Abstract
STUDY QUESTION
Does age at natural menopause increase with increasing of number of childbirths?
SUMMARY ANSWER
Age at menopause increased with increasing number of childbirths up to three childbirths; however, we found no further increase in age at menopause beyond three childbirths.
WHAT IS KNOWN ALREADY
Pregnancies interrupt ovulation, and a high number of pregnancies have therefore been assumed to delay menopause. Previous studies have had insufficient statistical power to study women with a high number of childbirths. Thus, the shape of the association of number of childbirths with age at menopause remains unknown.
STUDY DESIGN, SIZE, DURATION
A retrospective population study of 310 147 women in Norway who were 50–69 years old at data collection.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The data were obtained by two self-administered questionnaires completed by women attending BreastScreen Norway, a population-based screening program for breast cancer. The associations of number of childbirths with age at menopause were estimated as hazard ratios by applying Cox proportional hazard models, adjusting for the woman’s year of birth, cigarette smoking, educational level, country of birth, oral contraceptive use and body mass index.
MAIN RESULTS AND THE ROLE OF CHANCE
Women with three childbirths had the highest mean age at menopause (51.36 years; 95% CI: 51.33–51.40 years), and women with no childbirths had the lowest (50.55 years; 95% CI: 50.48–50.62 years). Thus, women with no childbirths had higher hazard ratio of reaching menopause compared to women with three childbirths (reference group) (adjusted hazard ratio, 1.24; 95% CI: 1.22–1.27). Beyond three childbirths, we estimated no further increase in age at menopause. These findings were confirmed in sub-analyses among (i) women who had never used hormonal intrauterine device and/or systemic menopausal hormonal therapy; (ii) women who were born before 1950 and (iii) women who were born in 1950 or after.
LIMITATIONS, REASONS FOR CAUTION
Information about age at menopause was based on self-reports.
WIDER IMPLICATIONS OF THE FINDINGS
If pregnancies truly delay menopause, one would expect that women with the highest number of childbirths had the highest age at menopause. Our results question the assumption that interrupted ovulation during pregnancy delays menopause.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the South-Eastern Norway Regional Health Authority [2016112 to M.S.G.] and by the Norwegian Cancer Society [6863294-2015 to E.K.B.]. The authors declare no conflicts of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: childbirth, menopause, parity, population study, pregnancy, risk factors
Introduction
Women reach menopause at very different ages (Schoenaker et al., 2014). Although genetic factors play a role (Voorhuis et al., 2010), the underlying mechanisms for the large variation in age at natural menopause are not well understood (Ginsberg, 1991). Identification of factors that are associated with the timing of menopause may improve our understanding of the biological mechanisms that ultimately lead to menopause. A woman’s age at menopause is associated with her subsequent risk of disease. Late menopause increases the risk of breast cancer and endometrial cancer (Xu et al., 2004; Collaborative Group on Hormonal Factors in Breast Cancer, 2012) and reduces the risk of cardiovascular disease, osteoporosis and early death (Gallagher, 2007; Muka et al., 2016). Thus, knowledge about factors associated with age at menopause may also increase our understanding of the causes of common diseases in women.
Menopause occurs when the number of ovarian follicles reaches a critically low level (Faddy et al., 1992). Factors that influence the rate of follicle atresia may therefore influence the age at menopause. Since pregnancy interrupts ovulation, ovarian follicles are assumed to be preserved during pregnancy. Thus, it has been proposed that menopause is delayed by the number of pregnancies (Cramer et al., 1995; Cramer and Xu, 1996).
Some studies support the hypothesis that age at natural menopause increases by the number of childbirths (Stanford et al., 1987; Mishra et al., 2007). In several studies, women with two childbirths reached menopause later compared to women with no or one childbirth (Harlow and Signorello, 2000; Gold et al., 2001; Kaczmarek, 2007; Henderson et al., 2008; Gold, 2011; Pelosi et al., 2015; Wang et al., 2018; Roman Lay et al., 2020). However, beyond two childbirths, the association with age at menopause remains inconclusive (Gold et al., 2013). The results of a Chinese study suggested earlier menopause in women with three or more childbirths compared to women with two childbirths (Dorjgochoo et al., 2008). In a recent study from the USA, women with four or more childbirths had similar risk of early menopause (<45 years) as women with three childbirths (Langton et al., 2020). However, all these previous studies lacked statistical power to study women with a high number of childbirths.
We therefore included 310 147 women in Norway to study the association of number of childbirths with age at natural menopause. The women were born during the years 1936–1966.
Materials and methods
Study design, recruitment, data collection and study sample
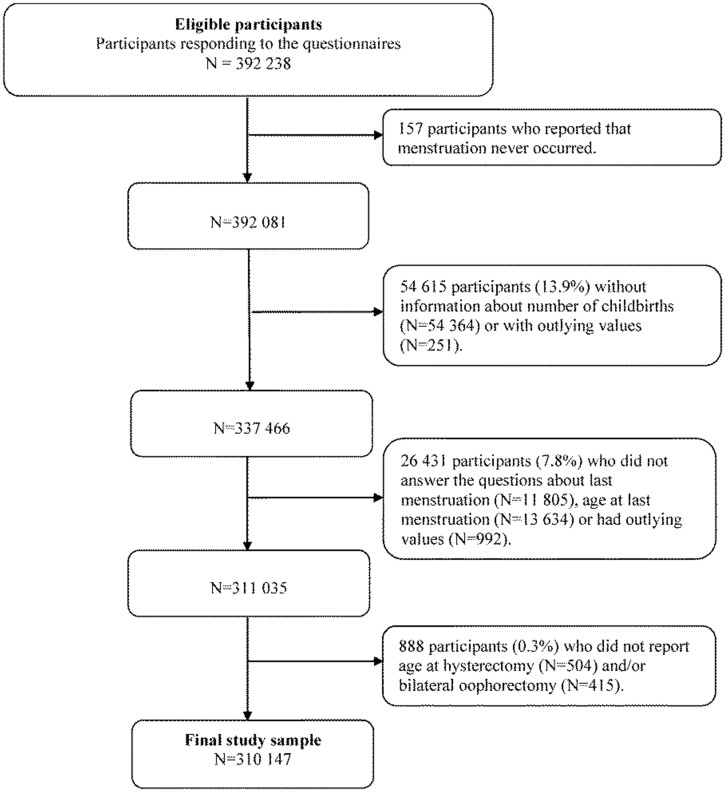
We performed a retrospective population study, using information from women who participated in the Norwegian breast cancer screening program, BreastScreen Norway (https://www.kreftregisteret.no/en/screening/mammografiprogrammet/breastscreen-norway/). This program offers breast cancer screening every second year to all women, aged 50–69 years, with residency in Norway. Of all women in the target group, 84% participated on at least one occasion during our recruitment period, 2006–2014 (Sebuødegård et al., 2016). The data in our study were collected by two self-administered questionnaires (Tsuruda et al., 2018). The first questionnaire included questions about sociodemographic factors, reproductive history and health prior to the age of 50 years. The second questionnaire included questions about current health, menstruation, and surgery on the ovaries or the uterus. A total of 392 238 women, who answered both questionnaires, were eligible for this study.
Of the 392 238 eligible women, we excluded women who reported that menstruation had never occurred (n = 157), or with missing or outlying information on number of childbirths (>19 childbirths) (n = 54 615) (Fig. 1). Thereafter, we excluded women with missing or outlying information on age at menopause (<15 and >71 years) (n = 26 431). We also excluded women who reported to have undergone removal of the uterus and/or both ovaries, but not did not report age at such surgery (n = 888). Thus, 310 147 women, aged 48–71 years, were included in our data analyses. They were born during the years 1936–1966.
Figure 1.
Flow chart of the study sample; 310 147 women in BreastScreen Norway, 2006–2014.
Main study factors
Our outcome variable was age at natural menopause. Age at menopause was based on the following two questions: ‘Are you still having menstrual periods?’ (yes; yes, but unregularly; no), and ‘If you no longer have menstrual periods, how old were you at your last menstrual period?’ We defined age at menopause as the attained age (in years) when the woman had had her last menstrual period.
Our main exposure variable was number of childbirths. The number of childbirths was based on the following questions: ‘Have you ever had a pregnancy that lasted longer than 6 months?’ (yes/no) and ‘If yes, how many?’ We categorized the number of childbirths as follows: 0, 1, 2, 3 (reference), 4, 5, 6 and ≥7 childbirths. In additional analyses, we used number of childbirths as a continuous variable (0–19 childbirths).
Other study factors
Based on literature search and the assumption that number of childbirths truly affects age at menopause, we assessed possible confounding factors by using directed acyclic graphs. We identified birth year, cigarette smoking, educational level, country of birth, oral contraceptive use and body mass index, since these factors may be associated with number of childbirths (Farrow et al., 2002; Jokela et al., 2007; Rogers, 2008; ESHRE Capri Workshop Group, 2010; Huber et al., 2010) and also with age at menopause (Gold, 2011; Schoenaker et al., 2014; Whitcomb et al., 2018; Zhu et al., 2018; Gottschalk et al., 2020; Roman Lay et al., 2020). Cigarette smoking was based on the following question: ‘Do you smoke cigarettes?’ (Yes/I have stopped/I have never smoked). We coded cigarette smoking as current smoker, former smoker, or never-smoker. Educational level was coded as <high school, high school, ≤4 years of college/university or >4 years of college/university. Country of birth was coded as Norway, other countries in Europe or countries outside Europe. The women’s year of birth was included in the data analyses as a continuous variable. The use of oral contraceptives was based on the question: ‘Have you ever used oral contraceptives?’ (yes/no) and coded as ever or never. Body mass index was based on self-reports of current weight and height and calculated as weight (kg)/height squared (m2). Body mass index was included in the data analyses as a continuous variable.
Statistical methods
Since not all women had reached menopause at the time of data collection, we used survival analyses to estimate mean age at menopause with 95% CI, and median age at menopause with interquartile ranges (IQRs) according to number of childbirths. The follow-up time was from the woman’s birth (age 0 years) until age at menopause or attained age at data collection (censoring). Thus, the women who still had menstrual periods (17.7%) or had irregular menstrual periods (8.2%), contributed with follow-up time until their attained age at data collection. Also, the women who had undergone hysterectomy (6.3%), bilateral oophorectomy (0.6%) or both surgeries (2.9%) prior to menopause were censored, and these women contributed with follow-up time until their attained age at surgery.
The association of number of childbirths (as a categorical variable) with age at menopause was estimated as hazard ratios (HR) by applying Cox proportional hazard models. HR >1.00 indicates earlier menopause compared to the reference group (women with three childbirths), and HR <1.00 indicates later menopause compared to the reference group. The Cox proportional hazard model assumes proportional hazards, and the proportional hazards assumption was evaluated by Schoenfeld residuals and by inspection of the log–log plots.
We estimated crude HRs (Model I). We also made adjustment for the women’s year of birth to account for any changes that may have occurred across birth cohorts in mean number of childbirths and mean age at menopause (Model II). In the final model, we made additional adjustments for cigarette smoking, educational level, country of birth, oral contraceptive use and body mass index (Model III).
We performed additional analyses, using number of childbirths as a continuous variable (0–19 childbirths), and in these analyses, we applied a Cox proportional hazard model with restricted cubic splines to allow for a non-linear association. The knots were placed at the 10th, the 50th and the 90th percentile of the childbirth distribution (1, 2 and 4 childbirths, respectively).
The use of systemic menopausal hormone therapy or hormonal intrauterine device may cause irregularity or absence of menstrual bleedings. Thus, women using such treatment in the years around menopause may report their age at natural menopause inaccurately. We therefore repeated the above analyses among women who had never used hormonal intrauterine device and/or systemic menopausal hormone therapy. To further check for consistency of our findings, we performed separate analyses among women born before 1950, among women born in 1950 or after, and also after exclusion of women who reported their last menstrual period within 1 year prior to data collection. We used Stata/SE version 14.2 for our data analyses (StataCorp, College Station, TX, USA).
Ethical considerations
This study was approved by the Regional Committee for Medical and Health Research Ethics (reference no. 2014/1711, REK South-East D). All women received written information about the study along with the invitation to BreastScreen Norway. By returning the questionnaires at the screening site, the woman agreed to participate in the study.
Results
At the time of data collection, the participants’ mean age was 56.4 years (SD 5.7 years, range 48–71 years), and 64.4% had undergone natural menopause. The mean number of childbirths was 2.2 (SD 1.3 childbirths, range 0–19 childbirths) (Table I). Further description of the study sample is presented in Table I and Supplementary Table SI.
Table I.
Characteristics of the study sample, 310 147 women in Norway, born during the years 1936–1966.
| No. women | Proportion (%) | Mean | SD | |
|---|---|---|---|---|
| Undergone natural menopause | 199 713 | 64.4 | ||
| Surgery on uterus or ovaries prior to menopause | ||||
| Hysterectomy | 19 562 | 6.3 | ||
| Bilateral oophorectomy | 1860 | 0.6 | ||
| Hysterectomy and bilateral oophorectomy | 9095 | 2.9 | ||
| Age at data collection, years | 56.4 | 5.7 | ||
| Age at first childbirth | 262 507 | 24.6 | 23.6 | 4.7 |
| Number of childbirths | 2.2 | 1.3 | ||
| Women born <1950 | 117 285 | 2.3 | 1.3 | |
| Women born ≥1950 | 192 862 | 2.1 | 1.2 | |
| Ever use of systemic menopausal hormone therapy | 92 822 | 29.9 | ||
| Ever use of hormonal intrauterine device | 64 279 | 20.7 | ||
| Ever use of oral contraceptives | 168 991 | 54.5 | ||
| Current body mass index | 285 062 | 25.8 | 4.6 | |
| Missing information | 25 085 | |||
| Cigarette smoking | ||||
| Current | 78 791 | 26.1 | ||
| Former | 100 078 | 33.1 | ||
| Never | 123 210 | 40.8 | ||
| Missing information | 8068 | |||
| Educational level | ||||
| <High school | 69 683 | 22.8 | ||
| High school | 128 136 | 41.9 | ||
| ≤4 years of college/university | 66 672 | 21.8 | ||
| >4 years of college/university | 41 544 | 13.6 | ||
| Missing information | 4112 | |||
| Country of birth | ||||
| Norway | 290 087 | 94.1 | ||
| Other countries in Europe | 11 987 | 3.9 | ||
| Countries outside Europe | 6184 | 2.0 | ||
| Missing information | 1889 | |||
Mean age at natural menopause was 51.17 years (95% CI: 51.15–51.19 years). The HR estimates of reaching menopause are presented in Table II. After adjustment for the women’s year of birth, the HR of reaching menopause was 1.23 (95% CI: 1.21–1.25) for women with no childbirth and HR was 1.12 (95% CI: 1.11–1.14) for women with one childbirth, using women with three childbirths as the reference (Model II, Table II). For any number of childbirths beyond three, the adjusted HR of reaching menopause were non-different from the reference group. Further adjustments for cigarette smoking, educational level, country of birth, oral contraceptive use and body mass index in the fully adjusted model did not alter the HRs estimates notably (Model III, Table II).
Table II.
The associations of number of childbirths with age at natural menopause among 310 147 women in Norway, born during the years 1936–1966.
| Model I |
Model II |
Model III |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. women | Mean | 95% CI | Crude HR | 95% CI | Adjusted HRa | 95% CI | Adjusted HRb | 95% CI | |
| Number of childbirths | |||||||||
| 0 | 30 630 | 50.55 | 50.48–50.62 | 1.21 | 1.19–1.23 | 1.23 | 1.21–1.25 | 1.24 | 1.22–1.27 |
| 1 | 34 825 | 50.94 | 50.88–51.00 | 1.10 | 1.09–1.12 | 1.12 | 1.11–1.14 | 1.10 | 1.08–1.12 |
| 2 | 130 340 | 51.25 | 51.22–51.28 | 1.02 | 1.01–1.04 | 1.03 | 1.02–1.04 | 1.03 | 1.02–1.04 |
| 3 | 82 287 | 51.36 | 51.33–51.40 | Reference | Reference | Reference | |||
| 4 | 23 046 | 51.23 | 51.16–51.29 | 1.03 | 1.01–1.05 | 1.01 | 0.99–1.03 | 1.00 | 0.99–1.02 |
| 5 | 5611 | 51.15 | 51.01–51.29 | 1.04 | 1.01–1.08 | 1.01 | 0.98–1.04 | 1.00 | 0.96–1.04 |
| 6 | 1446 | 51.12 | 50.86–51.38 | 1.06 | 1.00–1.12 | 1.01 | 0.95–1.07 | 0.99 | 0.92–1.06 |
| ≥7 | 1962 | 50.99 | 50.75–51.23 | 1.07 | 1.02–1.13 | 1.06 | 1.00–1.12 | 1.03 | 0.96–1.09 |
| Total | 310 147 | 51.17 | 51.15–51.19 | ||||||
HR, hazard ratio; IQR, interquartile range.
The associations were estimated as HRs by applying a Cox proportional hazard model. HR >1.00 indicates earlier menopause compared to the reference group (3 childbirths).
Adjustment was made for the women’s year of birth.
Adjustments were made for the women’s year of birth, cigarette smoking, educational level, country of birth, oral contraceptive use and body mass index. The study sample was restricted to women with information on all covariates, N = 266 304.
In additional analyses, using number of childbirths as a continuous variable in a Cox proportional hazard model with restricted cubic splines, we confirmed the non-linear association of number of childbirths with age at menopause (P for non-linearity < 0.001) (Fig. 2; Supplementary Table SII). The HR estimates were similar to the estimates using number of childbirths as a categorical variable. Adjustments were made for the women’s year of birth, cigarette smoking, educational level, country of birth, oral contraceptive use and current body mass index.
Figure 2.
The hazard ratios of reaching menopause (with 95% confidence intervals) according to number of childbirths among women born in Norway during the years 1936–1966. (A) Crude (N = 310 147); (B) adjusted for year of birth (N = 310 147); (C) adjusted for year of birth, cigarette smoking, educational level, country of birth, oral contraceptive use and current body mass index (N = 266 304). The hazard ratios were estimated by applying a Cox proportional hazard model with restricted cubic splines to allow for a non-linear association. Number of childbirths was included as a continuous variable.
We found similar results as in the sample as a whole among; (i) women who had never used a hormonal intrauterine device and/or systemic menopausal hormonal therapy; (ii) women who were born before 1950; (iii) women who were born in 1950 or after and (iv) after exclusion of women who reported their last menstrual period within 1 year prior to data collection (Supplementary Tables SIII, SIV and SV).
Discussion
Summary of findings
In this population study of 310 147 women in Norway, we found that age at natural menopause increased with increasing number of childbirths up to three childbirths. Beyond three childbirths, there was no further increase in age at menopause.
Strengths and limitations
To our knowledge, this study is the largest yet to assess the relationship between number of childbirths and age at menopause. Since we included a large number of women, we could estimate age at menopause among women with several childbirths and also study subgroups of women.
The age at menopause in our study was based on self-report. Age at menopause is reported with certainty at least 1 year after the last menstrual period (Soules et al., 2001). In our study, 2.7% reported that their last menstrual period occurred within the year prior to data collection, and menstrual periods could possibly reoccur in these women. It is unlikely, however, that the possible misclassification of age at menopause for 2.7% of the women is related to number of childbirths and thereby has biased our estimated associations of number of childbirths with age at menopause. Some women may have forgotten their exact age at menopause, particularly the oldest women in the study, since their menopause occurred a long time before data collection. However, previous studies have found high agreement between self-reported and true age at menopause (Rodstrom et al., 2005), and there is no reason to believe that any erroneous reporting of age at menopause was related to number of childbirths. We also performed separate analyses among women born before 1950 and among women born in 1950 or after, and we found similar results in both groups.
Use of oral contraceptives or systemic hormone therapy around menopause may induce menstrual bleedings beyond natural menopause. Thus, women in our study who used such treatment, may erroneously have reported too late menopause. Conversely, women who use a hormonal intrauterine device may have no menstrual bleedings despite being premenopausal, and these women may have reported too early menopause. In total, 29.9% of the women in our study had used systemic menopausal hormone therapy, and 20.7% had used hormonal intrauterine device. We excluded these women in supplemental analyses, and the results remained virtually unchanged.
We made adjustment for the women’s year of birth since the women in the oldest birth cohorts had higher mean number of childbirths and earlier mean age at menopause compared to the women in the youngest birth cohorts (Gottschalk et al., 2020). In the final model (Model III, Table II), we also made adjustment for cigarette smoking, educational level, country of birth, oral contraceptive use, and body mass index, and we found very similar results as in the model adjusted for birth year only. A true confounder is associated with and precede both the exposure and the outcome (VanderWeele, 2019). Thus, we did not make adjustment for variables that have unknown relation with either number of childbirths or age at menopause, such as physical activity and alcohol consumption. The use of hormonal contraceptives may influence women’s number of childbirths, and possibly the age at menopause (Roman Lay et al., 2020; Langton et al., 2021). Adjustment for oral contraceptive use did not alter the associations of number of childbirths with age at menopause in our study. Long-acting hormonal contraceptives, such as progestin-only injections or implants, may inhibit ovulation and could thus influence number of children and age at menopause. Unfortunately, our data did not include information about such contraception methods. Also, we had no information about health status and body mass index prior to first childbirth. As a proximate measure for body mass index, we made adjustment for body mass index at the time of data collection (Groenwold et al., 2021). Adjustment for body mass index did not alter the estimates notably. Although we made adjustment for several factors in our study, we cannot rule out residual confounding.
Comparison with other studies
Several studies have reported that women with no or one childbirth are at increased risk of early menopause (Gold et al., 2001; Gold, 2011; Pelosi et al., 2015; Langton et al., 2020; Roman Lay et al., 2020). We did not study the risk of early menopause, but our findings support that women with no or one childbirth are overrepresented among women with early menopause.
Whether a high number of childbirths increases the age at menopause, is insufficiently studied. Some previous studies applied linear regression models for their data analyses, and number of childbirths was included as a continuous variable (Stanford et al., 1987; Mishra et al., 2007; Roman Lay et al., 2020). Such models assume linear relations, and a non-linear association of number of childbirths with age at menopause cannot be detected. Several previous studies failed to report associations of age at menopause with any number of childbirths beyond four, possibly due to lack of statistical power (Gold et al., 2001; Kaczmarek, 2007; Mishra et al., 2007; Henderson et al., 2008; Li et al., 2012; Gold et al., 2013; Wang et al., 2018). A study of 3302 women in the USA included number of childbirths as a categorical variable (1, 2, 3 and ≥4) in their data analyses and reported no association with age at menopause (Gold et al., 2013). Yet, two studies may support a non-linear association (Dorjgochoo et al., 2008; Langton et al., 2020).
Previous studies of the association of number of childbirths with age at menopause have used different definitions of age at menopause (Gold et al., 2001; Kaczmarek, 2007; Mishra et al., 2007; Dorjgochoo et al., 2008; Henderson et al., 2008; Li et al., 2012; Gold et al., 2013; Wang et al., 2018). Comparison of results may therefore be challenging. In our study, all women who reported to have had their final menstrual period were defined as postmenopausal, also the few women with last menstrual period within the year prior to data collection. These women were included in the data analyses to ensure representative inclusion of menopausal women from recent cohorts.
Interpretations
In line with other studies, we found that women with no or one childbirth were youngest at menopause. It is conceivable that many women with no or one childbirth only have reduced fecundity (Laisk et al., 2019). If this is true, our findings may suggest that reduced fecundity is associated with early menopause.
We found no evidence of increasing age at menopause by number of childbirths among women with more than three childbirths. Menopause is caused by the exhaustion of the ovarian follicle reserve, and menopause is assumed to occur when less than 1000 follicles remain in the ovaries (Faddy et al., 1992). The ‘oocyte sparing’ hypothesis suggests that recruitment and atresia of ovarian follicles is suppressed during pregnancy, and that such suppression contributes to preservation of the ovarian follicle reserve, and thereby to delayed menopause (Cramer et al., 1995; McGee and Hsueh, 2000; Pelosi et al., 2015). If this hypothesis is true, age at menopause will increase by number of childbirths. Thus, our results do not support the ‘oocyte sparing’ hypothesis. On the contrary, our results indicate that the rate of follicle atresia is independent of number of childbirths. Nevertheless, our results are compatible with sparing of oocytes during the first three pregnancies, and thereby delayed menopause by number of childbirths up to three. In subsequent pregnancies, however, no further sparing of oocytes may take place, or other biological mechanisms counteract the effects of ‘oocyte sparing’ on age at menopause in multiparous women.
Also, oral contraceptive use has been assumed to reduce the recruitment and atresia of oocytes, and thereby delay menopause. However, recent studies report modest influence of oral contraceptive use on age at menopause, and these findings also challenge the ‘oocyte sparing’ hypothesis (Roman Lay et al., 2020; Langton et al., 2021).
The biological mechanisms underlying the large variation in age at menopause are not well understood (Laisk et al., 2019). For an individual woman, it may be important to know that a high number of pregnancies does not influence age at menopause, and possibly not ovarian aging. Whether the decrease in fertility rates seen in many parts of the world, will influence population mean age at menopause is yet to be studied.
Conclusions
In our study of 310 147 women, age at natural menopause increased with increasing number of childbirths up to three childbirths but did not increase beyond three childbirths. This finding does not support the hypothesis of an increasing age at menopause by number of childbirths.
Data availability
The data underlying this article are available in the article and in its supplementary material.
Authors’ roles
A.E., E.K.B. and M.S.G had the original idea for this study. M.S.G., A.E. and E.K.B. discussed the design and planned the data analytic approaches. M.S.G. and E.K.B. performed the data analyses. M.S.G., A.E. and E.K.B. interpreted the results and wrote the manuscript. S.H. contributed with interpretation of the results and critically revised the article. E.K.B. is the guarantor of the study. All authors had full access to the data and can take responsibility for the integrity of the data and the accuracy of the data analyses. All authors have approved the submitted version of the manuscript.
Funding
This work was supported by the South-Eastern Norway Regional Health Authority [2016112 to M.S.G.], and by the Norwegian Cancer Society [6863294-2015 to E.K.B.].
Conflict of interest
None to declare.
Supplementary Material
Contributor Information
Marthe S Gottschalk, Department of Obstetrics and Gynecology, Akershus University Hospital, Lørenskog, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Anne Eskild, Department of Obstetrics and Gynecology, Akershus University Hospital, Lørenskog, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Solveig Hofvind, Section of Mammographic Screening, Cancer Registry of Norway, Oslo, Norway; Faculty of Health Sciences, Oslo Metropolitan University, Oslo, Norway.
Elisabeth K Bjelland, Department of Obstetrics and Gynecology, Akershus University Hospital, Lørenskog, Norway; Faculty of Health Sciences, Oslo Metropolitan University, Oslo, Norway.
References
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012;13:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DW, Xu H, Harlow BL. Does “incessant” ovulation increase risk for early menopause? Am J Obstet Gynecol 1995;172:568–573. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Xu H. Predicting age at menopause. Maturitas 1996;23:319–326. [DOI] [PubMed] [Google Scholar]
- Dorjgochoo T, Kallianpur A, Gao Y-T, Cai H, Yang G, Li H, Zheng W, Shu XO. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause (New York, NY) 2008;15:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE Capri Workshop Group. Europe the continent with the lowest fertility. Hum Reprod Update 2010;16:590–602. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992;7:1342–1346. [DOI] [PubMed] [Google Scholar]
- Farrow A, Hull MG, Northstone K, Taylor H, Ford WC, Golding J. Prolonged use of oral contraception before a planned pregnancy is associated with a decreased risk of delayed conception. Hum Reprod 2002;17:2754–2761. [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause 2007;14:567–571. [DOI] [PubMed] [Google Scholar]
- Ginsberg J. What determines the age at the menopause? BMJ 1991;302:1288–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874. [DOI] [PubMed] [Google Scholar]
- Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013;178:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk MS, Eskild A, Hofvind S, Gran JM, Bjelland EK. Temporal trends in age at menarche and age at menopause: a population study of 312 656 women in Norway. Hum Reprod 2020;35:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenwold RHH, Palmer TM, Tilling K. To adjust or not to adjust? When a “confounder” is only measured after exposure. Epidemiology 2021;32:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3–9. [DOI] [PubMed] [Google Scholar]
- Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol 2008;167:1287–1294. [DOI] [PubMed] [Google Scholar]
- Huber S, Bookstein FL, Fieder M. Socioeconomic status, education, and reproduction in modern women: an evolutionary perspective. Am J Hum Biol 2010;22:578–587. [DOI] [PubMed] [Google Scholar]
- Jokela M, Kivimäki M, Elovainio M, Viikari J, Raitakari OT, Keltikangas-Järvinen L. Body mass index in adolescence and number of children in adulthood. Epidemiology 2007;18:599–606. [DOI] [PubMed] [Google Scholar]
- Kaczmarek M. The timing of natural menopause in Poland and associated factors. Maturitas 2007;57:139–153. [DOI] [PubMed] [Google Scholar]
- Laisk T, Tšuiko O, Jatsenko T, Hõrak P, Otala M, Lahdenperä M, Lummaa V, Tuuri T, Salumets A, Tapanainen JS. Demographic and evolutionary trends in ovarian function and aging. Hum Reprod Update 2019;25:34–50. [DOI] [PubMed] [Google Scholar]
- Langton CR, Whitcomb BW, Purdue-Smithe AC, Sievert LL, Hankinson SE, Manson JE, Rosner BA, Bertone-Johnson ER. Association of oral contraceptives and tubal ligation with risk of early natural menopause. Hum Reprod 2021;36:1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton CR, Whitcomb BW, Purdue-Smithe AC, Sievert LL, Hankinson SE, Manson JE, Rosner BA, Bertone-Johnson ER. Association of parity and breastfeeding with risk of early natural menopause. JAMA Netw Open 2020;3:e1919615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wu J, Pu D, Zhao Y, Wan C, Sun L, Shen CE, Sun W, Yuan Z, Shen Q et al. Factors associated with the age of natural menopause and menopausal symptoms in Chinese women. Maturitas 2012;73:354–360. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000;21:200–214. [DOI] [PubMed] [Google Scholar]
- Mishra G, Hardy R, Kuh D. Are the effects of risk factors for timing of menopause modified by age? Results from a British birth cohort study. Menopause 2007;14:717–724. [DOI] [PubMed] [Google Scholar]
- Muka T, Oliver-Williams C, Kunutsor S, Laven JSE, Fauser BCJM, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- Pelosi E, Simonsick E, Forabosco A, Garcia-Ortiz JE, Schlessinger D. Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biol Reprod 2015;92:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodstrom K, Bengtsson C, Lissner L, Bjorkelund C. Reproducibility of self-reported menopause age at the 24-year follow-up of a population study of women in Goteborg, Sweden. Menopause 2005;12:275–280. [DOI] [PubMed] [Google Scholar]
- Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res C Embryo Today 2008;84:1–15. [DOI] [PubMed] [Google Scholar]
- Roman Lay AA, do Nascimento CF, Horta BL, Dias Porto Chiavegatto Filho A. Reproductive factors and age at natural menopause: a systematic review and meta-analysis. Maturitas 2020;131:57–64. [DOI] [PubMed] [Google Scholar]
- Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014;43:1542–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebuødegård S, Sagstad S, Hofvind S. Oppmøte i Mammografiprogrammet [Attendance in the Norwegian Breast Cancer Screening Programme]. Tidsskriftet 2016;136:1448–1451. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW), Park City, Utah, July, 2001. Menopause 2001;8:402–407. [DOI] [PubMed] [Google Scholar]
- Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. J Chronic Dis 1987;40:995–1002. [DOI] [PubMed] [Google Scholar]
- Tsuruda KM, Sagstad S, Sebuødegård S, Hofvind S. Validity and reliability of self-reported health indicators among women attending organized mammographic screening. Scand J Public Health 2018;46:744–751. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol 2019;34:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhuis M, Onland-Moret NC, van der Schouw YT, Fauser BC, Broekmans FJ. Human studies on genetics of the age at natural menopause: a systematic review. Hum Reprod Update 2010;16:364–377. [DOI] [PubMed] [Google Scholar]
- Wang M, Gong W-W, Hu R-Y, Wang H, Guo Y, Bian Z, Lv J, Chen Z-M, Li L-M, Yu M. Age at natural menopause and associated factors in adult women: findings from the China Kadoorie Biobank study in Zhejiang rural area. PLoS One 2018;13:e0195658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb BW, Purdue-Smithe AC, Szegda KL, Boutot ME, Hankinson SE, Manson JE, Rosner B, Willett WC, Eliassen AH, Bertone-Johnson ER. Cigarette smoking and risk of early natural menopause. Am J Epidemiol 2018;87:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WH, Xiang YB, Ruan ZX, Zheng W, Cheng JR, Dai Q, Gao YT, Shu XO. Menstrual and reproductive factors and endometrial cancer risk: results from a population-based case-control study in urban Shanghai. Int J Cancer 2004;108:613–619. [DOI] [PubMed] [Google Scholar]
- Zhu D, Chung HF, Pandeya N, Dobson AJ, Kuh D, Crawford SL, Gold EB, Avis NE, Giles GG, Bruinsma F et al. Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. Eur J Epidemiol 2018;33:699–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its supplementary material.