Abstract
STUDY QUESTION
Is a single endometrial scratch prior to the second fresh IVF/ICSI treatment cost-effective compared to no scratch, when evaluated over a 12-month follow-up period?
SUMMARY ANSWER
The incremental cost-effectiveness ratio (ICER) for an endometrial scratch was €6524 per additional live birth, but due to uncertainty regarding the increase in live birth rate this has to be interpreted with caution.
WHAT IS KNOWN ALREADY
Endometrial scratching is thought to improve the chances of success in couples with previously failed embryo implantation in IVF/ICSI treatment. It has been widely implemented in daily practice, despite the lack of conclusive evidence of its effectiveness and without investigating whether scratching allows for a cost-effective method to reduce the number of IVF/ICSI cycles needed to achieve a live birth.
STUDY DESIGN, SIZE, DURATION
This economic evaluation is based on a multicentre randomized controlled trial carried out in the Netherlands (SCRaTCH trial) that compared a single scratch prior to the second IVF/ICSI treatment with no scratch in couples with a failed full first IVF/ICSI cycle. Follow-up was 12 months after randomization.
Economic evaluation was performed from a healthcare and societal perspective by taking both direct medical costs and lost productivity costs into account. It was performed for the primary outcome of biochemical pregnancy leading to live birth after 12 months of follow-up as well as the secondary outcome of live birth after the second fresh IVF/ICSI treatment (i.e. the first after randomization). To allow for worldwide interpretation of the data, cost level scenario analysis and sensitivity analysis was performed.
PARTICIPANTS/MATERIALS, SETTING, METHODS
From January 2016 until July 2018, 933 women with a failed first IVF/ICSI cycle were included in the trial. Data on treatment and pregnancy were recorded up until 12 months after randomization, and the resulting live birth outcomes (even if after 12 months) were also recorded.
Total costs were calculated for the second fresh IVF/ICSI treatment and for the full 12 month period for each participant. We included costs of all treatments, medication, complications and lost productivity costs. Cost-effectiveness analysis was carried out by calculating ICERs for scratch compared to control. Bootstrap resampling was used to estimate the uncertainty around cost and effect differences and ICERs. In the sensitivity and scenario analyses, various unit costs for a single scratch were introduced, amongst them, unit costs as they apply for the United Kingdom (UK).
MAIN RESULTS AND THE ROLE OF CHANCE
More live births occurred in the scratch group, but this also came with increased costs over a 12-month period. The estimated chance of a live birth after 12 months of follow-up was 44.1% in the scratch group compared to 39.3% in the control group (risk difference 4.8%, 95% CI −1.6% to +11.2%). The mean costs were on average €283 (95% CI: −€299 to €810) higher in the scratch group so that the point average ICER was €5846 per additional live birth. The ICER estimate was surrounded with a high level of uncertainty, as indicated by the fact that the cost-effectiveness acceptability curve (CEAC) showed that there is an 80% chance that endometrial scratching is cost-effective if society is willing to pay ∼€17 500 for each additional live birth.
LIMITATIONS, REASONS FOR CAUTION
There was a high uncertainty surrounding the effects, mainly in the clinical effect, i.e. the difference in the chance of live birth, which meant that a single straightforward conclusion could not be ascertained as for now.
WIDER IMPLICATIONS OF THE FINDINGS
This is the first formal cost-effectiveness analysis of endometrial scratching in women undergoing IVF/ICSI treatment. The results presented in this manuscript cannot provide a clear-cut expenditure for one additional birth, but they do allow for estimating costs per additional live birth in different scenarios once the clinical effectiveness of scratching is known. As the SCRaTCH trial was the only trial with a follow-up of 12 months, it allows for the most complete estimation of costs to date.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by ZonMW, the Dutch organization for funding healthcare research. A.E.P.C., F.J.M.B., E.R.G. and C.B. L. reported having received fees or grants during, but outside of, this trial.
TRIAL REGISTRATION NUMBER
Netherlands Trial Register (NL5193/NTR 5342).
Keywords: endometrial scratch, endometrial injury, endometrial scratch, ART, IVF, ICSI, cost-effectiveness, economic analysis, live birth
Introduction
In 2016, almost 2 million IVF and ICSI treatments were carried out globally—and this number is increasing every year—unfortunately not resulting in 2 million babies born (Adamson et al., 2016; Fauser, 2019). With pregnancy rates of maximally 35% per embryo transfer, embryo implantation is often regarded as the rate-limiting step in IVF/ICSI treatment (De Geyter et al., 2018). Ever since endometrial scratching was suggested as a promising add-on treatment to increase embryo implantation, its popularity has risen leading to widely accepted implementation into daily practice, even though its clinical effectiveness had not yet been proven and its cost-effectiveness had not been evaluated (Barash et al., 2003; Nastri et al., 2015; Lensen et al., 2016; Farquhar, 2019; Mol and Barnhart, 2019; van Hoogenhuijze et al., 2019).
It was this gap in knowledge that led us to conduct a large randomized controlled trial on the effect of endometrial scratching prior to IVF/ICSI on live birth rates. The SCRaTCH trial is a multicentre nationwide trial in the Netherlands that compared a single endometrial scratch to no scratch in women with one previously failed IVF/ICSI cycle and studied the live birth rates after the second fresh IVF/ICSI treatment as well as after 12 months of follow-up (van Hoogenhuijze et al., 2017b, 2020).
In the light of increasing healthcare costs, it is becoming ever more important to take costs of a new treatment into account alongside its effectiveness (OECD, 2013). It is for this reason that the SCRaTCH trial was set out to evaluate both the clinical effectiveness and the economic impact of endometrial scratching. Even though the SCRaTCH trial has not provided unequivocal evidence whether or not scratching improves live birth rates—a risk ratio of 1.13 (95% CI 0.97–1.32) after 12 months of follow-up was observed (van Hoogenhuijze et al., 2020)—it is essential to perform a formal economic evaluation to see if scratching would ever be a realistic add-on treatment. As cost-effectiveness in IVF/ICSI treatment is generally expected to result from a reduction in the total number of treatments needed to achieve a live birth, studying the long-term economic impact is most sensible. With its 12 months of follow-up, the SCRaTCH trial is—to date—the most suitable trial to provide insight into the economic implications of endometrial scratching in IVF/ICSI.
In this manuscript, we present the formal cost-effectiveness evaluation of the SCRaTCH trial, along with various scenarios that sketch how the economic impact would change with varying scratch prices or with alternative clinical effectiveness.
Materials and methods
A non-blinded randomized controlled trial comparing a single endometrial scratch prior to the second IVF/ICSI treatment to no scratch was conducted in the Netherlands between January 2016 and January 2020 (SCRaTCH trial). The trial received ethics approval from the institutional review board of the University Medical Centre Utrecht (METC 10-272). As it was a multicentre trial, each participating centre also obtained approval from the local board of directors. All women gave written informed consent, and the study was carried out in line with the WMA Declaration of Helsinki.
Study design, participants and study procedures
The study protocol and primary outcome of the study have been published previously (van Hoogenhuijze et al., 2017b, 2020). In short, women who had undergone one full IVF/ICSI cycle with at least one embryo transfer that did not result in a clinical pregnancy were eligible. Additional inclusion criteria were 18–44 years old, primary or secondary infertility and a normal transvaginal ultrasound. Exclusion criteria were endometriosis grade III/IV, untreated hydrosalpinx, oocyte donation and preimplantation genetic diagnosis.
Women were randomized 1:1 to either scratch or control. Women in the scratch group received a single endometrial scratch timed at 5–10 days before the expected menstrual or withdrawal bleeding, after which ovarian hyperstimulation was started. The scratch was performed with an endometrial biopsy catheter without additional anaesthetics or sedatives, and the residual tissue was not histologically or cytologically evaluated. Women in the control group did not receive a scratch or sham procedure and started directly with ovarian hyperstimulation. Thus, neither the participating women nor the medical staff were blinded.
Follow-up and outcomes
Follow-up was until 12 months after randomization. If participants had achieved an ongoing pregnancy (gestational age of 10 weeks) within 12 months we recorded data until live birth (i.e. follow-up could end before or beyond 12 months, depending on when ongoing pregnancy was achieved).
For the main article, which focussed on clinical effectiveness (published previously, van Hoogenhuijze et al., 2020), the primary outcome was ongoing pregnancy leading to live birth after the fresh cycle directly after randomization, and secondary outcomes included the ongoing pregnancy leading to live birth within the 12 months of follow-up. From a cost-effectiveness perspective in fertility research, the cost reduction from an effective treatment is expected to result from the reduced need for additional IVF/ICSI treatment. Thus, from a cost-effectiveness perspective, a long-term follow-up outcome is more informative than a single cycle analysis. As a simplification for the incurred costs, we opted for follow-up until biochemical pregnancy leading to live birth. Therefore, for this current cost-effectiveness analysis, the primary outcome was biochemical pregnancy leading to live birth within the full follow-up period (i.e. biochemical pregnancy must be reached within 10 months and 2 weeks—323 days—after randomization). The secondary outcome was biochemical pregnancy leading to live birth after just the fresh cycle directly after randomization.
Economic evaluation
The economic evaluation was performed from a healthcare (i.e. direct medical costs) and societal perspective (i.e. absence from work) and was expressed in European currency (Euro or EUR). In this manuscript, we adhere to the ISPOR guidelines for reporting economic evaluations as stated in the CHEERS checklist (Husereau et al., 2013).
Unit costs for medication were derived from the Dutch Formulary for medication (Zorginstituut Nederland), and unit costs for fertility treatments (cycle monitoring, interventions) and their complications were obtained from costs as determined by an expert panel on cost-effectiveness from the Dutch Consortium for Research in Women’s Health. This expert panel consists of gynaecologists, economists and a methodologist, and it determined the actual per unit medical costs from resources that were used in fertility studies within the Dutch Consortium and from two university hospitals and one general hospital in the Netherlands. For their final calculation, this expert panel used the average actual costs of these three hospitals. Through this approach, the average costs reflect actual clinical practice, for instance in IVF/ICSI or frozen embryo treatment where the actual number of ultrasound checks differ between individuals (Van Tilborg et al., 2017). In addition, lost productivity costs were determined for each participant based on data on hourly wage and labour costs, as derived from StatLine, the national organization for statistics on the Dutch society (Centraal Bureau voor Statistiek, CBS; Hakkaart-van Roijen et al., 2015). All prices were standardized for the year 2018 using consumer price index data (StatLine (Centraal Bureau voor Statistiek)).
Data on the number and type of treatments, and number and outcome of pregnancies were recorded in case report forms for all participants. The aim of this study was to calculate if endometrial scratching is cost-effective for reaching a biochemical pregnancy leading to a live birth. Therefore, for each participant, total costs for the second fresh IVF/ICSI as well as for the fertility treatments during the full follow-up period were calculated up to biochemical pregnancy. Costs after this time point, such as costs related to miscarriage, pregnancy complications or pregnancy leave were not included, nor were costs related to giving birth.
We thus included costs for a consultation with the physician, IVF/ICSI medication (downregulation, ovarian stimulation, ovulation trigger, luteal support), medication for frozen embryo transfer cycles, laboratory examinations, ultrasound scans, ovum pick-up, embryo transfer, costs for the ‘study-scratch’ as well as possible extra scratches, and costs associated with the complication ovarian hyperstimulation syndrome (OHSS; clinic visits, ultrasound scans, laboratory examinations, hospitalization). In addition, we included the lost-productivity costs for both the female patient and her partner for the IVF/ICSI treatment, frozen embryo transfers, and OHSS complication. The number of clinic visits related to treatment and complications was not recorded in the main SCRaTCH trial but were based on recorded data in the OPTIMIST trial, which was conducted in a similar population in similar hospitals in the Netherlands (Van Tilborg et al., 2017).
The full list of cost parameters that were included in the economic evaluation is presented in Table I.
Table I.
Unit costs for the intervention, infertility treatments and complications.
| Cost parameter | Unit costs (€)a | Source |
|---|---|---|
| Healthcare costs | ||
|
| ||
| Endometrial scratchb | 104.00 | Calculated estimatec |
| Materials | 10.77 | |
| Gynaecologist/fertility physician | 26.75 | |
| Other costs regarding medical staff | 50.87 | |
| General costs of outpatient clinic visit | 15.61 | |
| Treatment costsd | ||
| IVF/ICSI stimulation only | 532.64 | Dutch expert panel |
| IVF/ICSI stimulation and OPU, no lab-phase | 1296.62 | Dutch expert panel |
| IVF stimulation, OPU, lab-phase, with or without embryo transfer | 1449.73 | Dutch expert panel |
| ICSI stimulation, OPU, lab-phase, with or without embryo transfer | 1803.49 | Dutch expert panel |
| Frozen–thaw embryo transfer cycle | 585.15 | Dutch expert panel |
| Medication costs | ||
| FSH analogues (€/IU) | 0.34 | Farmacotherapeutisch kompas |
| GnRH antagonist (€/day) | 39.30 | Farmacotherapeutisch kompas |
| GnRH agonist (€/day) | 10.99 | Farmacotherapeutisch kompas |
| Ovulation trigger (€/10 000 IU) | 2.47 | Farmacotherapeutisch kompas |
| Progesterone (€/200 mg) | 0.21 | Farmacotherapeutisch kompas |
| Complicationse | ||
| Mild OHSSf | 200.29 | Dutch expert panel (Van Tilborg et al., 2017) |
| Severe OHSSg | 2847.95 | Dutch expert panel |
|
| ||
| Societal costs | ||
|
| ||
| Lost productivity costs | ||
| Female (€/h) | 32.68 | CBS |
| Male (€/h) | 39.20 | CBS |
IU, international unit; mg, milligram; OHSS, ovarian hyperstimulation syndrome; OPU, oocyte pick-up.
Calculated for the year 2018.
Based on a 15 min outpatient clinic visit, without analgesia.
Costs of a single scratch were estimated based on the average time and medical equipment needed for a scratch. Costs for placement of an intrauterine device (without the product costs), a regular ultrasound, and costs of an endometrial biopsy catheter contributed to determining the estimate.
The costs include a counselling consultation, ultrasound scans, OPU, laboratory phase and embryo transfer. The laboratory phase includes fertilization through IVF or ICSI, and selection, cryopreservation and thawing of embryos.
The costs include clinic visits, telephone consultations, laboratory diagnostics and ultrasound scans. For severe OHSS, also costs for hospitalization are included.
Mild OHSS was defined as clinical symptoms and hospital check-ups but no hospitalization.
Severe OHSS was defined as the need for hospitalization.
Farmacotherapeutisch kompas: Dutch formulary for medication (Zorginstituut Nederland).
CBS: Netherlands statistics, the national statistical office (Hakkaart-van Roijen et al., 2015; StatLine (Centraal Bureau voor Statistiek)).
Dutch expert panel: expert panel on cost-effectiveness established by the Dutch Consortium for Research in Women’s Health. This panel consists of gynaecologists, economists and a methodologist and determined the average unit costs based on other fertility studies and actual costs of two university hospitals and a general hospital in the Netherlands.
Statistical analysis
Primary economic analysis
A statistical analysis plan specifically for the economic evaluation was drafted prior to the start of analysis. All analyses were performed according to the intention-to-treat (ITT) principle and were of the cost-per-survival type—where in this case ‘survival’ is ‘live birth’. Discounting was not necessary, because of the 12-month horizon of this study. In contrast to the main publication on the clinical effectiveness, we did not perform an as-treated (AT) or AT for those patients with an embryo transfer (AT+ET) analysis. From a cost-effectiveness perspective, the ITT analysis is most informative as this resembles clinical practice best: even when endometrial scratching would be implemented in daily care, not all eligible patients would actually undergo scratching. We therefore limited the analysis to ITT only.
Total costs per participant were calculated as described above. As for this CEA, the primary outcome was cost-effectiveness after the full period of follow-up, we estimated the chance of a live birth after 12 months using the Kaplan–Meier method to account for the 4.7% of women who were lost to follow-up. Accounting for the artificially lower costs for participants lost to follow-up was resolved using inverse probability weighting for censoring (Wijeysundera et al., 2012). The 95% CIs of the cost and effect differences were estimated by bootstrap resampling with replacement for 5000 samples and were visualized in a cost-effectiveness plane for the primary and secondary outcome. Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the difference in mean total costs by the difference in chances of a live birth.
Cost-effectiveness acceptability curves (CEACs) with varying monetary values for the two live birth outcomes (direct and after follow-up) were constructed using the bootstrap samples following Fenwick et al. (2004), thereby visualizing the uncertainty around the ICERs.
Thus, the ICERs provide a point estimate for the costs for one additional live birth, and the CEACs visualize the probability that, given a range of monetary thresholds, scratching is cost-effective compared to control.
Sensitivity analyses
As the price of an endometrial scratch may vary between clinics and countries, a sensitivity analysis was undertaken for prices of a single endometrial scratch in the lowest (€50) and the highest (€350) range (Lensen et al., 2016). Total costs were calculated for all participants as described above, but with an endometrial scratch costing either €50 or €350. As a reference, for the primary analysis, an endometrial scratch was estimated at €104. For both scenarios, ICERs were calculated and CEACs were constructed to estimate the uncertainty around the ICERs. Sensitivity analyses were only performed for the primary outcome.
Scenario analysis
To illustrate how cost-effectiveness may vary between different countries, a scenario analysis was performed by carrying out the economic evaluation from a healthcare perspective for the United Kingdom (UK). For the healthcare perspective, direct medical costs were included but indirect costs due to the absence from work were not taken into account. Unit costs for the UK were derived from NHS data on treatment and medication prices for the year 2019 and expressed in Pound Sterling (GBP) (specification shown in Supplementary Table SI). We used the NHS price of £180 for an endometrial scratch. Total costs using GBP were then calculated for all participants for the full follow-up period, after which the ICER was calculated and a CEAC was constructed. The scenario analysis was only performed for the primary outcome.
Results
Study participants
Between January 2016 and July 2018, 933 women were randomized and included in the analyses (scratch group n = 467, control group n = 466) (van Hoogenhuijze et al., 2020). After randomization, seven women became lost to follow-up before they received a scratch and/or started their second fresh treatment (scratch n = 2, control n = 5). In the scratch group, 453 women started a second fresh treatment compared to 445 women in the control group, and 386 versus 368 women had a fresh transfer. For the women that did not start treatment, follow-up was still completed as they may have conceived naturally within 12 months. For the women without a fresh transfer, follow-up was also completed. Most of these women underwent frozen transfers or subsequent IVF/ICSI cycles during the follow-up period. During follow-up, women could continue with frozen–thaw embryo transfers (FET) and third, fourth or even fifth fresh IVF/ICSI cycles. In total, from randomization until end of follow-up, women in both groups underwent on average 1.4 fresh IVF/ICSI cycles and 1.9 FETs (van Hoogenhuijze et al., 2020). Additional information on baseline and treatment characteristics can be found in Supplementary Tables SII, SIII and SIV.
Data were complete for 925 of the 933 (99.1%, 465/467 in scratch and 458/466 in control group) participants for the outcome of live birth resulting from the treatment after randomization (i.e. second fresh IVF/ICSI cycle), and for 889 of the 933 (95.3%, 445/467 in scratch and 444/466 in control group) participants for the outcome of biochemical pregnancy leading to live birth within the 12-month follow-up period. A complete overview of the clinical outcomes is presented in van Hoogenhuijze et al. (2020).
Cost-effectiveness analysis for the Netherlands
The estimated chance (based on the Kaplan–Meier method) of a biochemical pregnancy leading to live birth within the 12-month follow-up period was 44.1% compared to 39.3% for the scratch and control group, respectively, corresponding with a 4.8% (95% CI: −1.6% to 11.2%) increased chance of live birth in the scratch group. The mean costs included both the healthcare and societal costs and were on average higher in the scratch group (€8477) compared to controls (€8194) with a cost difference of €283 (95% CI: −€299 to €810). The difference between groups in costs and the chance of live birth is visualized in a cost-effectiveness plane in Fig. 1. We found that in 18.5% of bootstrap samples scratch dominated no scratch (southeast), in 6.4% this was vice versa (northwest), in 0.9% scratch was less effective and less costly (southwest) and in the remaining 74.2% scratch was more effective and more costly (northeast). The ICER was €5846 per additional live birth. Because the cost-effectiveness plane overlaps all four quadrants, 95% CIs around the ICER are impossible to interpret.
Figure 1.
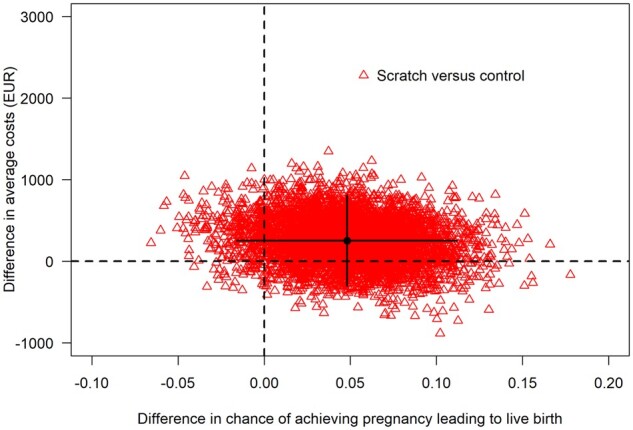
Cost-effectiveness acceptability plane of the chance of pregnancy leading to live birth after 12 months of follow-up.a The red triangles are the differences between scratch and control in the chance of achieving a live birth and the difference in average costs from individual bootstrap samples. The black lines indicate the 95% CI for these differences. The black dot represents the point estimate for the averages that is used to calculate the point estimate for the ICER. The spread of the triangles indicates the uncertainty around the differences in live birth and costs, and thus, in the ICER. While most triangles are in the upper right quadrant, indicating that scratching is more expensive and yields a higher chance of achieving a live birth, some triangles are also in the upper left quadrant (more expensive and less effective), bottom left quadrant (less expensive and less effective) and bottom right quadrant (less expensive and more effective). aThe ongoing pregnancy status had to be reached within 12 months after randomization; live birth could have resulted after 12 months of follow-up. EUR, European euro (€).
To show the uncertainty around the ICER, the CEAC (Fig. 2) shows that there is an 80% chance that endometrial scratching is cost-effective if society is willing to pay ∼€17 500 for each additional live birth.
Figure 2.
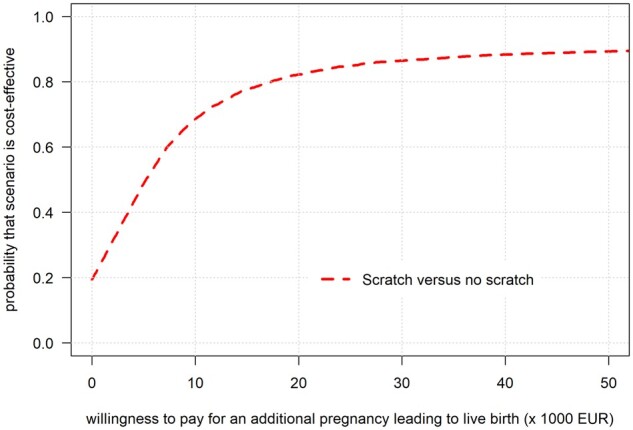
Cost-effectiveness acceptability curve for the chance of pregnancy leading to live birth after 12 months of follow-up.a The cost-effectiveness acceptability curve is shown for increasing monetary value for each additional live birth that society is willing to pay, with calculations ranging from €1 to €50 000. The line indicates the probability that endometrial scratching is cost-effective at varying monetary values for each additional live birth. aThe ongoing pregnancy status must be reached within 12 months after randomization; live birth could have resulted after 12 months of follow-up. EUR, European euro (€).
For the secondary outcome of live birth after the second fresh IVF/ICSI cycle, the calculated chance of a live birth was on average 23.6% for the scratch group and 19.1% for the control group, corresponding to an average difference in chances of 4.5% (95% CI: −0.8% to 9.5%). The calculated mean costs were higher in the scratch group (€5337) compared to controls (€4900) with a difference of €437 (95% CI: €253 to €614). The ICER was €9776 per additional live birth (Fig. 3). The CEAC (Fig. 4) shows that there is an 80% chance that endometrial scratching is cost-effective if society is willing to pay ∼€20 000 for each additional live birth from the fresh treatment directly after randomization.
Figure 3.
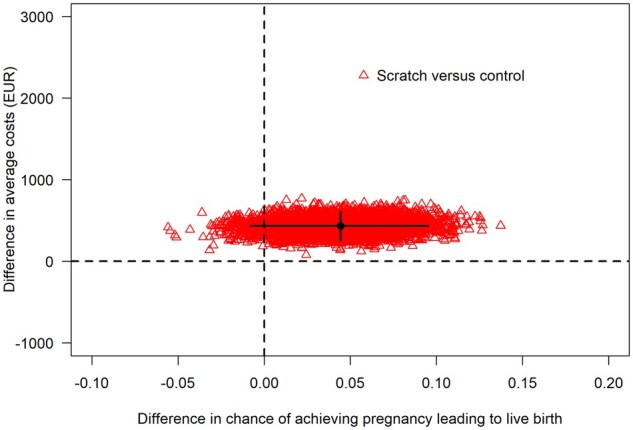
Cost-effectiveness acceptability plane of the chance of pregnancy leading to live birth from the 2nd fresh IVF/ICSI treatment. The red triangles are the differences between scratch and control in the chance of achieving a live birth and the difference in average costs from individual bootstrap samples. The black lines indicate the 95% CI for these differences. The black dot represents the point estimate for the averages that is used to calculate the point estimate for the ICER. The spread of the triangles indicates the uncertainty around the differences in live birth and costs, and thus, in the ICER. Although scratching was more expensive than control in all bootstrap samples, this did not always yield a positive difference in the chance of achieving a live birth. EUR, European euro (€).
Figure 4.
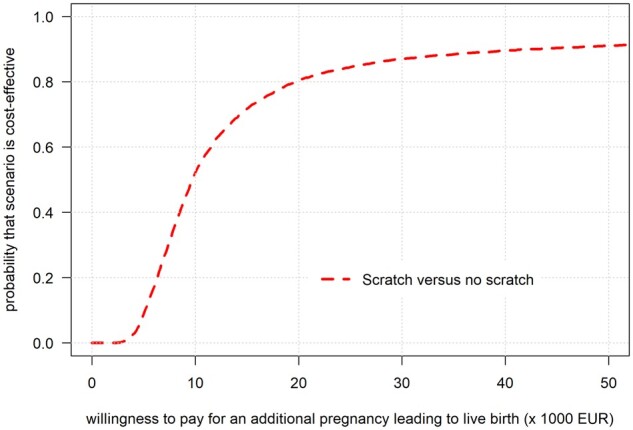
Cost-effectiveness acceptability curve for the chance of pregnancy leading to live birth from the second fresh IVF/ICSI treatment. The cost-effectiveness acceptability curve is shown for increasing monetary value for each additional live birth that society is willing to pay, with calculations ranging from €1 to €50 000. The line indicates the probability that endometrial scratching is cost-effective at varying monetary values for each additional live birth that originates from the second fresh IVF/ICSI treatment. EUR, European euro (€).
Sensitivity analysis
Mean total costs—i.e. both healthcare and societal—were calculated using prices for an endometrial scratch from the lowest ranges and from the highest ranges. Using a unit price of €50 for a single scratch, the mean costs during the full follow-up period were calculated at €8407 for the scratch group and €8206 for the control group with a mean cost difference of €201 (95% CI: −€349 to €765). The corresponding ICER was €4096 for each additional live birth. The CEAC (Supplementary Fig. S1) shows that there is an 80% chance that endometrial scratching is cost-effective if society is willing to pay ∼€13 000 for each additional live birth.
When an endometrial scratch is more expensive and would be offered at €350, the mean total costs would increase to €8707in the scratch and €8216 in the control group with a mean cost difference of €491 (95% CI: −€76 to €1067). The corresponding ICER then becomes €9935 for each additional live birth. As shown in the CEAC (Supplementary Fig. S1), there is an 80% chance that endometrial scratching—when offered at this price—is cost-effective if society is willing to pay ∼€26 500 for each additional live birth.
Scenario analysis
The mean healthcare costs based on the 12-month follow-up outcomes were calculated for the UK and showed that costs were higher for participants of the scratch group. On average, costs for a participant in the scratch group were £7065/€7738) compared to £6917/€7576 in the control group with a mean cost difference of £148/€162) (95% CI: −£337 to £619). The ICER was £3062/€3354 for each additional live birth. As is shown in the CEAC, there is an 80% chance that endometrial scratching is cost-effective if the UK is willing to pay ∼£12 000/€13 143 for each additional live birth (Supplementary Fig. S2).
Discussion
Principal findings
Within the SCRaTCH trial, we evaluated the economic impact of endometrial scratching alongside its clinical effectiveness. Based on these results, the estimated chance of a live birth after 12 months of follow-up was higher in the scratch group compared to controls, but this was also associated with slightly higher total costs (on average €283 higher). The expected costs within 12 months to achieve one additional live birth were €5846. On the short term, the costs to achieve one additional live birth within the second fresh IVF/ICSI cycle (i.e. directly after randomization) were €9776. These elevated costs were mainly caused by the total healthcare and societal expenditures associated with the scratch procedure itself and with the IVF/ICSI treatments.
Strengths and limitations
Important strengths of this trial are that it was designed to evaluate the live birth rate, which is the clinically most important outcome in infertility assessments, and that both the RCT and the economic evaluation were planned and registered prospectively. Importantly, we not only evaluated the cost-effectiveness for the ‘direct’ outcome (live birth after the second fresh cycle) but also the 12-month outcome, which better reflects clinical practice and is more valuable when considering cost-effectiveness for achieving a live birth. As applies for most medical treatments, a fertility trajectory not only has a financial impact in terms of direct healthcare costs but also affects societal costs through for example lost productivity. In infertility treatment, this is even more so as it (usually) concerns two individuals who are both of working age. We therefore provided a full-width analysis encompassing direct medical costs up to a detailed level and lost productivity costs for both the participant and her partner. Lastly, the scenario analyses for varying scratch unit costs and the sensitivity analysis for UK costs allow for interpretation of the economic impact beyond the Dutch system and indicate the ranges within which the costs of scratching are to be expected.
A limitation of this trial is that we did not include additional parameters from the perspective of patients such as travel expenses or quality adjusted life years (QALYs)—which traditionally is the outcome measure in cost-effectiveness analyses. However, QALYs are poorly defined in subfertile populations so that more often pregnancy outcomes such as live birth are used (Baird et al., 2015; Eijkemans et al., 2017). Still, while in infertility treatment the primary goal of live birth seems to be a very clear and measurable outcome, it could be argued that patient valuation of the treatment process is another important factor—perhaps especially so when a live birth is not reached.
Also, the analysis of the secondary outcome (live birth from the cycle following randomization) may have been compromised by a slight imbalance between the two arms in proceeding for embryo transfer. Of the randomized women, 82.7% in the scratch versus 79.0% in the control group received a fresh transfer. Baseline characteristics or ‘subjective’ reasons for cancelling the transfer did not explain this difference. The control group did ‘catch up’ during the follow-up period: the total number of started cycles and embryo transfers over the 12-month period was similar in both groups.
Another limitation is that interpretations of ICERs and their 95% CIs were complicated because bootstrap samples spanned all four quadrants of the CE plane. In that case, ICERs of the same sign can have two complementary meanings one in the favour of scratching and one in favour of not scratching (Fenwick et al., 2004). We thus do not present 95% CIs around ICER values and instead provide CEACs.
Our data showed that the elevated costs for the scratch group were partially caused by higher treatment costs. This may seem contradictory as one would expect a more effective treatment to result in fewer subsequent treatments. For the second fresh cycle only (i.e. directly after randomization), we observed that there was a small imbalance regarding continuation of the treatment: a slightly larger proportion in the control group cancelled their second (i.e. directly after randomization) IVF/ICSI cycle, thereby reducing both the direct medical and the lost productivity costs in this group. We could not objectify allocation-related reasons for cycle cancellation but we could also not exclude some form of confounding beyond our data recording (van Hoogenhuijze et al., 2020). Importantly, the total number of IVF/ICSI treatments during the 12 months follow-up (our primary outcome for this cost-effectiveness analysis) or FSH dosing did not differ between the groups.
Both direct medical costs and lost productivity costs were calculated up to biochemical pregnancy, meaning that costs associated with pregnancy complications or costs associated with a physiological pregnancy—such as pregnancy leave or giving birth—were not included in the current analysis. As the results of the clinical effectiveness of the SCRaTCH trial showed no difference in the occurrence of miscarriages, we expect that the additional costs associated with miscarriages would not differ between the scratch and control groups (van Hoogenhuijze et al., 2020). Similarly, late pregnancy complications do not seem to be affected by endometrial scratching (Lensen et al., 2019) and are therefore also not expected to affect the difference in costs between scratching and controls. Nonetheless, if all these costs—including costs associated with a physiological pregnancy—would have been included, it would have provided a more complete insight in the total costs associated with a live birth. These costs should then be regarded with the costs of a live birth after spontaneous conception in mind.
Relation to other studies
To our knowledge, this is the first economic evaluation on endometrial scratching in IVF/ICSI treatment. It is an important addition to the existing body of evidence that considers merely the clinical effectiveness of scratching, as in order for a treatment to become widely accessible, it should also be affordable. Obviously, the first question for a possible new treatment is whether it increases the chance of a live birth or not, but next we should consider the costs relative to the (possible) benefits. We should keep questioning ourselves what the minimal benefit and the maximal costs should be in order to maintain access to infertility care to the population.
In fertility treatment, it is difficult to determine what a cost-effectiveness threshold should be, as most economic evaluations determine costs per QALY, while fertility trials usually determine costs per pregnancy or per live birth as QALYs are poorly defined for the subfertile population (Baird et al., 2015; Goldhaber-Fiebert and Brandeau, 2015). Also, determining cost-effectiveness for most illnesses only involves the QALY of one person: the patient. In fertility treatment this is generally not the case as it (usually) involves both a couple and the unborn child (Baird et al., 2015; Goldhaber-Fiebert and Brandeau, 2015). Previous research has shown that, in the Western world, society is willing to pay approximately the gross domestic product pro capita for each QALY gained. For example, the NICE guidelines refer to a threshold of £20 000/€21 895 per QALY, and in the Netherlands, a threshold starting at €20 000 per QALY for the lowest category of disease burden is used (McCabe et al., 2008; Vijgen et al., 2018). The ESHRE Capri Workgroup has adequately pointed out that in a large, macro-economic perspective, IVF treatment itself is cost-effective if we assume that on average four cycles at a total of €20 000 are needed to ‘generate’ a single baby, while it is assumed that it improves the QALYs of the parents and also results in a future taxpayer (Baird et al., 2015). On the other hand, possible negative QALYs caused by unsuccessful IVF treatments were not taken into account, but one could also imagine that not even trying IVF/ICSI treatment may result in even more negative QALYs.
Future implications
The calculations based on the SCRaTCH trial illustrate that endometrial scratching comes at an additional cost to IVF/ICSI treatment—also when taking a 12-month (treatment) period into account. As scratching is a fairly safe and simple procedure that can be performed in the outpatient clinic, does not include high-tech material, the scratch itself should come at costs comparable to the costs of a single outpatient clinic visit. In other words, compared to the total costs of an IVF/ICSI treatment, the scratch itself should be relatively inexpensive. When including societal costs it is estimated that participants in the scratch group cost ∼€283 more than participants in the control group in the year following randomization. Given the increased chance of live birth of ∼4.8%, this came down to an ICER of €5846. However, the estimation of the ICER was surrounded with a high level of uncertainty, which compromises its interpretation. The currently ongoing individual participant data-analysis (IPD) on endometrial scratching is aiming to provide more accuracy in the treatment effect, which should also improve certainty in ICER estimates.
Taken together, we should now focus on determining with more certainty the clinical effectiveness of endometrial scratching. The IPD on this topic is well on its way and will soon provide the best available evidence as to whether scratching improves chances of a live birth but may be less suitable for a formal cost-effectiveness analysis due to the lack of long-term follow-up in most trials and due to large variations in data recording (van Hoogenhuijze et al., 2017a. Next steps will include determining how society values one additional live birth and whether the ICER falls below that: based on previous calculations as proposed by the ESHRE Capri Workshop Group this is estimated to be ∼€20 000 or £20 000 in Western societies (Baird et al., 2015). It could well be that a single endometrial scratch would result in an ICER below this threshold already at relatively low effectiveness rates, as in our example a 5% increase in live birth rates resulted in an ICER well below the €20 000. However, costs for IVF/ICSI and scratching vary between countries (as shown by our UK scenario). A similar clinical effect could thus have a different economic impact in different countries. In addition, reimbursement options vary widely, so that the ‘willingness to pay’ may also be weighted differently. Apart from these international variations that may affect decisions on whether or not to implement scratching into daily practice, we believe that at this moment it is premature to implement scratching in standard IVF/ICSI treatment. We should wait until its clinical effectiveness and the associated economic impact are established.
Furthermore, the current economic evaluation is focussed on a single endometrial scratch in the population with one previously failed IVF/ICSI cycle. If scratching appears to truly improve live birth rates, other scenarios in terms of timing and frequency of scratching should be evaluated both clinically and from an economic perspective, as well as (cost-)effectiveness in different populations.
Conclusion
In a population with one previous failed IVF/ICSI cycle, slightly higher expenditures but also higher live birth rates were observed in the scratch group compared to controls. The current uncertainty in both the clinical effect and the mean costs precludes a definite conclusion about the acceptability of the expenditure for one additional live birth. Studies such as the ongoing IPD on endometrial scratching should be awaited to provide a more precise clinical effect size and subsequently assess the costs for one additional live birth. As illustrated by the scenario analyses of the UK, country-specific prices and clinical practice need to be considered before generalizing these results to other countries.
Data availability
Data are available on request.
Supplementary Material
Acknowledgements
The authors thank all participating women, the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology, the trial collaborators as named in the author list, and the research nurses and other staff involved in recruitment of the participants.
Authors’ roles
N.E.v.H.: trial coordinator, statistical analyses, interpretation of results, drafting the manuscript. R.v.E.: statistical analyses, interpretation of results, revising the manuscript, final approval of the manuscript. F.J.M.B. and H.L.T.: trial design, obtaining funding, trial coordinator, interpretation of results, revising the manuscript, final approval of the manuscript. M.J.C.E.: supervising statistical analyses, revising the pre-final manuscript, final approval of the manuscript. M.v.W.: methodological advice, final approval of the manuscript. All other co-authors: principal investigators at participating sites, revising the pre-final manuscript, final approval of the manuscript.
Funding
The SCRaTCH trial was funded by the Dutch Organization for funding of healthcare research ZonMW. ZonMW-projectnumber 843002601. The sponsor of the SCRaTCH study was the University Medical Hospital Utrecht (UMCU), Heidelberglaan 100, 3584 CX Utrecht, The Netherlands. ZonMW was not involved in the study design, interpretation of data or writing of the report.
Conflict of interest
A.E.P.C. reports ‘other’ from Ferring BV, personal fees from Up to date Hyperthecosis, ‘other’ from Theramex BV, outside the submitted work. E.R.G. reports grants from Titus Health Care during the conduct of the study. F.J.M.B. reports personal fees as Member of the external advisory board for Ferring BV, The Netherlands, personal fees as Member of the external advisory board for Merck Serono, The Netherlands, personal fees as Member of the external advisory for Gedeon Richter, Belgium, personal fees from Educational activities for Ferring BV, The Netherlands, grants from Research support grant Merck Serono, grants from Research support grant Ferring, personal fees from Advisory and consultancy work Roche, outside the submitted work. C.B.L. reports grants from Merck, grants from Ferring, outside the submitted work.
Contributor Information
N E van Hoogenhuijze, Department of Gynaecology and Reproductive Medicine, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands.
R van Eekelen, Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology—NVOG Consortium 2.0, Amsterdam, The Netherlands.
F Mol, Amsterdam UMC, University of Amsterdam, Center for Reproductive Medicine, Reproduction and Development, Amsterdam, The Netherlands.
I Schipper, Division of Reproductive Endocrinology and Infertility, Department Obstetrics and Gynaecology, Erasmus Medical Centre Rotterdam, Rotterdam, The Netherlands.
E R Groenewoud, Department of Obstetrics, Gynaecology and Reproductive Medicine, Noordwest Ziekenhuisgroep, Den Helder, The Netherlands.
M A F Traas, Department of Gynaecology, Gelre Hospital, Apeldoorn, The Netherlands.
C A H Janssen, Department of Gynaecology, Groene Hart Hospital, Gouda, The Netherlands.
G Teklenburg, Isala Fertility Clinic, Isala Hospital, Zwolle, The Netherlands.
J P de Bruin, Department of Gynaecology and Obstetrics, Jeroen Bosch Hospital, Den Bosch, The Netherlands.
R H F van Oppenraaij, Department of Gynaecology, Maasstad Hospital, Rotterdam, The Netherlands.
J W M Maas, Department of Gynaecology, Maxima Medical Centre, Veldhoven, The Netherlands.
E Moll, Department of Gynaecology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands.
K Fleischer, Department of Obstetrics and Gynaecology, Radboud University Medical Centre, Nijmegen, The Netherlands.
M H A van Hooff, Department of Gynaecology, Franciscus Gasthuis en Vlietland, Rotterdam, The Netherlands.
C H de Koning, Department of Gynaecology, Tergooi Hospital, Hilversum, The Netherlands.
A E P Cantineau, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
C B Lambalk, Department of Reproductive Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
M Verberg, Fertility Clinic, Fertility Clinic Twente, Hengelo, The Netherlands.
A M van Heusden, Fertility Clinic, Medisch Centrum Kinderwens, Leiderdorp, The Netherlands.
A P Manger, Department of Gynaecology, Diakonessenhuis, Utrecht, The Netherlands.
M M E van Rumste, Department of Gynaecology, Catharina Hospital, Eindhoven, The Netherlands.
L F van der Voet, Department of Gynaecology, Deventer Hospital, Deventer, The Netherlands.
Q D Pieterse, Fertility Center, Haga Hospital, The Hague, The Netherlands.
J Visser, Department of Gynaecology and Obstetrics, Amphia Hospital, Breda, The Netherlands.
E A Brinkhuis, Department of Gynaecology and Obstetrics, Meander Hospital, Amersfoort, The Netherlands.
J E den Hartog, Department of Obstetrics and Gynaecology, Maastricht UMC+, Maastricht, The Netherlands.
M W Glas, Fertility Clinic, Wilhelmina Hospital Assen, Assen, The Netherlands.
N F Klijn, Department of Gynaecology, Leiden University Medical Centre, Leiden, The Netherlands.
M van der Zanden, Department of Gynaecology, Haaglanden Medical Centre, The Hague, The Netherlands.
M L Bandell, Department of Gynaecology, Albert Schweitzer Hospital, Sliedrecht, The Netherlands.
J C Boxmeer, Department of Gynaecology, Reinier de Graaf Gasthuis, Delft, The Netherlands.
J van Disseldorp, Department of Gynaecology and Obstetrics, St. Antonius Hospital, Nieuwegein, The Netherlands.
J Smeenk, Department of Reproductive Medicine, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands.
M van Wely, Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology—NVOG Consortium 2.0, Amsterdam, The Netherlands.
M J C Eijkemans, Julius Center for Health Sciences and Primary Care, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands.
H L Torrance, Department of Gynaecology and Reproductive Medicine, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands.
F J M Broekmans, Department of Gynaecology and Reproductive Medicine, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands.
References
- Adamson G, Mouzon JD, Chambers G, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S, Kupka M. No Title [Internet]. 2016. https://secureservercdn.net/198.71.233.206/3nz.654.myftpupload.com/wp-content/uploads/ICMART-ESHRE-WR2016-FINAL-20200901.pdf (1 August 2021, date last accessed).
- Baird DT, Barri PN, Bhattacharya S, Devroey P, Evers JLH, Gianaroli L, Somigliana E, Tapanainen JS, Wely MV, Crosignani PG et al. Economic aspects of infertility care: a challenge for researchers and clinicians. Hum Reprod 2015;30:2243–2248. [DOI] [PubMed] [Google Scholar]
- Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril 2003;79:1317–1322. [DOI] [PubMed] [Google Scholar]
- Eijkemans MJC, Kersten FAM, Lintsen AME, Hunault CC, Bouwmans CAM, Roijen LV, Habbema JDF, Braat DDM. Cost-effectiveness of “immediate IVF” versus “delayed IVF”: a prospective study. Hum Reprod 2017;32:999–1008. [DOI] [PubMed] [Google Scholar]
- Farquhar C. Endometrial scratching: how much evidence do you need to stop offering this to women having in vitro fertilization? Fertil Steril 2019;111:1092–1093. [DOI] [PubMed] [Google Scholar]
- Fauser BC. Towards the global coverage of a unified registry of IVF outcomes. Reprod Biomed Online 2019;38:133–137. [DOI] [PubMed] [Google Scholar]
- Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves—facts, fallacies and frequently asked questions. Health Econom 2004;13:405–415. [DOI] [PubMed] [Google Scholar]
- Geyter C, De Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V et al. ART in Europe, 2014: results generated from European registries by ESHRE. Hum Reprod 2018;33:1586–1601.30032255 [Google Scholar]
- Goldhaber-Fiebert JD, Brandeau ML. Evaluating cost-effectiveness of interventions that affect fertility and childbearing. Med Decis Making 2015;35:818–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkaart-van Roijen L, van der Linden N, Bouwmans C, Kanters T, Swan Tan S. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Diemen, The Netherlands: Zorginstituut Nederland, 2015. [Google Scholar]
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health 2013;16:231–250. [DOI] [PubMed] [Google Scholar]
- King’s Fertility. Full Price List V2.2. 2019. https://www.kingsfertility.co.uk/fertility-treatment-cost/ (27 July 2020, date last accessed).
- Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, Wilkinson J, Sadler L, Gupta D, Strandell A et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med 2019;380:325–334. [DOI] [PubMed] [Google Scholar]
- Lensen S, Sadler L, Farquhar C. Endometrial scratching for subfertility: everyone’s doing it. Hum Reprod 2016;31:1241–1244. [DOI] [PubMed] [Google Scholar]
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733–744. [DOI] [PubMed] [Google Scholar]
- Mol BW, Barnhart KT. Scratching the endometrium in in vitro fertilization-time to stop. N Engl J Med 2019;380:391–392. [DOI] [PubMed] [Google Scholar]
- Nastri C, Lensen S, Gibreel A, Raine-Fenning N, Ferriani R, Bhattacharya S, Martins W. Endometrial injury in women undergoing assisted reproductive techniques (review) summary of findings for the main comparison. Cochrane Database Syst Rev 2015; doi: 10.1002/14651858.CD009517.pub3. [DOI] [PubMed] [Google Scholar]
- NHS. (n.d.). National Schedule of NHS costs Year 2018-19 V2. https://www.england.nhs.uk/national-cost-collection/ (27 July 2020, date last accessed).
- OECD. Health at a Glance 2013 [Internet]. 2013. https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2013_health_glance-2013-en (3 December 2020, date last accessed).
- StatLine (Centraal Bureau voor Statistiek). Jaarmutatie consumentenprijsindex; vanaf 1963 (Year mutations consumer price index; as of 1963). https://opendata.cbs.nl/statline/#/CBS/nl/dataset/70936NED/table?fromstatweb (3 August 2020, date last accessed).
- University Hospitals Coventry and Warwickshire. Fee Schedule. 2019. https://www.uhcw.nhs.uk/download/clientfiles/files/Price List 2019 (GEN-PI-000211V31).doc (27 July 2020, date last accessed).
- van Hoogenhuijze NE, Kasius JC, Broekmans FJM, Bosteels J, Torrance HL. Endometrial scratching prior to IVF; does it help and for whom? A systematic review and meta-analysis. Hum Reprod Open 2019;2019:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoogenhuijze NE, Mol F, Laven JSE, Groenewoud ER, Traas MAF, Janssen CAH, Teklenburg G, Bruin JD, Oppenraaij RV, Maas JWM et al. Endometrial scratching in women with one failed IVF/ICSI cycle—outcomes of a randomised controlled trial (SCRaTCH). Hum Reprod 2020;36:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoogenhuijze N, Torrance H, Broekmans F, Lensen S, Farquhar C, van Hoogenhuijze N, Torrance H, Broekmans F, Lensen S, Farquhar C et al. International prospective register of systematic reviews study protocol: endometrial scratching in women trying to conceive from in vitro fertilization a collaborative individual participant data meta-analysis. Citation Review question Participants/population. 2017a;1–4. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017079120 (1 August 2021, date last accessed).
- van Hoogenhuijze NE, Torrance HL, Mol F, Laven JSE, Scheenjes E, Traas MAF, Janssen C, Cohlen B, Teklenburg G, Bruin JD et al. Endometrial scratching in women with implantation failure after a first IVF/ICSI cycle; does it lead to a higher live birth rate? The SCRaTCH study: a randomized controlled trial (NTR 5342). BMC Womens Health 2017b;17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilborg TC, Oudshoorn SC, Eijkemans MJC, Mochtar MH, Van Golde RJT, Hoek A, Kuchenbecker WKH, Fleischer K, De Bruin JP, Groen H et al. ; on behalf of the OPTIMIST study group. Individualized FSH dosing based on ovarian reserve testing in women starting IVF/ICSI: a multicentre trial and cost-effectiveness analysis. Human Reprod 2017;32:2485–2495. [DOI] [PubMed] [Google Scholar]
- van Tilborg T, Oudshoorn SC, Eijkemans MJC, Mochtar MH, Golde RV, Hoek A, Kuchenbecker WKH, Fleischer K, Bruin JD, Groen H et al. ; on behalf of the OPTIMIST study group. Individualized FSH dosing based on ovarian reserve testing in women starting IVF/ICSI: a multicentre trial and cost-effectiveness analysis. Hum Reprod 2017;32:2485–2495. [DOI] [PubMed] [Google Scholar]
- Vijgen S, Heesch FV, Obradovic M. Ziektelast in de praktijk. Dutch Natl Heal Care Inst. Diemen, The Netherlands: Zorginstituut Nederland, 2018, 1–34. https://www.zorginstituutnederland.nl/publicaties/rapport/2018/05/07/ziektelast-in-de-praktijk. [Google Scholar]
- Wijeysundera HC, Wang X, Tomlinson G, Ko DT, Krahn MD. Techniques for estimating health care costs with censored data: an overview for the health services researcher. Clinicoecon Outcomes Res 2012;4:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorginstituut Nederland. (n.d.). Farmacotherapeutisch Kompas (Dutch Formulary for medication). https://www.farmacotherapeutischkompas.nl/ (27 July 2020, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request.


