Abstract
STUDY QUESTION
Can transgender women cryopreserve germ cells obtained from their orchiectomy specimen for fertility preservation, after having used puberty suppression and/or hormonal treatment?
SUMMARY ANSWER
In the vast majority of transgender women, there were still immature germ cells present in the orchiectomy specimen, and in 4.7% of transgender women—who all initiated medical treatment in Tanner stage 4 or higher—mature spermatozoa were found, which would enable cryopreservation of spermatozoa or testicular tissue after having used puberty suppression and/or hormonal treatment.
WHAT IS KNOWN ALREADY
Gender affirming treatment (i.e. puberty suppression, hormonal treatment, and subsequent orchiectomy) impairs reproductive function in transgender women. Although semen cryopreservation is generally offered during the transition process, this option is not feasible for all transgender women (e.g. due to incomplete spermatogenesis when initiating treatment in early puberty, in case of inability to masturbate, or when temporary cessation of hormonal treatment is too disruptive). Harvesting mature spermatozoa, or testicular tissue harboring immature germ cells, from orchiectomy specimens obtained during genital gender-affirming surgery (gGAS) might give this group a chance of having biological children later in life. Previous studies on spermatogenesis in orchiectomy specimens showed conflicting results, ranging from complete absence of germ cells to full spermatogenesis, and did not involve transgender women who initiated medical treatment in early- or late puberty.
STUDY DESIGN, SIZE, DURATION
Histological and immunohistochemical analyses were performed on orchiectomy specimens from 214 transgender women who underwent gGAS between 2006 and 2018. Six subgroups were identified, depending on pubertal stage at initiation of medical treatment (Tanner stage 2-3, Tanner stage 4-5, adult), and whether hormonal treatment was continued or temporarily stopped prior to gGAS in each of these groups.
PARTICIPANTS/MATERIALS, SETTING, METHODS
All transgender women used a combination of estrogens and testosterone suppressing therapy. Orchiectomy specimen sections were stained with Mayer’s hematoxylin and eosin and histologically analyzed to assess the Johnsen score and the ratio of most advanced germ cell types in at least 50 seminiferous tubular cross-sections. Subsequently, immunohistochemistry was used to validate these findings using spermatogonia, spermatocytes or spermatids markers (MAGE-A3/A4, γH2AX, Acrosin, respectively). Possibilities for fertility preservation were defined as: preservation of spermatozoa, preservation of spermatogonial stem cells or no possibilities (in case no germ cells were found). Outcomes were compared between subgroups and logistic regression analyses were used to assess the association between the duration of hormonal treatment and the possibilities for fertility preservation.
MAIN RESULTS AND THE ROLE OF CHANCE
Mature spermatozoa were encountered in 4.7% of orchiectomy specimens, all from transgender women who had initiated medical treatment in Tanner stage 4 or higher. In 88.3% of the study sample orchiectomy specimens only contained immature germ cells (round spermatids, spermatocytes or spermatogonia, as most advanced germ cell type). In 7.0%, a complete absence of germ cells was observed, all these samples were from transgender women who had initiated medical treatment in adulthood. Cessation of hormonal treatment prior to gGAS did not affect the presence of germ cells or their maturation stage, nor was there an effect of the duration of hormonal treatment prior to gGAS.
LIMITATIONS, REASONS FOR CAUTION
Since data on serum hormone levels on the day of gGAS were not available, we were unable to verify if the transgender women who were asked to temporarily stop hormonal treatment 4 weeks prior to surgery actually did so, and if people with full spermatogenesis were compliant to treatment.
WIDER IMPLICATIONS OF THE FINDINGS
There may still be options for fertility preservation in orchiectomy specimens obtained during gGAS since a small percentage of transgender women had full spermatogenesis, which could enable cryopreservation of mature spermatozoa via a testicular sperm extraction procedure. Furthermore, the vast majority still had immature germ cells, which could enable cryopreservation of testicular tissue harboring spermatogonial stem cells. If maturation techniques like in vitro spermatogenesis become available in the future, harvesting germ cells from orchiectomy specimens might be a promising option for those who are otherwise unable to have biological children.
STUDY FUNDING/COMPETING INTEREST
None.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: transgender women, orchiectomy, fertility preservation, testicular tissue, spermatogenesis, hormonal treatment, azoospermia, germ cells
Introduction
Gender dysphoria refers to the distress experienced by people with an incongruence between their sex assigned at birth and their gender identity (APA, 2013). People assigned male at birth with a female gender identity are referred to as transgender women.
Many transgender women seek medical treatment to avoid (further) masculinization and induce feminization, and hereby align their physical characteristics with their gender identity. The preferred treatment protocol depends on the person’s age at time of start of medical treatment. For adolescents (<18 years), treatment can be initiated when a person reaches puberty (Tanner stage 2 or higher, determined by the development of secondary sex characteristics). It aims to suppress further pubertal development by administration of a gonadotropin-releasing hormone agonist (GnRHa) which reversibly inhibits the production of sex hormones. Hereby, adolescents have more time to explore options and to live in the experienced gender before deciding whether or not to proceed with additional, sometimes irreversible, treatments. At the age of approximately 16 years, treatment can be supplemented with estrogens to induce the development of female secondary sex characteristics (Hembree et al., 2017). For transgender women presenting at adult age (≥18 years), treatment usually does not consist of a phase of hormone suppression only, but immediately involves a combination of anti-androgens and estrogens, to achieve feminization. The combination of testosterone suppressing therapy and estrogen supplementation is referred to as gender affirming hormonal treatment (GAHT). Transgender women of 18 years or older who have used GAHT for at least one year, can opt for genital gender-affirming surgery (gGAS) if no surgical contraindications are present. gGAS may comprise vaginoplasty, gender confirming vulvoplasty or bilateral orchiectomy, depending on the desires of the individual (van der Sluis et al., 2021).
The use of testosterone suppressing therapy results in a severely impaired reproductive function, since spermatogenesis—the differentiation of spermatogonial stem cells into spermatozoa—requires adequate levels of intratesticular testosterone (Adeleye et al., 2019). This reproductive loss is permanent after gGAS. Although gender affirming treatment significantly improves quality of life, reproductive loss may be an unwanted consequence (Auer et al., 2018; Chen et al., 2018; Vyas et al., 2020). Therefore, it is important that (future) desire for biological children and the options for fertility preservation are discussed and offered prior to the start of medical treatment (Hembree et al., 2017).
The currently available option for fertility preservation in transgender women is cryopreservation of spermatozoa from a semen sample, obtained through ejaculation. Cryopreservation of surgically obtained spermatozoa through testicular sperm extraction (TESE) may serve as an alternative for those who are unable to ejaculate or in case of azoospermia (Wallace et al., 2014).
A complicating factor for contemporary fertility preservation in transgender female adolescents is the requirement of complete spermatogenesis, which only develops from Tanner stage 3 onwards, under the influence of increasing intratesticular testosterone levels. If puberty suppression is started in Tanner stage 2, full spermatogenesis is usually not present yet and therefore preservation of spermatozoa is not possible (Brik et al., 2019). The equipoise of commencing medical treatment to avoid progression of puberty and delaying treatment to enable semen cryopreservation as only option for biological children may be stressful, as puberty is accompanied by irreversible and often unwanted physical changes such as a lowering of the voice and facial hair growth. Severe genital dysphoria may pose another barrier for fertility preservation, since semen cryopreservation requires masturbation which is non-negotiable for some young transgender women (Brik et al., 2019). In addition, TESE, the currently available alternative to obtain spermatozoa for cryopreservation requires invasive procedures including surgery and (general) anesthesia.
For transgender women, cryopreservation of germ cells harvested from testicular tissue obtained during gGAS may serve as an alternative to keep the option for genetically related offspring open. How these germ cells can be used for procreation depends on their maturation phase. Spermatozoa can directly be used for ART. However, the use of immature germ cells relies on the feasibility of maturation techniques outside the human body, such as in vitro spermatogenesis. Unfortunately, complete in vitro spermatogenesis has only been successfully demonstrated in mouse models and is still unsuccessful in humans (Sato et al., 2011). If in vitro spermatogenesis becomes available in the future, cryopreservation of testicular tissue containing spermatogonial stem cells might be a promising option for fertility preservation in those who are otherwise unable to retain the possibility of having genetically related offspring.
Currently, limited data are available on the effect of GAHT on testicular histology and the most advanced germ cell type that can be harvested from testicular tissue obtained at time of gGAS. Previous studies conducted on this topic showed varying proportions of hyalinization of seminiferous tubules as well as conflicting results regarding spermatogenesis, ranging from a complete absence of germ cells to full spermatogenesis (Schneider et al., 2017; Matoso et al., 2018). Moreover, none of these studies focused on people who initiated medical treatment in early puberty.
The primary aim of this study is to evaluate the influence of puberty suppression and/or GAHT on exocrine testicular function, by determining the most advanced germ cell type in orchiectomy specimens obtained during gGAS. We aim to compare the outcome between people who started medical treatment as adult (≥18 years) and those who started as adolescent in early puberty (Tanner stage 2-3) or late puberty (Tanner stage 4-5). In addition, we will assess the influence of discontinuation of medical treatment 4 weeks prior to gGAS in each of these groups, and the association between the duration of hormonal treatment and the possibilities for fertility preservation. Hereby, we will get insights in the options for fertility preservation in orchiectomy specimens obtained during gGAS after having used puberty suppression and/or hormonal treatment.
Materials and methods
Study population and clinical data collection
For this study, we used orchiectomy specimens of transgender women who underwent bilateral orchiectomy combined with vaginoplasty at the Center of Expertise on Gender Dysphoria of Amsterdam UMC between 2006 and 2019. All participants provided written permission for the use of their body material and clinical data for research purposes. The Ethical Review Board of the Amsterdam UMC, location VUMC provided approval for conducting this study (METC2014322).
A total of 788 transgender women were identified. Data on medical history, age and Tanner stage at start of medical treatment, documented hormone use, date of gGAS, alcohol consumption, smoking, drug use, BMI at time of gGAS and last known serum hormone levels before gGAS, were collected from the medical files. Transgender women were categorized according to age and Tanner stage at initiation of medical treatment (Tanner stage 2-3, Tanner stage 4-5 or ≥18 years). Transgender women operated before 2017 discontinued GAHT 4 weeks prior to surgery, because of a presumed increased risk of perioperative thrombosis. As evidence suggested this risk is negligible, GAHT is continued in the perioperative period since July 2017. Six subgroups were created based on Tanner stage/age at start of medical treatment and continuation/discontinuation of GAHT prior to gGAS. People with an unknown age or Tanner stage at time of initiation of medical treatment were excluded. Other exclusion criteria were hard drug use, cryptorchidism, a medical history of receiving chemotherapy or genetic disorders which can all possibly impair spermatogenesis. Lastly, since the vast majority used estrogens combined with either triptorelin, or cyproterone acetate, people who used estrogen monotherapy and those who used spironolactone as anti-androgenic treatment were excluded to create a homogeneous study population. A maximum number of 80 transgender women were enrolled per group as this was deemed sufficient to answer the study questions. A random sample was drawn from groups that exceeded 80 individuals using STATA Statistical Software, version 15.1 (Statacorp, College Station, TX, USA). In total, 263 transgender women were selected for inclusion in the study cohort.
Testicular tissue preparation and analysis
Preparation for histology
Testicular tissue was obtained from the biobank of the Pathology Department of Amsterdam UMC, where orchiectomy specimens, obtained during gGAS, were stored after histopathological analysis for clinical purposes. Upon arrival at the Pathology Department, the orchiectomy specimens were fixed in 4% w/v paraformaldehyde and embedded in paraffin. For this study, seven slices of 5 μm thickness of one testicle were sectioned and mounted on microscope slides. From one slide of each specimen paraffin sections were deparaffinized and subsequently stained with Mayer’s hematoxylin and eosin, and at least one other slide was used for immunohistochemistry to confirm germ cell subtypes.
Histological analysis
Histological examination was conducted using a bright field microscope (Olympus BX41, OM Digital Solutions Americas, Bethlehem, PA, USA). From each specimen, at least 50 seminiferous tubules per slide were analyzed to assess spermatogenesis by determining the most advanced germ cell type from each seminiferous tubular cross-section based on their location within the tubule and nuclear morphology. The Modified Johnsen’s scoring system was used to assign a score to each tubule, and per slide a mean Johnsen’s score was calculated. The Modified Johnsen’s scoring system involves a 10-point Likert scale where score 1 corresponds to complete sclerosis without recognizable seminiferous epithelium, and score 10 implies the presence of more than 10 elongated spermatids without immature and apoptotic cells in the lumen (Supplementary Table SI) (Johnsen, 1970).
After assessment of spermatogenesis, overall testicular histology was assessed including the presence of a lumen in the seminiferous tubules and rate of seminiferous tubule hyalinization. The lumen was categorized as open, half-open or absent. Hyalinization was defined as a hyaline area separating the peritubular layer from the basal membrane of the seminiferous tubule.
Preparation for immunohistochemistry
In order to validate our findings, a second slide of each specimen was analyzed using immunohistochemistry. The primary antibodies were chosen based on the most advanced germ cell type that was identified during histological analysis, or on uncertainty regarding the presence of a germ cell type.
For the detection of spermatogonia, slides were stained for spermatogonial marker MAGE-A3/A4 using mouse monoclonal Anti-Mage A3/A4 antibody (clone 57B; Merck Millipore, Germany). Endogenous peroxidase activity was inactivated with 0.3% H2O2/phosphate-buffered saline (PBS) for 10 min at room temperature in the dark. Non-specific binding sites were then blocked with Superblock (ScyTek Lab, USA) for 1 h at room temperature in a humid slide box. Sections were subsequently incubated overnight at 4°C with Anti-Mage A3/A4 antibody diluted 1:2000 in BrightDiluent (Immunologic, the Netherlands). The next day, all slides were washed three times with PBS followed by 30 min incubation with Powervision goat-anti Mouse/Rabbit poly-horseradish peroxidase (DPVO110HRP, Immunologic, the Netherlands) secondary antibody at room temperature. After washing, the signal was visualized using Bright-DAB (3,3′-diaminobenzidine, Immunologic, the Netherlands) after which the sections were counterstained with Mayer’s hematoxylin. Finally, after dehydration in increasing ethanol concentrations and xylene, the slides were encapsulated with glass coverslips using Entellan® (Merck Millipore, Germany) for further microscopic analysis.
For the detection of spermatocytes, slides were stained for γH2AX using mouse monoclonal Anti-phospho-Histone H2A.X (Merck Millipore, Germany) antibody. Antigen retrieval was carried out by boiling tissue sections in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, pH = 9.0). The buffer was first heated until boiling in the microwave for 3 min at maximum Watt. After cooling down for 2 min at room temperature, the buffer was heated again in the microwave for 12 min at minimum Watt. Non-specific binding sites were blocked with 5% bovine serum albumin (BSA)/PBS/0.5% Triton X-100. This was followed by overnight incubation at 4°C with Anti-phospho-Histone H2A.X diluted 1:150 in 1% BSA/PBS/0.05% Tween. After incubation of the primary antibody, the same steps were performed as for the detection of spermatogonia with MAGE-A3/A4.
For the detection of round spermatids and spermatozoa, slides were stained for the presence of their Acrosin cap using rabbit polyclonal Acrosin antibody (ThermoFisher, PA5-61804). Antigen retrieval was carried out by boiling tissue sections in 0.01 M sodium citrate buffer (tri‐sodium citrate dihydrate Na3C6H5O7.2H2O, pH 6.0). The buffer was first heated until boiling in the microwave for 3 min at maximum Watt. After cooling down for 2 min at room temperature, the buffer was heated again in the microwave for 10 min at minimum Watt. Subsequently, the buffer was cooled down for 10 min at room temperature and placed under running tap water. After these steps, a standard immunohistochemical preparation protocol was followed, as described above.
For all three antibodies, slides with testicular tissue from a prostate cancer patient with normal spermatogenesis served as a positive control. Negative controls were carried out by replacing the first antibody by isotype IgG (Supplementary Fig. S1).
Immunohistochemical analysis
The immunohistochemically stained slides were examined using a bright field microscope (Olympus BX41) and assessed on the presence of the specifically targeted germ cell type. Outcome was then used to validate the Modified Johnsen’s scoring of the histologically analyzed slide of that same specimen. Results from the immunohistochemically stained slides were preferred if there was a difference between the two.
Statistical analyses
After completion of histological and immunohistochemical analyses, results were linked to clinical data and descriptive analyses were conducted for the total cohort and the six subgroups. Data are presented as means (SD) when normally distributed, as medians with interquartile ranges (IQRs) when non-normally distributed, or as numbers with percentage.
Progress of spermatogenesis, determined by the presence of the most advanced germ cell type per orchiectomy specimen, was used as main outcome measurement (no germ cells, spermatogonia, spermatocytes, round spermatids or spermatozoa). Secondary outcome measurements included mean Johnsen score per orchiectomy specimen, the degree of hyalinization and presence of a lumen.
To assess the possibilities for fertility preservation three categories were defined: preservation of spermatozoa; preservation of spermatogonial stem cells for those with round spermatids, spermatocytes or spermatogonia as most advanced germ cell type; and no possibilities for those with a complete absence of germ cells. Outcome was expressed as proportion with 95% confidence interval (95% CI) and compared between people who started medical treatment as an adult (>18 years) and those who started as adolescent in early puberty (Tanner stage 2-3) or late puberty (Tanner stage 4-5) (Newcombe, 1998). Since some categories contained no observations, we were not able to perform statistical tests. Therefore, differences between groups are shown in a figure. To assess the effect of cessation of GAHT prior to surgery, Fisher’s exact tests were used to compare outcome within each pubertal stage at initiation of medical treatment. The significance level was set at P < 0.05, and all tests were two-sided.
Lastly, logistic regression analyses were performed to assess the association between the duration of medical treatment and the possibility for preservation of spermatozoa, as well as the possibility for preservation of spermatogonial stem cells. Since the duration of medical treatment prior to gGAS, as well as progress of spermatogenesis both might be dependent on the age at start of medical treatment, a correction was performed for this factor. Odds ratios (ORs) with 95% CI were calculated.
All statistical analyses were performed using STATA Statistical Software, version 15.1 (Statacorp, College Station, TX, USA).
Results
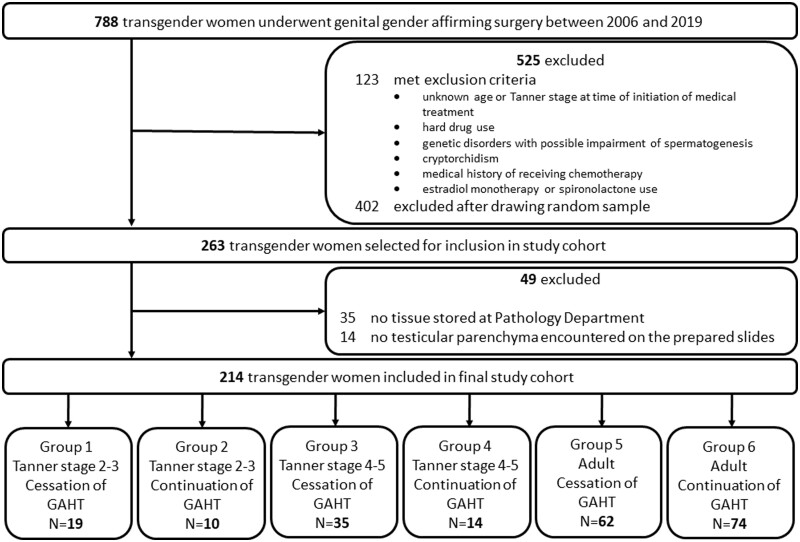
Initially, 263 transgender women were selected for inclusion in the study cohort. A total of 35 individuals were excluded when, upon preparation for analysis of the orchiectomy specimens, it became evident that for these transgender women no tissue was stored at the Pathology department of Amsterdam UMC. Another 14 transgender women were excluded because no testicular parenchyma was encountered on the prepared slides. Therefore, the final cohort consisted of 214 transgender women divided into 6 subgroups (Fig. 1).
Figure 1.
Study flowchart. GAHT, gender affirming hormonal treatment.
Characteristics at time of gGAS are presented in Table I. Mean age at gGAS was 29.6 years (SD 12.4) and was lower in people who started medical treatment in adolescence compared to those who started medical treatment in adulthood. Since adolescents started medical treatment with puberty suppressive therapy and had to wait until reaching the age of 18 years before being able to undergo gGAS, prior medical treatment duration was longer in the adolescent subgroups compared to those who initiated treatment at adult age. Different estradiol formulations were prescribed, including estradiol patches (50–150 µg/24 h twice weekly), estradiol gel (0.75–3.0 mg daily) and oral estradiol valerate or hemihydrate (2–6 mg daily). Testosterone suppressing therapy consisted of triptorelin injections (3.75 mg i.m./s.c. every 4 weeks or 11.25 mg i.m. every 12 weeks) for those who initiated treatment as adolescent, and cyproterone acetate (25–100 mg daily) for those who initiated treatment as adult. The last known serum hormone levels, median 189 days (IQR 96–340) before gGAS, showed that testosterone was adequately suppressed (median 0.7 nmol/l, IQR 0.5–1.0) and estradiol levels were in the female range (median 193 pmol/l, IQR 120–307). Furthermore, LH and FSH levels were suppressed. In transgender women with a cessation of GAHT 4 weeks prior to gGAS, estradiol levels were lower and testosterone and LH levels were higher, compared to those who continued GAHT until gGAS.
Table I.
Baseline characteristics at time of genital gender affirming surgery (gGAS).
| Total (n = 214) | Adolescent |
Adolescent |
Adult (n = 136) |
||||
|---|---|---|---|---|---|---|---|
| Tanner stage 2-3 (n = 29) |
Tanner stage 4-5 (n = 49) |
||||||
| Cessation of GAHT (n = 19) | Continuation of GAHT (n = 10) | Cessation of GAHT (n = 35) | Continuation of GAHT (n = 14) | Cessation of GAHT (n = 62) | Continuation of GAHT (n = 74) | ||
| Age (years)—mean (SD) | 29.6 (12.4) | 19.0 (1.5) | 19.6 (1.9) | 19.7 (1.2) | 19.3 (0.7) | 34.5 (12.3) | 36.2 (12.2) |
| Alcohol | |||||||
| Drinker—% (n) | 44 (82) | 43 (6) | 30 (3) | 60 (18) | 21 (3) | 56 (26) | 35 (26) |
| Non-drinker—% (n) | 56 (106) | 57 (8) | 70 (7) | 40 (12) | 79 (11) | 44 (20) | 65 (48) |
| Unknown—n | 26 | 5 | 0 | 5 | 0 | 16 | 0 |
| Smoking | |||||||
| Smoker—% (n) | 7 (12) | 0 | 0 | 8 (2) | 0 | 22 (10) | 0 |
| Non-smoker—% (n) | 93 (171) | 100 (15) | 100 (10) | 92 (24) | 100 (14) | 78 (34) | 100 |
| Unknown—n | 31 | 4 | 0 | 9 | 0 | 18 | 0 |
| Cannabis use | |||||||
| Yes—% (n) | 3 (5) | 0 | 0 | 4 (1) | 7 (1) | 6 (2) | 1 (1) |
| No—% (n) | 97 (166) | 100 (15) | 100 (10) | 96 (24) | 93 (13) | 94 (31) | 99 (73) |
| Unknown—n | 43 | 4 | 0 | 10 | 0 | 29 | 0 |
| BMI (kg/m2)—mean (SD) | 23.1 (3.3) | 22.0 (3.3) | 23.2 (2.8) | 21.6 (3.6) | 20.9 (3.6) | 23.9 (2.9) | 23.8 (3.0) |
| Mean duration of medical treatment (years) ∼ —(SD) | 3.3 (2.0) | 5.9 (1.4) | 6.8 (1.3) | 4.1 (1.8) | 2.8 (0.6) | 2.8 (1.9) | 2.3 (1.2) |
| Testosterone suppression | |||||||
| Triptorelin injections—% (n) | 36 (78) | 100 (19) | 100 (10) | 100 (35) | 100 (14) | 0 | 0 |
| Cyproterone acetate—% (n) | 64 (136) | 0 | 0 | 0 | 0 | 100 (62) | 100 (74) |
| Estrogen supplementation | |||||||
| Transdermal formulation—% (n) | 25 (54) | 11 (2) | 10 (1) | 0 | 0 | 40 (25) | 35 (26) |
| Oral formulation—% (n) | 75 (160) | 89 (17) | 90 (9) | 100 (35) | 100 (14) | 60 (37) | 65 (48) |
| Serum hormone levels before gGAS—Median (IQR)^ | |||||||
| Testosterone (nmol/) | 0.7 (0.5–1.0) | 1.0 (0.8–1.0) | 0.6 (0.5–0.8) | 1.0 (0.6–1.2) | 0.6 (0.5–1.1) | 0.7 (0.5–1.0) | 0.5 (0.5–0.8) |
| Estradiol (pmol/l) | 193 (120–307) | 95 (43–332) | 160 (141–392) | 120 (82–220) | 222 (100–281) | 219 (130–282) | 237 (151–341) |
| LH (U/l) | 0.1 (0.1–0.3) | 0.2 (0.1–0.4) | 0.3 (0.2–0.5) | 0.3 (0.2–0.4) | 0.2 (0.2–0.4) | 0.1 (0.1–0.3) | 0.1 (0.1–0.1) |
| FSH (U/l) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.4 (0.4–0.5) | 0.2 (0.1–0.5) | – | 0.3 (0.1–0.5) | 0.8 (0.1–3.0) |
GAHT, gender affirming hormone treatment; IQR, interquartile range.
Including GnRH agonist use, if applicable.
^Data were available for 201 (testosterone and LH), 200 (estradiol), and 53 (FSH) transgender women, respectively.
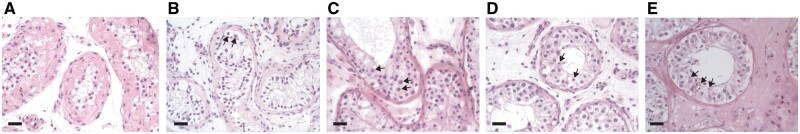
In 10 transgender women (4.7%) some seminiferous tubules contained full spermatogenesis, all of whom had initiated medical treatment in Tanner stage 4 or higher and it occurred in both the group that had continued GAHT until gGAS and in the group that had discontinued four weeks prior to gGAS (Table II, Fig. 2E). Complete absence of germ cells was encountered in 15 transgender women (7.0%) (Fig. 2A), all of whom had initiated medical treatment in adulthood. Also, mean Johnsen’s scores were lowest in the adult cohort. In the subgroup of transgender women who initiated medical treatment in Tanner stage 2 or 3, all specimens showed immature germ cells of which spermatogonia were most commonly observed (60–79%) (Fig. 2B–D). Supplementary Table SII shows the Modified Johnsen’s score for each individual separately.
Table II.
Results of histological and immunohistochemical analyses of orchiectomy specimens.
| Total (n = 214) | Adolescent |
Adolescent |
Adult (n = 136) |
||||
|---|---|---|---|---|---|---|---|
| Tanner stage 2–3 (n = 29) |
Tanner stage 4–5 (n = 49) |
||||||
| Cessation of GAHT (n = 19) | Continuation of GAHT (n = 10) | Cessation of GAHT (n = 35) | Continuation of GAHT (n = 14) | Cessation of GAHT (n = 62) | Continuation of GAHT (n = 74) | ||
| Spermatozoa | 4.7 (10) | 0 (0) | 0 (0) | 6 (2) | 22 (3) | 6 (4) | 1 (1) |
| Round spermatids | 0.5 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 0 (0) |
| Spermatocytes | 21.5 (46) | 21 (4) | 40 (4) | 31 (11) | 14 (2) | 23 (14) | 15 (11) |
| Spermatogonia | 66.3 (142) | 79 (15) | 60 (6) | 63 (22) | 64 (9) | 61 (38) | 70 (52) |
| No germ cells | 7.0 (15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (5) | 14 (10) |
|
| |||||||
| Mean Johnsen’s score—(SD) | 2.5 (0.8) | 2.6 (0.3) | 2.7 (0.4) | 2.8 (0.8) | 3.2 (1.4) | 2.5 (0.8) | 2.3 (0.6) |
| Hyalinization | 75.2 (161) | 47 (9) | 40 (4) | 63 (22) | 79 (11) | 76 (47) | 92 (68) |
| Lumen | |||||||
| Open | 8.4 (18) | 0 (0) | 0 (0) | 3 (1) | 22 (3) | 18 (11) | 4 (3) |
| Half-open | 25.2 (54) | 26 (5) | 10 (1) | 31 (11) | 14 (2) | 26 (16) | 26 (19) |
| Absent | 66.4 (142) | 74 (14) | 90 (9) | 66 (23) | 64 (9) | 56 (35) | 70 (52) |
Data are % (n) unless stated otherwise.
Figure 2.
Orchiectomy specimens with their most advanced germ cell type. (A) No germ cells present. (B) Spermatogonia. (C) Spermatocytes. (D) Round spermatids. (E) Spermatozoa. Arrows indicate most advanced germ cells. Bar represents 20 µm.
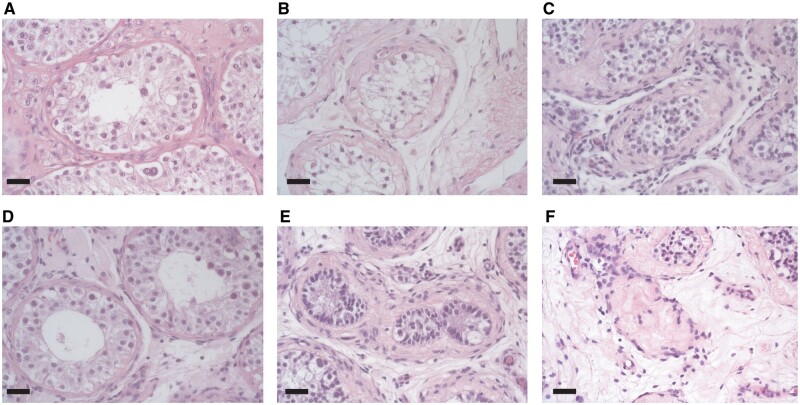
Hyalinization of seminiferous tubules was observed in 161 orchiectomy specimens (75.2%) and was most common in the adult subgroup (Fig. 3E and F). An open or half-open lumen of the seminiferous tubule was encountered in 8.4% and 25.2% of the orchiectomy specimens (Fig. 3A and B), respectively. The complete absence of a lumen was most common in those who initiated treatment in Tanner stage 2 or 3 (Fig. 3C).
Figure 3.
Different aspects of lumen and degrees of hyalinization of seminiferous tubules. (A) Open lumen. (B) Half-open lumen. (C) Absent lumen. (D) No hyalinization. (E) Mild hyalinization. (F) Severe hyalinization. Bar represents 20 µm.
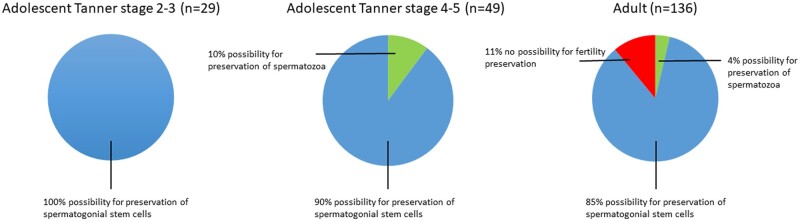
When comparing the options for fertility preservation, we found that for some transgender women it would still have been possible to harvest mature spermatozoa from testicular tissue obtained during gGAS (Fig. 4). This was the case for 4% (95% CI 2–8) of the adult subgroup and 10% (95% CI 4–22) of adolescents in the Tanner stage 4-5 subgroup, compared to 0% in the Tanner stage 2-3 subgroup. For 100% of people in the Tanner stage 2-3 subgroup, 90% (95% CI 78–96) of people in the Tanner stage 4-5 subgroup and 85% (95% CI 78–90) of the adult subgroup, preservation of testicular tissue containing spermatogonial stem cells would have been their only option for fertility preservation. Furthermore, for 11% (95% CI 7–17) of the adult subgroup no options for fertility preservation would have been available, compared to 0% of the two adolescent subgroups. No statistically significant differences were found between those who had continued GAHT until gGAS and those with four weeks cessation of GAHT prior to gGAS.
Figure 4.
Comparison of the possibilities for fertility preservation between people who started medical treatment as adolescent in early puberty (Tanner stage 2-3) or late puberty (Tanner stage 4-5), and those who started as an adult (>18 years).
Lastly, logistic regression analyses showed no association between the duration of GAHT and the possibility for preservation of spermatozoa (OR 0.75, 95% CI 0.47–1.18) or spermatogonial stem cells (OR 1.03, 95% CI 0.81–1.31).
Discussion
The results of our study imply that there may be options for fertility preservation for transgender women who are unable to pursue semen cryopreservation, by using testicular tissue from orchiectomy specimens obtained during gGAS. In a small percentage of transgender women who initiated medical treatment in Tanner stage 4 or higher, complete spermatogenesis was observed in the orchiectomy specimen. For this group, it would theoretically be possible to perform TESE and cryopreserve the harvested spermatozoa from this specimen. Furthermore, the vast majority of transgender women still had immature germ cells in their orchiectomy specimen. This is the first study to report on people who initiated medical treatment in Tanner stage 2-3, and it was found that in 100% of their orchiectomy specimens immature germ cells were present. If maturation techniques like in vitro spermatogenesis become available in the future, cryopreservation of testicular tissue containing spermatogonial stem cells might be a promising option for this group to retain the possibility to have biological children. A complete absence of germ cells was only observed in transgender women who commenced GAHT as adult. Cessation of GAHT prior to gGAS did not affect the possibilities for fertility preservation, neither was there an effect of the duration of GAHT prior to gGAS.
Although some previous studies have been conducted on the influence of GAHT on spermatogenesis and testicular architecture, this is the first study taking age and pubertal stage at time of initiation of medical treatment into account. Between 1970 and 1990, several small studies were conducted reporting on 4–11 transgender women per study (Rodriguez-Rigau et al., 1977; Lu and Steinberger, 1978; Payer et al., 1979; Sapino et al., 1987; Schulze, 1988; Venizelos and Paradinas, 1988). Therefore, no strong conclusions could be drawn, but results showed high proportions of tubular hyalinization and reduced spermatogenesis in all transgender women. The first large cohort study on this topic was performed in 2015 and assessed orchiectomy specimens of 108 transgender women from three clinics with different preoperative treatment protocols (6 weeks, 2 weeks or no discontinuation of GAHT prior to gGAS) (Schneider et al., 2015). Their results on testicular histology and spermatogenic state were highly heterogeneous and did not show a relation with treatment strategy. Remarkably, a high number of transgender women (24% of their study cohort) had complete spermatogenesis at time of gGAS. This finding was confirmed by Jiang et al. (2019) who even observed complete spermatogenesis in 40% of the 72 included transgender women. However, several other recent studies found lower percentages of complete spermatogenesis ranging from 0% to 11% of the study cohort (Jindarak et al., 2018; Kent et al., 2018; Matoso et al., 2018; Vereecke et al., 2021). It must be noted that hormonal and preoperative treatment protocols vary considerably within, and between, the different studies conducted on this topic. Therefore, for the current study, it was decided to only include transgender women who used estradiol in combination with testosterone suppressing therapy (triptorelin when initiated in adolescence, cyproterone acetate when initiated in adulthood), and to report results for those who continued GAHT until gGAS separate from those who discontinued four weeks prior to gGAS.
Since a study performed by Vereecke et al. (2021) also adhered strict in- and exclusion criteria that are similar to those in our adult subgroup, their results allow for the most accurate comparison. In addition, their method of analysis using immunohistochemistry to determine the most advanced germ cell type is similar to our study. In their cohort of 97 transgender women, 12.4% had a complete absence of germ cells which is in line with the observed 11% in our cohort. However, none of their orchiectomy specimens showed complete spermatogenesis, as opposed to 4% of orchiectomy specimens in the adult subgroup of our cohort. Vereecke et al. (2021) also assessed the relationship between serum hormone levels and spermatogenic state in their cohort. They found that higher serum testosterone levels were associated with more advanced maturation, and higher serum estradiol levels were associated with a lower number of spermatogonia. However, the hormone levels were not measured on the day of gGAS, but at the last visit in the outpatient clinic 91.0 (57.5–152.5) days before surgery (Vereecke et al., 2021). In contrast, Schneider et al. (2015) did collect serum and intratesticular testosterone levels on the day of gGAS but did not find an obvious correlation with spermatogenic state. In our gender identity clinic, hormone levels are not determined on the day of gGAS and laboratory results from the last visit in the outpatient clinic likely do not adequately reflect hormonal status during gGAS because of the preoperative cessation of GAHT 4 weeks prior to surgery. It was therefore decided not to assess this relationship in our cohort.
An interesting observation in the current study is that testicular histology and spermatogenesis seemed more negatively affected by GAHT in the adult subgroup compared to the adolescent subgroups, despite the lower mean duration of medical treatment in the former prior to gGAS. A higher percentage of hyalinization of the seminiferous tubules was observed in the adult subgroup, as well as a complete absence of germ cells in 15 orchiectomy specimens. The difference between the adult subgroup and the adolescent subgroups might be explained by age, lifestyle (a higher percentage of smokers and alcohol drinkers), higher dosages of estradiol or the use of cyproterone acetate instead of GnRHa as testosterone suppressing therapy. Whereas GnRHa only leads to inhibition of gonadotropin secretion, cyproterone acetate also has progestative effects and acts as a direct antagonist of the androgen receptor. It hereby inhibits the influence of androgens on the androgen-dependent organs, among which the testes. The latter might have more profound and irreversible effects on testicular tissue. Because of unwanted side-effects of cyproterone acetate (e.g. increased risk for meningioma), transgender women commencing GAHT in our clinic above the age of 18 years now receive GnRHa as testosterone suppressing therapy instead of cyproterone acetate. The potential consequence of irreversible infertility might be an extra reason to not prescribe cyproterone acetate anymore. In a future study, it would be interesting to assess if differences in testicular histology and spermatogenesis between adults and adolescents are still observed when they both receive GnRHa as testosterone suppressing therapy.
Cessation of GAHT prior to gGAS did not affect the possibilities for fertility preservation. In our study, the preoperative cessation of GAHT involved a period of four weeks, whereas the differentiation of spermatogonial stem cells into spermatozoa generally takes 10–12 weeks (Muciaccia et al., 2013). Therefore, the period of cessation was most likely not long enough to influence the options for fertility preservation. If transgender women would be willing to discontinue GAHT for at least 12 weeks prior to gGAS, this might positively influence the chances of finding mature spermatozoa in the orchiectomy specimen. Moreover, they could even consider an attempt for cryopreservation of spermatozoa from a semen sample, obtained through ejaculation. However, it is unknown if spermatogenesis can recover if GAHT is stopped and how much time is needed for this purpose. Furthermore, it should not be underestimated that cessation of GAHT will result in increased testosterone levels which is likely to have negative physical and psychological consequences, and that masturbation is often not an option in transgender women due to severe genital dysphoria. A disadvantage of spermatozoa that are harvested from testicular tissue, is that they are not suitable for a minimally invasive and inexpensive IUI and can only be used for ICSI (Ombelet et al., 2014). In addition, such ICSI treatments using surgically obtained spermatozoa are not always successful, since the cumulative ongoing pregnancy rate per cycle has been reported to be 22.8% and the live birth rate 22.3% (Meijerink et al., 2016). Therefore, cryopreservation of a semen sample prior to initiation of GAHT remains the preferred method of fertility preservation in transgender women and harvesting germ cells from orchiectomy specimens might only be considered an alternative in those for whom this is not an option.
The lumina of the seminiferous tubules in those who initiated medical treatment in Tanner stage 2-3 were all either half-open, or absent. This observation might be explained by the immaturity of testicular tissue in early puberty, since an open lumen develops parallel to the development of spermatogenesis under the influence of increasing intratesticular testosterone levels. The fact that germ cells were encountered in all orchiectomy specimens from transgender women who initiated medical treatment as adolescent, is reassuring. Decision-making about fertility can be very difficult for adolescents since their intellectual, emotional and social immaturity may impede assessment and prediction of future desires regarding fertility and family planning. A recent study among transgender youth showed that 67% of young transgender women expressed a desire for future parenthood, but only 7% indicated to be frustrated if biological parenthood would not be feasible (Chiniara et al., 2019). Another study, however, reported that 48% of transgender adolescents acknowledged that their desires regarding parenthood might change over time (Strang et al., 2017). Reduced levels of gender dysphoria and improved mental health might result in an improved capability to establish romantic relationships and consider future family building. Our observation that immature germ cells remain present in testicular tissue during GAHT suggest that transgender adolescents still have potential options for fertility preservation after initiation of treatment by cryopreserving testicular tissue from orchiectomy specimen obtained during gGAS.
Cryopreservation of testicular tissue containing spermatogonial stem cells is mostly offered to pre-pubertal boys with cancer, prior to undergoing gonadotoxic therapies such as chemo- and radiotherapy, but some clinics also offer this option to transgender adolescents (Pang et al., 2020). In the absence of complete spermatogenesis, the purpose of spermatogonial stem cell preservation in cisgender adolescents is to transplant these cells back into the testes years later, via injection into the rete testis space that is contiguous with all seminiferous tubules. Spermatogonial stem cells have the potential to colonize the testicular niche and regenerate spermatogenesis (David and Orwig, 2020). However, re-transplantation is not a feasible option for transgender women, as they will most likely use lifelong GAHT and many will undergo bilateral orchiectomy. Therefore, spermatogonial stem cell preservation will only be a viable method for fertility preservation in transgender women when other options for maturation become available, such as de novo testicular morphogenesis or in vitro spermatogenesis. Although these techniques are successful in animal models, they are still experimental and far from the clinical realm (Pelzman et al., 2020). Continuing research in this area will hopefully make these techniques available so that transgender adolescents, who are otherwise unable to have genetically related children, will be able to retain this possibility by cryopreserving testicular tissue containing spermatogonial stem cells. Furthermore, future research should focus on how GAHT influences the quality of germ cells and the safety of using cells harvested from orchiectomy specimens, for reproductive techniques. Lastly, it is important to examine how transgender women feel about fertility preservation options in orchiectomy specimens obtained during gGAS.
A limitation of this study is the lack of data on serum hormone levels on the day of gGAS. We were therefore unable to verify if the transgender women who were asked to temporarily stop hormonal treatment four weeks prior to surgery actually did so, and if people with complete spermatogenesis were compliant to treatment. However, the last known serum testosterone levels before gGAS were suppressed in all participants. Furthermore, despite our efforts to create a homogeneous study population, by excluding people who used estrogen monotherapy and those who used spironolactone as anti-androgenic treatment, participants still used varying formulations of estrogens and switched between different formulations over time. We were therefore unable to assess if different estrogen formulations have different effects on testicular histology and spermatogenesis. Strengths of our study include the large sample size of 214 transgender women, and the creation of six subgroups to allow for comparison between different preoperative protocols before gGAS and pubertal stage at initiation of medical treatment. Hereby, this study provides novel information about the influence of starting medical treatment in early puberty on testicular function, and its consequences for the possibilities for fertility preservation at time of gGAS. This is relevant because we are seeing a global increase of the number of referrals of adolescents to gender identity clinics (Handler et al., 2019; Kaltiala et al., 2020). At the same time, there is increasing controversy over the provision of GAHT to adolescents, with the negative effect on fertility often cited as an argument for limiting adolescents’ access to gender-affirming care (The Economist, 2020). Our observation that the spermatogonial stem cell pool is still intact in people who initiated GAHT during adolescence is therefore valuable information in this debate.
Conclusion
Counseling of transgender women about the effect of medical treatment on fertility and the currently available options for fertility preservation remains essential. However, for some transgender women with a wish for fertility preservation, there are barriers that prevent the use of semen cryopreservation. For example, some initiate medical treatment in early puberty before the development of complete spermatogenesis, some are unable to masturbate, and some feel that a temporary cessation of GAHT would be too psychologically and physically disruptive. The results of this study show that there may still be options for fertility preservation using orchiectomy specimens obtained during gGAS. In a small percentage of transgender women who initiated medical treatment in Tanner stage 4 or higher, spermatozoa could have been harvested from the orchiectomy specimen at time of gGAS. In addition, the vast majority (>85%) of transgender women in our cohort could still opt for cryopreservation of testicular tissue harboring spermatogonial stem cells. A complete absence of germ cells was only observed in a small number (7%) of transgender women in our cohort, who all commenced GAHT as adult. The possibilities for fertility preservation seem irrespective of preoperative cessation of GAHT and the duration of GAHT prior to gGAS.
Initiation of medical treatment in early pubertal adolescents (Tanner stage 2-3) limits the ability to retrieve mature spermatozoa that can directly be used for assisted reproductive techniques. However, if maturation techniques like in vitro spermatogenesis become available in the future, harvesting germ cells from orchiectomy specimens might be a promising option for those who are otherwise unable to have biological children.
Data availability
Part of the data underlying this article are available in the article and in its online supplementary material, the rest of the data will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
We would like to thank Cindy de Winter-Korver and Saskia van Daalen for their technical assistance during this project.
Authors’ roles
I.d.N.—conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript. C.L.M.—conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for intellectual content. A.M.—analysis and interpretation of data, critical revision of the manuscript for intellectual content. Y.S.—acquisition of data, analysis and interpretation of data, drafting of manuscript. E.M.H.—acquisition of data, drafting of manuscript. W.B.v.d.S.—acquisition of data, critical revision of the manuscript for intellectual content. S.E.H.—analysis and interpretation of data, critical revision of the manuscript for intellectual content. M.d.H.—conception and design, analysis and interpretation of data, critical revision of the manuscript for intellectual content. J.H.—conception and design, analysis and interpretation of data, critical revision of the manuscript for intellectual content. A.M.M.v.P.—conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for intellectual content. N.M.v.M.—conception and design, analysis and interpretation of data, critical revision of the manuscript for intellectual content. All authors approved the final version of the manuscript.
Funding
None.
Conflict of interest
None.
Contributor Information
I de Nie, Department of Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Center of Expertise on Gender Dysphoria, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Obstetrics and Gynecology, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
C L Mulder, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, University of Amsterdam, Amsterdam, The Netherlands.
A Meißner, Department of Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Center of Expertise on Gender Dysphoria, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Obstetrics and Gynecology, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Urology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Y Schut, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, University of Amsterdam, Amsterdam, The Netherlands.
E M Holleman, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, University of Amsterdam, Amsterdam, The Netherlands.
W B van der Sluis, Center of Expertise on Gender Dysphoria, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Plastic, Reconstructive and Hand Surgery, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
S E Hannema, Center of Expertise on Gender Dysphoria, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Pediatrics, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
M den Heijer, Department of Endocrinology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Center of Expertise on Gender Dysphoria, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
J Huirne, Department of Obstetrics and Gynecology, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
A M M van Pelt, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, University of Amsterdam, Amsterdam, The Netherlands.
N M van Mello, Center of Expertise on Gender Dysphoria, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Obstetrics and Gynecology, Amsterdam UMC, Amsterdam Reproduction & Development Research Institute, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
References
- Adeleye AJ, Reid G, Kao CN, Mok-Lin E, Smith JF. Semen parameters among transgender women with a history of hormonal treatment. Urology 2019;124:136–141. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- Auer MK, Fuss J, Nieder TO, Briken P, Biedermann SV, Stalla GK, Beckmann MW, Hildebrandt T. Desire to have children among transgender people in Germany: a cross-sectional multi-center study. J Sex Med 2018;15:757–767. [DOI] [PubMed] [Google Scholar]
- Brik T, Vrouenraets L, Schagen SEE, Meissner A, de Vries MC, Hannema SE. Use of fertility preservation among a cohort of transgirls in the Netherlands. J Adolesc Health 2019;64:589–593. [DOI] [PubMed] [Google Scholar]
- Chen D, Matson M, Macapagal K, Johnson EK, Rosoklija I, Finlayson C, Fisher CB, Mustanski B. Attitudes toward fertility and reproductive health among transgender and gender-nonconforming adolescents. J Adolesc Health 2018;63:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiniara LN, Viner C, Palmert M, Bonifacio H. Perspectives on fertility preservation and parenthood among transgender youth and their parents. Arch Dis Child 2019;104:739–744. [DOI] [PubMed] [Google Scholar]
- David S, Orwig KE. Spermatogonial stem cell culture in oncofertility. Urol Clin North Am 2020;47:227–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler T, Hojilla JC, Varghese R, Wellenstein W, Satre DD, Zaritsky E. Trends in referrals to a pediatric transgender clinic. Pediatrics 2019;144:e20191368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017;102:3869–3903. [DOI] [PubMed] [Google Scholar]
- Jiang DD, Swenson E, Mason M, Turner KR, Dugi DD, Hedges JC, Hecht SL. Effects of estrogen on spermatogenesis in transgender women. Urology 2019;132:117–122. [DOI] [PubMed] [Google Scholar]
- Jindarak S, Nilprapha K, Atikankul T, Angspatt A, Pungrasmi P, Iamphongsai S, Promniyom P, Suwajo P, Selvaggi G, Tiewtranon P. Spermatogenesis abnormalities following hormonal therapy in transwomen. Biomed Res Int 2018;2018:7919481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen SG. Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1970;1:2–25. [DOI] [PubMed] [Google Scholar]
- Kaltiala R, Bergman H, Carmichael P, de Graaf NM, Rischel KE, Frisen L, Schorkopf M, Suomalainen L, Waehre A. Time trends in referrals to child and adolescent gender identity services: a study in four Nordic countries and in the UK. Nord J Psychiatry 2020;74:40–44. [DOI] [PubMed] [Google Scholar]
- Kent MA, Winoker JS, Grotas AB. Effects of feminizing hormones on sperm production and malignant changes: microscopic examination of post orchiectomy specimens in transwomen. Urology 2018;121:93–96. [DOI] [PubMed] [Google Scholar]
- Lu CC, Steinberger A. Effects of estrogen on human seminiferous tubules: light and electron microscopic analysis. Am J Anat 1978;153:1–13. [DOI] [PubMed] [Google Scholar]
- Matoso A, Khandakar B, Yuan S, Wu T, Wang LJ, Lombardo KA, Mangray S, Mannan A, Yakirevich E. Spectrum of findings in orchiectomy specimens of persons undergoing gender confirmation surgery. Hum Pathol 2018;76:91–99. [DOI] [PubMed] [Google Scholar]
- Meijerink AM, Cissen M, Mochtar MH, Fleischer K, Thoonen I, de Melker AA, Meissner A, Repping S, Braat DD, van Wely M et al. Prediction model for live birth in ICSI using testicular extracted sperm. Hum Reprod 2016;31:1942–1951. [DOI] [PubMed] [Google Scholar]
- Muciaccia B, Boitani C, Berloco BP, Nudo F, Spadetta G, Stefanini M, de Rooij DG, Vicini E. Novel stage classification of human spermatogenesis based on acrosome development. Biol Reprod 2013;89:60. [DOI] [PubMed] [Google Scholar]
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17:857–872. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Dhont N, Thijssen A, Bosmans E, Kruger T. Semen quality and prediction of IUI success in male subfertility: a systematic review. Reprod Biomed Online 2014;28:300–309. [DOI] [PubMed] [Google Scholar]
- Pang KC, Peri AJS, Chung HE, Telfer M, Elder CV, Grover S, Jayasinghe Y. Rates of fertility preservation use among transgender adolescents. JAMA Pediatr 2020;174:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer AF, Meyer WJ 3rd, Walker PA. The ultrastructural response of human Leydig cells to exogenous estrogens. Andrologia 1979;11:423–436. [DOI] [PubMed] [Google Scholar]
- Pelzman DL, Orwig KE, Hwang K. Progress in translational reproductive science: testicular tissue transplantation and in vitro spermatogenesis. Fertil Steril 2020;113:500–509. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rigau LJ, Tcholakian RK, Smith KD, Steinberger E. In vitro steroid metabolic studies in human testes I: effects of estrogen on progesterone metabolism. Steroids 1977;29:771–786. [DOI] [PubMed] [Google Scholar]
- Sapino A, Pagani A, Godano A, Bussolati G. Effects of estrogens on the testis of transsexuals: a pathological and immunocytochemical study. Virchows Arch A Pathol Anat Histopathol 1987;411:409–414. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011;471:504–507. [DOI] [PubMed] [Google Scholar]
- Schneider F, Kliesch S, Schlatt S, Neuhaus N. Andrology of male-to-female transsexuals: influence of cross-sex hormone therapy on testicular function. Andrology 2017;5:873–880. [DOI] [PubMed] [Google Scholar]
- Schneider F, Neuhaus N, Wistuba J, Zitzmann M, Heß J, Mahler D, van Ahlen H, Schlatt S, Kliesch S. Testicular functions and clinical characterization of patients with gender dysphoria (GD) undergoing sex reassignment surgery (SRS). J Sex Med 2015;12:2190–2200. [DOI] [PubMed] [Google Scholar]
- Schulze C. Response of the human testis to long-term estrogen treatment: morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell Tissue Res 1988;251:31–43. [DOI] [PubMed] [Google Scholar]
- Strang JF, Jarin J, Call D, Clark B, Wallace GL, Anthony LG, Kenworthy L, Gomez-Lobo V. Transgender youth fertility attitudes questionnaire: measure development in nonautistic and autistic transgender youth and their parents. J Adolesc Health 2018;62:128–135. [DOI] [PubMed] [Google Scholar]
- The Economist. 2020. A new push to ban medical treatments for transgender children. The Economist. Washinton, DC. [Google Scholar]
- van der Sluis WB, de Nie I, Steensma TD, van Mello NM, Lissenberg-Witte BI, Bouman MB. Surgical and demographic trends in genital gender-affirming surgery in transgender women: 40 years of experience in Amsterdam. Br J Surg 2021; doi: 10.1093/bjs/znab213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venizelos ID, Paradinas FJ. Testicular atrophy after oestrogen therapy. Histopathology 1988;12:451–454. [DOI] [PubMed] [Google Scholar]
- Vereecke G, Defreyne J, Van Saen D, Collet S, Van Dorpe J, T'Sjoen G, Goossens E. Characterisation of testicular function and spermatogenesis in transgender women. Human Reproduction 2021;36:5–15. [DOI] [PubMed] [Google Scholar]
- Vyas N, Douglas CR, Mann C, Weimer AK, Quinn MM. Access, barriers, and decisional regret in pursuit of fertility preservation among transgender and gender-diverse individuals. Fertil Steril 2020:1029–1034. [DOI] [PubMed] [Google Scholar]
- Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol 2014;30:868–871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Part of the data underlying this article are available in the article and in its online supplementary material, the rest of the data will be shared on reasonable request to the corresponding author.