Renal disease (n, %) | 1,463 (29.1) | 574 (26.6) | 889 (31.1) | 0.001 | 949 (13.4) | 151 (16.1) | 798 (13.0) | 0.011 |
| Rheumatic disease (n, %) | 211 (4.2) | 84 (3.9) | 127 (4.4) | 0.375 | 165 (2.3) | 21 (2.2) | 144 (2.3) | 0.934 |
| Diabetes (%) | 1,665 (33.2) | 675 (31.3) | 990 (34.6) | 0.014 | 2,129 (30.1) | 283 (30.2) | 1,846 (30.1) | 0.971 |
| Charlson comorbidity index | 6 (4, 8) | 6 (4, 8) | 6 (4, 8) | 0.082 | 3 (2, 5) | 4 (3, 6) | 3.00 (2, 5) | <0.001 |
| GCS | 14 (10, 15) | 13 (7, 15) | 14 (10, 15) | <0.001 | 8 (6, 10) | 4 (3, 8) | 9 (6, 10) | <0.001 |
| Highest WBC (×109/L) | 14.1 (9.8, 19.9) | 14.7 (9.8, 21.3) | 13.8 (9.9, 18.9) | <0.001 | 11.9 (8.7, 16.4) | 14.7 (9.7, 20.5) | 11.7 (8.6, 15.8) | <0.001 |
| Lowest hemoglobin (g/L) | 9.2 (8.0, 10.6) | 9.1 (7.9, 10.6) | 9.3 (8.1, 10.6) | 0.002 | 9.7 (8.4, 11.3) | 9.3 (7.9, 10.8) | 9.8 (8.5, 11.4) | <0.001 |
| Lowest platelets (×109/L) | 143 (89, 213) | 136 (75, 212) | 147 (100, 215) | <0.001 | 167 (113, 233) | 141 (76, 212) | 170 (118, 236) | <0.001 |
| Highest creatinine (mg/dL) | 1.3 (0.9, 2.3) | 1.6 (1.0, 2.7) | 1.2 (0.8, 1.9) | <0.001 | 1.1 (0.7, 1.8) | 1.7 (1.0, 3.0) | 1.0 (0.7, 1.6) | <0.001 |
| Highest anion gap (mEq/L) | 15.0 (13.0, 19.0) | 17.0 (14.0, 22.0) | 14.0 (12.0, 17.0) | <0.001 | 10.0 (8.0, 14.0) | 13.0 (10.0, 18.0) | 10.0 (7.7, 13.0) | <0.001 |
| Lowest pH level | 7.3 (7.3, 7.4) | 7.3 (7.2, 7.4) | 7.3 (7.3, 7.4) | <0.001 | 7.4 (7.3, 7.4) | 7.3 (7.2, 7.4) | 7.4 (7.3, 7.4) | <0.001 |
| Lowest PaO2 (mmHg) | 80 (53, 106) | 75 (48, 99) | 84 (60, 110) | <0.001 | 85 (69, 115) | 78.00 (61, 102) | 86 (69, 117) | <0.001 |
| Highest PaCO2 (mmHg) | 45 (39, 51) | 44 (38, 52) | 45 (40, 50) | 0.837 | 43 (37, 49) | 42 (36, 52) | 43 (37, 49) | 0.977 |
| Lowest base excess (mEq/L) | −3.0 (−7.0, 0.0) | −4.0 (−10.0, 0.0) | −2.0 (−5.0, 0.0) | <0.001 | −1.0 (−5.4, 2.0) | −5.5 (−12.4,0.6) | −0.6 (−5.0, 2.2) | <0.001 |
| Highest heart rate (/min) | 103 (90, 119) | 108 (94, 124) | 100 (88, 115) | <0.001 | 103 (90, 118) | 112 (97, 129) | 102.00 (89, 116) | <0.001 |
| Highest respiratory rate (/min) | 27 (23, 31) | 29 (24, 33) | 26 (22, 30) | <0.001 | 25 (21, 31) | 30.00 (25, 35) | 25.00 (21, 30) | <0.001 |
| Lowest MAP (mmHg) | 60 (54, 65) | 59 (52, 64) | 60 (56, 66) | <0.001 | 65 (57, 73) | 59 (49, 68) | 66 (59, 74) | <0.001 |
| Highest body temperature (°C) | 37.4 (37.0, 38.1) | 37.5 (36.9, 38.2) | 37.4 (37.1, 37.9) | 0.021 | 37.4 (37.0, 37.9) | 37.4 (36.9, 38.1) | 37.4 (37.1, 37.9) | 0.362 |
| Lowest SPO2 | 94 (91, 96) | 93 (89, 95) | 94 (92, 97) | <0.001 | 94 (91, 97) | 91 (82, 94) | 94 (91, 97) | <0.001 |
| Highest PEEP (cmH2O) | 7 (5, 10) | 9 (5, 12) | 6 (5, 10) | <0.001 | 5 (5, 8) | 5 (5, 10) | 5.00 (5, 6) | <0.001 |
| Lowest tidal volume (ml) | 397 (328, 455) | 395 (325 452) | 398 (329 459) | 0.154 | 422 (343, 497) | 423.50 (356, 493) | 422.00 (340, 498) | 0.591 |
| Lowest OI | 174 (105, 250) | 152 (91, 232) | 188 (120, 260) | <0.001 | 206 (144, 282) | 161 (98, 227) | 212 (150, 288) | <0.001 |
| Highest FiO2 (%) | 50 (40, 80) | 50 (40, 90) | 50 (40, 80) | <0.001 | 50 (40, 100) | 60 (40, 100) | 50 (40, 80) | <0.001 |
| Antibiotic duration (day) | 1 (1, 4) | 2 (1, 5) | 1 (1, 3) | <0.001 | 0 (0, 2) | 1 (0, 4) | 0 (0, 2) | <0.001 |
| CRRT duration (day) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | <0.001 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | <0.001 |
| IMV duration (day) | 1.5 (0.6, 3.7) | 1.9 (0.7, 4.4) | 1.2 (0.6, 3.2) | <0.001 | 2.0 (1.0, 5.0) | 3.0 (2.0, 6.0) | 2.0 (1.0, 5.0) | <0.001 |
| Urine output (ml/kg/h) | 0.6 (0.2, 1.2) | 0.5 (0.1, 1.1) | 0.7 (0.4, 1.3) | <0.001 | 0.6 (0.3, 1.1) | 0.3 (0.1, 0.8) | 0.64 (0.3, 1.1) | <0.001 |
| Vasopressor used 1 day before weaning (n, %) | 3,882 (77.3) | 1,712 (79.3) | 2,170 (75.8) | 0.004 | 2,337 (33.0) | 511 (54.5) | 1,826 (29.7) | <0.001 |
Values are presented as median and interquartile range (IQR); BMI, body mass index; GCS, Glasgow coma scale; WBC, white blood cell count; PaO2, arterial oxygen partial pressure; PaCO2, arterial carbon dioxide partial pressure; MAP, mean arterial pressure; SPO2, pulse oxygen saturation; PEEP, positive end expiratory pressure; OI, oxygenation index; FIO2, fraction inspired oxygen concentration; CRRT, continuous renal replacement therapy; IMV, invasive mechanical ventilation.
Comparison and Explanation of Models
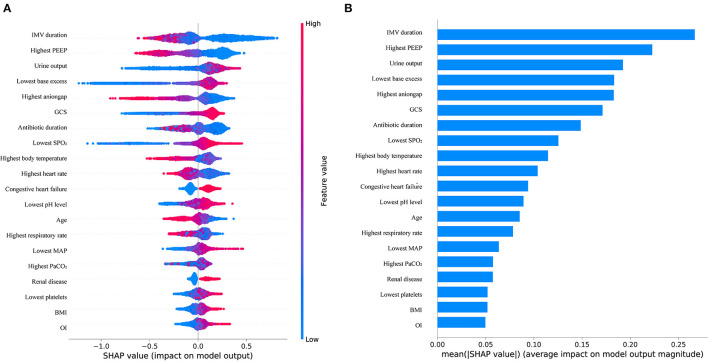
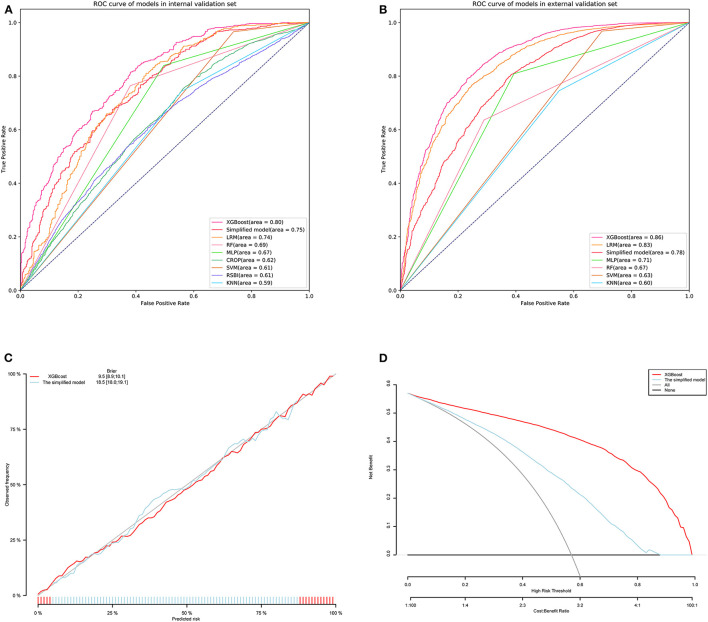
We trained the models using the training set from MIMIC-IV. As shown in Table 2, the XGBoost model with all available variables had a striking AUROC of 0.80 [95% confidence interval (CI): 0.77–0.82 in the internal validation set, and 0.86 (95% CI: 0.85–0.87)] in the external validation set, while the other five representative models had the highest AUROC, 00.74 (95% CI: 0.71–0.77) in the internal validation set, and 0.83 (95% CI: 0.82–0.84) in the external validation set. The final hyperparameter settings for XGBoost are listed in Supplementary Table 3. The SHAP values for the XGBoost model were assessed and are shown in Figure 3A. The importance of the variables was sorted by the gap value and is shown in Figure 3B. Figures 4A,B show the comparison between the XGBoost model and the other five models or predictive factors. As can be seen, the XGBoost model significantly outperformed the other five models or predictive factors in both the internal validation and external validation. Due to the extensive missing data, we did not show the ROC curves of CROP and RSBI in the external validation set. In addition, we performed a decision curve analysis (Figure 4C) and a calibration plot (Figure 4D) to illustrate the performance of the XGBoost model.
Table 2.
The predictive performance of models in the internal and external validation sets.
| Models | Internal validation set | External validation set | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUROC | Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUROC | Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| XGBoost | 0.80 (0.77–0.82) |
0.57 | 0.75 (0.71–0.78) |
0.71 (0.66–0.75) |
0.81 (0.79–0.84) |
0.62 (0.58–0.65) |
0.86 (0.85–0.87) |
0.51 | 0.96 (0.95–0.96) |
0.36 (0.34–0.38) |
0.79 (0.79–0.80) |
0.77 (0.74–0.79) |
| The simplified model | 0.75 (0.72–0.77) |
0.58 | 0.74 (0.70–0.78) |
0.61 (0.56–0.65) |
0.68 (0.65–0.70) |
0.68 (0.64–0.72) |
0.78 (0.77–0.79) |
0.54 | 0.93 (0.93–0.94) |
0.32 (0.30–0.34) |
0.80 (0.79–0.80) |
0.63 (0.60–0.65) |
| MLP | 0.67 (0.64–0.74) |
1 | 0.84 (0.81–0.87) |
0.50 (0.46–0.55) |
0.84 (0.81–0.86) |
0.50 (0.47–0.54) |
0.71 (0.69–0.72) |
1 | 0.93 (0.92–0.94) |
0.33 (0.30–0.35) |
0.81 (0.80–0.81) |
0.61 (0.58–0.63) |
| RF | 0.69 (0.66–0.72) |
1 | 0.73 (0.69–0.77) |
0.66 (0.61–0.70) |
0.76 (0.74–0.79) |
0.62 (0.58–0.65) |
0.67 (0.66–0.68) |
1 | 0.93 (0.93–0.94) |
0.23 (0.21–0.25) |
0.64 (0.63–0.64) |
0.71 (0.68–0.74) |
| SVM | 0.61 (0.58–0.64) |
1 | 0.64 (0.61–0.67) |
0.85 (0.77–0.90) |
0.97 (0.95–0.98) |
0.26 (0.24–0.29) |
0.63 (0.62–0.65) |
1 | 0.90 (0.89–0.91) |
0.59 (0.55–0.64) |
0.97 (0.96–0.97) |
0.30 (0.28–0.32) |
| LR | 0.74 (0.71–0.77) |
0.55 | 0.72 (0.68–0.75) |
0.68 (0.63–0.73) |
0.81 (0.78–0.83) |
0.57 (0.53–0.59) |
0.83 (0.82–0.84) |
0.58 | 0.95 (0.94–0.96) |
0.33 (0.31–0.35) |
0.77 (0.76–0.77) |
0.75 (0.73–0.78) |
| KNN | 0.59 (0.56–0.62) |
1 | 0.65 (0.61–0.68) |
0.56 (0.50–0.61) |
0.74 (0.72–0.77) |
0.44 (0.41–0.48) |
0.59 (0.58–0.61) |
1 | 0.89 (0.89–0.91) |
0.21 (0.19–0.23) |
0.75 (0.74–0.75) |
0.45 (0.42–0.48) |
XGBoost, eXtremely gradient boosting; MLP, multilayer perceptron; RF, random forest; SVM, support vector machine; LR, logistic regression; KNN, KNearest neighbor; AUROC, area under the receiver operating characteristic curves; PPV, positive predictive value; NPV, negative predictive value.
Figure 3.
(A) Distribution of the impacts of each variable on the output of the XGBoost model estimated using the SHAP values. (B) Ranking of variables importance.
Figure 4.
Receiver operating characteristic curves (ROCs) of the XGBoost, LRM, RF, MLP, SVM, KNN, and simplified model. (A) Internal validation set. (B) External validation set. (C) Decision curve analysis of the XGBoost and simplified model. (D) Calibration curve of the XGBoost and simplified model. XGBoost, eXtremely gradient boosting; KNN, KNearest neighbor; MLP, multi-layer perceptron; RF, random forest; SVM, support vector machine; LRM, logistic regression; RSBI, rapid shallow breathing Index; CROP, compliance, oxygenation, respiratory rate, pressure index.
SHAP Values Depending on Variables
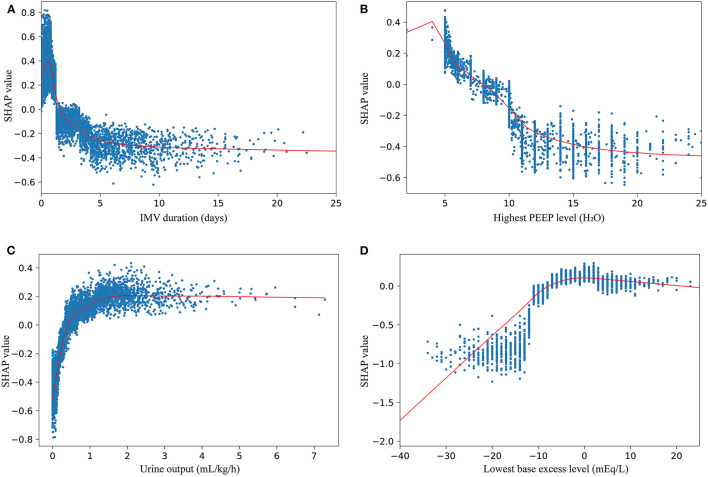
The probability of successful weaning increases with an increase in the following indicators: urine output, lowest base excess, GCS, lowest SPO2, congestive heart failure, lowest pH, lowest map, highest PaCO2, renal disease, lowest platelet count, BMI, and OI (Figure 3A). The contribution of each feature in the internal validation set is shown in Figure 3B in order of importance. Finally, we performed a partial dependency plot of the four contributing continuous variables to explain the impact of the change in value of each variable on the patients with WS, as shown in Figure 5. The remaining variables are shown in Supplementary Figures 2, 3. As shown in the partial dependency plot, feature values are indicated by a blue scatter plot, with the linear relationship represented by a red curve, where SHAP values represent an increase in the probability of WS when the value is positive and vice versa.
Figure 5.
SHAP dependency plots of IMV duration (A), highest PEEP level (B), urine output (C), and lowest base excess level (D).
Figure 5 shows the change trend of WS probability with the change in variables. For some variables, there is a discernable trend where the WS probability increases with an increase in the value of variables. These variables are urine output (Figure 5C), lowest BE (Figure 5D), GCS (Supplementary Figure 2B), and lowest SPO2 (Supplementary Figure 2D). In contrast, the decrease in some indicators, such as the highest PEEP level (Figure 5B), the highest anion gap level (Supplementary Figure 2A), and age (Supplementary Figure 2H), suggests a decrease in WS probability. Additionally, some variables seem to have a reasonable value, and the best cut-off value for these variables can be roughly judged by the LOWESS curve (Figure 5A; Supplementary Figures 2C,E–G). To obtain the best WS possibility, the values of these variables should be kept close to the cut-off value.
The Web-Based Tool and an Example Scenario
We simplified the previous XGBoost model according to the variable importance. The four variables (IMV duration, highest PEEP level, urine output, and lowest base excess) with the highest importance were used to develop a simplified model; the performance of the simplified model is shown in Figure 4 and Table 2. As shown, although the predictive performance of the simplified model decreased slightly, the model was greatly simplified. Subsequently, a web-based tool was developed for clinicians to use a simplified model. The tool can be accessed at http://49.235.211.121/frontdoc/sepweaning.html. After submitting the required data, the probability of weaning was calculated based on the simplified model.
When patients with sepsis are ready to be weaned from invasive mechanical ventilation, clinicians can make a quick decision using the SHAP dependency plots. For scenarios that require precise calculation, the web tool can provide an accurate probability of successful weaning based on the input indicators.
Discussion
This retrospective analysis included two large study cohorts from MIMIC-IV and eICU-CRD. First, we compared mortality and length of ICU stay from WS and WF in patients with sepsis using an effective balance method. Unfortunately, we found significant differences in mortality between patients with WS and WF. Therefore, the XGBoost model and the other five models were applied and evaluated sequentially to identify the beneficial factors associated with WS of patients with sepsis in the ICU. To our knowledge, this is the first study to predict weaning in patients with sepsis based on extensive public data. The difference from previous studies is that we developed an integrated machine learning model with high performance. In addition, we fully evaluated our model using another equally large public dataset. Finally, we explained the main variables and described the effects of their changing trends on weaning.
The matched results showed that there was a significant difference in the length of stay in the ICU between the WS and WF groups (Figure 1B, p = 0.002). This trend was not confirmed in the external validation set (Supplementary Figure 4C). Nevertheless, successful weaning of patients with sepsis significantly reduced mortality (Figure 1A, p < 0.001), and the matched result from the external validation set showed a similar trend (Supplementary Figure 4B). These results were comparable to those of previous studies (18). Therefore, successful weaning is a key factor in improving survival and potentially shortening ICU stay.
In both the internal validation set and the external validation set, our model showed excellent reliability and prospective generalization ability, and the prediction performance was significantly better than that of the traditional method (Figure 4C). In the model, the contribution to the prediction of WS varied with the variables. Previous studies have shown that the duration of IMV plays an important role in weaning (19–21). However, because IMV duration is the strongest variable in our model, the contribution of IMV duration to these models is inconsistent. We only included patients with sepsis admitted to the ICU in our study. Therefore, population diversity could lead to this difference. Moreover, it was observed that the best IMV duration was maintained for about 1 day according to the LOWESS curve (Figure 5A). Other variables with prospective cut-off values were antibiotic duration (Supplementary Figure 2C), highest body temperature (Supplementary Figure 2E), highest heart rate (Supplementary Figure 2F), highest respiratory rate (Supplementary Figure 3A), and highest PaCO2 (Supplementary Figure 3C). Although these variables have also been mentioned in previous studies (19, 21, 22), the trends and specific contributions of these variables have not been clarified.
In our study, age showed a clear downward trend of successful weaning ability with increasing value, especially in patients aged >75 years (Supplementary Figure 2H). This trend is supported by increasing evidence (19, 23). Interestingly, BMI and congestive heart failure as low-contributing variables were proportional to WS probability in our study. This finding is supported by previous studies (24, 25). However, there is also an opposite conclusion (19). In a study on viral infections, excessive BMI may lead to worse clinical outcomes (26). However, in another study, even a reasonably high BMI can help patients improve their disease (27). In summary, these differences may be due to the diversity of the population and the diversity of diseases.
PEEP, urine output, and SPO2, as the most commonly measured indices, play a key role in predicting WS. In this study, a low PEEP strategy was found to be more beneficial for patients with sepsis weaning from ventilation. Although the low-level PEEP strategy did not have a significant effect on improving ventilator weaning in a cohort study (28), it still has the potential to promote ventilator weaning (29). There is increasing evidence that high levels of PEEP are a risk factor in reducing the likelihood of WS (19, 30). As we have considered, high levels of PEEP lead to lung congestion and increase the respiratory burden of patients (31), which may explain why high levels of PEEP play a positive role in the weaning process (Figure 5B). Urinary output is the primary means for humans to maintain fluid balance, and excellent fluid management strategies could significantly improve patient survival (32). Polyuria has been shown to have no negative impact on weaning (33), but negative fluid balance significantly impedes weaning success (34, 35). These findings are consistent with those of the present study (Figure 5C). The pH was an interesting finding in our study. As the most important indicator of acid-base balance, an abnormally high or low pH had a detrimental effect on weaning in our study. Nevertheless, low pH was more likely to cause weaning failure (Supplementary Figure 2G). This finding is consistent with previous studies, as low pH of extracellular fluid compromises the immune function of the body in patients with sepsis (36). BE and PaCO2 are indicators closely related to pH. Early studies have shown that removing PaCO2 from the body effectively improves the success rate of weaning (37, 38). The relationship between BE and weaning has not yet been studied. In any case, extracellular acid-base balance is susceptible to the interaction of several variables. For the three variables of BE, PaCO2, and pH, we used an interactive effects plot to explain the relationship between the three variables. As can be seen in Supplementary Figure 3, the tendency of these three variables to predict weaning tended to be consistent, which also shows the rationality of using this model.
In clinical practice, weaning should be a medical behavior that needs careful consideration. Inappropriate weaning may lead to worsening of disease, higher mortality, longer hospital stays, and higher hospital costs (7, 39, 40). Therefore, a simple and effective prediction method is needed. Compared with the traditional prediction model, our model has better prediction performance (11, 41). Although some new weaning models have been proposed in recent years (19, 20, 42, 43), the performance of the model varies according to the target population. In patients with sepsis, the developed model was able to predict weaning well, as reflected by a high AUROC value of 0.80 and 0.86 in the internal and external validation sets, respectively. Interestingly, the performance of the model was better in patients with pulmonary infections than in the validation sets (Supplementary Figure 5). Obviously, the performance of our model was better in the external validation set. Apart from the good generalization ability of our model, we believe that the different data sources could explain this phenomenon, which also occurs in other studies (44). Finally, we simplified our model and developed a web-based tool that allows convenient use of the model.
There are still potential limitations in our research. First, because of the limitation of the database, other variables that could have predictive value, such as lactate and central venous pressure, with excessively high error rates, were not included in the model. Considering the availability of comprehensive clinical indicators, such as SOFA and SAPS, were not included in the model, although these indicators could improve the predictive performance of the model (19, 45, 46). Second, although our model was validated using data from multiple data sources, we still need additional data sources to further demonstrate the generalizability of the model. Third, in our study, the positive predictive value (PPV) and negative predictive value (NPV) were 0.81, 0.62, 0.79, and 0.77 in the internal and external validation sets, respectively, which means that the model still has some degree of false-positive and false-negative rates. More valuable variables and dynamic prediction models could improve the performance.
In conclusion, weaning success is independent of short-term mortality in patients with sepsis. We developed a prospective model for weaning from invasive mechanical ventilation using the XGBoost algorithm. This model included 35 conventional clinical variables and proved to be more interpretable and predictive. In addition, the model was simplified, and a web-based tool was developed for better clinical application.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://physionet.org/about/database/.
Author Contributions
MY and WL carried out the concepts, design, data acquisition, and manuscript preparation. GT, ZL, and YZ carried out literature search and manuscript preparation. WX, JZ, YL, and TH performed manuscript review, including revision of key technical content and English expression. All authors have read and approved the content of the manuscript.
Funding
This work is supported by National Natural Science Foundation of China (Grant No. 82072134).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.814566/full#supplementary-material
References
- 1.Zilberberg MD, Nathanson BH, Ways J, Shorr AF. Characteristics, hospital course, and outcomes of patients requiring prolonged acute versus short-term mechanical ventilation in the United States, 2014-2018. Crit Care Med. (2020) 48:1587–94. 10.1097/CCM.0000000000004525 [DOI] [PubMed] [Google Scholar]
- 2.Amoateng-Adjepong Y, Jacob BK, Ahmad M, Manthous CA. The effect of sepsis on breathing pattern and weaning outcomes in patients recovering from respiratory failure. Chest. (1997) 112:472–7. 10.1378/chest.112.2.472 [DOI] [PubMed] [Google Scholar]
- 3.Nin N, Lorente JA, Fernández-Segoviano P, De Paula M, Ferruelo A, Esteban A. High-tidal volume ventilation aggravates sepsis-induced multiorgan dysfunction in a dexamethasone-inhibitable manner. Shock. (2009) 31:429–34. 10.1097/SHK.0b013e318188b720 [DOI] [PubMed] [Google Scholar]
- 4.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Zheng H, et al. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep*. Crit Care Med. (2014) 42:e491–500. 10.1097/CCM.0000000000000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motta-Ribeiro GC, Hashimoto S, Winkler T, Baron RM, Grogg K, Paula LFSC, et al. Deterioration of Regional Lung Strain and Inflammation during Early Lung Injury. Am J Respir Crit Care Med. (2018) 198:891–902. 10.1164/rccm.201710-2038OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Zamora MD, Gordillo-Brenes A, Banderas-Bravo E, Arboleda-Sánchez JA, Hinojosa-Pérez R, Aguilar-Alonso E, et al. Prolonged mechanical ventilation as a predictor of mortality after cardiac surgery. Respir Care. (2018) 63:550–7. 10.4187/respcare.04915 [DOI] [PubMed] [Google Scholar]
- 7.Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. (2011) 26:502–9. 10.1016/j.jcrc.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 8.Saiphoklang N, Auttajaroon J. Incidence and outcome of weaning from mechanical ventilation in medical wards at Thammasat University Hospital. PLoS ONE. (2018) 13:e0205106. 10.1371/journal.pone.0205106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. (2006) 130:1664–71. 10.1378/chest.130.6.1664 [DOI] [PubMed] [Google Scholar]
- 10.Sassoon CS, Mahutte CK. Airway occlusion pressure and breathing pattern as predictors of weaning outcome. Am Rev Respir Dis. (1993) 148:860–6. 10.1164/ajrccm/148.4_Pt_1.860 [DOI] [PubMed] [Google Scholar]
- 11.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. (1991) 324:1445–50. 10.1056/NEJM199105233242101 [DOI] [PubMed] [Google Scholar]
- 12.Hilbert G, Gruson D, Portel L, Vargas F, Gbikpi-Benissan G, Cardinaud JP. Airway occlusion pressure at 01 s (P01) after extubation: an early indicator of postextubation hypercapnic respiratory insufficiency. Intensive Care Med. (1998) 24:1277–82. 10.1007/s001340050762 [DOI] [PubMed] [Google Scholar]
- 13.Jubran A, Tobin MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. (1997) 155:906–15. 10.1164/ajrccm.155.3.9117025 [DOI] [PubMed] [Google Scholar]
- 14.Moschietto S, Doyen D, Grech L, Dellamonica J, Hyvernat H, Bernardin G. Transthoracic Echocardiography with Doppler Tissue Imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care. (2012) 16:R81. 10.1186/cc11339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:775–87. 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schönhofer B, Geiseler J, Dellweg D, Fuchs H, Moerer O, Weber-Carstens S, et al. Prolonged weaning: S2k guideline published by the German Respiratory Society. Respiration. (2020) 2020:1–102. 10.1159/000510085 [DOI] [PubMed] [Google Scholar]
- 17.Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model. (1986) 7:1393–512. 10.1016/0270-0255(86)90088-629883322 [DOI] [Google Scholar]
- 18.Yang CH, Hsiao JL, Wu MF, Lu MH, Chang HM, Ko WS, et al. The declined levels of inflammatory cytokines related with weaning rate during period of septic patients using ventilators. Clin Respir J. (2018) 12:772–8. 10.1111/crj.12593 [DOI] [PubMed] [Google Scholar]
- 19.Zhao QY, Wang H, Luo JC, Luo MH, Liu LP, Yu SJ, et al. Development and validation of a machine-learning model for prediction of extubation failure in intensive care units. Front Med. (2021) 8:676343. 10.3389/fmed.2021.676343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel N, Chakraborty M, Watkins WJ, Banerjee S. Predicting extubation outcomes-a model incorporating heart rate characteristics index. J Pediatr. (2018) 195:53–58.e1. 10.1016/j.jpeds.2017.11.037 [DOI] [PubMed] [Google Scholar]
- 21.Thille AW, Boissier F, Ben Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C. Risk factors for and prediction by caregivers of extubation failure in ICU patients: a prospective study. Crit Care Med. (2015) 43:613–20. 10.1097/CCM.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 22.Minozzi S, Pifferi S, Brazzi L, Pecoraro V, Montrucchio G, D'Amico R. Topical antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving mechanical ventilation. Cochrane Database Syst Rev. (2021) 1:CD000022. 10.1002/14651858.CD000022.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warnke C, Heine A, Müller-Heinrich A, Knaak C, Friesecke S, Obst A, et al. Predictors of survival after prolonged weaning from mechanical ventilation. J Crit Care. (2020) 60:212–7. 10.1016/j.jcrc.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 24.Keng LT, Liang SK, Tseng CP, Wen YF, Tsou PH, Chang CH, et al. Functional status after pulmonary rehabilitation as a predictor of weaning success and survival in patients requiring prolonged mechanical ventilation. Front Med. (2021) 8:675103. 10.3389/fmed.2021.675103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen Q, Coghlan K, Hong Y, Nagendran J, MacArthur R, Lam W. Factors associated with early extubation after cardiac surgery: a retrospective single-center experience. J Cardiothorac Vasc Anesth. (2021) 35:1964–70. 10.1053/j.jvca.2020.11.051 [DOI] [PubMed] [Google Scholar]
- 26.Chand S, Kapoor S, Orsi D, Fazzari M, Tanner T, Umeh G, et al. COVID-19-associated critical illness-report of the first 300 patients admitted to intensive care units at a New York City Medical Center. J Intensive Care Med. (2020) 35:963–70. 10.1177/0885066620946692 [DOI] [PubMed] [Google Scholar]
- 27.Sakr Y, Alhussami I, Nanchal R, Wunderink RG, Pellis T, Wittebole X, et al. Being overweight is associated with greater survival in ICU patients: results from the intensive care over nations audit. Crit Care Med. (2015) 43:2623–32. 10.1097/CCM.0000000000001310 [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Su L, Ding X, Chen H, Zhang H, Wang J, et al. The risk factors for weaning failure of mechanically ventilated patients with COVID-19: a retrospective study in national medical team work. Front Med. (2021) 8:678157. 10.3389/fmed.2021.678157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Writing Committee and Steering Committee for the RELAx Collaborative Group Algera AG Pisani L Serpa Neto A den Boer SS Bosch FFH . Effect of a lower vs higher positive end-expiratory pressure strategy on ventilator-free days in ICU patients without ARDS: a randomized clinical trial. JAMA. (2020) 324:2509–20. 10.1001/jama.2020.23517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ionescu F, Zimmer MS, Petrescu I, Castillo E, Bozyk P, Abbas A, et al. Extubation failure in critically ill COVID-19 patients: risk factors and impact on in-hospital mortality. J Intensive Care Med. (2021) 36:1018–24. 10.1177/08850666211020281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retamal J, Bugedo G, Larsson A, Bruhn A. High PEEP levels are associated with overdistension and tidal recruitment/derecruitment in ARDS patients. Acta Anaesthesiol Scand. (2015) 59:1161–9. 10.1111/aas.12563 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Zheng B, Liu N. Individualized fluid administration for critically ill patients with sepsis with an interpretable dynamic treatment regimen model. Sci Rep. (2020) 10:17874. 10.1038/s41598-020-74906-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliyali M, Sharifpour A, Abedi S, Spahbodi F, Namarian N, Zarea A, et al. The ability of polyuria in prediction of weaning outcome in critically ill mechanically ventilated patients. Tanaffos. (2019) 18:74–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Maezawa S, Kudo D, Miyagawa N, Yamanouchi S, Kushimoto S. Association of body weight change and fluid balance with extubation failure in intensive care unit patients: a single-center observational study. J Intensive Care Med. (2021) 36:175–81. 10.1177/0885066619887694 [DOI] [PubMed] [Google Scholar]
- 35.Lin MY, Li CC, Lin PH, Wang JL, Chan MC, Wu CL, et al. Explainable machine learning to predict successful weaning among patients requiring prolonged mechanical ventilation: a retrospective cohort study in Central Taiwan. Front Med. (2021) 8:663739. 10.3389/fmed.2021.663739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. (2001) 69:522–30. 10.1189/jlb.69.4.522 [DOI] [PubMed] [Google Scholar]
- 37.Abrams DC, Brenner K, Burkart KM, Agerstrand CL, Thomashow BM, Bacchetta M, et al. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. (2013) 10:307–14. 10.1513/AnnalsATS.201301-021OC [DOI] [PubMed] [Google Scholar]
- 38.Braune S, Sieweke A, Brettner F, Staudinger T, Joannidis M, Verbrugge S, et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med. (2016) 42:1437–44. 10.1007/s00134-016-4452-y [DOI] [PubMed] [Google Scholar]
- 39.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. (2011) 39:2612–8. 10.1097/CCM.0b013e3182282a5a [DOI] [PubMed] [Google Scholar]
- 40.Perren A, Previsdomini M, Llamas M, Cerutti B, Györik S, Merlani G, et al. Patients' prediction of extubation success. Intensive Care Med. (2010) 36:2045–52. 10.1007/s00134-010-1984-4 [DOI] [PubMed] [Google Scholar]
- 41.Hendra KP, Bonis PA, Joyce-Brady M. Development and prospective validation of a model for predicting weaning in chronic ventilator dependent patients. BMC Pulm Med. (2003) 3:3. 10.1186/1471-2466-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vassilakopoulos T, Routsi C, Sotiropoulou C, Bitsakou C, Stanopoulos I, Roussos C, et al. The combination of the load/force balance and the frequency/tidal volume can predict weaning outcome. Intensive Care Med. (2006) 32:684–91. 10.1007/s00134-006-0104-y [DOI] [PubMed] [Google Scholar]
- 43.Jia Y, Kaul C, Lawton T, Murray-Smith R, Habli I. Prediction of weaning from mechanical ventilation using convolutional neural networks. Artif Intell Med. (2021) 117:102087. 10.1016/j.artmed.2021.102087 [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Jiang S, Lu Z, Xue D, Xia L, Lu J, et al. Development and verification of a nomogram for prediction of recurrence-free survival in clear cell renal cell carcinoma. J Cell Mol Med. (2020) 24:1245–55. 10.1111/jcmm.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brix N, Sellmer A, Jensen MS, Pedersen LV, Henriksen TB. Predictors for an unsuccessful INtubation-SURfactant-Extubation procedure: a cohort study. BMC Pediatr. (2014) 14:155. 10.1186/1471-2431-14-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaber S, Quintard H, Cinotti R, Asehnoune K, Arnal JM, Guitton C, et al. Risk factors and outcomes for airway failure versus non-airway failure in the intensive care unit: a multicenter observational study of 1514 extubation procedures. Crit Care. (2018) 22:236. 10.1186/s13054-018-2150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://physionet.org/about/database/.