Highlights
-
•
GLP-1 receptor agonists are neuroprotective and disease-modifying in PD animal models.
-
•
This is true for both histological measures and motor outcomes.
-
•
There is sufficient evidence for the creation of more human clinical trials.
Keywords: Parkinson’s Disease, GLP-1 receptor agonist, Type 2 Diabetes Mellitus
Abstract
Background
Parkinson’s Disease (PD) is a progressive neurodegenerative condition associated with significant morbidity. Currently, there are limited pharmacological options and none of the therapies available are disease-modifying. This systematic review and meta-analysis considers a novel drug class through the research question – in pre-clinical rodent models of PD, is GLP-1 receptor agonist therapy neuroprotective when compared to vehicle controls?
Methods
A literature search was conducted to locate relevant pre-clinical studies. Two separate outcomes were considered. The primary outcome was indicators of dopaminergic neurotransmission. The secondary outcome was indicators of motor symptoms. Untreated PD models were compared to PD-models treated with GLP-1 receptor agonists. The final meta-analysis was conducted using the Cochrane RevMan software and represented continuous data using the inverse variance statistical method and random effects analysis model. The final study statistic was represented as an SMD value with a p-value < 0.05 considered statistically significant. Study heterogeneity and publication bias was assessed using I2 values and funnel plots respectively.
Results
Eleven studies fit the inclusion criteria and were included in the final analysis. For the primary outcome (n = 128), there was a statistically significant relative improvement of dopaminergic neurotransmission (SMD 1.71, 95% CI = 0.74–2.68, p = 0.0005, I2 = 76%). For the secondary outcome (n = 280), there was a statistically significant improvement in motor outcomes (SMD 2.11, 95% CI = 1.14–3.09, p < 0.0001, I2 = 89%).
Conclusions
GLP-1 receptor agonist therapy is neuroprotective in pre-clinical models of PD. This study provides the clinical foundation and research support for the design of rigorous clinical trials to further investigate these results in human PD populations.
1. Introduction
Parkinson’s Disease (PD) is a progressive neurodegenerative condition characterised by the cardinal motor symptoms of bradykinesia, rigidity, tremor and postural stability [1]. PD is pathologically characterised by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the midbrain resulting in depletion of striatal dopamine and a disruption of the direct and indirect nigrostriatal motor pathways [2]. Vitally, the condition is associated with significant morbidity, motor disability and loss of independence.
Currently, there are no proven effective disease-modifying treatments and even the most potent options are purely symptomatic. None of the available therapeutic strategies impact the extent of dopaminergic cell loss or the underlying pathological processes of PD. Consequently, they are not disease-modifying, do not alter the prognostic course of the disease and are less effective against the non-motor features of PD [3]. In response to the therapeutic challenges of PD, alternative novel treatments are highly sought after and investigated. Accordingly, this systematic review and meta-analysis will consider the pre-clinical data available for the neuroprotective efficacy of the glucagon-like peptide-1 (GLP-1) receptor agonist drug class.
Within the Australian context, GLP-1 receptor agonists are currently approved by the Therapeutic Goods Administration (TGA) for the use of type 2 diabetes mellitus (T2DM). GLP-1 receptor agonists are well tolerated, and the side effects are predominantly gastrointestinal and injection-site reactions [4]. However, the mechanism of action of GLP-1 is unknown and the neuroprotective effects of GLP-1 is still being established. The hypothesis for the efficacy of GLP-1 receptor agonists as neuroprotective agents is founded on the evidence of the shared pathophysiology in PD and T2DM and the expression of GLP-1 receptors in brain tissue [5], [6], [7]. In line with the hypothesis regarding the potential neuroprotective effects of GLP-1 receptor agonists, many pre-clinical animal studies have been conducted over the last decade. Systematic reviews have inconclusively summarised the main findings of these studies and, to this date, no meta-analyses have been conducted to quantify the overall effect size from these various independent studies. It is important to note that whilst meta-analyses are uncommonly performed on pre-clinical data, they are invaluable in providing succinct information that can guide decision making in the study design of human trials.
Accordingly, this systematic review and meta-analysis will address the research question – in pre-clinical rodent models of Parkinson’s Disease, is GLP-1 receptor agonist therapy neuroprotective when compared to vehicle controls? Two outcomes will be considered. The primary outcome will focus on pathological evidence of preservation or improvement of dopaminergic neurotransmission. The second outcome will evaluate study motor outcomes. The results of each outcome will be pooled separately to determine overall effect sizes for the pre-clinical research that is currently available. Implications for future practice will be discussed in accordance.
2. Methodology
2.1. Literature search strategy
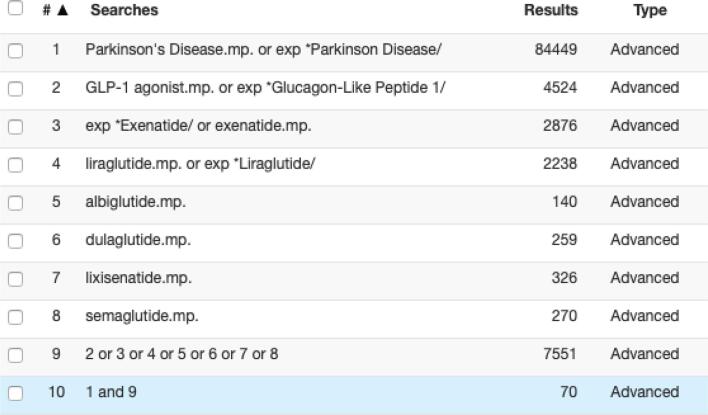
This meta-analysis considers the research question – in pre-clinical rodent models of Parkinson’s Disease, is GLP-1 receptor agonist therapy neuroprotective when compared to vehicle controls? Relevant studies were identified through database searches in Ovid Medline, PubMed, Clinicaltrials.gov, World Health Organisation International Clinical Trials Registry, Cochrane Library and Embase. Relevant search terms included: Parkinson’s Disease, GLP-1 agonist, exenatide, liraglutide, albiglutide, dulaglutide, lixisenatide and semaglutide. An example of the search strategy is presented in Fig. 1. The search was limited to articles available in English but was not limited by date of publication. Further manual searching involved screening individual articles for relevant references and the 500 most relevant items in Google Scholar. Study abstracts were reviewed for the relevance to the use of GLP-1 receptor agonists in PD. Duplicates were removed. Full text articles were then thoroughly assessed for eligibility based on the following selection criteria.
Fig. 1.
Search strategy in Ovid Medline.
2.2. Selection criteria
Only pre-clinical original research articles were considered. The final inclusion criteria were: 1) GLP-1 administration must be a primary intervention, 2) the GLP-1 receptor agonist specified must be Therapeutic Goods Administration (TGA) approved for T2DM, 3) the PD model must be a toxin-only model that involved administration of either 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone or 6-hydroxydopamine (6-OHDA), 4) PD toxin administration must occur prior to or simultaneous to drug therapy, 5) the PD model must be a full-lesion model, and 6) the rodent population must be normal-weight and non-diabetic.
2.3. Data extraction and critical appraisal
Two separate outcomes were considered. The primary outcome was indicators of dopaminergic neurotransmission. This included measures of striatal dopamine, tyrosine hydroxylase (TH) positive neurons in the substantia nigra pars compacta (SNpc) and the TH+ optical density in the SNpc. The secondary outcome was indicators of motor behavioural assessments. This included open-field tests, rotarod tests, catalepsy tests and apomorphine-induced rotations. For both outcomes, data was extracted from the article text, tables and figures. The data was recorded as the mean ± standard error of the mean (SEM) prior to statistical analysis. For papers where the data was not numerically presented and the authors did not return correspondence, the mean and SEM were extracted from figures using the Web Plot Digitizer software [8].
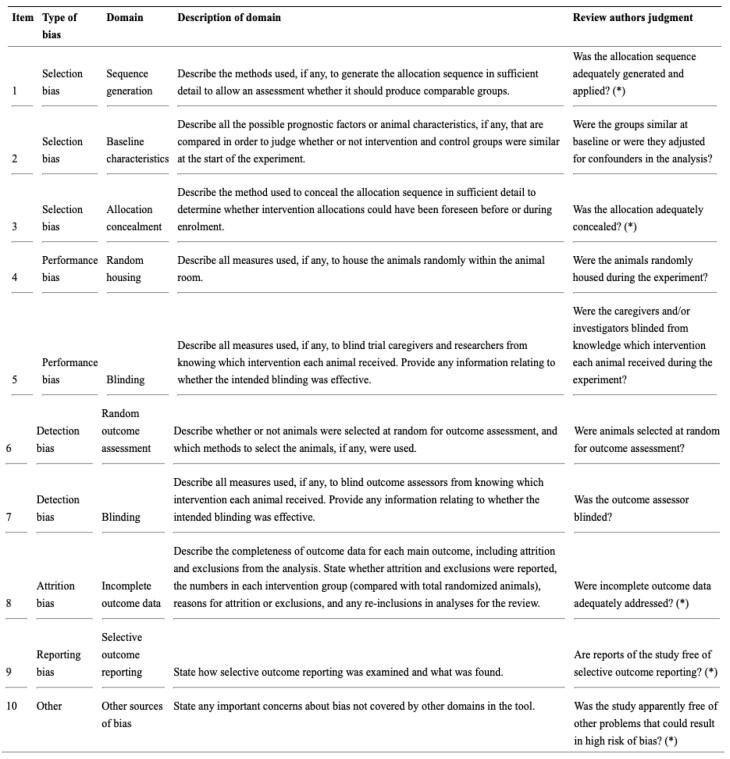
Studies were critically appraised for bias using the SYRCLE’s Risk of Bias (RoB) tool (Fig. 6), developed for animal studies as an adapted version of the Cochrane RoB tool [9]. The tool is qualitative and includes evaluation of selection bias, performance bias, detection bias, attrition bias and reporting bias. For the purposes of this meta-analysis, bias in each domain was qualitatively assessed as ‘high,’ ‘low,’ or ‘unclear.’ Studies with more than two ‘high’ bias characteristics were excluded.
Fig. 6.
SYRCLE’s Risk of Bias tool [9].
2.4. Statistical analysis
The meta-analysis was conducted using the Cochrane Review Manager (RevMan) Computer Program, Version 5.4 [10]. Continuous data was represented using the inverse variance statistical method and random effects analysis model to account for differences in study design. The effect measure chosen was standard mean difference (SMD) with 95% confidence intervals (CI). Mean and SD values were entered in RevMan for forest plot generation. For studies that compared multiple GLP-1 intervention groups to the PD control group for the same outcome, an overall intervention group mean and SD was calculated using the RevMan calculator function. For studies with the same intervention groups measuring multiple outcomes, the single most appropriate outcome from each study was chosen. This outcome was chosen with the investigator blinded to the results to prevent selection bias, and the rational for the outcomes is explained in Table 1, Table 2. This was done to prevent over-estimation of the weight of each individual study through non-independent study outcomes. Sub-group analysis was not statistically significant and was not included for either outcome. The final data was represented in separate forest plots for the outcomes. To test for study heterogeneity, the I2 value was referred to with an I2 value > 50% considered high heterogeneity. Publication bias was identified as a potential source of error through funnel plots, generated by RevMan. The final study statistic was represented as an SMD value with a p-value < 0.05 considered statistically significant.
Table 1.
Study characteristics for the primary outcome – indicators of dopaminergic neurotransmission.
| Study | Rodent | PD model | GLP-1 receptor agonist intervention | Outcome (unit of measurement) | Included/not included in meta-analysis with rationale |
|---|---|---|---|---|---|
| Zhang et al. 2019 | Mouse | MPTP (20 mg/kg/day i.p. for 30 days) | Simultaneous to PD-model induction:Semaglutide (25 nmol/kg every two days i.p. for 30 days);Liraglutide (25 nmol/kg/day i.p. for 30 days) | TH + neurons in the SNpc (cell count) | Yes – primary outcome within the study |
| Badawi et al. 2019 | Rat | Rotenone (3 mg/kg/day, s.c. for 10 days) | Following PD-model induction:Liraglutide (50 μg/kg/day s.c. for 16 days) | Striatal dopamine content (ng/mg of protein) | Yes – primary outcome within the study |
| Zhang et al. 2018 | Mouse | MPTP (20 mg/kg/day i.p. for 7 days) | Following PD-model induction:Semaglutide (25 nmol/kg every two days i.p. for 30 days);Liraglutide (25 nmol/kg/day i.p. for 30 days) | TH + neurons in the SNpc (% of non-PD vehicle control) | Yes – PD symptoms primarily caused by degeneration of dopaminergic neurons in the SNpc |
| TH + striatal optical density (% of non-PD vehicle control) | No – non-independent secondary study outcome | ||||
| Aksoy et al. 2017 | Rat | Rotenone (stereotaxical infusion of 3ug/ul in the left SNpc and the ventral tegmental area) | Following PD-model induction:Exenatide (30 ug/kg/day i.p. for 28 days) | No relevant outcomes | No – no relevant outcomes |
| Badawi et al. 2017 | Rat | Rotenone (3 mg/kg/day s.c. for 10 days) | Following PD-model induction:Liraglutide (50 μg/kg/day s.c. for 16 days) | Striatal dopamine content (ng/mg of protein) | No – non-independent secondary study outcome |
| TH + neurons in the SNpc (% of non-PD vehicle control) | Yes – PD symptoms primarily caused by degeneration of dopaminergic neurons in the SNpc | ||||
| Hansen et al. 2016 | Rat | 6-OHDA (stereotaxical infusion of 13.5 ug in the right medial forebrain bundle) | Following PD-model induction:Liraglutide (500 ug/kg/day s.c for 6 weeks) | TH + neurons in the SNpc (cell count) | Yes – primary outcome within the study |
| Harkavyi et al. 2008 | Rat | 6-OHDA (stereotaxical infusion of 8ug/4ul in the right medial forebrain bundle) | Following PD-model induction:Exendin-4 (0.5 ug/kg twice daily i.p. for 7 days) | Striatal dopamine content (pg/g of protein) | Yes – primary outcome within the study |
| Zhang et al. 2020 | Rat | 6-OHDA (stereotaxical infusion of 5uL in the right medial forebrain bundle) | Following PD-model induction:Exendin-4 (10 nmol/kg/day i.p. for 30 days) | Striatal dopamine content (pg/mg of protein) | No – non-independent secondary study outcome |
| TH + neurons in the SNpc (cell count) | Yes – PD symptoms primarily caused by degeneration of dopaminergic neurons in the SNpc | ||||
| Feng et al. 2018 | Mouse | MPTP (25 mg/kg/2h i.p. for 8 h) | Following PD-model induction:Liraglutide (25 nmol/kg/day i.p. for 6 days) | TH + density in the SNpc (optical density) | Yes – primary outcome within the study |
| Yuan et al. 2017 | Mouse | MPTP (25 mg/kg/day i.p. for 7 days) | Following PD-model induction:Liraglutide (25 nmol/kg/day i.p. for 7 days) | TH + density in the SNpc (optical density) | Yes – PD symptoms primarily caused by degeneration of dopaminergic neurons in the SNpc |
| TH + density in the striatum (optical density) | No – non-independent secondary study outcome | ||||
| Liu et al. 2015 | Mouse | MPTP (20 mg/kg/day i.p. for 7 days) | Simultaneous to PD-model induction:Liraglutide (25 nmol/kg/day i.p. for 14 days);Lixisenatide (10 nmol/kg/day i.p. for 14 days);Exendin-4 (10 nmol/kg/day i.p. for 14 days) | TH + neurons in the SNpc (cell count) | Yes – PD symptoms primarily caused by degeneration of dopaminergic neurons in the SNpc |
| TH + density in the striatum (optical density) | No – non-independent secondary study outcome |
Table 2.
Distribution of study characteristics for the primary outcome.
| Number of studies | ||
| PD model |
6-OHDA | 3 |
| MPTP | 5 | |
| Rotenone | 2 | |
| GLP-1 receptor agonist intervention | Liraglutide | 8 |
| Exenatide/exendin-4 | 3 | |
| Semaglutide | 2 | |
| Lixisenatide | 1 | |
| Outcome | TH + neurons in the SNpc | 6 |
| Striatal dopamine content | 2 | |
| TH + density in the SNpc | 2 | |
3. Results
3.1. Search results
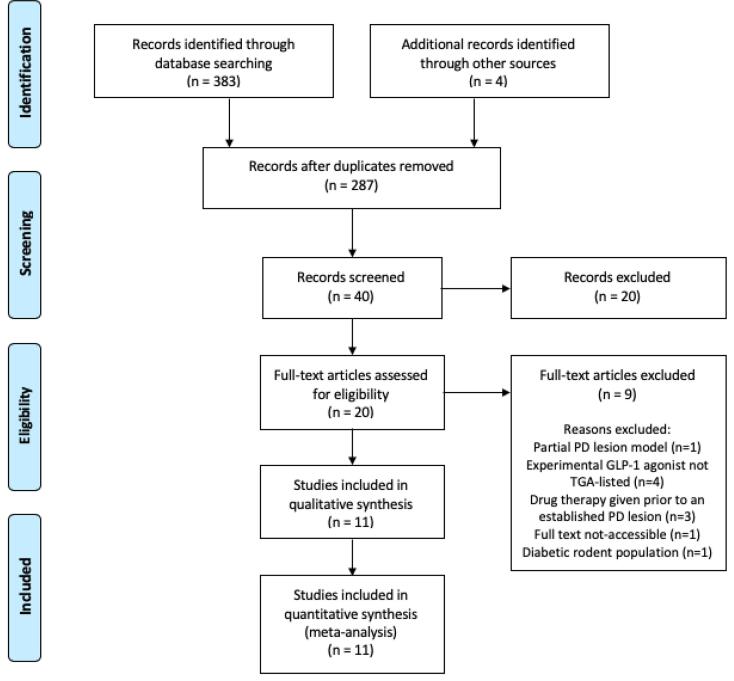
The search strategy resulted in 387 articles. 40 relevant records were screened for their relevance to GLP-1 receptor agonist use in PD. The final number of studies included in the analysis was 11 (Fig. 2) [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Articles were mainly excluded due to study design variations. Partial PD lesion models and study designs in which drug therapy was given prior to establishing a PD lesion were eliminated as the study is specific for the effect of GLP-1 receptor agonists on established PD lesions. Diabetic rodent populations were excluded due to potential additional mechanistic factors. Experimental GLP-1 receptor agonists with an unknown human safety profile that are not TGA-listed were excluded to optimise the applicability of this review to Phase IV human clinical trials.
Fig. 2.
PRISMA flow chart of search strategy and study selection [30].
3.2. Characteristics of included studies
3.2.1. Dopaminergic neurotransmission outcomes
Table 1 displays the study characteristics of studies included in the primary outcome measuring indicators of dopaminergic neurotransmission. As the pathogenesis of PD is typically characterised by dopaminergic neuron degeneration in the SNpc, study outcomes were chosen accordingly. TH is the rate-limiting enzyme in the synthesis of dopamine and is hence used as the marker for dopaminergic neurons in pre-clinical studies [2]. Chosen study outcomes thus included cell count of TH + neurons in the SNpc and optical density of TH + density in the SNpc. In studies that lacked a measure of SNpc dopaminergic neurotransmission, striatal dopamine was considered.
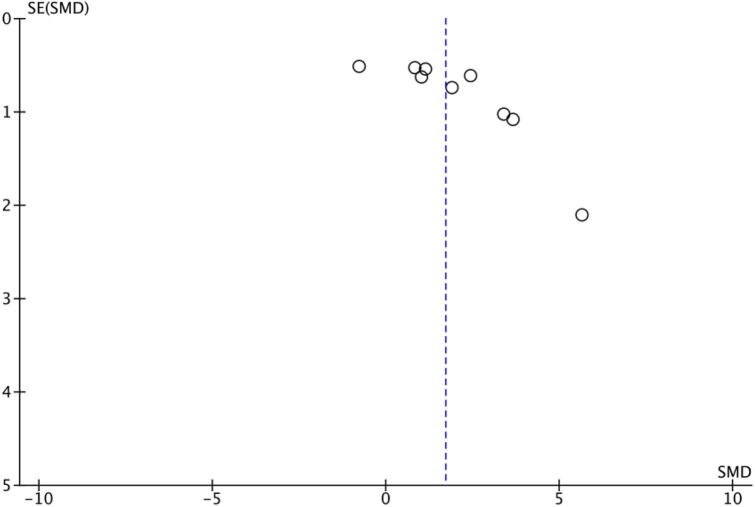
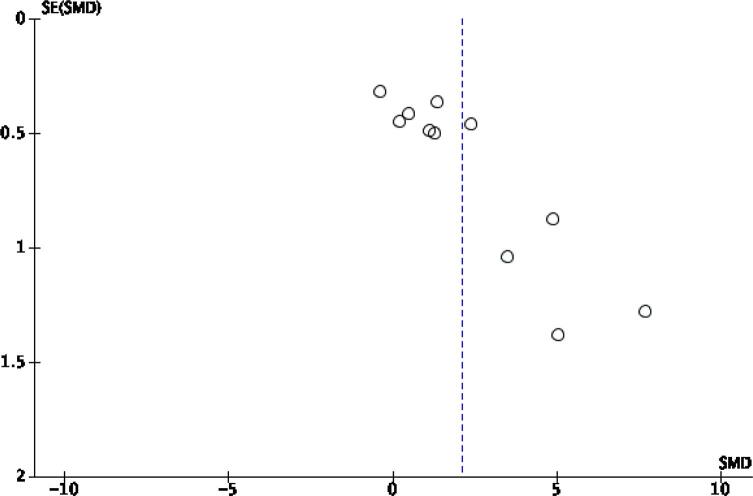
Table 2 summarises the distribution of variables within the studies – PD model choice, GLP-1 receptor agonist therapy choice and dopaminergic neurotransmission outcomes. To determine the publication bias of studies, the funnel plot generated for these outcomes is displayed in Fig. 3. Visual inspection of the funnel plot shows apparent asymmetry, suggesting the presence of likely publication bias or high study heterogeneity.
Fig. 3.
Funnel plot for the primary outcome.
3.2.2. Motor assessment outcomes
Table 3 displays the study characteristics of studies included in the secondary outcome considering motor symptoms. The study outcomes considered include the open-field test, the rotarod test, the apomorphine-induced rotations test and the catalepsy test. The open-field test is a measure of general locomotor activity, recording ambulations over a period of time [11]. The rotarod test is a more specific measure of motor coordination, specifically bradykinesia and imbalance [11]. Similarly, the catalepsy test is also a more specific test focusing on the symptoms of akinesia and rigidity [15]. The apomorphine-induced rotations test does not represent a specific Parkinsonian symptom but is the best indicator of motor impairment in unilateral full lesion models by determining the severity of the lesion [16]. Other tests were less commonly performed and were excluded from the meta-analysis.
Table 3.
Study characteristics for the secondary outcome – indicators of motor symptoms.
| Study | Rodent | PD model | GLP-1 receptor agonist intervention | Outcome (unit of measurement) | Included/not included in meta-analysis with rationale |
|---|---|---|---|---|---|
| Zhang et al. 2019 | Mouse | MPTP (20 mg/kg/day i.p. for 30 days) | Simultaneous to PD-model induction:Semaglutide (25 nmol/kg every two days i.p. for 30 days);Liraglutide (25 nmol/kg/day i.p. for 30 days) | Open field test (metres/10 min) | Yes – indicator of general locomotor and exploratory activity |
| Rotarod test (s) | No – non-independent secondary study outcome | ||||
| Grip strength (N) | No – non-independent secondary study outcome | ||||
| Stride variability (cm) | No – non-independent secondary study outcome | ||||
| Badawi et al. 2019 | Rat | Rotenone (3 mg/kg/day, s.c. for 10 days) | Following PD-model induction:Liraglutide (50 μg/kg/day s.c. for 16 days) | Open field test (ambulations/5 min) | Yes – primary outcome within the study |
| Zhang et al. 2018 | Mouse | MPTP (20 mg/kg/day i.p. for 7 days) | Following PD-model induction:Semaglutide (25 nmol/kg every two days i.p. for 7 days);Liraglutide (25 nmol/kg/day i.p. for 7 days) | Open field test (cm/10 min) | Yes – indicator of general locomotor and exploratory activity |
| Rotarod test (s) | No – non-independent secondary study outcome | ||||
| Stride variability (cm) | No – non-independent secondary study outcome | ||||
| Aksoy et al. 2017 | Rat | Rotenone (stereotaxical infusion of 3ug/ul in the left SNpc and the ventral tegmental area) | Following PD-model induction:Exenatide (30 ug/kg/day i.p. for 28 days) | Apomorphine-induced rotations (turns/10 min) | Yes – primary outcome within the study |
| Badawi et al. 2017 | Rat | Rotenone (3 mg/kg/day s.c. for 10 days) | Following PD-model induction:Liraglutide (50 μg/kg/day s.c. for 16 days) | Cylindrical test (rears/5 min) | No – non-independent secondary study outcome |
| Catalepsy test (s) | Yes – the catalepsy test is better reflective of Parkinsonian symptoms | ||||
| Hansen et al. 2016 | Rat | 6-OHDA (stereotaxical infusion of 13.5 ug in the medial forebrain bundle) | Following PD-model induction:Liraglutide (500 ug/kg/day s.c for 6 weeks) | Apomorphine-induced rotations (turns/15 min) | Yes – primary outcome within the study |
| Harkavyi et al. 2008 | Rat | 6-OHDA (stereotaxical infusion of 8ug/4ul in the right medial forebrain bundle) | Following PD-model induction:Exendin-4 (0.5 ug/kg twice daily i.p. for 7 days) | Apomorphine-induced rotations (turns/15 min) | Yes – primary outcome within the study |
| Zhang et al. 2020 | Rat | 6-OHDA (stereotaxical infusion of 5uL in the right medial forebrain bundle) | Following PD-model induction:Exendin-4 (10 nmol/kg/day i.p. for 30 days) | Open field test (cm/10 min) | No – non-independent secondary study outcome |
| Apomorphine-induced rotations (turns/30 min) | Yes – the apomorphine-induced rotations test is a better indicator of PD severity in unilateral lesion models | ||||
| Feng et al. 2018 | Mouse | MPTP (25 mg/kg/2h i.p. for 8 h) | Following PD-model induction:Liraglutide (25 nmol/kg/day i.p. for 6 days) | Rotarod test (s) | Yes – primary outcome within the study |
| Yuan et al. 2017 | Mouse | MPTP (25 mg/kg/day i.p. for 7 days) | Following PD-model induction:Liraglutide (25 nmol/kg/day i.p. for 7 days) | Rotarod test (s) | Yes – primary outcome within the study |
| Liu et al. 2015 | Mouse | MPTP (20 mg/kg/day i.p. for 7 days) | Simultaneous to PD-model induction:Liraglutide (25 nmol/kg/day i.p. for 14 days);Lixisenatide (10 nmol/kg/day i.p. for 14 days);Exendin-4 (10 nmol/kg/day i.p. for 14 days) | Open field test (metres/10 min) | No – non-independent secondary study outcome |
| Rotarod test (s) | Yes – data best presented for this variable (average for multiple trials given) | ||||
| Catalepsy test (s) | No – non-independent secondary study outcome |
Table 4 summarises the distribution of variables within the studies – PD model choice, GLP-1 receptor agonist therapy choice and motor test. The funnel plot generated for these outcomes is displayed in Fig. 4. Like the primary outcome, the funnel plot for the secondary outcome is visually asymmetrical indicating the presence of publication bias or high study heterogeneity.
Table 4.
Distribution of study characteristics for the secondary outcome.
| Number of studies | ||
| PD model | 6-OHDA | 3 |
| MPTP | 5 | |
| Rotenone | 3 | |
| GLP-1 receptor agonist intervention | Liraglutide | 8 |
| Exenatide/exendin-4 | 4 | |
| Semaglutide | 2 | |
| Lixisenatide | 1 | |
| Outcome | Open-field test | 3 |
| Rotarod test | 3 | |
| Apomorphine-induced rotations | 4 | |
| Catalepsy test | 1 | |
Fig. 4.
Funnel plot for the secondary outcome.
3.3. Meta-analysis for dopaminergic neurotransmission outcomes
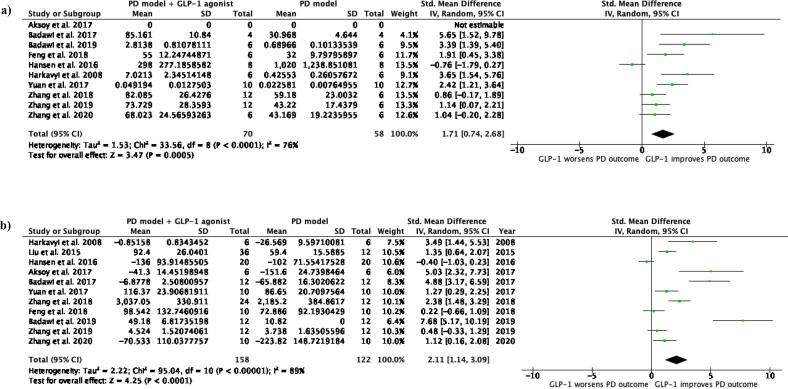
Based on 10 studies (n = 128), the forest plot for the primary outcome (Fig. 5a) revealed that GLP-1 receptor agonist therapy was neuroprotective in pre-clinical PD models, seen through a statistically significant relative improvement of dopaminergic neurotransmission (SMD 1.71, 95% CI = 0.74–2.68, p = 0.0005). The study heterogeneity is high (I2 76%, p < 0.0001) and subgroup analysis was statistically insignificant.
Fig. 5.
Forest plots comparing a) dopaminergic neurotransmission outcomes, and b) motor outcomes between PD models and PD models treated with a GLP-1 receptor agonist.
3.4. Meta-analysis for motor assessment outcomes
Based on 11 studies (n = 280), the forest plot for the secondary outcome (Fig. 5b) revealed that GLP-1 receptor agonist therapy was also neuroprotective in pre-clinical PD models when comparing motor outcomes (SMD 2.11, 95% CI = 1.14–3.09, p < 0.0001). Study heterogeneity is higher than the primary outcome (I2 89%, p < 00001) and subgroup analysis was statistically insignificant.
4. Discussion
4.1. Main findings
GLP-1 receptor agonist therapy is neuroprotective in pre-clinical rodent models of PD. As demonstrated in the forest plots, the overall effect size is positive for both motor outcomes and indicators of dopaminergic neurotransmission despite variations in study outcomes and study design. GLP-1 receptor agonist therapy was shown to increase both the presence of dopaminergic neurons and striatal dopamine indicating an improvement in the neurotransmission of the entire nigrostriatal pathway. The clinical impact of these pathological findings was considered through the secondary outcome assessing change in motor outcomes. There was a general trend of improvement reported for each separate motor outcome in most studies. Combining these parameters indicated an overall improvement in motor activity that encapsulates the varying motor symptoms of PD.
In some studies, the impact of GLP-1 receptor agonist therapy given following PD model induction showed a restoration of dopaminergic neurons comparable to the non-Parkinsonian vehicle controls. For instance, the 2018 study conducted by Zhang et al. evaluated the effect of liraglutide and semaglutide on the MPTP mouse model. On histological assessment, TH + neuron count was recorded and expressed as a percentage of the non-Parkinsonian vehicle control. Whilst the PD model without intervention had a mean dopaminergic neuronal percentage of 59.18% (SEM 9.391, p < 0.001 compared to non-PD control), this value increased to 75.61% (SEM 13.319, p < 0.01 compared to MPTP group) and 88.56% (SEM 7.869, p < 0.001 compared to MPTP group) with liraglutide and semaglutide treatment respectively [13]. In studies such as this one, GLP-1 receptor agonist therapy was initiated following the establishment of a PD lesion indicating that GLP-1 receptor agonist therapy may be disease-modifying and could alter the prognostic course and slow the progression of PD.
4.2. Study limitations
This review has limitations related to the statistical analysis of animal studies. First, the pooled forest plots for both outcomes have high heterogeneity values that are not resolved through subgroup analyses. The purpose of this meta-analysis was to consider a broader, clinically relevant research question than those proposed by the individual studies alone. For instance, considering the motor outcomes for rotarod test performance only, whilst resolving study heterogeneity values, is not as relevant for a clinician interested in overall motor improvement. Further, whilst many clinical symptoms in human trials are often standardised against rating scales and set protocols, animal studies are more flexible and less consistent in their study design. As a consequence, animal studies are likely to be inherently more heterogenous when considering variances in animal models, intervention protocols and chosen study outcomes [22]. In this review, three different PD model toxins were considered over two rodent species with variations in the study design for PD model induction and GLP-1 receptor agonist therapy. Further, distinct but related study outcomes were integrated into a more general assessment of behavioural and histological improvements. As a consequence, the high heterogeneity for these studies is explained by slight variations in study design but is considered appropriate given the clinical relevance of the broader questions asked. The high heterogeneity values also likely accounts for the asymmetry of the funnel plots [23]. However, publication bias in animal studies and studies performed with small sample sizes is common. Publication bias, consequently, must be considered as an influencing factor in the interpretation of the results of this meta-analysis.
Whilst being a necessary step in the general scheme of research, another limitation of animal studies is their shortcomings in modelling human pathology. Ideally, the model should reflect the main pathophysiological changes in PD. However, none of the included PD models in this meta-analysis completely replicate these pathologies [24], [25]. Further, animal models are also expected to mimic the symptomology of PD. Some motor symptoms were not directly translatable or assessable, such as tremor and postural stability. Non-motor symptoms were disregarded altogether as any animal study design would be unreliable in measuring factors such as mood and sleep disturbance. Hence, statistically significant results in animal studies may not translate to clinical significance due to limitations of the PD model. Nonetheless, animal studies are an invaluable resource that indicate the opportunity for further research and are often the first stage to constructing studies that confirm a clinically measurable effect of an intervention in human populations.
4.3. Implications for clinical practice
This meta-analysis, despite its limitations, has demonstrated that GLP-1 receptor agonist therapy is effective in reducing motor impairments and improving dopaminergic transmission in rodent models of PD. Importantly, the safety profiles of GLP-1 receptor agonists have already been identified and the drug class has been approved for human use. Consequently, there is sufficient research to justify human trials to determine the clinical outcomes of GLP-1 receptor agonist therapy in a human PD population.
Currently, the UCL Queen Square Institute of Neurology has been the only centre to address this available field of research and publish the outcomes of human clinical trials. Consistent with the results of this meta-analysis, the research team found that there was a statistically and clinically significant improvement observed for motor symptoms [26], [27], [28]. However, the evidence at this stage is insufficient to change clinical practice and further trials must be performed. If the results of future trials are successful, the approved use of GLP-1 receptor agonist therapy could be extended to include PD patients and GLP-1 receptor agonist therapy could become the first disease-modifying pharmacological option for PD.
4.4. Gaps in the literature
Currently, there are many gaps in the available literature that must be addressed before GLP-1 receptor agonist therapy is approved for use in PD patients. Primarily, there is a lack of information regarding long-term neurological outcomes. The longest pre-clinical study was run by Hansen et al. in 2016 and was conducted over 6 weeks [16]. Similarly, the longest human trial was conducted in 2013 and involved the administration of exenatide over 12 months, followed by a 12-week wash-out and follow-up outcomes measured at 24 months [26], [27]. There is also a lack of information regarding the side effect profile of GLP-1 receptor agonist use in non-diabetic populations. Weight loss was a significant finding in the published human trials and was seen to occur during administration and to reverse following discontinuation [27]. However, this evidence is limited. Some potential side effects, such as hypoglycaemia, have not been studied at all. Conversely, there is only a single pre-clinical study and no human clinical studies looking at the impact of the therapy in patients that have both T2DM and PD [29]. Both conditions are common, have a shared pathophysiological basis and are likely to co-exist. Further, given that the side effect profile is known to be tolerable in these patients, this would be a valuable sector of research. Within the Australian context, many patients may already be eligible for GLP-1 receptor agonist therapy under the Pharmaceutical Benefits Scheme (PBS) guidelines and if the drug class is proven to be effective, adjustment of their diabetic medications would be a simple task. Finally, studies that are available have limited information regarding non-motor symptom outcomes and are composed of small study numbers. Thus, to establish the efficacy of GLP-1 receptor agonist therapy in PD, a large double-blinded RCT must be conducted that administers the therapy over a longer period of time with long-term follow-up data. There must be a larger range of outcomes measured with an extensive analysis of recorded side effects.
References
- 1.K.L. Chou, Clinical manifestations of Parkinson disease. <https://www.uptodate.com/contents/clinical-manifestations-of-parkinson-disease?search=parkinsons%20disease%20adult&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1#H8> (accessed 01.09.2020).
- 2.J. Jankovic, Etiology and pathogenesis of Parkinson Disease. <https://www.uptodate.com/contents/etiology-and-pathogenesis-of-parkinson-disease?search=parkinsons%20disease%20adult&source=search_result&selectedTitle=5∼150&usage_type=default&display_rank=5> (accessed 01.09.2020).
- 3.Smith Y., Wichmann T., Factor S.A., DeLong M.R. Parkinson's disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology. 2012;37:213–246. doi: 10.1038/npp.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.H. Kelly, eTG complete > Type 2 diabetes in adults. <https://tgldcdp-tg-org-au.wwwproxy1.library.unsw.edu.au/viewTopic?topicfile=type-2-diabetes-in-adults§ionId=dbg1-c09-s4#tdbg1-c09-tbl2> (accessed 01.09.2020).
- 5.Khalilnezhan A., Taskiran D. The investigation of protective effects of glucagon-like peptide-1 (GLP-1) analogue exenatide against glucose and fructose-induced neurotoxicity. Int J Neurosci. 2019;129:481–491. doi: 10.1080/00207454.2018.1543671. [DOI] [PubMed] [Google Scholar]
- 6.Yue X., Li H., Yan H., Zhang P., Chang L., Tong L. Risk of Parkinson Disease in Diabetes Mellitus: An Updated Meta-Analysis of Population-Based Cohort Studies. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cereda E., Barichella M., Pedrolli C., Klersy C., Cassani E., Caccianlanza R., Pezzoli G. Diabetes and risk of Parkinson's disease: a systematic review and meta-analysis. Diabetes Care. 2011;34:2614–2623. doi: 10.2337/dc11-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A. Rohatgi, WebPlotDigitzer. <https://automeris.io/WebPlotDigitizer/citation.html> (accessed 01.09.2020).
- 9.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Lagendam M.W. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.[Computer Program]. Review Manager (RevMan) Version 5.4. The Cochrane Collaboration, 2020.
- 11.Zhang L., Zhang LingYu, Li L., Hölscher C. Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson's Disease. J Parkinsons Dis. 2019;9(1):157–171. doi: 10.3233/JPD-181503. [DOI] [PubMed] [Google Scholar]
- 12.Badawi G.A., Abd El Fattah M.A., Zaki H.F., El Sayed M.I. Sitagliptin and Liraglutide Modulate L-dopa Effect and Attenuate Dyskinetic Movements in Rotenone-Lesioned Rats. Neurotox Res. 2019;35(3):635–653. doi: 10.1007/s12640-019-9998-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Zhang L., Li L., Hölscher C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson's disease mouse model. Neuropeptides. 2018;71:70–80. doi: 10.1016/j.npep.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Aksoy D., Solmaz V., Çavuşoğlu T., Meral A., Ateş U., Erbaş O. Neuroprotective Effects of Eexenatide in a Rotenone-Induced Rat Model of Parkinson's Disease. Am J Med Sci. 2017;354(3):319–324. doi: 10.1016/j.amjms.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Badawi G.A., Abd El Fattah M.A., Zaki H.F., El Sayed M.I. Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson's disease. Inflammopharmacology. 2017;25(3):369–382. doi: 10.1007/s10787-017-0331-6. [DOI] [PubMed] [Google Scholar]
- 16.Hansen H.H., Fabricius K., Barkholt P., Mikkelsen J.D., Jelsing J., Pyke C., Knudsen L.B., Vrang N. Characterization of liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in rat partial and full nigral 6-hydroxydopamine lesion models of Parkinson's disease. Brain Res. 2016;1646:354–365. doi: 10.1016/j.brainres.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Harkavyi A., Abuirmeileh A., Lever R., Kingsbury A.E., Biggs C.S., Whitton P.S. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J Neuroinflammation. 2008;5:19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Jin Q., Lin L. The GLP-1/GIP dual receptor agonist DA5-CH is superior to Exendin-4 in protecting neurons in the 6-OHDA rat Parkinson Model. ResearchGate. 2020 [Google Scholar]
- 19.Feng P., Zhang X., Li D., Ji C., Yuan Z., Wang R., Xue G., Li G., Hölscher C. Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson's disease. Neuropharmacology. 2018;133:385–394. doi: 10.1016/j.neuropharm.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Z., Li D., Feng P., Xue G., Ji C., Li G., Hölscher C. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson's disease. Eur J Pharmacol. 2017;812:82–90. doi: 10.1016/j.ejphar.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Liu W., Jalewa J., Sharma M., Li G., Li L., Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience. 2015;303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Hooijmans C.R., IntHout J., Ritskes-Hoitinga M., Rovers M.M. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. Ilar j. 2014;55:418–426. doi: 10.1093/ilar/ilu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.J.A.C. Sterne, A.J. Sutton, J.P.A. Ionnaidis, N. Terrin, D.R. Jones, J. Lau, J. Carpenter, G. Rucker, R.M. Harbord, C.H. Schmid, J. Tetzlaff, J.J. Deeks, J. Peters, P. Macaskill, G. Schwarzer, S. Duval, D.G. Altman, D. Moher, J.P.T. Higgins, Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials, BMJ 343 (2011) d4002. [DOI] [PubMed]
- 24.Potashkin J.A., Blume S.R., Runkle N.K. Limitations of animal models of Parkinson's disease. Parkinsons Dis. 2010;2011 doi: 10.4061/2011/658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konnova E.A., Swanberg M. In: Parkinson’s Disease: Pathogenesis and Clinical Aspects. Stoker T.B., Greenland J.C., editors. Codon Publications; 2018. Animal Models of Parkinson’s Disease; pp. 83–106. [Google Scholar]
- 26.Aviles-Olmos I., Dickson J., Kefalopoulou Z., Djamshidian A., Ell P., Soderlund T., Whitton P., Wyse R., Isaacs T., Lees A., Limousin P., Foltynie T. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123(6):2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aviles-Olmos I., Dickson J., Kefalopoulou Z., Djamshidian A., Kahan J., Ell P., Whitton P., Wyse R., Isaacs T., Lees A., Limousin P., Foltynie T. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson's disease. J Parkinsons Dis. 2014;4(3):337–344. doi: 10.3233/JPD-140364. [DOI] [PubMed] [Google Scholar]
- 28.Athauda D., Maclagan K., Skene S.S., Bajwa-Joseph M., Letchford D., Chowdhury K., Hibbert S., Budnik N., Zampedri L., Dickson J., Li Y., Aviles-Olmos I., Warner T., Limousin P., Lees A.J., Greig N.H., Tebbs S., Foltynie T. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. The Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbassuoni E.A., Ahmed R.F. Mechanism of the neuroprotective effect of GLP-1 in a rat model of Parkinson's with pre-existing diabetes. Neurochem Int. 2019;131 doi: 10.1016/j.neuint.2019.104583. [DOI] [PubMed] [Google Scholar]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]