Abstract
Ferroptosis, a newly discovered form of regulatory cell death (RCD), has been demonstrated to be distinct from other types of RCD, such as apoptosis, necroptosis, and autophagy. Ferroptosis is characterized by iron-dependent lipid peroxidation and oxidative perturbation, and is inhibited by iron chelators and lipophilic antioxidants. This process is regulated by specific pathways and is implicated in diverse biological contexts, mainly including iron homeostasis, lipid metabolism, and glutathione metabolism. A large body of evidence suggests that ferroptosis is interrelated with various physiological and pathological processes, including tumor progression (neuro)degenerative diseases, and hepatic and renal failure. There is an urgent need for the discovery of novel effective ferroptosis-modulating compounds, even though some experimental reagents and approved clinical drugs have been well documented to have anti- or pro-ferroptotic properties. This review outlines recent advances in molecular mechanisms of the ferroptotic death process and discusses its multiple roles in diverse pathophysiological contexts. Furthermore, we summarize chemical compounds and natural products, that act as inducers or inhibitors of ferroptosis in the prevention and treatment of various diseases. Herein, it is particularly highlighted that natural products show promising prospects in ferroptosis-associated (adjuvant) therapy with unique advantages of having multiple components, multiple biotargets and slight side effects.
Keywords: iron homeostasis, lipid peroxidation, redox signaling, cancer, neurodegenerative diseases, natural products
Introduction
Along with other biological processes, cell death is of great significance in various molecular physiological processes of mammalian development, homeostasis and disease (Thompson, 1995; Fuchs and Steller, 2011). Generally, cell death can be divided into “accidental cell death” (ACD) and “regulatory cell death” (RCD). ACD is the actual structural collapse of cells when exposed to extreme physicochemical or mechanical stimuli. However, RCD is initiated by a genetically encoded apparatus and can be altered by pharmacologic or genetic interventions. RCD plays an integral role throughout the normal development and lifespan of humans, eliminating irreversibly impaired, dysfunctional or infected cells to protect the tissue or organ system from environmental injury or infection. Based on different biochemical phenomena, RCD is identified as several subtypes, including apoptosis, necrosis, autophagy and so on (Galluzzi et al., 2015). In 2003, Dolma et al. discovered a non-apoptotic cell death process when exploring the selective toxicity of erastin (a small molecule) in cancer cells (Dolma et al., 2003). In 2007, Yagoda et al. determined mitochondrial voltage-dependent anion channels 2 and 3 (VDAC2/3) as specific targets of erastin and VDAC2/3 also induced a non-apoptotic cell death, which depends on the RAS-RAF-MEK pathway (Yagoda et al., 2007). In 2008, Yang and Stockwell et al. identified two compounds, RSL3 and RSL5, via a high-throughput method that selectively induced iron-dependent, non-apoptotic cell death in cancer cells with mutated oncogenic RAS subtype genes (Yang and Stockwell, 2008). In 2012, Dr. Brent R Stockwell et al. treated the human fibrosarcoma cell line HT-1080 with erastin and found that the subunit of the amino acid cysteine transporter on the cell surface was suppressed. Restriction of cystine import, glutathione (GSH) depletion and phospholipid peroxidase glutathione peroxidase 4 (GPX4) inactivation could result in cell death. Thus, the term “ferroptosis” was formally coined (Dixon et al., 2012). As numerous studies have reported, ferroptosis usually refers to a novel iron-dependent, lipid peroxidation-driven RCD, that is obviously distinct from apoptosis, necrosis and autophagy at the morphological, biochemical, and genetic levels (Xie et al., 2016a; Stockwell et al., 2017). For example, morphological features during ferroptosis include smaller mitochondria, increased membrane density, crest density and decrease/disappearance, plasma membrane blistering, and increased cytoplasmic and lipid peroxidation. Ferroptosis is not limited to neoplastic diseases, and has been mostly linked to other iron- or redox-associated pathophysiological conditions, such as neurodegeneration, ischemia/reperfusion (I/R) injury, diabetes, and immune dysfunction (Skouta et al., 2014). A large number of studies have demonstrated that ferroptosis plays an indispensable role in the occurrence, development and phenotypic shift of a variety of diseases, which may provide new diagnostic and therapeutic strategies to regulate cell survival and death. Hence, it is instructive to discover novel compounds and drugs regulating ferroptosis to expand the development of potential therapeutic approaches. In recent decades, many clinical drugs and experimental compounds have been exploited to modulate ferroptosis by preclinical and clinical studies so as to achieve therapeutic purposes. As studies have progressed, natural products have attracted sufficient attention. Natural products and their analogs, including traditional Chinese medicines (TCMs) are unneglectable and conventional sources for the discovery and development of modern drugs. Due to their natural origin and potential beneficial efficacy, natural products and herbal medicines have been used globally to promote human health concerns for thousands of years, especially for the therapy of many chronic diseases, such as cancer, cardiovascular and (neuro)degenerative diseases (Shu-Feng Zhou et al., 2007; Kibble et al., 2015). Emerging evidence has demonstrated solid ferroptotic bioactivities in natural compounds and herbal medicines. In this review, we discuss emerging molecular mechanisms and biological processes of ferroptosis and its role in various diseases. We summarize synthetic compounds and natural products with anti- or pro-ferroptotic properties, and the great potential of natural products for ferroptosis-associated (adjuvant) therapy is highlighted. Overall, the underlying mechanisms of ferroptosis and corresponding pharmacological agents can provide insights into the discovery and development of promising therapeutic strategies in terms of ferroptosis.
Regulatory Networks and Signaling Pathways Associated With Ferroptosis
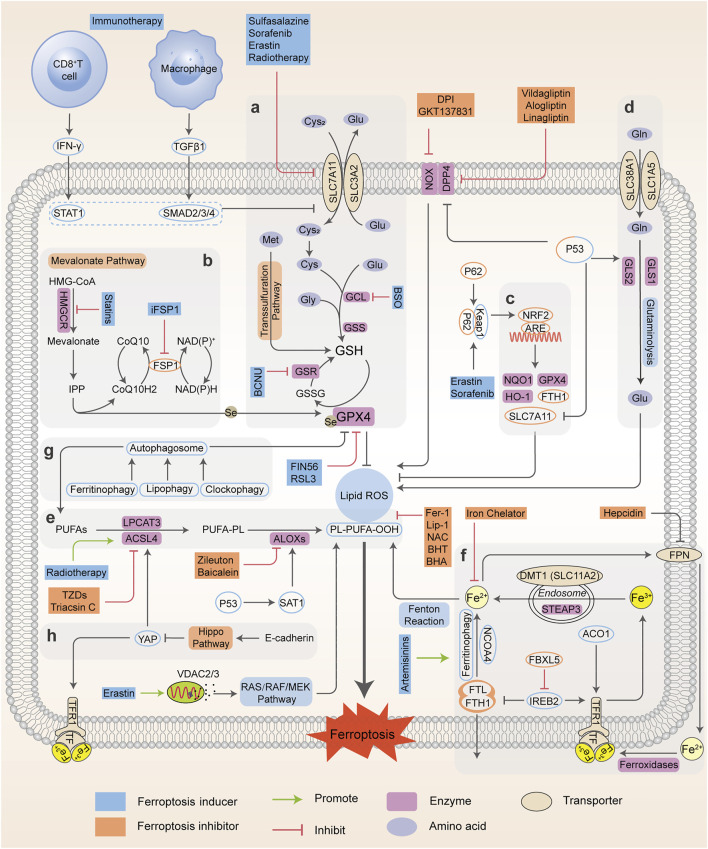
In recent years, the regulatory and metabolic mechanisms involved in ferroptosis have been emphatically elucidated and summarized by numerous original articles and reviews in detail. Hence, in this section, we briefly introduce the modulatory mechanisms and biological processes of ferroptosis, mainly including iron homeostasis, lipid metabolism, glutathione metabolism and other pathways, and illustrate them in Figure 1. Understanding the molecular mechanisms and signaling pathways of ferroptosis may open up new therapeutic opportunities to regulate cell fate in human diseases.
FIGURE 1.
Regulatory networks and signaling pathways associated with ferroptosis. Ferroptosis is primarily activated by iron-dependent lipid peroxidation and redox perturbation, which mainly occurs through two major pathways, the extrinsic or transporter-dependent pathway, and the intrinsic or enzyme-regulated pathway. (A) The cyst(e)ine/GSH/GPX4 antioxidative axis. (B) The mevalonate pathway (IPP/FSP1/CoQ10 system). (C) NRF2-regulated ARE defence. (D) The glutaminolysis pathway. (E) The lipid peroxidation process; (F) The absorption, export, storage and utilization of iron. (G) The autophagy cascade. (H) The EMT-related pathway. Processes favoring or counteracting ferroptotic cell death are labeled with red and green arrows, respectively (Cys2, cystine; Cys, cysteine; Glu, glutamate; Gly, glycine Gln, glutamine; BCNU, 1,3-bis-(2-chloroethyl)-1-nitrosourea; CoQ10H2, ubiquinol; HMG-CoA, 3-hydroxy-3-methyl glutaryl coenzyme A; GSR, glutathione-disulfide reductase; BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene).
Iron Homeostasis
Iron is of great physiological and biological functions such as the transport and storage of oxygen and energy metabolism. Normally, the intracellular iron maintains a balance within a narrow range. However, the disordered distribution and content of iron perturb physiological processes and biological survival. Excessive iron can provoke subsequent lipid peroxidation either by the iron-mediated Fenton reaction to produce reactive oxygen species (ROS, a group of molecules derived from molecular oxygen, mainly including hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide anion (O2 •–) and hydroxyl radicals (•OH)), or the activation of enzymes containing iron (for example, lipoxygenases) (Xie et al., 2016a; Dixon and Stockwell, 2014). In general, the iron atom (Fe3+) binds to transferrin (TF) and binds with transferrin receptor (TFRC) through blood circulation to form the double iron-TF-TFRC complex, which is then endocytosed into the nucleus and reduced to ferrous (Fe2+) by endosomal six-transmembrane epithelial antigen of prostate 3 (STEAP3). Subsequently, Fe2+ is released from the nuclear endosome to the labile iron pool (LIP) in the cytoplasm via divalent metal transporter 1 (DMT1, SLC11A2). Excessive iron is stored in ferritin (the primary iron storage protein) which consists of ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1). Finally, iron is exported from cells to blood through ferroportin (FPN, encoded by SLC40A1, the only known iron-efflux protein) in the cell membrane, and Fe2+ can be reoxidized by ferroxidases (such as ceruloplasmin or hephaestin) to Fe3+ (Torti and Torti, 2013). Alternatively, Fe2+ can be exported as ferritin through exosomes. Variable iron levels on account of interventions at multiple levels (such as iron absorption, storage, utilization, export and iron chelators) affect ferroptosis via an integrated signaling pathway (Figure 1). At the post-transcriptional level, iron regulatory protein 1 and 2 (IRP1; also known as ACO1, IRP2; also known as IREB2) can regulate the expression of DMT1, TFRC, ferritin and FPN (Torti and Torti, 2013) and thereby influence ferroptotic activity. Initiation of IREB2 promotes erastin-induced cytotoxicity, while inhibition of F-box and leucine-rich repeats protein 5 (FBXL5), an endogenous IREB2 antagonist, can sensitize cells to erastin (Reed and Pellecchia, 2012). Iron chelators, such as deferoxamine (DFO) (Dixon et al., 2012; Chen X. et al., 2020), dexrazoxane (DXZ) (Fang et al., 2019) and deferiprone (DFP) (Wu et al., 2020), can directly chelate iron and have been used in clinical treatment to reduce iron availability. As a result, these iron chelators are capable of relieving ferroptosis intrinsically or caused by proferroptotic reagents. Ferroptosis was originally identified as an autophagy-independent RCD (Xie et al., 2016a; Najafov et al., 2017). According to current studies, autophagy plays an important role in ferroptosis through the regulation of cellular iron levels. Autophagy recognizes and degrades ferritin into autophagosomes for lysosome-dependent degradation by using nuclear receptor coactivator 4 (NCOA4) as a selective cargo receptor. This process called “ferritinophagy”, which leads to increased iron accumulation and subsequent oxidative damage and ferroptosis. On the contrary, abrogating ferritinophagy by knockdown of autophagy-related genes or NCOA4 or inhibition of lysosomal function by specific inhibitors suppresses the availability of labile iron and decreases ferroptotic sensitivity (Mancias et al., 2014; Hou et al., 2016). In addition to NCOA4-mediated ferritinophagy, other forms of selective autophagy, including lipophagy, clockophagy, and chaperone-mediated autophagy, have been demonstrated to trigger ferroptosis in cells (Gao et al., 2016). Thus, intracellular iron homeostasis directly or indirectly targets ferroptosis, and the crosstalk of ferroptosis and autophagy provides new perspectives and contexts for the regulation of RCD.
Lipid Peroxidation Metabolism
Lipid peroxidation has been regarded as the hallmark in the context of ferroptosis. As materials of lipid synthesis, polyunsaturated fatty acids (PUFAs), especially arachidonic acid (AA) and adrenic acid (AdA), are vulnerable to the peroxidation process and incorporated into membrane phospholipids (PLs), which destroy the lipid bilayer to trigger the ferroptotic process (Stockwell et al., 2017; Kagan et al., 2017; Conrad and Pratt, 2019). Acyl coenzyme A synthase long-chain member 4 (ACSL4) connects coenzyme A (CoA) to long-chain PUFAs, preferentially AA or AdA, to form arachidonic acid-CoA (AA-CoA) or adrenic acid-CoA (AdA-CoA), respectively. Then PUFA-CoAs are esterified by lysophosphatidylcholine acyltransferase 3 (LPCAT3) to produce PUFA-containing phospholipids (PUFA-PLs). Particularly, phosphatidylethanolamines (PEs) with both AA and AdA (AA/AdA-PEs) are the significant PUFA-PLs for ferroptosis, and are susceptible to free radical trigged oxidation catalyzed by lipoxygenases (ALOXs). ALOXs are nonheme iron-containing dioxygenases that catalyze the oxidation of PUFAs to produce fatty acid hydroperoxides, with a context-dependent role in mediating lipid peroxidation (Kagan et al., 2017; Doll et al., 2017) (Figure 1). Based on this, many reports have uncovered the implications of ACSL4, LPCAT3 and ALOXs during ferroptosis and exploited some candidate compounds with ferroptotic activities (Ou et al., 2016; Colakoglu et al., 2018). ACSL4 knockdown or inhibition can significantly lower the levels of AA-CoA and AdA-CoA to prevent the destruction of membrane structure, and confer ferroptosis resistance. Conversely, supplementation with AA or other PUFAs can block the original ACSL4-mediated reaction, which promotes the occurrence of ferroptosis or sensitizes cells to ferroptosis induced by erastin or RSL3 (Yang et al., 2016; Shintoku et al., 2017). However, the actual driver of lipid peroxidation and subsequent ferroptosis is still controversial, as it has been shown that ALOX15 is not essential for ferroptosis in a mouse model of GPX4-mediated acute renal failure (Friedmann Angeli et al., 2014).
As a master repressor of ferroptosis, selenoenzyme glutathione peroxidase 4, GPX4, consumes two molecules of reductive GSH after being combined with lipids to reduce toxic phospholipid hydroperoxide (LOOH) to nontoxic phospholipid lipid alcohol (LOH), which simultaneously produces oxidative glutathione (GSSG) as a byproduct, and ultimately inhibits phospholipid lipid peroxidation. Once they enter the membrane, PUFAs which are peroxidized and not cleared up by GPX4, eventually drive a large amount of lipid peroxidation and ferroptosis (Yang et al., 2016; Kagan et al., 2017). Further studies have shown that GPX4 inactivation is observed in the ferroptotic death process in vitro and in vivo, whereas deletion of GPX4 with pharmacological or genetic approaches can induce ferroptosis execution (Hangauer et al., 2017; Guo et al., 2018a). Besides western blot or RT-PCR evidence, many studies have confirmed the function and activity of GPX4 in the regulation of ferroptosis using cell or mouse models (e.g. GPX4 knockout or overexpression) (Alvarez et al., 2017; Hangauer et al., 2017; Viswanathan et al., 2017). For example, GSH-depleting reagents (erastin and buthionine sulfoximine (BSO)) inhibits GPX activity to induce ferroptosis and the total activity of GPXs in cells is examined using tert-butylhydroperoxide (tBuOOH) as a substrate. Furthermore, 7a-cholesterol-OOH is a specific substrate for GPX4; no other GPX enzyme can catalyze the reduction of 7a-cholesterol-OOH (1S, 3R)-RSL3, a ferroptosis inducer, has been demonstrated to inhibit enzyme activity of GPX4, indicated by unchanged level of 7a-cholesterol-OOH (Yang et al., 2014). Additionally, it is shown that FINO2 indirectly inhibits GPX4 enzymatic function while FIN56 directly depletes GPX4 enzymatic function using 1H-15N heteronuclear single quantum coherence (HSQC) NMR spectroscopy, ultimately causing widespread lipid peroxidation and ferroptosis (Gaschler et al., 2018a). It should be noted that the GPX4 defense system is also linked to other non-ferroptotic RCDs such as apoptosis and necroptosis, suggesting that lipid peroxidation lies at the crossroads of these pathways with diverse functions and different downstream effectors (Ran et al., 2007; Canli et al., 2016). Additionally, GPX4 is necessary for tumor recurrence, indicating that eradicating drug-resistant cancer cells by targeting GPX4 to induce cellular ferroptosis is a promising clinical strategy (Rennekamp, 2017).
Glutathione Metabolism
The cystine-glutamate antiporter system xc --mediated GSH synthesis, representative of the capacity of antioxidation, plays a significant role in the accumulation of lipid peroxides and the occurrence of ferroptosis. The cystine-glutamate antiporter, a transmembrane transport protein, composed of SLC7A11 (xCT) and SLC3A2 (4F2hc), takes up cystine from outside the cell and correspondingly outputs glutamate for exchange at a 1:1 ratio (Dixon et al., 2014). Usually, cysteine under reductive extracellular conditions is transported directly into cells through ASC (a neutral amino acid transport system), while cystine under oxidative extracellular conditions is reduced to cysteine after entering cells through system xc -. Subsequently, cysteine, glutamate, and glycine are catalyzed by γ-glutamylcysteine synthetase (GCLC) and glutathione synthase (GSS) respectively to form GSH, the major intracellular antioxidant (Doll and Conrad, 2017; Liang et al., 2019). The functional activity of GPX4 to reduce lipid hydroperoxides is dependent on GSH biosynthesis, because GSH acts as a cofactor for GPX4. GSH depletion leads to GPX4 inactivation, thereby triggering intracellular lipid peroxidation and ferroptosis. Therefore, system xc - (SLC7A11) is a transportation hub that regulates intracellular redox balance and ferroptotic damage (Yang and Stockwell, 2016; Stockwell et al., 2017). The expression and activity of SLC7A11 is further positively modulated by nuclear factor erythroid 2-related factor 2 (NRF2), and negatively modulated by tumor suppressor genes, such as p53 and BAP1 (Carbone et al., 2013; Zhang Y. et al., 2018). This dual regulation constitutes a fine-tuning mechanism to delicately control and maintain GSH levels in ferroptosis. Consequently, ferroptosis is driven by GSH depletion either by restricting GSH biosynthesis (e.g., BSO inhibits GCLC) or by blocking cystine uptake from extracellular environment (e.g., erastin or sulfasalazine (SAS) inhibits system xc -). As an alternative way apart from system xc -, the transsulfuration pathway can supply cysteine as a compensation system under cystine deprivation, which is negatively regulated by cysteinyl-tRNA synthase (CARS). Loss of CARS has been proven to upregulate the transsulfuration pathway and inhibit erastin-induced ferroptosis (McBean, 2012; Hayano et al., 2016). Taken together, any link of GSH synthesis, representative of antioxidant capacity, can be a mediator of the ferroptotic cascade.
Other Regulatory (co)factors and Signaling Pathways
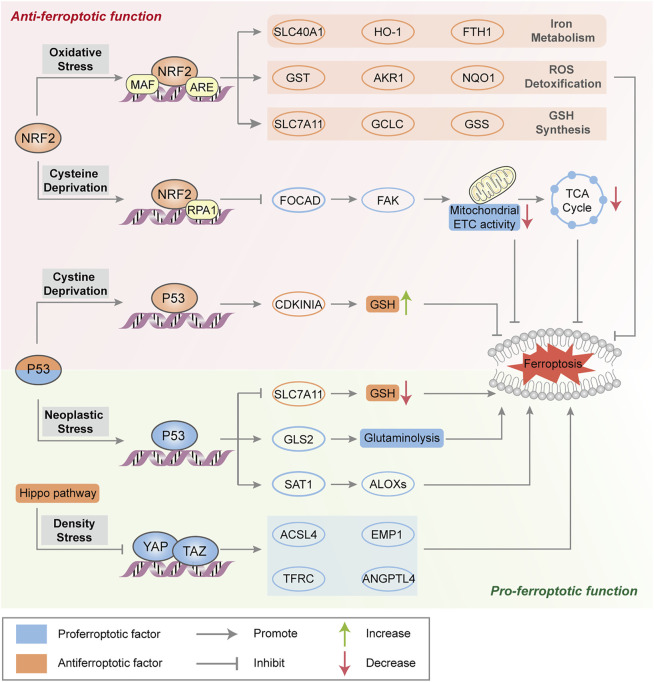
p53
Emerging evidence suggests the two contradictory functions of the tumor suppressor p53 in the regulation of ferroptosis (Figure 2). On the one hand, p53 transcriptionally suppresses the expression of SLC7A11 in some types of cells (e.g., human breast or lung cancer cells) to trigger ferroptosis, which induces GSH depletion and relieves the specific inhibition of the ALOX12 enzyme to suppress tumor growth (Jiang et al., 2015; Chu et al., 2019). Moreover, p53-mediated transcriptional activation of spermidine/spermine N1-acetyltransferase 1 (SAT1) may indirectly increase ALOX15 expression, thus inducing lipid peroxidation and sensitizing cells to ferroptotic death (Ou et al., 2016). On the other hand, activation of p53 may antagonize ferroptosis in human colorectal cancer (CRC) cells and fibrosarcoma cells through various mechanisms, such as promoting the localization of dipeptidyl peptidase 4 (DPP4) to the nuclear enzyme-free pool in a transcription-independent manner, increasing the expression of CDKN1A (encoding p21) and favoring the preservation of GSH (Jennis et al., 2016; Xie et al., 2017). Considering that the transcription-dependent or independent function of p53 seems to be cell type-specific, it is necessary to evaluate the role of p53 in ferroptosis according to different circumstances.
FIGURE 2.
Major transcription factors in the regulation of ferroptosis. NRF2 transactivates a range of cytoprotective genes under endogenous oxidative stress to drive antiferroptotic function. Moreover, NRF2 represses FOCAD expression and FAK activity and further reduces sensitivity to cysteine deprivation-induced ferroptosis. Generally, p53 triggers ferroptosis to suppress tumorigenesis by transcriptional regulation of ferroptosis-related genes. In some circumstances, p53 suppresses metabolic stress-induced ferroptosis to preserve cell survival in certain cancer cells. The Hippo pathway negatively regulates a series of proferroptotic genes via YAP/TAZ transcription factors.
NRF2
As a master protective regulator to maintain cellular redox homeostasis, NRF2 is unleashed from its ligand kelch-like ECH-associated protein 1 (Keap1) binding and translocates into the nucleus under oxidative stress. In the nucleus, NRF2 participates in the transcription of a series of antioxidant response element (ARE)-related genes that are implicated in iron metabolism (such as SLC40A1, heme oxygenase‐1 (HO-1) and FTH1), GSH synthesis (such as SLC7A11, GCLC and GSS) and ROS detoxification (such as aldosterone reductase family 1 (AKR1), glutathione thiotransferase (GST) and quinone oxidoreductase‐1 (NQO1)), resulting in the suppression of ROS- and/or iron-related ferroptosis (Sun et al., 2016a; Roh et al., 2017) (Figure 2). The p62-Keap1-NRF2 axis is engaged in oxidative stress-associated ferroptosis via the competitive binding of p62 to Keap1, which leads to the activation of NRF2 and downstream effectors. ARF is a crucial tumor suppressor, that activates the p53 pathway during carcinogenic stress (Sherr, 2006). Studies have shown that ARF blocks CBP (transcription coactivator)-dependent NRF2 acetylation and binds to its cognate transcriptional promoter, which inhibits NRF2-mediated transactivation (including SLC7A11) and leads to ferroptosis (Chen et al., 2017). In addition, NRF2 negatively regulates FOCAD gene expression in human non-small-cell lung cancer (NSCLC) cells, which is dependent on the NRF2-replication protein A1 (RPA1)-ARE complex. FOCAD is essential for focal adhesion kinase (FAK) activity, which further enhances the sensitivity of NSCLC cells to cysteine deprivation-induced ferroptosis via promoting the tricarboxylic acid (TCA) cycle and the activity of Complex I in the mitochondrial electron transport chain (ETC) (Liu P. et al., 2020). The role of NRF2 in ferroptosis sensitiviy and the therapeutic potential of NRF2 inhibitors need further investigation in ferroptosis-associated therapy.
Mitochondrial-Mediated Pathway
The contribution of mitochondria to the ferroptotic pathway is under intense debate. Some studies have shown that erastin alters the permeability of the outer mitochondrial membrane and directly binds to VDAC2/3 after cysteine deprivation (with erastin or cystine-free medium), leading to increased mitochondrial membrane potential (ΔΨ) and lipid peroxides (Bock and Tait, 2019; Gao et al., 2019). Other studies have shown that ROS production by the mitochondrial electron transport chain is not involved in the activation of ferroptosis (Gaschler et al., 2018b). Mitochondrial free iron accumulation can aggravate erastin-mediated ferroptosis and glutaminolysis is required for ferroptosis under cystine deprivation. Glutamine is degraded to glutamate catalyzed by glutaminase 1 and 2 (GLS1 and GLS2) in mitochondria and produces alpha-ketoglutaric acid (α-KG), which can effectively modulate ferroptotic death (Figure 1). Moreover, p53 can favor glutaminolysis by increasing GLS2 expression, which promotes ferroptosis through glutamate accumulation. Excessive extracellular glutamate decreases intracellular cysteine levels and eventually results in ferroptosis via inhibiting system xc -. Correspondingly, upregulation of GLS2 facilitates p53-dependent ferroptosis (Gao et al., 2015).
Mevalonate Pathway
The implication of the mevalonate pathway and ferroptosis has been well established, with two important products, isopentenyl pyrophosphate (IPP) and coenzyme Q10 (CoQ10) (Figure 1). Remarkably, IPP is essential for cholesterol biosynthesis, CoQ10 production and selenocysteine (Se)-tRNA function, which are responsible for the efficient translation of GPX4. Blocking 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), the rate-limiting enzyme of the mevalonate pathway with statins can downregulate GPX4 activity and consequently induce the occurrence of ferroptosis (Shimada et al., 2016a; Viswanathan et al., 2017). Ferroptosis suppressor protein 1 (FSP1), previously known as ‘‘apoptosis-inducing factor mitochondria associated 2 (AIFM2)’’, promotes the production of CoQ10, an endogenous ferroptosis suppressor to restrain lipid peroxidation, which is independent of GPX4. Indeed, the FSP1-CoQ10-NAD(P)H signaling axis acts as an independent parallel system, that coordinates with GPX4 and GSH to combat oxidative stress and confer ferroptosis resistance (Bersuker et al., 2019; Doll et al., 2019).
Pentose Phosphate Pathway
Nicotinamide adenine dinucleotide phosphate (NADPH) serves as a principal reducing agent for biosynthesis across many biological processes, and is essential for maintaining intracellular GSH levels. Indeed, basal levels of NADPH are considered as a biomarker of ferroptosis susceptibility in several cancer cells, and NADPH depletion facilitates the ferroptotic cascade (Ding et al., 2020). The reduction in NADPH enhances erastin-, RSL3-and FIN56-induced ferroptosis (Shimada et al., 2016b). However, the pentose phosphate pathway (PPP) also produces NADPH as a substrate for NADPH oxidases (NOXs), which promote lipid peroxidation in subsequent ferroptosis. Direct inhibition of PPP or knockdown of related enzymes (glucose-6-phosphate dehydrogenase (G6PD) and phosphoglycerate dehydrogenase (PGD)) can enhance antioxidative activity to inhibit ferroptosis (Dixon et al., 2012; Hassannia et al., 2019). NADPH may play a dual role in ferroptotic regulation, which requires intensive explorations.
Epithelial-Mesenchymal Transition
Epithelial-mesenchymal transition (EMT) is identified that the epithelial phenotype with polarity and intercellular adhesion abilities progressively converts into the mesenchymal phenotype with migratory and invasive properties. EMT is an important cellular program during tumor migration, invasion and metastasis, contributing to drug resistance (Yang J. et al., 2020). EMT-related tumor metastasis and treatment resistance are provoked by transcription factors, such as SNAI1, TWIST1 and ZEB1, which are all potential targets in cancer therapy (van Staalduinen et al., 2018; Wu et al., 2019). Additionally, EMT has been demonstrated to be important for ferroptosis promotion. A highly mesenchymal-like cell state in human cancer cell lines and organoids confers a selective vulnerability to ferroptosis. Ferroptosis inhibitors, including RSL3, and statins have been reported to target the mesenchymal state for cancer therapy (Viswanathan et al., 2017). A CD44-dependent increase in iron endocytosis promotes the activity of iron-dependent demethylases, which increase the expression of genes related to EMT signaling, thereby sensitizing breast cancer cells to ferroptosis (Müller et al., 2020). Collectively, ferroptosis-based treatment may be effective in certain types of cancer, requiring careful consideration of cell type- and differentiation status-related pathways that may determine ferroptosis sensitivity and resistance.
Hippo Pathway
The Hippo pathway is usually believed to control cell number, organ size, tissue development and tumor growth, which negatively regulates the activity of several transcription factors, including transcription coactivators (e.g., yes-related protein 1 (YAP1) and WW domain containing transcription regulator 1 (WWTR1, also known as TAZ). As mentioned above, increased cell adhesion confers resistance to ferroptosis. In epithelial cells, neighboring cells interact through E-cadherin (ECAD) to activate NF2 (still identified as merlin) and the Hippo signaling pathway (Merlin-Hippo signaling) to promote ECAD-dependent cell adhesion, leading to ACSL4 transcriptional downregulation and ferroptosis resistance (Wu et al., 2019). In contrast, the activation of transcription factors involved in the Hippo pathway promotes ferroptosis in human renal cell carcinoma or ovarian cancer cells by regulating the expression of ferroptosis modulators (e.g., YAP-mediated ACSL4 and TFRC expression, TAZ-mediated epithelial membrane protein 1 (EMP1) and angiopoietin-like 4 (ANGPTL4) expression) to increase iron accumulation and lipid peroxidation (Yang et al., 2019; Yang Y. H. et al., 2020) (Figure 2).
Ferroptosis and Its Implication in Diseases
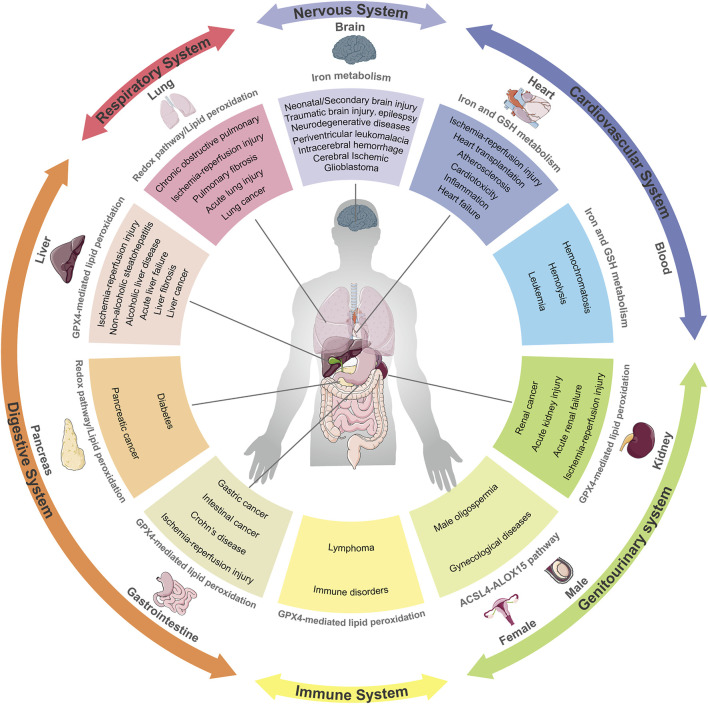
The link between ferroptosis and human diseases has been mainly verified by either the impact of physiopathology on ferroptosis or the impact of ferroptosis on physiopathology. Indicators or hallmarks of ferroptosis (e.g., lipid peroxidation) in some pathological models are obviously altered consistently with the ferroptosis pathway. Alternatively, the pathological state varies with the induction or inhibition of ferroptosis. Either way, molecular mechanisms and signaling pathways involved in ferroptosis help to expand understanding of human health and diseases. Ferroptosis has been interrelated with multiple pathological and physiological environments, with a context-dependent role (Figure 3). On the one hand, ferroptosis, like other forms of RCD, plays a beneficial role in the maintenance of normal physiological functions in the body through the removal of excessively damaged or disordered cells during the response to environmental injury/infection. On the other hand, the death of useful or functional cells aggravates the initiation and progression of diseases, consequently leading to certain pathological states, such as cardiovascular disease and neurodegeneration. Hence, pathologies in diverse tissues or organ systems interrelated with ferroptosis and relevant research models are summarized in Table 1.
FIGURE 3.
Ferroptosis and its implication in different pathophysiological contexts. Ferroptosis has been linked to pathological processes in diverse human body systems, including the nervous system, digestive system, respiratory system, circulatory system and urinary system. Abnormal pathways that contribute to diseases are recapitulatively presented.
TABLE 1.
The relevance of ferroptosis in diseases.
| Diseases | Disease subtype | Test models | Impact of ferroptosis | Related effects and important findings in diseases | Refs |
|---|---|---|---|---|---|
| Cancer | Head and neck cancer (HNC) | A dozen of HNC cells; HN3R, HN9, HN9R HN10 xenograft mice Normal oral keratinocytes or fibroblasts obtained from patients | Ferroptosis of cancer cells inhibiting diseases | GPX4 inhibitors, (1S, 3R)-RSL 3 and ML-162 induce ferroptosis; Accumulated mitochondrial iron and lipid ROS promote ferroptosis | Roh et al. (2017), Kim et al. (2018), Shin et al. (2018) |
| Breast Cancer | MDA-MB-231, T47D, HCC-1806, BT549, MCF-7(X) cells; TUBO, 4T1 xenograft mice; Patients’ samples | TRFC is a candidate marker of a subgroup of ER+/luminal-like breast cancer with poor outcome and tamoxifen resistance; GPX4-ACSL4 DKO cells show marked resistance to ferroptosis; Siramesine and lapatinib combination increase intracellular iron and ROS levels, and initially induce ferroptosis | Tonik et al. (1986), Habashy et al. (2010), Lanzardo et al. (2016), Ma et al. (2017), Yu et al. (2019) | ||
| Hepatocellular carcinoma (HCC) | A dozen of HCC cells; THLE-3, HL-7702 primary human hepatocytes (PHH); Hepa1-6, Bel-7402 xenograft mice; DEN/CCl4-liver cancer model mice; Patients’ samples | The p62-Keap1-NRF2 pathway prevents ferroptosis and reduced GSH promote ferroptosis in liver cancer cells; Metallothionein-1 (MT-1), which inhibits lipid peroxidation, are associated with drug resistance and reduced overall survival; Ferroptosis inhibits liver tumorgenesis and is suppressed in liver cancer; XCT expression is higher, inversely related to the patient’s overall survival rate and disease-free survival rate | Kinoshita et al. (2013), Sun et al. (2016a), Sun et al. (2016b), Houessinon et al. (2016), Zhang X. et al. (2019), Bai et al. (2019) | ||
| Lung cancer | A dozen of lung cancer cells; Mouse metastatic lung tumors | Lung adenocarcinomas select for expression of a pathway that confers resistance to high oxygen tension and protects cells from ferroptosis; Erastin upregulates p53 and inhibits SLC7A11, which induce ROS accumulation and ferroptosis | Wang S. J.et al. (2016), Alvarez et al. (2017) | ||
| Gastric cancer (GC) | AGS, SGC7901, MGC803, MKN45 cells; BGC823 cells and xenograft mice; Patients’ samples | Cysteine dioxygenase 1 (CDO1) uptakes cysteine competitively, thereby restricting GSH synthesis and promoting ferroptosis; Suppression of CDO1 restores GSH level, prevents ROS production, upregulates GPX4 expression, and ultimately blocks lipid peroxidation and ferroptosis | Hao et al. (2017) | ||
| Colorectal cancer (CRC) | TP53+/+ and TP53−/− HCT116 cells and mice | Loss of p53 restricts the nuclear accumulation of DPP4 and thus facilitates plasma membrane-associated DPP4-dependent lipid peroxidation, which eventually leads to ferroptosis | Xie et al. (2017) | ||
| Pancreatic cancer | MIAPaCa-2, CFPAC-1, BxPC-3, (resistant) PANC-1 cells | Ferroptosis inducer increases ROS production and activates ferroptosis; STAT3 is a positive regulator of ferroptosis and STAT3 silencing blocks erastin-induced ferroptosis | Kasukabe et al. (2016), Gao et al. (2018), Yamaguchi et al. (2018) | ||
| Ovarian cancer | A dozen of ovarian cancer cells; HEY1 and HEY2 spheroids; ID8 cells and xenograft mice; Ovarian cancer cells isolated from patients | IFNγ cooperated with cyst(e)inase to increase lipid peroxidation and induce ferroptosis | Greenshields et al. (2017), Wang W. et al. (2019) | ||
| Melanoma | SK-MEL-28 cells; A375, G-361, B16 cells and xenograft mice; Human melanoma cell lines established from patient biopsies | Inhibition of mitochondrial complex I triggers ROS production, lipid peroxidation and ferroptosis; Melanoma dedifferentiation increases sensitivity to ferroptosis; Depletion of cyst(e)ine and inhibition of system xc − promote lipid peroxidation and ferroptosis; Expression of system xc− is negatively associated with CD8+ T cell signature, IFNγ expression and patient outcome | Basit et al. (2017), Luo et al. (2018), Tsoi et al. (2018), Wang W. et al. (2019) | ||
| Glioblastoma | F98, U87 cells; Glioblastoma patients | NRF2 level is inversely related to clinical outcome and overall survival; Fostered NRF2 expression and conversely Keap1 inhibition promote resistance to ferroptosis | Fan et al. (2017) | ||
| Leukemia | Dozens of leukemia cells; Patient-derived xenografts (PDXs) of leukemia cells | High level of ACSL4 mRNA is expressed and is sensitive to ferroptosis; Low expression of FPN results in the susceptibility via increased iron levels; ROS produced by free ferrous iron leads to increased oxidative stress and ferroptosis | Yuan et al. (2016), Probst et al. (2017), Trujillo-Alonso et al. (2019) | ||
| DLBCL; Renal cell carcinoma (RCC) | Dozens of DLBCL and RCC cells | DLBCL and RCC are particularly susceptible to GPX4-regulated ferroptosis; GPX4 is an essential mediator of ferroptotic cell death | Yang et al. (2014) | ||
| Adrenocortical carcinoma (ACC) | NCI-H295R, HEK cells | Elevated expression of GPX4 and higher sensitivity to ferroptosis are found in ACCs | Belavgeni et al. (2019) | ||
| Neuro-degenerative diseases | Alzheimer’s disease (AD) | AD Patients; Brain tissues from GPX4BIKO mice; Tauopathy model mice | Ferroptosis of useful or functional cells inducing diseases | Iron-induced lipid peroxidation is abnormally elevated in the brain; Cerebrospinal fluid ferritin level is negatively correlated with cognitive ability; Ferroptosis inhibitors prevent neuronal damage | Ayton et al. (2015), Hambright et al. (2017), Zhang Y.-H. et al. (2018) |
| Parkinson’s disease (PD) | PD Patients; LUHMES cells; Human brain tissues; MPTP-induced PD model mice | Iron concentration in the SN is related to the degree of disease progression and DFP improves related symptoms; Levels of MDA and lipid hydroperoxide are increased in the SN. | Devos et al. (2014), Pyatigorskaya et al. (2015), Do Van et al. (2016) | ||
| Huntington’s disease (HD) | R6/2 HD mice; HD Patients | Plasma MDA, 4-hydroxynonenal (4-HNE) and lipid peroxidation are increased; IRPs 1/2, TFRC and GPX are decreased and FPN is increased | Klepac et al. (2007), Lee et al. (2011), Chen et al. (2013) | ||
| Periventricular leukomalacia (PVL) | Oligodendrocytes | Fer-1 increases the number of healthy spinous neurons and inhibits oxidized lipid damage and ferroptosis | Skouta et al. (2014) | ||
| Brain diseases | Neonatal brain injury | Organotypic hippocampal slice cultures (OHSCs); Neonatal hypoxia-ischemia rats | Free iron is accumulated, TFRC expression is increased and ferritin expression is reduced | Lu et al. (2015) | |
| Traumatic Brain Injury (TBI) | TBI model HT22 cells; TBI model mice | AA/AdA-PE are increased; ALOX15, ACSL4 and GSH are exhausted; Ferroptosis inducers and mechanical stretch injury cause cell death | Kenny et al. (2019) | ||
| Secondary brain injury (SBI) | Mouse brain astrocytes; ICH rats | GPX4 is downregulated in brain after ICH; GPX4 contributes to SBI following ICH by mediating ferroptosis; Induction of NRF2 expression serves as an adaptive self-defense mechanism | Cui et al. (2016), Zhang Z. et al. (2018) | ||
| Intracerebral hemorrhage (ICH) | ICH mice; OHSCs; Human induced pluripotent stem cell (iPSC)-derived neurons | Fer-1 reduces iron accumulation, prostaglandin-endoperoxide synthase 2 (PTGS2) expression, lipid ROS and protects hemorrhagic brain from neuronal death | Li et al. (2017) | ||
| Cerebral ischemia | MCAO mice and rats; Transient forebrain ischemia (TRI) rats | Ferritin, TFRC and iron accumulation are increased, and infarct focus is strengthened; The leaking blood-brain barrier (BBB) increases the iron level; Targeting iron-mediated oxidative stress holds extended therapeutic time window against an ischemic event | Park et al. (2011), Tuo et al. (2017) | ||
| Heart diseases | Ischemia-reperfusion (I/R) | Isolated hearts of mice; Cardiomyocytes | GSH level is significantly reduced and ROS level is increased; Inhibition of glutamate breakdown reduces I/R-induced heart damage; DFO improves function and reduces in myocardial infarcts size | Gao et al. (2015), Baba et al. (2018), Fang et al. (2019) | |
| Heart failure | Isolated adult cardiomyocytes; FPN knockout mice; Mice with cardiomyocyte-specific deletion of FTH1, hepcidin, or knock-in of hepcidin-resistant FPN | DXZ relieves myocardial toxicity; FTH1 deficiency leads to a decrease in cardiac iron level and an increase in oxidative stress; FPN knockout causes iron deposits in the myocardium and impairs cardiac function | Lakhal-Littleton et al. (2015), Lakhal-Littleton et al. (2016), Fang et al. (2020) | ||
| Inflammation | Several immune deficient mice; Heart transplantation mice | Ferroptosis orchestrates neutrophil recruitment to injured myocardium by promoting adhesion of neutrophils to coronary vascular endothelial cells through TLR4/TRIF signaling pathway, which inhibited by Fer-1 | Li W. et al. (2019) | ||
| Atherosclerosis | Overexpressing GPX4 and control Apolipoprotein E (ApoE)−/− mice | Iron accumulation causes ROS accumulation and death in macrophages; Increased antioxidant capacity can reduce the ferroptosis of macrophages; GPX4 overexpression inhibits plaque formation by inhibiting oxidized lipid modification and reduces mid-advanced aortic sinus lesions | Guo et al. (2008) | ||
| Blood diseases | Hemolysis | J774 cells; RBC transfusion and clearance model mice | Increased red blood cells (RBCs)through phagocytosis lead to iron degeneration, ROS accumulation and lipid peroxidation in splenic red plasma macrophage (RPMs), which can be ameliorated by Fer-1; Ferroptosis may be clinically relevant to transfusion-related immunomodulation and impaired host immunity | Youssef et al. (2018) | |
| Hereditary hemo-chromatosis (HH) | Primary hepatocytes; Bone marrow-derived macrophage (BMDMs); SLC7A11−/− mice; HH model mice | Iron overload is sufficient to trigger ferroptosis both in vitro and in vivo; SLC7A11 confers protection against ferroptosis during iron overload; SLC7A11 depletion facilitates ferroptosis onset specifically under high-iron conditions | Wang et al. (2017a) | ||
| Lung diseases | Chronic obstructive pulmonary (COPD) | Human bronchial epithelial cells (HBECs); BEAS-2B, A549 cells; GPX4 deficient or transgenic mice | Cigarette triggers NCOA4-mediated ferritinophagy; Iron accumulation and lipid peroxidation are increased, which can be reversed by GPX4 knockout, DFO and Fer-1 | Yoshida et al. (2019) | |
| Pulmonary I/R | Pulmonary I/R model mice; Hypoxia-reoxygenation model A549 cells | ACSL4 expression is enhanced and GPX4 expression is reduced; Ferroptotic features emerge after lung I/R injury, which is prevented by liproxstatin-1 (Lip-1) | Xu et al. (2020) | ||
| Liver diseases | Acute liver failure | ACSL4 KO mice; acetaminophen (APAP)-induced acute liver failure mice | APAP administration induces hepato-toxicity, lipid peroxidation, PTGS2 upregulation and GSH depletion, which are markedly suppressed by Fer-1and DFO. | Yamada et al. (2020b), Yamada et al. (2020c) | |
| Non-alcoholic steatohepatitis (NASH) | Several NASH model mice; CCl4 induced liver injury mice | Enhanced AA metabolism, iron-mediated lipid ROS accumulation, mitochondrial morphological changes are alleviated by ferroptosis inhibitors | Tsurusaki et al. (2019), Li X. et al. (2020) | ||
| Alcoholic liver disease (ALD) | ALD patients | Serum hepcidin is decreased; Iron, ferritin and FPN are upregulated | Dostalikova-Cimburova et al. (2014) | ||
| Hepatic I/R; Living donor liver transplantation (LDLT) | Hepatic I/R model mice; Hepatic I/R injury in pediatric LDLT | A high serum ferritin level, a marker of iron overload, is an independent risk factor for liver damage after LT; Liver damage, lipid peroxidation, and upregulation of PTGS2 are induced by I/R | Yamada et al. (2020a) | ||
| Pancreas diseases | Diabetes mellitus and its complications | NRK-52E cells; Type 2 diabetes (T2DM) mice; Diabetic nephropathy mice | Depleted GSH and downregulated GPX4 induce oxidative stress in pancreatic tissue of T2DM molding; ACSL4 is increased and GPX4 is decreased in DN mice | Li D. et al. (2020), Wang et al. (2020) | |
| Gastrointestinal diseases | Intestinal I/R | Caco-2 cells; Intestinal I/R model mice | ACSL4 and cyclooxygenase 2 (COX2) are increased while GPX4 and FTH1 are reduced in I/R-induced intestinal injury | Li Y. et al. (2019) | |
| Crohn’s disease (CD) | GPX4 deficient intestinal epithelial cells (IECs); GPX4+/−IEC mice; CD patients | IECs in CD exhibit impaired GPX4 activity and signs of lipid peroxidation | Mayr et al. (2020) | ||
| Kidney diseases | Acute kidney injury; Acute renal failure (ARF) | Human renal proximal tubule epithelial cells (HRPTEpiCs); GPX4−/− Pfa1 cells; GPX4−/− mice | Mitochondrial lipid phosphatidylcholine (PC), PE and cardiolipin are heavily oxidized; Ferroptosis inhibitor, SRS16-86 strongly protects kidneys | Friedmann Angeli et al. (2014), Skouta et al. (2014) | |
| Immune diseases | Immune disorders | GPX4-deficient T cells; T cell-specific GPX4 deficient mice; Peripheral blood mononuclear cell (PBMCs) | GPX4 deficiency causes T cells to fail to protect against viruses and infections, which can be rescued by vitamin E; Rapid accumulation of membrane lipid peroxides induces ferroptosis; Erastin-induced lipid peroxidation promotes PBMCs proliferation and differentiation into B cells and natural killer cells | Matsushita et al. (2015), Wang D. et al. (2018) | |
| Other diseases | Age-related macular degeneration (AMD) | ARPE-19 cells | Oxidative stress-mediated senescence upon GSH depletion and subsequent death of photoreceptors are observed in AMD. | Sun et al. (2018) |
Cancer
Cancer is a multifactorial and complicated disease that contributes to a major health burden worldwide, despite substantial efforts and considerable improvement. In general, there are two key challenges in cancer treatment. One is how to kill cancer cells effectively without affecting healthy cells. Second, cancer cells in advanced tumors often exhibit multiple genetic variations with high oxidative stress and enhanced antioxidant capacity, which confer drug resistance (Trachootham et al., 2009). Ferroptosis, as a novel form of cell death, offers unique possibilities efficiently against a variety of cancers. A higher iron requirement than their non-malignant counterparts has been observed to support sustained proliferation and immortalized replication in certain cancer cells, along with FPN downregulation and TFRC upregulation. The strong dependency on iron makes cancer cells more susceptible to iron overload and ROS-mediated lipid peroxidation than noncancerous cells. Epidemiological investigations have also revealed that high dietary iron intake increases the risk of several cancer types (such as hepatocellular carcinoma (HCC) and breast cancer) (Fonseca-Nunes et al., 2014). Ferroptosis inducers have been proven effective in suppressing the occurrence and development of cancers derived from iron-rich tissues, such as HCC, breast cancer and pancreatic ductal adenocarcinoma (PDAC) (Gao et al., 2015; Friedmann Angeli et al., 2019). According to a sensitivity profiling in 177 cancer cell lines, renal cell carcinomas (RCCs) and diffuse large B cell lymphomas (DLBCLs) are particularly susceptible to GPX4-regulated ferroptotic cell death (Yang et al., 2014). From another perspective, it is well acknowledged that the activation of the apoptosis cascade is the most prevalent way to eliminate cancer cells. Unfortunately, the apoptotic pathway of most mutant cancer cells is frequently blocked, which confers robust drug resistance (Amable, 2016). Cancer cells resistant to some forms of death may still be vulnerable to the induction of other forms of cell death, suggesting bypass of apoptotic resistance is a considerable approach to combating resistant cancers. A large amount of evidence has confirmed that ferroptosis induction plays a predominant role against certain chemoresistant cancer cells that are in a high mesenchymal state or escaping drug treatment (Holohan et al., 2013). Recent research has showed that cisplatin can induce both ferroptosis and apoptosis in the NSCLC A549 cells and colon cancer HCT116 cells, while drug resistance is observed in cisplatin-induced apoptosis, rather than ferroptosis (Guo et al., 2018a). Additionally, some drug-resistant cancer cells with activated EMT signaling (upregulation of mesenchymal markers and downregulation of epithelial markers) become specifically sensitive to ferroptosis-based therapies (Viswanathan et al., 2017; Müller et al., 2020). Beyond acting as a monotherapy, ferroptosis induction also participates in combination with conventional therapies. For instance, erastin, a classical ferroptosis inducer, has been shown to enhance the chemotherapeutic efficacy of traditional anticancer drugs, such as temozolomide and cisplatin in specific cancer cell lines (Yamaguchi et al., 2013; Chen et al., 2015). Accordingly, deficiencies in a host of NRF2 targets, including GPX4, SLC7A11 and FTH1/FTL, are also capable of predisposing cells to succumb to proferroptotic agents in many cancer types (Hassannia et al., 2019).
Currently, discovered proferroptotic compounds mainly spotlight on key elements of redox homeostasis (e.g., system xc - and GPX4) to disrupt the existing redox balance and trigger the ferroptotic cascade in cancers whose growth and survival are highly dependent on the uptake of amino acids. Lack of SLC7A11 and knockdown of GPX4 have been demonstrated to increase ROS-mediated lipid peroxidation and induce subsequent ferroptosis in even resistant cancer cells, indicating that deficiency in antiferroptotic function enhances cancer cell death (Yang et al., 2014; Roh et al., 2016). Furthermore, increased expression of SLC7A11, GPX4 or their upstream regulatory gene NRF2 not only promotes ferroptosis resistance, but is also correlated with a poorer prognosis and lower survival rate in many types of cancers, such as liver, lung and ovarian cancers (Fan et al., 2017; Daher et al., 2019; Lim et al., 2019). However, the role of ferroptosis in tumorigenesis and progression remains elusive, as ferroptosis appears to have a dual role. There has been evidence that deficiency in NRF2 favors early tumorigenesis in several cancers (Rojo de la Vega et al., 2018). Another critical mediator of the proferroptotic cascade, ACSL4, is downregulated in bladder, brain, breast, leukemia and lung cancer but upregulated in colorectal, head and neck, kidney, myeloma and liver cancer. Prognostic analysis shows that colorectal cancer patients with high ACSL4 expression have a low survival rate. In contrast, brain, breast and lung cancer patients with low ACSL4 expression have a poor survival, suggesting that ACSL4 plays different roles in different cancer types (Chen et al., 2016).
With a comprehensive understanding, ferroptosis has also been implicated in cancers undergoing immunotherapy and radiotherapy. Immunotherapy with immune checkpoint blockade, which activates an effective cytotoxic T cell-triggered antitumor immune-response, has a revolutionary impact on clinical cancer treatment. Mechanistically, interferon gamma (IFNγ) released from CD8+ T cells can impair cystine uptake by cancer cells upon downregulating the expression of SLC3A2 and SLC7A11 (two subunits of the cystine-glutamate antiporter system xc -) and consequently promote lipid peroxidation and ferroptosis in ovarian cancer, melanoma and fibrosarcoma (Tsoi et al., 2018; Wang W. et al., 2019; Lang et al., 2019). Radiotherapy acts as frontline therapy for approximately half of patients with many cancers. Recently, ferroptosis has also been proven to be interrelated with cancer radiotherapy, which induces ACSL4 expression and enhances oxidative damage in a variety of cancers, including NSCLC, melanoma and esophageal cancer (Lang et al., 2019; Lei et al., 2020). Indeed, immunotherapy and radiotherapy synergistically activate tumoral lipid oxidation and ferroptosis.
However, ferroptosis also triggers severe damage and toxicity in organisms, including bone marrow injury and cisplatin-induced acute kidney injury. How to modulate ferroptosis pathway to obtain the highest clinical benefit in clinical oncology is a critical question that deserves further investigation.
Neurodegeneration and Brain Injury
A large body of evidence suggests that low levels of GSH/GSSG, elevated iron levels and lipid peroxidation are implicated in a variety of neurodegenerative diseases, along with abnormal levels of ferroptosis-associated modulators (Praticò and Sung, 2004; Belaidi and Bush, 2016). Alzheimer’s disease (AD) is the most common neurodegenerative disease and is characterized by synaptic loss and neuronal death. In AD patients, there is abnormally elevated iron in the brain tissue, and ferritin levels are negatively correlated with cognitive ability (Ayton et al., 2015). Excessive ROS generation triggers lipid peroxidation, which contributes to severe oxidative stress damage and is observed in GPX4-deficient AD mouse models, while ferroptosis inhibitors ameliorate neurodegeneration and behavioral dysfunction by blocking iron overload, lipid peroxidation and inflammation (Hambright et al., 2017; Zhang Y.-H. et al., 2018). Parkinson’s disease (PD) is characterized by progressive dopaminergic neuronal loss in the substantia nigra (SN) pars compacta. Epidemiological investigations have shown that high iron levels lead to a high risk of PD, associated with enhanced basal lipid peroxidation and elevated malondialdehyde (MDA) levels, a toxic byproduct of lipid peroxidation (Pyatigorskaya et al., 2015). Given the implication of iron in PD, preclinical experiments and clinical trials have revealed that the U.S. Food and Drug Administration (FDA)-approved iron chelators improve dopamine activity and prevent iron-mediated oxidative damage, by exerting a certain protective effect in PD patients (Devos et al., 2014; Chen X. et al., 2020). Several features of Huntington’s disease (HD) physiopathology are consistent with the ferroptosis pathway, including a lower GSH level, a higher lipid oxidation level and abnormal iron metabolism. Accordingly, administration of DFO significantly improves striatal pathology and motor phenotype in an R6/2 HD mouse model (Klepac et al., 2007; Lee et al., 2011; Chen et al., 2013). Additionally, ferrostatin-1 (Fer-1, classical ferroptosis inhibitor) and its analogs inhibit lipid peroxidation damage and increase the number of healthy spinous process neurons in the HD model. Apart from common neurodegeneration, other neurodegenerative disorders are also characterized by perturbations in iron homeostasis and accumulation, such as periventricular leukomalacia (PVL) (Skouta et al., 2014), amyotrophic lateral sclerosis (ALS) (Southon et al., 2020), multiple sclerosis (MS) (Jhelum et al., 2020), Friedreich’s ataxia (FRDA) (Cotticelli et al., 2019). Affected tissue regions share features of elevated iron, low GSH, decreased GPX4, as well as increased lipid peroxidation, consistent with the salient features of ferroptosis. Although there are few relevant studies, some ferroptosis-associated factors appear to be potential markers and therapeutic targets in these disorders (Hu et al., 2019; Seco-Cervera et al., 2020).
Besides leading to neurodegenerative diseases, the activation of the of ferroptotic cascade has also been determined in a wide variety of neuronal injuries. Neonatal brain injury, as well as traumatic brain injury (TBI), is usually accompanied by a series of biochemical processes, such as dysfunctional iron metabolism, decreased GPX activity, ROS accumulation and disordered ferroptosis-related mediators in vitro and in vivo (Lu et al., 2015; Kenny et al., 2019). Oxidative stress damage induced by specific GPX4 deletion in the brain is observed during the acute phase of intracerebral hemorrhage (ICH) and subsequent secondary brain injury (SBI) and finally leads to hemorrhagic stroke which can be relieved by an increasing GPX4 levels. The application of N-acetylcysteine (NAC, a ROS scavenger) or Fer-1 has been documented to prevent neuronal mortality after ICH and improve the prognosis of patients, by decreasing iron accumulation and lipid ROS (Li et al., 2017; Zhang Z. et al., 2018). Dysfunction of tau protein contributes to age-dependent, iron-mediated neurotoxicity in ischemic stroke tissue. Tau is capable of blocking ferroptosis by promoting iron export against middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia-reperfusion injury. Ferroptosis has been recognized as a main cause of neuronal death after ischemic stroke, and ferroptosis inhibitors significantly improve the prognosis of patients (Tuo et al., 2017). Actually, ferroptosis inhibitors have been successfully applied in animal models of I/R-associated tissue injuries including heart (Fang et al., 2019), lung (Xu et al., 2020), liver (Yamada et al., 2020a), intestine (Li Y. et al., 2019) and kidney (Friedmann Angeli et al., 2014).
Overall, these studies suggest that ferroptosis irreversibly drives neuronal loss and tissue damage linked with central and/or the peripheral neuronal pathologies. The effectiveness of ferroptosis inhibition as a therapeutic approach has been mostly validated by cellular and animal experiments. However, there seems to be a lack of strong evidence in clinical trials. Providing comprehensive treatments for neurological diseases remains a largely insurmountable challenge.
Cardiovascular Diseases
As “iron-induced heart disease” was first reported, iron-induced ferroptosis has been gradually identified as playing a detrimental role in the occurrence and aggravation of cardiovascular conditions in both animal models and human patients (Sullivan, 1981). Abnormal myocardial iron accumulation plays a pathogenic role in the myocardium which leads to cardiotoxicity and cardiac dysfunction. Studies have shown that ferroptosis mediates myocardial damage caused by doxorubicin (DOX) or I/R mainly by HO-1 upregulation, and subsequently degrading heme to release free iron and generate mitochondrial membrane oxidized lipids. Iron deposition in the myocardium of FPN knockout mice which results in severe damage to cardiac function has been recognized as a risk factor for coronary artery diseases (Bagheri et al., 2013; Lakhal-Littleton et al., 2015). Iron chelators, such as DFO and DFP have been shown to counteract the pathogenic effects of iron overload, indicating a promising therapeutic strategy against ferroptosis-driven cardiac abnormalities (Gao et al., 2015; Fang et al., 2019). Nonetheless, cardiomyocyte iron deficiency can also lead to fatal contractile and metabolic dysregulation as a consequence (Lakhal-Littleton et al., 2016). This findings supports compelling evidence that the influence of ferroptosis on cardiac diseases depends on proper iron homeostasis, rather than absolutely low iron levels. Ferroptosis has also been involved in inflammatory responses after heart transplantation, which promotes the adhesion of neutrophils to coronary endothelial cells and myocardial I/R damage. Fer-1 can alleviate the loss of cardiomyocytes and limit the recruitment of neutrophils by the TLR4/TRIF signaling pathway (Li W. et al., 2019). As the master regulator of systemic iron homeostasis, hepcidin is derived primarily from the liver, and it inhibits the iron exporter FPN to decrease iron availability in blood (Lakhal-Littleton et al., 2016). Due to an inherited genetic disorder or receiving multiple blood transfusions, the depletion of hepcidin induces ferroptosis as a result of iron overload in the body. As an iron overload disease that blocks hepcidin biosynthesis, hemochromatosis can be ameliorated by either iron chelators or genetic regulation of ferroptosis, such as targeting SLC7A11 (Wang H. et al., 2017).
Although the link with cardiovascular diseases still requires further evaluation by clinical evidence, iron-induced ferroptosis has been well-established as a master trigger of several cardiovascular pathologies. Chelation therapy has been considered as an efficient preventative measure and treatment approach and the key is how to maintain relatively precise iron homeostasis.
In addition to common diseases mentioned above, more pathologies associated with ferroptosis are summarized in Table 1 and Figure 3.
Pharmacological Approaches to Ferroptosis Modulation
A more profound understanding of the regulatory and metabolic mechanisms of ferroptosis in a variety of diseases provides attractive approaches to diagnosis and therapy. As mentioned above, the positive or negative role of ferroptosis depends on the context of diseases with different pathological and physiological processes. In cancer therapy, it aims to slow or suppress tumor growth by inducing ferroptosis in cancer cells without affecting non-malignant cells. Thus, the activation of ferroptosis appears to be a promising therapeutic strategy to block pro-carcinogenic alterations, especially those are resistant to other modes of cell death. In degenerative or traumatic diseases, such as neurodegenerative disorders and I/R injury, the therapeutic goal is to protect functional cells from ferroptosis-induced injury. As such, ferroptosis inhibitors are expected to reverse or mitigate oxidative damage and disease progression and even achieve curative effect. Besides individual effects, multiple ferroptosis regulators can also act synergistically with some conventional drugs. Many kinds of synthetic compounds and natural products have been identified as inducers and inhibitors of ferroptosis to overcome diseases by modulating ferroptotic or mixed death.
Synthetic Compounds and Their Modulation on Ferroptosis
Currently, numerous chemical reagents with ferroptotic properties have been continuously exploited and demonstrated in preclinical and clinical experiments. Although the underlying mechanism needs more investigation, ferroptosis inducers are reckoned as a novel therapeutic strategy against various cancers, especially those resistant to conventional drugs. Indeed, ferroptosis was first discovered by studying reaction mechanisms of two small RAS-selective molecule compounds, erastin and RSL3, including their derivatives, that have been confirmed to suppress tumor growth in many types of cancers in vitro and in vivo (Dixon et al., 2012; Yang et al., 2014; Viswanathan et al., 2017; Zhang Y. et al., 2019). Originally, ferroptosis-inducing reagents were divided into two mechanistic classes: 1) inhibitors of cystine via system xc - (e.g., erastin, sorafenib, SAS and glutamate), which subsequently drive GSH depletion, and 2) direct inhibitors of GPX4 (e.g., RSL3 (1S, 3R)-RSL3). More recently, the small molecule FIN56 has been reported to degrade GPX4 and to deplete CoQ10, an endogenous inhibitor of lipid peroxidation, through the mevalonate pathway. Moreover, FINO2 can either oxidize ferrous iron directly, or deactivate GPX4 indirectly (Shimada et al., 2016a; Gaschler et al., 2018a). On the contrary, ferroptosis also participates in other nonneoplastic lesions and a variety of compounds can pharmacologically inhibit ferroptosis, most of which are iron chelators or antioxidants. Iron chelators (e.g., DFO and DXZ) or regulators of iron metabolism suppress ferroptosis through lowering free iron levels (Fang et al., 2019; Chen X. et al., 2020). In addition, inhibitors of lipid peroxidation, including ACSL4 inhibitors (e.g., thiazolidinediones (TZDs)), lipophilic antioxidants (e.g., α-tocopherol), and agonists of the antioxidant system (e.g., ROS scavengers (NAC) and system xc- inducers (β-mercaptoethanol)) inhibit ferroptosis via single or complex mechanisms (Dixon et al., 2012; Karuppagounder et al., 2018).
Fortunately, some of these pro- and anti-ferroptotic compounds are clinical drugs approved by the FDA, and are expected to be novel therapeutic applications based on ferroptotic mechanisms. Mechanistically, SAS, sorafenib and lanperisone have been broadly documented to inhibit cysteine-glutamate antiporter and cystine absorption, leading to the attenuation of GSH, and ultimately resulting in the death of certain types of cancer cells, including HCC, lymphoma and prostate cancer (Lachaier et al., 2014; Sun et al., 2016a; Sun et al., 2016b). Siramesine, lapatinib and salinomycin (ironomycin) can trigger ferroptosis by elevating iron levels in breast cancer cells and leukemia cells (Hamaï et al., 2017; Ma et al., 2017; Mai et al., 2017). Iron chelators are usually used as antidotes for iron poisoning in clinical practice, and new indications seem to be available based on their antiferroptotic properties. TZDs, a class of typical antidiabetic compounds, have been proven to act as ACSL4 inhibitors to restore GPX4 expression and reduce COX2 expression, and finally prevent lipid peroxidation and ferroptosis to ameliorate intestinal and pulmonary I/R injuries in vitro and in vivo (Doll et al., 2017; Li Y. et al., 2019; Xu et al., 2020). Furthermore, chelation therapy targeting iron dyshomeostasis has shown promise against neurodegenerative disorders in preclinical studies and clinical trials. Especially, DXZ is currently the only FDA-approved drug for preventing and treating DOX-induced cardiotoxicity in cancer patients (Fang et al., 2019). DFP has been recently used clinically for systemic iron overload dysfunctions and investigated as a conventional or modified treatment in several clinical trials against PD. It has shown reduced iron deposition in the SN and an improvement in motor function, as well as slowed disease progression and relieved neurological symptoms (Devos et al., 2014). Currently, many studies on ferroptosis relevance in neurodegenerative diseases are attempting to use ferroptosis inhibitors, especially iron chelators, to slow disease progression in preclinical animal models, most of which have achieved an potent efficacy (Guo et al., 2016; Rao et al., 2021). Nevertheless, there are still many restrictions and problems associated with chelation therapy. When applied in human clinical trials, iron chelators appears to be far from satisfactory (Grolez et al., 2015). DFP is well tolerated, achieved target engagement (lowering of iron) in patients with neurodegeneration. It seems to somewhat slow disease progression and improve quality of life, but they have not reach significance (Martin-Bastida et al., 2017; Klopstock et al., 2019). Although some of iron chelators, such as DFO, DFP and deferasirox (DFS) are recommended as iron overloaded first-line treatments, they all have their own definite side effects, such as gastrointestinal disorders, hepatotoxicity, nephrotoxicity and visual and auditory neurotoxicity (Vichinsky et al., 2011; Aydinok et al., 2015; Maggio et al., 2020). Furthermore, poor pharmacokinetic properties of DFO and DFS limit their availabilities in the central nervous system, as they are prevented from crossing the blood-brain barrier (BBB) (Sripetchwandee et al., 2016; Crielaard et al., 2017). More appropriate iron chelators that regulate the ferroptotic process need to be further explored and developed for their potential to treat neurodegenerative diseases in preclinical and clinical studies.
Therefore, it is worth noting that nanotechnology has emerged to achieve anticancer effects by improving the pharmacokinetic properties of existing proferroptotic agents or developing novel nano-inducers of ferroptosis. The FDA-approved iron-supplementing agent ferumoxytol (Feraheme) has been proven to have an anti-leukaemia effect in vitro and in vivo via increasing intracellular iron and ROS production, leading to enhanced oxidative stress and ferroptotic cell death (Trujillo-Alonso et al., 2019). Cisplatin (CDDP)-loaded Fe3O4/Gd2O3 hybrid nanoparticles with the conjugation of lactoferrin and RGD dimer (RGD2) (FeGd-HN@Pt@LF/RGD2), which are able to cross the BBB, have been developed for therapy of orthotopic brain tumors based on ferroptosis mechanisms (Shen et al., 2018). In view of the oxidative stress regulation ability of p53 and inducibility of the metal-organic network (MON) on the Fenton reaction, synthesized MON encapsulated with p53 plasmid (MON-p53) can trigger both ferroptosis and apoptosis pathways to synergistically kill cancer cells (Zheng et al., 2017).
Collectively, we have listed FDA-approved synthetic drugs that have been reported to modulate ferroptosis in Table 2 and experimental chemical compounds in Supplementary Tables S1 and Supplementary Tables S2.
TABLE 2.
FDA-approved synthetic drugs associated with ferroptosis.
| Drugs | Functional targets | Impact on ferroptosis | Diseases | Test models | Mechanisms/Effects | Refs |
|---|---|---|---|---|---|---|
| Sulfasalazine (SAS) | System xc - | Induce | Fibrosarcoma; Breast cancer; Pancreatic cancer | MDA-MB-231, T47D, BT549, MCF7, CFPAC1 cells; PANC1, HT-1080 cells and xenograft mice; Panc02 orthotopic mice | GSH depletion; SLC7A11 downregulation; Lipid peroxidation | Dixon et al. (2012), Zhu et al. (2017), Wang W. et al. (2019), Yu et al. (2019) |
| Sorafenib | System xc - | Induce | Hepatocellular carcinoma (HCC) | HepaG2, Hep3B cells; Hepatocytes from HCC patients | Lipid peroxidation; GSH depletion; Increasing ROS level | Lachaier et al. (2014), Sun et al. (2016a), Sun et al. (2016b) |
| Lanperisone | System xc - | Induce | K-ras-driven tumors | K-ras-mutant mouse embryonic fibroblasts (MEFs) and xenograft mice | GSH depletion; Increasing ROS level | Shaw et al. (2011) |
| Glutamate; Glutamine | System xc - | Induce | Fibrosarcoma | OHSCs, MEFs; HT-1080, HT22 cells; Primary cortical neurons | Inhibiting cystine import; GSH depletion | Dixon et al. (2012), Gao et al. (2015), Bueno et al. (2020) |
| Statins (fluvastatin, simvastatin, lovastatin acid) | GPX4 | Induce | Fibrosarcoma | HT-1080 cells | Downregulating GPX4 level, mevalonate pathway and selenoprotein biosynthesis; Lipid peroxidation | Viswanathan et al. (2017) |
| Altretamine | GPX4 | Induce | DLBCL | OCI-LY3, OCI-LY7, U-2932 cells | GPX4 inactivation; Decreased PC-OOH level; Lipid ROS accumulation | Woo et al. (2015) |
| Ferumoxytol (Feraheme) | Iron | Induce | Leukemia | 19 kinds of leukemia cells; PDXs of leukemia cells | FPN downregulation; Increasing intracellular iron and ROS levels | Trujillo-Alonso et al. (2019) |
| Salinomycin (ironomycin) | Iron | Induce | Breast cancer | Human breast cancer stem cells (CSCs); PDXs | Iron accumulation and sequestration in lysosomes; Degraded ferritin, the iron storage protein; Increasing ROS and TFRC | Mai et al. (2017) |
| Ferric ammonium citrate (FAC) | Iron | Induce | Fibrosarcoma | HT-1080 cells | Iron overloading; Oxidative damage; Activating ALOXs; Increasing ROS production | Fang et al. (2018) |
| Cisplatin | GSH | Induce | NSCLC; Human colon cancer | A549, HCT116 cells | GSH depletion; Increasing ROS level; Inducing both ferroptosis and apoptosis | Guo et al. (2018a), Guo et al. (2018b) |
| Haloperidol | Sigma 1 receptor | Induce | HCC | HepG2, Huh-7 cells | Increasing cellular levels of HO-1 | Bai et al. (2017) |
| Busulfan | NRF2; GPX4 | Induce | — | Oligospermia mice | Downregulating expressions of NRF2, GPX4 and FPN | Zhao et al. (2020) |
| Siramesine and lapatinib | Iron; FPN | Induce | Breast cancer | MDA-MB-231, SKBR3 cells | Iron and ROS accumulation; Upregulating TF; Downregulating FPN and ferritin | Ma et al. (2017) |
| Ciclopirox olamine (CPX) | Iron | Inhibit | Neuro-degenerative diseases | HT1080 cells; OHSCs | Removing excess iron; Rescuing cells from erastin-induced ferroptosis | Dixon et al. (2012) |
| Deferoxamine (DFO) | Iron | Inhibit | Neuro-degenerative diseases; Ageing | HT-1080, Calu-1, BJeLR, PC12, MEF cells; Ageing model mice | Removing excess iron; Rescuing from erastin-induced ferroptosis | Dixon et al. (2012), Chen X. et al. (2020) |
| Dexrazoxane (DXZ) | Iron | Inhibit | Cardiomyopathy | DOX-induced cardiomyopathy model mice; Acute and chronic I/R model mice | Reducing DOX- cardiomyopathy; Maintaining mitochondrial function | Fang et al. (2019) |
| Deferiprone (DFP) | Iron | Inhibit | Neuro-degenerative diseases | Primary hippocampal neurons and hippocampus of developing rats and aged mice after general anaesthesia | Iron depletion; Slowing disease progression and improving motor function; Protecting cells against ferroptosis | Wu et al. (2020) |
| Thiazolidinediones (TZDs) Rosiglitazone pioglitazone troglitazone | ACSL4 | Inhibit | Intestinal and pulmonary I/R | ACSL4-knockout MEFs GPX4-knockout mice; Caco-2 cells; Intestinal and pulmonary I/R model mice; Hypoxia-reoxygenation (HR) model A549 cells | Inhibiting ACSL4 and COX2 expression; Restoring GPX4 expression Inhibiting lipid peroxidation and ferroptosis; Improving tissue death | Doll et al. (2017), Li Y. et al. (2019), Xu et al. (2020) |
| N-acetylcysteine (NAC) | ALOX5 | Inhibit | Hemorrhagic stroke | HT-1080 cells; Primary cortical neuronal cultures; ICH model mice and rats | Increasing GSH; Inhibiting active lipids; Neutralizing toxic lipids | Dixon et al. (2012), Karuppagounder et al. (2018) |
| Zileuton | ALOX5 | Inhibit | Neuro-degenerative diseases; Iron overload related diseases | Molt-4, Jurket, HT22, HT-1080 cells | Decreasing ROS production; Rescuing from glutamate oxidative toxicity; Neuroprotective effect | Liu et al. (2015), Probst et al. (2017), Fang et al. (2018) |
| Vildagliptin; Alogliptin; Linagliptin | DPP4 | Inhibit | — | TP53+/+ and TP53−/− HCT116 cells and mice | Blocking DPP4-mediated lipid peroxidation; Attenuating the anticancer activity of erastin on TP53−/−CRC cells | Xie et al. (2017) |
| Dopamine | Neuro-transmitter | Inhibit | Neuro-degenerative diseases | HT-22, HT-1080, PANC-1, HEY, HEK293, MEF cells | Reducing GSH depletion; Increasing GPX4, iron accumulation and MDA production; Protecting cells against lipid peroxidation | Wang D. et al. (2016), Tang and Tang, (2019) |
| Cycloheximide (CHX) | Protein synthesis | Inhibit | Neuro-degenerative diseases | HT1080, Calu-1, BJeLR, MEF cells | Inhibiting protein synthesis; Preventing erastin-induced ferroptosis | Dixon et al. (2012) |
| Tocotrienols: Vitamin E; α-tocopherols | Lipid peroxidation | Inhibit | Acute lymphocytic chorio-meningitis virus and Leishmania major parasite infections; Hepatic I/R | HT-1080, BJeLR cells; GPX4-deficient T cells and mice; Hepatic I/R model mice | Eliminating peroxygen free radicals; Preventing lipid peroxidation | Yang et al. (2014), Matsushita et al. (2015), Yamada et al. (2020a) |
| Coenzyme Q10 (CoQ10); Idebenone | Mevalonate pathway | Inhibit | — | Four engineered BJ cell lines (BJeLR, DRD, BJeHLT, BJeH); HT-1080 cells | Inhibiting ROS production | Shimada et al. (2016a) |
| α-Lipoic acid | Unknown | Inhibit | Alzheimer’s disease (AD) | P301S Tau transgenic mice | Increasing FPN, xCT and GPX4; Decreasing TFRC, iron and ROS generation | Zhang Y. -H. et al. (2018) |
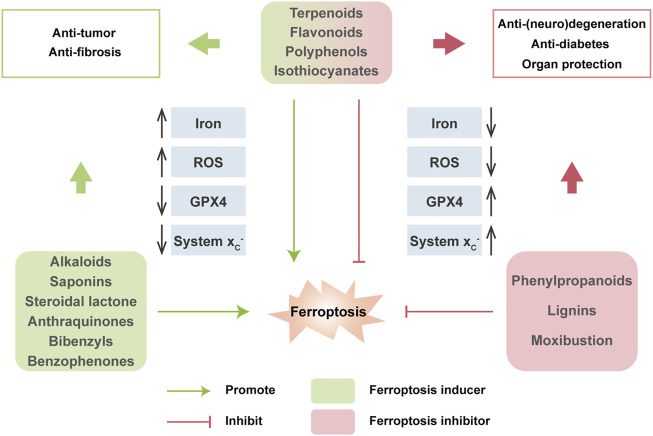
Natural Products and Their Modulation on Ferroptosis
Nowadays, natural medicines are usually used alone or, in most cases, in combination with conventional drugs in clinical in the hope of enhancing the efficacies and/or neutralizing the toxicities of chemical drugs. In addition to clinical (adjuvant) medicines, natural products are widely consumed as supplements in alternative strategies (Shu-Feng Zhou et al., 2007; Kibble et al., 2015). Growing evidence has uncovered the solid regulatory properties of ingredients with bioactive functions on ferroptosis (Figure 4). Hence, we focus on natural compounds that show a pharmacological potential for ferroptosis-associated (adjuvant) therapy.
FIGURE 4.
Multiple effects of natural compounds regulating ferroptosis. Alkaloids, saponins, steroidal lactone, anthraquinones, bibenzyls and benzophenones can induce ferroptosis with anticancer and antifibrosis functions. In contrast, phenylpropanoids and ligins alleviate (neuro)degeneration and prevent irreparable organic damage via antagonizing ferroptosis. Interestingly, terpenoids, flavonoids, polyphenols and isothiocyanates have dual effects on ferroptosis which depends on different disease contexts.
Terpenoids
Terpenoids are important sources for the research of natural products and the development of modern drugs with a great deal of pharmacological functions such as inflammation, neurodegeneration and cancers. Artemisinin and its derivatives (Artemisinins, including artesunate, artemether, dihydroartemisinin, etc.) are sesquiterpene lactone ingredients derived from Artemisia annua L., and are well known globally for their antimalarial bioactivity (Dondorp et al., 2005). Beyond the antimalarial efficacy, artemisinins have been well documented to be potently effective in diverse diseases in vitro and in vivo. Artemisinins have been extensively demonstrated to induce ferroptosis for cancer treatment through various mechanisms, including regulating iron-related genes, promoting ROS production, depleting intracellular GSH, and increasing intracellular iron levels and activating ferritinophagy. Artesunate (ART), a water-soluble derivative of artemisinin, has been approved as a ferroptosis inducer to selectively promote ROS- and lysosomal iron-dependent cell death in KRAS-transformed PDAC cells, which are highly resistant to apoptosis. Of note, ART exerts no effect on nonneoplastic human pancreatic ductal epithelial cells (Eling et al., 2015). Surprisingly, ART induces ferroptosis via the accumulation of iron, leading to elevated lipid peroxides and reduced antioxidant capacity in several other cancers, such as ovarian cancer, head and neck cancer and Burkitt’s lymphoma (Greenshields et al., 2017; Roh et al., 2017; Wang N. et al., 2019). ART has undergone several clinical trials against various cancers, including metastatic breast cancer and cervical intraepithelial neoplasia, and has been proven to be safe and well-tolerated in patients (von Hagens et al., 2017). Dihydroartemisinin (DHA), one of the semisynthetic derivatives of artemisinin, can trigger ferroptosis to directly kill cancer cells or synergistically sensitize the efficacy of chemotherapeutic agents via multiple functions, including autophagic degradation of ferritin, direct binding to free iron and disturbing iron homeostasis in a cohort of cancer cells (Chan et al., 2013; Du et al., 2019; Chen G. -Q. et al., 2020). Interestingly, DHA activates the feedback pathway of ferroptosis in glioma cells. That is, under endoplasmic reticulum stress induced by DHA, the subsequent increase in GPX4 expression counteracts lipid peroxidation-mediated ferroptosis (Chen et al., 2019). In addition to antineoplastic activities, artemisinins also exhibit hepatoprotective effects via the execution of ferroptosis. ART and artemether have been proven to trigger p53-dependent ferroptosis by promoting the accumulation of iron and lipid peroxides, which can inactivate hepatic stellate cells (HSCs) and ameliorate hepatic fibrosis (Wang L. et al., 2019; Kong et al., 2019). Danshen (Salvia miltiorrhiza) is a commonly used traditional Chinese medicinal herb for the clinical therapy of many diseases. However, two diterpenoid compounds, dihydroisotanshinone I (DT) and cryptotanshinone isolated from Danshen show opposite functions in cancer cells. DT treatment significantly induces ferroptosis to suppress breast carcinoma through downregulating GPX4 protein expression in cell and xenograft models (Lin et al., 2019). In contrast, cryptotanshinone definitely blocks erastin-induced ferroptosis in PDAC cells upon antagonizing STAT3, a positive modulator of ferroptosis (Gao et al., 2018). Glycyrrhizin (GLY) and magnesium isoglycyrrhizinate (MgIG) are natural ingredients extracted from the root of Glycyrrhiza with neuroprotective activity. Nevertheless, current studies have demonstrated that GLY and MgIG protect hepatic function through different modulations of ferroptosis in different subtypes of hepatic cells. MgIG remarkably induces HSC ferroptosis through iron accumulation and ROS production, and finally ameliorates CCl4-induced hepatic fibrosis where HO-1 plays an important role (Sui et al., 2018). Yet, GLY, a high mobility group protein B1 (HMGB1) inhibitor, has been shown to reduce the degree of ferroptosis of hepatocytes during acute liver failure (ALF) via activating NRF2/HO-1/HMGB1 pathway to antagonize oxidative stress (Wang Y. et al., 2019).
Flavonoids
Flavonoids are extensively distributed in the plant kingdom, with multifaceted pharmacological properties and diversified molecular structures. Due to powerful free radical scavenging and antioxidant action, many flavonoids have been reported to be potential ferroptosis inhibitors. Baicalein (also termed 5,6,7-trihydroxyflavone) is a flavonoid originally isolated from the whole root of Scutellaria baicalensis, and has been shown to be effective against cancer, bacterial infections, organ dysfunctions and oxidative stress diseases. Before ferroptosis was formally coined, solid evidence has revealed that baicalein acted as a strong iron chelating agent, which inhibited the Fenton reaction and lipid peroxidation by forming an iron-baicalein complex (Perez et al., 2009). It is reasonable to speculate that baicalein plays its pharmacological role through the above ferroptosis-associated mechanisms. Emerging studies have shown that baicalein is a selective inhibitor of ALOX12/15, which in turn strongly downregulates prostaglandin-endoperoxide synthase 2 (PTGS2) and upregulates GPX4, and significantly suppresses ferroptosis to exhibit a neuroprotective role in post-traumatic epileptic seizures (Li Q. et al., 2019). Furthermore, as a natural ferroptosis inhibitor, baicalein limits iron production, GSH depletion, GPX4 degradation and lipid peroxidation to reverse erastin- or RSL3-induced ferroptosis in pancreatic cancer cells (Xie et al., 2016b; Probst et al., 2017). Quercetin (3,30,40,5,7-pentahydroxyflavone) is one of the most widely distributed flavonoids in edible and medicinal plants, such as onion, tea and ginkgo biloba, and displays a variety of biological activities, especially antioxidative capacity. A recent study has demonstrated that type 2 diabetes (T2DM) molding depletes GSH, downregulates GPX4, and induces oxidative stress in pancreatic β cells (PBCs), indicating that ferroptosis contributes to the loss and dysfunction of PBCs. Indeed, quercetin protects PBCs from pancreatic iron deposition to alleviate ferroptosis in T2DM (Li D. et al., 2020). Moreover, both quercetin and its metabolite quercetin Diels-Alder anti-dimer (QDAD) prevent bone marrow-derived mesenchymal stem cells (bmMSCs) from erastin-induced ferroptosis possibly through the antioxidant pathway, which in turn converts quercetin into QDAD, an inferior ferroptosis inhibitor and antioxidant (Li X. et al., 2020). Despite that flavonoids discussed above are usually approved as strong antioxidants, there are some other flavonoids functioning as ferroptosis inducers, including amentoflavone (AF), a polyphenolic flavonoid widely found in Selaginella and typhaneoside (TYP), a major flavonoid in the extract of Pollen Typhae. Both AF and TYP trigger ferroptosis in an autophagy-dependent manner via AMPK signaling, contributing to ferritin degradation, ROS accumulation and ultimately ferroptotic cell death in acute myeloid leukemia (AML) and glioma (Zhu et al., 2019; Chen Y. et al., 2020).
Polyphenols
Phenolic compounds isolated from plants are known as a fighter against various diseases related to oxidative stress, such as cancer, inflammation, obesity, organic damage and neuronal disorders (Curti et al., 2017). Curcumin, a polyphenol extracted from the rhizome of turmeric and other plants of the family Zingiberaceae, is a powerful antioxidant compound with a great deal of pharmacological activities. Notably, curcumin has been demonstrated to enhance antioxidative capacity as a ferroptosis inhibitor. In renal tubular cells, curcumin inhibits myoglobin (Mb)-induced ferroptosis and ameliorates renal injury associated with rhabdomyolysis by inhibiting the TLR4/NF-κB axis and activating the cytoprotective enzyme HO-1 (Guerrero-Hue et al., 2019). Proanthocyanidins (PACs), widely found in plants in nature, are potent free radical scavengers. PAC treatment significantly decreases the levels of iron, ACSL4, and ALOX15B, while increasing the levels of GSH, GPX4, NRF2, and HO-1 in the traumatic spinal cord, eventually rescues ferroptosis-induced injuries and improves the locomotive function of spinal cord injury (SCI) mice (Zhou et al., 2020). Similarly, Epigallocatechin gallate (EGCG), the most abundant catechin in tea, is a polyphenol with various pharmacological benefits. Both curcumin and EGCG have been demonstrated to prevent ferroptosis against iron-induced oxidative damage in mouse MIN6 pancreatic β cells through neutralizing iron, reducing GSH consumption as well as preventing GPX4 inactivation (Kose et al., 2019). Beyond the organ protection effect, the anticancer efficacy of polyphenols has been well established by accumulated clinical experience, where ferroptosis is believed to be involved. EGCG is a direct inhibitor of heat shock 70 kDa protein 5 (HSPA5), which has been proven to negatively regulate ferroptosis. Further research shows that EGCG can prevent the formation of the anti-ferroptotic HSPA5-GPX4 complex and subsequent lipid peroxidation, which enhances gemcitabine sensitivity by disinhibiting ferroptosis in PDAC in vitro and in vivo (Zhu et al., 2017). Interestingly, EGCG either prevents ferroptosis in normal pancreatic β cells, or induces ferroptosis in cancerous PDAC cells, implying context-dependent bidirectional regulation and special pancreatic for pancreas protection of EGCG.
Isothiocyanates
Isothiocyanates (ITCs) are abundant in cruciferous vegetables, exhibiting chemopreventive and chemotherapeutic properties to tackle many types of diseases. Two ITCs, phenylethyl isothiocyanate (PEITC) and sulforaphane have been employed in small human clinical trials against various diseases ranging from cancer to autism, either within cruciferous food or as single agents (Palliyaguru et al., 2018). PEITC is a dietary anticancer compound with pharmacokinetic behaviors of rapid absorption and high bioavailability (Ji et al., 2005). It has been documented that PEITC triggers multiple forms of cell death, namely ferroptosis, apoptosis, and autophagy in both murine and human osteosarcoma models. PEITC drives oxidative stress as a consequence of GSH depletion-induced ROS generation and lipid peroxidation. ROS generation is proven to be the major cause of PEITC-induced cell death, accompanied by altered iron metabolism and activation of the MAPK signaling pathway (Lv H. -H. et al., 2020; Lv H. et al., 2020). However, another ITC, sulforaphane, acting as a ferroptosis inhibitor, reduces busulfan-induced damage to prevent oligospermia by activating the upregulation of GPX4 and FPN1 protein expression (Zhao et al., 2020).
Alkaloids
Alkaloids are one type of important active component in Chinese herbal medicine and display a broad range of biological properties, such as neurogenesis promotion, pro-oxidant activities, and anti-infection. Trigonelline is an alkaloid derived from a traditional Chinese herbal, Trigonella foenum-graecum L., present in coffee and fenugreek seeds in considerable amounts (Sun et al., 2016a). Trigonelline has been believed to be of great benefits in the prevention and treatment of diabetes and central nervous system disorders (Zhou et al., 2012). Recent studies have revealed that trigonelline exerts antitumor efficiency as a pharmacological inhibitor of NRF2. The inhibition of NRF2 by trigonelline enhances the anticancer activity of classical ferroptosis inducers, such as erastin, sorafenib and RSL3, and sensitizes chemoresistant cancer cells to ferroptosis both in vitro and in vivo models of HCC and HNC (Sun et al., 2016a; Shin et al., 2018). Solasonine isolated from Solanum melongena has various pharmacological benefits. Solasonine has been proven to inhibit the growth and migration of HCC cells. Detailed studies elucidate that solasonine increases lipid ROS levels via GPX4-induced destruction of the GSH redox system, leading to the promotion of ferroptosis (Jin et al., 2020).
Saponins
Saponins are the major active ingredients of many Chinese herbal medicines, such as ginseng, licorice, and bupleurum, processing valuable biological activities including antipyretic, anticancer properties, as well as immunoregulation. Ruscogenin, is initially extracted from Ruscus aculeatus and is a main component of Radix Ophiopogon japonicas, with a wide range of biological functions (Song et al., 2020). Acting as a promising ferroptosis inducer, ruscogenin is found to increase ferrous iron and aberrant ROS production and regulate the levels of TF and FPN, which triggers ferroptosis against pancreatic cancer in vitro and in vivo. Ardisiacrispin B, a natural oleanane-type tritepene saponin isolated from the fruit of Ardisia kivuensis Taton (Myrsinaceae), displays a significant cytotoxic effect in a panel of 9 cancer cell lines (including various sensitive and drug-resistant phenotypes) depending on ROS-mediated ferroptosis (Mbaveng et al., 2018a).
Others
Actually, many kinds of natural compounds serve as potential modulators of ferroptosis. Withaferin A (WA), a major constituent of Withania somnifera extract, has been well documented to provide pharmacological benefits. WA can obviously induce ferroptosis to suppress high-risk neuroblastoma via a double-edged mechanism. On the one hand, WA decreases the protein level and activity of GPX4, which resembles the canonical ferroptosis-inducing pathway. On the other hand, WA induces excessive lipid peroxidation by increasing the labile Fe (II) pool upon HO-1 activation through direct targeting of Keap1, which represents for a novel noncanonical ferroptosis pathway (Hassannia et al., 2018). Erianin, a natural product isolated from a famous TCM, Dendrobium chrysotoxum Lindl, has been reported to exert anticancer effects on several cancer types. Erianin has shown to inhibit the growth and migration of lung cancer cells via Ca2+/CaM-mediated ferroptosis, accompanied by ROS accumulation, GSH depletion and lipid peroxidation (Chen P. et al., 2020). Cullen corylifolium is a famous TCM known as “Buguzhi” and listed officially in Chinese pharmcopoeia, containing various phytochemical compounds such as flavonoids and coumarines. The extracts have been approved to be effective for its anti-inflammatory, antioxidation, neuroprotection properties. Compound 3 (psoralidin) isolated from Cullen corylifolium displays tremendous inhibitory affinity for ALOX and Keap1-NRF2 protein-protein interactions, two important ferroptosis-related targets, and shows anti-ferroptotic activities on erastin-treated hippocampal HT22 cells (Yaseen et al., 2021). Nordihydroguaiaretic acid (NDGA), a lignin contained in Larrea tridentata, is a potent antioxidant. As a pan-ALOX inhibitor, NDGA effectively prevented lipid peroxidation, ROS generation and ferroptosis induced by proferroptotic reagents in BT-474, AML12 and acute lymphoblastic leukemia (ALL) cells (Hangauer et al., 2017; Probst et al., 2017; Fang et al., 2018).
Cooperation of Multiple Natural Compounds
In addition to single compound, cooperation of multiple (two or more) natural products or direct extracts of herbals and TCM formulations that are rich in various compounds, actually exhibits pro- or anti-ferroptosis pharmaco-activities. Cotylenin A (CN-A, plant growth regulator) combined with PEITC in the treatment of pancreatic cancer can synergistically induce ROS production and lead to cell death mainly due to the induction of ferroptosis, while CN-A or PEITC alone fails to induce ferroptosis (Kasukabe et al., 2016). Betula etnensis Raf (Birch Etna) belongs to the Betulaceae family, which contains multiple antineoplastic ingredients in bark, including polyphenols, terpenoids, betulin, betulinic acid, and ursolic acid. Methanolic extract of B. etnensis Raf. promotes ROS production and an oxidative cellular microenvironment, which results in the death of colon cancer cells dependent on ferroptosis mediated by HO-1 hyper-expression and lipid peroxidation (Malfa et al., 2019). Brown rice is known as an antioxidant-rich food with a high level of vitamin E. Methanol extracts of brown rice have been reported to be a ferroptosis inhibitor and protect cells against lipid peroxidation via compensating for GPX4 loss, suggesting that brown rice is useful for the pathologies of vascular diseases driven by lipid peroxidation (Sakai et al., 2017). Naotaifang is a compound traditional Chinese herbal medicine consisting of four herbs, namely, Radix Astragali (Huangqi), Rhizoma chuanxiong (Chuangxiong), Pheretima (Dilong) and Bombyx batryticatus (Jiangcan). Its extract (NTE) has clinical benefits for the neurological improvement of patients with acute cerebral ischemia. NTE can ameliorate neuronal ferroptosis in MCAO rats through the TFRC/DMT1 and SCL7A11/GPX4 pathways (Lan et al., 2020).
Clinical Therapeutic Potential of Natural Products Targeting Ferroptosis
Iron homeostasis, lipid peroxidation and GSH metabolism are three mainstays in the modulation of ferroptosis, and are supposed to be promising in ferroptosis-associated diseases. Based on physiopathological characteristics, proferroptotic compounds are potent anticancer candidates, whereas anti-ferroptotic agents are organo-protective therapies. Apart from chemical compounds, natural products, including TCM resources, which are rich in nature, provide abundant bioactive ingredients that modulate ferroptosis. Indeed, preclinical and clinical studies have demonstrated that many natural compounds with ferroptotic activity exhibit definite regulatory effects on the cyst(e)ine/GSH/GPX4 antioxidative axis (Figure 4). Of note, many of them, including artemisinin and its derivatives, β-elemene, SFN, baicalein, kaempferol, quercetin, curcumin and EGCG, have been evaluated in extensive clinical trials for various pharmacological effects (Wang Y. et al., 2017; Luo et al., 2019; Parvez and Rishi, 2019). Except for antimalarial effects, artemisinin and its derivatives seem to be good proferroptotic candidates and have been evidenced for their immune surveillance to inhibit carcinogenesis, tumor progression and metastasis (Kiani et al., 2020). For cancer therapy, a large body of studies have indicated that artemisinin and its derivatives trigger ferroptosis through overproducing ROS and regulating the xc -/GPX4 system axis in various cancer cells, which has undergone several clinical trials and is proven to be safe and well-tolerated in patients with metastatic breast cancer or advanced-stage solid tumors (von Hagens et al., 2017). Additionally, β-elemene (a sesquiterpene) injection has been approved recently as adjuvant therapy by China’s State Food and Drug Administration (SFDA) for lung cancer (Wang et al., 2012). Mechanistically, combinative treatment with β-elemene and cetuximab induces ferroptosis and suppresses CRC migration by regulating EMT through both GPX4-dependent and-independent pathways (Chen P. et al., 2020). Two ITCs, PEITC and sulforaphane protect cells from ferroptosis via limiting iron accumulation and preventing GPX4 degradation and have been investigated in clinical trials against various diseases ranging from cancer to autism, either alone or in combination with other drugs (Palliyaguru et al., 2018). In addition to proferroptotic cancer treatment, bioactive ferroptosis inhibitors, which are usually antioxidants that restore GSH/GPX4 levels, have also been clinically investigated to treat other diseases. EGCG, a famous antioxidant has been evaluated in numerous clinical trials for various disorders for decades, such as metabolic syndromes, neurodegenerative diseases and cardiovascular diseases. EGCG has been proven to protect pancreatic β cells against iron toxicity and erastin-induced ferroptosis through neutralizing iron and preventing GPX4 inactivation (Kose et al., 2019). Moreover, EGCG can also act as an iron chelator to regulate the TFRC/FTH expression and to prevent GPX4 activation, resulting in the inhibition of ferroptosis (Md Nesran et al., 2020). In addition to its antioxidative properties, EGCG is evidenced by clinical experience as an anticancer agent that modulates GPX4 levels to promote ferroptosis (Zhu et al., 2017). Owing to the context-dependent regulation of ferroptosis based on GPX4, EGCG has been recognized as a promising effective ferroptosis-modulating compound for clinical application or herbal supplementation.
Many natural compounds that are already in clinical use or have a strong potential for clinical translation are known to promote or block ferroptosis. Compared with classical ferroptosis regulators, TCM has the characteristics of many regulatory targets, stable structure, high safety, low cost and easy availability. However, it remains to be determined whether their biological activities rely on ferroptosis-regulating properties. The lack of specific biomarkers and molecular mechanisms for ferroptosis blocks the clinical transformation of novel ferroptosis-associated treatments mediated by natural product candidates. Due to starting late and accumulating less, more studies should be conducted to avoid side effects and to develop individualized and precise therapy.
Apart from the above discussed representative compounds, more natural products that have been reported to be able to induce/inhibit ferroptosis are classified and summarized in Tables 3, 4.
TABLE 3.
Natural products induced ferroptosis.
| Classification | Compounds | Functional targets | Diseases | Test models | Mechanisms/Effects | Refs |
|---|---|---|---|---|---|---|
| Terpenoids | Artemisinin | Iron | Osteosarcoma | D-17 cells | Decreasing iron levels | Isani et al. (2019) |
| Artesunate | NRF2; p62; FTH1; Activating transcript-tion factor 4 (ATF4) | Head and neck cancer (HNC); Pancreatic cancer; Ovarian cancer; Burkitt’s lymphoma; Liver fibrosis | Several HNC/PDAC/ovarian cancer cells and xenograft mice; DAUDI and CA-46 cells and xenograft mice; Mouse HSCs; LX2 cells | Decreasing GSH level; Increasing iron and lipid ROS levels; Increasing GRP78 levels; Inducing ATF4-CHOP-CHAC1 pathway | Eling et al. (2015), Greenshields et al. (2017), Roh et al. (2017), Wang N. et al. (2019), Wang K. et al. (2019), Kong et al. (2019) | |
| Dihydroartemisinin (DHA) | FTH1; GPX4 | Several types of cancers, including glioma, head and neck carcinoma; Acute myeloid leukemia | Dozens of multiple cancer cells; Human umbilical vein endothelial cells (HUVECs), MEFs; U251, U373, HL60, H292 xenograft mice; Patient-derived glioma cells | Inducing lysosomal degradation of ferritin; Inducing iron and ROS accumulation; Inhibiting GPX4 expression; Activating the feedback path of ferroptosis | Chan et al. (2013), Lin et al. (2016), Chen et al. (2019), Du et al. (2019), Chen G. -Q. et al. (2020) | |
| Artemether | Iron | Liver fibrosis | HSC-T6 cells; CCl4-induced hepatic fibrosis model mice | Promoting accumulation of iron and lipid peroxides; Inducing p53-dependent ferroptosis of HSC; Ameliorating CCl4-induced hepatic fibrosis | Wang L. et al. (2019) | |
| Dihydro-isotanshinone I (DT) | GPX4 | Breast cancer | MDA-MB-231 cells; MCF-7 cells and xenograft mice; Patients | Reducing GSH/GSSG ratio and GPX4 activity; Inducing apoptosis and ferroptosis | Lin et al. (2019) | |
| Magnesium isoglycyrrhizinate (MgIG) | HO-1 | Liver fibrosis | CCl4-induced liver fibrosis model rats; HSC-T6 cells | Increasing HO-1, TF, TFRC expression and nuclear abundance; Reducing GSH level and FPN expression; Increasing levels of ROS, iron and lipid peroxides | Sui et al. (2018) | |
| Ferroptocide, a compound from pleuromutilin | Thioredoxin | Many cancer types | A dozen of cancer cell lines and primary cancer cells | Inhibiting thioredoxin; Inducing lipid ROS accumulation | Llabani et al. (2019) | |
| β-elemene | Unknown | Colorectal cancer | HCT116, LOVO, Caco-2 cells; Orthotopic HCT116 mice | Inducing iron-mediated ROS accumulation, GSH depletion, lipid peroxidation; Upregulating HO-1 and TF and downregulating GPX4, FTH1, GLS, SLC7A11, SLC40A1 | Chen et al. (2020e) | |
| Pseudolaric acid B (PAB) | p53; TFRC; NOX4; xCT | Glioblastoma | SHG-44, U87, U251 cells; C6 cells and xenograft mice | Upregulating TFRC and NOX4; Increasing ferrous and lipid peroxidation; Inducing GSH exhaustion by xCT inhibition | Wang Z. et al. (2018) | |
| Flavonoids | Amentoflavone | FTH1; AMPK/mTOR signaling | Glioblastoma | Normal human astrocytes; U373 cells; U251 cells and xenograft mice | Increasing intracellular levels of iron, MDA and lipid ROS; Reducing levels of GSH, FTH1 and the mitochondrial membrane potential | Chen et al. (2020c) |
| Typhaneoside (TYP) | AMPK/mTOR signaling | Acute myeloid leukemia | Kas-1, NB4, K562, 293T cells HL60 cells and xenograft mice | Triggering autophagy by activating AMPK signaling; Inducing ferritin degradation, ROS accumulation and ferroptosis | Zhu et al. (2019) | |
| Polyphenols | Epigallocatechin gallate (EGCG) | HSPA5 | Pancreatic cancer | PANC1, CFPAC1 cells | Inhibiting GPX4 activity; Enhancing erastin-induced MDA production and ferroptosis | Zhu et al. (2017) |
| Gallic acid | GPX4 | Cervical cancer; Lung cancer Neuroblastoma; Breast cancer; Melanoma | HeLa, H446, SH-SY5Y, MDA-MB-231, MCF10A, A375 cells; Human dermal fibroblasts (HDF) | Decreasing GPX4 activity; Promoting ROS generation and lipid peroxidation | Tang and Cheung, (2019), Khorsandi et al. (2020) | |
| Iso-thiocyanates | β-Phenethyl isothiocyanate (PEITC) | MAPK signaling pathway | Osteosarcoma | K7M2, U-2 OS, MG-63, 143B cells; MNNG/HOS cells and xenograft mice; Orthotopic osteosarcoma mice | Triggering ROS accumulation; Inducing GSH depletion | Lv H. -H. et al. (2020), Lv H. et al. (2020) |
| Alkaloids | Trigonelline | NRF2 | Hepatocellular carcinoma; (HCC); HNC | HepG2, SNU-182, Hep3B cells; Hepa1-6 cells and xenograft mice; Several HNC cells; Cisplatin-resistant HNC xenograft mice | Blocking NRF2; Inducing GSH depletion and ROS production; Increasing iron level | Sun et al. (2016a), Shin et al. (2018) |
| Piperlongumine (PL) | GSH; GSTP1; Thioredoxin reductase (TrxR) | Pancreatic cancer | MIAPaCa-2, PANC-1, CFPAC-1, BxPC-3 cells | Increasing ROS level; Decreasing GSH level | Yamaguchi et al. (2018) | |
| Ungeremine | Unknown | Breast cancer; Leukemia; Glioblastoma; Colon cancer; Liver cancer | Several cell models including sensitive and resistant counterparts | Increasing ROS production; Inducing apoptosis, necrosis and ferroptosis | Mbaveng et al. (2019) | |
| Solasonine | GPX4 | HCC | HepRG cells; HepG2 cells and xenograft mice | Inhibiting GPX4 and GSS expressions; Increasing lipid ROS | Jin et al. (2020) | |
| Saponins | Ruscogenin | TF; FPN | Pancreatic cancer | SW 1990, PANC-1, AsPC-1, HPDE6-C7 cells; BxPC-3 cells and xenograft mice | Increasing ferrous irons and ROS production | Song et al. (2020) |
| Ardisiacrispin B | Iron | Leukemia; Breast cancer; Colon cancer; Glioblastoma | Several cell models including sensitive and their resistant counterparts | Increasing ROS production; Inducing ferroptosis and apoptosis | Mbaveng et al. (2018a) | |
| Albiziabioside A derivative, Compounds D13 | p53 | Colon cancer | HCT116 cells and xenograft mice | Activating p53; Reducing mitochondrial membrane potential and GPX4 expression; Inducing ROS production and lipid peroxidation | Wei et al. (2018) | |
| N-acetylglycoside of oleanolic acid (aridanin) | Unknown | Many kinds of cancer | 18 cancer cell lines with sensitive and drug-resistant phenotypes; Metastasizing B16/F10, HepG2, AML12 cells | Increasing ROS levels and mitochondrial membrane potential (MMP) breakdown | Mbaveng et al. (2020) | |
| Steroidal lactone | Withaferin A | GPX4; NRF2 | Neuroblastoma | A dozen of neuroblastoma cells; IMR-32 cells and xenograft mice | Inducing lipid peroxidation; Reducing GPX4 activity; Activating HO-1 | Hassannia et al. (2018) |
| Anthra-quinones | Physcion 8-O-β-glucopyranoside | GLS2 | Gastric cancer | MKN-45 cells; MGC-803 cells and xenograft mice | Increasing levels of ROS, Fe2+ and MDA; Inducing ferroptosis via miR-103a-3p/GLS2 axis | Niu et al. (2019) |
| Bibenzyls | Erianin | Ca2+/CaM signaling | Lung caner | H1299 cells; H460 cells and orthotopic mice | Inducing ROS generation, lipid peroxidation and GSH exhaustion | Chen et al. (2020d) |
| Benzo- phenones | Epunctanone | Unknown | Many kinds of cancer | 9 cancer cell lines including sensitive and drug-resistant cell lines | Increasing ROS levels and MMP breakdown | Mbaveng et al. (2018b) |
| Multiple | Cotylenin A+ PEITC | Unknown | Pancreatic cancer | (resistant)PANC-1, MIAPaCa-2 cells | Inducing ROS production | Kasukabe et al. (2016) |
| Methanolic extract of Betula etnensis Raf. bark | HO-1 | Colon cancer | Caco-2 cells | Increasing ROS and lipid peroxidation; Reducing HO-1 activity | Malfa et al. (2019) | |
| Actinidia chinensis Planch (ACP) | EMT; GPX4; xCT | Gastric cancer | HGC-27 cells and zebrafish xenografts | Inhibiting GPX4 and xCT proteins; Inducing ROS accumulation | Gao et al. (2020) | |
| Bromelain | ACSL4 | Colorectal cancer | Caco-2, NCI-H508, HCT116, DLD1, G13D, G12D cells; DSS-treated KRAS mutant mice | Increasing ACSL4 level, lipid biosynthesis and fatty acid degradation | Park et al. (2018) |
TABLE 4.
Natural products inhibited ferroptosis.
| Classification | Compounds | Functional targets | Diseases | Test models | Mechanisms/Effects | Refs |
|---|---|---|---|---|---|---|
| Terpenoids | Cryptotanshinone | STAT3 | Pancreatic cancer | PANC-1, CFPAC1 cells | Inhibiting STAT3; Blocking erastin-induced ferroptosis | Gao et al. (2018) |
| Glycyrrhizin (GLY) | HMGB1 | Acute liver failure (ALF) | L02 hepatocytes; ALF mice | Reducing levels of Fe2+, MDA, ROS and lactic dehydrogenase (LDH); Increasing NRF2, HO-1 GPX4 and GSH levels; Hepatoprotective effect | Wang Y. et al. (2019) | |
| Bakuchiol and 3-hydroxybakuchiol, isolated from Cullen corylifolium | Unknow | Ferroptosis-related diseases | HT22 cells | Inhibiting erastin-induced ferroptosis | Yaseen et al. (2021) | |
| Flavonoids | Baicalein | ALOX12; ALOX15; PTGS2; GPX4 | Post-traumatic epilepsy (PTE); Ferroptosis-induced diseases | HT22, PANC-1, BxPc3, Molt-4, Jurkat cells; FeCl3-induced PTE mice | Decreasing ROS, 4-HNE, PTGS2 and ALOX12/15; Inhibiting GSH depletion and increasing GPX4 expression | (Xie et al., 2016b; Probst et al., 2017; Li Q. et al., 2019) |
| Quercetin; quercetin Diels-Alder anti-dimer (QDAD) | Antioxidant pathway | T2DM; Degenerative diseases | Bone marrow-derived mesenchymal stem cells (BMSCs); T2DM mice | Lowering iron level; Upregulating GSH, GPX4 and antioxidant pathway | (Li D. et al., 2020; Li X. et al., 2020) | |
| Puerarin | NOX4; GPX4; FTH1 | Heart failure | H9c2 cells; Heart failure model rats | Inhibiting NOX4 expression; Increasing GPX4 and FTH1 expression | Liu et al. (2018) | |
| Morachalcone D, Morachalcone E | GPX4; NRF2; SLC7A11 | Neuro-degenerative diseases | HT22 cells | Preventing ROS production, GSH depletion and iron accumulation; Increasing SLC7A11, GPX4, NRF2 and HO-1 levels; Neuroprotective effect | Wen et al. (2020) | |
| Butein; (s) -Butin | Antioxidant pathway | Degenerative diseases | BMSCs | Making cells resistant to ferroptosis | Liu J. et al. (2020) | |
| Sterubin | Unknown | Neuro-degenerative diseases | HT22, BV2, MC65 cells | Activating NRF2/ATF4 Signaling; Protecting against oxytosis/ferroptosis by maintaining GSH levels | Fischer et al. (2019), Maher et al. (2020) | |
| 7-O-Esters of taxifolin: 7-O-cinnamoyl-taxifolin; 7-O-feruloyl-taxifolin | GSH; NRF2 | Alzheime’s disease (AD) | BV-2, HT22 cells; AD model mice | Scavenging free radical; Maintaining GSH under stress conditions; Increasing NRF2 level; Neuroprotective effect | Gunesch et al. (2020) | |
| 6 flavonoids isolated from Cullen corylifolium | Unknow | Ferroptosis-related diseases | HT22 cells | Inhibiting erastin-induced ferroptosis | Yaseen et al. (2021) | |
| Kaempferol, Kaempferide | ARE; ROS | Neuro-degenerative diseases | HT22 cells | Inducing ARE activity; Suppressing intracellular ROS and mitochondrial superoxide anion production | Takashima et al. (2019) | |
| Polyphenols | Proanthocyanidin (PACs) | Unknown | Spinal cord injury (SCI) | SCI mice | Reducing iron, ACSL4 and ALOXs levels and oxidative stress; Increasing GSH, GPX4, NRF2 and HO-1 levels | Zhou et al. (2020) |
| Curcumin | Iron; GPX4 | Acute kidney injury; Pancreatic damage | HK-2, MIN6 cells; Proximal murine tubular epithelial cells (MCTs) Rhabdomyolysis mice | Activating HO-1; Reducing inflammation and oxidative stress; Inhibiting TLR4/NF-κB axis; Decreasing iron level, GPX4 inactivation, GSH depletion, and lipid peroxidation | Guerrero-Hue et al. (2019), Kose et al. (2019) | |
| Epigallocatechin gallate (EGCG) | Iron; GPX4 | Pancreatic damage | Mouse MIN6 pancreatic β cells | Decreasing iron level, GPX4 inactivation, GSH depletion, and lipid peroxidation | Kose et al. (2019) | |
| Iso-thiocyanates | Sulforaphane | NRF2 | Oligospermia | Oligospermia mice | NRF2 agonist; Up-regulating GPX4 and FPN protein expression | Zhao et al. (2020) |
| Phenyl-propanoids | Psoralidin | ALOX5; Keap1-NRF2 pathway | Ferroptosis-related diseases | HT22 cells | Inhibiting ALOX5 and Keap1-NRF2 protein-protein interactions; Neuroprotective effect | Yaseen et al. (2021) |
| Artepillin C | ROS | Neuro-degenerative diseases | HT22 cells | Reducing intracellular ROS and mitochondrial superoxide anion production | Takashima et al. (2019) | |
| Lignins | Nordi-hydroguaiaretic acid (NDGA) | ALOX5 | Iron overload related diseases | BT474, HT-1080, Molt-4, Jurkat, AML12 cells | Reversing ferroptosis caused by GPX4 inhibition; Protecting cells from iron overload, lipid peroxidation, ROS generation | Hangauer et al. (2017), Probst et al. (2017), Fang et al. (2018) |
| Multiple | Brown Rice extract | GPX4 | Vascular endothelial disorders | HUVECs | Compensating GPX4 loss and preventing LDH release; Decreasing lipid peroxides | Sakai et al. (2017) |
| Naotaifang Extract | SLC11A2 (DMT1); Iron; GPX4 | Acute brain injury | MCAO rats | Decreasing TFRC and SLC11A2 expressions; Reducing ROS, MDA and iron accumulations; Increasing SLC7A11, GPX4 expressions and GSH level; Neuroprotective effect | Lan et al. (2020) | |
| Moxibustion | GPX4; FTH1 | Parkinson’s disease (PD) | PD model rats | Upregulating GPX4 and FTH1 levels; Neuroprotective effect | Lu et al. (2019) |
Conclusion and Perspectives
Nowadays, numerous studies have shown an ever-growing enthusiasm in exploring the role of ferroptosis in the occurrence and progression of various human diseases. A better understanding of the regulatory mechanisms underlying ferroptosis occurrence and susceptibility will facilitate the identification of better targets for the treatment of diversified diseases and the optimization of clinical prevention, diagnostics, prognostics and treatment. Therefore, the activation of ferroptosis-related sites can induce not only the death of ordinary tumor cells, but also the death of drug-resistant tumor cells. In other diseases, inhibiting the occurrence of ferroptosis can effectively alleviate the disease, offering a novel concept for future drug treatment and development. Despite the rapid growth of ferroptosis research, there are some insurmountable and inevitable challenges that remain to be resolved. First, many puzzles lie in ferroptosis-associated complicated mechanisms and signaling pathways that need to be further explored, although they indeed provide some constructive and meaningful answers for a multitude of questions regarding the survival and death of cells. It should be noted that ferroptosis also leads to unexpected toxicity and injury, raising awareness that such ferroptosis-modulating therapies need careful consideration and conduction. Additionally, robust and unique biomarkers of ferroptosis, analogous to active caspase-3 for apoptosis, have not yet been discovered and identified until now to guide the appropriate therapy for human pathologies. Instead, evaluating and validating a whole cascade of biochemical and genetic/proteic changes to facilitate the detection and tracking ferroptosis will be necessary to definitively distinguish between distinct forms of RCD.
Although some progress has been made, inconsistent findings are frequently observed in cells, organoids, animals, and even patients (Conrad and Pratt, 2019). As mentioned above, most clinical trials on administering iron chelators and antioxidants have showed only moderate treatment effect, compared to corresponding preclinical studies (Grolez et al., 2015). DFP seems to somewhat slow disease progression and improve quality of life, but they have not reach significance (Martin-Bastida et al., 2017; Klopstock et al., 2019). Unlike mice with a deadly global GPX4-deficiency, SLC7A11-deficient mice have a normal lifespan and do not display any major phenotype develop (Sato et al., 2005; Conrad and Pratt, 2019). However, almost all cells isolated from these mice die rapidly on account of cysteine starvation, GSH depletion, lipid peroxidation and ferroptotic effects that be induced by erastin or other system xc --inhibitors. Actully, cysteine is mainly present in the culture medium in its oxidized dimeric form, cystine which can be transported into cells only by system xc -. Whereas, cysteine is still available in its reduced monomeric form in plasma and other extracellular body fluids in a whole organism, which can be uptaken by cells via neutral amino acid transporters instead of system xc - (Conrad and Pratt, 2019). Collectively, the relevance of results from various models require for careful evaluation, owing to the physiological, histological and enzymological differences on specific conditions.
As listed and discussed, some synthetic drugs and natural products have suggested to overcome and treat diseases by regulating ferroptosis or mixed death mainly in preclinical models. However, a general lack of animal experiments and clinical trials limit their further development as future targeted drugs with ferroptotic properties. Particularly, we have shed light on the potential of natural compounds with multiple targets, multiple mechanisms and relatively safe profiles as ferroptosis inducers or inhibitors for (adjuvant) therapy of diseases from the perspective of integration. Although there is growing evidence that natural products are ideal templates for both single ingredient and multiple-component modern drugs, they are faced with many dilemmas and challenges, including productivity problems, poor pharmacokinetics, and unclear pharmacology. Further studies should be conducted to elaborate the pharmacokinetic properties both in vitro and in vivo to optimize the best functional ingredients and dosage for clinical therapy.
Author Contributions
YZ, DF, and JA designed the framework and content of the review. CG, SZ and HM wrote the original review draft. SZ, ZT, XH, CX and JZ revised the article with feedback from all the co-authors. All authors approved this work for publication.
Funding
This work was supported by the National Natural Science Foundation of China (No.81803624, 81803622), China Postdoctoral Science Foundation (No. 2018M640498) and the Jiangsu postdoctoral grant program (No. 2018Z092).
Conflict of Interest
DF was employed by Nanjing Southern Pharmaceutical Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.774957/full#supplementary-material
References
- Alvarez S. W., Sviderskiy V. O., Terzi E. M., Papagiannakopoulos T., Moreira A. L., Adams S., et al. (2017). NFS1 Undergoes Positive Selection in Lung Tumours and Protects Cells from Ferroptosis. Nature 551, 639–643. 10.1038/nature24637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amable L. (2016). Cisplatin Resistance and Opportunities for Precision Medicine. Pharmacol. Res. 106, 27–36. 10.1016/j.phrs.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Aydinok Y., Kattamis A., Cappellini M. D., El-Beshlawy A., Origa R., Elalfy M., et al. (2015). Effects of Deferasirox-Deferoxamine on Myocardial and Liver Iron in Patients with Severe Transfusional Iron Overload. Blood 125, 3868–3877. 10.1182/blood-2014-07-586677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S., Faux N. G., Faux N. G., Bush A. I. (2015). Ferritin Levels in the Cerebrospinal Fluid Predict Alzheimer's Disease Outcomes and Are Regulated by APOE. Nat. Commun. 6, 6760. 10.1038/ncomms7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y., Higa J. K., Shimada B. K., Horiuchi K. M., Suhara T., Kobayashi M., et al. (2018). Protective Effects of the Mechanistic Target of Rapamycin against Excess Iron and Ferroptosis in Cardiomyocytes. Am. J. Physiology-Heart Circulatory Physiol. 314, H659–H668. 10.1152/ajpheart.00452.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri B., Shokrzadeh M., Mokhberi V., Azizi S., Khalilian A., Akbari N., et al. (2013). Association between Serum Iron and the Severity of Coronary Artery Disease. Int. Cardiovasc. Res. J. 7, 95–98. [PMC free article] [PubMed] [Google Scholar]
- Bai T., Lei P., Zhou H., Liang R., Zhu R., Wang W., et al. (2019). Sigma‐1 Receptor Protects against Ferroptosis in Hepatocellular Carcinoma Cells. J. Cel Mol Med 23, 7349–7359. 10.1111/jcmm.14594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T., Wang S., Zhao Y., Zhu R., Wang W., Sun Y. (2017). Haloperidol, a Sigma Receptor 1 Antagonist, Promotes Ferroptosis in Hepatocellular Carcinoma Cells. Biochem. biophysical Res. Commun. 491, 919–925. 10.1016/j.bbrc.2017.07.136 [DOI] [PubMed] [Google Scholar]
- Basit F., van Oppen L. M., Schöckel L., Bossenbroek H. M., van Emst-de Vries S. E., Hermeling J. C., et al. (2017). Mitochondrial Complex I Inhibition Triggers a Mitophagy-dependent ROS Increase Leading to Necroptosis and Ferroptosis in Melanoma Cells. Cell Death Dis 8, e2716. 10.1038/cddis.2017.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaidi A. A., Bush A. I. (2016). Iron Neurochemistry in Alzheimer's Disease and Parkinson's Disease: Targets for Therapeutics. J. Neurochem. 139 (Suppl. 1), 179–197. 10.1111/jnc.13425 [DOI] [PubMed] [Google Scholar]
- Belavgeni A., Bornstein S. R., von Mässenhausen A., Tonnus W., Stumpf J., Meyer C., et al. (2019). Exquisite Sensitivity of Adrenocortical Carcinomas to Induction of Ferroptosis. Proc. Natl. Acad. Sci. USA 116, 22269–22274. 10.1073/pnas.1912700116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K., Hendricks J. M., Li Z., Magtanong L., Ford B., Tang P. H., et al. (2019). The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 575, 688–692. 10.1038/s41586-019-1705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock F. J., Tait S. W. G. (2019). Mitochondria as Multifaceted Regulators of Cell Death. Nat. Rev. Mol. Cel Biol. 21, 85–100. 10.1038/s41580-019-0173-8 [DOI] [PubMed] [Google Scholar]
- Bueno D. C., Canto R. F. S., de Souza V., Andreguetti R. R., Barbosa F. A. R., Naime A. A., et al. (2020). New Probucol Analogues Inhibit Ferroptosis, Improve Mitochondrial Parameters, and Induce Glutathione Peroxidase in HT22 Cells. Mol. Neurobiol. 57, 3273–3290. 10.1007/s12035-020-01956-9 [DOI] [PubMed] [Google Scholar]
- Canli Ö., Alankuş Y. B., Grootjans S., Vegi N., Hültner L., Hoppe P. S., et al. (2016). Glutathione Peroxidase 4 Prevents Necroptosis in Mouse Erythroid Precursors. Blood 127, 139–148. 10.1182/blood-2015-06-654194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M., Yang H., Pass H. I., Krausz T., Testa J. R., Gaudino G. (2013). BAP1 and Cancer. Nat. Rev. Cancer 13, 153–159. 10.1038/nrc3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. W., Singh N. P., Lai H. C. (2013). Cytotoxicity of Dihydroartemisinin toward Molt-4 Cells Attenuated by N-Tert-Butyl-Alpha-Phenylnitrone and Deferoxamine. Anticancer Res. 33, 4389–4393. [PubMed] [Google Scholar]
- Chen D., Tavana O., Chu B., Erber L., Chen Y., Baer R., et al. (2017). NRF2 Is a Major Target of ARF in P53-independent Tumor Suppression. Mol. Cel. 68, 224–232. e4. 10.1016/j.molcel.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Li D., Sun H. Y., Wang W. W., Wu H., Kong W., et al. (2020). Relieving Ferroptosis May Partially Reverse Neurodegeneration of the Auditory Cortex. Febs J. 287, 4747–4766. 10.1111/febs.15266 [DOI] [PubMed] [Google Scholar]
- Chen G.-Q., Benthani F. A., Wu J., Liang D., Bian Z.-X., Jiang X. (2020). Artemisinin Compounds Sensitize Cancer Cells to Ferroptosis by Regulating Iron Homeostasis. Cell Death Differ 27, 242–254. 10.1038/s41418-019-0352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li N., Wang H., Wang N., Peng H., Wang J., et al. (2020). Amentoflavone Suppresses Cell Proliferation and Induces Cell Death through Triggering Autophagy-dependent Ferroptosis in Human Glioma. Life Sci. 247, 117425. 10.1016/j.lfs.2020.117425 [DOI] [PubMed] [Google Scholar]
- Chen P., Wu Q., Feng J., Yan L., Sun Y., Liu S., et al. (2020). Erianin, a Novel Dibenzyl Compound in Dendrobium Extract, Inhibits Lung Cancer Cell Growth and Migration via Calcium/calmodulin-dependent Ferroptosis. Sig Transduct Target. Ther. 5, 51. 10.1038/s41392-020-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Li X., Zhang R., Liu S., Xiang Y., Zhang M., et al. (2020). Combinative Treatment of β-elemene and Cetuximab Is Sensitive to KRAS Mutant Colorectal Cancer Cells by Inducing Ferroptosis and Inhibiting Epithelial-Mesenchymal Transformation. Theranostics 10, 5107–5119. 10.7150/thno.44705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Marks E., Lai B., Zhang Z., Duce J. A., Lam L. Q., et al. (2013). Iron Accumulates in Huntington's Disease Neurons: protection by Deferoxamine. PLoS One 8, e77023. 10.1371/journal.pone.0077023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li X., Liu L., Yu B., Xue Y., Liu Y. (2015). Erastin Sensitizes Glioblastoma Cells to Temozolomide by Restraining xCT and Cystathionine-γ-Lyase Function. Oncol. Rep. 33, 1465–1474. 10.3892/or.2015.3712 [DOI] [PubMed] [Google Scholar]
- Chen W.-C., Wang C.-Y., Hung Y.-H., Weng T.-Y., Yen M.-C., Lai M.-D. (2016). Systematic Analysis of Gene Expression Alterations and Clinical Outcomes for Long-Chain Acyl-Coenzyme A Synthetase Family in Cancer. PLoS One 11, e0155660. 10.1371/journal.pone.0155660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Mi Y., Zhang X., Ma Q., Song Y., Zhang L., et al. (2019). Dihydroartemisinin-induced Unfolded Protein Response Feedback Attenuates Ferroptosis via PERK/ATF4/HSPA5 Pathway in Glioma Cells. J. Exp. Clin. Cancer Res. 38, 402. 10.1186/s13046-019-1413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B., Kon N., Chen D., Li T., Liu T., Jiang L., et al. (2019). ALOX12 Is Required for P53-Mediated Tumour Suppression through a Distinct Ferroptosis Pathway. Nat. Cel Biol 21, 579–591. 10.1038/s41556-019-0305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colakoglu M., Tuncer S., Banerjee S. (2018). Emerging Cellular Functions of the Lipid Metabolizing Enzyme 15-Lipoxygenase-1. Cel Prolif. 51, e12472. 10.1111/cpr.12472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M., Pratt D. A. (2019). The Chemical Basis of Ferroptosis. Nat. Chem. Biol. 15, 1137–1147. 10.1038/s41589-019-0408-1 [DOI] [PubMed] [Google Scholar]
- Cotticelli M. G., Xia S., Lin D., Lee T., Terrab L., Wipf P., et al. (2019). Ferroptosis as a Novel Therapeutic Target for Friedreich's Ataxia. J. Pharmacol. Exp. Ther. 369, 47–54. 10.1124/jpet.118.252759 [DOI] [PubMed] [Google Scholar]
- Crielaard B. J., Lammers T., Rivella S. (2017). Targeting Iron Metabolism in Drug Discovery and Delivery. Nat. Rev. Drug Discov. 16, 400–423. 10.1038/nrd.2016.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Zhong Z., Yang Y., Wang B., Sun Y., Sun Q., et al. (2016). Ferrous Iron Induces Nrf2 Expression in Mouse Brain Astrocytes to Prevent Neurotoxicity. J. Biochem. Mol. Toxicol. 30, 396–403. 10.1002/jbt.21803 [DOI] [PubMed] [Google Scholar]
- Curti V., Di Lorenzo A., Dacrema M., Xiao J., Nabavi S. M., Daglia M. (2017). In Vitro polyphenol Effects on Apoptosis: An Update of Literature Data. Semin. Cancer Biol. 46, 119–131. 10.1016/j.semcancer.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Daher B., Parks S. K., Durivault J., Cormerais Y., Baidarjad H., Tambutte E., et al. (2019). Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res. 79, 3877–3890. 10.1158/0008-5472.can-18-3855 [DOI] [PubMed] [Google Scholar]
- Devos D., Moreau C., Devedjian J. C., Kluza J., Petrault M., Laloux C., et al. (2014). Targeting Chelatable Iron as a Therapeutic Modality in Parkinson's Disease. Antioxid. Redox Signaling 21, 195–210. 10.1089/ars.2013.5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C.-K. C., Rose J., Sun T., Wu J., Chen P.-H., Lin C.-C., et al. (2020). MESH1 Is a Cytosolic NADPH Phosphatase that Regulates Ferroptosis. Nat. Metab. 2, 270–277. 10.1038/s42255-020-0181-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Patel D. N., Welsch M., Skouta R., Lee E. D., Hayano M., et al. (2014). Pharmacological Inhibition of Cystine-Glutamate Exchange Induces Endoplasmic Reticulum Stress and Ferroptosis. Elife 3, e02523. 10.7554/eLife.02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., et al. (2012). Ferroptosis: an Iron-dependent Form of Nonapoptotic Cell Death. Cell 149, 1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Stockwell B. R. (2014). The Role of Iron and Reactive Oxygen Species in Cell Death. Nat. Chem. Biol. 10, 9–17. 10.1038/nchembio.1416 [DOI] [PubMed] [Google Scholar]
- Do Van B., Gouel F., Jonneaux A., Timmerman K., Gelé P., Pétrault M., et al. (2016). Ferroptosis, a Newly Characterized Form of Cell Death in Parkinson's Disease that Is Regulated by PKC. Neurobiol. Dis. 94, 169–178. 10.1016/j.nbd.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Doll S., Freitas F. P., Shah R., Aldrovandi M., da Silva M. C., Ingold I., et al. (2019). FSP1 Is a Glutathione-independent Ferroptosis Suppressor. Nature 575, 693–698. 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- Doll S., Conrad M. (2017). Iron and Ferroptosis: A Still Ill‐defined Liaison. IUBMB Life 69, 423–434. 10.1002/iub.1616 [DOI] [PubMed] [Google Scholar]
- Doll S., Proneth B., Tyurina Y. Y., Panzilius E., Kobayashi S., Ingold I., et al. (2017). ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 13, 91–98. 10.1038/nchembio.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma S., Lessnick S. L., Hahn W. C., Stockwell B. R. (2003). Identification of Genotype-Selective Antitumor Agents Using Synthetic Lethal Chemical Screening in Engineered Human Tumor Cells. Cancer cell 3, 285–296. 10.1016/s1535-6108(03)00050-3 [DOI] [PubMed] [Google Scholar]
- Dondorp A., Nosten F., Stepniewska K., Day N., White N. (2005). Artesunate versus Quinine for Treatment of Severe Falciparum Malaria: a Randomised Trial. Lancet 366, 717–725. 10.1016/S0140-6736(05)67176-0 [DOI] [PubMed] [Google Scholar]
- Dostalikova-Cimburova M., Balusikova K., Kratka K., Chmelikova J., Hejda V., Hnanicek J., et al. (2014). Role of Duodenal Iron Transporters and Hepcidin in Patients with Alcoholic Liver Disease. J. Cel Mol Med 18, 1840–1850. 10.1111/jcmm.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Wang T., Li Y., Zhou Y., Wang X., Yu X., et al. (2019). DHA Inhibits Proliferation and Induces Ferroptosis of Leukemia Cells through Autophagy Dependent Degradation of Ferritin. Free Radic. Biol. Med. 131, 356–369. 10.1016/j.freeradbiomed.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Eling N., Reuter L., Hazin J., Hamacher-Brady A., Brady N. R. (2015). Identification of Artesunate as a Specific Activator of Ferroptosis in Pancreatic Cancer Cells. Oncoscience 2, 517–532. 10.18632/oncoscience.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Wirth A.-K., Chen D., Wruck C. J., Rauh M., Buchfelder M., et al. (2017). Nrf2-Keap1 Pathway Promotes Cell Proliferation and Diminishes Ferroptosis. Oncogenesis 6, e371. 10.1038/oncsis.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S., Yu X., Ding H., Han J., Feng J. (2018). Effects of Intracellular Iron Overload on Cell Death and Identification of Potent Cell Death Inhibitors. Biochem. biophysical Res. Commun. 503, 297–303. 10.1016/j.bbrc.2018.06.019 [DOI] [PubMed] [Google Scholar]
- Fang X., Cai Z., Wang H., Han D., Cheng Q., Zhang P., et al. (2020). Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 127, 486–501. 10.1161/circresaha.120.316509 [DOI] [PubMed] [Google Scholar]
- Fang X., Wang H., Han D., Xie E., Yang X., Wei J., et al. (2019). Ferroptosis as a Target for protection against Cardiomyopathy. Proc. Natl. Acad. Sci. USA 116, 2672–2680. 10.1073/pnas.1821022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Currais A., Liang Z., Pinto A., Maher P. (2019). Old Age-Associated Phenotypic Screening for Alzheimer's Disease Drug Candidates Identifies Sterubin as a Potent Neuroprotective Compound from Yerba Santa. Redox Biol. 21, 101089. 10.1016/j.redox.2018.101089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Nunes A., Jakszyn P., Agudo A. (2014). Iron and Cancer Risk-A Systematic Review and Meta-Analysis of the Epidemiological Evidence : a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive. Cancer Epidemiol. Biomarkers Prev. 23, 12–31. 10.1158/1055-9965.epi-13-0733 [DOI] [PubMed] [Google Scholar]
- Friedmann Angeli J. P., Krysko D. V., Conrad M. (2019). Ferroptosis at the Crossroads of Cancer-Acquired Drug Resistance and Immune Evasion. Nat. Rev. Cancer 19, 405–414. 10.1038/s41568-019-0149-1 [DOI] [PubMed] [Google Scholar]
- Friedmann Angeli J. P., Schneider M., Proneth B., Tyurina Y. Y., Tyurin V. A., Hammond V. J., et al. (2014). Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cel Biol 16, 1180–1191. 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Steller H. (2011). Programmed Cell Death in Animal Development and Disease. Cell 147, 742–758. 10.1016/j.cell.2011.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Bravo-San Pedro J. M., Vitale I., Aaronson S. A., Abrams J. M., Adam D., et al. (2015). Essential versus Accessory Aspects of Cell Death: Recommendations of the NCCD 2015. Cel Death Differ 22, 58–73. 10.1038/cdd.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Bai Y., Jia Y., Zhao Y., Kang R., Tang D., et al. (2018). Ferroptosis Is a Lysosomal Cell Death Process. Biochem. biophysical Res. Commun. 503, 1550–1556. 10.1016/j.bbrc.2018.07.078 [DOI] [PubMed] [Google Scholar]
- Gao M., Monian P., Pan Q., Zhang W., Xiang J., Jiang X. (2016). Ferroptosis Is an Autophagic Cell Death Process. Cell Res 26, 1021–1032. 10.1038/cr.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. (2015). Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cel. 59, 298–308. 10.1016/j.molcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Yi J., Zhu J., Minikes A. M., Monian P., Thompson C. B., et al. (2019). Role of Mitochondria in Ferroptosis. Mol. Cel. 73, 354–363. e3. 10.1016/j.molcel.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Deng G., Li Y., Huang H., Sun X., Shi H., et al. (2020). Actinidia Chinensis Planch Prevents Proliferation and Migration of Gastric Cancer Associated with Apoptosis, Ferroptosis Activation and Mesenchymal Phenotype Suppression. Biomed. Pharmacother. 126, 110092. 10.1016/j.biopha.2020.110092 [DOI] [PubMed] [Google Scholar]
- Gaschler M. M., Andia A. A., Liu H., Csuka J. M., Hurlocker B., Vaiana C. A., et al. (2018a). FINO2 Initiates Ferroptosis through GPX4 Inactivation and Iron Oxidation. Nat. Chem. Biol. 14, 507–515. 10.1038/s41589-018-0031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschler M. M., Hu F., Feng H., Linkermann A., Min W., Stockwell B. R. (2018b). Determination of the Subcellular Localization and Mechanism of Action of Ferrostatins in Suppressing Ferroptosis. ACS Chem. Biol. 13, 1013–1020. 10.1021/acschembio.8b00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenshields A. L., Shepherd T. G., Hoskin D. W. (2017). Contribution of Reactive Oxygen Species to Ovarian Cancer Cell Growth Arrest and Killing by the Anti-malarial Drug Artesunate. Mol. Carcinog. 56, 75–93. 10.1002/mc.22474 [DOI] [PubMed] [Google Scholar]
- Grolez G., Moreau C., Sablonnière B., Garçon G., Devedjian J.-C., Meguig S., et al. (2015). Ceruloplasmin Activity and Iron Chelation Treatment of Patients with Parkinson's Disease. BMC Neurol. 15, 74. 10.1186/s12883-015-0331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Hue M., García-Caballero C., Palomino-Antolín A., Rubio-Navarro A., Vázquez-Carballo C., Herencia C., et al. (2019). Curcumin Reduces Renal Damage Associated with Rhabdomyolysis by Decreasing Ferroptosis-Mediated Cell Death. Faseb j 33, 8961–8975. 10.1096/fj.201900077R [DOI] [PubMed] [Google Scholar]
- Gunesch S., Hoffmann M., Kiermeier C., Fischer W., Pinto A. F. M., Maurice T., et al. (2020). 7-O-Esters of Taxifolin with Pronounced and Overadditive Effects in Neuroprotection, Anti-neuroinflammation, and Amelioration of Short-Term Memory Impairment In Vivo . Redox Biol. 29, 101378. 10.1016/j.redox.2019.101378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Hao L.-J., Yang Z.-H., Chai R., Zhang S., Gu Y., et al. (2016). Deferoxamine-mediated Up-Regulation of HIF-1α Prevents Dopaminergic Neuronal Death via the Activation of MAPK Family Proteins in MPTP-Treated Mice. Exp. Neurol. 280, 13–23. 10.1016/j.expneurol.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Guo J., Xu B., Han Q., Zhou H., Xia Y., Gong C., et al. (2018a). Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 50, 445–460. 10.4143/crt.2016.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Xu B., Han Q., Zhou H., Xia Y., Gong C., et al. (2018b). Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 50, 445–460. 10.4143/crt.2016.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Ran Q., Roberts L. J., Zhou L., Richardson A., Sharan C., et al. (2008). Suppression of Atherogenesis by Overexpression of Glutathione Peroxidase-4 in Apolipoprotein E-Deficient Mice. Free Radic. Biol. Med. 44, 343–352. 10.1016/j.freeradbiomed.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashy H. O., Powe D. G., Staka C. M., Rakha E. A., Ball G., Green A. R., et al. (2010). Transferrin Receptor (CD71) Is a Marker of Poor Prognosis in Breast Cancer and Can Predict Response to Tamoxifen. Breast Cancer Res. Treat. 119, 283–293. 10.1007/s10549-009-0345-x [DOI] [PubMed] [Google Scholar]
- Hamaï A., Cañeque T., Müller S., Mai T. T., Hienzsch A., Ginestier C., et al. (2017). An Iron Hand over Cancer Stem Cells. Autophagy 13, 1465–1466. 10.1080/15548627.2017.1327104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambright W. S., Fonseca R. S., Chen L., Na R., Ran Q. (2017). Ablation of Ferroptosis Regulator Glutathione Peroxidase 4 in Forebrain Neurons Promotes Cognitive Impairment and Neurodegeneration. Redox Biol. 12, 8–17. 10.1016/j.redox.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer M. J., Viswanathan V. S., Ryan M. J., Bole D., Eaton J. K., Matov A., et al. (2017). Drug-tolerant Persister Cancer Cells Are Vulnerable to GPX4 Inhibition. Nature 551, 247–250. 10.1038/nature24297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Yu J., He W., Huang Q., Zhao Y., Liang B., et al. (2017). Cysteine Dioxygenase 1 Mediates Erastin-Induced Ferroptosis in Human Gastric Cancer Cells. Neoplasia 19, 1022–1032. 10.1016/j.neo.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia B., Vandenabeele P., Vanden Berghe T. (2019). Targeting Ferroptosis to Iron Out Cancer. Cancer cell 35, 830–849. 10.1016/j.ccell.2019.04.002 [DOI] [PubMed] [Google Scholar]
- Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y. Y., et al. (2018). Nano-targeted Induction of Dual Ferroptotic Mechanisms Eradicates High-Risk Neuroblastoma. J. Clin. Invest. 128, 3341–3355. 10.1172/jci99032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano M., Yang W. S., Corn C. K., Pagano N. C., Stockwell B. R. (2016). Loss of Cysteinyl-tRNA Synthetase (CARS) Induces the Transsulfuration Pathway and Inhibits Ferroptosis Induced by Cystine Deprivation. Cel Death Differ 23, 270–278. 10.1038/cdd.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohan C., Van Schaeybroeck S., Longley D. B., Johnston P. G. (2013). Cancer Drug Resistance: an Evolving Paradigm. Nat. Rev. Cancer 13, 714–726. 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- Hou W., Xie Y., Song X., Sun X., Lotze M. T., Zeh H. J., et al. (2016). Autophagy Promotes Ferroptosis by Degradation of Ferritin. Autophagy 12, 1425–1428. 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houessinon A., François C., Sauzay C., Louandre C., Mongelard G., Godin C., et al. (2016). Metallothionein-1 as a Biomarker of Altered Redox Metabolism in Hepatocellular Carcinoma Cells Exposed to Sorafenib. Mol. Cancer 15, 38. 10.1186/s12943-016-0526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. L., Nydes M., Shanley K. L., Morales Pantoja I. E., Howard T. A., Bizzozero O. A. (2019). Reduced Expression of the Ferroptosis Inhibitor Glutathione Peroxidase‐4 in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Neurochem. 148, 426–439. 10.1111/jnc.14604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isani G., Bertocchi M., Andreani G., Farruggia G., Cappadone C., Salaroli R., et al. (2019). Cytotoxic Effects of Artemisia Annua L. And Pure Artemisinin on the D-17 Canine Osteosarcoma Cell Line. Oxid Med. Cel Longev 2019, 1615758. 10.1155/2019/1615758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennis M., Kung C.-P., Basu S., Budina-Kolomets A., Leu J. I.-J., Khaku S., et al. (2016). An African-specific Polymorphism in the TP53 Gene Impairs P53 Tumor Suppressor Function in a Mouse Model. Genes Dev. 30, 918–930. 10.1101/gad.275891.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhelum P., Santos-Nogueira E., Teo W., Haumont A., Lenoël I., Stys P. K., et al. (2020). Ferroptosis Mediates Cuprizone-Induced Loss of Oligodendrocytes and Demyelination. J. Neurosci. 40, 9327–9341. 10.1523/jneurosci.1749-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Kuo Y., Morris M. E. (2005). Pharmacokinetics of Dietary Phenethyl Isothiocyanate in Rats. Pharm. Res. 22, 1658–1666. 10.1007/s11095-005-7097-z [DOI] [PubMed] [Google Scholar]
- Jiang L., Kon N., Li T., Wang S.-J., Su T., Hibshoosh H., et al. (2015). Ferroptosis as a P53-Mediated Activity during Tumour Suppression. Nature 520, 57–62. 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Shi C., Li T., Wu Y., Hu C., Huang G. (2020). Solasonine Promotes Ferroptosis of Hepatoma Carcinoma Cells via Glutathione Peroxidase 4-induced Destruction of the Glutathione Redox System. Biomed. Pharmacother. 129, 110282. 10.1016/j.biopha.2020.110282 [DOI] [PubMed] [Google Scholar]
- Kagan V. E., Mao G., Qu F., Angeli J. P. F., Doll S., Croix C. S., et al. (2017). Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 13, 81–90. 10.1038/nchembio.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder S. S., Alin L., Chen Y., Brand D., Bourassa M. W., Dietrich K., et al. (2018). N-acetylcysteine Targets 5 Lipoxygenase-Derived, Toxic Lipids and Can Synergize with Prostaglandin E2to Inhibit Ferroptosis and Improve Outcomes Following Hemorrhagic Stroke in Mice. Ann. Neurol. 84, 854–872. 10.1002/ana.25356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasukabe T., Honma Y., Okabe-Kado J., Higuchi Y., Kato N., Kumakura S. (2016). Combined Treatment with Cotylenin A and Phenethyl Isothiocyanate Induces strong Antitumor Activity Mainly through the Induction of Ferroptotic Cell Death in Human Pancreatic Cancer Cells. Oncol. Rep. 36, 968–976. 10.3892/or.2016.4867 [DOI] [PubMed] [Google Scholar]
- Kenny E. M., Fidan E., Yang Q., Anthonymuthu T. S., New L. A., Meyer E. A., et al. (2019). Ferroptosis Contributes to Neuronal Death and Functional Outcome after Traumatic Brain Injury*. Crit. Care Med. 47, 410–418. 10.1097/ccm.0000000000003555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorsandi K., Kianmehr Z., Hosseinmardi Z., Hosseinzadeh R. (2020). Anti-cancer Effect of Gallic Acid in Presence of Low Level Laser Irradiation: ROS Production and Induction of Apoptosis and Ferroptosis. Cancer Cel Int 20, 18. 10.1186/s12935-020-1100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani B. H., Kayani W. K., Khayam A. U., Dilshad E., Ismail H., Mirza B. (2020). Artemisinin and its Derivatives: a Promising Cancer Therapy. Mol. Biol. Rep. 47, 6321–6336. 10.1007/s11033-020-05669-z [DOI] [PubMed] [Google Scholar]
- Kibble M., Saarinen N., Tang J., Wennerberg K., Mäkelä S., Aittokallio T. (2015). Network Pharmacology Applications to Map the Unexplored Target Space and Therapeutic Potential of Natural Products. Nat. Prod. Rep. 32, 1249–1266. 10.1039/c5np00005j [DOI] [PubMed] [Google Scholar]
- Kim E. H., Shin D., Lee J., Jung A. R., Roh J.-L. (2018). CISD2 Inhibition Overcomes Resistance to Sulfasalazine-Induced Ferroptotic Cell Death in Head and Neck Cancer. Cancer Lett. 432, 180–190. 10.1016/j.canlet.2018.06.018 [DOI] [PubMed] [Google Scholar]
- Kinoshita H., Okabe H., Beppu T., Chikamoto A., Hayashi H., Imai K., et al. (2013). Cystine/glutamic Acid Transporter Is a Novel Marker for Predicting Poor Survival in Patients with Hepatocellular Carcinoma. Oncol. Rep. 29, 685–689. 10.3892/or.2012.2162 [DOI] [PubMed] [Google Scholar]
- Klepac N., Relja M., Klepac R., Hećimović S., Babić T., Trkulja V. (2007). Oxidative Stress Parameters in Plasma of Huntington's Disease Patients, Asymptomatic Huntington's Disease Gene Carriers and Healthy Subjects. J. Neurol. 254, 1676–1683. 10.1007/s00415-007-0611-y [DOI] [PubMed] [Google Scholar]
- Klopstock T., Tricta F., Neumayr L., Karin I., Zorzi G., Fradette C., et al. (2019). Safety and Efficacy of Deferiprone for Pantothenate Kinase-Associated Neurodegeneration: a Randomised, Double-Blind, Controlled Trial and an Open-Label Extension Study. Lancet Neurol. 18, 631–642. 10.1016/s1474-4422(19)30142-5 [DOI] [PubMed] [Google Scholar]
- Kong Z., Liu R., Cheng Y. (2019). Artesunate Alleviates Liver Fibrosis by Regulating Ferroptosis Signaling Pathway. Biomed. Pharmacother. 109, 2043–2053. 10.1016/j.biopha.2018.11.030 [DOI] [PubMed] [Google Scholar]
- Kose T., Vera-Aviles M., Sharp P. A., Latunde-Dada G. O. (2019). Curcumin and (-)- Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals (Basel) 12, 26. 10.3390/ph12010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaier E., Louandre C., Godin C., Saidak Z., Baert M., Diouf M., et al. (2014). Sorafenib Induces Ferroptosis in Human Cancer Cell Lines Originating from Different Solid Tumors. Anticancer Res. 34, 6417–6422. [PubMed] [Google Scholar]
- Lakhal-Littleton S., Wolna M., Chung Y. J., Christian H. C., Heather L. C., Brescia M., et al. (2016). An Essential Cell-Autonomous Role for Hepcidin in Cardiac Iron Homeostasis. Elife 5. 10.7554/eLife.19804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal-Littleton S., Wolna M., Carr C. A., Miller J. J. J., Christian H. C., Ball V., et al. (2015). Cardiac Ferroportin Regulates Cellular Iron Homeostasis and Is Important for Cardiac Function. Proc. Natl. Acad. Sci. USA 112, 3164–3169. 10.1073/pnas.1422373112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan B., Ge J. W., Cheng S. W., Zheng X. L., Liao J., He C., et al. (2020). Extract of Naotaifang, a Compound Chinese Herbal Medicine, Protects Neuron Ferroptosis Induced by Acute Cerebral Ischemia in Rats. J. Integr. Med. 18, 344–350. 10.1016/j.joim.2020.01.008 [DOI] [PubMed] [Google Scholar]
- Lang X., Green M. D., Wang W., Yu J., Choi J. E., Jiang L., et al. (2019). Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 9, 1673–1685. 10.1158/2159-8290.cd-19-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzardo S., Conti L., Rooke R., Ruiu R., Accart N., Bolli E., et al. (2016). Immunotargeting of Antigen xCT Attenuates Stem-like Cell Behavior and Metastatic Progression in Breast Cancer. Cancer Res. 76, 62–72. 10.1158/0008-5472.can-15-1208 [DOI] [PubMed] [Google Scholar]
- Lee J., Kosaras B., Del Signore S. J., Cormier K., McKee A., Ratan R. R., et al. (2011). Modulation of Lipid Peroxidation and Mitochondrial Function Improves Neuropathology in Huntington's Disease Mice. Acta Neuropathol. 121, 487–498. 10.1007/s00401-010-0788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G., Zhang Y., Koppula P., Liu X., Zhang J., Lin S. H., et al. (2020). The Role of Ferroptosis in Ionizing Radiation-Induced Cell Death and Tumor Suppression. Cel Res 30, 146–162. 10.1038/s41422-019-0263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Han X., Lan X., Gao Y., Wan J., Durham F., et al. (2017). Inhibition of Neuronal Ferroptosis Protects Hemorrhagic Brain. JCI insight 2, e90777. 10.1172/jci.insight.90777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Feng D., Wang Z., Zhao Y., Sun R., Tian D., et al. (2019). Ischemia-induced ACSL4 Activation Contributes to Ferroptosis-Mediated Tissue Injury in Intestinal Ischemia/reperfusion. Cel Death Differ 26, 2284–2299. 10.1038/s41418-019-0299-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Feng G., Gauthier J. M., Lokshina I., Higashikubo R., Evans S., et al. (2019). Ferroptotic Cell Death and TLR4/Trif Signaling Initiate Neutrophil Recruitment after Heart Transplantation. J. Clin. Invest. 129, 2293–2304. 10.1172/jci126428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li Q.-Q., Jia J.-N., Sun Q.-Y., Zhou H.-H., Jin W.-L., et al. (2019). Baicalein Exerts Neuroprotective Effects in FeCl3-Induced Posttraumatic Epileptic Seizures via Suppressing Ferroptosis. Front. Pharmacol. 10, 638. 10.3389/fphar.2019.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang T. X., Huang X., Li Y., Sun T., Zang S., et al. (2020). Targeting Ferroptosis Alleviates Methionine‐choline Deficient (MCD)‐diet Induced NASH by Suppressing Liver Lipotoxicity. Liver Int. 40, 1378–1394. 10.1111/liv.14428 [DOI] [PubMed] [Google Scholar]
- Li D., Jiang C., Mei G., Zhao Y., Chen L., Liu J., et al. (2020). Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 12. 10.3390/nu12102954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zeng J., Liu Y., Liang M., Liu Q., Li Z., et al. (2020). Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder Anti-dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells. Antioxidants (Basel) 9, 205. 10.3390/antiox9030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Zhang X., Yang M., Dong X. (2019). Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 31, e1904197. 10.1002/adma.201904197 [DOI] [PubMed] [Google Scholar]
- Lim J. K. M., Delaidelli A., Minaker S. W., Zhang H.-F., Colovic M., Yang H., et al. (2019). Cystine/glutamate Antiporter xCT (SLC7A11) Facilitates Oncogenic RAS Transformation by Preserving Intracellular Redox Balance. Proc. Natl. Acad. Sci. USA 116, 9433–9442. 10.1073/pnas.1821323116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Zhang Z., Chen L., Zhou Y., Zou P., Feng C., et al. (2016). Dihydroartemisinin (DHA) Induces Ferroptosis and Causes Cell Cycle Arrest in Head and Neck Carcinoma Cells. Cancer Lett. 381, 165–175. 10.1016/j.canlet.2016.07.033 [DOI] [PubMed] [Google Scholar]
- Lin Y.-S., Shen Y.-C., Wu C.-Y., Tsai Y.-Y., Yang Y.-H., Lin Y.-Y., et al. (2019). Danshen Improves Survival of Patients with Breast Cancer and Dihydroisotanshinone I Induces Ferroptosis and Apoptosis of Breast Cancer Cells. Front. Pharmacol. 10, 1226. 10.3389/fphar.2019.01226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Zhao C., Li H., Chen X., Ding Y., Xu S. (2018). Puerarin Protects against Heart Failure Induced by Pressure Overload through Mitigation of Ferroptosis. Biochem. Biophysical Res. Commun. 497, 233–240. 10.1016/j.bbrc.2018.02.061 [DOI] [PubMed] [Google Scholar]
- Liu P., Wu D., Duan J., Xiao H., Zhou Y., Zhao L., et al. (2020). NRF2 Regulates the Sensitivity of Human NSCLC Cells to Cystine Deprivation-Induced Ferroptosis via FOCAD-FAK Signaling Pathway. Redox Biol. 37, 101702. 10.1016/j.redox.2020.101702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li X., Cai R., Ren Z., Zhang A., Deng F., et al. (2020). Simultaneous Study of Anti-ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin. Molecules 25, 674. 10.3390/molecules25030674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang W., Li Y., Xiao Y., Cheng J., Jia J. (2015). The 5-Lipoxygenase Inhibitor Zileuton Confers Neuroprotection against Glutamate Oxidative Damage by Inhibiting Ferroptosis. Biol. Pharm. Bull. 38, 1234–1239. 10.1248/bpb.b15-00048 [DOI] [PubMed] [Google Scholar]
- Llabani E., Hicklin R. W., Lee H. Y., Motika S. E., Crawford L. A., Weerapana E., et al. (2019). Diverse Compounds from Pleuromutilin lead to a Thioredoxin Inhibitor and Inducer of Ferroptosis. Nat. Chem. 11, 521–532. 10.1038/s41557-019-0261-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Liu X., Tian Y., Li H., Ren Z., Liang S., et al. (2019). Moxibustion Exerts a Neuroprotective Effect through Antiferroptosis in Parkinson's Disease. Evid. Based Complement. Alternat Med. 2019, 2735492. 10.1155/2019/2735492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Harris V. A., Rafikov R., Sun X., Kumar S., Black S. M. (2015). Nitric Oxide Induces Hypoxia Ischemic Injury in the Neonatal Brain via the Disruption of Neuronal Iron Metabolism. Redox Biol. 6, 112–121. 10.1016/j.redox.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Vong C. T., Chen H., Gao Y., Lyu P., Qiu L., et al. (2019). Naturally Occurring Anti-cancer Compounds: Shining from Chinese Herbal Medicine. Chin. Med. 14, 48. 10.1186/s13020-019-0270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Wu L., Zhang K., Wang H., Zhang T., Gutierrez L., et al. (2018). miR-137 Regulates Ferroptosis by Targeting Glutamine Transporter SLC1A5 in Melanoma. Cel Death Differ 25, 1457–1472. 10.1038/s41418-017-0053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H.-H., Zhen C.-x., Liu J.-y., Shang P. (2020). PEITC Triggers Multiple Forms of Cell Death by GSH-Iron-ROS Regulation in K7M2 Murine Osteosarcoma Cells. Acta Pharmacol. Sin 41, 1119–1132. 10.1038/s41401-020-0376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Zhen C., Liu J., Shang P. (2020). β-Phenethyl Isothiocyanate Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the MAPK Signaling Pathway. Oxid Med. Cel Longev 2020, 5021983. 10.1155/2020/5021983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Dielschneider R. F., Henson E. S., Xiao W., Choquette T. R., Blankstein A. R., et al. (2017). Ferroptosis and Autophagy Induced Cell Death Occur Independently after Siramesine and Lapatinib Treatment in Breast Cancer Cells. PLoS One 12, e0182921. 10.1371/journal.pone.0182921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio A., Kattamis A., Felisi M., Reggiardo G., El-Beshlawy A., Bejaoui M., et al. (2020). Evaluation of the Efficacy and Safety of Deferiprone Compared with Deferasirox in Paediatric Patients with Transfusion-dependent Haemoglobinopathies (DEEP-2): a Multicentre, Randomised, Open-Label, Non-inferiority, Phase 3 Trial. Lancet Haematol. 7, e469–e478. 10.1016/s2352-3026(20)30100-9 [DOI] [PubMed] [Google Scholar]
- Maher P., Fischer W., Liang Z., Soriano-Castell D., Pinto A. F. M., Rebman J., et al. (2020). The Value of Herbarium Collections to the Discovery of Novel Treatments for Alzheimer's Disease, a Case Made with the Genus Eriodictyon. Front. Pharmacol. 11, 208. 10.3389/fphar.2020.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai T. T., Hamaï A., Hienzsch A., Cañeque T., Müller S., Wicinski J., et al. (2017). Salinomycin Kills Cancer Stem Cells by Sequestering Iron in Lysosomes. Nat. Chem 9, 1025–1033. 10.1038/nchem.2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfa G. A., Tomasello B., Acquaviva R., Genovese C., La Mantia A., Cammarata F. P., et al. (2019). Betula Etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int. J. Mol. Sci. 20, 2723. 10.3390/ijms20112723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias J. D., Wang X., Gygi S. P., Harper J. W., Kimmelman A. C. (2014). Quantitative Proteomics Identifies NCOA4 as the Cargo Receptor Mediating Ferritinophagy. Nature 509, 105–109. 10.1038/nature13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bastida A., Ward R. J., Newbould R., Piccini P., Sharp D., Kabba C., et al. (2017). Brain Iron Chelation by Deferiprone in a Phase 2 Randomised Double-Blinded Placebo Controlled Clinical Trial in Parkinson's Disease. Sci. Rep. 7, 1398. 10.1038/s41598-017-01402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M., Freigang S., Schneider C., Conrad M., Bornkamm G. W., Kopf M. (2015). T Cell Lipid Peroxidation Induces Ferroptosis and Prevents Immunity to Infection. J. Exp. Med. 212, 555–568. 10.1084/jem.20140857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr L., Grabherr F., Schwärzler J., Reitmeier I., Sommer F., Gehmacher T., et al. (2020). Dietary Lipids Fuel GPX4-Restricted Enteritis Resembling Crohn's Disease. Nat. Commun. 11, 1775. 10.1038/s41467-020-15646-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbaveng A. T., Bitchagno G. T. M., Kuete V., Tane P., Efferth T. (2019). Cytotoxicity of Ungeremine towards Multi-Factorial Drug Resistant Cancer Cells and Induction of Apoptosis, Ferroptosis, Necroptosis and Autophagy. Phytomedicine 60, 152832. 10.1016/j.phymed.2019.152832 [DOI] [PubMed] [Google Scholar]
- Mbaveng A. T., Chi G. F., Bonsou I. N., Abdelfatah S., Tamfu A. N., Yeboah E. M. O., et al. (2020). N-acetylglycoside of Oleanolic Acid (Aridanin) Displays Promising Cytotoxicity towards Human and Animal Cancer Cells, Inducing Apoptotic, Ferroptotic and Necroptotic Cell Death. Phytomedicine 76, 153261. 10.1016/j.phymed.2020.153261 [DOI] [PubMed] [Google Scholar]
- Mbaveng A. T., Ndontsa B. L., Kuete V., Nguekeu Y. M. M., Çelik İ., Mbouangouere R., et al. (2018a). A Naturally Occuring Triterpene Saponin Ardisiacrispin B Displayed Cytotoxic Effects in Multi-Factorial Drug Resistant Cancer Cells via Ferroptotic and Apoptotic Cell Death. Phytomedicine 43, 78–85. 10.1016/j.phymed.2018.03.035 [DOI] [PubMed] [Google Scholar]
- Mbaveng A. T., Fotso G. W., Ngnintedo D., Kuete V., Ngadjui B. T., Keumedjio F., et al. (2018b). Cytotoxicity of Epunctanone and Four Other Phytochemicals Isolated from the Medicinal Plants Garcinia Epunctata and Ptycholobium Contortum towards Multi-Factorial Drug Resistant Cancer Cells. Phytomedicine 48, 112–119. 10.1016/j.phymed.2017.12.016 [DOI] [PubMed] [Google Scholar]
- McBean G. J. (2012). The Transsulfuration Pathway: a Source of Cysteine for Glutathione in Astrocytes. Amino Acids 42, 199–205. 10.1007/s00726-011-0864-8 [DOI] [PubMed] [Google Scholar]
- Md Nesran Z. N., Shafie N. H., Md Tohid S. F., Norhaizan M. E., Ismail A. (2020). Iron Chelation Properties of Green Tea Epigallocatechin-3-Gallate (EGCG) in Colorectal Cancer Cells: Analysis on Tfr/Fth Regulations and Molecular Docking. Evid. Based Complement. Alternat Med. 2020, 7958041. 10.1155/2020/7958041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S., Sindikubwabo F., Cañeque T., Lafon A., Versini A., Lombard B., et al. (2020). CD44 Regulates Epigenetic Plasticity by Mediating Iron Endocytosis. Nat. Chem. 12, 929–938. 10.1038/s41557-020-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafov A., Chen H., Yuan J. (2017). Necroptosis and Cancer. Trends Cancer 3, 294–301. 10.1016/j.trecan.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Zhang J., Tong Y., Li J., Liu B. (2019). Physcion 8-O-β-Glucopyranoside Induced Ferroptosis via Regulating miR-103a-3p/GLS2 axis in Gastric Cancer. Life Sci. 237, 116893. 10.1016/j.lfs.2019.116893 [DOI] [PubMed] [Google Scholar]
- Ou Y., Wang S.-J., Li D., Chu B., Gu W. (2016). Activation of SAT1 Engages Polyamine Metabolism with P53-Mediated Ferroptotic Responses. Proc. Natl. Acad. Sci. USA 113, E6806–E6812. 10.1073/pnas.1607152113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliyaguru D. L., Yuan J. M., Kensler T. W., Fahey J. W. (2018). Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 62, e1700965. 10.1002/mnfr.201700965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Oh J., Kim M., Jin E.-J. (2018). Bromelain Effectively Suppresses Kras-Mutant Colorectal Cancer by Stimulating Ferroptosis. Anim. Cell Syst. 22, 334–340. 10.1080/19768354.2018.1512521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park U. J., Lee Y. A., Won S. M., Lee J. H., Kang S.-H., Springer J. E., et al. (2011). Blood-derived Iron Mediates Free Radical Production and Neuronal Death in the Hippocampal CA1 Area Following Transient Forebrain Ischemia in Rat. Acta Neuropathol. 121, 459–473. 10.1007/s00401-010-0785-8 [DOI] [PubMed] [Google Scholar]
- Parvez M. K., Rishi V. (2019). Herb-Drug Interactions and Hepatotoxicity. Cdm 20, 275–282. 10.2174/1389200220666190325141422 [DOI] [PubMed] [Google Scholar]
- Perez C. A., Wei Y., Guo M. (2009). Iron-binding and Anti-Fenton Properties of Baicalein and Baicalin. J. Inorg. Biochem. 103, 326–332. 10.1016/j.jinorgbio.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò D., Sung S. (2004). Lipid Peroxidation and Oxidative Imbalance: Early Functional Events in Alzheimer's Disease. Jad 6, 171–175. 10.3233/jad-2004-6209 [DOI] [PubMed] [Google Scholar]
- Probst L., Dächert J., Schenk B., Fulda S. (2017). Lipoxygenase Inhibitors Protect Acute Lymphoblastic Leukemia Cells from Ferroptotic Cell Death. Biochem. Pharmacol. 140, 41–52. 10.1016/j.bcp.2017.06.112 [DOI] [PubMed] [Google Scholar]
- Pyatigorskaya N., Sharman M., Corvol J.-C., Valabregue R., Yahia-Cherif L., Poupon F., et al. (2015). High Nigral Iron Deposition in LRRK2 and Parkin Mutation Carriers Using R2* Relaxometry. Mov Disord. 30, 1077–1084. 10.1002/mds.26218 [DOI] [PubMed] [Google Scholar]
- Ran Q., Liang H., Ikeno Y., Qi W., Prolla T. A., Roberts L. J., et al. (2007). Reduction in Glutathione Peroxidase 4 Increases Life Span through Increased Sensitivity to Apoptosis. Journals Gerontol. Ser. A: Biol. Sci. Med. Sci. 62, 932–942. 10.1093/gerona/62.9.932 [DOI] [PubMed] [Google Scholar]
- Rao S. S., Lago L., Volitakis I., Shukla J. J., McColl G., Finkelstein D. I., et al. (2021). Deferiprone Treatment in Aged Transgenic Tau Mice Improves Y-Maze Performance and Alters Tau Pathology. Neurotherapeutics 18, 1081–1094. 10.1007/s13311-020-00972-w [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reed J. C., Pellecchia M. (2012). Ironing Out Cell Death Mechanisms. Cell 149, 963–965. 10.1016/j.cell.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Rennekamp A. J. (2017). The Ferrous Awakens. Cell 171, 1225–1227. 10.1016/j.cell.2017.11.029 [DOI] [PubMed] [Google Scholar]
- Roh J.-L., Kim E. H., Jang H. J., Park J. Y., Shin D. (2016). Induction of Ferroptotic Cell Death for Overcoming Cisplatin Resistance of Head and Neck Cancer. Cancer Lett. 381, 96–103. 10.1016/j.canlet.2016.07.035 [DOI] [PubMed] [Google Scholar]
- Roh J.-L., Kim E. H., Jang H., Shin D. (2017). Nrf2 Inhibition Reverses the Resistance of Cisplatin-Resistant Head and Neck Cancer Cells to Artesunate-Induced Ferroptosis. Redox Biol. 11, 254–262. 10.1016/j.redox.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo de la Vega M., Chapman E., Zhang D. D. (2018). NRF2 and the Hallmarks of Cancer. Cancer cell 34, 21–43. 10.1016/j.ccell.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai O., Yasuzawa T., Sumikawa Y., Ueta T., Imai H., Sawabe A., et al. (2017). Role of GPx4 in Human Vascular Endothelial Cells, and the Compensatory Activity of Brown rice on GPx4 Ablation Condition. Pathophysiology 24, 9–15. 10.1016/j.pathophys.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., et al. (2005). Redox Imbalance in Cystine/glutamate Transporter-Deficient Mice. J. Biol. Chem. 280, 37423–37429. 10.1074/jbc.m506439200 [DOI] [PubMed] [Google Scholar]
- Seco-Cervera M., Gonzalez-Cabo P., Pallardo F. V., Roma-Mateo C., Garcia-Gimenez J. L. (2020). Thioredoxin and Glutaredoxin Systems as Potential Targets for the Development of New Treatments in Friedreich's Ataxia. Antioxidants (Basel) 9, 1257. 10.3390/antiox9121257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. T., Winslow M. M., Magendantz M., Ouyang C., Dowdle J., Subramanian A., et al. (2011). Selective Killing of K-Ras Mutant Cancer Cells by Small Molecule Inducers of Oxidative Stress. Proc. Natl. Acad. Sci. 108, 8773–8778. 10.1073/pnas.1105941108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z., Liu T., Li Y., Lau J., Yang Z., Fan W., et al. (2018). Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS nano 12, 11355–11365. 10.1021/acsnano.8b06201 [DOI] [PubMed] [Google Scholar]
- Sherr C. J. (2006). Divorcing ARF and P53: an Unsettled Case. Nat. Rev. Cancer 6, 663–673. 10.1038/nrc1954 [DOI] [PubMed] [Google Scholar]
- Shimada K., Skouta R., Kaplan A., Yang W. S., Hayano M., Dixon S. J., et al. (2016a). Global Survey of Cell Death Mechanisms Reveals Metabolic Regulation of Ferroptosis. Nat. Chem. Biol. 12, 497–503. 10.1038/nchembio.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Hayano M., Pagano N. C., Stockwell B. R. (2016b). Cell-Line Selectivity Improves the Predictive Power of Pharmacogenomic Analyses and Helps Identify NADPH as Biomarker for Ferroptosis Sensitivity. Cel Chem. Biol. 23, 225–235. 10.1016/j.chembiol.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Kim E. H., Lee J., Roh J.-L. (2018). Nrf2 Inhibition Reverses Resistance to GPX4 Inhibitor-Induced Ferroptosis in Head and Neck Cancer. Free Radic. Biol. Med. 129, 454–462. 10.1016/j.freeradbiomed.2018.10.426 [DOI] [PubMed] [Google Scholar]
- Shintoku R., Takigawa Y., Yamada K., Kubota C., Yoshimoto Y., Takeuchi T., et al. (2017). Lipoxygenase‐mediated Generation of Lipid Peroxides Enhances Ferroptosis Induced by Erastin and RSL3. Cancer Sci. 108, 2187–2194. 10.1111/cas.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu-Feng Zhou S. F., Charlie Changli Xue C. C., Xue-Qing Yu X. Q., Guangji Wang G. (2007). Metabolic Activation of Herbal and Dietary Constituents and its Clinical and Toxicological Implications: an Update. Cdm 8, 526–553. 10.2174/138920007781368863 [DOI] [PubMed] [Google Scholar]
- Skouta R., Dixon S. J., Wang J., Dunn D. E., Orman M., Shimada K., et al. (2014). Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J. Am. Chem. Soc. 136, 4551–4556. 10.1021/ja411006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Xiang X., Li J., Deng J., Fang Z., Zhang L., et al. (2020). Ruscogenin Induces Ferroptosis in Pancreatic Cancer Cells. Oncol. Rep. 43, 516–524. 10.3892/or.2019.7425 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Southon A., Szostak K., Acevedo K. M., Dent K. A., Volitakis I., Belaidi A. A., et al. (2020). Cu II (Atsm) Inhibits Ferroptosis: Implications for Treatment of Neurodegenerative Disease. Br. J. Pharmacol. 177, 656–667. 10.1111/bph.14881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripetchwandee J., Wongjaikam S., Krintratun W., Chattipakorn N., Chattipakorn S. C. (2016). A Combination of an Iron Chelator with an Antioxidant Effectively Diminishes the Dendritic Loss, Tau-Hyperphosphorylation, Amyloids-β Accumulation and Brain Mitochondrial Dynamic Disruption in Rats with Chronic Iron-Overload. Neuroscience 332, 191–202. 10.1016/j.neuroscience.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Stockwell B. R., Friedmann Angeli J. P., Bayir H., Bush A. I., Conrad M., Dixon S. J., et al. (2017). Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285. 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui M., Jiang X., Chen J., Yang H., Zhu Y. (2018). Magnesium Isoglycyrrhizinate Ameliorates Liver Fibrosis and Hepatic Stellate Cell Activation by Regulating Ferroptosis Signaling Pathway. Biomed. Pharmacother. 106, 125–133. 10.1016/j.biopha.2018.06.060 [DOI] [PubMed] [Google Scholar]
- Sullivan J. (1981). Iron and the Sex Difference in Heart Disease Risk. The Lancet 317, 1293–1294. 10.1016/s0140-6736(81)92463-6 [DOI] [PubMed] [Google Scholar]
- Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., et al. (2016a). Activation of the P62-Keap1-NRF2 Pathway Protects against Ferroptosis in Hepatocellular Carcinoma Cells. Hepatology 63, 173–184. 10.1002/hep.28251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Niu X., Chen R., He W., Chen D., Kang R., et al. (2016b). Metallothionein-1G Facilitates Sorafenib Resistance through Inhibition of Ferroptosis. Hepatology 64, 488–500. 10.1002/hep.28574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Zheng Y., Wang C., Liu Y. (2018). Glutathione Depletion Induces Ferroptosis, Autophagy, and Premature Cell Senescence in Retinal Pigment Epithelial Cells. Cel Death Dis 9, 753. 10.1038/s41419-018-0794-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima M., Ichihara K., Hirata Y. (2019). Neuroprotective Effects of Brazilian green Propolis on Oxytosis/ferroptosis in Mouse Hippocampal HT22 Cells. Food Chem. Toxicol. 132, 110669. 10.1016/j.fct.2019.110669 [DOI] [PubMed] [Google Scholar]
- Tang H. M., Cheung P. C. K. (2019). Gallic Acid Triggers Iron-dependent Cell Death with Apoptotic, Ferroptotic, and Necroptotic Features. Toxins (Basel) 11, 492. 10.3390/toxins11090492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. M., Tang H. L. (2019). Cell Recovery by Reversal of Ferroptosis. Biol. Open 8, bio043182. 10.1242/bio.043182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B. (1995). Apoptosis in the Pathogenesis and Treatment of Disease. Science 267, 1456–1462. 10.1126/science.7878464 [DOI] [PubMed] [Google Scholar]
- Tonik S. E., Shindelman J. E., Sussman H. H. (1986). Transferrin Receptor Is Inversely Correlated with Estrogen Receptor in Breast Cancer. Breast Cancer Res. Tr 7, 71–76. 10.1007/bf01806791 [DOI] [PubMed] [Google Scholar]
- Torti S. V., Torti F. M. (2013). Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 13, 342–355. 10.1038/nrc3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D., Alexandre J., Huang P. (2009). Targeting Cancer Cells by ROS-Mediated Mechanisms: a Radical Therapeutic Approach. Nat. Rev. Drug Discov. 8, 579–591. 10.1038/nrd2803 [DOI] [PubMed] [Google Scholar]
- Trujillo-Alonso V., Pratt E. C., Zong H., Lara-Martinez A., Kaittanis C., Rabie M. O., et al. (2019). FDA-approved Ferumoxytol Displays Anti-leukaemia Efficacy against Cells with Low Ferroportin Levels. Nat. Nanotechnol. 14, 616–622. 10.1038/s41565-019-0406-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi J., Robert L., Paraiso K., Galvan C., Sheu K. M., Lay J., et al. (2018). Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-dependent Oxidative Stress. Cancer cell 33, 890–904. 10.1016/j.ccell.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurusaki S., Tsuchiya Y., Koumura T., Nakasone M., Sakamoto T., Matsuoka M., et al. (2019). Hepatic Ferroptosis Plays an Important Role as the Trigger for Initiating Inflammation in Nonalcoholic Steatohepatitis. Cel Death Dis 10, 449. 10.1038/s41419-019-1678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo Q.-z., Lei P., Jackman K. A., Li X.-l., Xiong H., Li X.-l., et al. (2017). Tau-mediated Iron export Prevents Ferroptotic Damage after Ischemic Stroke. Mol. Psychiatry 22, 1520–1530. 10.1038/mp.2017.171 [DOI] [PubMed] [Google Scholar]
- van Staalduinen J., Baker D., Ten Dijke P., van Dam H. (2018). Epithelial-mesenchymal-transition-inducing Transcription Factors: New Targets for Tackling Chemoresistance in Cancer. Oncogene 37, 6195–6211. 10.1038/s41388-018-0378-x [DOI] [PubMed] [Google Scholar]
- Vichinsky E., Bernaudin F., Forni G. L., Gardner R., Hassell K., Heeney M. M., et al. (2011). Long-term Safety and Efficacy of Deferasirox (Exjade) for up to 5 Years in Transfusional Iron-Overloaded Patients with Sickle Cell Disease. Br. J. Haematol. 154, 387–397. 10.1111/j.1365-2141.2011.08720.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V. S., Ryan M. J., Dhruv H. D., Gill S., Eichhoff O. M., Seashore-Ludlow B., et al. (2017). Dependency of a Therapy-Resistant State of Cancer Cells on a Lipid Peroxidase Pathway. Nature 547, 453–457. 10.1038/nature23007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hagens C., Walter-Sack I., Goeckenjan M., Osburg J., Storch-Hagenlocher B., Sertel S., et al. (2017). Prospective Open Uncontrolled Phase I Study to Define a Well-Tolerated Dose of Oral Artesunate as Add-On Therapy in Patients with Metastatic Breast Cancer (ARTIC M33/2). Breast Cancer Res. Treat. 164, 359–369. 10.1007/s10549-017-4261-1 [DOI] [PubMed] [Google Scholar]
- Wang B., Peng X.-x., Sun R., Li J., Zhan X.-r., Wu L.-j., et al. (2012). Systematic Review of β-Elemene Injection as Adjunctive Treatment for Lung Cancer. Chin. J. Integr. Med. 18, 813–823. 10.1007/s11655-012-1271-9 [DOI] [PubMed] [Google Scholar]
- Wang S.-J., Li D., Ou Y., Jiang L., Chen Y., Zhao Y., et al. (2016). Acetylation Is Crucial for P53-Mediated Ferroptosis and Tumor Suppression. Cel Rep. 17, 366–373. 10.1016/j.celrep.2016.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Peng Y., Xie Y., Zhou B., Sun X., Kang R., et al. (2016). Antiferroptotic Activity of Non-oxidative Dopamine. Biochem. biophysical Res. Commun. 480, 602–607. 10.1016/j.bbrc.2016.10.099 [DOI] [PubMed] [Google Scholar]
- Wang H., An P., Xie E., Wu Q., Fang X., Gao H., et al. (2017). Characterization of Ferroptosis in Murine Models of Hemochromatosis. Hepatology 66, 449–465. 10.1002/hep.29117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Q., Li C., Lu L., Zhang Q., Zhu R., et al. (2017). A Review of Chinese Herbal Medicine for the Treatment of Chronic Heart Failure. Curr. Pharm. Des. 23, 5115–5124. 10.2174/1381612823666170925163427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Xie N., Gao W., Kang R., Tang D. (2018). The Ferroptosis Inducer Erastin Promotes Proliferation and Differentiation in Human Peripheral Blood Mononuclear Cells. Biochem. biophysical Res. Commun. 503, 1689–1695. 10.1016/j.bbrc.2018.07.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ding Y., Wang X., Lu S., Wang C., He C., et al. (2018). Pseudolaric Acid B Triggers Ferroptosis in Glioma Cells via Activation of Nox4 and Inhibition of xCT. Cancer Lett. 428, 21–33. 10.1016/j.canlet.2018.04.021 [DOI] [PubMed] [Google Scholar]
- Wang W., Green M., Choi J. E., Gijón M., Kennedy P. D., Johnson J. K., et al. (2019). CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 569, 270–274. 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Zeng G.-Z., Yin J.-L., Bian Z.-X. (2019). Artesunate Activates the ATF4-CHOP-CHAC1 Pathway and Affects Ferroptosis in Burkitt's Lymphoma. Biochem. biophysical Res. Commun. 519, 533–539. 10.1016/j.bbrc.2019.09.023 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang Z., Li M., Wang F., Jia Y., Zhang F., et al. (2019). P53-dependent Induction of Ferroptosis Is Required for Artemether to Alleviate Carbon Tetrachloride-Induced Liver Fibrosis and Hepatic Stellate Cell Activation. IUBMB Life 71, 45–56. 10.1002/iub.1895 [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen Q., Shi C., Jiao F., Gong Z. (2019). Mechanism of Glycyrrhizin on Ferroptosis during Acute Liver Failure by Inhibiting Oxidative Stress. Mol. Med. Rep. 20, 4081–4090. 10.3892/mmr.2019.10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Zhang Z., Wang M., Cao X., Qi J., Wang D., et al. (2019). Role of GRP78 Inhibiting Artesunate-Induced Ferroptosis in KRAS Mutant Pancreatic Cancer Cells. Dddt 13, 2135–2144. 10.2147/dddt.s199459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Bi R., Quan F., Cao Q., Lin Y., Yue C., et al. (2020). Ferroptosis Involves in Renal Tubular Cell Death in Diabetic Nephropathy. Eur. J. Pharmacol. 888, 173574. 10.1016/j.ejphar.2020.173574 [DOI] [PubMed] [Google Scholar]
- Wei G., Sun J., Hou Z., Luan W., Wang S., Cui S., et al. (2018). Novel Antitumor Compound Optimized from Natural Saponin Albiziabioside A Induced Caspase-dependent Apoptosis and Ferroptosis as a P53 Activator through the Mitochondrial Pathway. Eur. J. Med. Chem. 157, 759–772. 10.1016/j.ejmech.2018.08.036 [DOI] [PubMed] [Google Scholar]
- Wen L., Shi D., Zhou T., Tu J., He M., Jiang Y., et al. (2020). Identification of Two Novel Prenylated Flavonoids in mulberry Leaf and Their Bioactivities. Food Chem. 315, 126236. 10.1016/j.foodchem.2020.126236 [DOI] [PubMed] [Google Scholar]
- Woo J. H., Shimoni Y., Yang W. S., Subramaniam P., Iyer A., Nicoletti P., et al. (2015). Elucidating Compound Mechanism of Action by Network Perturbation Analysis. Cell 162, 441–451. 10.1016/j.cell.2015.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Minikes A. M., Gao M., Bian H., Li Y., Stockwell B. R., et al. (2019). Intercellular Interaction Dictates Cancer Cell Ferroptosis via NF2-YAP Signalling. Nature 572, 402–406. 10.1038/s41586-019-1426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Yang J.-J., Cao Y., Li H., Zhao H., Yang S., et al. (2020). Iron Overload Contributes to General Anaesthesia-Induced Neurotoxicity and Cognitive Deficits. J. Neuroinflammation 17, 110. 10.1186/s12974-020-01777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., et al. (2016a). Ferroptosis: Process and Function. Cel Death Differ 23, 369–379. 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Song X., Sun X., Huang J., Zhong M., Lotze M. T., et al. (2016b). Identification of Baicalein as a Ferroptosis Inhibitor by Natural Product Library Screening. Biochem. biophysical Res. Commun. 473, 775–780. 10.1016/j.bbrc.2016.03.052 [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhu S., Song X., Sun X., Fan Y., Liu J., et al. (2017). The Tumor Suppressor P53 Limits Ferroptosis by Blocking DPP4 Activity. Cel Rep. 20, 1692–1704. 10.1016/j.celrep.2017.07.055 [DOI] [PubMed] [Google Scholar]
- Xu Y., Li X., Cheng Y., Yang M., Wang R. (2020). Inhibition of ACSL4 Attenuates Ferroptotic Damage after Pulmonary Ischemia‐reperfusion. FASEB j. 34, 16262–16275. 10.1096/fj.202001758r [DOI] [PubMed] [Google Scholar]
- Yagoda N., von Rechenberg M., Zaganjor E., Bauer A. J., Yang W. S., Fridman D. J., et al. (2007). RAS-RAF-MEK-dependent Oxidative Cell Death Involving Voltage-dependent Anion Channels. Nature 447, 864–868. 10.1038/nature05859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Karasawa T., Wakiya T., Sadatomo A., Ito H., Kamata R., et al. (2020a). Iron Overload as a Risk Factor for Hepatic Ischemia‐reperfusion Injury in Liver Transplantation: Potential Role of Ferroptosis. Am. J. Transpl. 20, 1606–1618. 10.1111/ajt.15773 [DOI] [PubMed] [Google Scholar]
- Yamada N., Komada T., Ohno N., Takahashi M. (2020b). Acetaminophen-induced Hepatotoxicity: Different Mechanisms of Acetaminophen-Induced Ferroptosis and Mitochondrial Damage. Arch. Toxicol. 94, 2255–2257. 10.1007/s00204-020-02722-5 [DOI] [PubMed] [Google Scholar]
- Yamada N., Karasawa T., Kimura H., Watanabe S., Komada T., Kamata R., et al. (2020c). Ferroptosis Driven by Radical Oxidation of N-6 Polyunsaturated Fatty Acids Mediates Acetaminophen-Induced Acute Liver Failure. Cel Death Dis 11, 144. 10.1038/s41419-020-2334-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Hsu J. L., Chen C.-T., Wang Y.-N., Hsu M.-C., Chang S.-S., et al. (2013). Caspase-independent Cell Death Is Involved in the Negative Effect of EGF Receptor Inhibitors on Cisplatin in Non-small Cell Lung Cancer Cells. Clin. Cancer Res. 19, 845–854. 10.1158/1078-0432.ccr-12-2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Kasukabe T., Kumakura S. (2018). Piperlongumine Rapidly Induces the Death of Human Pancreatic Cancer Cells Mainly through the Induction of Ferroptosis. Int. J. Oncol. 52, 1011–1022. 10.3892/ijo.2018.4259 [DOI] [PubMed] [Google Scholar]
- Yang W.-H., Ding C.-K. C., Sun T., Rupprecht G., Lin C.-C., Hsu D., et al. (2019). The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cel Rep. 28, 2501–2508. 10.1016/j.celrep.2019.07.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Antin P., Berx G., Blanpain C., Brabletz T., Bronner M., et al. (2020). Guidelines and Definitions for Research on Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cel Biol 21, 341–352. 10.1038/s41580-020-0237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.-H., Huang Z., Wu J., Ding C.-K. C., Murphy S. K., Chi J.-T. (2020). A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol. Cancer Res. 18, 79–90. 10.1158/1541-7786.mcr-19-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., Kim K. J., Gaschler M. M., Patel M., Shchepinov M. S., Stockwell B. R. (2016). Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 113, E4966–E4975. 10.1073/pnas.1603244113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., SriRamaratnam R., Welsch M. E., Shimada K., Skouta R., Viswanathan V. S., et al. (2014). Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 156, 317–331. 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., Stockwell B. R. (2016). Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biology 26, 165–176. 10.1016/j.tcb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., Stockwell B. R. (2008). Synthetic Lethal Screening Identifies Compounds Activating Iron-dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 15, 234–245. 10.1016/j.chembiol.2008.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen A., Yang F., Zhang X., Li F., Chen B., Faraag A. H. I., et al. (2021). Ferroptosis Inhibitory Constituents from the Fruits of Cullen Corylifolium. Nat. Prod. Res. 35, 5364–5368. 10.1080/14786419.2020.1762188 [DOI] [PubMed] [Google Scholar]
- Yoshida M., Minagawa S., Araya J., Sakamoto T., Hara H., Tsubouchi K., et al. (2019). Involvement of Cigarette Smoke-Induced Epithelial Cell Ferroptosis in COPD Pathogenesis. Nat. Commun. 10, 3145. 10.1038/s41467-019-10991-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef L. A., Rebbaa A., Pampou S., Weisberg S. P., Stockwell B. R., Hod E. A., et al. (2018). Increased Erythrophagocytosis Induces Ferroptosis in Red Pulp Macrophages in a Mouse Model of Transfusion. Blood 131, 2581–2593. 10.1182/blood-2017-12-822619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Yang C., Jian L., Guo S., Chen R., Li K., et al. (2019). Sulfasalazine-induced F-erroptosis in B-reast C-ancer C-ells I-s R-educed by the I-nhibitory E-ffect of E-strogen R-eceptor on the T-ransferrin R-eceptor. Oncol. Rep. 42, 826–838. 10.3892/or.2019.7189 [DOI] [PubMed] [Google Scholar]
- Yuan H., Li X., Zhang X., Kang R., Tang D. (2016). Identification of ACSL4 as a Biomarker and Contributor of Ferroptosis. Biochem. biophysical Res. Commun. 478, 1338–1343. 10.1016/j.bbrc.2016.08.124 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shi J., Liu X., Feng L., Gong Z., Koppula P., et al. (2018). BAP1 Links Metabolic Regulation of Ferroptosis to Tumour Suppression. Nat. Cel Biol 20, 1181–1192. 10.1038/s41556-018-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-H., Wang D.-W., Xu S.-F., Zhang S., Fan Y.-G., Yang Y.-Y., et al. (2018). α-Lipoic Acid Improves Abnormal Behavior by Mitigation of Oxidative Stress, Inflammation, Ferroptosis, and Tauopathy in P301S Tau Transgenic Mice. Redox Biol. 14, 535–548. 10.1016/j.redox.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wu Y., Yuan S., Zhang P., Zhang J., Li H., et al. (2018). Glutathione Peroxidase 4 Participates in Secondary Brain Injury through Mediating Ferroptosis in a Rat Model of Intracerebral Hemorrhage. Brain Res. 1701, 112–125. 10.1016/j.brainres.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Zhang X., Du L., Qiao Y., Zhang X., Zheng W., Wu Q., et al. (2019). Ferroptosis Is Governed by Differential Regulation of Transcription in Liver Cancer. Redox Biol. 24, 101211. 10.1016/j.redox.2019.101211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tan H., Daniels J. D., Zandkarimi F., Liu H., Brown L. M., et al. (2019). Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cel Chem. Biol. 26, 623–633. e9. 10.1016/j.chembiol.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Liu Z., Gao J., Li H., Wang X., Li Y., et al. (2020). Inhibition of Ferroptosis Attenuates Busulfan-Induced Oligospermia in Mice. Toxicology 440, 152489. 10.1016/j.tox.2020.152489 [DOI] [PubMed] [Google Scholar]
- Zheng D.-W., Lei Q., Zhu J.-Y., Fan J.-X., Li C.-X., Li C., et al. (2017). Switching Apoptosis to Ferroptosis: Metal-Organic Network for High-Efficiency Anticancer Therapy. Nano Lett. 17, 284–291. 10.1021/acs.nanolett.6b04060 [DOI] [PubMed] [Google Scholar]
- Zhou H., Yin C., Zhang Z., Tang H., Shen W., Zha X., et al. (2020). Proanthocyanidin Promotes Functional Recovery of Spinal Cord Injury via Inhibiting Ferroptosis. J. Chem. Neuroanat. 107, 101807. 10.1016/j.jchemneu.2020.101807 [DOI] [PubMed] [Google Scholar]
- Zhou J., Chan L., Zhou S. (2012). Trigonelline: a Plant Alkaloid with Therapeutic Potential for Diabetes and central Nervous System Disease. Cmc 19, 3523–3531. 10.2174/092986712801323171 [DOI] [PubMed] [Google Scholar]
- Zhu H.-Y., Huang Z.-X., Chen G.-Q., Sheng F., Zheng Y.-S. (2019). Typhaneoside Prevents Acute Myeloid Leukemia (AML) through Suppressing Proliferation and Inducing Ferroptosis Associated with Autophagy. Biochem. biophysical Res. Commun. 516, 1265–1271. 10.1016/j.bbrc.2019.06.070 [DOI] [PubMed] [Google Scholar]
- Zhu S., Zhang Q., Sun X., Zeh H. J., Lotze M. T., Kang R., et al. (2017). HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 77, 2064–2077. 10.1158/0008-5472.can-16-1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.